Cancer is one of the most common causes of mortality
worldwide. However, it is not only a serious threat to public
health, but also a global socioeconomic burden (1). An estimated 2,814,000 cases of
cancer-related death and 4,292,000 new cancer cases occurred in
China in 2015 (2). Based on GLOBOCAN
(a global cancer statistics database), in 2018 the number of cases
of cancer-related death was 9.6 million, and the number of new
cancer cases was 18.1 million worldwide (3). However, data also indicate a decline in
the number of new cases, which may be associated with lifestyle
changes or reduced exposure to high-risk environmental factors in
developed countries (4).
Accumulating evidence also suggests that the proteins encoded by a
variety of aberrantly-expressed regulatory genes promote
tumorigenesis; these include anti-apoptotic proteins, transcription
factors, growth factors and their respective receptors (5–7).
Tumorigenesis is a multistep process characterized by numerous
abnormalities, rather than a single mutation, during cancer
initiation, promotion and progression; therefore, a single target
agent is unlikely to inhibit cancer growth (8,9).
Currently, the primary treatment strategies against tumors include
the following: Surgery, chemoradiotherapy, immunotherapy, molecular
targeted therapy and Traditional Chinese Medicine. Although
chemotherapy has been proven to improve survival in patients with
cancer, drug resistance and severe adverse side effects, such as
damage to liver function, bone marrow suppression and
neurotoxicity, are major obstacles that cause treatment failure
(10,11). There is therefore an urgent need to
develop novel and more effective drugs with fewer side effects for
various types of cancer.
Due to their selective molecular targets, novel
bioactive components from plant sources have emerged as new and
reliable therapeutic elements for treating various types of human
cancer (12,13). Indeed, over the past half century,
numerous plant derivatives and secondary metabolites have been used
in clinical practice for the treatment of cancer (14,15). For
example, pentacyclic triterpenes constitute a group of promising
anticancer drugs that comprise the lupane, oleanane and ursane
groups (16,17). Since Pisha et al (18) first reported in 1995 that betulinic
acid (19), a plant secondary
metabolite, is a highly promising anticancer drug, experimental
studies have largely focused on the cytotoxic effects of betulinic
acid and other types of triterpenes, particularly their
apoptosis-inducing mechanisms, initially in melanoma cell lines
in vitro and in vivo (20–22). The
cytotoxic effects of betulinic acid were subsequently confirmed in
other cell lines, such as those derived from breast (23), colon and lung cancer (24), as well as neuroblastoma (25). In the last decade, triterpenes were
also found to have additional effects on cancer through several
modes of action, such as induction of apoptosis and enhancement of
endoplasmic reticulum (ER) stress (23–25).
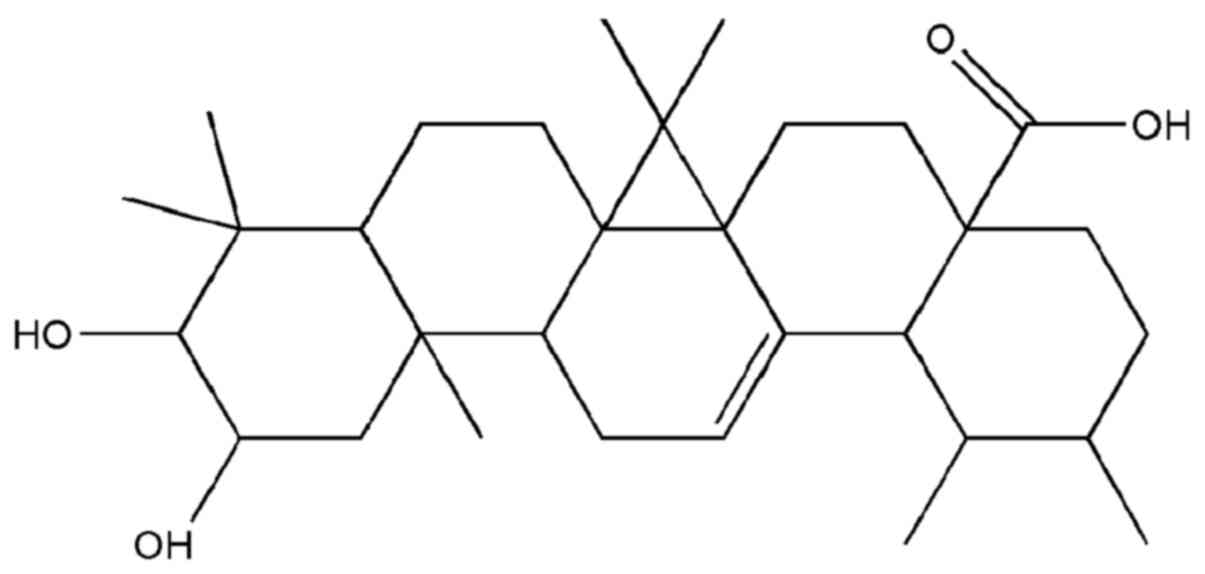
Corosolic acid, also known as 2α-hydroxyursolic
acid, has a molecular formula of
C30H48O4, and a molecular weight
of 472.70 g/mol (Fig. 1). As a
prevalent pentacyclic triterpenoid and the principal component of
Banaba leaves, corosolic acid has received a great deal of
attention due to its anti-diabetic properties (26). Corosolic acid is known as a
‘phyto-insulin’ or ‘botanical insulin’ (27). It is the principal component of
Lagerstroemia speciosa leaves (also called Banaba), a
tropical plant found in the Philippines, Vietnam, Malaysia and
Southern China (28,29). Table I
lists the plant species able to biosynthesize corosolic acid
(28–50). Corosolic acid has also been isolated
from European and South American plants.
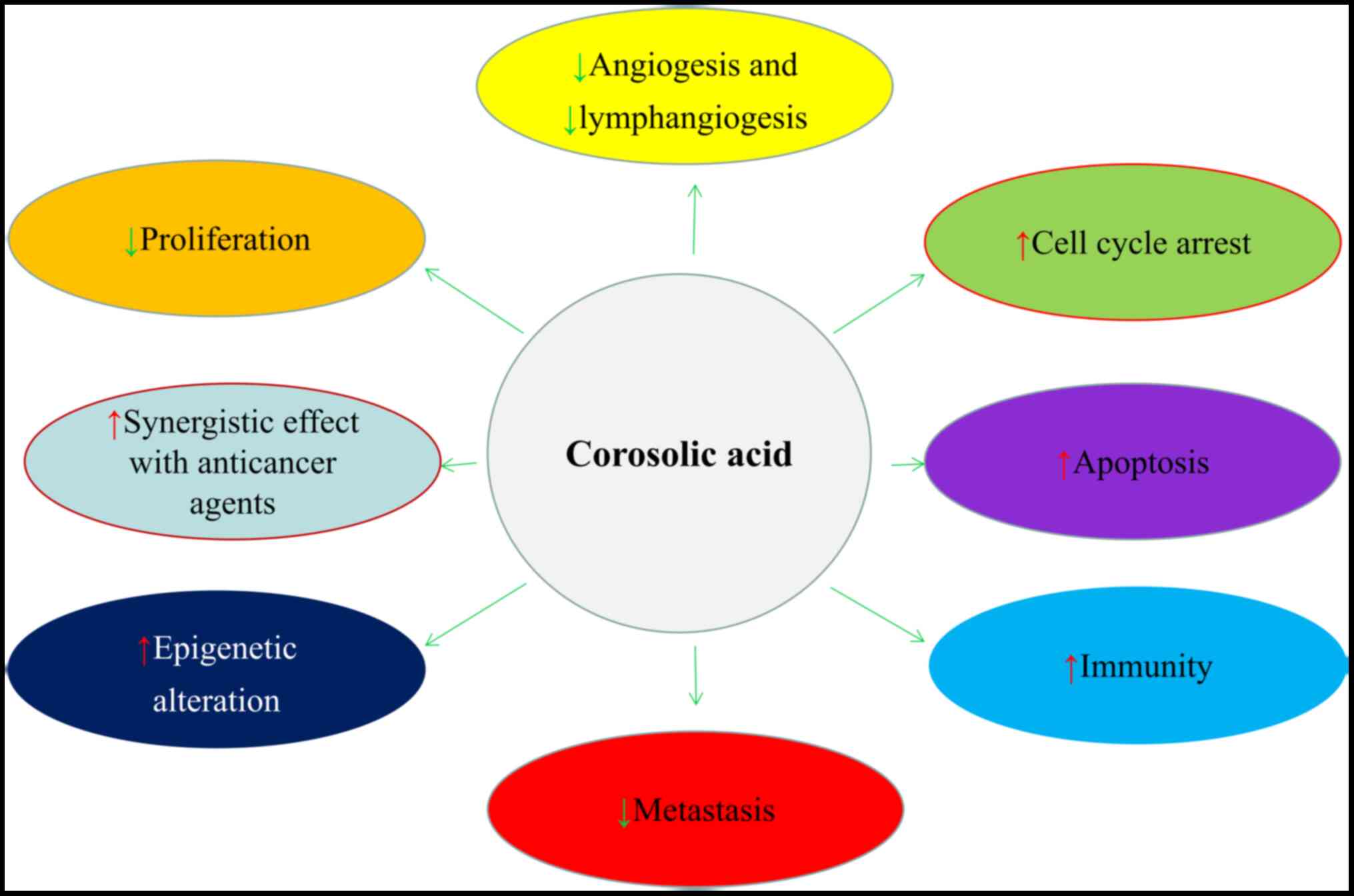
Experimental studies have indicated that corosolic
acid plays a pivotal anticancer role in several tumorigenic
processes in vitro and in vivo, including cellular
proliferation, apoptosis, angiogenesis, lymphangiogenesis,
metastasis and tumor immunity, and it exerts a synergistic effect
when administered with other anticancer agents (Fig. 2) (51–53). In
addition, corosolic acid has the ability to modulate multiple
cancer-related signaling pathways and processes, such as the
nuclear factor kappa-B (NF-κB), phosphatidylinositol 3
kinase/protein kinase B (PI3K/Akt) and Wnt/β-catenin pathways,
apoptosis, nuclear factor erythroid 2-related factor 2 (Nrf2) and
several other components associated with cellular proliferation or
mortality (Table II) (49,51,54,55).
However, more research is required to determine its potential in
human clinical trials. The most recent registry data from
Surveillance, Epidemiology and End Results shows that the morbidity
of liver and intrahepatic bile duct cancers have risen on average
3.0% each year between 2004 and 2013 in the United States (56). In particular, hepatocellular
carcinoma (HCC) is an aggressive cancer with a poor prognosis.
Chronic liver diseases, such as hepatitis B and C virus infections,
alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD)
and cirrhosis are the most common underlying causes of HCC
(41). NAFLD in particular, has been
recognized as one of the leading etiologies for the development of
HCC (57,58). NAFLD encompasses a spectrum of
chronic liver diseases, ranging from simple steatosis to liver
injury, which are closely associated with metabolic syndrome (MS)
and are characterized by conditions such as obesity, diabetes and
dyslipidemia (59–61). The understanding of the pathogenesis
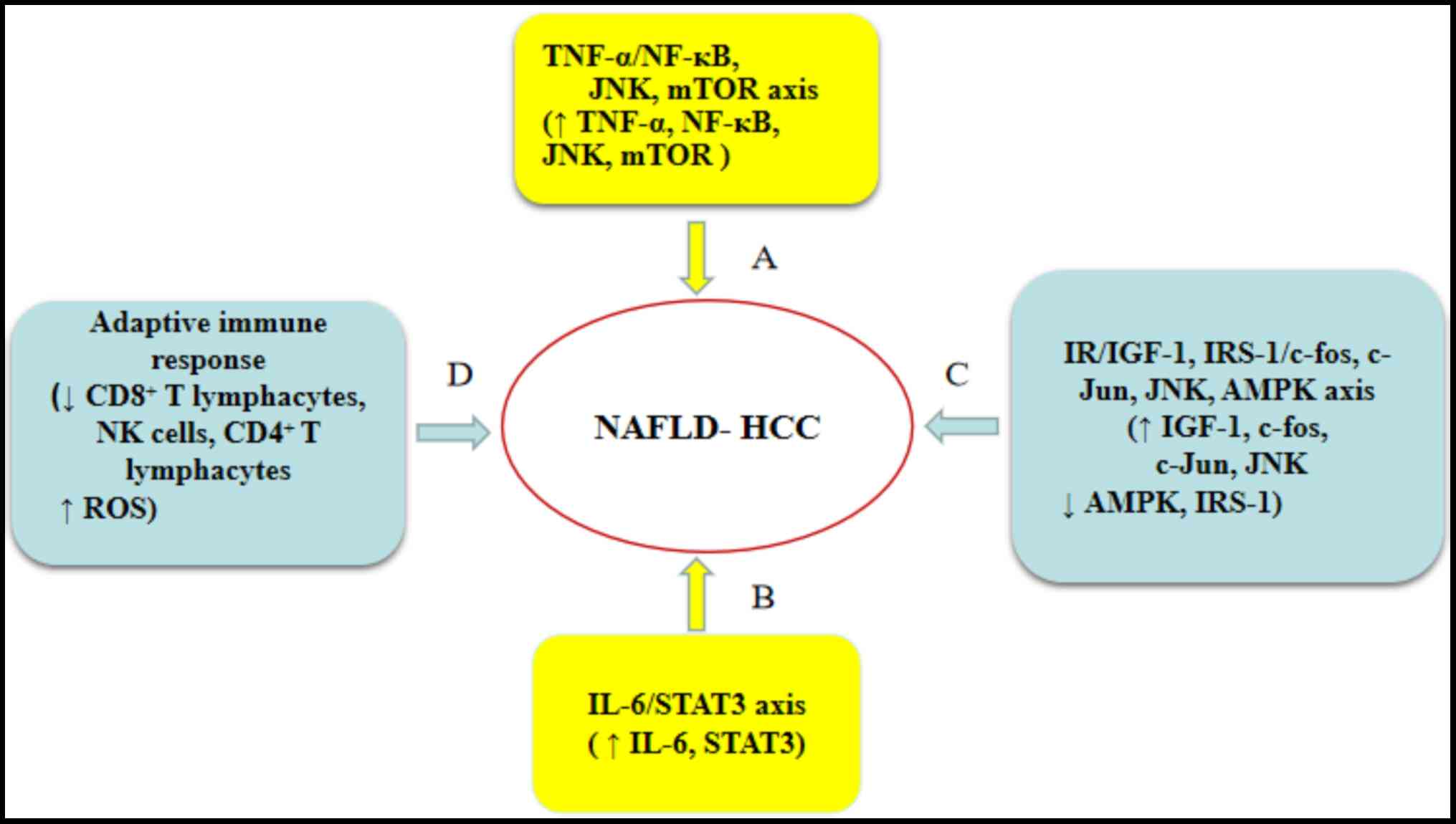
of NAFLD-related HCC is limited, and several possible mechanisms of
NAFLD-related HCC have been described, including obesity-induced
inflammation (62–64), insulin resistance (IR) (65–68),
oxidative stress (69,70) and adaptive immune responses (71,72).
Accumulating experimental evidence has suggested
that corosolic acid possesses a variety of biological properties,
exerting anti-diabetic, anti-obesity, anti-hyperlipidemic,
anti-viral, anti-inflammatory and anticancer effects (26,73,74).
Therefore, the present review describes the anticancer effects and
related molecular mechanisms of corosolic acid, highlighting its
ability to inhibit NAFLD progression, and providing guidelines for
future research on its use as an agent in NAFLD-related HCC
prevention and treatment.
Cancer cell migration is a critical process in tumor
development and metastasis (75,76), and
is closely associated with vascular growth factor receptor (VEGFR)
signaling (57,58); thus, the inhibition of VEGFR, and
VEGFR2 in particular, is considered an important treatment approach
for HCC and prevent HCC metastasis (77–79). Ku
et al (48) showed that the
half-maximal inhibitory concentration (IC50) for
corosolic acid was 2.5 µM for migratory ability, and 50 µM for
cytotoxicity on the HCC Huh7 cell line. In addition, corosolic acid
treatment resulted in a decrease in Huh7 cell migration in a
dose-dependent manner, and corosolic acid at a dose of 2.5 µM
induced low cytotoxicity for 24 h (IC50
cytotoxicity/IC50 migration=20), compared to the
untreated control (48). The authors
further demonstrated that the cytotoxic effects observed with
corosolic acid might be associated with the markedly suppression of
the VEGFR2/steroid receptor coactivator/focal adhesion kinase
(FAK)/cell division cycle42 (cdc42) signaling pathway and the
inhibition of the kinase activity of VEGFR2. On the other hand, Xu
et al (80) reported that
corosolic acid had reduced efficacy in treating liver cancer, since
it accelerated the degradation of the transcription factors of
Yes-associated protein (YAP) by enhancing large tumor suppressor
gene 1-induced phosphorylation and β-transductin repeat containing
protein (βTrCP)-dependent ubiquitination. However, Xu et al
(80) also demonstrated that
corosolic acid-induced apoptosis of liver cancer cells was enhanced
by combined treatment with actinomycin D, which resulted in
elevated YAP protein levels and decreased βTrCP protein activity.
This study suggests that the effectiveness of liver cancer
treatment with corosolic acid (at a final concentration of 10 µM)
might be improved by its combined administration with 5 µg/ml
actinomycin D for 24 h (80).
In gastric cancer cells, corosolic acid has been
shown to effectively inhibit the progression of carcinogenesis
through multiple mechanisms, including targeting of the adenosine
monophosphate-activated protein kinase (AMPK)-mammalian target of
rapamycin (mTOR) signaling pathway, the inhibition of the NF-κB
pathway, the downregulation of EGFR2/neu oncogene, the promotion of
the anticancer activities of 5-fluorouracil (5-FU) via mTOR
inhibition, and the reduction of 5-FU chemoresistance through the
activation of the AMPK pathway (49,81,82). In
human gastric cancer NCI-N87 cells, corosolic acid has been shown
to inhibit the expression of human epidermal growth factor receptor
2 (HER2) and AMPK-mTOR signal phosphorylated proteins, such as Akt
and extracellular signal-regulated protein kinase (ERK), which are
involved in signaling pathways downstream of HER2, with the
inhibitory effect of corosolic acid being both dose- and
time-dependent (25 µM for 12, 24 and 48 h, and 50 µM for 24 h)
(81). Furthermore, corosolic acid
has been found to induce G0/G1 arrest, which
was associated with the induction of cyclin-dependent kinase
inhibitor 1B and the downregulation of cyclin D1 (81). In addition, Lee et al
(81) found that corosolic acid
could effectively inhibit cell proliferation in both a dose- and
time-dependent manner (1, 5, 10 and 50 µM for 24 h, and 25 µM for
3, 6, 12, 24 and 48 h). Furthermore, corosolic acid has been shown
to induce cell cycle arrest and apoptosis through the
downregulation of the HER2/neu oncogene, suggesting that it may
play a role in patients with HER2-amplified gastric cancers
(81). Moreover, at an
IC50 value of 16.9±2.9 µM, corosolic acid has been shown
to inhibit the proliferation of human gastric cancer SNU-601 cells
via AMPK-mTOR signaling (82).
Another study has reported that corosolic acid treatment at a
concentration of 10, 20, 40 and 80 mg/ml for 72 h induces apoptosis
in human gastric cancer BGC823 cells in a dose-dependent manner
(49). This effect is achieved by
inhibiting the NF-κB (p65 subunit) pathway, by decreasing the mRNA
and protein expression of p65, apoptosis antigen 1 (Fas), second
mitochondria derived activator of caspase, and B-cell lymphoma-2
(Bcl-2), whilst increasing that of Bcl-2 associated X (Bax),
inhibitor of NF-κB (IκB) α and survivin (49). In addition, the experimental data of
Sung et al (83) provides
insights into the molecular mechanisms through which corosolic acid
induces the apoptosis of colorectal cancer cells. Corosolic acid,
at an IC50 value of 24 µM for 24 h, inhibits the
viability of colorectal cancer HCT116 cells by inducing apoptotic
cell death in a dose-dependent manner, through a molecular
mechanism associated with the upregulation of the proapoptotic
proteins Bax, Fas and Fas ligand (FasL), and the downregulation of
the anti-apoptotic proteins Bcl-2 and survivin. Of note, corosolic
acid was proven to be an ideal antagonist of the Wnt/β-catenin
pathway (51). Corosolic acid
decreased the level of intracellular β-catenin and suppressed the
proliferation of colon cancer HCT-15 and DLD-1 cells with an APC
mutation in a dose-dependent manner (20, 40 and 60 µM for 8 h),
which was achieved by promoting N-terminal phosphorylation and
degrading the proteasomes of β-catenin (Table II) (51).
Accumulating evidence has suggested that activated
Nrf2 plays a critical role in the proliferation and survival of
tumor cells, making its inhibition a promising therapeutic strategy
for cancer treatment (84–87). A previous report on several Nrf2
inhibitors showed that these are promising therapeutic agents
(88). Of note, corosolic acid at a
concentration of 0.25–32 µM for 3 or 5 days inhibited the
proliferation of TRAMP-C1 cells, a type of anchorage-independent
human prostate cancer (PCa) cell line with increased levels of mRNA
and protein expression of Nrf2, heme oxygenase-1 (HO-1) and
nicotinamide adenine dinucleotide phosphate quinone oxidoreductase
1; however, corosolic acid did not exert the same inhibitory effect
in Nrf2-knockout TRAMP-C1 cells (54). These findings indicate that the
significant cytotoxic effect of corosolic acid might be associated
with its ability to restore the expression of Nrf2 via epigenetic
modification (54). In addition, in
the PCa, PC-3 and DU145 cell lines, (ER) stress was activated by 0,
5, 10 and 15 µM corosolic acid after 24 and 48 h, through two
proapoptotic signaling pathways: The inositol-requiring
ER-to-nucleus signal kinase 1/apoptosis signal regulating kinase
1/Jun N-terminal kinase (JNK) pathway and the protein kinase
RNA-like ER kinase/eukaryotic initiation factor 2 α/activating
transcription factor 4/C/EBP-homologous protein signaling pathway,
which induced apoptosis and suppressed cell proliferation (89). However, Woo et al (90) found that the corosolic acid-induced
death of human renal carcinoma Caki cells (at 10 µM for 24 h) was
inhibited by the use of α-tocopherol (a hydrophobic anti-oxidant
that prevents free radical damage), but was not inhibited by
benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (an apoptosis
inhibitor), necrostatin-1 (a necroptosis inhibitor), ferrostatin-1
or deferoxamine (ferroptosis inhibitors) (90). Futhermore, corosolic acid induces
lipid oxidation, and α-tocopherol markedly prevents corosolic
acid-induced lipid peroxidation and cell death.
Anti-chemotherapeutic effects of α-tocopherol are dependent on
inhibition of lipid oxidation rather than inhibition of ROS
production (90). It was therefore
speculated that corosolic acid induced the non-apoptotic cell death
associated with lipid peroxidation in cancer cells (90). Furthermore, in renal carcinoma ACHN
and A498 cells, treatment with 10 µM corosolic acid for 24 h
induced non-apoptotic cell death (90). Xu et al (91) reported that treating human cervix
adenocarcinoma HeLa cells with 40 µM corosolic acid for 24 h could
induce cell cycle arrest at the S phase, and promote apoptosis by
activating caspases-8, −9 and −3 and disrupting mitochondrial
membrane potential (91). In another
report on CaSki human cervical cancer cells, the results indicated
that 10, 50 and 100 µM corosolic acid treatment for 12, 24 and 48 h
effectively inhibited proliferation in a dose- and time-dependent
manner (55). In addition, the
results revealed that the cytotoxic effects of corosolic acid
inhibited tumor cell proliferation by inducing apoptosis and cell
cycle arrest, and suppressing the PI3K/Akt signaling pathway
(55). It has also been demonstrated
that in epithelial ovarian cancer (92), glioma and lymphoma (93,94)
cells, the activation of signal transducer and activator of
transcription 3 (STAT3) was induced by co-culturing the cells with
M2, but not M1 macrophages. However, Fujiwara et al
(95) demonstrated that corosolic
acid, at a minimum of 30 µM for 48 h, suppressed STAT3 activation
in co-culture experiments with epithelial ovarian cancer ES-2 cells
treated with bromodeoxyuridine (used to abrogate macrophage
differentiation into the M2 phenotype), and that STAT3 inhibition
was associated with the prevention of M2 macrophage polarization.
In addition, the epithelial ovarian cancer cell line SKOV3 treated
with 20 µM corosolic acid for 24 h, showed no effect on the
viability of these cells, suggesting that corosolic acid have no
anticancer properties at this concentration. By contrast, 20 µM
corosolic acid enhanced the inhibitory effect of paclitaxel (PTX;
10 µM) on the proliferation of the epithelial ovarian cancer cell
lines SKOV3, RMG-1 and ES-2. These results demonstrated that
corosolic acid enhances the anticancer activity of anticancer drugs
such as PTX in epithelial ovarian carcinoma cells (95). Notably, the combination of 20 µM
corosolic acid and 10 µM paclitaxel for 24 h also inhibited STAT3
activity in the epithelial ovarian cancer cells, but corosolic acid
alone or PTX alone had lesser effects on the STAT3 activity
(95). These data suggested that
corosolic acid enhanced cancer cell chemosensitivity and
effectively inhibited cancer cell proliferation, which was also
found to be associated with the prevention of M2 polarization via
the suppression of STAT3 activation (95). These findings were similar to those
showing that corosolic acid (30 µM for 1 h) suppressed the M2
macrophage polarization and proliferation of U373 and T98G
glioblastoma cells in parallel with inhibiting both STAT3 and NF-κB
activation (Table II) (96).
The response of osteosarcoma MG-63 cells to
corosolic acid treatment has been previously reported (97,98). The
results shared by both studies indicate that the viability of
osteosarcoma MG-63 cells was significantly inhibited by corosolic
acid (35 µM for 12 h, and 20, 30 and 40 µM for 24 h), and that
corosolic acid induced apoptosis through the activation of
caspases-3 and −9 to cause mitochondrial dysfunction (97,98).
Moreover, using human osteosarcoma Saos2 and HSOS-1 cell lines and
the murine osteosarcoma LM8 cell line, Horlad et al
(52) reported that treatment with
30 µM corosolic acid for 24 h inhibited lung metastasis by
inhibiting STAT3 activation, increasing the number of infiltrating
lymphocytes in the tumor tissues and abrogating the
immunosuppressive effect of myeloid-derived suppressor cells
(MDSCs) through the decreased expression of cyclooxygenase-2
(COX-2) and chemokine (C-C motif) ligand 2 (CCL2) mRNA in these
MDSCs (52) (Table II).
Corosolic acid (10–40 µM for 6–48 h) had a
significant inhibitory effect on A549 cells, a human lung
adenocarcinoma cell line, in a concentration- and time-dependent
manner (99). Exposure to corosolic
acid induced cell cycle arrest at the sub-G1 stage and
caused apoptotic death in A549 cells (99). In addition, corosolic acid also
activated caspases-3/-7, −8 and −9 and poly (ADP-ribose)
polymerase, and increased the levels of reactive oxygen species
(ROS). Corosolic acid-induced apoptosis was inhibited by exposure
to the ROS scavenger N-acetylcysteine (99). These results indicate that corosolic
acid induced apoptosis through mitochondria-mediated and
caspase-dependent processes in a ROS-dependent manner (99). In addition, under
CoCl2-stimulated hypoxic conditions, corosolic acid
(IC50 of 12.5 µg/ml for 48 h) induced marked
cytotoxicity in cancerous cells, and its action was associated with
the suppressed expression of hypoxia-inducible factor-1 α and β and
its downstream target genes (Table
II) (100).
The response of human retinoblastoma Y-79 cells to
corosolic acid was investigated (101). The results showed that corosolic
acid (10 µM for 24 h) could induce cell cycle arrest and apoptosis
through its cytotoxic activity (IC50 of 4.15 µM for 24 h
or 3.37 µM for 48 h) in a dose-dependent manner (101). The results also showed that the
transcriptional activity of forkhead box M1 (FoxM1) was
self-induced or driven by maternal embryonic leucine-zipper kinase
(MELK), and that corosolic acid inhibited the expression levels of
MELK and FoxM1 (101). The report
established a promising therapeutic target of human retinoblastoma
via MELK-FoxM1 signaling (Table II)
(101).
Accumulating experimental evidence has highlighted
the pivotal role of STAT3 activation in the resistance to
chemotherapy and radiotherapy in the thyroid cancer-derived CD133+
cells (103) and human epithelial
ovarian cancer cells (104). It is
thought that inhibiting STAT3 might be effective for treating
patients with malignant tumors (103–105).
A report by Fujiwara et al (95) suggested that 20 µM corosolic acid, as
a selective STAT3 inhibitor, is able to increase sensitivity to
chemotherapeutic agents, including paclitaxel (10 µM), cisplatin
(10 µM) and doxorubicin (10 µM), in epithelial ovarian cancer
SKOV3, RMG-1 and ES-2 cell lines for 24 h. In addition, the results
of a study by Lee et al (81)
showed that 25 µM corosolic acid enhances the inhibitory effect on
human gastric cancer NCI-N87 cell proliferation when combined with
adriamycin (0.01 to 2 mg/ml) and 5-FU (0.1 to 50 mg/ml), but not
with docetaxel (0.01 to 2 mg/ml) or paclitaxel (0.01 to 6 mg/ml).
Lee et al (106) indicated
that corosolic acid (50 µM) enhances the anticancer activity of
5-FU (20 µg) after 24 h in human gastric carcinoma SNU-620 cells in
an mTOR inhibition-dependent manner. In addition, a report by
Fujiwara et al (102) showed
that corosolic acid (20 µM) also displayed synergistic effects with
anticancer agents, such as adriamycin (10 µM) and cisplatin (10 µM)
24 h. Furthermore, in a study by Park et al (107), a 5-FU-resistant gastric cancer cell
line (SNU-620/5-FUR) was established, which had a marked reduced
AMPK phosphorylation when compared with the parental cell line,
SNU-620. Cell treatment with 25 µM corosolic acid for 24 h was
found to enhance the chemosensitivity of 5-FU-resistant gastric
cancer cells, and the reduction of AMPK phosphorylation by compound
c (AMPK inhibitor) was revealed to be associated with increased
5-FU-resistant cell viability (107). Corosolic acid treatment
significantly reduced cell viability while compound c reversed
corosolic acid-induced cell growth inhibition (107). The corosolic acid-induced AMPK
activation was markedly increased by additional 5-FU treatment,
while compound c reversed AMPK phosphorylation (107). These results imply that corosolic
acid can activate AMPK and sensitize gastric cancer to 5-FU (150
µM; Table II).
The characteristics of NAFLD include obesity, IR,
hypertension and dyslipidemia, which are also the most common
characteristics observed in livers affected by MS (112). Furthermore, the development of
NAFLD-related HCC is increasingly recognized, since patients with
NAFLD are at high risk of developing HCC (112). NAFLD-associated HCC has been
estimated to account for 10–12% of HCC cases in Western populations
and 1–6% of HCC cases in Asian populations from 42 sites in 14
countries from 2005 to 2012 (58).
Based on multiple studies, accumulated evidence has suggested that
type 2 diabetes mellitus (T2DM) and obesity are independent risk
factors for the development of HCC in patients with NAFLD.
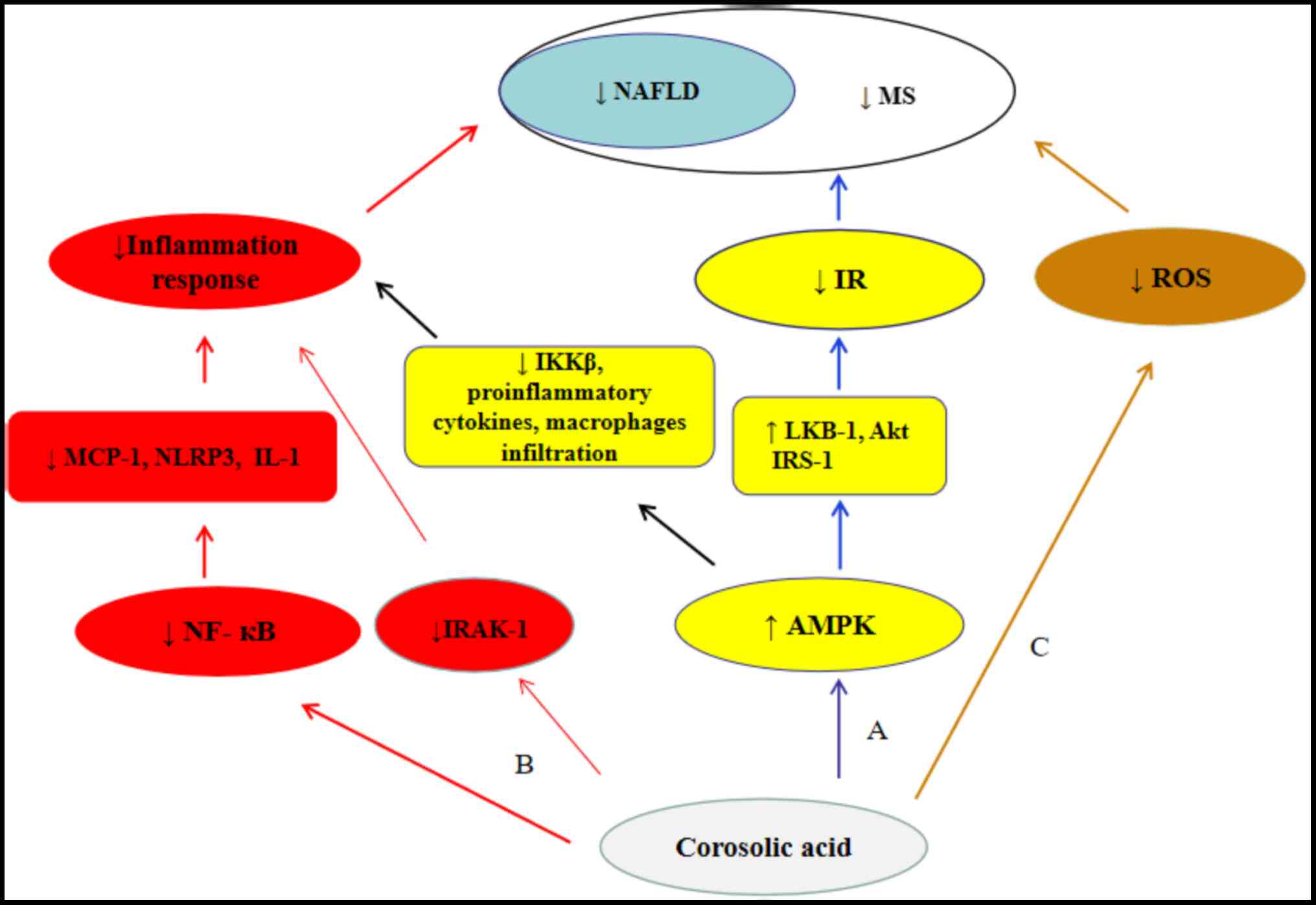
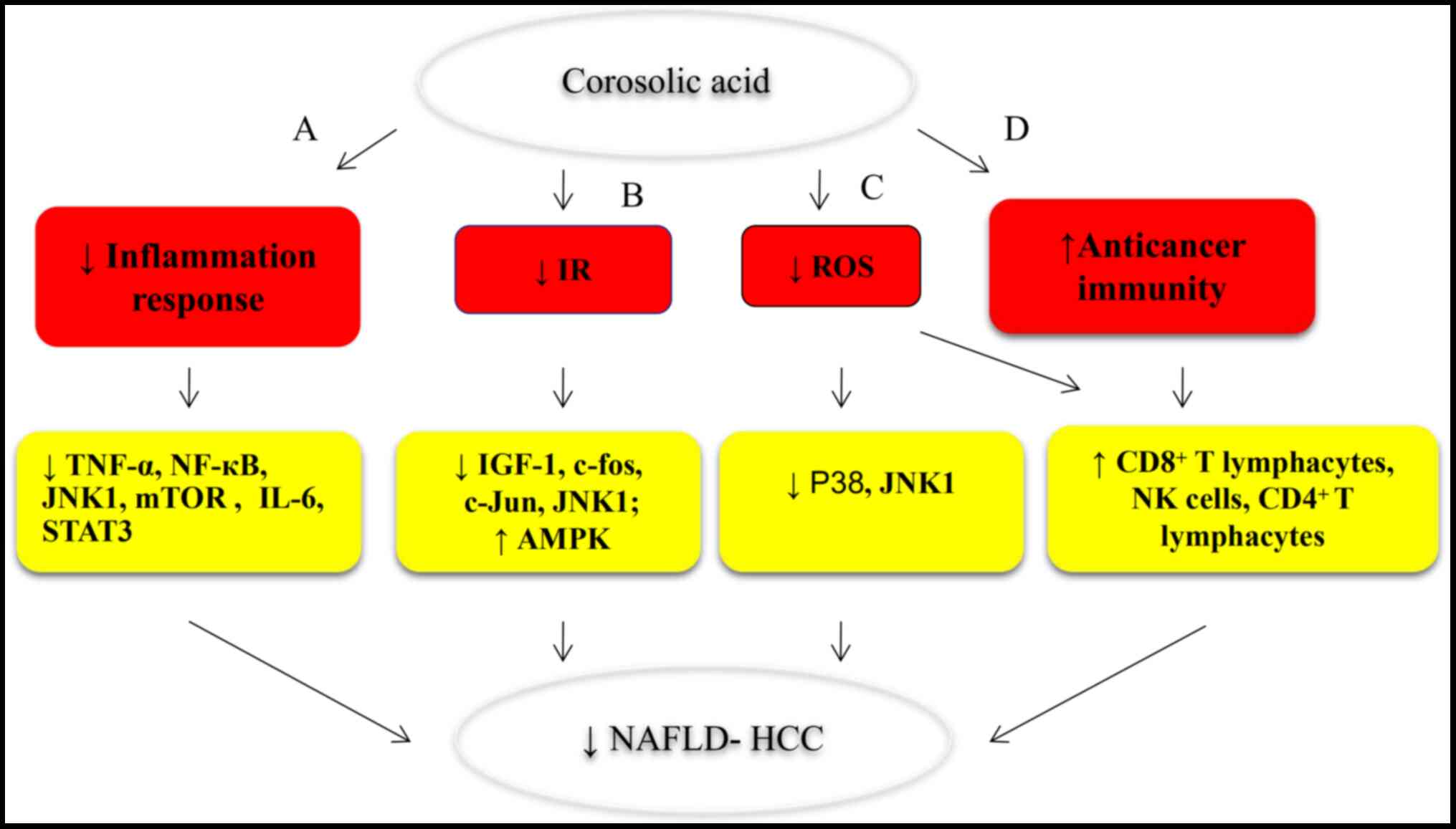
The present review summarizes current advancements
in our understanding of the anticancer activity and mechanisms of
corosolic acid in vitro and in vivo. Due to the
ability of corosolic acid to target multiple components of cancer
cells, it acts not only as an anticancer agent but also as a
synergistic adjuvant when administered alongside chemotherapeutic
drugs, even in drug-resistant cells. In addition, parts of the same
corosolic acid mechanism that ameliorates MS also induces
anticancer activity and suppresses the progression of NAFLD-related
HCC. Therefore, corosolic acid, a potential tool in MS treatment,
is being considered as a possible agent in NAFLD-related HCC
prevention and treatment (Figs. 3
and 5).
Not applicable.
This work was supported by the Fund for Science
& Technology Development of Jilin Province (grant nos.
20200201544JC, 20160101060JC and 20150101108JC), the National Key
R&D Program of China (grant nos. 2017YFD0502200 and
2016YFD0501302), and the Project of the Education Department of
Jilin Province (grant no. 2016444).
Not applicable.
JZ, HZ, YA, KS and LY participated in the design
and interpretation of the studies, the revision of the manuscript.
JZ, HZ, YA and KS wrote the review. All authors read and approved
the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WQ, Li H, Sun KX, Zheng RS, Zhang SW,
Zeng HM, Zou XN, Gu XY and He J: Report of cancer incidence and
mortality in China, 2014. Zhonghua Zhong Liu Za Zhi. 40:5–13.
2018.(In Chinese). PubMed/NCBI
|
|
3
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Islami F, Goding Sauer A, Miller KD,
Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J,
Soerjomataram I, et al: Proportion and number of cancer cases and
deaths attributable to potentially modifiable risk factors in the
United States. CA Cancer J Clin. 68:31–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khan M, Maryam A, Qazi JI and Ma T:
Targeting apoptosis and multiple signaling pathways with icariside
II in cancer cells. Int J Biol Sci. 11:1100–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Millimouno FM, Dong J, Yang L, Li J and Li
X: Targeting apoptosis pathways in cancer and perspectives with
natural compounds from mother nature. Cancer Prev Res (Phila).
7:1081–1107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan M, Maryam A, Zhang H, Mehmood T and
Ma T: Killing cancer with platycodin D through multiple mechanisms.
J Cell Mol Med. 20:389–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faivre S, Djelloul S and Raymond E: New
paradigms in anticancer therapy: Targeting multiple signaling
pathways with kinase inhibitors. Semin Oncol. 33:407–420. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shu L, Cheung KL, Khor TO, Chen C and Kong
AN: Phytochemicals: Cancer chemoprevention and suppression of tumor
onset and metastasis. Cancer Metastasis Rev. 29:483–502. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye B, Li J, Li Z, Yang J, Niu T and Wang
S: Anti-tumor activity and relative mechanism of ethanolic extract
of Marsdenia tenacissima (Asclepiadaceae) against human hematologic
neoplasm in vitro and in vivo. J Ethnopharmacol. 153:258–267. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan Y, Xu Z, Dai S, Qian L, Sun L and Gong
Z: Targeting autophagy to sensitive glioma to temozolomide
treatment. J Exp Clin Cancer Res. 35:232016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rengarajan T, Nandakumar N, Rajendran P,
Haribabu L, Nishigaki I and Balasubramanian MP: D-pinitol promotes
apoptosis in MCF-7 cells via induction of p53 and Bax and
inhibition of Bcl-2 and NF-κB. Asian Pac J Cancer Prev.
15:1757–1762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montaser R and Luesch H: Marine natural
products: A new wave of drugs? Future Med Chem. 3:1475–1489. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Efferth T: From ancient herb to modern
drug: Artemisia annua and artemisinin for cancer therapy. Semin
Cancer Biol. 46:65–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng CS, Chen J, Tan HY, Wang N, Chen Z
and Feng Y: Scutellaria baicalensis and cancer treatment: Recent
progress and perspectives in biomedical and clinical studies. Am J
Chin Med. 46:25–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Oleanolic acid and its synthetic
derivatives for the prevention and therapy of cancer: Preclinical
and clinical evidence. Cancer Lett. 346:206–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salvador JAR, Leal AS, Valdeira AS,
Gonçalves BMF, Alho DPS, Figueiredo SAC, Silvestre SM and Mendes
VIS: Oleanane-, ursane-, and quinone methide friedelane-type
triterpenoid derivatives: Recent advances in cancer treatment. Eur
J Med Chem. 142:95–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pisha E, Chai H, Lee IS, Chagwedera TE,
Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown
DM, et al: Discovery of betulinic acid as a selective inhibitor of
human melanoma that functions by induction of apoptosis. Nat Med.
1:1046–1051. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knowles J and Gromo G: A guide to drug
discovery: Target selection in drug discovery. Nat Rev Drug Discov.
2:63–69. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strüh CM, Jäger S, Schempp CM, Scheffler A
and Martin SF: A novel triterpene extract from mistletoe induces
rapid apoptosis in murine B16.F10 melanoma cells. Phytother Res.
26:1507–1512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, He J, Li J, Peng X, Wang X, Dong
Z, Zhao E, Liu Y, Wu Z and Cui H: Demethylzeylasteral inhibits cell
proliferation and induces apoptosis through suppressing MCL1 in
melanoma cells. Cell Death Dis. 8:e31332017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu W, Liu X, Pan Z, Wang D, Li M, Chen X,
Zhou L, Xu M, Li D and Zheng Q: Ailanthone induces cell cycle
arrest and apoptosis in melanoma B16 and A375 cells. Biomolecules.
9:2752019. View Article : Google Scholar
|
|
23
|
Tiwari R, Puthli A, Balakrishnan S, Sapra
BK and Mishra KP: Betulinic acid-induced cytotoxicity in human
breast tumor cell lines MCF-7 and T47D and its modification by
tocopherol. Cancer Invest. 32:402–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mullauer FB, van Bloois L, Daalhuisen JB,
Ten Brink MS, Storm G, Medema JP, Schiffelers RM and Kessler JH:
Betulinic acid delivered in liposomes reduces growth of human lung
and colon cancers in mice without causing systemic toxicity.
Anticancer Drugs. 22:223–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fulda S, Jeremias I, Steiner HH, Pietsch T
and Debatin KM: Betulinic acid: A new cytotoxic agent against
malignant brain-tumor cells. Int J Cancer. 82:435–441. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sivakumar G, Vail DR, Nair V,
Medina-Bolivar F and Lay JO Jr: Plant-based corosolic acid: Future
anti-diabetic drug? Biotechnol J. 4:1704–1711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Leng J, Li JJ, Tang JF, Li Y, Liu
BL and Wen XD: Corosolic acid inhibits adipose tissue inflammation
and ameliorates insulin resistance via AMPK activation in high-fat
fed mice. Phytomedicine. 23:181–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ulbricht C, Dam C, Milkin T, Seamon E,
Weissner W and Woods J: Banaba (Lagerstroemia
speciosa L.): An evidence-based systematic review by the
natural standard research collaboration. J Herb Pharmacother.
7:99–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park C and Lee JS: Banaba: The natural
remedy as antidiabetic drug. Biomed Res. 22:127–131. 2011.
|
|
30
|
Kim E, Sy-Cordero A, Graf TN, Brantley SJ,
Paine MF and Oberlies NH: Isolation and identification of
intestinal CYP3A inhibitors from cranberry (Vaccinium
macrocarpon) using human intestinal microsomes. Planta Med.
77:265–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aguirre MC, Delporte C, Backhouse N, Erazo
S, Letelier ME, Cassels BK, Silva X, Alegría S and Negrete R:
Topical anti-inflammatory activity of 2alpha-hydroxy pentacyclic
triterpene acids from the leaves of Ugni molinae. Bioorg Med Chem.
14:5673–5677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou W, Li Y, Zhang Q, Wei X, Peng A, Chen
L and Wei Y: Triterpene acids isolated from Lagerstroemia
speciosa leaves as alpha-glucosidase inhibitors. Phytother Res.
23:614–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu C, Chen L, Xin Y and Cai Q:
Determination of corosolic acid in Eriobotrya japonica leaves by
reversed-phase high performance liquid chromatography. Se Pu.
24:492–494. 2006.(In Chinese). PubMed/NCBI
|
|
34
|
Lv H, Chen J, Li WL and Zhang HQ: Studies
on the triterpenes from loquat leaf (Eriobotrya japonica). Zhong
Yao Cai. 31:1351–1354. 2008.(In Chinese). PubMed/NCBI
|
|
35
|
Lu H, Xi C, Chen J and Li W: Determination
of triterpenoid acids in leaves of Eriobotrya japonica collected at
in different seasons. Zhongguo Zhong Yao Za Zhi. 34:2353–2355.
2009.(In Chinese). PubMed/NCBI
|
|
36
|
Rollinger JM, Kratschmar DV, Schuster D,
Pfisterer PH, Gumy C, Aubry EM, Brandstötter S, Stuppner H, Wolber
G and Odermatt A: 11beta-Hydroxysteroid dehydrogenase 1 inhibiting
constituents from Eriobotrya japonica revealed by
bioactivity-guided isolation and computational approaches. Bioorg
Med Chem. 18:1507–1515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Banno N, Akihisa T, Tokuda H, Yasukawa K,
Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa J and
Nishino H: Triterpene acids from the leaves of Perilla frutescens
and their anti-inflammatory and antitumor-promoting effects. Biosci
Biotechnol Biochem. 68:85–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim DH, Han KM, Chung IS, Kim DK, Kim SH,
Kwon BM, Jeong TS, Park MH, Ahn EM and Baek NI: Triterpenoids from
the flower of Campsis grandiflora K. Schum. as human acyl-CoA:
Cholesterol acyltransferase inhibitors. Arch Pharm Res. 28:550–556.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Na M, Yang S, He L, Oh H, Kim BS, Oh WK,
Kim BY and Ahn JS: Inhibition of protein tyrosine phosphatase 1B by
ursane-type triterpenes isolated from Symplocos paniculata. Planta
Med. 72:261–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thuong PT, Min BS, Jin W, Na M, Lee J,
Seong R, Lee YM, Song K, Seong Y, Lee HK, et al: Anti-complementary
activity of ursane-type triterpenoids from Weigela subsessilis.
Biol Pharm Bull. 29:830–833. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee MS and Thuong PT: Stimulation of
glucose uptake by triterpenoids from Weigela subsessilis. Phytother
Res. 24:49–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang NY, Duan JA, Li P and Qian SH:
Chemical Constituents of Glechoma longituba. Yao Xue Xue Bao.
41:431–434. 2006.PubMed/NCBI
|
|
43
|
Shen Y, Wang QH, Lin HW, Shu W, Zhou JB
and Li ZY: Study on chemical constituents of Potentilla chinensis
Ser. Zhong Yao Cai. 29:237–239. 2006.(In Chinese). PubMed/NCBI
|
|
44
|
Kang SH, Shi YQ and Yang CX: Triterpenoids
and steroids of root of Rubus biflorus. Zhong Yao Cai.
31:1669–1671. 2008.(In Chinese). PubMed/NCBI
|
|
45
|
Liu P, Teng J, Zhang YW, Takaishi Y and
Duan HQ: Chemical constituents from rhizome of Phlomis umbrosa. Yao
Xue Xue Bao. 42:401–404. 2007.(In Chinese). PubMed/NCBI
|
|
46
|
Li YL, Dai HN, Ma GX, Zhang W, Wu TY, Wang
YQ, Zou JM, Zhong XQ, Zhou YL, Yuan JQ, et al: A new triterpenic
acid from the roots of Rosa laevigata. Yao Xue Xue Bao. 52:425–429.
2017.(In Chinese). PubMed/NCBI
|
|
47
|
Huang XY, Ma GX, Zhong XQ, Zhou YL, Dai
HN, Wu HF, Zhu YD, Yang JS, Yuan JQ and Xu XD: Triterpene
constituents from Rosa cymosa Tratt. Zhongguo Zhong Yao Za Zhi.
39:4637–4641. 2014.(In Chinese). PubMed/NCBI
|
|
48
|
Ku CY, Wang YR, Lin HY, Lu SC and Lin JY:
Corosolic acid inhibits hepatocellular carcinoma cell migration by
targeting the VEGFR2/Src/FAK pathway. PLoS One. 10:e01267252015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheng QL, Li HL, Li YC, Liu ZW, Guo XH and
Cheng YJ: CRA(Crosolic Acid) isolated from Actinidia valvata Dunn.
Radix induces apoptosis of human gastric cancer cell line BGC823 in
vitro via down-regulation of the NF-κB pathway. Food Chem Toxicol.
105:475–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Manayi A, Saeidnia S, Ostad SN,
Hadjiakhoondi A, Ardekani MR, Vazirian M, Akhtar Y and Khanavi M:
Chemical constituents and cytotoxic effect of the main compounds of
Lythrum salicaria L. Z Naturforsch C J Biosci. 68:367–375. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim JH, Kim YH, Song GY, Kim DE, Jeong YJ,
Liu KH, Chung YH and Oh S: Ursolic acid and its natural derivative
corosolic acid suppress the proliferation of APC-mutated colon
cancer cells through promotion of β-catenin degradation. Food Chem
Toxicol. 67:87–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Horlad H, Fujiwara Y, Takemura K, Ohnishi
K, Ikeda T, Tsukamoto H, Mizuta H, Nishimura Y, Takeya M and
Komohara Y: Corosolic acid impairs tumor development and lung
metastasis by inhibiting the immunosuppressive activity of
myeloid-derived suppressor cells. Mol Nutr Food Res. 57:1046–1054.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yoo KH, Park JH, Lee DY, Hwang-Bo J, Baek
NI and Chung IS: Corosolic acid exhibits anti-angiogenic and
anti-lymphangiogenic effects on in vitro endothelial cells and on
an in vivo CT-26 colon carcinoma animal model. Phytother Res.
29:714–723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang J, Wu R, Li W, Gao L, Yang Y, Li P
and Kong AN: The triterpenoid corosolic acid blocks transformation
and epigenetically reactivates Nrf2 in TRAMP-C1 prostate cells. Mol
Carcinog. 57:512–521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yong QX, Jian HZ and Xing SY: Corosolic
acid induces potent anti-cancer effects in CaSki cervical cancer
cells through the induction of apoptosis, cell cycle arrest and
PI3K/Akt signalling pathway. Bangladesh J Pharmacol. 11:453–459.
2016. View Article : Google Scholar
|
|
56
|
National Cancer Institute (NIH), .
Surveillance, Epidemiology, and End Results (SEER) Program. NIH.
2016.simplehttps://seer.cancer.gov/
|
|
57
|
Baffy G: Hepatocellular carcinoma in
non-alcoholic fatty liver disease: Epidemiology, pathogenesis, and
prevention. J Clin Transl Hepatol. 1:131–137. 2013.PubMed/NCBI
|
|
58
|
Wong SW, Ting YW and Chan WK: Epidemiology
of non-alcoholic fatty liver disease-related hepatocellular
carcinoma and its implications. JGH Open. 2:235–241. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rinella ME: Nonalcoholic fatty liver
disease: A systematic review. JAMA. 313:2263–2273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Starley BQ, Calcagno CJ and Harrison SA:
Nonalcoholic fatty liver disease and hepatocellular carcinoma: A
weighty connection. Hepatology. 51:1820–1832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Welzel TM, Graubard BI, Zeuzem S, El-Serag
HB, Davila JA and Mcglynn KA: Metabolic syndrome increases the risk
of primary liver cancer in the United States: A study in the
SEER-medicare database. Hepatology. 54:463–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hotamisligil GS: Inflammation and
metabolic disorders. Nature. 444:860–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Stickel F and Hellerbrand C: Non-alcoholic
fatty liver disease as a risk factor for hepatocellular carcinoma:
Mechanisms and implications. Gut. 59:1303–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Park EJ, Lee JH, Yu GY, He G, Ali SR,
Holzer RG, Osterreicher CH, Takahashi H and Karin M: Dietary and
genetic obesity promote liver inflammation and tumorigenesis by
enhancing IL-6 and TNF expression. Cell. 140:197–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ish-Shalom D, Christoffersen CT, Vorwerk
P, Sacerdoti-Sierra N, Shymko RM, Naor D and De Meyts P: Mitogenic
properties of insulin and insulin analogues mediated by the insulin
receptor. Diabetologia. 40 (Suppl 2):S25–S31. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Buzzelli G, Dattolo P, Pinzani M, Brocchi
A, Romano S and Gentilini P: Circulating growth hormone and
insulin-like growth factor-I in nonalcoholic liver cirrhosis with
or without superimposed hepatocarcinoma: Evidence of an altered
circadian rhythm. Am J Gastroenterol. 88:1744–1748. 1993.PubMed/NCBI
|
|
67
|
Hirosumi J, Tuncman G, Chang L, Görgün CZ,
Uysal KT, Maeda K, Karin M and Hotamisligil GS: A central role for
JNK in obesity and insulin resistance. Nature. 420:333–336. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chang Q, Zhang Y, Beezhold KJ, Bhatia D,
Zhao H, Chen J, Castranova V, Shi X and Chen F: Sustained JNK1
activation is associated with altered histone H3 methylations in
human liver cancer. J Hepatol. 50:323–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang S, Zhu H, Li Y, Lin H, Gabrielson K,
Trush MA and Diehl AM: Mitochondrial adaptations to obesity-related
oxidant stress. Arch Biochem Biophys. 378:259–268. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ikura Y, Mita E and Nakamori S:
Hepatocellular carcinomas can develop in simple fatty livers in the
setting of oxidative stress. Pathology. 43:167–168. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ma C, Kesarwala AH, Eggert T,
Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor
V, ElGindi M, et al: NAFLD causes selective CD4(+) T lymphocyte
loss and promotes hepatocarcinogenesis. Nature. 531:253–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wolf MJ, Adili A, Piotrowitz K, Abdullah
Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M,
Wohlleber D, et al: Metabolic activation of intrahepatic CD8+ T
cells and NKT cells causes nonalcoholic steatohepatitis and liver
cancer via cross-talk with hepatocytes. Cancer Cell. 26:549–564.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Stohs SJ, Miller H and Kaats GR: A review
of the efficacy and safety of banaba (Lagerstroemia speciosa
L.) and corosolic acid. Phytother Res. 26:317–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sharma H, Kumar P, Deshmukh RR, Bishayee A
and Kumar S: Pentacyclic triterpenes: New tools to fight metabolic
syndrome. Phytomedicine. 50:166–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fife CM, McCarroll JA and Kavallaris M:
Movers and shakers: Cell cytoskeleton in cancer metastasis. Br J
Pharmacol. 171:5507–5523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chiang AC and Massagué J: Molecular basis
of metastasis. N Engl J Med. 359:2814–2823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang L, Wang JN, Tang JM, Kong X, Yang
JY, Zheng F, Guo LY, Huang YZ, Zhang L, Tian L, et al: VEGF is
essential for the growth and migration of human hepatocellular
carcinoma cells. Mol Biol Rep. 39:5085–5093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lang SA, Brecht I, Moser C, Obed A, Batt
D, Schlitt HJ, Geissler EK and Stoeltzing O: Dual inhibition of Raf
and VEGFR2 reduces growth and vascularization of hepatocellular
carcinoma in an experimental model. Langenbecks Arch Surg.
393:333–341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang C, Wu X, Zhang M, Zhu L, Zhao R, Xu
D, Lin Z, Liang C, Chen T, Chen L, et al: Small molecule R1498 as a
well-tolerated and orally active kinase inhibitor for
hepatocellular carcinoma and gastric cancer treatment via targeting
angiogenesis and mitosis pathways. PLoS One. 8:e652642013.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xu Y, Zhao Y, Xu Y, Guan Y, Zhang X, Chen
Y, Wu Q, Zhu G, Chen Y, Sun F, et al: Blocking inhibition to YAP by
ActinomycinD enhances anti-tumor efficacy of Corosolic acid in
treating liver cancer. Cell Signal. 29:209–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lee MS, Cha EY, Thuong PT, Kim JY, Ahn MS
and Sul JY: Down-regulation of human epidermal growth factor
receptor 2/neu oncogene by corosolic acid induces cell cycle arrest
and apoptosis in NCI-N87 human gastric cancer cells. Biol Pharm
Bull. 33:931–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lee MS, Lee CM, Cha EY, Thuong PT, Bae K,
Song IS, Noh SM and Sul JY: Activation of AMP-activated protein
kinase on human gastric cancer cells by apoptosis induced by
corosolic acid isolated from Weigela subsessilis. Phytother Res.
24:1857–1861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sung B, Kang YJ, Kim DH, Hwang SY, Lee Y,
Kim M, Yoon JH, Kim CM, Chung HY and Kim ND: Corosolic acid induces
apoptotic cell death in HCT116 human colon cancer cells through a
caspase-dependent pathway. Int J Mol Med. 33:943–949. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ohta T, Iijima K, Miyamoto M, Nakahara I,
Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T,
et al: Loss of Keap1 function activates Nrf2 and provides
advantages for lung cancer cell growth. Cancer Res. 68:1303–1309.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
DeNicola GM, Karreth FA, Humpton TJ,
Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES,
et al: Oncogene-induced Nrf2 transcription promotes ROS
detoxification and tumorigenesis. Nature. 475:106–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mitsuishi Y, Taguchi K, Kawatani Y,
Shibata T, Nukiwa T, Aburatani H, Yamamoto M and Motohashi H: Nrf2
redirects glucose and glutamine into anabolic pathways in metabolic
reprogramming. Cancer Cell. 22:66–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jia Y, Wang H, Wang Q, Ding H, Wu H and
Pan H: Silencing Nrf2 impairs glioma cell proliferation via
AMPK-activated mTOR inhibition. Biochem Biophys Res Commun.
469:665–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhu J, Wang H, Chen F, Fu J, Xu Y, Hou Y,
Kou HH, Zhai C, Nelson MB, Zhang Q, et al: An overview of chemical
inhibitors of the Nrf2-ARE signaling pathway and their potential
applications in cancer therapy. Free Radic Biol Med. 99:544–556.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ma B, Zhang H, Wang Y, Zhao A, Zhu Z, Bao
X, Sun Y, Li L and Zhang Q: Corosolic acid, a natural triterpenoid,
induces ER stress-dependent apoptosis in human castration resistant
prostate cancer cells via activation of IRE-1/JNK, PERK/CHOP and
TRIB3. J Exp Clin Cancer Res. 37:2102018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Woo SM, Seo SU, Min KJ, Im SS, Nam JO,
Chang JS, Kim S, Park JW and Kwon TK: Corosolic acid induces
non-apoptotic cell death through generation of lipid reactive
oxygen species production in human renal carcinoma caki cells. Int
J Mol Sci. 19:13092018. View Article : Google Scholar
|
|
91
|
Xu Y, Ge R, Du J, Xin H, Yi T, Sheng J,
Wang Y and Ling C: Corosolic acid induces apoptosis through
mitochondrial pathway and caspase activation in human cervix
adenocarcinoma HeLa cells. Cancer Lett. 284:229–237. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Takaishi K, Komohara Y, Tashiro H, Ohtake
H, Nakagawa T, Katabuchi H and Takeya M: Involvement of
M2-polarized macrophages in the ascites from advanced epithelial
ovarian carcinoma in tumor progression via Stat3 activation. Cancer
Sci. 101:2128–2136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Komohara Y, Horlad H, Ohnishi K, Ohta K,
Makino K, Hondo H, Yamanaka R, Kajiwara K, Saito T, Kuratsu J and
Takeya M: M2 macrophage/microglial cells induce activation of Stat3
in primary central nervous system lymphoma. J Clin Exp Hematop.
51:93–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Komohara Y, Horlad H, Ohnishi K, Fujiwara
Y, Bai B, Nakagawa T, Suzu S, Nakamura H, Kuratsu J and Takeya M:
Importance of direct macrophage-tumor cell interaction on
progression of human glioma. Cancer Sci. 103:2165–2172. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fujiwara Y, Takaishi K, Nakao J, Ikeda T,
Katabuchi H, Takeya M and Komohara Y: Corosolic acid enhances the
antitumor effects of chemotherapy on epithelial ovarian cancer by
inhibiting signal transducer and activator of transcription 3
signaling. Oncol Lett. 6:1619–1623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fujiwara Y, Komohara Y, Ikeda T and Takeya
M: Corosolic acid inhibits glioblastoma cell proliferation by
suppressing the activation of signal transducer and activator of
transcription-3 and nuclear factor-kappa B in tumor cells and
tumor-associated macrophages. Cancer Sci. 102:206–211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cai X, Zhang H, Tong D, Tan Z, Han D, Ji F
and Hu W: Corosolic acid triggers mitochondria and
caspase-dependent apoptotic cell death in osteosarcoma MG-63 cells.
Phytother Res. 25:1354–1361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Jia Y, Yuan H, Shan S, Xu G, Yu J, Zhao C
and Mou X: Corosolic acid inhibits the proliferation of
osteosarcoma cells by inducing apoptosis. Oncol Lett. 12:4187–4194.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Nho KJ, Chun JM and Kim HK: Corosolic acid
induces apoptotic cell death in human lung adenocarcinoma A549
cells in vitro. Food Chem Toxicol. 56:8–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bahadori MB, Vandghanooni S, Dinparast L,
Eskandani M, Ayatollahi SA, Ata A and Nazemiyeh H: Triterpenoid
corosolic acid attenuates HIF-1 stabilization upon cobalt (II)
chloride-induced hypoxia in A549 human lung epithelial cancer
cells. Fitoterapia. 134:493–500. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang K, Zhu X, Yao Y, Yang M, Zhou F and
Zhu L: Corosolic acid induces cell cycle arrest and cell apoptosis
in human retinoblastoma Y-79 cells via disruption of MELK-FoxM1
signaling. Oncol Rep. 39:2777–2786. 2018.PubMed/NCBI
|
|
102
|
Fujiwara Y, Takeya M and Komohara Y: A
novel strategy for inducing the antitumor effects of triterpenoid
compounds: Blocking the protumoral functions of tumor-associated
macrophages via STAT3 inhibition. Biomed Res Int. 2014:3485392014.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tseng LM, Huang PI, Chen YR, Chen YC, Chou
YC, Chen YW, Chang YL, Hsu HS, Lan YT, Chen KH, et al: Targeting
signal transducer and activator of transcription 3 pathway by
cucurbitacin I diminishes self-renewing and radiochemoresistant
abilities in thyroid cancer-derived CD133+ cells. J Pharmacol Exp
Ther. 341:410–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang X, Liu P, Zhang B, Wang A and Yang
M: Role of STAT3 decoy oligodeoxynucleotides on cell invasion and
chemosensitivity in human epithelial ovarian cancer cells. Cancer
Genet Cytogenet. 197:46–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Page BD, Ball DP and Gunning PT: Signal
transducer and activator of transcription 3 inhibitors: A patent
review. Expert Opin Ther Pat. 21:65–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lee HS, Park JB, Lee MS, Cha EY, Kim JY
and Sul JY: Corosolic acid enhances 5-fluorouracil-induced
apoptosis against SNU-620 human gastric carcinoma cells by
inhibition of mammalian target of rapamycin. Mol Med Rep.
12:4782–4788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Park JB, Lee JS, Lee MS, Cha EY, Kim S and
Sul JY: Corosolic acid reduces 5-FU chemoresistance in human
gastric cancer cells by activating AMPK. Mol Med Rep. 18:2880–2888.
2018.PubMed/NCBI
|
|
108
|
Nelson AT, Camelio AM, Claussen KR, Cho J,
Tremmel L, Digiovanni J and Siegel D: Synthesis of oxygenated
oleanolic and ursolic acid derivatives with anti-inflammatory
properties. Bioorg Med Chem Lett. 25:4342–4346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yamaguchi Y, Yamada K, Yoshikawa N,
Nakamura K, Haginaka J and Kunitomo M: Corosolic acid prevents
oxidative stress, inflammation and hypertension in SHR/NDmcr-cp
rats, a model of metabolic syndrome. Life Sci. 79:2474–2479. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chen H, Yang J, Zhang Q, Chen LH and Wang
Q: Corosolic acid ameliorates atherosclerosis in apolipoprotein
E-deficient mice by regulating the nuclear factor-κB signaling
pathway and inhibiting monocyte chemoattractant protein-1
expression. Circ J. 76:995–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kim SJ, Cha JY, Kang HS, Lee JH, Ji YL,
Park JH, Bae JH, Song DK and Im SS: Corosolic acid ameliorates
acute inflammation through inhibition of IRAK-1 phosphorylation in
macrophages. BMB Rep. 49:276–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Festa A, D'Agostino R Jr, Howard G,
Mykkänen L, Tracy RP and Haffner SM: Chronic subclinical
inflammation as part of the insulin resistance syndrome: The
insulin resistance atherosclerosis study (IRAS). Circulation.
102:42–47. 2000. View Article : Google Scholar : PubMed/NCBI
|