Introduction
Skin cancer is one of the major causes of
cancer-associated mortality in humans, with 232,000 new cases
worldwide in 2012, resulting in 55,000 deaths (1) There are two major types of non-melanoma
skin cancer, named basal cell carcinoma and squamous cell carcinoma
(SCC) (1,2). A number of known chemical carcinogens,
such as arsenide, and exposure to hazardous UV rays can cause the
development of SCC through mediation of free radical production,
resulting in a healthy epithelial cell being transformed in a
malignant one (3,4). As the prevalence of skin cancer is
increasing, with skin cancer having the fastest-growing cancer
incidence among all malignancies in recent years with an annual
growth rate of 3–5% per year, it is necessary to investigate the
molecular mechanisms underlying SCC and develop novel treatment
options for therapies (4). However,
it is well known that chemotherapy drugs for patients with skin
cancer are highly toxic compounds that target fast-growing healthy
cells and have numerous side effects. Therefore, investigation into
novel drugs with high efficiency and low toxicity for the treatment
of skin cancer is highly important.
Apoptosis is a process whereby dysregulated cells in
the body are removed and the balance is maintained between the
generation of new cells and the removal of old ones (5). Cell adhesion to the extracellular
matrix serves a key role in the regulation of cellular migration,
proliferation and differentiation, which is controlled by important
regulators for cell adhesion and migration, such as matrix
metalloproteinases (MMPs) (6). It
has been reported that polyphenols can generate reactive oxygen
species (ROS) in cancer cells, leading to the induction of
apoptosis, as well as downregulation of proliferation and migration
via the modulation of several signaling pathways, such as the
EGFR/MAPK signaling pathways, and MMPs, suggesting that cell
apoptosis and migration serve key roles in cancer regulation and
prevention (7). Mitochondria are
important organelles of energy production and survival signaling
cascades, as well as cell death (8).
Mitochondrial-associated abnormalities may be associated with an
imbalance of mitochondrial metabolism and the enhancement of
antimitochondrial apoptosis (8).
Mitochondrial apoptosis is also known as intrinsic apoptosis
(8). Previous studies have indicated
that decreased mitochondrial membrane potential can induce
apoptosis and increase ROS levels in colorectal cancer cells,
suggesting that the mitochondrial apoptotic pathway serves a
pivotal role in the initiation and progression of cancer (9,10).
The p53 signaling pathway, which is composed of
numerous genes, responds to multiple stress signals by inducing
apoptosis, cell senescence or cell cycle arrest, allowing to
prevent or repair damage (11).
Previous studies revealed that the p53 signaling pathway is closely
associated with the development of SCC and epigenetic abnormalities
in this process (12,13). Ataxia telangiectasia-mutated (ATM) is
a phosphoinositide 3-kinase-related kinase associated with cellular
processes, including cell proliferation and DNA repair (14). ATM expression is induced by DNA
damage, where ATM undergoes auto-phosphorylation on Ser1981 and is
recruited to sites of DNA damage, where it initiates a signaling
cascade through multiple DNA damage response proteins, including
p53 and other ligases (15). A
previous study revealed that shallots and licorice induce apoptosis
through the induction of ATM/p53 signaling in human cervical
carcinoma HeLa cells, suggesting that ATM/p53 signaling may promote
or prevent the development of cancer (16). However, to the best of our knowledge,
there is no evidence on whether ATM/p53 signaling can regulate
apoptosis and DNA damage in skin cancer.
Traditional Chinese medicines (TCMs), which are rich
in biologically active metabolites with low toxicity, are widely
used in the clinic for healthcare and disease control (17). Some natural products, including
matrine and flavonolignans from medicinal plants, have potent
effects against skin cancer (18,19).
Therefore, it is reasonable to seek effective natural products from
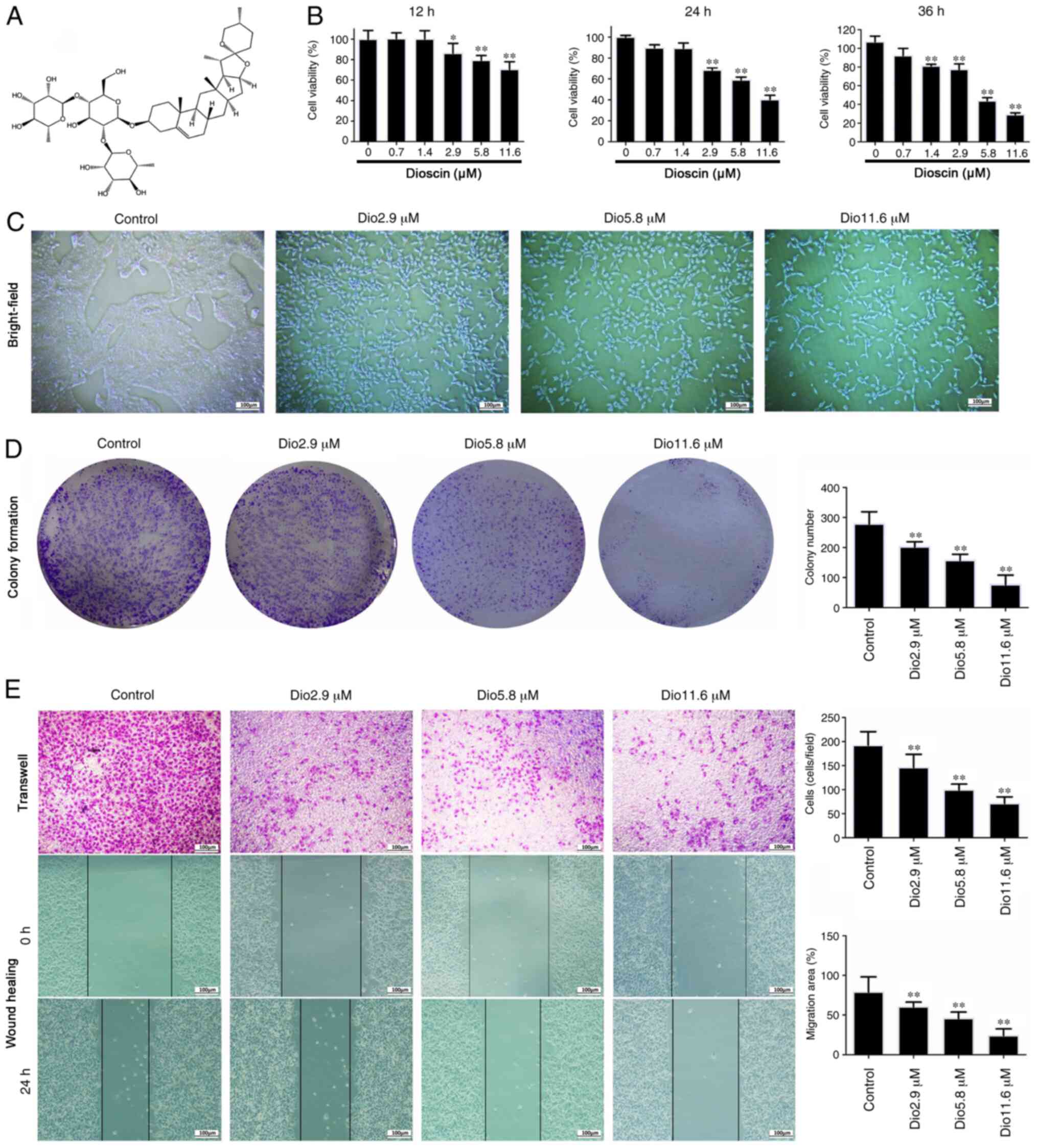
TCMs to treat skin cancer. Dioscin (Fig.
1A), an active natural product from numerous medicinal plants,
has exhibited potent activity against liver damage and renal injury
(20–30), as well as antioxidant and
anti-inflammatory effects in previous studies, including
antifungal, antiviral, hypoglycemic and immune regulatory effects
(31,32). Increasing efforts have been made to
investigate the anticancer activities of dioscin on several types
of human cancer cell lines, such as Hep-2, SMM7721 and PC3 cells
(12,33–37). An
increasing amount of evidence has revealed that dioscin can inhibit
tumor cell metastasis in lung cancer, breast cancer, melanoma and
laryngeal cancer (38,39). A mechanistic study revealed that
dioscin exerts an antimetastatic effect through connexin 43,
suppressing tumor cell malignancy and activating macrophage
sensitivity in melanoma (39). In
addition, targeted daunorubicin and dioscin co-delivery liposomes
can increase the inhibitory effects of daunorubicin on A549 cells
and decrease tumor metastasis by downregulation of MMP2 and TGF-β
(40). However, to the best of our
knowledge, there are currently no studies on the anticancer effects
of dioscin on A431 cells, and the mechanisms underlying the action
of dioscin against skin cancer remain unknown. Therefore, the aim
of the present study was to investigate the effects of dioscin
against skin cancer and its potential mechanisms in A4431 cells
in vitro.
Materials and methods
Chemicals and reagents
Dioscin was obtained from the Chengdu Research
Institute of Biology of the Chinese Academy of Sciences. MTT was
obtained from Roche Diagnostics. The Protein Extraction kit,
penicillin and streptomycin were purchased from Nanjing KeyGen
Biotech Co., Ltd. The BCA protein assay kit was obtained from
Beyotime Institute of Biotechnology. Carboxymethylcellulose-Na,
Tris and SDS were purchased from Sigma-Aldrich (Merck KGaA). The
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were obtained from Gibco (Thermo Fisher Scientific, Inc.).
DAPI was purchased from Sigma-Aldrich (Merck KGaA). The comet assay
kit was purchased from Cell Biolabs, Inc. The terminal
deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
was performed using the TransDetect® In situ
Fluorescein TUNEL Cell Apoptosis Detection kit (TransGen Biotech
Co., Ltd.). Primary antibodies were purchased from ProteinTech
Group, Inc., and Wuhan Boster Biological Technology, Ltd. (Table SI). Secondary antibodies were
purchased from ProteinTech Group, Inc. Lipofectamine®
2000 was purchased from Thermo Fisher Scientific, Inc., and p53
small interfering (si)RNAs were purchased from Guangzhou RiboBio
Co., Ltd. Z-VAD-FMK/pan-caspase inhibitor was purchased from
MedChemExpress.
Cell lines and culture
The human skin carcinoma A431 cell line was
purchased from Wuhan Boster Biological Technology, Ltd. The A431
cells were cultured in DMEM with 10% FBS, supplemented with 100
U/ml penicillin and 100 g/ml streptomycin, in a humidified 5%
CO2 atmosphere at 37°C.
MTT assay
The cells were plated in 96-well plates
(5×104 cells/well) and incubated at 37°C for 24 h. After
100 µl of medium was removed, various concentrations of dioscin
(0.0, 0.7, 1.4, 2.9, 5.8 or 11.6 µM) were added into the plates and
then incubated for 6, 12 or 24 h at 37°C. Subsequently, 10 µl MTT
stock solution (5 mg/ml) was added, and the plates were incubated
for another 4 h at 37°C. The formazan crystals were dissolved using
150 µl DMSO (150 µl/well). The absorbance was measured using a
microplate reader (Thermo Fisher Scientific, Inc.) at 490 nm, and
the cell morphology was observed using a phase contrast light
microscope (Nikon Corporation) with bright-field at ×200
magnification.
Colony-forming assay
A total of 500 cells/well were seeded into 6-well
plates. The cells were treated with dioscin (0.0, 2.9, 5.8 or 11.6
µM) for 3 days, and after treatment the drugs were replaced with
medium. The treated cells were maintained in culture medium for 10
days. Finally, the cells were stained with 0.1% crystal violet at
room temperature for 20 min and >50 cells were considered as a
colony. The colony formation numbers were analyzed using ImageJ
Software v1.3 (National Institutes of Health).
Wound-healing assay
A431 cells were plated into 6-well plates at
3×105 cells/well and cultured for 24 h at 37°C. Wounds
were scratched using a pipette tip and washed with PBS to remove
detached cells in serum-free medium. The cells were treated with
dioscin (0.0, 2.9, 5.8 and 11.6 µM) at 37°C for 24 h. After the
dead cells were washed away with PBS, the migration images were
captured using a bright-field light microscope at ×200
magnification and analyzed using ImageJ Software v1.3 (National
Institutes of Health).
Transwell invasion assay
The invasion of A431 cells was measured using 8-µm
Transwell chambers and the filter membrane was coated with 60 µl
Matrigel at 37°C for 24 h (BD Biosciences). A total of
6×104 cells in serum-free medium (200 µl) were added
into the top invasion chambers, while the lower chamber was filled
with 500 µl medium containing 15% FBS. After incubation with
dioscin (0.0, 2.9, 5.8 and 11.6 µM) at 37°C for 24 h, the cells
were fixed with 4% formaldehyde at 37°C for 20 min and stained with
hematoxylin at 37°C for 20 min. A phase-contrast light microscope
(Nikon Corporation) was used to count cells in five randomly
selected fields at ×200 magnification.
TUNEL assay
Cell apoptosis was analyzed using the
TransDetect® In situ Fluorescein TUNEL Cell
Apoptosis Detection kit based on the manufacturer's protocol. A431
cells in 24-well plates were washed with PBS, fixed with 4%
paraformaldehyde at 37°C for 20 min and permeabilized with 0.5%
Triton-100. Subsequently, 150 µl green fluorescein-labeled dUTP
solution was added on the surface of samples and incubated at 37°C
for 1 h. Then, 50 µl 50% glycerinum was used as the mounting
medium. Finally, the images were captured using a fluorescence
microscope (Olympus Corporation; magnification, ×200) and cells
were counted under the microscope in five random fields. The data
were analyzed using ImageJ Software v1.4 (National Institutes of
Health).
Comet assay
The extent of DNA damage was determined using the
Comet assay kit according to the manufacturer's protocol (Cell
Biolabs, Inc.). A total of 2×105 cells/well of A431
cells were incubated in 6-well plates. After being treated with
different concentrations of dioscin (2.9, 5.8 and 11.6 µM) at 37°C
for 24 h, cell images were captured using a fluorescence microscope
at ×200 magnification (Olympus Corporation). Finally, the Comet
Assay Software Project v1.2.2 (CaspLab) was used to analyze the
selected cells (50 cells from each of the two replicate
slides).
Immunofluorescence assay
A431 cells were incubated in a moist chamber at 4°C.
After pretreatment, the cells were fixed with 4% paraformaldehyde
for 20 min at 37°C and incubated with 3% BSA (Beijing Solarbio
Science & Technology Co., Ltd.) for 2 h at 37°C. Subsequently,
the cells were incubated overnight with rabbit anti-p53, cleaved
PARP and cleaved-caspase-3 antibodies (1:1,00), and p-ATM (1:50)
(Table SI). After washing with PBS
three times, the samples were incubated with a FITC-conjugated goat
anti-rabbit IgG (1:2,000; cat. no. SA00001-2; ProteinTech Group,
Inc.) at 37°C for 1 h, and then the cell nuclei were stained with
DAPI (5 µg/ml) at 37°C for 24 h. The sample images were captured
using a fluorescence microscope (Olympus Corporation) at a
magnification of ×200.
Western blot assay
The proteins from A431 cells treated with dioscin
(0, 2.9, 5.8 and 11.6 µM at 37°C for 24 h) were extracted using the
Protein Extraction kit. After measuring the protein concentrations
using the BCA protein assay kit, 50 µg/lane of protein samples were
separated via 10–15% SDS-PAGE and transferred to a PVDF membrane
(EMD Millipore). Subsequently, the membranes were incubated with 5%
milk for 3 h at 37°C and were then incubated with primary
antibodies (Table SI) at 4°C
overnight, followed by incubation with horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no.
SA00001-2; ProteinTech Group, Inc.) for 2 h at room temperature.
Finally, detection of protein bands was performed using an enhanced
chemiluminescence system on a ChemiDoc XRS imaging system (Bio-Rad
Laboratories, Inc.), and the data were normalized to GAPDH
expression and analyzed using GraphPad Prism 6.01 (GraphPad
Software, Inc.). The protein bands were quantified using ImageJ
Software v1.4 (National Institutes of Health).
Inhibition of p53 and caspase
experiments
A431 cells (2×105 cells/well) were plated
in 6-well plates for 24 h 37°C. For inhibition of caspase, the
cells were pretreated with 20 µM Z-VAD-FMK for 1 h at 37°C before
addition of dioscin (0, 2.9, 5.8 and 11.6 µM) at 37°C for 24 h, and
the expression levels of cleaved-caspase-3/9 were detected as
aforementioned. For inhibition of p53, transfection was applied to
knock down p53 expression using p53-targeted siRNAs (siRNA1,
5′-GCACAGAGGAAGAGAAUCUTT-3′; siRNA2, 5′-GCGCACAGAGGAAGAGAAUTT-3′;
siRNA3, 5′-GAAAUUUGCGUGUGGAGUATT-3′) and a scrambled siRNA
(5′-GCTTCGGCAGCACATATACTA-3′). Subsequently, p53-siRNA and
transfection reagent (Lipofectamine® 2000) were mixed
for 20 min. The A431 cells were then transfected at 37°C for 5 h
with the siRNA liposomes in antibiotic-free cell medium. Finally,
cell proliferation, apoptosis, colony formation and migration, and
the expression levels of p53, procaspase-3/9, cleaved caspase-3/9,
cleaved PARP, Bax, Bcl-2, RHO and cdc42 were detected as
aforementioned. After 24 h of transfection, cells were treated with
11.6 µM dioscin or PBS for 24 h.
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc.) was
used to analyze the data, which are presented as the mean ± SD.
Comparisons between 2 groups were performed using unpaired
Student's t-test, while a one-way ANOVA followed by Tukey's post
hoc test was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cytotoxicity of dioscin in A431
cells
The MTT results demonstrated that dioscin treatment
at the concentrations of 2.9, 5.8 and 11.6 µM for 24 h
significantly inhibited cell viability to 79, 58 and 39%,
respectively, compared with the control (Fig. 1B). The viability rates at
concentrations of 2.9, 5.8 and 11.6 µM for 12 h were 81, 78 and
65%, respectively, while those for 36 h were 66, 41 and 23%,
respectively (Fig. 1B). For
subsequent experiments, cells were exposed to 2.9, 5.8 and 11.6 µM
dioscin for 24 h as the change of inhibition rate was the most
obvious. As presented in Fig. 1C,
bright-field images suggested that cell death was induced by
dioscin.
Effects of dioscin on proliferation,
invasion and migration of A431 cells
As presented in Fig.
1D, dioscin (2.9, 5.8 and 11.6 µM) significantly decreased
colony formation in A431 cells compared with the control. Cell
migration and invasion assays in Fig.
1E suggested that dioscin (2.9, 5.8 and 11.6 µM) suppressed the
invasive and migratory capabilities of A431 cells. In addition, the
effects of wound-healing on A431 cells were significantly inhibited
by dioscin (2.9, 5.8 and 11.6 µM) in a dose-dependent manner. These
results suggested that dioscin significantly inhibited the
proliferation, migration and invasion of A431 cells.
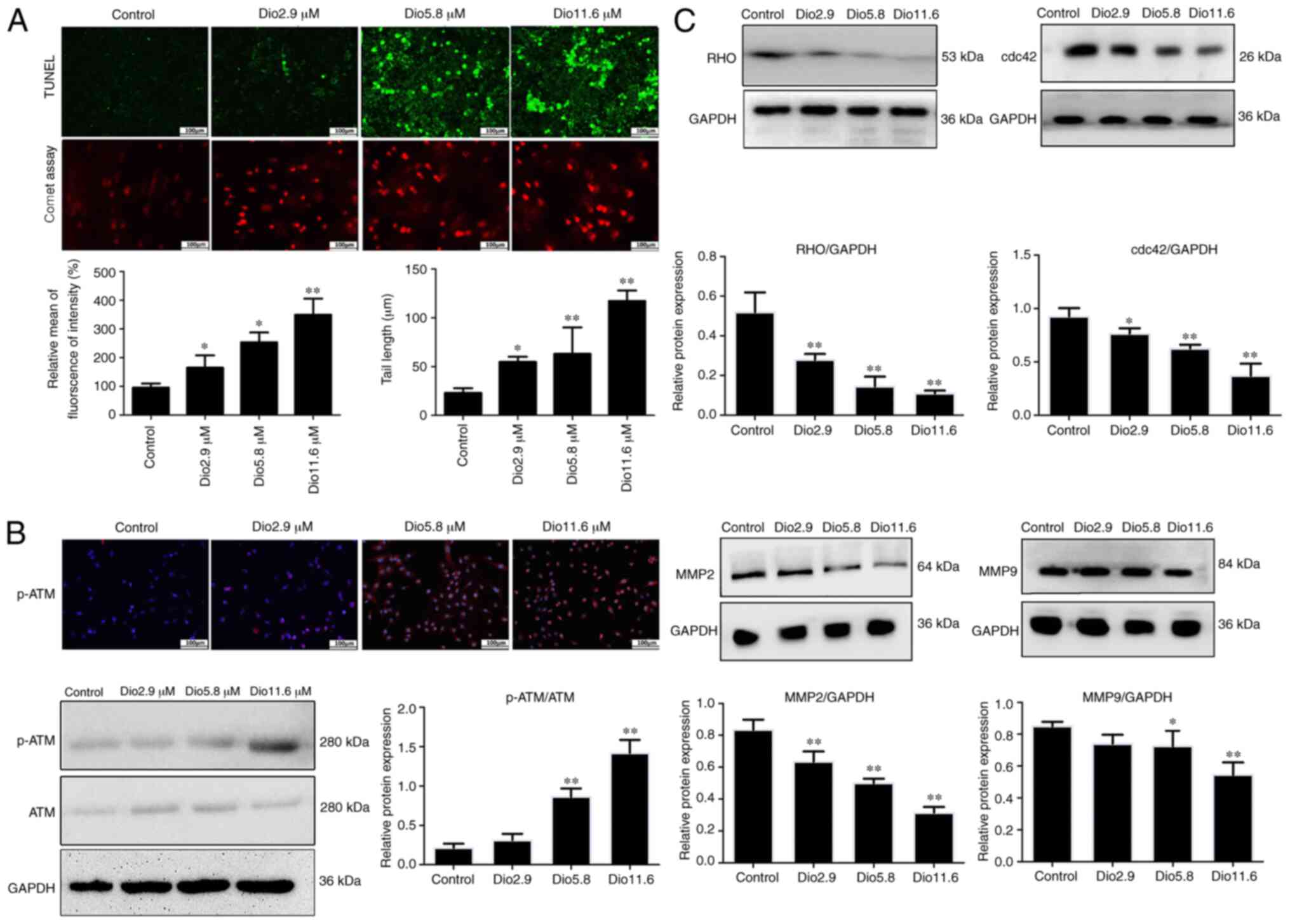
Dioscin induces apoptosis and DNA
damage in A431 cells
In order to investigate the inhibitory effects of
dioscin on apoptosis, apoptosis was measured using a TUNEL assay.
As presented in Fig. 2A,
TUNEL-positive cells were significantly increased by dioscin
compared with the control. In a comet assay, dioscin decreased the
contents of head DNA and significantly increased the length of DNA
migration smears (the comet tails) compared with the control group
(Fig. 2A), which demonstrated that
dioscin significantly caused DNA damage in A431 cells
Dioscin activates ATM
autophosphorylation in vitro
In order to investigate the impact of dioscin on
ATM, A431 cells were treated with different concentrations of
dioscin and the expression levels of p-ATM were assessed via
immunofluorescence. The results presented in Fig. 2B indicated that, compared with the
control group, p-ATM expression was increased by dioscin. In
addition, the western blot analysis results indicated that 5.8 and
11.6 µM dioscin significantly upregulated the expression levels of
p-ATM compared with the control group (Fig. 2B).
Dioscin inhibits the expression levels
of MMP2/9 and Rho GTPase family protein
As presented in Fig.
2C, dioscin significantly decreased the expression levels of
MMP2/9, RHO and cdc42 compared with the control group. These
results indicated that dioscin may suppress cell invasion and
migration via inhibiting MMP2/9, RHO and cdc42 in human A431
cells.
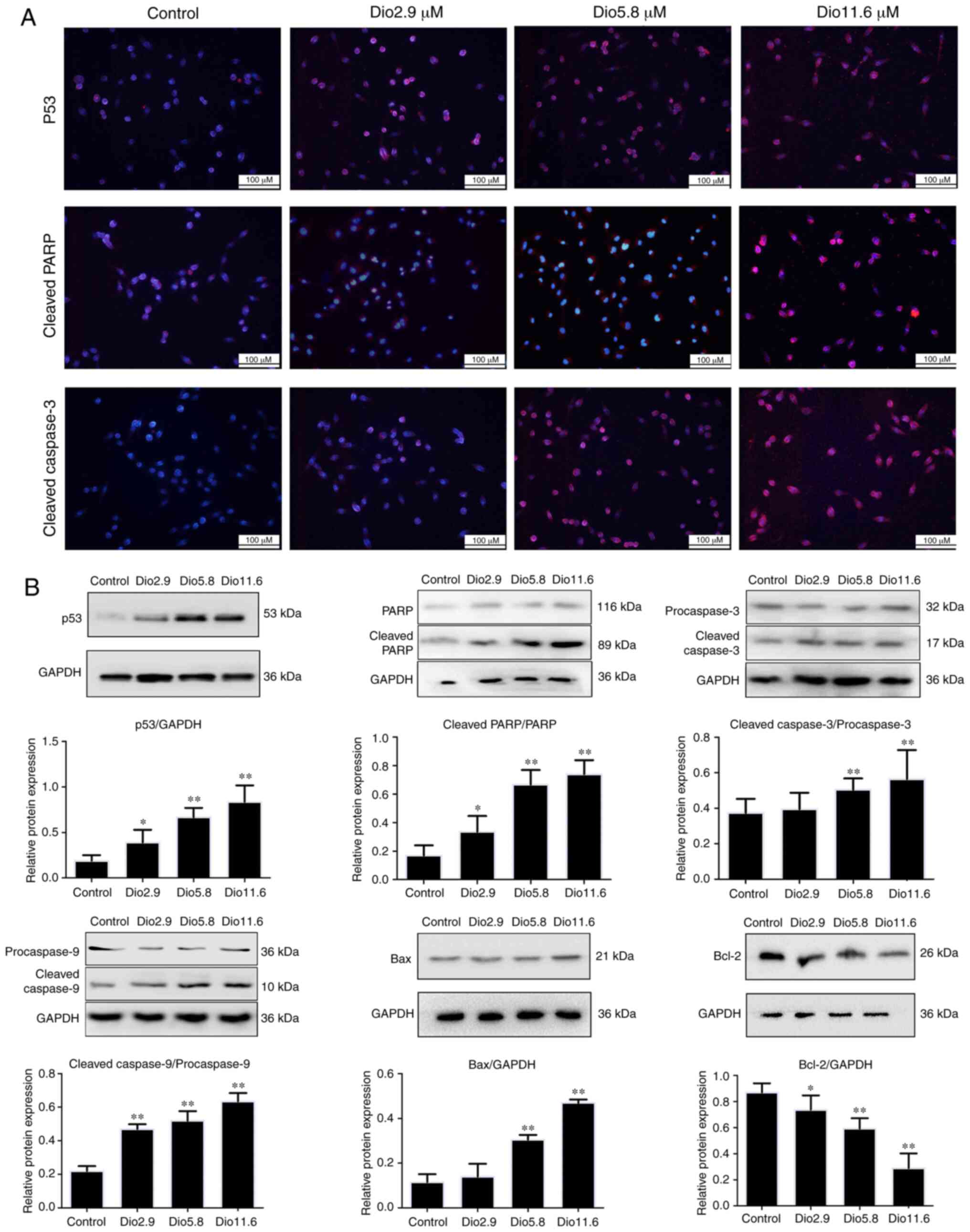
Dioscin activates p53 signal pathway
associated with cell apoptosis
As presented in Fig. 3A
and B, immunofluorescence and western blot analysis revealed
that the expression levels of p53, cleaved-PARP and
cleaved-caspase-3 were significantly upregulated by dioscin
compared with those in the control group. Furthermore, compared
with the control group, dioscin significantly increased the
expression levels of cleaved-caspase-9 and Bax, and significantly
inhibited Bcl-2 expression (Fig.
3B). These data suggested that dioscin may induce apoptosis of
A431 cells via the p53 signaling pathway.
Effect of p53 siRNA and caspase
inhibitor on dioscin-induced apoptosis, migration and DNA damage in
cells
In order to investigate the effect of p53 in the
anticancer activity of dioscin, p53-siRNA transfection experiments
were performed in the present study. Knockdown of p53 was performed
using 3 siRNAs, and p53 siRNA2 was selected for subsequent
experiments since it induced the most significant decrease in p53
protein expression (Fig. S1). As
presented in Fig. 4A, compared with
the control group, dioscin significantly inhibited the viability of
A431 cells, which was then ameliorated by p53 siRNA. Notably,
compared with the dioscin group, the group treated with dioscin
plus p53 siRNA exhibited increased viability of cancer cells, but
this was not significantly different compared with the p53 siRNA
group, which indicated that p53-siRNA restored the inhibitory
effect of dioscin on A431 cells. Furthermore, p53 siRNA ameliorated
the effects of dioscin on colony formation (Fig. 4B), migration and apoptosis in A431
cells (Figs. 4C and D and S2. Furthermore, the expression levels of
p53, cleaved caspase-3/9, cleaved PARP and Bax were all notably
decreased compared with the dioscin group, and the Bcl-2 expression
level was markedly increased compared with the dioscin group, while
the protein levels of RHO and cdc42 were markedly upregulated
following transfection with p53-siRNA with or without dioscin
compared with the dioscin group (Fig.
4E). Additionally, the levels of caspase-3/9 were downregulated
after treatment with Z-VAD compared with the dioscin group
(Fig. S3). These data suggested
that p53-siRNA transfection abrogated the effect of dioscin on the
p53 signaling pathway.
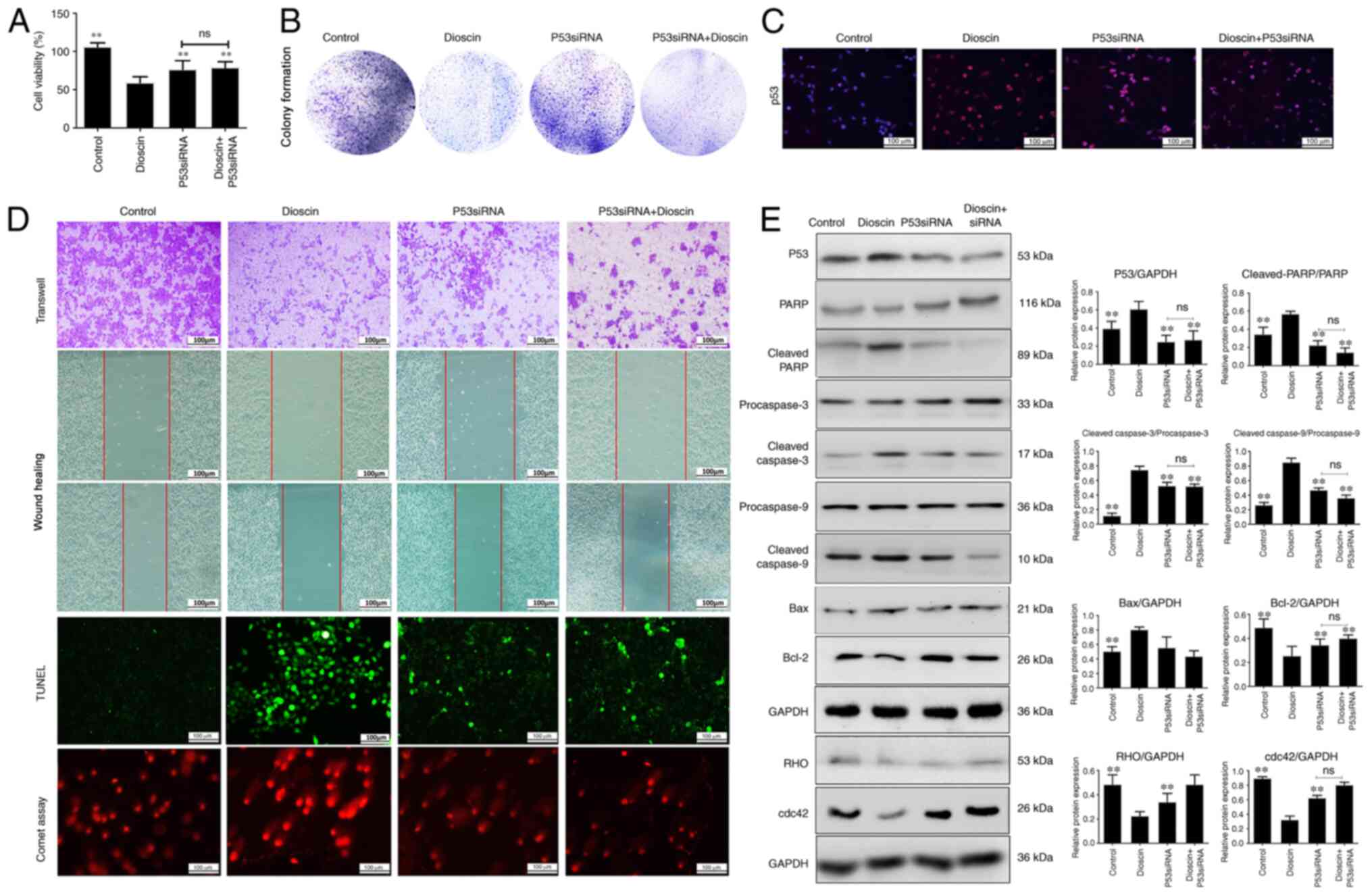 | Figure 4.p53-siRNA abrogates the inhibitory
effects of dioscin on A431 cells. (A) A431 cells were transfected
with p53-siRNA and the viability of cells was measured. Effects of
dioscin and p53-siRNA on (B) cell colony formation ability, (C)
cell migration, apoptosis and DNA damage, (D) p53 expression via
immunofluorescence and (E) protein expression levels of
cleaved-PARP, cleaved caspase-3/9, Bax, Bcl-2, RHO and cdc42. Scale
bar, 100 µm. Data are presented as the mean ± SD (n=3). **P<0.01
vs. dioscin group. PARP, poly (ADP-ribose) polymerase; ns, not
significant; siRNA, small interfering RNA. |
Discussion
Skin cancer is one of the most prevalent
malignancies in the world, and ~20% of the population will develop
skin cancer at some point during their lifetime (41). In recent years, as the specific
pathogenesis of skin cancer remains unclear, and the clinical
treatment of skin cancer is also limited, the incidence of skin
cancer has been increasing by 3–5% per year (4). As an active ingredient in herbs,
dioscin has been reported to exert beneficial actions against liver
cancer, head and neck cancer, pancreatic cancer and lung cancer in
previous studies (13,36). In addition, it has been reported that
dioscin-loaded mixed micelles exhibit antitumor activities, which
may benefit from the significant increase in cellular uptake of
dioscin in MCF-7 cancer cells, suggesting that dioscin-loaded mixed
micelles may be a potential nano-drug delivery system for cancer
chemotherapy (42). However, to the
best of our knowledge, there is currently no study available that
describes the anticancer effects of dioscin on skin cancer.
Therefore, the inhibitory effect of dioscin on A431 cells was
investigated in the present study, and the results demonstrated
that cell proliferation and colony formation of A431 cells were
markedly inhibited by dioscin. The current results suggested that
dioscin may have the potential to treat skin cancer.
DNA damage response leads to DNA repair, which is
closely associated with cell survival or cell death. The stress of
DNA damage activates ATM signaling pathways, including ATM
autophosphorylation and phosphorylation of p53 (43). p53 is a nuclear transcription factor
that becomes stabilized and activated by a variety of cellular
stresses, such as DNA damage, oxidative stress and hypoxia
(44). Once activated, ATM
phosphorylates various downstream molecules, such as p53 and H2AX,
resulting in cell death (45). A
study has reported that kaempferol induces ROS production,
phosphorylation of ATM and activation of p53 protein levels
(46). In the present study, the
comet assay revealed that following dioscin treatment, the DNA
content was transferred to the tail of the comet, suggesting that
DNA damage was increased, and the number of apoptotic cells was
increased by dioscin treatment. In addition, it was revealed that
dioscin markedly upregulated the levels of p-ATM and p53 using
western blotting and immunofluorescence assays. The present data
indicated that dioscin had potent activity against skin cancer
cells in vitro via regulating DNA damage and apoptosis.
Mitochondrial apoptotic signaling is important in
apoptotic cell death. The BCL-2 family contains 2 classes of
anti-apoptotic and pro-apoptotic members (47). BCL-2 is an anti-apoptotic member,
while BAX is a pro-apoptotic member that is primarily distributed
on the mitochondrial membrane, and the loss of mitochondrial
membrane potential is closely associated with the release of
cytochrome c (48). Caspases
are vital in cells for apoptosis. Activation of both the exogenous
promoters caspase-8 and the mitochondria-associated caspase-9
activates downstream caspases, such as caspase-3, leading to cell
apoptosis (49). In the present
study, it was revealed that dioscin markedly decreased the levels
of Bcl-2, and upregulated the levels of Bax, cleaved-PARP and
cleaved caspase-3/9. Overall, the aforementioned results indicated
that dioscin induced its effects in A431 cells via ATM/p53
signaling to activate mitochondrial-mediated apoptosis.
MMPs are a family of proteins involved in the
degradation of the extracellular matrix and the basement membrane
component, and they are regarded as zinc-dependent proteinases that
promote the invasion and metastasis of malignant tumors (50). Rho GTPases are a class of small
GTP-binding proteins that belong to the Ras superfamily, and serve
a crucial role in regulating cell migration, proliferation and
apoptosis (51). The dysregulation
of GTPases occurs in numerous types of human cancer, such as
hepatocellular carcinoma, gastric cancer and prostate cancer, which
contributes to local cancer cell proliferation and distant
metastasis (52,53). Notably, the results in the present
study revealed that the expression levels of RHO, cdc42 and MMP2/9
were decreased by dioscin, which may inhibit the development and
progression of tumor metastasis. Further transfection experiments
using p53-siRNA in vitro revealed that p53-siRNA reversed
the effects of dioscin on A431 cell colony formation, migration,
apoptosis and DNA damage. In addition, p53 knockdown abrogated the
effects of dioscin on the expression levels of cleaved caspase-3/9,
cleaved-PARP, Bax, Bcl-2, RHO and cdc42, indicating that dioscin
exerted anticancer effects against skin cancer cells via regulating
p53 signaling.
Overall, the present study demonstrated that dioscin
inhibited cell proliferation and migration, and induced apoptosis
and DNA damage in human A431 cells through ATM/p53 signaling.
Further research is required to investigate the underlying
mechanisms, drug targets and clinical applications of dioscin
against skin cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant nos. 81973735
and 81774184).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW and CL designed the experiments and wrote the
manuscript. PW and CW performed the cell experiments. PW performed
the western blot assays. PW and CW performed the gene transfection
experiments. PW and CL edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trakatelli M, Ulrich C, del Marmol V,
Euvrard S, Stockfleth E and Abeni D: Epidemiology of nonmelanoma
skin cancer (NMSC) in Europe: Accurate and comparable data are
needed for effective public health monitoring and interventions. Br
J Dematol. 156 (Suppl 3):S1–S7. 2007. View Article : Google Scholar
|
|
2
|
Karia PS, Han J and Schmults CD: Cutaneous
squamous cell carcinoma: Estimated incidence of disease, nodal
metastasis, and deaths from disease in the United States, 2012. J
Am Acad Dermatol. 68:957–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Peng YS, Chen PJ, Wang ML, Cao C,
Xiong H, Zhang J, Chen MH, Peng XB and Zeng K: Peroxisome
proliferator-activated receptor-γ agonist-mediated inhibition of
cell growth is independent of apoptosis in human epidermoid
carcinoma A431 cells. Oncol Lett. 15:6578–6584. 2018.PubMed/NCBI
|
|
4
|
Zhai XX, Ding JC, Tang ZM, Li JG, Li YC,
Yan YH, Sun JC and Zhang CX: Effects of resveratrol on the
proliferation, apoptosis and telomerase ability of human A431
epidermoid carcinoma cells. Oncol Lett. 11:3015–3018. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li H, Tang Z, Chu P, Song Y, Yang Y, Sun
B, Niu M, Qaed E, Shopit A, Han G, et al: Neuroprotective effect of
phosphocreatine on oxidative stress and mitochondrial dysfunction
induced apoptosis in vitro and in vivo: Involvement of dual
PI3K/Akt and Nrf2/HO-1 pathways. Free Radic Biol Med. 120:228–238.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Afaq F and Katiyar SK: Polyphenols: Skin
photoprotection and inhibition of photocarcinogenesis. Mini Rev Med
Chem. 11:1200–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sajadimajd S, Bahramsoltani R, Iranpanah
A, Kumar Patra J, Das G, Gouda S, Rahimi R, Rezaeiamiri E, Cao H,
Giampieri F, et al: Advances on natural polyphenols as anticancer
agents for skin cancer. Pharmacol Res. 151:1045842020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Xu Y, Qi Y, Xu L, Song S, Yin L,
Tao X, Zhen Y, Han X, Ma X, et al: Protective effects of dioscin
against doxorubicin-induced nephrotoxicity via adjusting
FXR-mediated oxidative stress and inflammation. Toxicology.
378:53–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia Y, Jia C, Xue Q, Jiang J, Xie Y, Wang
R, Ran Z, Xu F, Zhang Y and Ye T: Antipsychotic drug
trifluoperazine suppresses colorectal cancer by inducing G0/G1
arrest and apoptosis. Front Pharmacol. 10:10292019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao H, Sun Y, Song S, Qi Y, Tao X, Xu L,
Yin L, Han X, Xu Y, Li H, et al: Protective effects of dioscin
against lipopolysaccharide-induced acute lung injury through
inhibition of oxidative stress and inflammation. Front Pharmacol.
8:1202017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li XJ, Li ZF, Wang JJ, Han Z, Liu Z and
Liu BG: Effects of microRNA-374 on proliferation, migration,
invasion, and apoptosis of human SCC cells by targeting Gadd45a
through P53 signaling pathway. Biosci Rep. 37:BSR201707102017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao Z, Han X, Chen D, Xu Y, Xu L, Yin L,
Sun H, Qi Y, Fang L, Liu K and Peng J: Potent effects of dioscin
against hepatocellular carcinoma through regulating TP53-induced
glycolysis and apoptosis regulator (TIGAR)-mediated apoptosis,
autophagy and DNA damage. Br J Pharmacol. 176:919–937. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Si L, Xu L, Yin L, Qi Y, Han X, Xu Y, Zhao
Y, Liu K and Peng J: Potent effects of dioscin against pancreatic
cancer via miR-149-3P-mediated inhibition of the Akt1 signaling
pathway. Br J Pharmacol. 174:553–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun D, Yu M, Li Y, Xing H, Gao Y, Huang Z,
Hao W, Lu K, Kong C, Shimozato O, et al: Histone deacetylase 2 is
involved in DNA damage-mediated cell death of human osteosarcoma
cells through stimulation of the ATM/p53 pathway. FEBS Open Bio.
9:478–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lavin MF and Kozlov S: ATM activation and
DNA damage response. Cell Cycle. 6:931–942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu YL, Chia CC, Chen PJ, Huang SE, Huang
SC and Kuo PL: Shallot and licorice constituent isoliquiritigenin
arrests cell cycle progression and induces apoptosis through the
induction of ATM/p53 and initiation of the mitochondrial system in
human cervical carcinoma HeLa cells. Mol Nutr food Res. 53:826–835.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao X, Sun X, Yin L, Han X, Xu L, Qi Y, Xu
Y, Li H, Lin Y, Liu K and Peng J: Dioscin ameliorates cerebral
ischemia/reperfusion injury through the downregulation of TLR4
signaling via HMGB-1 inhibition. Free Radical Bio Med. 84:103–115.
2015. View Article : Google Scholar
|
|
18
|
Sun M, Ren J, Du H, Zhang Y, Zhang J, Wang
S and He L: A combined A431 cell membrane chromatography and online
high performance liquid chromatography /mass spectrometry method
for screening compounds from total alkaloid of Radix Caulophylli
acting on the human EGFR. J Chromatogr B Analyt Technol Biomed Life
Sci. 878:2712–2718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pliskova M, Vondracek J, Kren V, Gazak R,
Sedmera P, Walterova D, Psotova J, Simanek V and Machala M: Effects
of silymarin flavonolignans and synthetic silybin derivatives on
estrogen and aryl hydrocarbon receptor activation. Toxicology.
215:80–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Tao X, Qi Y, Xu L, Yin L and Peng
J: Protective effect of dioscin against doxorubicin-induced
cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial
oxidative stress. Redox Bio. 16:189–198. 2018. View Article : Google Scholar
|
|
21
|
Yao H, Tao X, Xu L, Qi Y, Yin L, Han X, Xu
Y, Zheng L and Peng J: Dioscin alleviates non-alcoholic fatty liver
disease through adjusting lipid metabolism via SIRT1/AMPK signaling
pathway. Pharmacol Res. 131:51–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Mao Z, Xu L, Yin L, Tao X, Tang Z,
Qi Y, Sun P and Peng J: Protective effect of dioscin against
intestinal ischemia/reperfusion injury via adjusting
miR-351-5p-mediated oxidative stress. Pharmacol Res. 137:56–63.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng L, Han X, Hu Y, Zhao X, Yin L, Xu L,
Qi Y, Xu Y, Han X, Liu K and Peng J: Dioscin ameliorates intestinal
ischemia/ reperfusion injury via adjusting
miR-351-5p/MAPK13-mediated inflammation and apoptosis. Pharmacol
Res. 139:431–439. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiao Y, Xu L, Tao X, Yin L, Qi Y, Xu Y,
Han X, Tang Z, Ma X, Liu K and Peng J: Protective effects of
dioscin against fructose-induced renal damage via adjusting
Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and
inflammation. Toxicol Lett. 284:37–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng L, Yin L, Xu L, Qi Y, Li H, Xu Y,
Han X, Liu K and Peng J: Potective effect of dioscin against
thioacetamide-induced acute liver injury via FXR/AMPK signaling
pathway in vivo. Biomed Pharmacother. 97:481–488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin L, Qi Y, Xu Y, Xu L, Han X, Tao X,
Song S and Peng J: Dioscin inhibits HSC-T6 cell migration via
adjusting SDC-4: Insights from iTRAQ-based quantitative proteomics.
Front Pharmacol. 8:6652017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Tao X, Yin L, Xu L, Xu Y, Qi Y,
Han X, Song S, Zhao Y, Lin Y, et al: Protective effects of dioscin
against cisplatin-induced nephrotoxicity via regulating
miR-34a/Sirt1 signal pathway. Br J Pharmacol. 174:2512–2527. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao H, Xu Y, Yin L, Tao X, Xu L, Qi Y, Han
X, Sun P, Liu K and Peng J: Dioscin protects ANIT-induced
intrahepatic cholestasis through regulating transporters, apoptosis
and oxidative stress. Front Pharmacol. 8:1162017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Tao X, Han X, Xu L, Yin L, Qi Y,
Zhao Y, Xu Y, Wang C and Peng J: Dioscin attenuates gastric
ischemia/reperfusion injury through the down-regulation of
PKC/ERK1/2 signaling via PKCα and PKCβ2 inhibition. Chem Bio
Interact. 258:234–244. 2016. View Article : Google Scholar
|
|
30
|
Qi M, Yin L, Xu L, Tao X, Qi Y, Han X,
Wang C, Xu Y, Sun H, Liu K and Peng J: Dioscin alleviates
lipopolysaccharide-induced inflammatory kidney injury via the
microRNA let-7i/TLR4/MyD88 signaling pathway. Pharmacol Res.
111:509–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tao X, Yin L, Xu L and Peng J: Dioscin: A
diverse acting natural compound with therapeutic potential in
metabolic diseases, cancer, inflammation and infections. Pharmacol
Res. 137:259–269. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Yin L, Fang L, Xu L, Sun P, Xu M,
Liu K and Peng J: Protective effects of dioscin against systemic
inflammatory response syndromevia adjusting TLR2/MyD88/NF-κb signal
pathway. Int Immunopharmacol. 65:458–469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv L, Zheng L, Dong D, Xu L, Yin L, Xu Y,
Qi Y, Han X and Peng J: Dioscin, a natural steroid saponin, induces
apoptosis and DNA damage through reactive oxygen species: A
potential new drug for treatment of glioblastoma multiforme. Food
Chem Toxicol. 59:657–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Si L, Zheng L, Xu L, Yin L, Han X, Qi Y,
Xu Y, Wang C and Peng J: Dioscin suppresses human laryngeal cancer
cells growth via induction of cell-cycle arrest and MAPK-mediated
mitochondrial-derived apoptosis and inhibition of tumor invasion.
Eur J Pharmacol. 774:105–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tao X, Xu L, Yin L, Han X, Qi Y, Xu Y,
Song S, Zhao Y and Peng J: Dioscin induces prostate cancer cell
apoptosis through activation of estrogen receptor-β. Cell Death
Dis. 8:e29892017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei Y, Xu Y, Han X, Qi Y, Xu L, Xu Y, Yin
L, Sun H, Liu K and Peng J: Anti-cancer effects of dioscin on three
kinds of human lung cancer cell lines through inducing DNA damage
and activating mitochondrial signal pathway. Food Chem Toxicol.
59:118–128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao X, Tao X, Xu L, Yin L, Qi Y, Xu Y,
Han X and Peng J: Dioscin induces apoptosis in human cervical
carcinoma HeLa and SiHa cells through ROS-mediated DNA damage and
the mitochondrial signaling pathway. Molecules. 21:7302016.
View Article : Google Scholar
|
|
38
|
Zhao X, Xu L, Zheng L, Yin L, Qi Y, Han X,
Xu Y and Peng J: Potent effects of dioscin against gastric cancer
in vitro and in vivo. Phytomedicine. 23:274–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kou Y, Ji L, Wang H, Wang W, Zheng H, Zhou
J, Liu L, Qi X, Liu Z, Du B and Lu L: Connexin 43 upregulation by
dioscin inhibits melanoma progression via suppressing malignancy
and inducing M1 polarization. Int J Cancer. 141:1690–1703. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Fu M, Liu J, Yang Y, Yu Y, Li J,
Pan W, Fan L, Li G, Li X and Wang X: Inhibition of tumor metastasis
by targeted daunorubicin and dioscin codelivery liposomes modified
with PFV for the treatment of non-small-cell lung cancer. Int J
Nanomedicine. 14:4071–4090. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zeng N, Hongbo T, Xu Y, Wu M and Wu Y:
Anticancer activity of caffeic acid nbutyl ester against A431 skin
carcinoma cell line occurs via induction of apoptosis and
inhibition of the mTOR/PI3K/AKT signaling pathway. Mol Med Rep.
17:5652–5657. 2018.PubMed/NCBI
|
|
42
|
Zhao J, Xu Y, Wang C, Ding Y, Chen M, Wang
Y, Peng J, Li L and Lv L: Soluplus/TPGS mixed micelles for dioscin
delivery in cancer therapy. Drug Dev Ind Pharm. 43:1197–1204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T,
He TC, Du W and Yuan CS: Genistein induces G2/M cell cycle arrest
and apoptosis via ATM/p53-dependent pathway in human colon cancer
cells. Int J Oncol. 43:289–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ling G, Ahmadian A, Persson A, Unden AB,
Afink G, Williams C, Uhlén M, Toftgård R, Lundeberg J and Pontén F:
PATCHED and p53 gene alterations in sporadic and hereditary basal
cell cancer. Oncogene. 53:7770–7778. 2001. View Article : Google Scholar
|
|
45
|
Chang IY, Kim JH, Park KH and Yoon SP:
Experimental varicocele induces p53-dependent germ cell apoptosis
through activation of γ-H2AX. Urol Int. 85:216–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee CF, Yang JS, Tsai FJ, Chiang NN, Lu
CC, Huang YS, Chen C and Chen FA: Kaempferol induces
ATM/p53-mediated death receptor and mitochondrial apoptosis in
human umbilical vein endothelial cells. Int J Oncol. 48:2007–2014.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maira F, Catania A, Candido S, Russo AE,
McCubrey JA, Libra M, Malaponte G and Fenga C: Molecular targeted
therapy in melanoma: A way to reverse resistance to conventional
drugs. Curr Drug Deliv. 9:17–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu J, Yin S, Reddy N, Spencer C and Sheng
S: Bax mediates the apoptosis-sensitizing effect of maspin. Cancer
Res. 64:1703–1711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nikolaou V, Stratigos A, Bafaloukos D and
Katsambas A: Antiangiogenic and antiapoptotic treatment in advanced
melanoma. Clin Dermatol. 31:257–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sarig-Nadir O and Seliktar D: The role of
matrix metalloproteinases in regulating neuronal and nonneuronal
cell invasion into PEGylated fibrinogen hydrogels. Biomaterials.
31:6411–6416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhu Y, Zhou J, Xia H, Chen X, Qiu M, Huang
J, Liu S, Tang Q, Lang N, Liu Z, et al: The Rho GTPase RhoE is a
p53-regulated candidate tumor suppressor in cancer cells. Int J
Oncol. 44:896–904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Takeba Y, Matsumoto N, Watanabe M,
Takenoshita-Nakaya S, Ohta Y, Kumai T, Takagi M, Koizumi S, Asakura
T and Otsubo T: The Rho kinase inhibitor fasudil is involved in
p53-mediated apoptosis in human hepatocellular carcinoma cells.
Cancer Chemother Pharmacol. 69:1545–1555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dornan D, Shimizu H, Mah A, Dudhela T, Eby
M, O'rourke K, Seshagiri S and Dixit VM: ATM engages
autodegradation of the E3 ubiquitin ligase COP1 after DNA damage.
Science. 313:1122–1126. 2006. View Article : Google Scholar : PubMed/NCBI
|