Introduction
Metastasis is an important driver of the high
mortality rate of patients with cancer (1). Research on the impact of abnormal tumor
cell metabolism on metastasis has gained attention in recent years.
Most cancer cells rely on aerobic glycolysis for energy generation,
which features high glucose uptake and high lactate production; a
phenomenon widely known as the Warburg effect (2). To avoid the accumulation of large
amounts of toxic lactic acid, cancer cells necessitate mechanisms
that actively transport lactate out of cells. Two transporters of
the monocarboxylate transporter (MCT) family, MCT1 and MCT4, have
been shown to be associated with lactate transport (3). Their localization on the plasma
membrane depends on the chaperone protein Basigin (CD147) (4).
Studies have shown that MCT1 and/or MCT4 are highly
expressed in various types of cancer tissue, such as breast, lung
and colorectal cancer tissues, and may serve as targets for cancer
intervention (5–10). Moreover, when tumors progress to a
higher grade or metastasis, a higher expression of MCT1 or MCT4 is
typically observed (11–13), which suggests that their expression
is associated with the migration or invasion of cancer cells. It
has also been suggested that excess lactic acid that is secreted by
cancer cells acidifies the tumor microenvironment and serves as a
signal to promote the invasion and migration of cancer cells
(14).
AZD3965, which is a specific inhibitor of MCT1 and
MCT2, has been reported to induce the accumulation of intracellular
lactate, which leads to the death of small cell lung cancer cells
(15). Non-specific inhibitors of
MCT transporters, including AR-C155858 and DIDS, can also block the
function of MCT1 or MCT4 and lead to the death of breast and lung
cancer cells, respectively (16,17).
Knocking down the expression of MCT4 was also been reported to
inhibit the proliferation of gastric cancer cells (18).
In previous years, it has been reported that the
expression of MCT in cancer cells is related to cell migration or
invasiveness, but the results have been inconsistent among
different studies. Studies have shown that knocking down the
expression or inhibiting the function of MCT1 or MCT4 decreases the
migration and invasion of breast cancer cells and nasopharyngeal
carcinoma cells (19,20). However, other studies have reported
that the knockdown of MCT1 or MCT4 expression is only associated
with invasion, but not migration (17,21).
Silencing or knocking down MCT expression is
difficult to standardize, since transfection is typically
incomplete and silencing is transient (22,23).
Moreover, the transfection procedure itself is harmful to the cells
due to the cytotoxicity of the transfection reagents (24). In this study, human MCT1 or MCT4 was
overexpressed via stable transfection in L929 cells to evaluate
their impact on cellular migration and invasion, which are two
major traits of metastasis (25).
Metastatic cancer cells are commonly characterized
by the enhancement of metalloproteinase (MMP) activity (26). Migration and invasion are regulated
by the classical hepatocyte growth factor (HGF)/c-Met or epidermal
growth factor (EGF)/EGF receptor (R)-mediated signal transduction
pathways (27,28). In the present study, the effects of
MCT1 or MCT4 high expression on the migratory and invasive
abilities of non-tumorigenic L929 cells were investigated.
Preliminary investigations into the molecular mechanisms of MCT4
overexpression on migration and invasion were also performed.
Materials and methods
Plasmids preparation
The coding regions of human MCT1, MCT4, MCT4-R278Q
or enhanced green fluorescent protein (EGFP) were synthesized with
EcoRI and HindIII restriction sites (Genewiz, Inc.). These gene
fragments and pcDNA3.0 vectors (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) were treated with EcoRI and HindIII
restriction endonuclease (Takara Bio, Inc.) at 37°C for 4 h and
connected using DNA ligase (Takara Bio, Inc.) at 16°C for 12 h. The
respective gene sequence post-recombination was confirmed by
Genewiz, Inc.
Transfection and generation of stable
monoclonal cells
L929 [CCL-1™; American Type Culture Collection
(ATCC)], is a non-carcinogenic murine cell line and was cultured in
Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS)
(Sigma-Aldrich; Merck KGaA) in T-flasks. EGFP-L929, MCT1-L929,
MCT4-L929 and MCT4-R278Q-L929 cells were established by
transfecting respective plasmids into host L929 cells via
electroporation. Electroporation was conducted at room temperature
in 0.4 cm gapped electroporation cuvettes (aluminum electrode; 1×1
cm) using an electroporator (Gene Pulser XcellTM; Bio-Rad
Laboratories, Inc.) according to manufacturer's instructions.
Briefly, 1 µg/µl plasmids and 1.0×107 L929 cells were
mixed in the electroporation cuvette at a total volume of 800 µl.
Cell medium used for transfection was CSC03 medium with an
osmolarity of 280–320 mOsm/kg (Vbiosci Co., Ltd.). Electroporation
condition used an exponential protocol and was set at 900 µF and
300 V. After electroporation, the entire content in the
electroporation cuvette was added into 200 ml of pre-warmed CSC03
medium with 10% FBS and mixed well. A total of 100 µl of the cell
suspension was then added into each well of 96-well plates
(Corning, Inc.), which corresponds to ~5,000 cells/well. Plates
were then incubated at 37°C for 24 h. An additional 100 µl of CSC03
medium with 10% FBS and 2 mg/ml G418 (Vazyme Biotech Co., Ltd.)
were added into each well. Plates are then returned to the
incubator for 2 to 3 weeks. Cells that formed a single colony in
the plate wells were scaled up into 24- and 6-well plates (Corning,
Inc.) in DMEM with 10% FBS. Clones were sub-cultured in 6-well
plates with 1:5 split ratio every 3–4 days. Clones were screened
for EGFP or MCT expression using flow cytometric analysis and
confirmed by immunoblotting and immunofluorescence assays. Clones
of interest were sub-cultured in T25 or T65 flasks (Corning, Inc.)
in DMEM with 10% FBS. Three high-expressing clones for each
transfected L929 cells were used for the subsequent
experiments.
Fluorescence-activated cell sorting
(FACS)
To screen for MCT expression, cells were detached
from 6-well plates with trypsin (Sigma-Aldrich; Merck KGaA) and
permeabilized with 0.1% Triton-X-100, as the primary antibody
targeted the intracellular portions of the MCTs. Cells were
incubated for 2 h at room temperature with primary antibodies
against MCT1 (1:1,000; cat. no. ab85021; Abcam) or MCT4 (1:1,000;
22787-1-AP; ProteinTech Group, Inc.), and then with secondary
fluorescein-conjugated goat anti-mouse IgG (1:5,000; cat. no.
A-11001; Thermo Fisher Scientific, Inc.). Labeled cells were
analyzed with a FACSCalibur flow cytometer (BD Biosciences) and
Flowjo v7.6.1 program (FlowJo LLC). Importantly, EGFP-expressing
cells were screened without labeling.
Western blot analysis
Proteins from whole cell lysates of L929, CHO, MCF7,
HEK293, H526, A549, HeLa, U-87MG, and DMS114 (all purchased from
ATCC; Fig. S1) were obtained using
RIPA lysis buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) with a protease inhibitor cocktail (Sigma-Aldrich; Merck
KGaA). Proteins at 10 µg/lane were loaded onto a 12% acrylamide
gel, resolved using SDS-PAGE; and were then transferred to a
polyvinylidene difluoride membrane (Merck KGaA). After blocking
with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 2 h at
room temperature, primary antibodies against MCT1, MCT4, CD147
(1:1,000; cat. no. ab119020; Abcam), or matrix metalloproteinase
(MMP)2 (1:2,000; cat. no. 66366-1-Ig; ProteinTech Group, Inc.) were
incubated overnight at 4°C and then washed with PBS-Tween20 (0.05%
Tween-20) and incubated with the secondary horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG (1:5,000; cat. no. A-31430;
Thermo Fisher Scientific, Inc.) at room temperature for 1 h.
Enhanced chemiluminescence (ECL) reagent (Vazyme Biotech Co., Ltd.)
was used to detect the proteins, and the immunoblots were developed
using the Image Scanner ChemiDoc MP (Bio-Rad Laboratories, Inc.)
and analyzed by ImageJ v1.5.1 (National Institutes of Health).
Reverse transcription-PCR
Total RNA from whole cell lysates of L929, CHO,
MCF7, HEK293, H526, A549, HeLa, U-87MG, and DMS114 (all purchased
from ATCC; Fig. S1) was extracted
using RNA purification Kit (CWBIO Co., Ltd.) according to the
manufacturer's protocols. RNA concentration and purity were
determined with a Nanodrop 2000 Analyzer (Thermo Fisher Scientific,
Inc.). Purified RNA was reverse transcribed using the HiFiScript
cDNA Synthesis Kit (CWBio). PCR was performed using the following
thermocycling conditions: 94°C for 30 sec, 55°C for 30 sec and 72°C
for 30 sec on a PCR instrument (Mastercycler nexus, Eppendorf)
using Super Multiplex PCR Mix (CWBio) according to the
manufacturer's protocol. The primers for each gene were shown in
Fig. S1C.
Immunofluorescence
MCT-transfected L929 cells were fixed in 4%
paraformaldehyde for 30 min and permeabilized with 0.1%
Triton-X-100 for 15 min. Cells were incubated with the
aforementioned primary antibodies against MCT1, MCT4 or CD147 for 2
h and then treated with secondary fluorescein-conjugated antibodies
for 30 min. Nuclei were stained with DAPI (1:20; Thermo Fisher
Scientific, Inc.) for 15 min. The expression levels of MCT1, MCT4,
or CD147 were observed under a fluorescence microscope (×20
magnification; DMI3000 B; Leica Microsystems GmbH) and images were
captured. All operations were performed at room temperature.
Lactate transport assay
MCT or EGFP transfected L929 cells were seeded into
96-well plates at a density of 5.0×104 cells/well. A
final concentration of 1, 5, 10, 30, 50, 100, 300 and 500 nM
AZD3965 in DMSO or pure DMSO (control) was added 24 h later, and
the culture supernatant was sampled 72 h later. Lactate
concentration was measured using a biosensor analyzer (Shandong
Baisheng Biotechnology Co., Ltd.). Relative lactate concentrations
were calculated by the ratio of lactate concentration between
AZD3965-treated cells and pure DMSO treated samples (controls). The
experiments were performed in triplicate.
Cell counting assay
L929 cells were seeded into 96-well plates at a
density of 5.0×104 cells/well and cultured for 3 days.
Cell proliferation was measured at 24, 48 and 72 h using a Cell
Counting Kit-8 (Gen-view Scientific, Inc.) according to the
manufacturer's protocol. Relative cell proliferation was calculated
based on the proliferation of EGFP-L929 cells at 24 h. The
experiments were performed in triplicate.
Wound healing assay for migration
MCT or EGFP transfected L929 cells were seeded into
24-well plates at a density of 1.0×106 cells/well and
cultured until ~90% confluency. A scratch was made using a sterile
200-µl pipette tip, and detached cells were washed off using DMEM
and replaced with new DMEM. For the EGFR inhibitor OSI-744 or c-Met
inhibitor INCB28060 (both Selleck Chemicals), the medium was
supplemented with 10 µM OSI-744 or 5 µM INCB28060 dissolved in
DMSO, while the same volume of DMSO without inhibitors was used as
the control. Wound healing was observed 48 h later under a phase
contrast microscope (×4 magnification; Leica Microsystems GmbH) and
the cell migration rate was calculated by the percentage of wound
closure. The EGFP transfected cells were used as negative controls
for the MCT transfected cells. The experiments were performed in
triplicate.
Transwell assays for migration and
invasion
MCT or EGFP transfected L929 cells in serum-free
medium were seeded at 1.0×105 cells/well in the inserts
of 24 Transwell plates. For the migration analysis, these cells
were seeded directly into the inserts; meanwhile, for the invasion
assay, 50 µl of a 1:9 mixture of Matrigel (BD Biosciences) and
culture medium were added to each upper compartment of the inserts
and cured at 37°C for 24 h prior to cell seeding. Then, 10%
FBS-containing medium was added to the bottom chamber of the
Transwell plates. Cells on the lower surface of the insert were
fixed in 4% paraformaldehyde at room temperature for 30 min,
stained with 0.1% crystal violet at room temperature for 30 min and
examined under a microscope after 24 h (for migration) or 36 h of
incubation (for invasion) at 37°C. For the inhibition studies,
either 10 µM OSI-744 or 5 µM INCB28060 was added to the cell
culture medium prior to seeding the cells into inserts, while the
same volume of DMSO without inhibitors was used as the control. The
average number of migrated or invading cells from five designated
optical fields under a phase contrast microscope (×40
magnification) was counted and averaged. The EGFP transfected cells
were used as negative controls for the MCT transfected cells. The
experiments were performed in triplicate.
Gelatin zymography
L929 cells were seeded into 6-well plates at a
density of 5.0×106 cells/well and then incubated with
serum-free medium at 37°C for 24 h. Culture supernatants were
collected and electrophoresed using 10% SDS-PAGE containing 2%
gelatin (Corning, Inc.). After washing, the gel was cultured with a
buffer that contains 5 mM CaCl2 and 1 µM
ZnCl2 at 37°C for 48 h. Afterwards, the gel was stained
with Coomassie Blue R250 at room temperature for 3 h and
decolorized with a solution that contained 5% methanol and 10%
acetic acid. The band was scanned using Image Scanner (ChemiDoc MP;
Bio-Rad, Laboratories, Inc.) and analyzed using ImageJ v1.5.1
(National Institutes of Health).
Statistical analysis
All statistical analysis was performed using
GraphPad Prism 7.0 (GraphPad Software, Inc.). Two-tailed Welch's
t-tests were used to compare results in two groups. One-way ANOVA
or two-way ANOVA with Tukey's post hoc test was used to compare
results in more than two groups. P<0.05 was considered to
indicate a statistically significant difference. The P-values were
calculated from the results of three independent experiments.
Results
Expression of EGFP, MCT1 or MCT4 in
L929 cells
L929 is a non-carcinogenic murine cell line with
undetectable murine MCT1 or MCT4 expression, analyzed using western
blotting and PCR (Fig. S1A and B).
The impact of overexpression of human MCT1 or MCT4 in L929 cells on
cellular migration and invasion was investigated. To avoid using a
pool of cells with both transfected and non-transfected cells,
transfected cells were cloned to select only high-expressing
clones. To overcome clone-to-clone variations, a panel of three
stable clones was used for each genetic construct. The negative
controls were three clones of L929 cells with high expression
levels of the mock EGFP.
For transfection, pcDNA3.0-EGFP, pcDNA3.0-MCT1 and
pcDNA3.0-MCT4 plasmids were constructed and verified (Fig. S2A-C). The plasmids were then used to
transfect L929 cells and transfected cells were single-cell cloned.
Clones were ranked by FACS (Fig.
S2D-F) and western blotting (Fig.
S2G-I) for MCT1, MCT4 or EGFP expression. Three high MCT1
expressing clones (MCT1-L929 5B8, 5E6 and 6E6), three high MCT4
expressing clones (MCT4-L929 3E10, 4D11 and 8E4), and three EGFP
expressing clones (EGFP-L929 1H9, 2H6 and 5C1) were chosen for
subsequent investigations. Throughout the investigation, the
expression of MCT1, MCT4 or EGFP in the nine chosen clones were
monitored concurrently. As shown in Fig.
1A and B, high expression of MCT1, MCT4 or EGFP was well
maintained during the investigation. Human CD147, which is the
molecular chaperone of MCT1 and MCT4 that assists in guiding MCT
proteins to the membrane (4), was
not co-transfected. However, mouse CD147 was present in all clones
and showed similar distributions as those of MCT1 (5B8) and MCT4
(3E10) (Fig. 1C). Other clones of
MCT1- and MCT4-L929 showed similar results. Overexpression of MCT1
or MCT4 did not increase lactate secretion of L929 cells (Fig. 1D). Nevertheless, it rendered L929
cells more resistant to AZD3965, which is an inhibitor of MCT1 and
MCT2 (Fig. 1E). At a concentration
of 10 nM AZD3965 or higher, both MCT1-L929 and MCT4-L929 cells had
a significant reduction on lactate secretion compared with
EGFP-L929 cells. The lactate secretion of EGFP-L929 cells was
reduced by ~50%; in contrast, the lactate secretion of MCT1-L929
cells was reduced by only 30% and that of MCT4-L929 cells was not
reduced at all. These observations suggested that lactate
metabolism and transportation of L929 cells are tightly regulated
and may not be affected by overexpression of MCT1 or MCT4. However,
once lactate transportation was disrupted, the overexpression of
MCT1 or MCT4 partially or completely rescued the normal
phenotype.
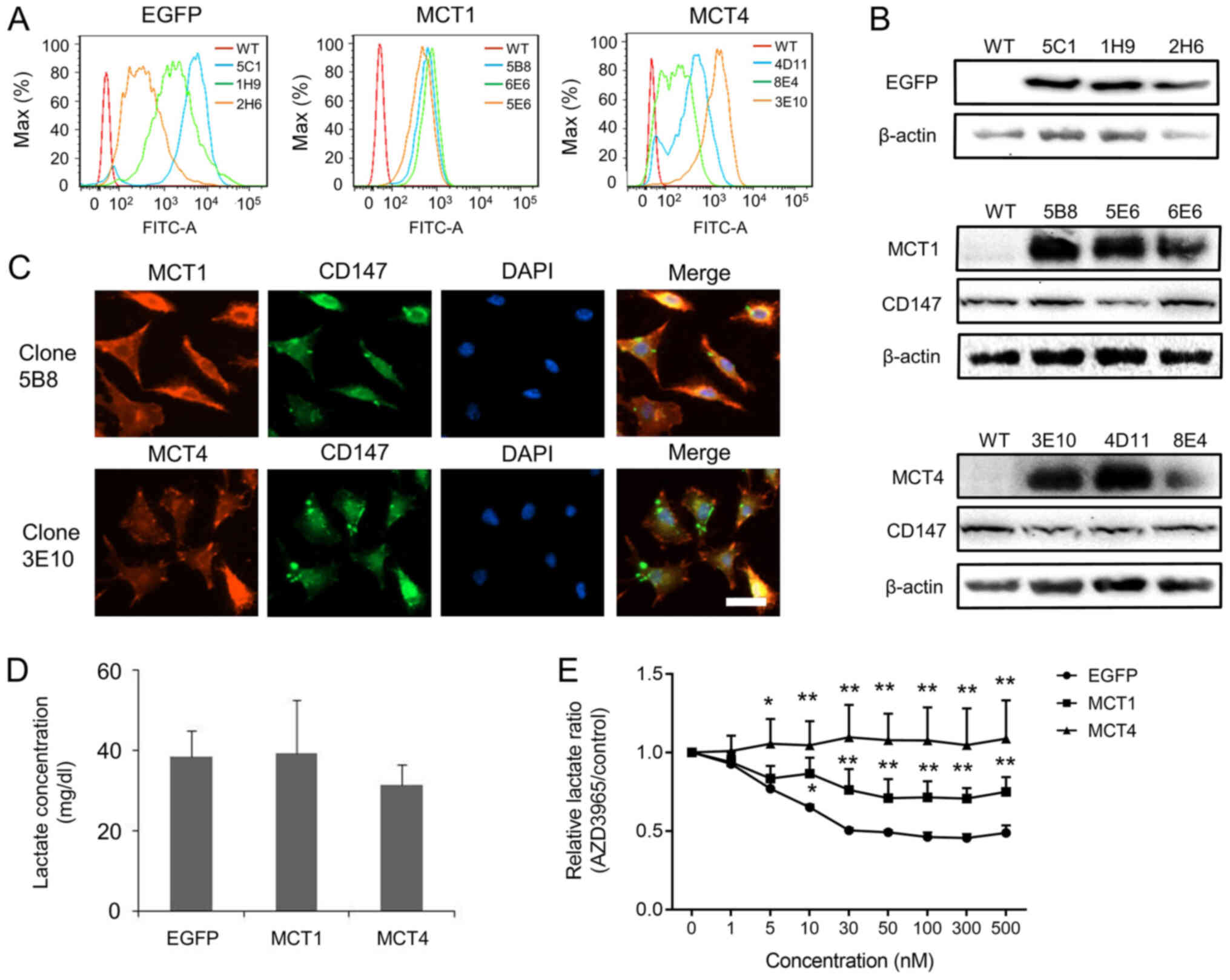 | Figure 1.Overexpression of EGFP, MCT1 and MCT4
in L929 cells. (A) Overexpression of EGFP, MCT1 and MCT4 was
confirmed using flow cytometry for EGFP-L929, MCT1-L929 and
MCT4-L929 clones. (B) Overexpression of EGFP, MCT1, and MCT4, as
well as expression of endogenous CD147 were confirmed using western
blotting. (C) Immunofluorescence was performed to investigate the
expression of transfected MCT1 or MCT4. Co-expression of MCT and
CD147 was observed in clone 5B8 of MCT1-L929 and clone 3E10 of
MCT4-L929 cells. Scale bar, 30 µm. (D) Lactate concentration in the
culture medium of MCT1-, MCT4- and EGFP-transfected cells. No
significant differences were observed among different panel of
clones. Data were analyzed using a two-tailed Welch's t-test. (E)
Normalized lactate concentration in the culture medium of
AZD3965-treated cells to respective control cells that received
only AZD3965 solvent (DMSO). The MCT1/2 inhibitor, AZD3965, reduced
lactate secretion of EGFP-L929 cells in a dose-dependent manner,
but MCT1-L929 and MCT4-L929 cells were partially or completely
resistant to AZD3965. The results in (D) and (E) were the average
of three independent experiments using three different clones, and
each error bar indicates one standard deviation. P-values shown in
(E) were calculated using one-way ANOVA with a Tukey's post hoc
test. *P<0.05, **P<0.01 vs. EGFP. EGFP, enhanced green
fluorescent protein; MCT, monocarboxylate transporter. |
Migration and invasion of MCT1- or
MCT4-transfected L929 cells
Migration and invasion are two key features of
metastasis (25). Migration was
analyzed using wound healing and Transwell migration assays, while
invasion was analyzed using a Transwell invasion assay. As shown in
Fig. 2A, the migration rate of
MCT4-L929 clones was significantly faster compared with that of the
EGFP control clones (P<0.01), while the MCT1-L929 clones were
not different from the control in terms of migration rate. A
Transwell migration assay confirmed these results. As shown in
Fig. 2B, more than twice as many
cells passed through Transwell membranes for the
MCT4-overexpression clones (P<0.01), while MCT1 overexpression
clones behaved similar to the control clones. In addition, the
invasiveness of L929 cells was increased for MCT4-expressing cells,
and not MCT1-overexpressing cells (P<0.05; Fig. 2C). Approximately twice as many cells
passed through the Matrigel base for MCT4-L929 cells compared with
both control and MCT1-L929 cells. These results indicated that
MCT4, but not MCT1, promoted both migration and invasion of L929
cells.
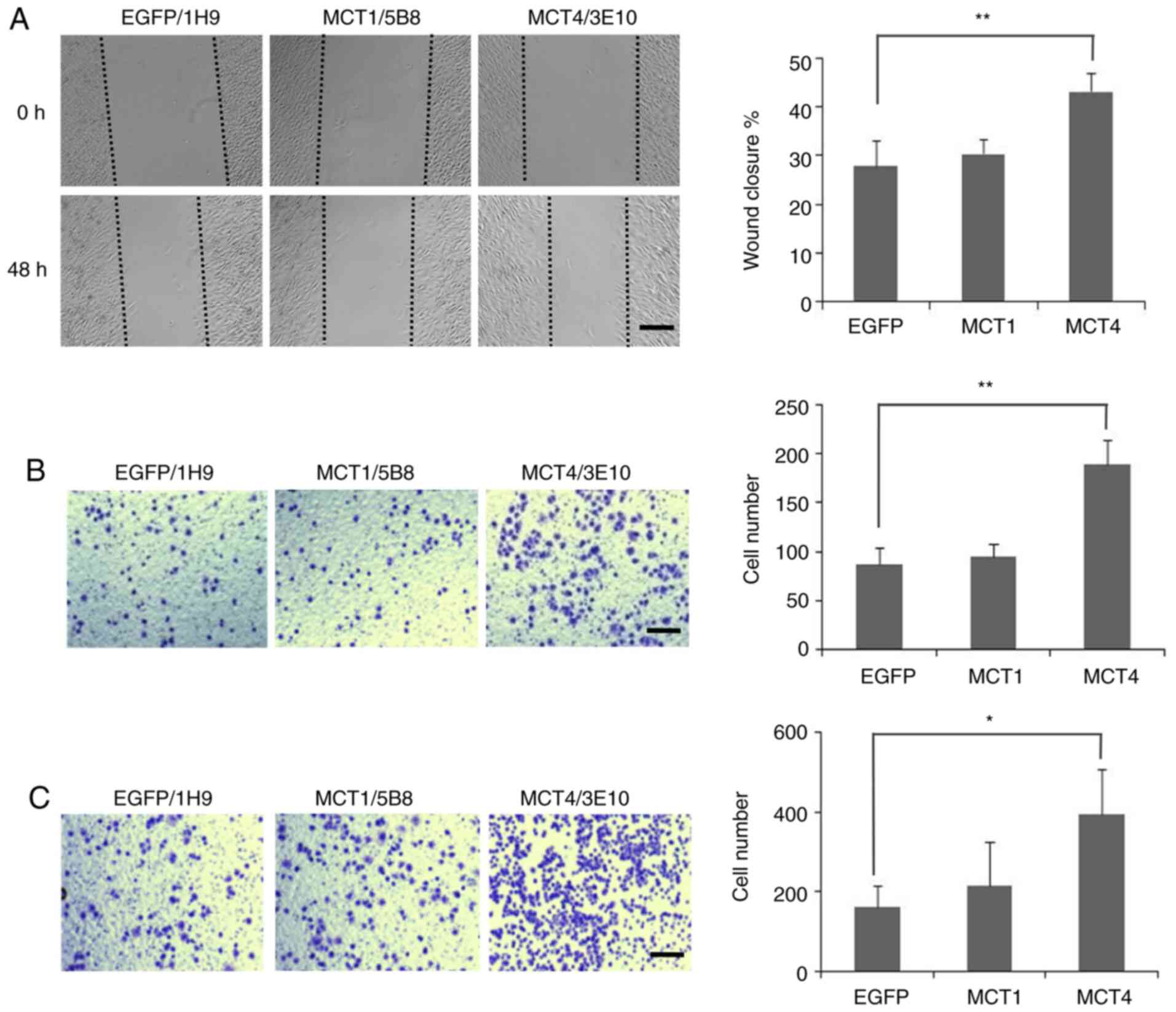 | Figure 2.Migration and invasion of MCT1- and
MCT4-transfected L929 cells. (A) Wound healing assay.
Representative images of scratch closing of EGFP-L929 (1H9),
MCT1-L929 (5B8) and MCT4-L929 (3E10) cells at 0 and 48 h
post-scratch are shown. Scale bar, 300 µm. Percentage of wound
closure, calculated as the average of three parallel clones, at 48
h post scratch is shown to the right. (B) Migration assay.
Representative images of EGFP-L929 (1H9), MCT1-L929 (5B8) and
MCT4-L929 (3E10) cells that migrated through a migration filter are
shown after the cells were seeded on the top chambers for 36 h. The
average number of cells that adhere to the lower chamber is
presented to the right. (C) Invasion assay. Cells were plated onto
Matrigel invasion chambers for 36 h. Representative images of
EGFP-L929 (1H9), MCT1-L929 (5B8) and MCT4-L929 (3E10) cells that
invaded through the Matrigel invasion filters are shown. The
average number of cells that adhere to the lower chamber is
presented to the right. The average results in (A), (B) and (C)
were from three independent experiments using three different
clones, and each error bar represents one standard deviation.
P-values shown in (A), (B) and (C) were calculated using one-way
ANOVA with a Tukey's post hoc test. *P<0.05, **P<0.01 vs.
EGFP. EGFP, enhanced green fluorescent protein; MCT,
monocarboxylate transporter. |
Migration and invasion of
dysfunctional MCT4-transfected L929 cells
It was investigated whether the function of MCT4 is
required for its promotion of migration and invasion of L929 cells.
A single amino acid mutation, known as R278Q, causes MCT4 to be
completely dysfunctional (29). A
panel of L929 clones transfected with the MCT4-R278Q gene
were screened using FACS and western blotting (Fig. S3A and B). Three MCT4-R278Q-L929
high-expressing clones (8E4R, 8D6 and 9G2) were selected, as shown
in Fig. 3A-C. It was first confirmed
that the MCT4-R278Q mutants lost their ability to engage in lactate
transportation. As shown in Fig. 3D,
the lactate secretion of MCT4-R278Q-L929 cells was reduced by
AZD3965 by ~50%, which was similar to that of the negative control
(EGFR-L929) cells, whereas AZD3965 treatment of MCT4-L929 cells
showed no inhibitory effect. Fig. 3D
indicated that MCT4-R278Q-L929 cells could not compensate for the
inhibition of AZD3965, in contrast to the wild-type MCT4-L929
cells, and behaved similarly to the negative control cells,
indicating that the R278Q mutation completely annihilated the
lactate transportation function of MCT4. The promotion of migration
and invasion by MCT4 was lost with the R278Q mutation, as shown in
Fig. 3E-G, where MCT4-R278Q cells
behaved similarly to the negative control, EGFP-L929 cells, but not
to the MCT4-L929 cells with active lactate transportation function.
As the expression level of MCT4 and MCT4-R278Q on respective cells
was similar, our observation suggests that cellular migration and
invasion were associated with the transportation function of
MCT4.
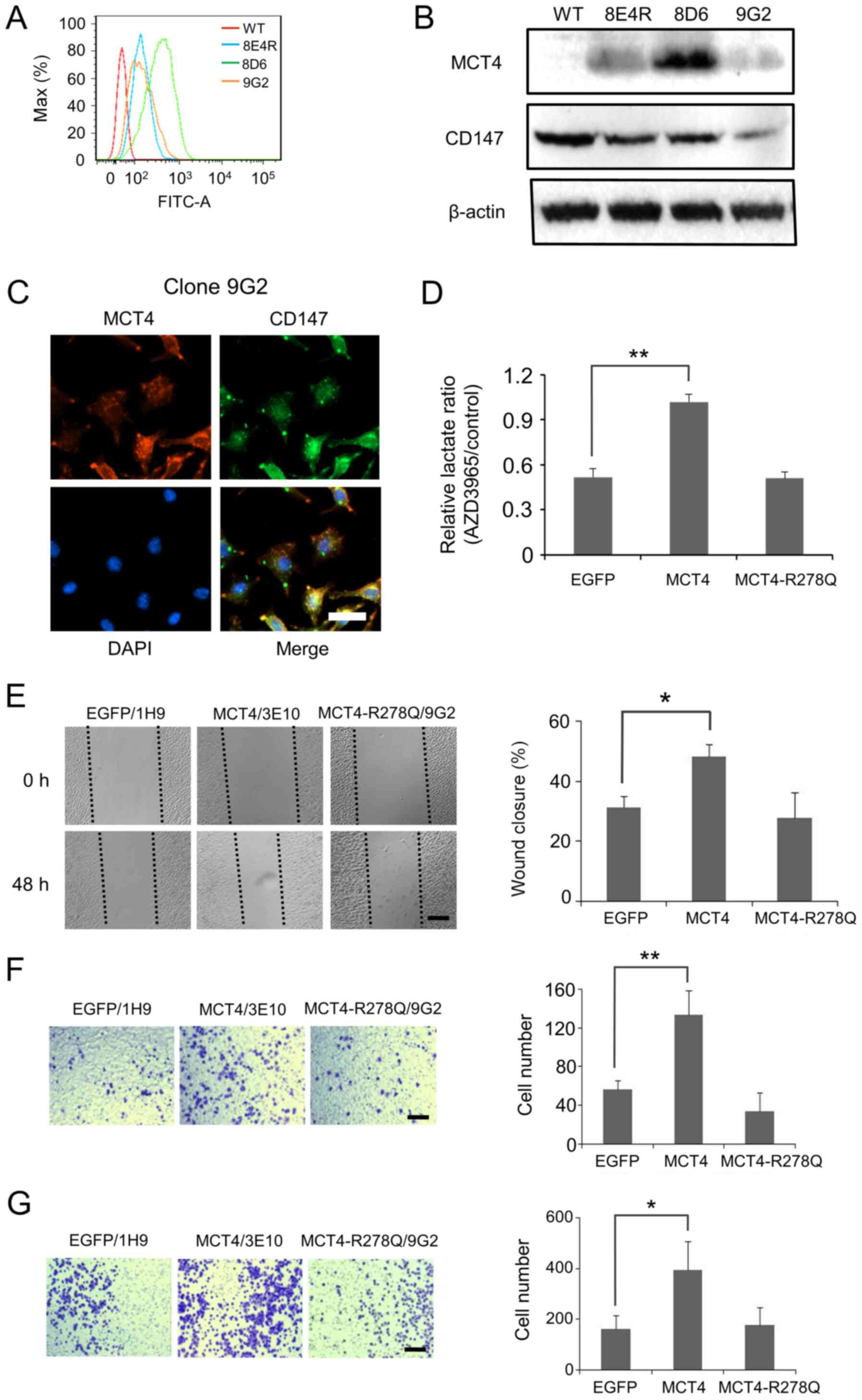 | Figure 3.Mutated MCT4 without lactate
transportation activity does not promote migration or invasion of
L929 cells. A panel of three clones with high expression of
MCT4-R278Q was obtained, as evidenced by (A) flow cytometry and a
(B) western blotting. (C) Co-expression of MCT4-R278Q and CD147 was
observed with clone 9G2. Scale bar, 30 µm. (D) Normalized
concentration of lactate in the culture medium of AZD3965-treated
cells compared with that of the corresponding cells that are
treated with only the solvent. MCT4-R278Q-L929 cells lost the
ability to compensate for the inhibition mediated by AZD3965 in
L929 cells compared with MCT4-L929 cells. (E) Wound healing assay.
MCT4-R278Q-L929 (9G2) cells showed a similar migration rate as
EGFP-L929 (1H9) cells, which was much slower compared with that of
wild-type MCT4-L929 (3E10) cells. Scale bar, 300 µm. The average
percentage of wound closure at 48 h is shown to the right. (F)
Representative images of EGFP-L929 (1H9), MCT4-L929 (3E10) and
MCT4-R278Q-L929 (9G2) cells that crossed through migration filters
36 h post seeding are shown. The average number of cells that
adhered to the lower chamber is shown to the right. (G)
Representative images of EGFP-L929 (1H9), MCT4-L929 (3E10) and
MCT4-R278Q-L929 (9G2) cells that invaded through the
Matrigel-coated filters 36 h after seeding are shown. The average
number of cells that adhered to the lower chamber is presented to
the right. The average is from three independent experiments that
use three different clones, and each error bar represents one
standard deviation. P-values shown in (D), (E), (F), and (G) were
calculated using one-way ANOVA with a Tukey's post hoc test.
*P<0.05, **P<0.01 vs. EGFP. EGFP, enhanced green fluorescent
protein; MCT, monocarboxylate transporter. |
The EGF/EGFR pathway in migration and
invasion is promoted by MCT4
EGF/EGFR- and HGF/c-Met-mediated signaling pathways
are two classical pathways that are associated with the regulation
of cell migration and invasion (30,31). It
was investigated whether these two pathways were involved in the
promotion of migration and invasion of MCT4-L929 cells. Both
MCT4-L929 cells and control EGFP-L929 cells were treated with
either the EGFR inhibitor OSI-744 or the c-Met inhibitor INCB28060
to assess their impact on migration and invasion. Treatment with
the EGFR inhibitor OSI-744 decreased the migration rate of
MCT4-L929 cells, as demonstrated by both the wound healing and
Transwell migration assays (P<0.01 and P<0.05; Fig. 4A and B, respectively). However, the
c-Met inhibitor had no impact, as shown in Fig. 4A and 4B. Similar phenomena were observed in the
invasion study. Fig. 4C shows that
the invasiveness of MCT4-L929 cells was significantly decreased by
OSI-744 (P<0.01), but not INCB28060. These results indicated
that the EGF/EGFR pathway may be involved in the metastatic
transformation of L929 cells by MCT4.
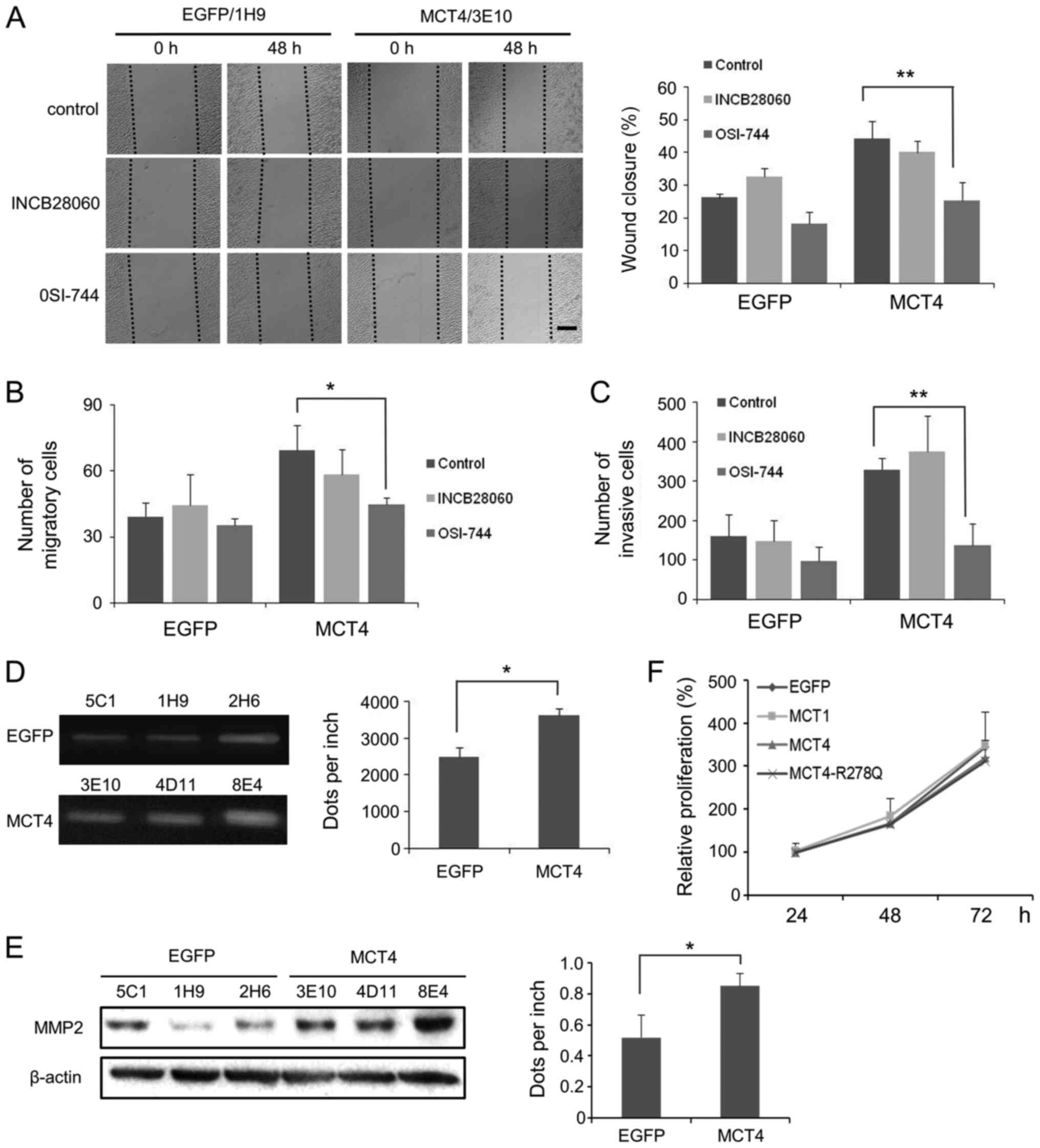 | Figure 4.The EGF/EGFP-mediated signaling
pathway may be associated with the enhanced migration and invasion
of MCT4-L929 cells. (A) Representative wound healing images of
EGFP-L929 (1H9) and MCT4-L929 (3E10) cells that were treated with
the EGFP inhibitor OSI-744 or the c-Met inhibitor INCB28060 at 0
and 48 h post-scratching are shown. Scale bar, 300 µm. The average
percentage of wound closure at 48 h is presented to the right. (B)
EGFP inhibitor OSI-744- or the c-Met inhibitor INCB28060-treated
MCT4-L929 cells were plated directly onto migration chambers for 36
h. The average numbers of cells that adhere to the lower chamber
are presented. (C) EGFP inhibitor OSI-744- or c-Met inhibitor
INCB28060-treated MCT4-L929 cells were plated onto Matrigel
invasion chambers and cultured for 36 h. The average number of
cells that adhered to the lower chamber is presented. (D) MMP2
activity of the EGFP-L929 and MCT4-L929 cells was detected by a
gelatin zymography assay. The gray value of the strip is presented
to the right. (E) MMP2 levels were determined using western
blotting. The gray value of the MMP2 bands is presented to the
right. (F) The cell viability of EGFP-L929, MCT1-L929, MCT4-L929,
and MCT4-R278Q-L929 cells during a 3-day culture. The average is of
three independent experiments using three different clones, and
each error bar represents one standard deviation. P-values in (A),
(B) and (C) were calculated using two-way ANOVA with a Tukey's post
hoc test, and P-values in (D) and (E) were calculated using
two-tailed Welch's t-test. *P<0.05, **P<0.01 vs. OSI-744 or
EGFP. EGFP, enhanced green fluorescent protein; MCT,
monocarboxylate transporter; MMP, matrix metalloproteinase. |
As MMPs are commonly highly expressed with highly
active in metastatic cells, presumably via the EGF/EGFR pathway
(32), the activity and expression
of MMPs in MCT4-L929 cells were investigated through gelatin
zymography and western blotting. As shown in Fig. 4D, MMP2 activity in the culture medium
of MCT4-L929 cells was increased compared with EGFP-L929 cells
(P<0.05). MMP2 levels in MCT4-L929 cells were also elevated
(P<0.05; Fig. 4E), which is
considered to be related to cell invasion. However, MMP9 expression
was not detected in any L929 clone (data not shown). Cell
proliferation rate of MCT4-L929 cells was similar to that of MCT1-,
MCT4-R278Q-, and the negative control EGFP-L929 cells (Fig. 4F), hence the possibility of the
higher migration and invasion rate of MCT4-L929 cells being a
result of higher cellular proliferation rate could be ruled
out.
Discussion
Transfected human MCT1 or MCT4 is active in murine
L929 cells. Although overexpression of MCT1 and MCT4 does not alter
lactate metabolism or transportation, such overexpression was found
to counteract the effects of the MCT1/2 inhibitor AZD3965 (Fig. 1D and E). CD147, which is the
chaperone protein that helps direct MCT transporters to the plasma
membrane (4), was not
co-transfected. Nevertheless, the function of endogenous murine
CD147 seemed to be sufficient to direct recombinant MCT proteins to
the plasma membrane and support their function.
It has been reported that MCF7 cells that are
transfected with MCT1 show both higher tumorigenicity in nude mice
and enhanced in vitro invasion (33). Since the present screening study
demonstrated that MCF7 cells express MCT4, albeit at low levels,
MCF7 cells were not used in the present study.
A panel of three high expression clones was used for
each genetic construct. The negative control was a panel of three
clones with high expression of EGFP, which was a mock protein that
underwent the same clone selection process. Studies on these
transgenic cell lines demonstrated that overexpression of human
MCT4, but not human MCT1, promoted the migration and invasion of
the noncarcinogenic L929 cell line. In addition, it was observed
that the activity of MCT4 is required for migration and invasion
promotion. When L929 cells were transfected with an inactive MCT4
mutant, neither migration nor invasion increased. Inactive MCT4 has
a single amino acid mutation, known as R278Q, which was previously
reported to be inactive (29).
Moreover, Fig. 3D shows that the
R278Q mutant lost its ability to counteract the function of
AZD3965. This observation suggested that the MCT4 protein needs to
be functional to promote cellular migration and invasion. No
previous studies have evaluated the impact of inactivated MCT4 or
MCT1 on cell migration and invasion, to the best of our knowledge.
However, MCT1 has been suggested to promote metastasis of human
SiHa cells independent of its activity (34), whereby a MCT1 inhibitor blocks
lactate transportation, but does not reduce the migration or
invasion rate of SiHa cells. It is unknown if the discrepancy on
cell migration and invasion between the present findings and those
of the study on SiHa cells is due to differences in cell lines,
methods or differences between MCT1 and MCT4. It would be
worthwhile to investigate whether other substrates, besides
lactate, that are also transported by MCT4, but not by MCT1,
contribute to enhanced migration and invasion.
To investigate possible mechanisms between
overexpression of MCT4 and migration or invasion, two signaling
pathways were examined: The HGF/c-Met and EGF/EGFR signaling
pathways. The migration and invasion of MCT4-L929 cells were
decreased to the same level as control cells by the EGFR inhibitor
OSI-744, but not by the c-Met inhibitor INCB28060. Activated EGFR
has been suggested to promote the invasiveness of MCF7 cells by
upregulating MMP2 and MMP9 (35).
Although MMP9 expression was not detected in L929 cells, it was
demonstrated that MMP2 activity was slightly upregulated in the
MCT4-L929 cells.
In conclusion, the present study reported the
complex impact of the overexpression of MCT transporters on cell
migration and invasion. The finding that the overexpression of MCT4
promotes both migration and invasion is consistent with those of
previous studies, while the finding that the overexpression of MCT1
promotes migration and invasion is inconsistent. Second, it was
demonstrated that, while MCT4 activity is critical for the
migration and invasion of L929 cells, the activity of MCT1, which
is similar to MCT4 in respect to lactate transportation function,
had no impact on L929 migration and invasion. Finally, it was
revealed that overexpression of active human MCT4 increased
migration and invasion capability of non-carcinogenic L929 cells.
Inhibition of MCT4 activity may serve as a potential therapeutic
strategy to eliminate metastatic tumor cells. As such, the stably
transfected MCT1- and MCT4-L929 cell lines may be used as valuable
model cell lines both in vitro and in vivo to further
investigate the mechanisms between overexpression of MCT1 and MCT4
and cell signaling, metabolism and tumorigenicity.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Yu Yang and Ms.
Xiaonan Ma (Shenyang Pharmaceutical University) for their technical
support and instruction.
Funding
This work was funded by the Doctoral Start-up
Foundation of Liaoning Province (grant no. 20170520316), the Junior
Teacher Career Development Support Plan Foundation of Shenyang
Pharmaceutical University (grant no. ZQN2016019), the Department of
Education of Liaoning Province (grant no. 201610163L24), and
Significant New Drug Development Project of Ministry of Science and
Technology (grant no. 2019ZX09732-001) of China.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL was a major contributor in the writing of the
manuscript and was responsible for the statistical analysis. XZ and
YL contributed to the development of all MCT and EGFP
overexpressing L929 cell lines. JF and HH contributed to the
migration and invasion experiment. JY contributed to the western
blotting, fluorescence-activated cell sorting and
immunofluorescence assays. XL and LW assisted with the design of
the study. NM contributed to the conception and design of the study
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MCT
|
monocarboxylate transporter
|
|
EGFP
|
enhanced green fluorescent protein
|
|
MMP
|
metalloproteinase
|
|
EGF
|
epidermal growth factor
|
|
EGFR
|
epidermal growth factor receptor
|
|
HGF
|
hepatocyte growth factor
|
|
c-Met
|
cellular mesenchymal to epithelial
transition factor
|
|
FBS
|
fetal bovine serum
|
|
FACS
|
fluorescence-activated cell
sorting
|
References
|
1
|
Reuben JM, Krishnamurthy S, Woodward W and
Cristofanilli M: The role of circulating tumor cells in breast
cancer diagnosis and prediction of therapy response. Expert Opin
Med Diagn. 2:339–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ngo H, Tortorella SM, Ververis K and
Karagiannis TC: The Warburg effect: Molecular aspects and
therapeutic possibilities. Mol Biol Rep. 42:825–834. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halestrap AP: The monocarboxylate
transporter family-Structure and functional characterization. IUBMB
Life. 64:1–9. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kirk P, Wilson MC, Heddle C, Brown MH,
Barclay AN and Halestrap AP: CD147 is tightly associated with
lactate transporters MCT1 and MCT4 and facilitates their cell
surface expression. EMBO J. 19:3896–3904. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Z, Wu Q and Sun S, Wu J, Li J, Zhang Y,
Wang C, Yuan J and Sun S: Monocarboxylate transporters in breast
cancer and adipose tissue are novel biomarkers and potential
therapeutic targets. Biochem Biophys Res Commun. 501:962–967. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pinheiro C, Reis RM, Ricardo S,
Longatto-Filho A, Schmitt F and Baltazar F: Expression of
monocarboxylate transporters 1, 2, and 4 in human tumours and their
association with CD147 and CD44. J Biomed Biotechnol.
2010:4276942010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeon JY, Lee M, Whang SH, Kim JW, Cho A
and Yun M: Regulation of acetate utilization by monocarboxylate
transporter 1 (MCT1) in hepatocellular carcinoma (HCC). Oncol Res.
26:71–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pinheiro C, Longatto-Filho A,
Azevedo-Silva J, Casal M, Schmitt FC and Baltazar F: Role of
monocarboxylate transporters in human cancers: State of the art. J
Bioenerg Biomembr. 44:127–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pinheiro C, Longatto-Filho A, Scapulatempo
C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA,
Schmitt F and Baltazar F: Increased expression of monocarboxylate
transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch.
452:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruan Y, Zeng F, Cheng Z, Zhao X, Fu P and
Chen H: High expression of monocarboxylate transporter 4 predicts
poor prognosis in patients with lung adenocarcinoma. Oncol Lett.
14:5727–5734. 2017.PubMed/NCBI
|
|
11
|
Kim Y, Choi JW, Lee JH and Kim YS:
Expression of lactate/H+ symporters MCT1 and MCT4 and
their chaperone CD147 predicts tumor progression in clear cell
renal cell carcinoma: Immunohistochemical and The Cancer Genome
Atlas data analyses. Hum Pathol. 46:104–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi JW, Kim Y, Lee JH and Kim YS:
Prognostic significance of lactate/proton symporters MCT1, MCT4,
and their chaperone CD147 expressions in urothelial carcinoma of
the bladder. Urology. 84:245.e9–245.e15. 2014. View Article : Google Scholar
|
|
13
|
Gerlinger M, Santos CR, Spencer-Dene B,
Martinez P, Endesfelder D, Burrell RA, Vetter M, Jiang M, Saunders
RE, Kelly G, et al: Genome-wide RNA interference analysis of renal
carcinoma survival regulators identifies MCT4 as a Warburg effect
metabolic target. J Pathol. 227:146–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marchiq I and Pouysségur J: Hypoxia,
cancer metabolism and the therapeutic benefit of targeting
lactate/H+ symporters. J Mol Med (Berl). 94:155–171.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Polański R, Hodgkinson CL, Fusi A, Nonaka
D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE,
et al: Activity of the monocarboxylate transporter 1 inhibitor
AZD3965 in small cell lung cancer. Clin Cancer Res. 20:926–937.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guan X, Bryniarski MA and Morris ME: In
vitro and in vivo efficacy of the monocarboxylate transporter 1
inhibitor AR-C155858 in the murine 4T1 breast cancer tumor model.
AAPS J. 21:32018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Izumi H, Takahashi M, Uramoto H, Nakayama
Y, Oyama T, Wang KY, Sasaguri Y, Nishizawa S and Kohno K:
Monocarboxylate transporters 1 and 4 are involved in the invasion
activity of human lung cancer cells. Cancer Sci. 102:1007–1013.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JY, Lee I, Chang WJ, Ahn SM, Lim SH,
Kim HS, Yoo KH, Jung KS, Song HN, Cho JH, et al: MCT4 as a
potential therapeutic target for metastatic gastric cancer with
peritoneal carcinomatosis. Oncotarget. 7:43492–43503. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morais-Santos F, Granja S,
Miranda-Gonçalves V, Moreira AH, Queirós S, Vilaça JL, Schmitt FC,
Longatto-Filho A, Paredes J, Baltazar F, et al: Targeting lactate
transport suppresses in vivo breast tumour growth. Oncotarget.
6:19177–19189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang P, Ma J, Gao J, Liu F, Sun X, Fang
F, Zhao S and Liu H: Downregulation of monocarboxylate transporter
1 inhibits the invasion and migration through suppression of the
PI3K/Akt signaling pathway in human nasopharyngeal carcinoma cells.
J Bioenerg Biomembr. 50:271–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong SC, Nøhr-Nielsen A, Zeeberg K,
Reshkin SJ, Hoffmann EK, Novak I and Pedersen SF: Monocarboxylate
transporters MCT1 and MCT4 regulate migration and invasion of
pancreatic ductal adenocarcinoma cells. Pancreas. 45:1036–1047.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rybakovsky E, Valenzano MC, DiGuilio KM,
Buleza NB, Moskalenko DV, Harty RN and Mullin JM: Improving
transient transfection efficiency in a differentiated, polar
epithelial cell layer. J Biomol Tech. 30:19–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rao DD, Vorhies JS, Senzer N and
Nemunaitis J: siRNA vs. shRNA: Similarities and differences. Adv
Drug Deliv Rev. 61:746–759. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romøren K, Thu BJ, Bols NC and Evensen Ø:
Transfection efficiency and cytotoxicity of cationic liposomes in
salmonid cell lines of hepatocyte and macrophage origin. Biochim
Biophys Acta. 1663:127–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeung KT and Yang J:
Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol.
11:28–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pachmayr E, Treese C and Stein U:
Underlying mechanisms for distant metastasis-molecular biology.
Visc Med. 33:11–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meirson T and Gil-Henn H: Targeting
invadopodia for blocking breast cancer metastasis. Drug Resist
Updat. 39:1–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sasaki S, Kobayashi M, Futagi Y, Ogura J,
Yamaguchi H, Takahashi N and Iseki K: Crucial residue involved in
L-lactate recognition by human monocarboxylate transporter 4
(hMCT4). PLoS One. 8:e676902013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsai PC, Hsieh CY, Chiu CC, Wang CK, Chang
LS and Lin SR: Cardiotoxin III suppresses MDA-MB-231 cell
metastasis through the inhibition of EGF/EGFR-mediated signaling
pathway. Toxicon. 60:734–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shojaei F, Simmons BH, Lee JH, Lappin PB
and Christensen JG: HGF/c-Met pathway is one of the mediators of
sunitinib-induced tumor cell type-dependent metastasis. Cancer
Lett. 320:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Navarini NF, Araújo VC, Brown AL,
Passador-Santos F, Souza IF, Napimoga MH, Araújo NS and Martinez
EF: The EGF signaling pathway influences cell migration and the
secretion of metalloproteinases by myoepithelial cells in
pleomorphic adenoma. Tumour Biol. 36:205–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Levenson AS, Thurn KE, Simons LA,
Veliceasa D, Jarrett J, Osipo C, Jordan VC, Volpert OV, Satcher RL
Jr and Gartenhaus RB: MCT-1 oncogene contributes to increased in
vivo tumorigenicity of MCF7 cells by promotion of angiogenesis and
inhibition of apoptosis. Cancer Res. 65:10651–10656. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Payen VL, Hsu MY, Rädecke KS, Wyart E,
Vazeille T, Bouzin C, Porporato PE and Sonveaux P: Monocarboxylate
transporter MCT1 promotes tumor metastasis independently of its
activity as a lactate transporter. Cancer Res. 77:5591–5601. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Majumder A, Ray S and Banerji A: Epidermal
growth factor receptor-mediated regulation of matrix
metalloproteinase-2 and matrix metalloproteinase-9 in MCF-7 breast
cancer cells. Mol Cell Biochem. 452:111–121. 2019. View Article : Google Scholar : PubMed/NCBI
|


















