Introduction
Breast cancer is one of the most common malignancies
and is the most common cancer in women. In 2018, 2.1 million new
cases of breast cancer were diagnosed worldwide (1). Approximately 75% of breast cancer is
luminal subtype (ER+), and the number of young women with estrogen
receptor-positive breast cancer has gradually increased (2). Currently, surgery and chemotherapy are
the primary treatment methods for breast cancer, and medical
oncology has been employed to treat early-stage breast cancer, but
treatments for breast cancer remain expensive, especially in
developing countries. In addition, due to the high frequency of
metastasis and drug resistance in breast cancer, the recurrence and
mortality rate of breast cancer are increasing rapidly (3). Therefore, it is urgently important to
elucidate the molecular pathogenesis of breast cancer and identify
new candidate therapeutic targets and biomarkers for this disease
to improve breast cancer detection and treatment.
Many standard clinical drugs, such as
cyclophosphamide, capecitabine and doxorubicin, have been
determined to have inhibitory effects on breast cancer (4–7). 6-TG
has been used as an anticancer and immunosuppressant in medical
practice for over 50 years (8).
Initially, 6-TG was only employed to treat acute and chronic
myeloid leukemia (9). Following
further research, 6-TG has proven to have therapeutic effects on
inflammatory bowel disease, children's gliomas and pancreatic
cancer (9–11). In a previous study, 6-TG was shown to
inhibit MCF-7 breast cancer cell proliferation by increasing FAS
expression, thereby activating the apoptosis pathway (12). Therefore, regarding the treatment of
breast cancer with 6-TG, the mechanism underlying the effects of
this drug warrant further exploration.
Non-coding (nc)RNAs are a group of RNA molecules
that do not encode proteins. The subtypes of ncRNAs include
highlyconserved RNAs, such as micro (mi)RNAs and circular
(circ)RNAs, and other RNAs lacking conservation across species,
such as long non-coding (lnc)RNAs, which constitute 60% of
transcriptional outputs in human cells (13). These ncRNAs have gradually been
associated with human diseases and especially play important roles
in the occurrence and development of cancer (14). Numerous studies have shown that
lncRNAs, as ceRNAs, are involved in the occurrence and development
of a variety of cancer types, such as esophageal, breast, liver and
pancreatic cancer (15–18). The ceRNA was proposed as a novel
regulatory mechanism between ncRNA and coding RNA (19). The types of ceRNAs include mRNAs,
lncRNAs and circRNAs. These ceRNAs act as molecular sponges of
miRNAs through their MREs, thereby regulating the target genes of
the corresponding miRNAs (20).
Complex crosstalk of the ceRNA network has been observed in
different cancer types (21). Wang
et al (22) selected 322
muscle-invasive bladder cancer tissues and 19 non-tumour bladder
tissues from The Cancer Genome Atlas (TCGA) and further analysed
the RNA profiles in these tissues. Next, a lncRNA-associated ceRNA
network was constructed, and novel lncRNAs were identified as
candidate prognostic biomarkers for muscle-invasive bladder cancer.
Zheng et al (23) constructed
a STARD13-correlated ceRNA network to explore the effects of this
network on breast cancer stemness and demonstrated that activation
of the STARD13-correlated ceRNA network was accompanied by
inhibition of the activity of YAP/TAZ in regulating breast cancer
stem cell traits. Fan et al (24) analyzed RNA sequencing data of
patients with breast cancer from TCGA database and established a
lncRNA-miRNA-mRNA ceRNA network. Finally, four lncRNA biomarkers
were identified as prognostic signatures in breast cancer. Taken
together, the findings of these studies show that dysregulated
lncRNAs in the ceRNA network can directly interact by causing miRNA
sponges to regulate mRNA expression, thereby contributing to cancer
initiation and progression (25).
Nevertheless, there are significant differences among the breast
cancer subtypes regarding their molecular profiles and responses to
therapy, and very little information is available on breast cancer
ceRNAs.
A previous study determined that 6-TG can inhibit
the proliferation of the MCF-7 breast cancer cell line. The changes
in the ceRNA network that may be caused by 6-TG through the
mediation of lncRNAs requires further exploration. In the present
study, differentially expressed lncRNAs and mRNAs were determined
by RNA-seq in untreated MCF-7 cells and 6-TG-treated MCF-7 cells
and predicted their common interactional miRNAs. Subsequently, the
lncRNA-miRNA-mRNA ceRNA network was successfully constructed by
comparing, predicting and integrating differentially expressed
RNAs. This study elucidated the lncRNA-mediated ceRNA regulatory
mechanism underlying the effects of 6-TG treatment on MCF-7 breast
cancer cells and identified potential diagnostic biomarkers that
may contribute to the early diagnosis, treatment and prognosis of
luminal subtype breast cancer.
Materials and methods
Cell culture and cell viability
assay
The breast cancer cell line MCF-7 was purchased from
the Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences (Beijing, China). MCF-7 cells were maintained at 37°C in a
cell incubator with 5% CO2. The cells were cultured in
high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Biological Industries), 1% MEM
nonessential amino acids (Gibco; Thermo Fisher Scientific, Inc.),
1% GluMAX and 1% penicillin-streptomycin solution (Gibco; Thermo
Fisher Scientific, Inc.). The effects of 6-TG on cell viability
were determined through the Cell Counting Kit-8 assay, and MCF-7
cells were treated with a half-maximal inhibitory concentration of
6-TG (12).
Data processing and differential
expression analysis
The sequencing data of samples from the MCF-7
control and 6-TG groups were obtained from a previous study
(12). The raw data were available
from the NCBI GEO database (https://www.ncbi.nlm.nih.gov/geo/) (GSE130161)
(12). The differential expression
of lncRNAs were confirmed by performing normalization and
log2 conversion. The lncRNAs with |log2FC|≥2
and P≤0.05 were selected as candidate lncRNAs.
Weighted gene correlation network analysis (WGCNA).
A WGCNA network was generated for several subsets of the data. The
WGCNA package (v1.67) (https://github.com/paytonyau/WGCNA) was installed for
co-expression analysis using BiocManager (http://bioconductor.org/biocLite.R). The soft
threshold method was employed to carry out Pearson correlation
analysis on the expression profiles to determine the connection
strength between two genes, thereby constructing a weighted
network. Based on the topological overlap difference of network
connection strength, average link hierarchical clustering was
performed on group transcripts. To obtain the correct module number
and clarify gene interactions, the restricted minimum gene number
was set to 5 for each module, and a threshold of 0.25 was used to
merge the similar modules. Next, the significant differentially
expressed lncRNAs (sDELs) were collaborated with the hub genes of
candidate pathways in the module that were considered to be
potential targets for the therapeutic mechanism of 6-TG.
Prediction of lncRNA-miRNA and
miRNA-mRNA interactions
To predict the interaction of lncRNA and miRNA, the
data were downloaded from the starBase V2.0 database (http://starbase.sysu.edu.cn/). In addition, the miRNA
target prediction online tools Targetscan7.1 (http://www.targetscan.org/), miRDB (http://www.mirdb.org/miRDB/) and miRTarBas (http://mirtarbase.mbc.nctu.edu.tw) were employed
to predict the miRNA of mRNA. Finally, a Venn diagram analysis
through TBtools (https://github.com/CJ-Chen/TBtools/releases) was used
to obtain the common target miRNAs of differential expression
lncRNAs and mRNAs.
Construction of the lncRNA-miRNA-mRNA
ceRNA network
Cytoscape (www.cytoscape.org/) is a visual source software
platform for constructing molecular interaction networks and
biological pathways and integrating these networks with annotation,
gene expression profiles, and other data (26). Based on the associations among
differentially expressed lncRNAs, miRNAs and mRNAs, Cytoscape3.7.2
was employed to construct and visualize the lncRNA-miRNA-mRNA ceRNA
network, where the nodes represented mRNAs, lncRNAs or miRNAs and
the edges represented their interactions.
RNA isolation and reverse
transcription-quantitative (RT-q)PCR verification
Total RNA was extracted from MCF-7 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. For the lncRNA
verification, reverse transcription reactions with TransScript
All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (TRAN,
China) were performed in two steps: 15 min at 42°C and 5 sec at
85°C. The qPCR assay was performed in 96-well optical reaction
plates using SYBR® Premix Ex Taq™ reagents (Takara
Biotechnology Co., Ltd.) and a Light Cycle® 96 Real-Time
PCR System (Roche Diagnostics). The qPCR steps were as follows: 30
sec denaturation at 95°C, 45 cycles of PCR for the quantitative
analysis (95°C for 5 sec and 60°C for 30 sec), one cycle for the
melting curve analysis (95°C for 5 sec, 60°C for 1 min, and 95°C
for 1 sec) and cooling at 4°C. For miRNA verification, reverse
transcription reactions using the miRcute Plus miRNA First-Strand
cDNA kit (Tiangen Biotech Co., Ltd.) were performed in two steps:
60 min at 42°C and 3 min at 95°C. The quantitative amplification of
miRNA cDNA was performed with the miRcute Plus miRNA qPCR Detection
kit (Tiangen Biotech Co., Ltd.). The qPCR conditions were as
follows: 15 min denaturation at 95°C, 45 cycles of PCR for the
quantitative analysis (94°C for 20 sec and 60°C for 34 sec), one
cycle for the melting curve analysis (95°C for 5 sec, 60°C for 1
min, and 95°C for 1 sec) and cooling at 4°C. The primer sequences
of lncRNAs were as follows: MIR22HG-forward (F),
TGCGTGTGGGACAGTGGTAGAG; MIR22HG-reverse (R), GCGAGGGCTGGAGGGAGATG;
LINC00324-F, GGGTTGGGCATTAGGAACGG; LINC00324-R,
GTGCCATTCCGTAACCTGGG; and GAPDH-F, AAGGTGAAGGTCGGAGTC and GAPDH-R,
GGGTCATTGATGGCAACA. The validated primers of miRNAs were purchased
from Tiangen Biotech Co., Ltd. and were as follows: Hsa-miR-370-3p,
cat. no. CD201-0107; hsa-miR-424-5p, cat. no. CD201-0119; and
hsa-U6, cat. no. CD201-0145.
Survival analysis of lncRNAs and
miRNAs
In order to assess the association between days and
surviving percentages of clinical patients, data regarding the
expression of lncRNAs and miRNAs and the survival of patients with
cancer were analyzed by Cox regression analysis of survival
packages and Kaplan-Meier curves on the OncoLnc (https://www.oncolnc.org) and Kaplan-Meier-plotter
(Breast Cancer) (http://kmplot.com/analysis/).
Statistical analysis
All data are presented as the mean ± SD, and the
qPCR data were analyzed with Prism 7 (GraphPad Software, Inc.) by
the unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001.
Results
Inhibitory effect of 6-TG on MCF-7
cells and identification of differentially expressed lncRNAs
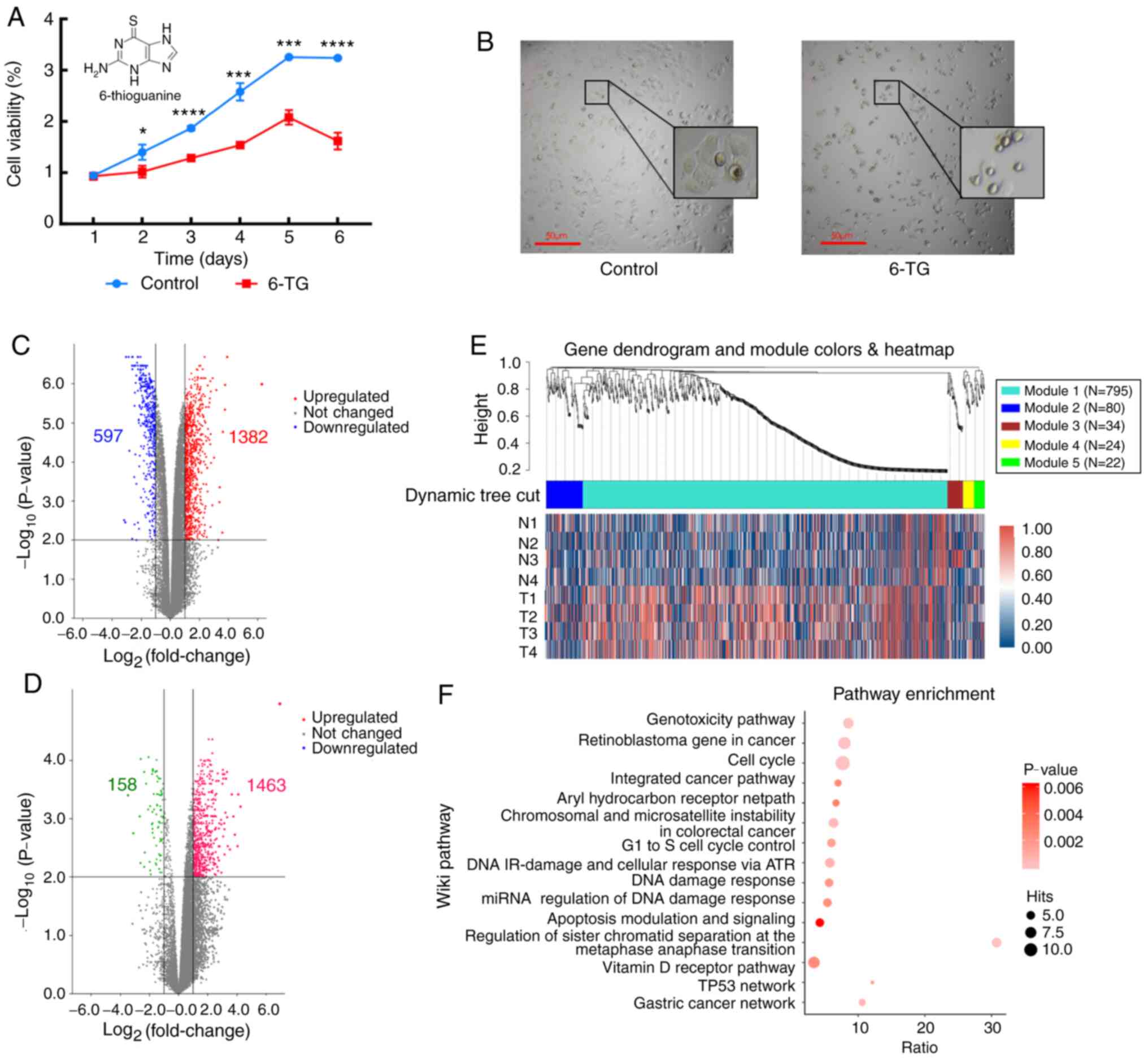
The cell proliferation curve showed that compared
with the control group, the cell proliferation activity of the 6-TG
treatment group was significantly decreased (Fig. 1A). The MCF-7 cells of the untreated
control group proliferated well and were highly confluent.
Following 6-TG treatment, the cell morphology changed, cell volume
shrank, nuclei contracted and shattered, intercellular connections
were broken, gaps between cells became higher, and the growth state
was notably poor (Fig. 1B). Next,
mRNA and lncRNA expression profiles between the control group and
the 6-TG treatment group were identified. From the volcano plot, it
can be observed that 1382 mRNAs were upregulated and 597 mRNAs were
downregulated (Fig. 1C). Moreover, a
total of 1621 differentially expressed lncRNAs were identified,
including 1463 upregulated lncRNAs and 158 downregulated lncRNAs
(Fig. 1D). Using |logFC|>2.0 and
P<0.05 as criteria for candidate lncRNAs, 21 differentially
expressed lncRNAs were screened out (Table I). To determine whether the
identified co-expression modules were associated with 6-TG
treatment in the MCF-7 cells, WGCNA was performed with the mRNA
samples, and all significant differentially expressed genes (sDEGs)
were merged into five modules according to the degree of
co-expression across the WGCNA dataset. Gene dendrogram analysis
showed that the turquoise module contained the most co-expressed
genes, and it contained 795 genes in total. The integrated heatmap
further showed that these genes were upregulated genes, accounting
for more than half of the total upregulated genes, and they were
the major groups responding to 6-TG (Fig. 1E). The pathway enrichment results of
co-expressed genes showed that only the genes in the turquoise
module could be enriched in the Wikipedia pathway database. The
enrichment results showed that the enrichment genes of the cell
cycle pathway were the most enriched, while the enrichment degree
of the apoptosis pathway was the most significant. The regulation
of sister chromatid separation at the metaphase-anaphase
transition, TP53 network and gastric cancer network were the three
pathways with the highest enrichment ratio (Fig. 1F).
 | Table I.Differentially expressed long
non-coding RNA of |logFC|≥2.0 and P≤0.05 in MCF-7 breast cancer
cells. |
Table I.
Differentially expressed long
non-coding RNA of |logFC|≥2.0 and P≤0.05 in MCF-7 breast cancer
cells.
| Symbol | Ensemble ID | |logFC| | P-value | Regulation |
|---|
| RP11-278H7.4 |
ENSG00000229960 | 3.652546701 | 0.000385 | Up |
| LINC01176 |
ENSG00000281404 | 2.899046519 | 0.0411 | Up |
| RP11474O21.5 |
ENSG00000272482 | 2.698056836 | 0.00465 | Up |
| RP11791G15.2 |
ENSG00000272275 | 2.672211342 | 0.0139 | Up |
| LINC02626 |
ENSG00000236799 | 2.519380254 | 0.00152 | Up |
| LINC00322 |
ENSG00000237864 | 2.491851412 | 0.000696 | Up |
| AC003088.1 |
ENSG00000226965 | 2.395515003 | 0.000463 | Up |
| CTC-296K1.3 |
ENSG00000267505 | 2.316408448 | 0.000331 | Up |
| MIR22HG |
ENSG00000186594 | 2.239789252 | 0.000252 | Up |
| RP11310E22.5 |
ENSG00000231829 | 2.229351767 | 0.00273 | Up |
| RP11308B16.2 |
ENSG00000248783 | 2.226968124 | 0.00174 | Up |
| RP11533E19.7 |
ENSG00000272906 | 2.226393831 | 0.000348 | Up |
| RP11114H24.7 |
ENSG00000261244 | 2.163565587 | 0.00169 | Up |
| C17orf82 |
ENSG00000187013 | 2.156767197 | 0.000102 | Up |
| RP11-92K15.3 |
ENSG00000272264 | 2.141591947 | 0.00486 | Up |
| LINC00324 |
ENSG00000178977 | 2.119767138 | 0.000173 | Up |
| AC005786.5 |
ENSG00000267231 | 2.118808646 | 0.00168 | Up |
| PURPL |
ENSG00000250337 | 2.108714038 | 0.00298 | Up |
| LINC01164 |
ENSG00000189275 | 2.051611959 | 0.0000988 | Up |
| LINC02672 |
ENSG00000227121 | 2.032007338 | 0.00365 | Up |
| RP11763B22.6 |
ENSG00000235887 | 2.029273852 | 0.0112 | Up |
Matching of lncRNAs-miRNAs and
prediction of mRNAs-miRNA interaction
Based on the online database of StarBase V2.0, the
lncRNA-miRNA pairs were predicted and seven candidate lncRNAs and
125 miRNAs were compared. Both pathway enrichment and the results
of a previous study (12)
demonstrated that apoptosis and the p53 signaling pathway were the
two main pathways induced by 6-TG in MCF-7 cells, and the aberrant
expression of mRNAs from these two pathways is depicted in Table II. Next, the target miRNAs of these
differentially expressed mRNAs were predicted using TargetScan,
miRDB and miRTarBas online software. Finally, 4 lncRNAs, 10 miRNAs
and 25 mRNAs were successfully matched though StarBase
verification. The results of this analysis are shown in Table III.
 | Table II.Differentially expressed mRNA of p53
signaling pathway and apoptosis pathway. |
Table II.
Differentially expressed mRNA of p53
signaling pathway and apoptosis pathway.
| p53 signaling
pathway mRNA | Apoptosis pathway
mRNA |
|---|
| STEAP3, ZMAT3,
RRM2B | XIAP, NFKBIA,
NFKB1 |
| CCNG1, SESN2,
CCNG2 | AKT1, ACTG1,
FOS |
| SESN1, CDKN1A,
EI24 | TNFRSF1A, PIK3CA,
DIABLO |
| TP53I3, PPM1D,
CCND3 | TUBA1A, PIK3R3,
TUBA1B |
| TSC2, DDB2,
MDM2 | AKT2, PIK3R2,
ACTB |
| IGFBP3, BBC3,
BAX | CFLAR, MAP2K2,
RELA |
| GADD45G, TP53,
PIDD1 | CAPN2, DDIT3,
CAPN1 |
| FAS, GADD45A | MAPK1, TNFRSF10C,
CTSK |
|
| JUN, IKBKG,
MAPK3 |
|
| IKBKB, IL3RA,
SPTAN1 |
|
| CTSF, TNFRSF10B,
BBC3 |
|
| AX, GADD45G,
TP53 |
|
| PIDD1, FAS,
GADD45A |
 | Table III.Targeting associations of
lncRNA-miRNA-mRNA. |
Table III.
Targeting associations of
lncRNA-miRNA-mRNA.
| LncRNA-ID | miRNA-ID | mRNA-ID |
|---|
| LINC01176 | hsa-miR-155-5p | FOS |
| LINC01176 | hsa-miR-155-5p | PIK3CA |
| LINC00324 | hsa-miR-15b-5p | CCND3 |
| LINC00324 | hsa-miR-15b-5p | ZMAT3 |
| LINC00324 | hsa-miR-16-5p | PPM1D |
| MIR22HG | hsa-miR-24-3p | PPM1D |
| MIR22HG | hsa-miR-24-3p | ZMAT3 |
| MIR22HG | hsa-miR-24-3p | PIK3R3 |
| MIR22HG | hsa-miR-24-3p | SESN1 |
| LINC00324 | hsa-miR-497-5p | CCND3 |
| LINC00324 | hsa-miR-497-5p | GADD45G |
| LINC00324 | hsa-miR-497-5p | SESN2 |
| LINC00324 |
hsa-miR-6838-5p | ZMAT3 |
| LINC00324 |
hsa-miR-6838-5p | GADD45G |
| LINC00324 |
hsa-miR-6838-5p | SESN2 |
| LINC00324 |
hsa-miR-6838-5p | SESN1 |
| MIR22HG |
hsa-miR-6893-3p | CDKN1A |
| PURPL | hsa-miR-19a-3p | FAS |
| PURPL | hsa-miR-19a-3p | TP53 |
| PURPL | hsa-miR-19a-3p | MAPK1 |
| PURPL | hsa-miR-19a-3p | ZMAT3 |
| PURPL | hsa-miR-19a-3p | PIK3CA |
| PURPL | hsa-miR-19a-3p | PIK3R3 |
| PURPL | hsa-miR-19a-3p | IGFBP3 |
| LINC00324 | hsa-miR-424-5p | CCND3 |
| LINC00324 | hsa-miR-424-5p | PPM1D |
| LINC00324 | hsa-miR-424-5p | ZMAT3 |
| LINC00324 | hsa-miR-424-5p | GADD45G |
| LINC00324 | hsa-miR-424-5p | TUBA1A |
| LINC00324 | hsa-miR-424-5p | IKBKB |
| LINC00324 | hsa-miR-424-5p | SESN2 |
| LINC00324 | hsa-miR-424-5p | SESN1 |
| MIR22HG | hsa-miR-370-3p | CDKN1A |
| MIR22HG | hsa-miR-370-3p | GADD45A |
| MIR22HG | hsa-miR-370-3p | STEAP3 |
| MIR22HG | hsa-miR-370-3p | MAP2K2 |
| MIR22HG | hsa-miR-370-3p | SESN2 |
| MIR22HG | hsa-miR-370-3p | AKT1 |
| MIR22HG | hsa-miR-370-3p | MAPK1 |
| MIR22HG | hsa-miR-370-3p | FOS |
| MIR22HG | hsa-miR-370-3p | TNFRSF1A |
| MIR22HG | hsa-miR-370-3p | CCND3 |
| MIR22HG | hsa-miR-370-3p | JUN |
| MIR22HG | hsa-miR-370-3p | IKBKG |
| MIR22HG | hsa-miR-370-3p | GADD45G |
| MIR22HG | hsa-miR-370-3p | PIK3CA |
| MIR22HG | hsa-miR-370-3p | MDM2 |
| MIR22HG | hsa-miR-370-3p | FAS |
| MIR22HG | hsa-miR-370-3p | PIK3R3 |
| MIR22HG | hsa-miR-370-3p | CTSF |
| MIR22HG | hsa-miR-370-3p | AKT2 |
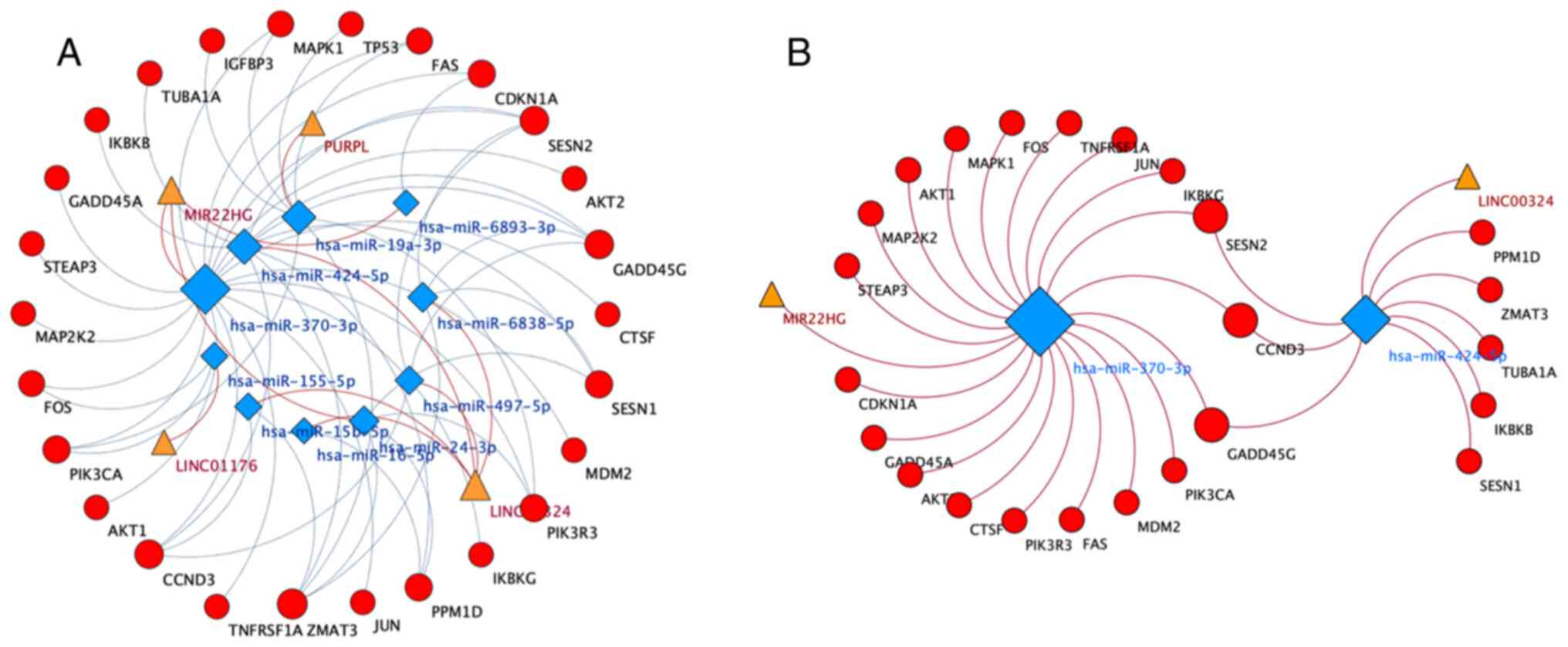
Construction of lncRNA-miRNA-mRNA
ceRNA network
Cytoscape 3.7.2 was used to visualize the
lncRNA-miRNA-mRNA ceRNA network. Based on the aforementioned data,
the lncRNA-miRNA-mRNA ceRNA network was constructed. As shown
Fig. 2A, 4 lncRNAs, 10 miRNAs and 25
mRNAs were involved in the ceRNA network. According to the network,
hsa-miR-370-3p (degree, 20) and hsa-miR-424-5p (degree, 9) were
identified as hub regulatory elements. Therefore, a more meaningful
ceRNA network was obtained based on the MIR22HG-hsa-miR-370-3p and
LINC0034-hsa-miR-424-5p pairs (Fig.
2B). This core ceRNA network included several hub genes, such
as FAS, CCND3 and CDKN1A, which played important roles in
6-TG-induced apoptosis in MCF-7 cells (12).
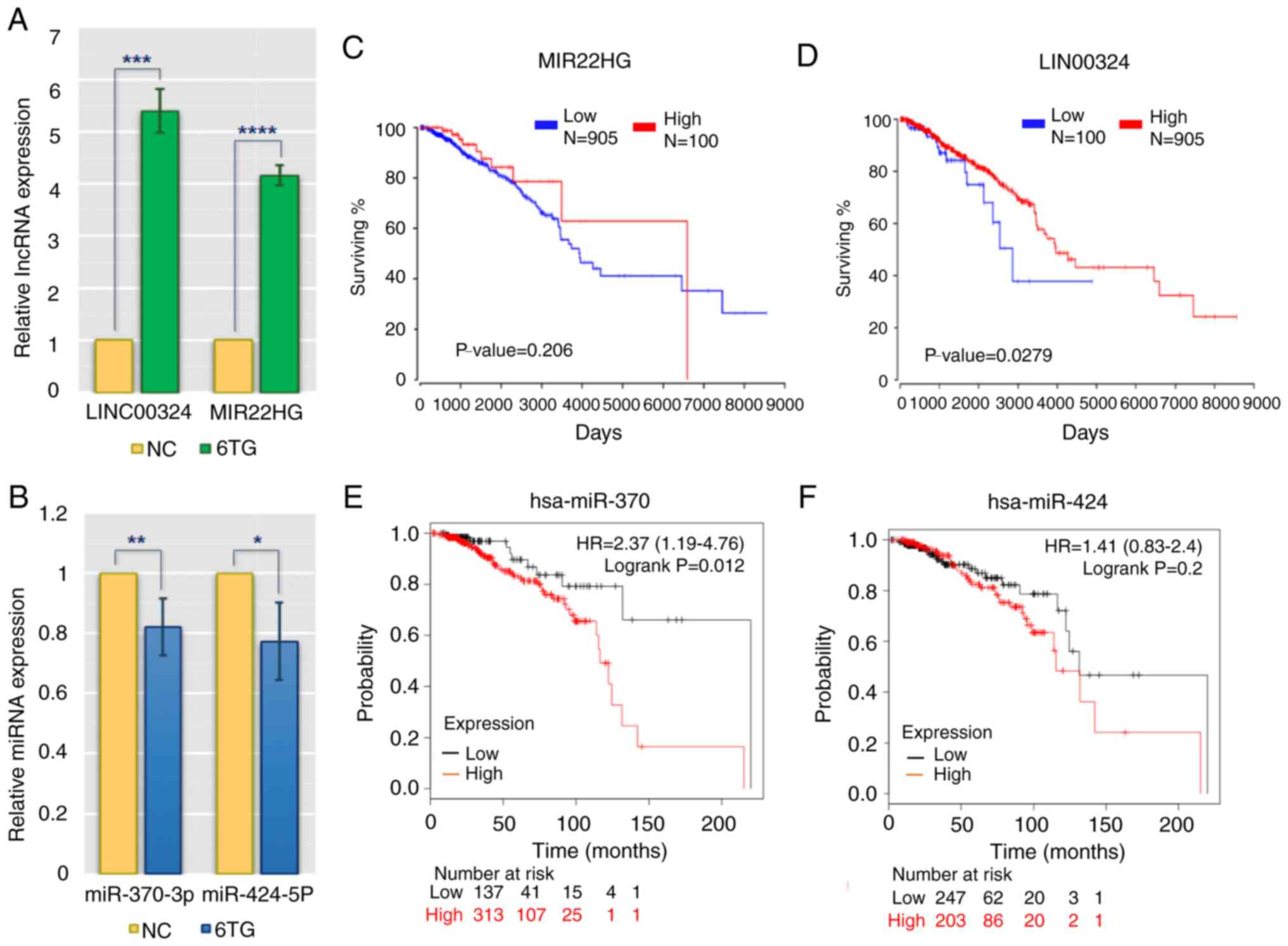
RT-qPCR verification and overall
survival assessment of specific lncRNAs and miRNAs
To validate the reliability and validity of the
aforementioned analysis data, RT-qPCR was applied to evaluate the
differences in the expression of main regulatory molecules between
the control and 6-TG treatment groups. The results showed that
hsa-miR-370-3p and hsa-miR-424-5p were downregulated in 6-TG
treatment group compared with the control group, while MIR22HG and
LINC0034 were upregulated in the 6-TG treatment group (Fig. 3A and B). These results were in
keeping with the bioinformatic results. The lncRNAs, such as
MIR22HG and LINC0034, may regulate the activities of hub genes by
sponging the target miRNAs (miR-370-3p and miR-424-5p). According
to the clinical patients' survival rates, the two specific lncRNAs
and two specific miRNAs from the ceRNA network were further
analyzed. High expression of LINC00324 and MIR22HG was associated
with high survival rates. Regarding LINC00324, the survival rates
of high- and low-expression groups were considered to be
significant (P<0.05). However, although there was no significant
difference in the survival rates of MIR22HG within five years, the
effect of this lncRNA on the survival rate of patients changed over
time (Fig. 3C and D). In addition,
low expression levels of two specific miRNAs (hsa-miR-370 and
hsa-miR-424) were associated with an extended survival time, and
the role played by hsa-miR-424 increased with the passage of time.
Approximately 50 months later, low expression of hsa-miR-424 was
associated with high survival rate (Fig.
3E and F).
Discussion
In recent years, the role played by lncRNA as a
ceRNA has become a highly studied topic in the field of tumor
research. Prior studies indicated that the lncRNA-miRNA-mRNA ceRNA
network was involved in the development of lung cancer, esophageal
cancer and gastric cancer (27–29).
However, as the classification of breast cancer is complicated and
the response of this cancer to treatment is highly variable,
further research is warranted to determine the role played by ceRNA
in breast cancer. Therefore, it is important to study the
differential expression of lncRNA, miRNA and mRNA in breast cancer
and characterize their regulatory association to identify potential
biomarkers and new drug targets for the diagnosis, prognosis and
treatment of breast cancer. In the present study, RNA-seq and
bioinformatic technology were employed to analyze the
differentially expressed lncRNA, the ceRNA network was constructed
and the regulatory mechanism underlying the role played by lncRNA
as ceRNA in MCF-7 breast cancer cells treated with 6-TG was
elucidated.
In the field of breast cancer ceRNA research, Jia
et al (30) constructed a
ceRNA network consisting of 44 miRNA-lncRNA interaction pairs and
two miRNA-mRNA interaction pairs, based on the information from 428
HR+/Her-2- and 113 triple-negative breast cancer samples from TCGA.
These researchers selected SFRP1, AC006449.1 and MUC2 as novel
clinical targets of breast cancer. Gao et al (26) constructed a ceRNA network including
90 lncRNAs, 18 miRNAs and 26 mRNAs from invasive breast cancer
samples and found that LINC00466, hsa-mir-204 and NTRK2 were
associated with the prognosis of breast cancer. In the present
study, by RNA-seq, differentially expressed lncRNAs and mRNAs were
obtained from MCF-7 breast cancer cells with and without 6-TG
treatment, and their common target miRNAs were predicted. A ceRNA
network consisting of 4 lncRNAs, 10 miRNAs, and 25 mRNAs was
successfully constructed by screening, predicting and matching
differentially expressed RNAs. In a previous study (31), the criteria for screening
differential lncRNA were |logFC|>1.0 and P<0.05. In contrast
with the previous studies, our screening criteria were |logFC|≥2.0
and P≤0.05. Therefore, the differentially expressed lncRNAs
selected in the present study were more likely to be prognostic
markers of breast cancer.
A previous study showed that PURPL regulated the
growth of cancer cells by inhibiting the mutual binding of p53 and
the p53-activating protein MYBBP1A in colon cancer (32). A study in liver cancer also showed
that PURPL and p53 mRNA expression levels were negatively
correlated in hepatocellular carcinoma cells and promoted the
proliferation of liver cancer cells by regulating p53 expression
(33). In the present study, PURPL
was also screened by the integrated bioinformatic analyses, which
indicated that this lncRNA played an important role in multiple
tumors, including breast cancer. However, the present results
showed that PURPL was highly expressed and positively correlated
with p53 expression in MCF-7 cells after the 6-TG treatment. It was
hypothesized that PURPL might act as ceRNA by inhibiting its
targeted miR-19a-3p to regulate p53 expression and induce apoptosis
in MCF-7 cells. Prior studies showed that LINC00324 acted as a
ceRNA to regulate AKT1 expression by inhibiting miR-615-5p in lung
adenocarcinomas, and LINC00324 promoted gastric cancer cell
proliferation by binding to RNA binding protein HuR to regulate
FAM83B protein expression in gastric cancer (34,35). Ni
et al (36) measured the
expression level of LINC00324 and miR-214-3p in CRC cells by
RT-qPCR and proved that LINC00324 regulated CRC cell proliferation
by sponging miR-214-3p. In the present study, LINC00324 was
selected as a hub lncRNA in the ceRNA network and was observed to
target miR-424-5p to regulate downstream mRNA. Meanwhile, the
results of qPCR analysis verified that the expression of LINC00324
was increased, while the expression of miR-424-5p was decreased, in
MCF-7 cells after 6-TG treatment. Therefore, LINC00324 may inhibit
the proliferation and induce apoptosis of MCF-7 cells by sponging
miR-424-5p to exert ceRNA regulation in the breast cancer cells.
The function of MIR22HG as a tumor suppressor has been confirmed in
basic research of liver, lung and thyroid cancer, and it plays a
key role in the progression of many tumors by inhibiting tumor cell
proliferation, invasion and metastasis (37–39). Wu
et al (40) verified that
knockdown of MIR22HG promoted growth, migration and invasion of
hepatocellular carcinoma cells (HCC) and demonstrated that MIR22HG
functioned as a ceRNA to inhibit the Wnt/β-catenin pathway, by
sponging miR-10a-5p in the HCC. Su et al (39) reported that MIR22HG was downregulated
in lung cancer and suggested that silencing of MIR22HG increased
lung cancer cells proliferation, migration and invasion. However,
to the best of our knowledge, no relevant research has been
conducted in breast cancer. The results of the present study
indicated that MIR22HG, which is highly expressed in MCF-7 cells
treated with 6-TG, served as a hub lncRNA to regulate downstream
target genes by targeted inhibition of miR-370-3p to induced
apoptosis. Therefore, MIR22HG is likely to be a potential
therapeutic target for breast cancer.
The miRNA is the core factor of the ceRNA network
and is an important tumor marker. Zhang et al (41) indicated that high expression of
miR-424-5p increased the proliferation and invasion of gastric
cancer cells. Liu et al (42)
also found that miR-424-5p promoted lung metastasis ability of
thyroid cancer cells in lung colonization models in vivo.
Therefore, miR-424-5p served as an oncogene in several cancer types
such as gastric, thyroid and colorectal cancer (41–43). Hou
et al (44) reported that
miR-370-3p expression was increased in cerebral aneurysm tissues
compared with normal tissues, and miR-370-3p was involved in the
development of cerebral aneurysm by targeting the KDR/AKT signaling
pathway. Lyu et al (45)
suggested that miR-370-3p, as a core molecular, played important
role in breast cancer. The pseudogene HLA-DPB2 upregulated HLA-DPB1
through sponging miR-370-3p to exert antitumor effect by recruiting
tumor-infiltrating immune cells into the breast tumor
microenvironment. In the present study, miR-424-5p and miR-370-3p
were identified as hub regulatory elements in the ceRNA network,
and the qPCR results showed that the expression levels of these two
key miRNAs were decreased in MCF-7 cells after 6-TG treatment. The
results indicated that miR-424-5p and miR-370-3p were also crucial
biomarkers in breast cancer. Furthermore, miRNAs affected gene
expression primarily by regulating targeted mRNAs. GADD45G has been
implicated in a variety of processes, such as apoptosis, DNA repair
and cell cycle checkpoints (46).
The previous studies (47,48) showed that GADD45G inhibited the
migration of esophageal squamous cell carcinoma (ESCC) cells and
was associated with ESCC patients' survival. CCND3 has been
verified to regulate cell cycle progression. It was confirmed that
CCND3 could bind to CDKs and formed complex compounds, and the
upregulation of cyclin D-CDK compounds was observed to inhibit cell
cycle progression in multiple myeloma (49). In our ceRNA network, GADD45G and
CCND3, as the terminal effector molecule, were commonly targeted
mRNA by both miR-424-5p and miR-370-3p. Meanwhile, pathway
enrichment analysis indicated that differential expression mRNAs
were mainly enriched in the cell cycle and apoptosis pathway.
Therefore, GADD45G and CCND3 might play important roles in
apoptosis and cell cycle regulation of breast cancer.
In conclusion, a ceRNA network was constructed based
on the screening criteria of |logFC|≥2.0 and P≤0.05 and used
bioinformatics to analyze the regulatory mechanism by which lncRNAs
function as ceRNA molecules in MCF-7 breast cancer cells.
Differentially expressed lncRNAs with potential research value were
obtained, such as MIR22HG and LINC00324, which may provide new
prognostic biomarkers for the treatment of breast cancer. The
present study helps to establish a theoretical basis for further
research elucidating the molecular mechanism of breast cancer
regulation and screening potential therapeutic targets.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
R&D Program of China (grant no. 2017YFA0104400), the Program
for Changjiang Scholars and Innovative Research Team in University
(grant no. IRT_16R32) and The Central Finance Forestry Science and
Technology Promotion Demonstration Fund Project and the National
Key Research and Development Program of China Stem Cell and
Translational Research (grant no. 2017YFA0105101).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL, ZL and HY conducted the analyses and interpreted
the data. QL and XA performed the experiments. HL was a major
contributor in designing the study and writing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
6-TG
|
6-thioguanine
|
|
ceRNAs
|
competing endogenous RNAs
|
|
ncRNAs
|
non-coding RNAs
|
|
lncRNAs
|
long non-coding RNAs
|
|
miRNAs
|
microRNAs
|
|
circRNAs
|
circular RNAs
|
|
MREs
|
miRNA binding sites
|
|
TCGA
|
The Cancer Genome Atlas
|
|
WGCNA
|
weighted gene co-expression network
analysis
|
|
ER+
|
estrogen receptor-positive
|
|
RNA-seq
|
RNA sequencing
|
References
|
1
|
Fahad Ullah M: Breast cancer: Current
perspectives on the disease status. Adv Exp Med Biol. 1152:51–64.
2019. View Article : Google Scholar
|
|
2
|
Jeong SB, Im JH, Yoon JH, Bui QT, Lim SC,
Song JM, Shim Y, Yun J, Hong J and Kang KW: Essential role of
polo-like kinase 1 (Plk1) oncogene in tumor growth and metastasis
of tamoxifen-resistant breast cancer. Mol Cancer Ther. 17:825–837.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
4
|
Geng C, Tang P, Zhang Y and Gao W:
Hyponatremia induced by low-dose cyclophosphamide in two patients
with breast cancer. Breast J. 20:442–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Natori A, Ethier JL, Amir E and Cescon DW:
Capecitabine in early breast cancer: A meta-analysis of randomised
controlled trials. Eur J Cancer. 77:40–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shafei A, El-Bakly W, Sobhy A, Wagdy O,
Reda A, Aboelenin O, Marzouk A, El Habak K, Mostafa R, Ali MA and
Ellithy M: A review on the efficacy and toxicity of different
doxorubicin nanoparticles for targeted therapy in metastatic breast
cancer. Biomed Pharmacother. 95:1209–1218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Liang Y, Cao J, Zhang N, Wei X, Tu
M, Xu F and Xu Y: The delivery of a Wnt pathway inhibitor toward
CSCs requires stable liposome encapsulation and delayed drug
release in tumor tissues. Mol Ther. 27:1558–1567. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karran P and Attard N: Thiopurines in
current medical practice: Molecular mechanisms and contributions to
therapy-related cancer. Nat Rev Cancer. 8:24–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Munshi PN, Lubin M and Bertino JR:
6-thioguanine: A drug with unrealized potential for cancer therapy.
Oncologist. 19:760–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dubinsky MC, Feldman EJ, Abreu MT, Targan
SR and Vasiliauskas EA: Thioguanine: A potential alternate
thiopurine for IBD patients allergic to 6-mercaptopurine or
azathioprine. Am J Gastroenterol. 98:1058–1063. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim I, Choi YS, Song JH, Choi EA, Park S,
Lee EJ, Rhee JK, Kim SC and Chang S: A drug-repositioning screen
for primary pancreatic ductal adenocarcinoma cells identifies
6-thioguanine as an effective therapeutic agent for TPMT-low cancer
cells. Mol Oncol. 12:1526–1539. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, An X, Zhang D, Li Q, Zhang N, Yu H
and Li Z: Transcriptomics analysis of the tumor-inhibitory pathways
of 6-Thioguanine in MCF-7 cells via silencing DNMT1 activity. Onco
Targets Ther. 13:1211–1223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Almeida RA, Fraczek MG, Parker S,
Delneri D and O'Keefe RT: Non-coding RNAs and disease: The
classical ncRNAs make a comeback. Biochem Soc Trans. 44:1073–1078.
2016. View Article : Google Scholar
|
|
15
|
Dong Z, Zhang A, Liu S, Lu F, Guo Y, Zhang
G, Xu F, Shi Y, Shen S, Liang J and Guo W: Aberrant
methylation-mediated silencing of lncRNA MEG3 Functions as a ceRNA
in esophageal cancer. Mol Cancer Res. 15:800–810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Conte F, Fiscon G, Chiara M, Colombo T,
Farina L and Paci P: Role of the long non-coding RNA PVT1 in the
dysregulation of the ceRNA-ceRNA network in human breast cancer.
PLoS One. 12:e01716612017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Huo X, Yang XR, He J, Cheng L,
Wang N, Deng X, Jin H, Wang N, Wang C, et al: STAT3-mediated
upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer
metastasis by regulating SOX4. Mol Cancer. 16:1362017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Wang X, Wen C, Huo Z, Wang W, Zhan
Q, Cheng D, Chen H, Deng X, Peng C and Shen B: Long noncoding RNA
NORAD, a novel competing endogenous RNA, enhances the
hypoxia-induced epithelial-mesenchymal transition to promote
metastasis in pancreatic cancer. Mol Cancer. 16:1692017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J, Li Y, Lu J, Pan T, Ding N, Wang Z,
Shao T, Zhang J, Wang L and Li X: The mRNA related ceRNA-ceRNA
landscape and significance across 20 major cancer types. Nucleic
Acids Res. 43:8169–8182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Niu L, Jiang S, Zhai J, Wang P,
Kong F and Jin X: Comprehensive analysis of aberrantly expressed
profiles of lncRNAs and miRNAs with associated ceRNA network in
muscle-invasive bladder cancer. Oncotarget. 7:86174–86185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng L, Xiang C, Li X, Guo Q, Gao L, Ni
H, Xia Y and Xi T: STARD13-correlated ceRNA network-directed
inhibition on YAP/TAZ activity suppresses stemness of breast cancer
via co-regulating Hippo and Rho-GTPase/F-actin signaling. J Hematol
Oncol. 11:722018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan CN, Ma L and Liu N: Systematic
analysis of lncRNA-miRNA-mRNA competing endogenous RNA network
identifies four-lncRNA signature as a prognostic biomarker for
breast cancer. J Transl Med. 16:2642018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karreth FA and Pandolfi PP: ceRNA
cross-talk in cancer: When ce-bling rivalries go awry. Cancer
Discov. 3:1113–1121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao C, Li H, Zhuang J, Zhang H, Wang K,
Yang J, Liu C, Liu L, Zhou C and Sun C: The construction and
analysis of ceRNA networks in invasive breast cancer: A study based
on the cancer genome atlas. Cancer Manag Res. 11:1–11. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sui J, Li YH, Zhang YQ, Li CY, Shen X, Yao
WZ, Peng H, Hong WW, Yin LH, Pu YP and Liang GY: Integrated
analysis of long non-coding RNAassociated ceRNA network reveals
potential lncRNA biomarkers in human lung adenocarcinoma. Int J
Oncol. 49:2023–2036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue WH, Fan ZR, Li LF, Lu JL, Ma BJ, Kan
QC and Zhao J: Construction of an oesophageal cancer-specific ceRNA
network based on miRNA, lncRNA, and mRNA expression data. World J
Gastroenterol. 24:23–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chu A, Liu J, Yuan Y and Gong Y:
Comprehensive analysis of aberrantly expressed ceRNA network in
gastric cancer with and without H. pylori infection. J Cancer.
10:853–863. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia X, Shi Y, Zhu Y, Meng W, He L, Jia Y
and Tong Z: Integrated analysis of mRNA-miRNA-lncRNA ceRNA network
in human HR+/Her-2-breast cancer and triple negative breast cancer.
J Comput Biol. 27:1055–1066. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao Y, Zhang T, Qi L, Zhou C, Wei J, Feng
F, Liu R and Sun C: Integrated analysis of co-expression and ceRNA
network identifies five lncRNAs as prognostic markers for breast
cancer. J Cell Mol Med. 23:8410–8419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li XL, Subramanian M, Jones MF, Chaudhary
R, Singh DK, Zong X, Gryder B, Sindri S, Mo M, Schetter A, et al:
Long noncoding RNA PURPL suppresses basal p53 levels and promotes
tumorigenicity in colorectal cancer. Cell Rep. 20:2408–2423. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu X, Wang Y, Wu G, Zhang W, Xu S and Wang
W: Long noncoding RNA PURPL promotes cell proliferation in liver
cancer by regulating p53. Mol Med Rep. 19:4998–5006.
2019.PubMed/NCBI
|
|
34
|
Pan ZH, Guo XQ, Shan J and Luo SX:
LINC00324 exerts tumor-promoting functions in lung adenocarcinoma
via targeting miR-615-5p/AKT1 axis. Eur Rev Med Pharmacol Sci.
22:8333–8342. 2018.PubMed/NCBI
|
|
35
|
Zou Z, Ma T, He X, Zhou J, Ma H, Xie M,
Liu Y, Lu D, Di S and Zhang Z: Long intergenic non-coding RNA 00324
promotes gastric cancer cell proliferation via binding with HuR and
stabilizing FAM83B expression. Cell Death Dis. 9:7172018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ni X, Xie JK, Wang H and Song HR:
Knockdown of long non-coding RNA LINC00324 inhibits proliferation,
migration and invasion of colorectal cancer cell via targeting
miR-214-3p. Eur Rev Med Pharmacol Sci. 23:10740–10750.
2019.PubMed/NCBI
|
|
37
|
Zhang DY, Zou XJ, Cao CH, Zhang T, Lei L,
Qi XL, Liu L and Wu DH: Identification and functional
characterization of long non-coding RNA MIR22HG as a tumor
suppressor for hepatocellular carcinoma. Theranostics. 8:3751–3765.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qin L, Luo JZ, Tang XL and Han CG:
Identification of long noncoding RNA MIR22HG as a novel biomarker
in thyroid cancer. Pathol Oncol Res. 25:703–710. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su W, Feng S, Chen X, Yang X, Mao R, Guo
C, Wang Z, Thomas DG, Lin J, Reddy RM, et al: Silencing of long
noncoding RNA MIR22HG triggers cell survival/death signaling via
oncogenes YBX1, MET, and p21 in lung cancer. Cancer Res.
78:3207–3219. 2018.PubMed/NCBI
|
|
40
|
Wu Y, Zhou Y, Huan L, Xu L, Shen M, Huang
S and Liang L: LncRNA MIR22HG inhibits growth, migration and
invasion through regulating the miR-10a-5p/NCOR2 axis in
hepatocellular carcinoma cells. Cancer Sci. 110:973–984. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Liu H, Hou L, Wang G, Zhang R,
Huang Y, Chen X and Zhu J: Circular RNA_LARP4 inhibits cell
proliferation and invasion of gastric cancer by sponging miR-424-5p
and regulating LATS1 expression. Mol Cancer. 16:1512017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu X, Fu Y, Zhang G, Zhang D, Liang N, Li
F, Li C, Sui C, Jiang J, Lu H, et al: miR-424-5p promotes anoikis
resistance and lung metastasis by inactivating Hippo signaling in
thyroid cancer. Mol Ther Oncolytics. 15:248–260. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dai W, Zhou J, Wang H, Zhang M, Yang X and
Song W: miR-424-5p promotes the proliferation and metastasis of
colorectal cancer by directly targeting SCN4B. Pathol Res Pract.
216:1527312020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hou WZ, Chen XL, Wu W and Hang CH:
MicroRNA-370-3p inhibits human vascular smooth muscle cell
proliferation via targeting KDR/AKT signaling pathway in cerebral
aneurysm. Eur Rev Med Pharmacol Sci. 21:1080–1087. 2017.PubMed/NCBI
|
|
45
|
Lyu L, Yao J, Wang M, Zheng Y, Xu P, Wang
S, Zhang D, Deng Y, Wu Y, Yang S, et al: Overexpressed pseudogene
HLA-DPB2 promotes tumor immune infiltrates by regulating HLA-DPB1
and indicates a better prognosis in breast cancer. Front Oncol.
10:12452020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Salvador JM, Brown-Clay JD and Fornace AJ
Jr: Gadd45 in stress signaling, cell cycle control, and apoptosis.
Adv Exp Med Biol. 793:1–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li T, Xu L, Teng J, Ma Y, Liu W, Wang Y,
Chi X, Shao S, Dong Y, Zhan Q and Liu X: GADD45G Interacts with
E-cadherin to suppress the migration and invasion of esophageal
squamous cell carcinoma. Dig Dis Sci. 65:1032–1041. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo W, Zhu T, Dong Z, Cui L, Zhang M and
Kuang G: Decreased expression and aberrant methylation of Gadd45G
is associated with tumor progression and poor prognosis in
esophageal squamous cell carcinoma. Clin Exp Metastasis.
30:977–992. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Misiewicz-Krzeminska I, Sarasquete ME,
Vicente-Dueñas C, Krzeminski P, Wiktorska K, Corchete LA, Quwaider
D, Rojas EA, Corral R, Martín AA, et al: Post-transcriptional
modifications contribute to the upregulation of cyclin D2 in
multiple myeloma. Clin Cancer Res. 22:207–217. 2016. View Article : Google Scholar : PubMed/NCBI
|