Introduction
The incidence and mortality rates of lung cancer are
one of the highest amongst all types of malignant tumors, which
affects the physical and mental health of patients (1). According to the pathological subtypes,
lung cancer is divided into non-small cell lung cancer (NSCLC) and
small-cell lung cancer (SCLC), of which ~15% of all cases are SCLC.
The common characteristics of this subtype include high malignancy,
a short tumor doubling time, rapid growth, and strong invasive
ability (1). Thus, patients with
SCLC are prone to distant metastasis in the early stage and poor
prognosis (2,3). Several histological and
immunohistochemical markers are used in the diagnosis of SCLC,
including thyroid transcription factor-1, cytokeratin 7, Leu-7,
chromogranin A and synaptophysin (4). These tests have guided significance and
are beneficial for the diagnosis of SCLC; however, they are
expensive, the waiting time is long, and they are only used as
diagnostic indicators, and so cannot be used to determine
prognosis. Thus, it remains critical to identify sensitive and
accurate indicators to assess prognosis and guide early clinical
treatment.
Hematological assessment is extensively used in
clinical settings due to its convenience, production of fast
results, low costs, and low invasiveness. Currently, several
studies have implemented serum indexes to predict the prognosis of
patients with SCLC, including carcinoembryonic antigen, neuron
specific enolase and gastrin releasing peptide precursor; however,
their sensitivity and specificity are poor (5–7).
Biochemical tests are commonly used for serum index tests, and the
results of serum cystatin C (Cys C), uric acid (UA) and lactate
dehydrogenase (LDH) are included in the biochemical tests.
Cys C is a non-glycosylated basic protein encoded by
the CST3 gene (8). It has been
hypothesized that Cys C may be overexpressed in tumor cells,
resulting in increased circulating levels (8,9). UA is a
product of xanthine oxidoreductase (XOR) oxidation between xanthine
and hypoxanthine (10). Ames et
al (10) hypothesized that UA is
a key antioxidant that acts against different types of human
cancer; however, Vona-Davis et al (11) proposed that the proinflammatory
effect of UA promotes the occurrence and development of cancer. LDH
catalyzes the conversion of pyruvate and lactate during glycolysis
and gluconeogenesis, respectively (12). A previous study has confirmed that
increased glycolysis promotes the progression of malignant tumors
(12).
It is well-known that Cys C, UA and LDH all affect
tumor growth and invasion (11,13,14);
however, the association between Cys C, UA and LDH and prognosis of
patients with SCLC remains unclear. Thus, the present study aimed
to investigate the association between serum concentrations of Cys
C, UA and LDH prior to chemotherapy and the prognosis of patients
with SCLC, with the potential to discover independent prognostic
factors. The results presented here can be applied in clinical
settings to assess patients at an early stage of the disease, and
provide novel strategies for identifying the changes in SCLC.
Materials and methods
Patients
The present study was a retrospective analysis that
selected patients pathologically diagnosis with SCLC at the
Affiliated Hospital of Qingdao University between April 2015 and
December 2018. The inclusion criteria were as follows: SCLC was
diagnosed by fiberoptic bronchoscopy or puncture biopsy and no
antitumor treatment was administered prior to diagnosis. The
exclusion criteria were as follows: i) SCLC was diagnosed by
postoperative pathology; ii) History of other malignancies; iii)
Patients with renal insufficiency (serum creatinine, >1.5 times
the upper limit of normal value or creatinine clearance rate <50
ml/min) and iv) Patients with liver dysfunction [alanine
aminotransferase (ALT)/aspartate aminotransferase (AST) >2.5
times upper limit of the normal value, or ALT/AST >5 times upper
limit of normal value in liver metastasis]. Based on these
criteria, 230 patients were selected and followed-up. A total of 25
patients were lost to follow-up, with a loss rate of 10.87%; thus,
205 patients with SCLC were included in the final cohort, including
161 men and 44 women (mean age, 62 years; age range, 41–76 years).
The present study was approved by the Ethics Committee of Qingdao
University Hospital (Shandong, China; approval. no. QYFYWZLL25870)
and informed consent was provided by all patients prior to the
study start.
Data collection
The clinicopathological data of the patients were
collected and analyzed at diagnosis, including sex, age, smoking
status, primary tumor site, stage (according to Veterans
Administration Lung Study Group) (15), Cys C, UA, LDH, urea nitrogen,
creatinine, alkaline phosphatase, adenosine deamination enzymes,
neutrophil to lymphocyte ratio and platelet to lymphocyte ratio.
During follow-up, the treatment plan was assessed, and first-line
chemotherapy was recorded. In addition, administration of
radiotherapy and the imaging assessment results were also recorded.
The clinicopathological characteristics of patients with SCLC are
presented in Table I.
 | Table I.Clinicopathological characteristics
of 205 patients with small-cell lung cancer. |
Table I.
Clinicopathological characteristics
of 205 patients with small-cell lung cancer.
| Characteristic | Number of patients,
% or median (range) |
|---|
| Sex |
|
|
Male | 161, 78.54 |
|
Female | 44, 21.46 |
| Age, years |
|
|
<60 | 87, 42.44 |
|
≥60 | 118, 57.56 |
| Smoking status |
|
|
Yes | 142, 69.27 |
| No | 63, 30.73 |
| Primary lesion |
|
| Right
lung | 126, 61.46 |
| Left
lung | 79, 38.54 |
| Clinical stage |
|
| LS | 107, 52.20 |
| ES | 98, 47.80 |
| First-line
chemotherapy |
|
|
Etoposide and platinum | 175, 85.37 |
|
Irinotecan and platinum | 30, 14.63 |
| Radiotherapy |
|
|
Yes | 110, 53.66 |
| No | 95, 46.34 |
| Cys C (mg/l) | 0.79
(0.34–1.77) |
| UA (umol/l) | 285.00
(77.30–579.00) |
| LDH (U/l) | 199.00
(102.30–837.00) |
| Urea nitrogen
(mmol/l) | 5.10
(1.53–11.77) |
| Creatinine
(umol/l) | 80.00
(31.00–126.10) |
| Alkaline
phosphatase (U/l) | 76.00
(38.00–248.67) |
| Adenosine deaminase
(U/l) | 10.00
(2.00–26.00) |
| Neutrophil to
lymphocyte ratio | 2.44
(0.56–20.94) |
| Platelet to
lymphocyte ratio | 158.60
(55.21–516.19) |
Progression-free survival (PFS) and overall survival
(OS) were the observation indicators that were recorded. Follow-up
methods included review of the electronic medical record system via
the telephone. The follow-up period was between September 2019 to
December 2019.
Statistical analysis
Statistical analysis was performed using SPSS v25.0
software (IBM Corp.). Receiver operating characteristic (ROC) curve
and the area under the curve (AUC) graphs were constructed to
determine diagnostic accuracy. The larger the area, the greater the
diagnostic accuracy is to determine the optimal cut-off value for
the serum index. Survival analysis was performed using the
Kaplan-Meier method and log-rank test. The Cox proportional hazards
model was used to identify factors associated with prognosis, while
univariate and multivariate analyses was performed to identify
independent prognostic factors. The χ2 test was used to
assess the association between UA and LDH concentrations and
different disease stages. P<0.05 was considered to indicate a
statistically significant difference.
By consulting the literature, it was found that the
mOS of patients with SCLC was 18 months (1). Disease progression is used as a
grouping standard to draw ROC curve. The patients were divided into
the disease progressive group and the non-progressive disease
group. In addition, according to the cut-off value of each index,
patients were also divided into the high value group and the low
value group.
Results
Clinicopathological
characteristics
As presented in Table
I, 205 patients diagnosed with SCLC were enrolled in this
retrospective study, including 161 men (78.54%) and 44 women
(21.46%). A total of 87 patients (42.44%) were <60 years old,
while 118 patients (57.56%) were ≥60 years old. In addition, 142
patients (69.27%) had a history of smoking, while 63 patients
(30.73%) did not. Primary lesions in the right lung was observed in
126 patients (61.46%), while 79 patients (38.54%) had primary
lesions in the left lung. At initial diagnosis, 107 patients
(52.20%) had limited stage, while 98 patients (47.80%) had
extensive stage SCLC. For the application of first-line
chemotherapy following diagnosis, 175 patients (85.37%) were
treated with etoposide and platinum, while 30 patients (14.63%)
were treated with irinotecan and platinum. During treatment, 110
patients (53.66%) received chest radiotherapy, while 95 patients
(46.34%) did not. Notably, detection of Cys C concentration was not
included in the routine assessment of patients before July 2016 at
the Affiliated Hospital of Qingdao University, thus Cys C
concentration was only measured in 152/205 patients included. Data
of the other indicators were collected from all 205 patients.
ROC and survival curve analyses
The optimal cut-off value for the serum index was
determined according to the most approximate index, and the optimal
sensitivity and specificity were exhibited. Disease progression was
used as the grouping standard, and patients were divided into
non-progressive disease, including 96 patients (46.83%) and disease
progressive groups, including 109 patients (53.17%). For the ROC
curve, the continuous variables, Cys C, UA and LDH were used, and
the binary variable was whether the disease was progressing. The
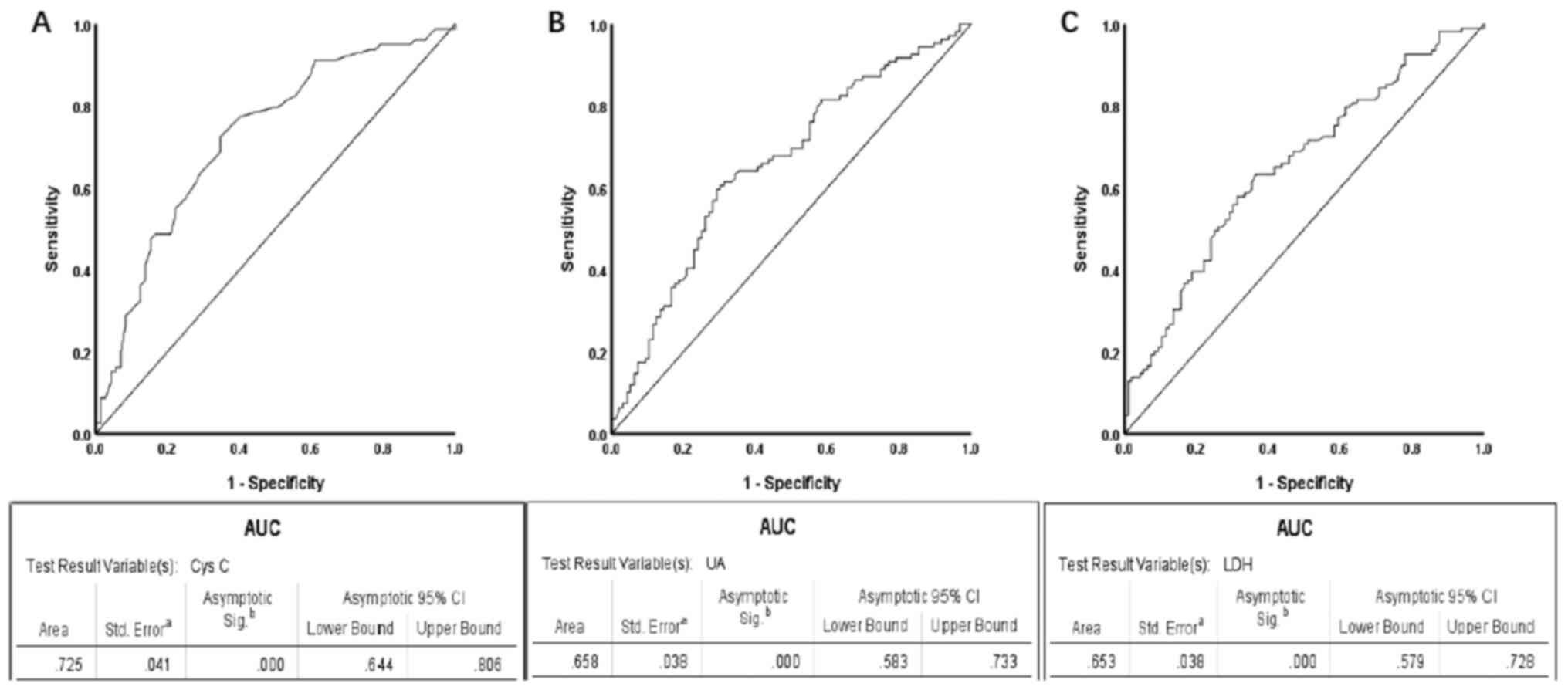
optimal cut-off value for serum Cys C was 0.775 mg/l (sensitivity,
0.725 and specificity, 0.653), while the AUC was 0.725 [95%
confidence interval (CI), 0.644–0.806; P<0.001; Fig. 1A]. Based on these values, Cys C
concentration was lower than the cut-off value in 69 patients
(45.39%), who were classified into the low Cys C group, while Cys C
concentration was higher than or equal to the cut-off value in 83
patients (54.61%), who were classified into the high Cys C group.
The optimal cut-off value for UA was 296.45 µmol/l (sensitivity,
0.596 and specificity, 0.708), while the AUC was 0.658 (95% CI,
0.583–0.733; P<0.001; Fig. 1B).
Based on these values, UA concentration was lower than the cut-off
value in 112 patients (54.63%), who were classified into the low UA
group, while UA concentration was higher than or equal to the
cut-off value in 93 patients (45.37%), who were classified into the
high UA group. The optimal cut-off value for LDH was 198.5 U/l
(sensitivity, 0.633 and specificity, 0.635), while the AUC was
0.653 (95% CI, 0.579–0.728; P<0.001; Fig. 1C). Based on these values, LDH
concentration was lower than the cut-off value in 101 patients
(49.27%), who were classified into the low LDH group, while LDH
concentration was higher than or equal to the cut-off value in 104
patients (50.73%), who were classified into the high LDH group
(Table II).
 | Table II.Diagnostic value of ROC curves for
Cys C, UA and LDH. |
Table II.
Diagnostic value of ROC curves for
Cys C, UA and LDH.
| Variable | AUC | P-value | 95% CI | Sensitivity | Specificity | Cut-off value | Low group, n
(%) | High group, n
(%) |
|---|
| Cys C | 0.725 | <0.001 | 0.644–0.806 | 0.725 | 0.653 | 0.775 mg/l | 69
(45.39) | 83 (54.61) |
| UA | 0.658 | <0.001 | 0.583–0.733 | 0.596 | 0.708 | 296.450 µmol/l | 112 (54.63) | 93 (45.37) |
| LDH | 0.653 | <0.001 | 0.579–0.728 | 0.633 | 0.635 | 198.500 U/l | 101 (49.27) | 104 (50.73) |
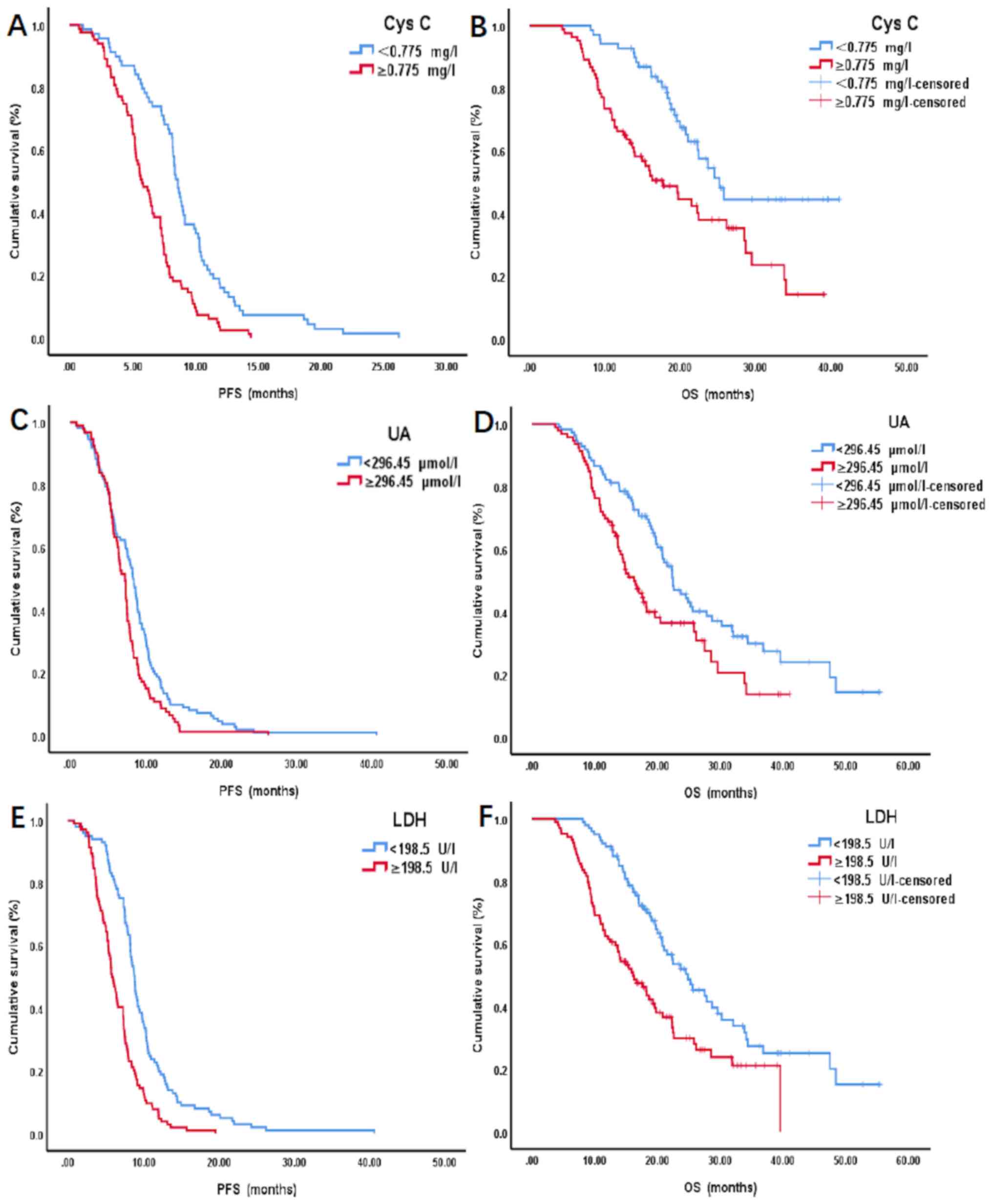
Survival analysis was performed using the
Kaplan-Meier method and log-rank test. The results demonstrated
that the survival time of patients in the high Cys C group (≥0.775
mg/l) was significantly shorter compared with the low Cys C group
(<0.775 mg/l), for both mean (m) PFS (5.70 months vs. 8.57
months; P<0.001) and mOS (14.67 months vs. 19.57 months;
P<0.001) (Fig. 2A and B).
Similarly, the survival time of patients in the high UA group
(≥296.45 µmol/l) was significantly shorter compared with the low UA
group (<296.45 µmol/l), for both mPFS (6.67 vs. 8.27 months;
P=0.010) and mOS (14.77 vs. 19.64 months; P=0.003) (Fig. 2C and D). In addition, the survival
time of patients in the high LDH group (≥198.5 U/l) was
significantly shorter compared with the low LDH group (<198.5
U/l), for both mPFS (5.75 vs. 8.73 months; P<0.001) and mOS
(14.64 vs. 19.60 months; P<0.001) (Fig. 2E and F).
The 1-, 2- and 3-year survival rates of the patients
were subsequently assessed. As presented in Table III, the survival rates of the low
value groups were higher compared with the high value groups, and
treatment in the low value groups was more effective compared with
that in the high value groups.
 | Table III.Survival rates of patients. |
Table III.
Survival rates of patients.
|
| OS (Low group) | OS (High
group) |
|---|
|
|
|
|
|---|
| Variable | >1 year, % | >2 years, % | >3 years, % | >1 year, % | >2 years, % | >3 years, % |
|---|
| Cys C | 92.75 | 26.09 | 8.70 | 66.27 | 20.48 | 2.41 |
| UA | 82.14 | 31.25 | 10.71 | 69.89 | 17.20 | 4.30 |
| LDH | 91.09 | 32.67 | 12.87 | 62.50 | 17.31 | 2.88 |
Univariate and multivariate
analyses
Univariate analysis of the clinicopathological
characteristics demonstrated that PFS was significantly associated
with: Smoking status (P=0.013), clinical stage (P<0.001),
first-line chemotherapy (P=0.012), radiotherapy (P<0.001), Cys C
(P<0.001), LDH (P<0.001) and alkaline phosphatase (P=0.001).
In addition, OS was significantly associated with: Smoking status
(P=0.011), clinical stage (P=0.001), radiotherapy (P<0.001), Cys
C (P=0.020), UA (P=0.027) and LDH (P<0.001) (Table IV).
 | Table IV.Univariate analysis of
clinicopathological characteristics of 205 patients with small-cell
lung cancer. |
Table IV.
Univariate analysis of
clinicopathological characteristics of 205 patients with small-cell
lung cancer.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Sex |
|
|
|
|
|
|
| Male
vs. Female | 0.079 | 0.741 | 0.530–1.035 | 0.137 | 0.724 | 0.473–1.109 |
| Age, years |
|
|
|
|
|
|
| <60
vs. ≥60 | 0.372 | 1.136 | 0.859–1.503 | 0.599 | 0.911 | 0.642–1.291 |
| Smoking status |
|
|
|
|
|
|
| Yes vs.
No | 0.013 | 0.685 | 0.507–0.924 | 0.011 | 0.598 | 0.402–0.890 |
| Primary lesion |
|
|
|
|
|
|
| Right
lung vs. Left lung | 0.552 | 1.089 | 0.822–1.445 | 0.345 | 0.840 | 0.585–1.206 |
| Clinical stage |
|
|
|
|
|
|
| LS vs.
ES | <0.001 | 1.756 | 1.324–2.330 | 0.001 | 1.775 | 1.251–2.519 |
| First-line
chemotherapy |
|
|
|
|
|
|
|
Etoposide vs. Irinotecan | 0.012 | 1.659 | 1.116–2.466 | 0.087 | 1.518 | 0.941–2.451 |
| Radiotherapy |
|
|
|
|
|
|
| Yes vs.
No | <0.001 | 2.289 | 1.727–3.035 | <0.001 | 2.054 | 1.442–2.926 |
| Cys C | <0.001 | 3.649 | 1.792–7.430 | 0.020 | 3.171 | 1.198–8.390 |
| UA | 0.068 | 1.001 | 1.000–1.003 | 0.027 | 1.002 | 1.000–1.004 |
| LDH | <0.001 | 1.005 | 1.004–1.007 | <0.001 | 1.005 | 1.003–1.007 |
| Urea nitrogen | 0.930 | 1.000 | 1.000–1.001 | 0.250 | 1.000 | 1.000–1.001 |
| Creatinine | 0.714 | 1.002 | 0.993–1.011 | 0.615 | 1.003 | 0.991–1.051 |
| Alkaline
phosphatase | 0.001 | 1.011 | 1.005–1.017 | 0.495 | 1.002 | 0.996–1.009 |
| Adenosine
deaminase | 0.346 | 1.019 | 0.980–1.059 | 0.570 | 1.014 | 0.967–1.063 |
| Neutrophil to
lymphocyte ratio | 0.909 | 1.003 | 0.956–1.052 | 0.382 | 1.026 | 0.968–1.088 |
| Platelet to
lymphocyte ratio | 0.258 | 1.001 | 0.999–1.003 | 0.989 | 1.000 | 0.998–1.002 |
These factors were included in the multivariate
analysis and the results demonstrated that smoking status
(P=0.033), radiotherapy (P<0.001), Cys C (P=0.005), LDH
(P<0.001) and alkaline phosphatase (P<0.001) were independent
prognostic factors for PFS, while radiotherapy (P=0.034) and LDH
(P<0.001) were independent prognostic factors for OS (Table V).
 | Table V.Multivariate analysis of
clinicopathological characteristics of 205 patients with small-cell
lung cancer. |
Table V.
Multivariate analysis of
clinicopathological characteristics of 205 patients with small-cell
lung cancer.
|
|
| PFS | OS |
|---|
|
|
|
|
|
|---|
| Variable |
| P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Smoking status | Yes vs. No | 0.033 | 0.675 | 0.471–0.968 | 0.271 | 0.750 | 0.449–1.252 |
| Clinical stage | LS vs. ES | 0.884 | 0.972 | 0.669–1.414 | 0.533 | 1.166 | 0.719–1.890 |
| First-line
chemotherapy | Etoposide vs.
Irinotecan | 0.478 | 1.184 | 0.742–1.891 | – | – | – |
| Radiotherapy | Yes vs. No | <0.001 | 2.234 | 1.551–3.218 | 0.034 | 1.648 | 1.039–2.615 |
| Cys C |
| 0.005 | 3.153 | 1.413–7.037 | 0.256 | 1.954 | 0.615–6.213 |
| UA |
| – | – | – | 0.522 | 0.999 | 0.996–1.002 |
| LDH |
| <0.001 | 1.004 | 1.002–1.005 | <0.001 | 1.005 | 1.003–1.007 |
| Alkaline
phosphatase |
| <0.001 | 1.012 | 1.006–1.019 | – | – | – |
χ2 test
Indicators such as LDH and UA are associated with
tumor burden (14,16). The χ2 test was used to
assess the association between the concentrations of UA or LDH, and
different disease stages. For UA, χ2=5.755, P=0.016, the
concentration was associated with the stage of the disease. The
concentration will increase in the ES. For LDH,
χ2=9.957, P=0.002, the concentration was also associated
with the stage of the disease. The concentration will increase in
the ES.
Discussion
The results of the present study confirmed that
elevated serum levels of Cys C, UA and LDH prior to chemotherapy
were significantly associated with shorter PFS and OS times in
patients with SCLC. The serum LDH concentration prior to
chemotherapy may be an independent prognostic factor for PFS and OS
in patients with SCLC, while Cys C concentration prior to
chemotherapy may be independent prognostic factor for PFS in
patients with SCLC.
LDH consists of two subunits, A and B, which are
encoded by LDH-A and LDH-B, respectively (17–19).
LDH-A has been identified as a direct target gene of c-Myc
oncogenic transcription factor (17–19). LDH
is a key enzyme in glycolysis and gluconeogenesis that catalyzes
the mutual conversion of pyruvate and lactic acid; thus, it is
essential for energy metabolism. LDH is released during tissue
damage and has been reported to be involved in tumor growth,
metastasis and metabolism (17).
Previous studies have demonstrated that LDH concentration is an
important prognostic factor for tumor progression and metastasis in
different types of cancer, including colon, nasopharyngeal, breast,
prostate, and germ cell cancers and melanoma (20–28).
In the present study, ROC curves were used to
determine the optimal cut-off value of LDH concentrations prior to
chemotherapy, and the patients were divided into high and low
groups for survival analysis. The results demonstrated that
patients in the high LDH group had significantly shorter mPFS and
mOS times compared with patients in the low LDH group. Thus,
increased LDH concentration prior to chemotherapy was associated
with shorter PFS and OS times in patients with SCLC. Univariate and
multivariate analyses demonstrated that LDH concentration prior to
chemotherapy may be an independent prognostic factor. Increased LDH
concentration was associated with rapid disease progression, a
short survival time and poor prognosis. This may be due to factors
such as LDH, hypoxia-inducible factor 1 (HIF-1) and vascular
endothelial growth factor (VEGF), which associate tumor metabolism
with angiogenesis (14,29–31).
Under the regulation of HIF-1 and VEGF, tumor cells rapidly
proliferate and contribute to disease progression (14,29–31). In
addition, previous studies focusing on malignant tumors have
demonstrated that increased LDH levels are associated with the
tolerance of patients to chemoradiotherapy (32,33).
Taken together, these results suggest that the concentration of LDH
prior chemotherapy is associated with the prognosis of patients
with SCLC.
Cys C is a non-glycosylated basic protein encoded by
the CST3 gene. Cys C is continuously transcribed and expressed in
all nucleated cells (8). It is not
affected by factors such as age, sex and weight, and does not
change under inflammatory conditions (8). Previous studies have reported an
association between Cys C and cancer; however, these findings are
inconsistent. For example, Ervin et al (34) demonstrated that Cys C plays an
important role in inhibiting melanoma lung metastasis, while
Naumnik et al (13) reported
that Cys C concentrations are significantly higher in patients with
NSCLC compared with healthy individuals. A previous study by Sevier
and Kaiser (35) demonstrated that
there are no significant differences in Cys C expression between
lung squamous cell carcinoma tissues and normal lung tissues. Thus,
the association between Cys C expression and the prognosis of
patients with SCLC was investigated in the present study. Notably,
the effect of Cys C concentration on the prognosis of patients
prior to treatment was assessed, thus Cys C levels were detected
following diagnosis. Given that the patients did not receive any
treatment, Cys C concentration was not affected by chemotherapy
drugs. Based on the ROC curve, the optimal cut-off value of Cys C
concentration prior to chemotherapy was 0.775 mg/l. Patients were
subsequently divided into high and low Cys C groups for survival
analysis. The results demonstrated that patients in the high Cys C
had significantly shorter mPFS and mOS times compared with patients
in the low Cys C group. Thus, increased Cys C concentration prior
to chemotherapy was associated with shorter PFS and OS times in
patients with SCLC. Univariate and multivariate analyses
demonstrated that Cys C concentration prior to chemotherapy may be
an independent prognostic factor for PFS of patients with SCLC.
Notably, Cys C concentration was not identified as an independent
prognostic factor for OS, and thus cannot be used alone to predict
patient survival. Increased Cys C concentration was associated with
rapid disease progression and poor prognosis. This may be due to
the following reasons, the process of tumor cell division and
proliferation requires folic acid as a coenzyme to participate in
the synthesis of nucleic acids, leading to the accumulation of
homocysteine in the body (36). Cys
C is a well-known cysteine protease inhibitor C, which can bind
with cysteine protease to inhibit homocysteine activity, which in
turn increases Cys C concentration (36). In addition, the imbalance between
cathepsin and protease inhibitor may lead to the invasion and
metastasis of cancer cells, thus further promoting the
concentration of Cys C (37).
Increased Cys C concentration is associated with tumor infiltration
and metastasis (37,38).
UA is a product of XOR oxidizing xanthine and
hypoxanthine (10). Previous studies
have reported an association between UA and cancer; however, these
findings are inconsistent. For example, Ames et al (10) hypothesized that UA, as a powerful
antioxidant, is a scavenger of free radicals, which can inhibit
lipid peroxidation under high concentrations and exert anticancer
effects. However, it has been demonstrated that UA promotes the
development of inflammation, and plays a key role in the
development of breast cancer (11).
Elevated UA expression increases the risk of colorectal, breast and
prostate cancers (39–43). In the present study, the optimal
cut-off value of UA concentration prior to chemotherapy was 312.75
µmol/l. Based on this value, patients were divided into high and
low UA groups for survival analysis and the results demonstrated
that patients in the high UA group had significantly shorter mPFS
and mOS times compared with patients in the low UA group. Notably,
univariate and multivariate analyses demonstrated that UA
concentration prior to chemotherapy was not an independent
prognostic factor for PFS and OS, and thus cannot be used alone to
predict disease progression and the survival time of patients with
SCLC. Increased UA concentration prior to chemotherapy was
associated with poor prognosis. This may be due to the
proinflammatory nature of UA (16).
Inflammation mediators and cellular effectors are an important part
of the tumor's local environment, whereby the inflammatory response
has been demonstrated to promote tumor proliferation and survival
(16). In addition, UA has the
ability to inhibit XOR expression, and decreased XOR expression
regulates the secretion of COX-2 and MMP-1, which in turn induce
the expression of differentiated protein inhibitors to increase the
aggressiveness of cancer cells (44). Increased UA and decreased XOR
expression levels contribute to the proliferation, migration and
survival of tumor cells (44).
Indicators such as LDH and UA are associated with
tumor burden (14,16). It is speculated that patients with
extensive stage SCLC exhibit poor prognosis compared with other
stages. The χ2 test was used to assess the association
between UA and LDH concentrations, and different disease stages
(Table VI). Patients were divided
into different groups based on their disease stage. Further
analysis demonstrated that there were no significant associations
between the assessed indexes and the prognosis of patients.
Multivariate analysis demonstrated that staging was not an
independent prognostic factor for PFS or OS; thus, staging analysis
was not performed in the present study.
 | Table VI.χ2 test of different
stages, and UA and LDH concentrations. |
Table VI.
χ2 test of different
stages, and UA and LDH concentrations.
|
| UA | LDH |
|---|
|
|
|
|
|---|
| Variable | Low group, n | High group, n | χ2 | P-value | Low group, n | High group, n | χ2 | P-value |
|---|
| LS-SCLC | 67 | 40 | 5.755 | 0.016 | 64 | 43 | 9.957 | 0.002 |
| ES-SCLC | 45 | 53 |
|
| 37 | 61 |
|
|
Given that SCLC is a tumor that is sensitive to
radiotherapy and chemotherapy (45),
the treatment-related factors were also assessed in the preset
study. Etoposide or irinotecan combined with platinum is the most
common chemotherapy regimen for first-line treatment of SCLC
(46). Univariate and multivariate
analyses demonstrated that there were no significant differences in
the effects of both schemes on PFS or OS of patients with SCLC.
Radiotherapy plays an important role in the treatment of SCLC
(47). Consistent with previous
findings (45,47), multivariate analysis in the present
study demonstrated that radiotherapy was an independent prognostic
factor for PFS and OS in patients with SCLC, which was associated
with favorable prognosis.
The present study is not without limitations. First,
all patients who participated were Chinese and predominantly from
coastal areas, thus this may cause selection bias. Secondly, the
sample size was relatively small, which may have also caused
selection bias. Thirdly, this was a retrospective study, which
cannot completely exclude selection bias and information bias. In
addition, even if some interfering factors were excluded, other
confounding factors associated with Cys C, UA and LDH, such as
dietary habits and lifestyle were not included as variables in the
present study. Thus, large-scale prospective and multicenter
studies are required to validate the results presented here.
In conclusion, the results of the present study
demonstrated that Cys C, UA and LDH concentrations in patients with
SCLC, prior to chemotherapy were associated with the prognosis of
patients. Patients with elevated concentrations exhibited shorter
PFS and OS times, and poor prognosis. Notably, high LDH
concentration prior to chemotherapy may be an independent risk
factor for patients with shorter PFS and OS times, while elevated
Cys C levels prior to chemotherapy may be an independent risk
factor for patients with a shorter PFS time. The identification of
these factors will assist with the prediction of the differences in
prognosis in different populations, and further provide new ideas
for determining the changes in SCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no. ZR2017MH062) and
the Science and Technology for People's Livelihood Project of
Qingdao (grant no. 17-3-3-33-nsh).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZY, HW, DS, YD and LZ conceived and designed the
present study. ZY provided administrative support. DS and YD
provided the study materials and patient samples. LZ collected and
assembled the data. HW and XY interpreted and analyzed the data.
HW, DS and YD revised the manuscript for important intellectual
content. All authors drafted the initial manuscript, and have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Qingdao University Hospital (Shandong, China; approval
no. QYFYWZLL25870) and informed consent was provided by all
patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-Cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amini A, Byers LA, Welsh JW and Komaki RU:
Progress in the management of limited-stage small cell lung cancer.
Cancer. 120:790–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiani A, Khosravi A, Moghaddam AS, Jabbari
H and Fakhri M: Long-Term survival of a small cell lung cancer
patient with proper endobronchial management. Pneumologia.
61:245–248. 2012.PubMed/NCBI
|
|
4
|
Poola I and Graziano SL: Expression of
neuron-specific enolase, chromogranin A, synaptophysin and Leu-7 in
lung cancer cell lines. J Exp Clin Cancer Res. 17:165–173.
1998.PubMed/NCBI
|
|
5
|
Yang X, Wang D, Yang Z, Qing Y, Zhang Z,
Wang G, Yang Z and Wang Z: CEA is an independent prognostic
indicator that is associated with reduced survival and liver
metastases in SCLC. Cell Biochem Biophys. 59:113–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Molina R, Auge JM, Escudero JM, Marrades
R, Viñolas N, Carcereny E, Ramirez J and Filella X: Mucins CA 125,
CA 19.9, CA 15.3 and TAG-72.3 as tumor markers in patients with
lung cancer: Comparison with CYFRA 21-1, CEA, SCC and NSE. Tumour
Biol. 29:371–380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winther B, Moi P, Paus E and Reubsaet JL:
Targeted determination of the early stage SCLC specific biomarker
pro-gastrin-releasing peptide (ProGRP) at clinical concentration
levels in human serum using LC-MS. J Sep Sci. 30:2638–2646. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakai K, Kikuchi M, Fujimoto K, Kaneko Y,
Omori S, Nakai K and Suwabe A: Serum levels of cystatin C in
patients with malignancy. Clin Exp Nephrol. 12:132–139. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kos J, Krasovec M, Cimerman N, Nielsen HJ,
Christensen IJ and Brünner N: Cysteine proteinase inhibitors stefin
A, stefin B, and cystatin C in sera from patients with colorectal
cancer: Relation to prognosis. Clin Cancer Res. 6:505–511.
2000.PubMed/NCBI
|
|
10
|
Ames BN, Cathcart R, Schwiers E and
Hochstein P: Uric acid provides an antioxidant defense in humans
against oxidant- and radical-caused aging and cancer: A hypothesis.
Proc Natl Acad Sci USA. 78:6858–6862. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vona-Davis L, Howard-McNatt M and Rose DP:
Adiposity, type 2 diabetes and the metabolic syndrome in breast
cancer. Obes Rev. 8:395–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dang CV and Lewis BC: Role of oncogenic
transcription factor c-myc in cell cycle regulation, apoptosis and
metabolism. J Biomed Sci. 4:269–278. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naumnik W, Niklińska W, Ossolińska M and
Chyczewska E: Serum cathepsin K and cystatin C concentration in
patients with advanced non-small-cell lung cancer during
chemotherapy. Folia Histochem Cytobiol. 47:207–213. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parks SK, Chiche J and Pouysségur J:
Disrupting proton dynamics and energy metabolism for cancer
therapy. Nat Rev Cancer. 13:611–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Micke P, Faldum A, Metz T, Beeh KM,
Bittinger F, Hengstler JG and Buhl R: Staging small cell lung
cancer: Veterans administration lung study group versus
international association for the study of lung cancer-what limits
limited disease? Lung Cancer. 37:271–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghaemi-Oskouie F and Shi Y: The role of
uric acid as an endogenous danger signal in immunity and
inflammation. Curr Rheumatol Rep. 13:160–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fantin VR, St-Pierre J and Leder P:
Attenuation of LDH-A expression uncovers a link between glycolysis,
mitochondrial physiology, and tumor maintenance. Cancer Cell.
9:425–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis BC, Shim H, Li Q, Wu CS, Lee LA,
Maity A and Dang CV: Identification of putative c-myc-responsive
genes: Characterization of rcl, a novel growth-related gene. Mol
Cell Biol. 17:4967–4978. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shim H, Dolde C, Lewis BC, Wu CS, Dang G,
Jungmann RA, Dalla-Favera R and Dang CV: C-Myc transactivation of
LDH-A: Implications for tumor metabolism and growth. Proc Natl Acad
Sci USA. 94:6658–6663. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, Gatter KC, Trarbach T, Folprecht G, Shi MM, Lebwohl D, Jalava T,
Laurent D, et al: Prognostic and predictive role of lactate
dehydrogenase 5 expression in colorectal cancer patients treated
with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin
Cancer Res. 17:4892–4900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turen S, Ozyar E, Altundag K, Gullu I and
Atahan IL: Serum lactate dehydrogenase level is a prognostic factor
in patients with locoregionally advanced nasopharyngeal carcinoma
treated with chemoradiotherapy. Cancer Invest. 25:315–321. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin Y, Ye X, Shao L, Lin BC, He CX, Zhang
BB and Zhang YP: Serum lactic dehydrogenase strongly predicts
survival in metastatic nasopharyngeal carcinoma treated with
palliative chemotherapy. Eur J Cancer. 49:1619–1626. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brown JE, Cook RJ, Lipton A and Coleman
RE: Serum lactate dehydrogenase is prognostic for survival in
patients with bone metastases from breast cancer: A retrospective
analysis in bisphosphonate-treated patients. Clin Cancer Res.
18:6348–6355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Halabi S, Small EJ, Kantoff PW, Kattan MW,
Kaplan EB, Dawson NA, Levine EG, Blumenstein BA and Vogelzang NJ:
Prognostic model for predicting survival in men with
hormone-refractory metastatic prostate cancer. J Clin Oncol.
21:1232–1237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
von Eyben FE, Madsen EL, Liu F, Amato R
and Fritsche H: Serum lactate dehydrogenase isoenzyme 1 as a
prognostic predictor for metastatic testicular germ cell tumours.
Br J Cancer. 83:1256–1259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gerlinger M, Wilson P, Powles T and
Shamash J: Elevated LDH predicts poor outcome of recurrent germ
cell tumours treated with dose dense chemotherapy. Eur J Cancer.
46:2913–2918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egberts F, Kotthoff EM, Gerdes S, Egberts
JH, Weichenthal M and Hauschild A: Comparative study of YKL-40,
S-100B and LDH as monitoring tools for stage IV melanoma. Eur J
Cancer. 48:695–702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agarwala SS, Keilholz U, Gilles E,
Bedikian AY, Wu J, Kay R, Stein CA, Itri LM, Suciu S and Eggermont
AM: LDH correlation with survival in advanced melanoma from two
large, randomised trials (Oblimersen GM301 and EORTC 18951). Eur J
Cancer. 45:1807–1814. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seagroves TN, Ryan HE, Lu H, Wouters BG,
Knapp M, Thibault P, Laderoute K and Johnson RS: Transcription
factor HIF-1 is a necessary mediator of the pasteur effect in
mammalian cells. Mol Cell Biol. 21:3436–3444. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schofield CJ and Ratcliffe PJ: Oxygen
sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 5:343–354.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ostergaard L, Tietze A, Nielsen T, Drasbek
KR, Mouridsen K, Jespersen SN and Horsman MR: The relationship
between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic
glycolysis. Cancer Res. 73:5618–5624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ryberg M, Nielsen D, Osterlind K,
Skovsgaard T and Dombernowsky P: Prognostic factors and long-term
survival in 585 patients with metastatic breast cancer treated with
epirubicin-based chemotherapy. Ann Oncol. 12:81–87. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brizel DM, Schroeder T, Scher RL, Walenta
S, Clough RW, Dewhirst MW and Mueller-Klieser W: Elevated tumor
lactate concentrations predict for an increased risk of metastases
in head-and-neck cancer. Int J Radiat Oncol Biol Physics.
51:349–353. 2001. View Article : Google Scholar
|
|
34
|
Ervin H and Cox JL: Late stage inhibition
of hematogenous melanoma metastasis by cystatin C over-expression.
Cancer Cell Int. 5:142005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sevier CS and Kaiser CA: Formation and
transfer of disulphide bonds in living cells. Nat Rev Mol Cell
Biol. 3:836–847. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi SW and Mason JB: Folate and
carcinogenesis: An integrated scheme. J Nutr. 130:129–132. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saleh Y, Sebzda T, Warwas M, Kopec W,
Ziólkowska J and Siewinski M: Expression of cystatin C in clinical
human colorectal cancer tissues. J Exp Ther Oncol. 5:49–53.
2005.PubMed/NCBI
|
|
38
|
Zhang X, Hou Y, Niu Z, Li W, Meng X, Zhang
N and Yang S: Clinical significance of detection of cathepsin X and
cystatin C in the sera of patients with lung cancer. Zhongguo Fei
Ai Za Zhi. 16:411–416. 2013.(In Chinese). PubMed/NCBI
|
|
39
|
Hammarsten J, Damber JE, Peeker R,
Mellström D and Högstedt B: A higher prediagnostic insulin level is
a prospective risk factor for incident prostate cancer. Cancer
Epidemiol. 34:574–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bjorge T, Lukanova A, Jonsson H, Tretli S,
Ulmer H, Manjer J, Stocks T, Selmer R, Nagel G, Almquist M, et al:
Metabolic syndrome and breast cancer in the me-can (metabolic
syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev.
19:1737–1745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rose DP, Haffner SM and Baillargeon J:
Adiposity, the metabolic syndrome, and breast cancer in
African-American and white American women. Endocr Rev. 28:763–777.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giovannucci E: Metabolic syndrome,
hyperinsulinemia, and colon cancer: A review. Am J Clin Nutr.
86:s836–s842. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Siddiqui AA: Metabolic syndrome and its
association with colorectal cancer: A review. Am J Med Sci.
341:227–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Linder N, Butzow R, Lassus H, Lundin M and
Lundin J: Decreased xanthine oxidoreductase (XOR) is associated
with a worse prognosis in patients with serous ovarian carcinoma.
Gynecol Oncol. 124:311–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Herrmann MK, Bloch E, Overbeck T, Koerber
W, Wolff HA, Hille A, Vorwerk H, Hess CF, Muller M, Christiansen H
and Pradier O: Mediastinal radiotherapy after multidrug
chemotherapy and prophylactic cranial irradiation in patients with
SCLC-treatment results after long-term follow-up and literature
overview. Cancer Radiother. 15:81–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Buyse M, Thirion P, Carlson RW,
Burzykowski T, Molenberghs G and Piedbois P; Mata-analysis Group in
Cancer, : Re: A model to select chemotherapy regimens for phase III
trials for extensive-stage small-cell lung cancer. J Natl Cancer
Inst. 93:399–401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Abdelwahab S, Abdulla H, Azmy A,
Abdelfatah A, Abdel-Aziz H, Margerges M, Riad A, Sharma V and
Dwedar I: Integration of irinotecan and cisplatin with early
concurrent conventional radiotherapy for limited-disease SCLC
(LD-SCLC). Int J Clin Oncol. 14:230–236. 2009. View Article : Google Scholar : PubMed/NCBI
|