Introduction
Breast cancer is the most frequent developing
malignancy among women with high mortality rates, worldwide
(1,2). Epidemiological data revealed that in
2018 more than 2 million women were diagnosed with breast cancer,
accounting for more than 600.000 cancer-associated deaths (2–4). Since
1980, the global prevalence of breast cancer provides a
consistently positive trend and it has been anticipated that the
tendency for the number of incidences to rise will continue in the
following years (2–4). Although the 60% of deaths occur in
developing countries, the breast cancer mortality rate has been
reduced in most developed countries due to the establishment of
novel methodologies in screening, diagnosis and cancer therapy
(5,6).
Over the last years, several studies have focused on
the analysis of numerous biomarkers, in order to improve the early
prognosis, diagnosis and the proper breast cancer treatment
(7–9). In particular, the effect of fatty
acid-binding protein 4 (FABP4) on breast disease gained increased
popularity, since high levels of the corresponding protein have
been observed in plasma of breast cancer patients, while exogenous
FABP4 significantly stimulates the proliferation of breast cancer
cells (10–12). FABP4 is a member of fatty
acid-binding proteins family (FABPs) which is implicated in the
intracellular transportation of fatty acids in several cellular
organelles (12). FABP4 is
constantly expressed in macrophage and adipocyte, while it is
involved in numerous cellular functions, including fatty acid
uptake and storage as well as in regulation of gene expression,
cell proliferation and differentiation (12,13). In
macrophages FABP4 regulates inflammatory responses via activation
of the NF-κB (nuclear factor κB) and JNK pathways (c-Jun N-terminal
kinase), while in adipocytes FABP4 stimulates lipolysis and
constraints lipogenesis through interaction with HSL
(hormone-sensitive lipase) and PPARγ (peroxisome
proliferator-activated receptor gamma) (14–16). In
regard to breast cancer development FABP4 is highly expressed in a
small subgroup of tumor associated macrophages (TAMs).
FABP4-positive TAM subgroup in turn assembles in the late stage of
mammary tumor, stimulating tumor growth, (17). An alternative pathway implicates
adipocytes in cases of obesity. In particular, during obesity
adipocytes release FABP4 which triggers oncogenic signaling
factors, including IL-6, STAT3 and ALDH1 in breast mammary tumor
cells, leading to tumor progression (14,16–18).
Recent analyses have focused on the implication of
retinol binding protein 4 (RBP4) in breast cancer development, as
well. RBP4 is an adipokine and member of the lipocalin family of
proteins (19,20). Lipocalin protein family consist of a
heterogeneous group of extracellular proteins, expressed in liver
and adipose tissue and they facilitate the transferring of small
hydrophobic molecules, including hormones, prostaglandins,
arachidonic acids and retinoids, while it is the major transporter
for retinol acid (Vitamin A) (20,21).
RBP4 along with retinol acid (Vitamin A) stimulate STRA6 that in
turn recruit and activate Janus kinase and the transcriptional
factors STAT3 or STAT5 (22).
Alternatively, RBP4 alone can activate pro-inflammatory responses
through JNK1, JNK2 or Toll-like receptors (23). The expression of RBP4 is associated
with insulin resistance as well as with cardiovascular risk
markers, including body mass index [BMI; calculated as weight (kg)
divided by height (m2)], waist to hip ratio and the
levels of triglyceride in serum (21). Furthermore, RBP4 is correlated with
numerous types of cancer, such as prostate cancer, colon adenoma,
ovarian cancer and oral cancer (24–26), but
its association with breast cancer growth remains rather vague
(27,28).
Finally, neutrophil gelatinase-associated lipocalin
(NGAL) is a member of the lipocalin family and it has been related
with breast disease, as well (20,29).
NGAL is a fundamental component of cytoplasmic granules of human
neutrophils and it contributes to the chelation of bacterial
siderophores, thus inhibiting iron availability and consequently it
prevents bacteria to establish infection (30,31).
Moreover, NGAL act as siderocalin and with sideropores and ferrous
iron (Fe++) contribute to iron uptake that is necessary
in regulating the iron-dependent growth pathways (32). Cancer cells have increased need for
intracellular iron, while iron uptake is pivotal in regulating cell
proliferation, invasion and metastasis (32,33).
NGAL expression is regarded as a considerable diagnostic and
prognostic biomarker in several diseases, including inflammation
and tumor growth (29,31,34). It
is noteworthy that NGAL generates a complex with
metalloproteinase-9 (MMP-9) that constraints MMP-9 autodegradation
and subsequently leads to increased activity of MMP-9 (31,34–36).
Previous studies have suggested that MMP-9/NGAL complex augments
cancer growth, including breast cancer (29,31,34–36).
MMP-9 is found in gelatinase granules and contribute to cancer
progression, invasion and metastasis in numerous neoplastic
diseases (29,37,38).
Notably, a previous analysis in Greek population demonstrated that
high levels of MMP-9, NGAL as well as increased levels of
MMP-9/NGAL complex are associated with breast disease (26).
Nowadays, no further analyses have been conducted in
order to better evaluate the influence of MMP-9/NGAL complex on the
development of breast malignancy, while little is known concerning
the impact of FABP4 and RBP4 on patients' susceptibility to the
development of breast cancer in Greek women. Towards this end, the
present study focused on the relationship of serum levels of FABP4,
RBP4, MMP-9/NGAL complex with the growth of breast cancer in the
Greek population. Moreover, we examined the association of FABP4,
RBP4, MMP-9/NGAL complex with the different breast cancer molecular
subtypes, body mass index (BMI), diabetes, menopausal status and
the social background of patients. Our goal was to elucidate
whether the examined proteins are implicated in breast cancer
development as well as to further investigate whether additional
factors may influence this association, providing valuable
information concerning the prognosis and diagnosis of breast
cancer.
Materials and methods
Breast samples
In the present analysis a total of 73 women were
examined. In particular, 53 women were diagnosed with breast cancer
and they were further classified into different molecular types
according to their immunehistochemistry (IHC) profile concerning ER
(estrogen receptor), PR (progesterone receptor), HER-2 (human
epidermal growth factor receptor 2) and ki67 (39,40). As
a result, patients were characterized as Luminal A (n=22), Luminal
B (n=8), Triple Negative-TN: ER negative, PR negative, HER-2
negative (n=15) and HER-2 positive: ER negative, PR negative, HER-2
positive (n=8). According to histopathological criteria all breast
cancer cases were characterized as invasive ductal carcinoma (IDC).
Moreover, the control group comprised 20 healthy women. The mean
age of the examined patients and controls was 62.8±14.12 and
58.7±7.9 years, respectively, while the mean age in Luminal 64±12
years old and the mean age in TN/HER-2 group was 61±14.12 years
old. In addition, 44 patients were postmenopausal and 9 were
premenopausal, while 45 breast cancer patients were diagnosed with
diabetes, as well. Finally, the mean BMI value of breast cancer
cases was 28.1±6 kg/m2, while the mean BMI value of
healthy women was 27.7±4.2 kg/m2. Clinical samples were
collected from Anticancer Oncology Hospital of Athens ‘Saint
Savvas’ between May 2017 and July 2018. All patients signed an
informed consent form, while the study was approved from the
Research Committee of the Hospital.
Sample preparation
Venus blood was collected from patients and controls
between 12:00 a.m. and 14:00 p.m. Sampling was performed directly
into serum vacuum tubes with clot activator, while prior to
centrifugation, tubes remained at room temperature for 20–30 min to
enable blood clotting. Subsequently, the samples were centrifuged
at 3,000 × g for 15 min at 8°C, the serum was isolated and divided
into aliquots parts, while all serums were stored at −80°C for
further use.
ELISA assay for the measurement of
serum levels of FABP4, RBP4 and MMP-9/NGAL complex
The evaluation of serum levels of FABP4, RBP4 and
MMP-9/NGAL complex in the examined clinical samples was conducted
for each individual protein in duplicates through enzyme-linked
immunosorbent assay (ELISA), using the respective Human FABP4, RBP4
and MMP-9/NGAL Quantikine® ELISA kit (R&D
Systems), according to the manufacturers instruction. Finally, the
acquired fluorescence data were analyzed using Multiscan™ FC
Microplate Photometer (Thermo Fisher Scientific, Inc.).
Statistical analysis
Data are presented with mean, standard deviation and
median, while statistical analysis was conducted considering
whether they followed a normal distribution or not, respectively.
Normality was examined through D'Agostino - Pearson, Shapiro-Wilk
or Kolmogorov-Smirnov tests. In particular, FABP4 data follow a non
normal distribution, whereas RBP4 and MMP-9/NGAL complex data
follow a normal distribution. Student's t-test or Mann-Whitney
non-parametric test were used to examine the association of the
respective proteins with breast cancer development, BMI, menopausal
status, diabetes and social background of patients. One way Anova
was used for statistical analysis of the expression of RBP4,
MMP-9/NGAL in Luminal, TN/HER-2 cancers and controls, followed by
the Tukey post hoc test for pair-wise comparison, while
Kruskal-Wallis non parametric test was performed for statistical
analysis of the expression of FABP4 in the respective breast cancer
molecular subtypes, followed by the Dunn multiple comparison post
hoc test. P values were regarded as statistically significant at
the 0.05 cut off level. All analyses were carried out with the
GraphPad Prism 6 (GraphPad Software, Inc.).
Results
FABP4, RBP4, MMP9/NGAL complex and
breast cancer
In the present analysis the serum levels of FABP4,
RBP4 and MMP9/NGAL complex were measured in a total of 73 women, 53
of which have been diagnosed with breast cancer, while 20 cases
were used as control group. The differences that occur at protein
levels between patients and controls were evaluated, in order to
investigate whether the concentrations of FABP4, RBP4 and MMP9/NGAL
complex are related with breast cancer in the studied population.
The mean and median serum concentrations of the respective proteins
are presented in Table I. According
to our results it was demonstrated that the median concentration of
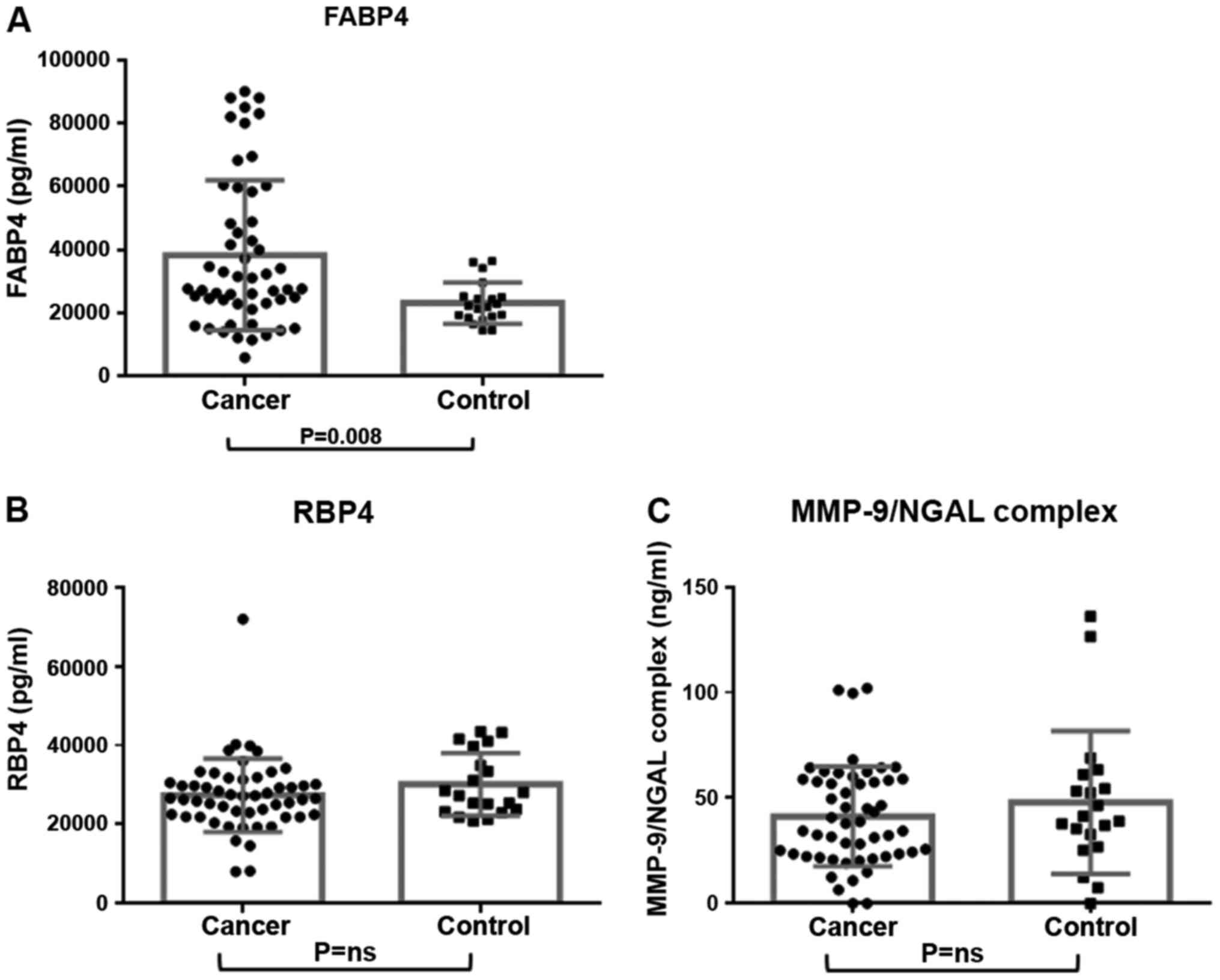
FABP4 in breast cancer cases was significantly higher than that of
healthy women (median concentration; 27,740 pg/ml vs. 22,115 pg/ml,
P=0.0078) (Tables I and II, Fig. 1).
However, no considerable association was recorded between cancer
cases and controls, regarding the serum levels of RBP4 and
MMP9/NGAL complex, respectively (Tables
I and II; Fig. 1).
 | Table I.Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of the examined
patients. |
Table I.
Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of the examined
patients.
| Group | FABP4 (pg/ml), mean
± SD | FABP4 (ng/ml),
median | RBP4 (pg/ml), mean
± SD | RBP4 (ng/ml),
median | MMP-9/NGAL(pg/ml),
mean ± SD | MMP-9/NGAL (ng/ml),
median |
|---|
| Breast cancer
(n=53) | 38,324±3,250 | 27,740 | 27,368±1,274 | 26,650 | 41.26±3.24 | 37.89 |
| Control (n=20) | 23,193±1,462 | 22,115 | 30,138±1,765 | 27,705 | 47.96±7.56 | 40.23 |
| Luminal (n=30) | 45,298±4,659 | 37,105 | 26,755±1,278 | 26,605 | 50.97±4.19 | 53.21 |
| TN/HER-2
(n=23) | 29,227±3,684 | 25,040 | 28,167±2,445 | 27,200 | 28.59±3.78 | 24.48 |
| Control (n=20) | 23,193±1,462 | 22,115 | 30,138±1,765 | 27,705 | 47.96±7.56 | 40.23 |
 | Table II.P-values were calculated to estimate
the differences in protein levels between patients with breast
cancer and controls, and among different breast cancer
subtypes. |
Table II.
P-values were calculated to estimate
the differences in protein levels between patients with breast
cancer and controls, and among different breast cancer
subtypes.
|
| P-values |
|---|
|
|
|
|---|
| Groups | FABP4 | RBP4 | MMP-9/NGAL |
|---|
| Breast cancer vs.
control | 0.008 | 0.260 | 0.420 |
| Luminal vs.
control | <0.001 | 0.130 | 0.700 |
| TN/HER-2 vs.
control | 0.420 | 0.320 | 0.030 |
| Luminal vs.
TN/HER-2 | 0.007 | 0.600 | <0.001 |
FABP4, RBP4, MMP9/NGAL complex and
molecular subtypes
The relationship of the corresponding proteins with
the development of breast disease was further examined considering
the molecular classification of breast cancer into different
molecular subtypes (34,35). Hence, the examined cases were
classified into two major groups, containing the Luminal cohort
(Luminal A and Luminal B) and TN/HER-2 group (HER-2 positive and
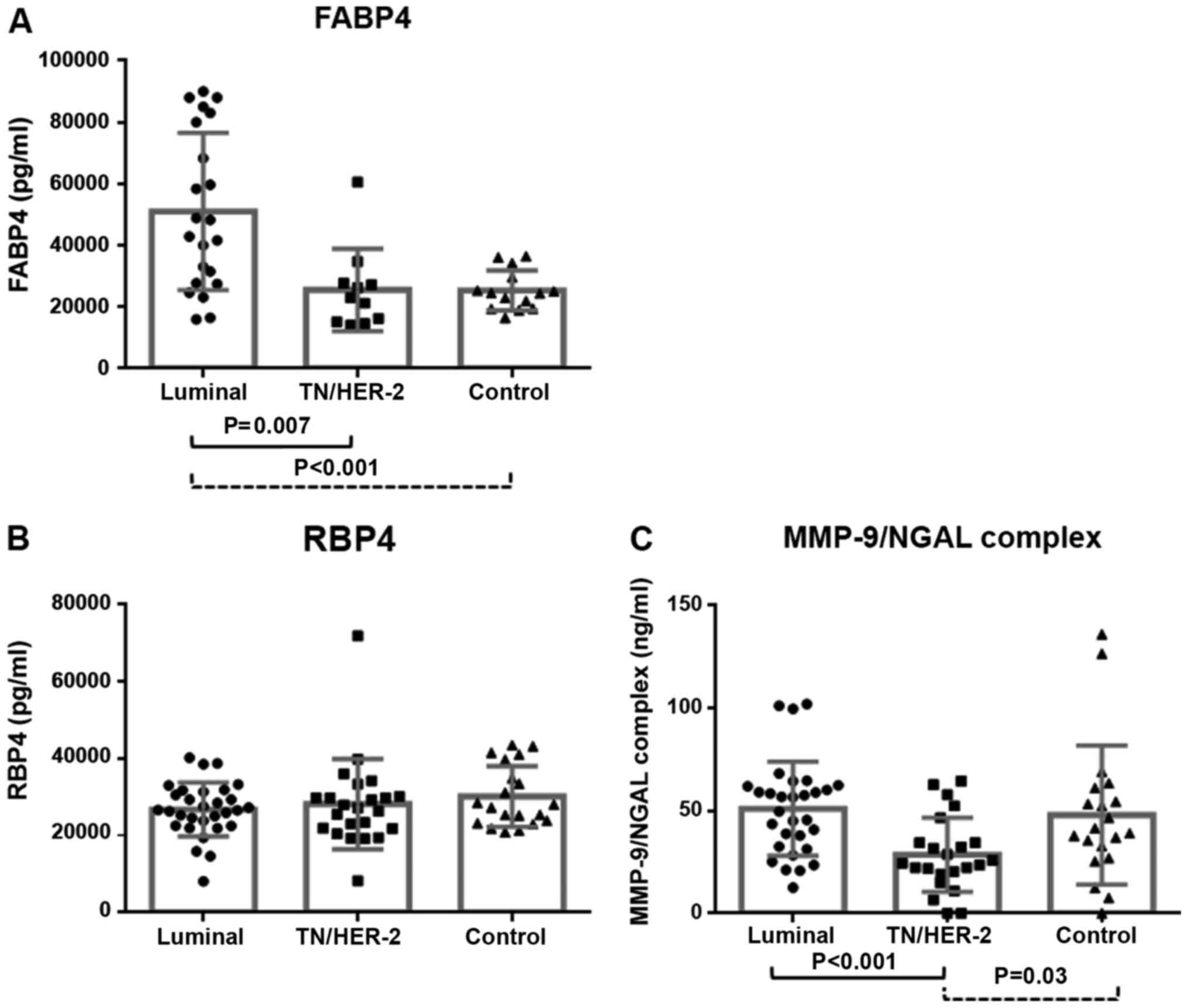
Triple Negative). In regard to FABP4 serum levels, it was
demonstrated that the median concentration of the respective
protein in Luminal cases was considerably higher than that of
healthy women (median concentration; 37,105 pg/ml vs. 22,115 pg/ml,
P=0.0002), while high levels of FABP4 were recorded in Luminal
group when compared with that of TN/HER-2 group (median
concentration; 37,105 pg/ml vs. 25,040 pg/ml, P=0.0073) (Tables I and II; Fig. 2).
Although the median concentration of FABP4 in TN/HER-2 cluster was
found to be increased compared to healthy women, the difference was
not considered as statistically significant (P=ns). In addition, no
significant association was recorded between the groups of breast
cancer cases and the serum levels of RBP4 protein (Tables I and II, Fig.
2).
Interestingly, differences in serum levels of
MMP9/NGAL complex were found between breast cancer subtypes. In
particular, slightly elevated levels of MMP9/NGAL complex were
recorded in Luminal cohort compared to control group, but this
association did not reach the limits of statistical significance
(mean concentration-ng/ml; 50.97±4.191 vs. 47.96±7.566, P=ns)
(Tables I and II). In contrast, the serum levels of
MMP-9/NGAL complex were found to be substantially decreased in
TN/HER-2 group. In particular, the mean concentration of MMP-9/NGAL
complex in TN/HER-2 group was considerably lower than that of
control group (mean concentration-ng/ml; 28.59±3.78 vs.
47.96±7.566, P=0.03) and Luminal cohort, as well (mean
concentration-ng/ml; 28.59±3.78 vs. 50.97±4.191, P=0.0002)
(Tables I and II; Fig.
2).
FABP4, RBP4, MMP9/NGAL complex and
BMI
A stratified analysis according to body mass index
(BMI) values was performed in order to evaluate whether the BMI in
combination with serum levels of FABP4, RBP4 and MMP9/NGAL complex,
may be used as valuable biomarkers for the development of breast
disease. In particular, breast cancer cases and controls were
classified into two groups, comprising specimens with BMI values
≥25 kg/m2 and specimens with BMI values <25
kg/m2 (Tables III and
IV). Our results indicate that in
clinical samples with BMI ≥25 kg/m2 the median
concentration of FABP4 was significantly higher when compared with
that of healthy women (median concentration 37,180 pg/ml vs. 24,445
pg/ml, P=0.0023) (Tables III and
IV). In contrast, in samples with
BMI <25 kg/m2 no significant alterations were
detected in FABP4 levels between cancer and control cases.
Moreover, no significant differences were recorded in RBP4 and
MMP9/NGAL serum levels between cancer cases and controls, neither
when BMI ≥25 kg/m2 nor when BMI <25 kg/m2.
Merging outcomes derived from BMI clusters and the different cancer
groups it was revealed that when BMI ≥25 kg/m2 the
median concentration of FABP4 was significantly augmented in
Luminal cases compared either to TN/HER-2 (median concentration
45,595 pg/ml vs. 22,960 pg/ml, P=0.018) or healthy women (median
concentration 45,595 pg/ml vs. 24,445 pg/ml, P=0.0009) (Tables III and IV). However, no considerable changes in
FABP4 levels were detected in cases with BMI lower than 25
kg/m2. In regard to RBP4 serum levels, no significant
associations were identified between the concentration of the
respective protein and BMI groups within the different molecular
subtypes of breast disease (Tables
III and IV). Finally,
considering results derived from MMP9/NGAL complex, it was observed
that in TN/HER-2 cases with BMI ≥25 kg/m2 the mean
concentration of the respective complex was significantly lower
than that of Luminal samples (mean concentration-ng/ml; 30.59±6.45
vs. 50.83±3.7, P=0.014) (Tables
III and IV). Nevertheless, no
further associations were found between the concentration of
MMP9/NGAL complex and BMI cohorts within the divergent subtypes of
breast malignancy (Tables III and
IV).
 | Table III.Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of patients
separated according to BMI values. |
Table III.
Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of patients
separated according to BMI values.
| A, BMI ≥25
kg/m2 |
|---|
|
|---|
| Cancer subtype | FABP4 (pg/ml), mean
± SD | FABP4 (pg/ml),
median | RBP4 (pg/ml), mean
± SD | RBP4 (pg/ml),
median | MMP-9/NGAL (pg/ml),
mean ± SD | MMP-9/NGAL (pg/ml),
median |
|---|
| Breast cancer
(n=33) | 42,509±4,378 | 37,180 | 25,846±1,203 | 25,900 | 44.09±3,65 | 45.11 |
| Luminal (n=22) | 51,005±5,442 | 45,595 | 26,571±1,391 | 26,090 | 50.83±3.75 | 53.21 |
| TN/HER-2
(n=11) | 25,516±4,038 | 22,960 | 24,396±2,332 | 23,050 | 30.59±6.44 | 31.69 |
| Control (n=14) | 25,345±1,728 | 24,445 | 30,368±2,081 | 27,885 | 52.92±9.88 | 40.23 |
|
| B, BMI <25
kg/m2 |
|
| Cancer
subtype | FABP4 (pg/ml),
mean ± SD | FABP4 (pg/ml),
median | RBP4 (pg/ml),
mean ± SD | RBP4 (pg/ml),
median | MMP-9/NGAL
(pg/ml), mean ± SD | MMP-9/NGAL
(pg/ml), median |
|
| Breast cancer
(n=33) | 31,419±4,396 | 26,050 | 29,878±2,686 | 28,875 | 36.59±6.13 | 25.5 |
| Luminal (n=30) | 29,603±6,701 | 28,555 | 27,262±3,058 | 31,560 |
51.36±12.53 | 42.9 |
| TN/HER-2
(n=23) | 32,629±6,021 | 25,595 | 31,623±4,014 | 27,600 | 26.75±4.42 | 24.05 |
| Control (n=20) | 18,172±1,331 | 18,185 | 29,602±3,618 | 26,015 | 36.37±9.72 | 40.13 |
 | Table IV.P-values were calculated to assess
the differences in protein levels between patients and controls,
and among the different breast cancer subtypes. |
Table IV.
P-values were calculated to assess
the differences in protein levels between patients and controls,
and among the different breast cancer subtypes.
| A, BMI ≥25
kg/m2 |
|---|
|
|---|
|
| P-values |
|---|
|
|
|
|---|
| Groups | FABP4 | RBP4 | MMP-9/NGAL |
|---|
| Breast cancer vs.
control | 0.002 | 0.070 | 0.300 |
| Luminal vs.
control | <0.001 | 0.100 | 0.800 |
| TN/HER-2 vs.
control | 0.540 | 0.069 | 0.070 |
| Luminal vs.
TN/HER-2 | 0.018 | 0.430 | 0.014 |
|
| B, BMI <25
kg/m2 |
|
|
|
P-values |
|
|
|
| Groups | FABP4 | RBP4 |
MMP-9/NGAL |
|
| Breast cancer vs.
control | 0.055 | 0.900 | 0.900 |
| Luminal vs.
control | 0.120 | 0.600 | 0.362 |
| TN/HER-2 vs.
control | 0.061 | 0.700 | 0.400 |
| Luminal vs.
TN/HER-2 | 0.900 | 0.250 | 0.090 |
FABP4, RBP4, MMP9/NGAL complex,
menopausal status, diabetes and social background
Finally, we further evaluate the relationship of the
tested serum proteins with menopausal status, diabetes, and social
background of the examined patients including smoking and exercise.
Considering our results, it was observed that serum FABP4 levels
are significantly increased in postmenopausal breast cancer
patients when compared with that of premenopausal patients (median
concentration 32,720 pg/ml vs. 14,550 pg/ml, P=0.0005) (Table V). In addition, in the cohort of
postmenopausal patients the concentration of FABP4 protein is
significantly higher in individuals with BMI ≥25 kg/m2
than patients with BMI <25 kg/m2 (median
concentration 40,050 pg/ml vs. 24,610 pg/ml, P=0.03) (Table VI). In addition, the link of FABP4
with diabetes reached the limits of statistical significance, as
the serum FABP4 concentration was found to be higher in breast
cancer patients with diabetes when compared with that of patients
with no diabetes (median concentration 54,310 pg/ml vs. 27,460
pg/ml, P=0.05) (Table VII).
 | Table V.Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of patients with
breast cancer separated according to menopausal status. |
Table V.
Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of patients with
breast cancer separated according to menopausal status.
|
| FABP4 | RBP4 | MMP-9/NGAL |
|---|
|
|
|
|
|
|---|
| Menopause
status | Mean ± SD
(pg/ml) | Median (pg/ml) | P-value | Mean ± SD
(pg/ml) | Median (pg/ml) | P-value | Mean ± SD
(pg/ml) | Median (pg/ml) | P-value |
|---|
| Postmenopause
(n=44) | 42,446±3,558 | 32,720 | <0.001 | 28,069±1,474 | 27,435 | 0.211 | 41.52±3.08 | 39.76 | 0.900 |
| Premenopause
(n=9) | 18,172±3,259 | 14,550 |
| 23,941±1,802 | 24,550 |
| 39.97±12.39 | 22.29 |
|
 | Table VI.Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of postmenopausal
women with breast cancer, divided according to BMI values. |
Table VI.
Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of postmenopausal
women with breast cancer, divided according to BMI values.
|
| FABP4 | RBP4 | MMP-9/NGAL |
|---|
|
|
|
|
|
|---|
| Postmenopause | Mean ± SD
(pg/ml) | Median (pg/ml) | P-value | Mean ± SD
(pg/ml) | Median (pg/ml) | P-value | Mean ± SD
(pg/ml) | Median (pg/ml) | P-value |
|---|
| BMI ≥25
kg/m2 (n=31) | 46,802±4471 | 40,050 | 0.030 | 27,334±1,320 | 27,590 | 0.440 | 46.21±3.52 | 45.56 | 0.016 |
| BMI <25
kg/m2 (n=13) | 32,058±7556 | 24,610 |
| 29,821±3,949 | 25,900 |
| 30.34±5.15 | 25.17 |
|
 | Table VII.Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of patients with
breast cancer, separated according to diabetes. |
Table VII.
Mean and median concentrations of
FABP4, RBP4 and MMP-9/NGAL complex in the serum of patients with
breast cancer, separated according to diabetes.
|
| FABP4 | RBP4 | MMP-9/NGAL |
|---|
|
|
|
|
|
|---|
| Diabetes | Mean ± SD
(pg/ml) | Median (pg/ml) | P-value | Mean ± SD
(pg/ml) | Median (pg/ml) | P-value | Mean ± SD
(pg/ml) | Median (pg/ml) | P-value |
|---|
| yes (n=45) | 51,184±7,827 | 54,310 | 0.050 | 32,323±6,011 | 26,605 | 0.100 | 31.71±5.64 | 27.90 | 0.200 |
| no (n=8) | 36,083±3,491 | 27,460 |
| 26,487±1,058 | 27,500 |
| 42.96±3.65 | 40.77 |
|
Although the serum levels of MMP9/NGAL complex are
not modified considering the menopausal status of patients, it was
observed that MMP9/NGAL complex in postmenopausal individuals is
significantly higher in patients with BMI ≥25 kg/m2 than
patients with BMI <25 kg/m2 (mean concentration
ng/ml; 46.21±3.51 vs. 30.34±5.15, P=0.016) (Table VI). Moreover, no significant
association was recorded between MMP9/NGAL complex and diabetes in
breast cancer patients as well as no significant association was
detected regarding RBP4 serum levels either with menopausal status
or diabetes (Table VII).
Furthermore, it was revealed that smoking and exercise do not
influence the serum levels of FABP4, RBP4 and MMP9/NGAL in breast
cancer patients.
Discussion
The stratification of breast cancer severity lies on
numerous factors, including TNM stage, tumor grade, lymphatic
invasion as well as the expression of the hormonal receptors ER, PR
and HER-2 (39,40). Although these factors offer crucial
information concerning the risk profile of patients and they
significantly contribute to select the most suitable therapeutic
approach (41,42), today, there is a growing interest in
the establishment of novel biomarkers that will enable the accurate
prognosis and diagnosis of breast disease (8,9,41,42).
Hence, the present analysis focused on the study of serum FABP4,
RBP4 and MMP9/NGAL complex levels in women diagnosed with breast
cancer along with healthy women, while results were further
associated with the different breast cancer molecular subtypes,
BMI, menopause status diabetes and social background of
patients.
In the present study differential serum levels of
FABP4, RBP4 and MMP9/NGAL complex were detected in breast cancer
cases, while different serum levels of the corresponding proteins
were observed between the different molecular subtypes of breast
disease, as well. In particular, increased serum concentration of
FABP4 was detected in the examined breast cancer cases compared to
control group, supporting previous findings (10–12,17,43,44).
Although substantially increased serum FABP4 levels were recorded
in Luminal breast cancer samples, the concentration of the
respective protein was found to be decreased in TN/HER-2 cases.
These results imply that FABP4 may be a key element in the
development of specific breast cancer molecular subtype and that
each subtype uses different metabolic pathways. Indeed, receptor
positive breast cancers are associated with gene signature involved
in de novo lipogenesis, fat acid mobilization and oxidation while
TNBC are associated with genes expressed in exogenous lipid uptake
(45). This may support the
hypothesis that specific metabolic lipid pathways are probably
accelerated or moderated depending on tumor ER and HER-2 status.
Interestingly, a previous study concerning the tissue analysis of
breast tumors showed that the concentration of FABP4 was higher in
HER-2 tumors and lower in Luminal A tumors, suggesting that this
discrepancy lies mainly on differences in lipid metabolic pathways
that have been observed in the different breast cancer subtypes
(43,46). Taking all these data into account it
was anticipated that the different serum FABP4 levels that were
detected in Luminal and TN/HER-2 breast cancers might be observed
due to a possible stronger association between lipid metabolic
status and breast cancer subtypes and therefore different levels of
FABP4 might be circulating in the serum of patients. However,
further analyses are required in order to confirm this
hypothesis.
In regard to RBP4, previous research studies have
recommended that RBP4 is not associated with breast cancer
development, while the expression of RBP4 gene seems to be down
regulated in breast cancer cases (27,47). In
contrast, a more recent study revealed for the first time that
increased serum levels of RBP4 are related with breast cancer in
menopausal women (28). In the
present analysis no significant association between the serum
levels of RBP4 and the development of breast cancer was observed in
the tested patients (27,47). Interestingly, a previous analysis by
Formelli et al (27)
suggested that low plasma RBP4 levels are found in postmenopausal
women (≥55 years old) and these patients exhibited poor prognosis
of breast disease. In the present analysis, no significant
association was recorded between serum levels of RBP4 and the
development of breast cancer, while no significant relationship was
described between RBP4 and menopausal status of the examined breast
cancer patients.
Nowadays, little is known concerning the impact of
MMP-9/NGAL complex on breast disease. A previous analysis derived
by our group observed for the first time significantly increased
serum levels of MMP-9/NGAL complex in breast cancer cases (29). In contrast to previous findings, we
observed low levels of MMP-9/NGAL complex in breast cancer cases
compared to healthy women but this association did not reach the
limits of statistical significance. It is important to underline
that significantly lower levels of MMP-9/NGAL complex were found in
TN/HER-2 group compared to either healthy women or Luminal breast
cancers (P<0.05). One possible explanation of the contradictory
results could be the age group of the examined population, since in
the previous study the mean age of the tested women was 52.8 years
old, while in the present analysis the mean age of the examined
patients was 62.8 years old (29).
As a result, the age of the tested population may influence the
MMP-9/NGAL complex levels (48,49). An
additional explanation could be the molecular subtype of breast
cancer cases. Notably, in the previous analysis by our group the
breast lesions were classified into four groups including sclerosis
adenosis, atypical ductal hyperplasia, ductal carcinoma in
situ and invasive carcinoma, while there was no available data
concerning the molecular subtype of breast cancer (29). As a consequence the composition of
breast cancer cases in TN/HER-2 subtype may influence the serum
levels of MMP-9/NGAL complex and consequently might affect the
prognostic value of MMP-9/NGAL complex in developing breast cancer.
As a consequence, more analyses are needed in order to examine
whether low serum MMP-9/NGAL complex levels might indicate the
development of TN/HER-2 breast cancer in the examined women.
Finally, in order to assess the impact of obesity on
the described associations, a stratified analysis based on BMI
values and the serum levels of the corresponding proteins was
carried out. According to our results it was revealed that FABP4 in
patients with high BMI values (BMI ≥25 kg/m2) is
considerably increased, supporting previous findings (11). Moreover, patients diagnosed with
Luminal breast cancer and BMI ≥25 kg/m2 exhibited
significantly higher levels of FABP4 (P<0.02). In addition, the
serum levels of RBP4, MMP-9/NGAL complex are not influenced by BMI
value, supporting previous studies (28,29).
However the concentration of MMP-9/NGAL complex was found to be
significantly decreased in patients with TN/HER-2 breast cancer and
BMI ≥25 kg/m2 when compared to patients with Luminal
subtype. It is significant to highlight that in patients with BMI
<25 kg/m2 the previously described associations did
not reach the limits of statistical significance. Taking all these
data into consideration it was suggested that BMI values regulate
the described associations and thus BMI should be taken into
consideration in order to draw more accurate conclusions concerning
the prognosis of breast disease.
In addition, when protein serum levels were
considered together with menopausal status, BMI and diabetes, it
was observed that FABP4 concentration is significantly increased in
postmenopausal patients with high BMI values as well as in breast
cancer patients with diabetes, supporting previous findings
(11,17,50).
Although FABP4 and estrogens have been associated with obesity and
obesity-associated breast cancer development, a recent analysis
proved that FABP4 and estrogens have an independent impact on
obesity related breast cancer growth (51). Interestingly, MMP9/NGAL complex was
significantly associated in breast cancer development in
postmenopausal women. As it was described above no considerable
association was found between MMP9/NGAL complex and breast cancer
development. However, this association reaches the limits of
statistical significance in postmenopausal women with BMI ≥25
kg/m2. As a consequence, further analyses are required
in order to confirm these outcomes as well as to examine whether
MMP9/NGAL complex in association with estrogens contribute to the
growth of breast cancer. To the best of our knowledge this is the
first study that implicates MMP9/NGAL complex to the development of
breast cancer in postmenopausal women.
Our results imply that there is a bidirectional
interaction between circulating FABP4 and ER/PR receptors in tumor
microenvironment. The relationship between lipid metabolism and
breast cancer molecular subtypes varies considerably regarding
menopausal status, BMI, presence of diabetes. Adipocytes and
secreted fatted acid proteins induce resistance against to hormonal
therapy, HER-2 targeting agents and radiotherapy (52). From a clinical perspective, the
therapeutic interventions targeting multiple molecular pathways
that are implicated in tumor establishment and progression could be
more effective. That is to say the specific blockade of FABP4 may
improve the efficacy of endocrine therapies and moderate
resistance. Further studies should be conducted in order to better
evaluate the complexity of lipid metabolism and the impact of
obesity-related proteins as a diagnostic, prognostic and
therapeutic tool in specific group of patients. This study has some
limitations. The number of participants was small, while all
specimens were collected from a single institute. As a consequence,
further analyses are required in a larger population scale derived
from different tertiary care Greek hospitals in order to reinforce
our findings.
In conclusion, our results support the initial
recommendation that FABP4 can be regarded as a powerful biomarker
of breast cancer development, while its strong association with
obesity indicates that FABP4 is a key therapeutic target for
treatment of obesity-related breast cancer (17). To the best of our knowledge this is
the first study that describes the association of serum FABP4 and
MMP-9/NGAL levels in Luminal and TN/HER-2 breast cancer subtypes,
respectively, suggesting that the measurement of these two factors
may provide significant information concerning not only breast
cancer development but also the growth of specific breast cancer
subtype, in order to better evaluate the interaction of the
respective circulating proteins in specific group of patients
(postmenopausal, obese, diabetes). The present analysis anticipated
that the metabolic profile of breast cancer subtype, the menopausal
status and BMI of the examined women should be taken into
consideration in order to select the most suitable biomarker,
offering crucial information regarding the personalized prognosis,
diagnosis and the proper therapy of breast disease.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the Hellenic
Anticancer Institute, a private non-profit organization. The
funding source had no involvement in study design, conduct of
research or manuscript publication.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on request.
Author's contribution
GB, EZ and FZ designed the present study. DB
collected and analyzed samples, and interpreted results. GB, DT and
EK analyzed and interpreted the data. DT and GB wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided their written informed consent
prior to enrollement. The current study was approved by the ‘Saint
Savvas’ General Anticancer Hospital Ethics Committee (approval no.
1617017278).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bertucci F and Birnbaum D: Reasons for
breast cancer heterogeneity. J Biol. 7:62008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Laversanne M, Brewster
DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E,
Swaminathan R, Antoni S, et al: Cancer incidence in five
continents: Inclusion criteria, highlights from Volume X and the
global status of cancer registration. Int J Cancer. 137:2060–2071.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harbeck N, Penault-Llorca F, Cortes J,
Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J and Cardoso F:
Breast cancer. Nat Rev Dis Primers. 5:662019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), : Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colomer R, Aranda-Lopez I, Albanell J,
García-Caballero T, Ciruelos E, López-García MÁ, Cortés J, Rojo F,
Martín M and Palacios-Calvo J: Biomarkers in breast cancer: A
consensus statement by the Spanish Society of Medical Oncology and
the Spanish Society of Pathology. Clin Transl Oncol. 20:815–826.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kashyap D and Kaur H: Cell-free miRNAs as
non-invasive biomarkers in breast cancer: Significance in early
diagnosis and metastasis prediction. Life Sci. 246:1174172020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bourgain C, Pourtau L, Mazouni C, Bungener
M and Bonastre EJ: Imperfect biomarkers for adjuvant chemotherapy
in early stage breast cancer with good prognosis. Soc Sci Med.
246:1127352020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guaita-Esteruelas S, Bosquet A, Saavedra
P, Gumà J, Girona J, Lam EW, Amillano K, Borràs J and Masana L:
Exogenous FABP4 increases breast cancer cell proliferation and
activates the expression of fatty acid transport proteins. Mol
Carcinog. 56:208–217. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guaita-Esteruelas S, Saavedra-Garcia P,
Bosquet A, Borràs J, Girona J, Amiliano K, Rodríguez-Balada M,
Heras M, Masana L and Gumà J: Adipose-derived fatty acid-binding
proteins plasma concentrations are increased in breast cancer
patients. Oncologist. 22:1309–1315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guaita-Esteruelas S, Guma J, Masana L and
Borras J: The peritumoural adipose tissue microenvironment and
cancer. The roles of fatty acid binding protein 4 and fatty acid
binding protein 5. Mol Cell Endocrinol. 462:107–118. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zimmerman AW and Veerkamp JH: New insights
into the structure and function of fatty acid-binding proteins.
Cell Mol Life Sci. 59:1096–1116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng J, Sauter ER and Li B: FABP4: A new
player in obesity-associated breast cancer. Trends Mol Med.
26:437–440. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prentice KJ, Saksi J and Hotamisligil GS:
Adipokine FABP4 integrates energy stores and counterregulatory
metabolic responses. J Lipid Res. 60:734–740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao H, Sekiya M, Ertunc ME, Burak MF,
Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, et
al: Adipocyte lipid chaperone AP2 is a secreted adipokine
regulating hepatic glucose production. Cell Metab. 17:768–778.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao J, Zhang Y, Yan X, Yan F, Sun Y, Zeng
J, Waigel S, Yin Y, Fraig MM, Egilmez NK, et al: Circulating
adipose fatty acid binding protein is a new link underlying
obesity-associated breast/mammary tumor development. Cell Metab.
28:689–705 e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peek ME, Bhatnagar A, McCarty NA and
Zughaier SM: Pyoverdine, the major siderophore in pseudomonas
aeruginosa, evades NGAL recognition. Interdiscip Perspect Infect
Dis. 2012:8435092012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flowe DR: The lipocalin protein family:
Structure and function. Biochem J. 318:1–14. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawaguchi R, Yu J, Honda J, Hu J,
Whitelegge J, Ping P, Wiita P, Bok D and Sun H: A membrane receptor
for retinol binding protein mediates cellular uptake of vitamin A.
Science. 315:820–825. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berry DC, Jin H, Majumdar A and Noy N:
Signaling by vitamin A and retinol-binding protein regulates gene
expression to inhibit insulin responses. Proc Natl Acad Sci USA.
108:4340–4345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Norseen J, Hosooka T, Hammarstedt A, Yore
MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ,
et al: Retinol-binding protein 4 inhibits insulin signaling in
adipocytes by inducing proinflammatory cytokines in macrophages
through a c-Jun N-terminal kinase- and toll-like receptor
4-dependent and retinol-independent mechanism. Mol Cell Biol.
32:2010–2019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abola MV, Thompson CL, Chen Z, Chak A,
Berger NA, Kirwan JP and Li L: Serum levels of retinol-binding
protein 4 and risk of colon adenoma. Endocr Relat Cancer. 22:L1–L4.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng Y, Liu C, Zhang N, Wang S and Zhang
Z: Proteomics analysis for finding serum markers of ovarian cancer.
Biomed Res Int. 2014:1790402014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Azman SN, Kerishnan JP, Zain RB,
Chen YN, Wong YL and Gopinath SC: Identification of host-immune
response protein candidates in the sera of human oral squamous cell
carcinoma patients. PLoS One. 9:e1090122014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Formelli F, Meneghini E, Cavadini E,
Camerini T, Di Mauro MG, De Palo G, Veronesi U, Berrino F and
Micheli A: Plasma retinol and prognosis of postmenopausal breast
cancer patients. Cancer Epidemiol Biomarkers Prev. 18:42–48. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiao C, Cui L, Ma A, Li N and Si H:
Elevated Serum Levels of Retinol-Binding Protein 4 Are Associated
with Breast Cancer Risk: A Case-Control Study. PLoS One.
11:e01674982016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Provatopoulou X, Gounaris A, Kalogera E,
Zagouri F, Flessas I, Goussetis E, Nonni A, Papassotiriou I and
Zografos G: Circulating levels of matrix metalloproteinase-9
(MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and
their complex MMP-9/NGAL in breast cancer disease. BMC Cancer.
9:3902009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barasch J, Hollmen M, Deng R, Hod EA,
Rupert PB, Abergel RJ, Allred BE, Xu K, Darrah SF, Tekabe Y, et al:
Disposal of iron by a mutant form of lipocalin 2. Nat Commun.
7:129732016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chakraborty S, Kaur S, Guha S and Batra
SK: The multifaceted roles of neutrophil gelatinase associated
lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta.
1826:129–169. 2012.PubMed/NCBI
|
|
32
|
Candido S, Abrams SL, Steelman LS,
Lertpiriyapong K, Fitzgerald TL, Martelli AM, Cocco L, Montalto G,
Cervello M, Polesel J, et al: Roles of NGAL and MMP-9 in the tumor
microenvironment and sensitivity to targeted therapy. Biochim
Biophys Acta. 1863:438–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roli L, Pecoraro V and Trenti T: Can NGAL
be employed as prognostic and diagnostic biomarker in human
cancers? A systematic review of current evidence. Int J Biol
Markers. 32:e53–e61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen YC, Chang SC, Huang YH, Lee YJ, Chang
CC, Liao JW and Hsu WL: Expression and the molecular forms of
neutrophil gelatinase-associated lipocalin and matrix
metalloproteinase 9 in canine mammary tumours. Vet Comp Oncol.
17:427–438. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan L, Borregaard N, Kjeldsen L and Moses
MA: The high molecular weight urinary matrix metalloproteinase
(MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil
gelatinase-associated lipocalin (NGAL). Modulation of MMP-9
activity by NGAL. J Biol Chem. 276:37258–37265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sung H, Choi JY, Lee SA, Lee KM, Han S,
Jeon S, Song M, Lee Y, Park SK, Yoo KY, et al: The association
between the preoperative serum levels of lipocalin-2 and matrix
metalloproteinase-9 (MMP-9) and prognosis of breast cancer. BMC
Cancer. 12:1932012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang H: Matrix Metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 27:32492018. View Article : Google Scholar
|
|
39
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members, : Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St. Gallen international expert consensus on the primary
therapy of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Waks AG and Winer EP: Breast cancer
treatment: A Review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fragomeni SM, Sciallis A and Jeruss JS:
Molecular Subtypes and Local-Regional Control of Breast Cancer.
Surg Oncol Clin N Am. 27:95–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim S, Lee Y and Koo JS: Differential
expression of lipid metabolism-related proteins in different breast
cancer subtypes. PLoS One. 10:e01194732015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu Q, Li B, Li Z, Li J and Sun S and Sun
S: Cancer-associated adipocytes: key players in breast cancer
progression. J Hematol Oncol. 12:952019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koundouros N and Poulogiannis G:
Reprogramming of fatty acid metabolism in cancer. Br J Cancer.
122:4–22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moreno-Sanchez R, Rodriguez-Enriquez S,
Marin-Hernandez A and Saavedra E: Energy metabolism in tumor cells.
FEBS J. 274:1393–1418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Merdad A, Karim S, Schulten HJ, Jayapal M,
Dallol A, Buhmeida A, Al-Thubaity F, GariI MA, Chaudhary AG,
Abuzenadah AM and Al-Qahtani MH: Transcriptomics profiling study of
breast cancer from Kingdom of Saudi Arabia revealed altered
expression of Adiponectin and Fatty Acid Binding Protein4: Is lipid
metabolism associated with breast cancer? BMC Genomics. 16 (Suppl
1):S112015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lanning NJ, Castle JP, Singh SJ, Leon AN,
Tovar EA, Sanghera A, MacKeigan JP, Filipp FV and Graveel CR:
Metabolic profiling of triple-negative breast cancer cells reveals
metabolic vulnerabilities. Cancer Metab. 5:62017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tayyari F, Gowda GAN, Olopade OF, Berg R,
Yang HH, Lee MP, Ngwa WF, Mittal SK, Raftery D and Mohammed SI:
Metabolic profiles of triple-negative and luminal A breast cancer
subtypes in African-American identify key metabolic differences.
Oncotarget. 9:11677–11690. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hancke K, Grubeck D, Hauser N, Kreienberg
R and Weiss JM: Adipocyte fatty acid-binding protein as a novel
prognostic factor in obese breast cancer patients. Breast Cancer
Res Treat. 119:367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li B, Hao J, Yan X, Kong M and Sauter ER:
A-FABP and oestrogens are independently involved in the development
of breast cancer. Adipocyte. 8:379–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Choi J, Cha YJ and Koo JS: Adipocyte
biology in breast cancer: From silent bystander to active
facilitator. Prog Lipid Res. 69:11–20. 2018. View Article : Google Scholar : PubMed/NCBI
|