Introduction
Despite declining incidence, gastric cancer remains
one of the leading causes of cancer-associated death worldwide,
with the proportion of deaths to newly diagnosed cases exceeding
75% in 2018 (1). The late onset of
clinical symptoms limits the curative role of surgical treatment
and standard chemotherapy. However, there is evidence of
improvements in patient survival resulting from the implementation
of targeted treatment. Among numerous monoclonal antibodies applied
in the treatment of gastric cancer to date, trastuzumab is still
the only standard agent that demonstrates significant efficacy
(2). Trastuzumab is a recombinant
humanized monoclonal antibody targeting human epidermal growth
factor receptor 2 (HER2) that has been demonstrated to improve
survival outcome in patients with HER2-positive gastric cancer
(2,3).
HER2, also known as ERBB2 and
HER2/neu, is a proto-oncogene located on the long arm of
chromosome 17. HER2 encodes the tyrosine kinase membrane
receptor HER2, whose phosphorylation initiates signaling pathways
resulting in cell division, proliferation, differentiation and the
suppression of apoptosis (4–7). HER2 is expressed in a variety of
tissues, including the breast and gastrointestinal tract, where it
is considered one of the key drivers of tumorigenesis (3–5). The
upregulation of HER2, or amplification of the HER2 gene, is
associated with significantly worse prognosis in patients with
breast cancer, both via enhanced local growth and metastasis
formation (5). In gastric cancer,
studies of the prognostic relevance of HER2
upregulation/amplification have generated inconsistent results, and
the association between HER2 status and gastric cancer prognosis
remain controversial (4,5).
Trastuzumab is used to target the extracellular
domain of HER2, which inhibits HER2-mediated downstream signal
activation (4,5). Trastuzumab was originally introduced to
treat HER2-positive metastatic breast cancer (5,6). In the
case of patients with advanced HER2-positive gastric
adenocarcinoma, it has also been acknowledged that the addition of
trastuzumab to the chemotherapy regimen increases the response rate
and prolongs both progression-free and overall survival time
(4).
The accurate evaluation of HER2 status is essential
for the appropriate use of anti-HER2 therapy (8,9). To
assess HER2 positivity, protein expression is evaluated by
immunohistochemistry (IHC) and if the result is equivocal (2+),
fluorescence in situ hybridization (FISH) is performed to
assess HER2 gene amplification (4–6).
Generally speaking, in situ hybridization is performed using
a single probe, in which absolute counts per cell determine the
scoring system, or with the use of a dual probe technique that
relies on the HER2/chromosome 17 centromere enumeration probe
(CEP17) ratio (4,10). In gastric cancer, the dual probe
hybridization method is strongly recommended (4); single probe methods are discouraged as
they are more affected by section thickness (4,10), tumor
mitotic index and abnormal chromosome copy number (10). However, in dual probe methods, the
increased number of CEP17 signals (often classified as polysomic)
may underrate the test results.
The negative impact of CEP17 copy number increase
(CNI) on prognosis has previously been revealed in cases of breast
cancer (11,12). Our previous study (13) demonstrated that CEP17 CNI may also be
a negative prognostic factor in gastric cancer, but the studied
group was relatively small. Thus, the aim of the present study was
to assess the frequency of CEP17 CNI occurrence and its effect on
HER2 protein expression in tumor cells, as well as treatment
outcome, in a larger group of patients with gastric cancer.
Materials and methods
Study design and participants
Our previous study was performed on 83 patients who
underwent surgery between July 2006 and January 2011 at the
Department of Surgical Oncology of Gdynia Oncology Centre (Poland)
(13). To increase the size of the
study group, patients that received surgery in the same center
between January 2011 and December 2013, and patients from the
Department of Oncological Surgery, Medical University of Gdańsk
(operated upon between July 2006 and December 2013), were also
included. Both inclusion and exclusion criteria, as well as IHC and
FISH methodology, were the same for both the old and new cohorts
(83 and 208 patients, respectively).
The archival primary tumor samples from the
additional 208 patients (who underwent major gastric resection for
adenocarcinoma) were retrospectively retrieved for both HER2
protein expression analysis by IHC, and HER2 gene
amplification using FISH. The combined study group consisted of 291
patients that underwent major gastric resection for adenocarcinoma
of the stomach. The only inclusion criterion for patients was major
resection for adenocarcinoma of the stomach in the study period.
The exclusion criterion was the coexistence of any other types of
malignancy, including stromal tumors, neuroendocrine cancer and
lymphoma.
The surgical and pathological reports were analyzed
and included the following study parameters: i) Range of stomach
resection (total or subtotal); ii) extent of lymphadenectomy; iii)
the total number of harvested lymph nodes; iv) pTNM stage of the
disease, according to the 7th edition of the American Society for
Clinical Pathology and the American Joint Committee on Cancer
Staging manual (14); v) depth of
tumor invasion into the stomach wall (pT); vi) presence of nodal
involvement (pN); vii) number of metastatic lymph nodes; viii)
presence of distant metastases (M); ix) Lauren histological type of
tumor; x) presence of mucinous component in the tumor tissue; xi)
tumor location in the stomach (cardia involvement); and xii)
survival outcome. Mortality data were acquired from the Polish
Ministry of Digitization on January 1st, 2019.
HER2 status was evaluated according to the
guidelines from the College of American Pathologists, American
Society for Clinical Pathology and the American Society of Clinical
Oncology (CAP/ASCP/ASCO) (4). HER2
status was considered positive in cases of IHC results of 3+, or 2+
with the presence of HER2 gene amplification
(FISH-positive). The associations between HER2 status, CEP17 CNI
and multiple clinicopathological parameters (including survival)
were assessed, as well as the relationship between CEP17 CNI and
HER2 protein upregulation.
For statistical reasons, patients with TNM stage I
or II disease were combined into one group, and those with TNM
stage III or IV into a second group. Similarly, pT1 and pT2 were
combined into one group and pT3 and pT4 into a second. Patients
with Lauren type II or III classification were classified as
‘diffuse type’.
Preoperative diagnosis and
surgery
The patients were preoperatively diagnosed by
endoscopy with histopathological examination. The stage of the
disease was routinely determined by abdominal CT and chest
radiography. The standard surgical procedure for gastric cancer in
both centers was total gastrectomy with appropriate
lymphadenectomy. The particular extent of gastric resection and
lymph node dissection was based on the disease stage and the
individual surgeon's judgement. Resection was routinely followed by
Roux-en-Y reconstruction. All procedures were performed by
laparotomy. Resected tissue was fixed in 10% neutral buffered
formalin at room temperature for at least 24 h.
IHC
IHC staining was conducted on 4-µm tissue sections
which were obtained from paraffin-embedded tissue blocks. The
sample collection took place between January and March 2018, and
the requirement for patient consent was waived by the Ethics
Committee of the Medical University of Gdańsk. The sections
containing the most representative tumor tissues, without signs of
necrosis, were selected and placed in silanized glasses. Then
deparaffinization with use of xylene), rehydration with use of
descending alcohol series (ethanol 99, 95 and 80%) and blocking
using of 3% hydrogen peroxide solution for 4 min at 36°C were
performed, and the glasses were incubated at 36°C for 24 h. For
HER2 staining, pre-diluted anti-HER2/neu (4B5) Rabbit Monoclonal
Primary Antibody (cat. no. 05278368001; Roche Diagnostics) was used
in an automatic machine (Roche Benchmark GX; Roche Diagnostics),
according to the manufacturer's instructions. The Benchmark machine
performed a fully automated heat induced epitope retrieval step
with use of pre-diluted ready to use Cell Conditioning 1 (cat. no.
950-124; Ventana; Roche Diagnostics) at 95°C for 35 min. The
antigen visualization was performed via the iVIEW DAB detection kit
(streptavidin-horseradish peroxidase conjugate; cat. no. 760-091;
Ventana; Roche Diagnostics) at 37°C for 32 min. The samples were
counterstained with hematoxylin II (cat. no. 760-2021; Ventana;
Roche Diagnostics) at room temperature for 4 min, blued with Bluing
Reagent (cat. no. 760-2037; Ventana; Roche Diagnostics) at room
temperature for 4 min in a fully automated way. The tissue sections
were then dehydrated in an ascending alcohol series (80, 95, 99 and
99%) and xylene and placed on coverslips. For evaluation, the
Olympus BX43 light microscope (magnification, ×40; Olympus
Corporation) was used according to the criteria recommended by
Hofmann et al (15), and the
evaluation was confirmed by Abrahao-Machado (Department of
Pathology, Barretos Cancer Hospital, Barretos, Brazil) and Rushoff
(Targos Molecular Pathology GmBH und Pathology Nordhessen, Kassel,
Germany) (6,7).
FISH
Molecular cytogenetic analysis was performed in all
cases (irrespective of IHC score) at the Molecular Oncology and
Genetics Department, IFM, Łukaszczyk Oncology Centre in Bydgoszcz;
4- and 6-µm sections from formalin-fixed paraffin-embedded tissue
blocks were used for FISH analysis. The most representative areas
of the tumor (without signs of necrosis) were selected, and
HER2 gene amplification was performed. The commercially
validated Vysis PathVysion HER2 FISH test (Vysis, Inc.; Abbott
Pharmaceutical) was used to evaluate gene amplification per the
manufacturer's protocol.
The tissue sections were deparaffinized, dehydrated
and air-dried. After immersion in 0.2N HCl, purified water and Wash
Buffer, the samples were pretreated with Pretreatment Solution at
80°C for 30 min. The sections were then immersed in Protease
Solution at 37°C for 34 min, followed by immersion in Wash Buffer
(70, 80 and 100% ethanol), and then subjected to hybridization. The
DNA probe mixture (10 ng/µl 226 kb HER2 probe and 20 ng/µl 9 kb
CEP17 probe) was applied to the target area of the slide and
covered with a glass coverslip (both probes were fragmented to
facilitate hybridization). After the probe mixture had spread
evenly under the coverslip, the slides were placed in a prewarmed
humidified hybridization chamber and incubated at 74°C for 5 min,
and then at 37°C overnight. Next, the slides were immersed in
post-hybridization wash buffer at room temperature for 15 min, and
then in prewarmed post-hybridization buffer at 72°C for 2 min.
After air-drying, 10 µl DAPI was added to the target area and a
glass coverslip was applied. The slides were stored in the dark
prior to signal enumeration.
A minimum of 60 cells in interphase were scored for
each sample using a fluorescence microscope (Eclipse 80i; Nikon
Corporation) and CAP/ASCP/ASCO 2016 HER2 standard recommendations
(4). A geneticist (Professor Marzena
Anna Lewandowska; Molecular Oncology and Genetics Department, IFM,
Łukaszczyk Oncology Centre in Bydgoszcz) reported the average copy
number of HER2 and CEP17, and the HER2/CEP17 ratio in each
case. FISH results were interpreted as positive with a ratio of
HER2 to CEP17 signal ≥2, and negative with a ratio <2. In
cases with an average of ≥3 CEP17 copies (CEP17 CNI) and a ratio
<2, the presence of >6 HER2 signals was interpreted as
a positive result, <4 HER2 signals was interpreted as a
negative result, and a signal number between 4 and 6 was
interpreted as an equivocal result. Cases with IHC examination
results of 2+ and an equivocal FISH result were considered as
undetermined HER2 status, and were not included in the statistical
analysis for the relationship between HER2 status and
clinicopathological parameters (Table
I). The 83 FISH results used in our previous study (13) were reinterpreted according to the
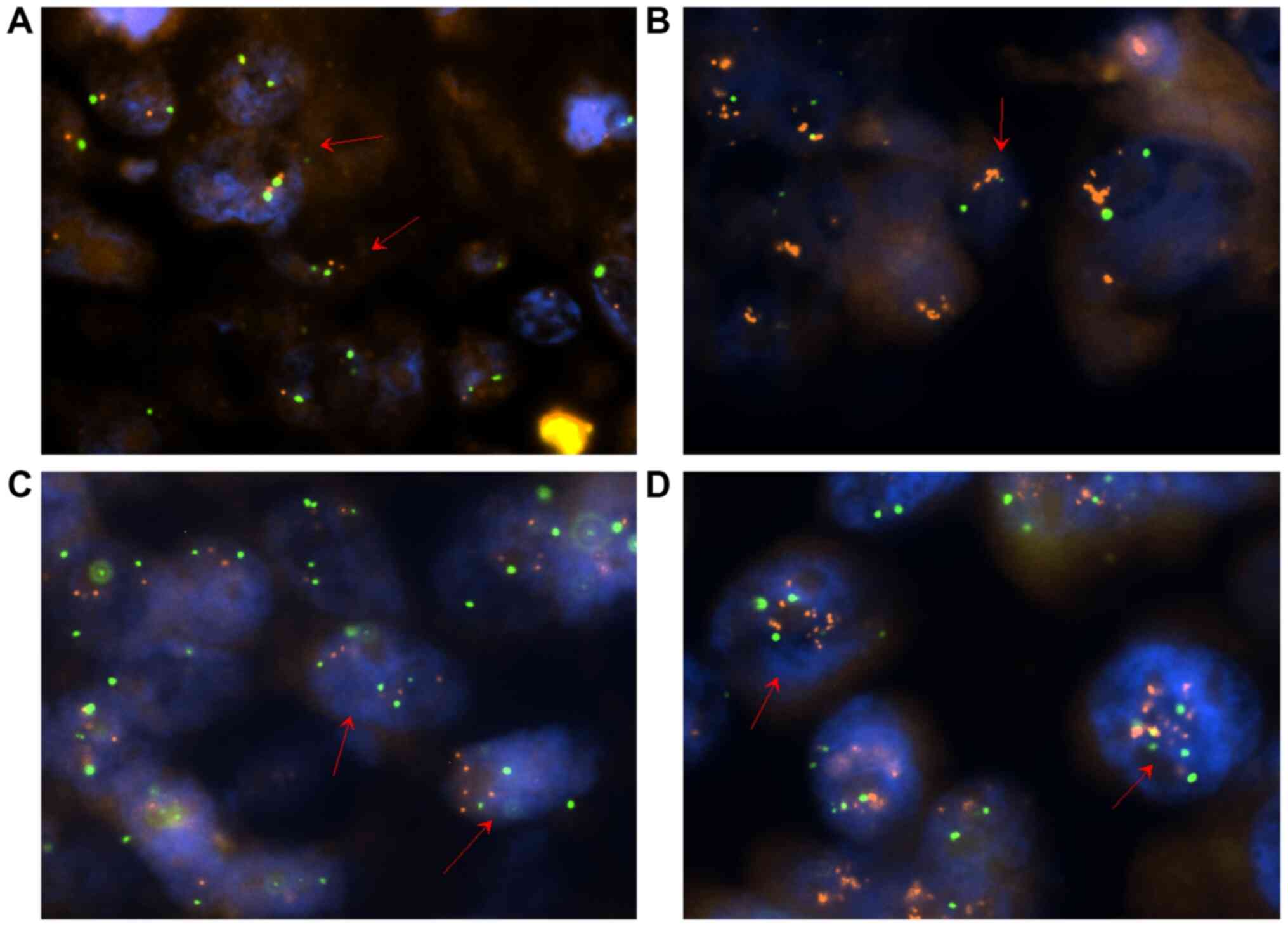
same recommendations. Images were captured using Lucia Cytogenetics
2 Laboratory Imaging software v.2.1, examples of which are
presented in Fig. 1.
 | Table I.Association between HER2 status or
CEP17 CNI and clinicopathological parameters. |
Table I.
Association between HER2 status or
CEP17 CNI and clinicopathological parameters.
| Clinicopathological
feature | CEP17 CNI (+),
n=42 | CEP17 CNI (−),
n=202 | P-value | HER2 (+), n=28 | HER2 (−),
n=212 | P-value |
|---|
| Rate of total
gastrectomy, % | 90.5 | 86.6 | 0.49 | 89.3 | 87.3 | 0.89 |
| Rate of D2-D1+
lymphadenectomy, % | 26.2 | 23.8 | 0.73 | 17.9 | 25.0 | 0.4 |
| Total number of
lymph nodes resected mean/median | 18.9/21.5 | 19.5/19.5 | 0.33 | 18.8/21.5 | 16.0/20.0 | 0.17 |
| pT3-pT4, % | 81.0 | 73.8 | 0.600 | 60.7 | 76.9 | 0.120 |
| pN+, % | 76.2 | 67.8 | 0.300 | 60.7 | 70.8 | 0.300 |
| Number of
metastatic lymph nodes, mean/median | 5.5/3 | 5.9/2 | 0.700 | 4.9/1.5 | 6.1/3 | 0.310 |
| Mucinous component,
% | 23.8 | 31.7 | 0.300 | 10.7 | 33.0 | 0.010a |
| Lauren diffuse
type, % | 45.2 | 50.0 | 0.600 | 25.0 | 52.8 | 0.005 |
| Cardia involvement,
% | 50.0 | 24.8 | 0.001 | 35.7 | 28.3 | 0.400 |
| Presence of distant
metastases, % | 14.3 | 8.4 | 0.200 | 14.3 | 9.0 | 0.300a |
| pTNM III–IV, % | 59.5 | 55.0 | 0.600 | 42.9 | 58.0 | 0.130 |
| Overall survival
mean/median (months) | 35.7/17.5 | 45.1/31.5 | 0.200 | 49.7/32.5 | 43.0/29.5 | 0.510 |
| 1-year survival,
% | 64.3 | 75.2 | 0.120 | 75.0 | 73.6 | 0.790 |
| 2-year survival,
% | 42.9 | 58.4 | 0.050 | 57.1 | 56.1 | 0.810 |
| 3-year survival,
% | 33.3 | 46.5 | 0.070 | 42.9 | 44.8 | 0.940 |
| 4-year survival,
% | 31.0 | 41.6 | 0.100 | 42.9 | 40.1 | 0.680 |
| 5-year survival,
% | 28.6 | 38.1 | 0.120 | 42.9 | 35.8 | 0.510 |
| HER2 positive,
% | 31.0 | 7.4 | 0.00001 | N/A |
|
|
| HER2 protein
upregulation: IHC 2+ and 3+, % | 47.6 | 16.3 | 0.000008 | N/A |
|
|
Statistical analysis
Statistical analysis was performed using Statistica
software, version 13 (StatSoft, Inc.; Dell). Survival analysis was
calculated using the Kaplan-Meier method, followed by the log-rank
test, to assess the differences between the groups. The
clinicopathological variables of the four patient groups were
compared using χ2, Fisher's exact or U Mann-Whitney
tests, as appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Data collection
The IHC or FISH assays were unsuccessful in 22
cases. The most common reasons were either inefficient material (in
the case of small tumors) or invalid material preservation, most
often within the 2006–2008 period, which was similar to the results
of our previous study (25 unsuccessful cases). Results were
ultimately obtained for 186 patients; the complete and successfully
tested group consisted of 58 old and 186 new cases (n=244).
Treatment details
Among the studied group, 213 patients (87.3%)
underwent total gastrectomy and 31 (12.7%) underwent subtotal
gastric resection. The range of lymphadenectomy was D2 in 34
(13.9%), D1+ in 25 (10.2%), D1 in 179 (73.4%) and D0 in 6 (2.5%)
cases (16). There were 200 (82.0%)
procedures with curative intent and 44 (18.0%) regarded as
palliative. The average number of resected lymph nodes was 21.1
(median, 19.5; and range, 0–78), and neoadjuvant chemotherapy was
administered to 8.2% of patients.
CEP17 CNI rate and its association
with clinicopathological features
CEP17 CNI was observed in 17.2% of cases. There were
no significant differences in the CEP17-positive and -negative
groups concerning the range of stomach resection (rate of total
gastrectomy, 90.5% vs. 86.6%), the extent of lymphadenectomy (rate
of D2-D1+, 26.2 vs. 23.8%), the total number of lymph nodes
resected (mean, 18.9 vs. 21.5; median, 19.5 vs. 19.5), pTNM stage,
the depth of tumor invasion into the stomach wall (pT), the
presence of nodal involvement (pN), the number of metastatic lymph
nodes, the presence of distant metastases (M), Lauren histological
type of the tumor or the presence of mucinous component in the
tumor cells (Table I). Among all of
the studied clinicopathological features, there was a significant
difference between the CEP17-positive and -negative groups with
regard to cardia involvement only (P=0.001; Table I). However, there was also a strong
association between CEP17 CNI and HER2 protein upregulation in the
tumor cells (P<0.0001; Table
I).
Association between CEP17 CNI and
patient survival
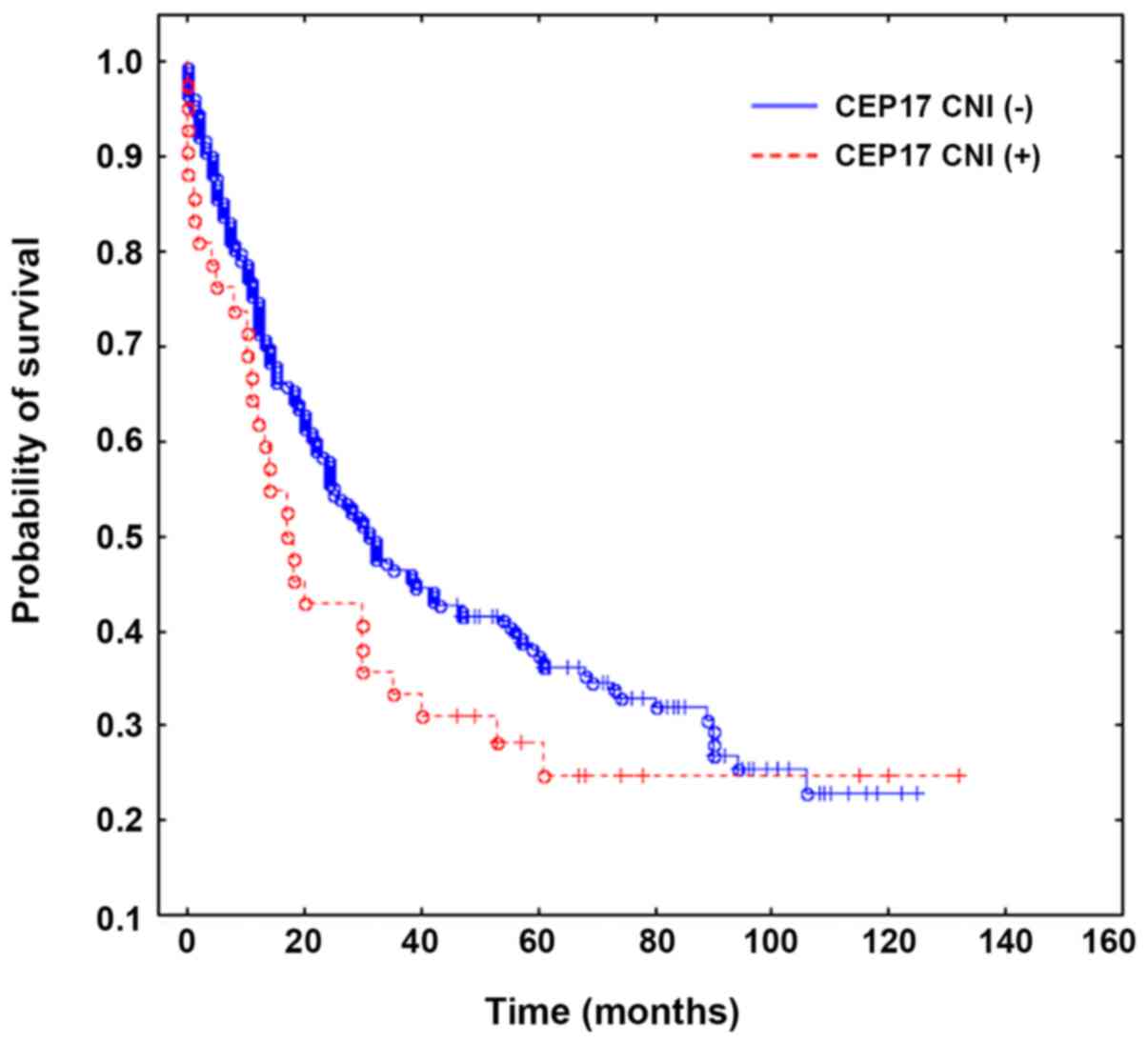
The Overall survival rate between the CEP17
CNI-positive and -negative groups was determined using the
Kaplan-Meier method followed by the log-rank test; no significant
difference was observed (P=0.17; Fig.
2). The two-year survival rate (also determined using the
Kaplan-Meier method and log-rank test; Table I) tended to statistical significance
in favor of CEP17 CNI-negative tumors (P=0.05).
HER2 positivity rate and the
association between HER2 status and clinicopathological
features
HER2 positivity was observed in 11.5% of cases and
was equivocal in 4 cases (undetermined HER2 status). Among the 42
CEP17 CNI-positive cases, 13 were assessed as HER2 positive, 3 as
equivocal and 26 as negative. There were no significant differences
between the HER2-positive and -negative groups regarding the range
of stomach resection (rate of total gastrectomy, 89.3% vs. 87.3%),
the extent of lymphadenectomy (rate of D2-D1+, 17.9% vs. 25%), the
total number of lymph nodes resected (mean, 18.8 vs. 21.5; median
16 vs. 20), tumor location in the stomach, pTNM stage, pT, pN, the
number of metastatic lymph nodes and the presence of distant
metastases (Table I). HER2 status
was significantly associated with intestinal type according to
Lauren classification, and lack of mucinous component of the tumor.
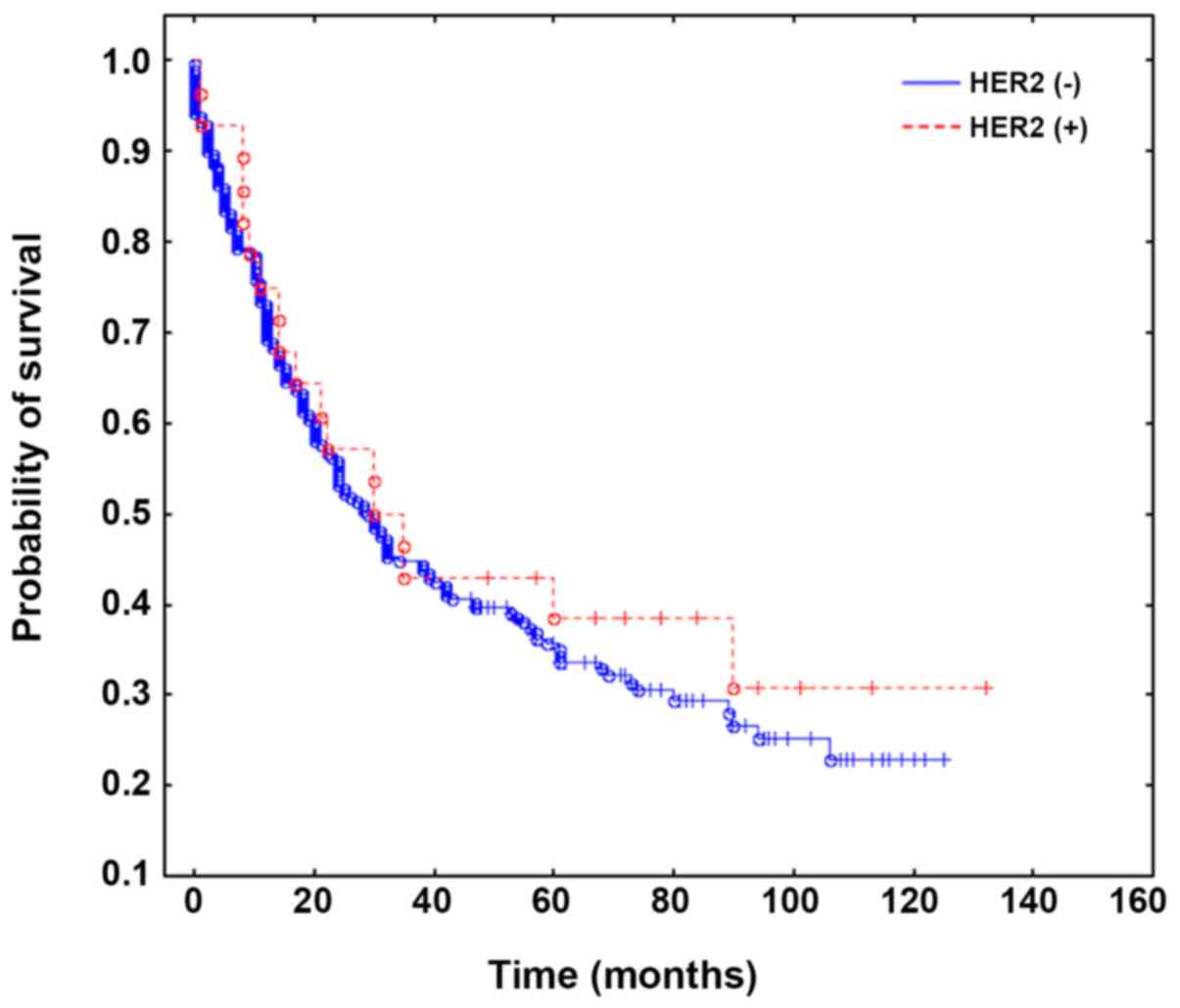
HER2 status was not associated with overall survival and survival
rates. The relationships between HER2 status, CEP17 CNI and
clinicopathological parameters are presented in Table I. The survival curves are presented
in Figs. 2 and 3.
Discussion
Approval for the use of trastuzumab in patients with
gastric cancer is as a result of HER2 upregulation, defined by an
IHC score of 3+, or 2+ confirmed by a positive FISH result. These
criteria have not changed over the years (4,17).
Positive HER2 status was observed in ~20% of patients with gastric
cancer (18), but ranged between 6.0
and 36.6% (19). The present study
revealed HER2 positivity in 11.5% of patients, as well as an
association between positive HER2 status and the intestinal type,
according to Lauren classification and lack of mucinous component
of the tumor. These findings are consistent with those from a
previous study (20), and other
studies have also indicated a relationship between HER2 positivity
and tumor location in the gastrointestinal junction (8,21).
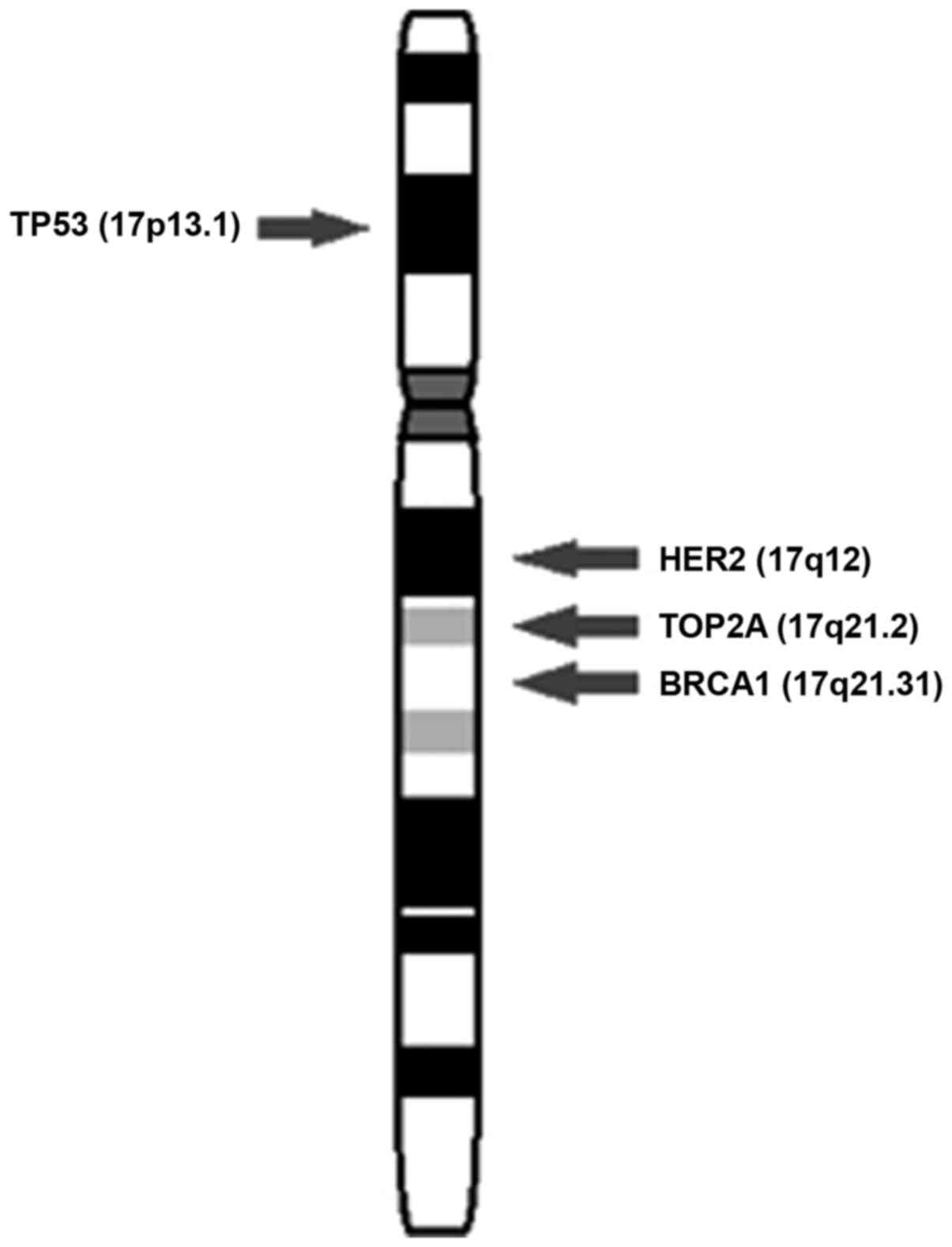
The HER2 oncogene is located on the long arm of the
chromosome 17, near the centromere (22), as shown in Fig. 4. FISH is performed with the use of
dual probes, one for the HER2 gene and another for the
centromere of chromosome 17 (23).
Since the results of FISH are based on the ratio between the number
of HER2 gene and chromosome 17 centromere signals, a higher
number of CEP17 signals translates to a lower HER2/CEP17 ratio. The
issue of chromosome 17 copy number change contributing to a high
percentage of inaccurate and equivocal results during HER2 status
assessment, has already been raised in breast cancer (9).
Originally, CEP17 CNI was reported as chromosome 17
polysomy, but it is now recognized that true chromosome 17
polysomy, which is defined by the presence of extra copies of the
whole chromosome, is an uncommon event in both breast and gastric
cancers (9,10,22–25).
Indeed; the use of molecular tools such as multiple ligation probe
amplification (MLPA) and array-comparative genomic hybridization
have confirmed that in the vast majority of cases, elevated CEP17
signals are caused by an amplification of the centromeric region of
the chromosome (10,11,23,25–27). In
this study, the HER2/CEP17 ratio was <1 in only a single case
among 244 (data not shown), suggesting that the usually amplified
centromeric region contains the HER2 gene locus on the long
arm of the chromosome. Varga et al (28) performed FISH on 14 breast cancer
specimens using multiple chromosome 17 probes, and demonstrated
that CEP17 amplification almost always involves the HER2
locus. A strong relationship between CEP17 CNI and HER2 protein
upregulation also suggests that an increased CEP17 signal is often
associated with increased levels of HER2 gene expression in
the cancer cell. The relationship between CEP17 CNI and IHC results
was also found in other studies concerning both gastric cancer
(19) and breast cancer (29). Therefore, the question of whether
increased CEP17 copy signals should underrate FISH results
arises.
In the present study, CEP17 CNI was found in 17.2%
of cases and tended to be associated with poorer 1- (P=0.12), 2-
(P=0,05), 3- (P=0.07), 4- (P=0.1) and 5-year (P=0.12) survival
rates. However, it must be stated that statistical analysis
surrounding the impact of CEP17 CNI on survival did not reveal any
significant differences. Apart from cardia involvement, CEP17 CNI
was not significantly associated with any of the other investigated
clinicopathological factors. The association between CEP17 CNI and
clinicopathological features has not been widely studied. However,
Onchi et al (30) revealed a
relationship between CEP17 CNI and lymph node involvement.
The impact of CEP17 multiplication on adverse
clinical outcomes or negative prognostic indicators has already
been demonstrated in breast cancer (11,12,31,32). Kim
et al (11) revealed worse
overall survival and disease free survival rates in breast cancer
patients with non-amplified HER2 expression, but with CEP17
multiplication. Lee et al (12) found CEP17 CNI to be an independent
adverse prognostic factor in the HER2-negative tumors from 945
cases of invasive breast cancer. In this study, CEP17 CNI was also
associated with multiple aggressive histological variables,
including higher T stage, higher histologic grade, lymphovascular
invasion, negative hormone receptor status, p53 upregulation and
high Ki-67 proliferative index.
In addition to HER2, chromosome 17 contains
other genes that participate in carcinogenic process, such as
TOP2A, DARPP32, BRCA1 and TP53 (25). The mechanisms facilitating the poor
outcomes of CEP17 CNI-positive patients is not known, though the
association between CEP17 CNI and HER2 upregulation begs us to
question whether patients with CEP17 CNI-positive gastric cancer
would benefit from anti-HER2 therapy. If the answer is positive, in
the study series of 244 patients, up to 29 (11.9%) polysomic, but
HER2-negative (n=26) or equivocal (n=3) patients might have been
denied eligible trastuzumab treatment.
In breast cancer, there is evidence that CEP17 CNI
may determine the response to trastuzumab treatment in tumors with
negative FISH results (33,34). Hofmann et al (33) studied the response to trastuzumab
first-line monotherapy in a group of 105 patients with
HER2-positive metastatic breast cancer. A partial or complete
response was observed in 19 of the 75 (25.3%) patients with IHC 3+
tumors, in 16 of 74 (21.6%) patients with FISH-positive tumors, and
in 6 of 26 (23.1%) patients with CEP17 CNI. Notably, two of the six
CNI-positive responders were FISH negative (HER2/CEP17 ratio
<2,0). In a randomized study by Kaufman et al (34), trastuzumab was added to paclitaxel
treatment in HER2-negative/CEP17 CNI-positive (CEP17 ≥2.2) patients
with metastatic breast cancer, and the response rate increased from
25 to 63%. To further complicate matters, it is hypothesized that
CEP17 CNI may serve different roles in the prediction of anti-HER2
treatment response for primary vs. metastatic breast cancer
(35). Nevertheless, these data
suggest that at least a proportion of patients with CEP17
CNI-positive breast cancer may potentially benefit from trastuzumab
treatment, in spite of negative HER2 status.
In gastric cancer, the issue of CEP17 CNI in HER2
testing interpretation appears to be overlooked. Numerous studies
have concluded that CEP17 CNI is infrequent and has limited impact
on HER2 status evaluation (19). In
the well-known ToGA trial (3) CEP17
CNI occurred in only 4.1% of the studied population. Similarly,
Gomez-Martin et al (17)
found only 2 (3.0%) CEP17 CNI-positive and concurrent amplified
cases among 66 patients fulfilling trastuzumab treatment criteria.
The authors studied the impact of the level of HER2 gene
amplification on the benefit to overall survival and the response
to treatment with trastuzumab-based chemotherapy. Both CEP17
CNI-positive patients showed some degree of clinical benefit.
To the best of our knowledge, there are no studies
concerning the response to trastuzumab in patients with
HER2-negative, CEP17 CNI-positive gastric cancer. Such studies are
highly anticipated to limit false-negative HER2 status assessment,
and to optimize patient selection for HER2-targeted treatments.
The primary limitation of the present study was the
lack of knowledge concerning systemic treatment of the studied
patients. It is unclear whether the CEP17 CNI-positive and
-negative groups were treated in the same manner. In the study
period, neoadjuvant chemotherapy was not as widely adopted as it is
now, and was received by only 8.2% of the patients. In the adjuvant
settings, patients eligible for postoperative treatment primarily
received chemoradiation in accordance with the MacDonald protocol
(36). Due to the lack of
standardized chemotherapy in recurrent and metastatic disease at
this time, patients underwent multiple chemotherapeutic regimens
according to the oncologist's judgement. In patients with
metastatic gastric cancer, the reimbursement of Trastuzumab
treatment costs by the Polish health care system began in on 1st
March 2014, thus it is assumed that few, if any HER2-positive
patients from this period in the study had received targeted
anti-HER2 therapy. There was also a lack of CEP17 CNI re-evaluation
using novel molecular droplet digital PCR, MLPA or array
techniques. On the other hand, the present study primarily focused
on routinely used in situ hybridization techniques rather
than technologies more frequently used in research.
In conclusion, the results of the present study
indicate that CEP17 CNI is strongly associated with HER2
upregulation on tumor cells, thus it is recommended that the
presence of CEP17 CNI be mentioned in routine histopathological
reports. These findings may represent a critical issue in HER2
testing, complementing the clinical value of the HER2/CEP17 ratio
for the prognosis and treatment of patients with gastric cancer.
The impact of CEP17 CNI on HER2 upregulation is evident, and the
eligibility for HER2-targeted agents in CEP17 CNI-positive patients
requires further recognition.
Acknowledgements
The authors wish to profoundly thank Professor
Marzena Anna Lewandowska from the Molecular Oncology and Genetics
Department, IFM, Łukaszczyk Oncology Centre, Bydgoszcz for the FISH
analyses and support in preparing the manuscript. Also, we
graciously acknowledge Professor Janusz Jaśkiewicz and Professor
Wojciech Biernat for their assistance in the study, as well as the
Ministry of Digital Development for the disclosure of patient
mortality dates.
Funding
The present study was financially supported by the
Medical University of Gdańsk (grant no. ST 02/130/07/308).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC conceived the presented idea, made major
contributions to design of the study and wrote the manuscript. MSz
analyzed the data, created the figures, and was involved in
drafting the manuscript. JW made major contributions to data
acquisition and interpretation. RP, RL and MSu performed all
histopathological examinations (immunohistochemistry) and specimen
preparation for FISH assessment. JZ and WJK made substantial
contributions to the conception and design of the study, as well as
the revision of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Independent
Ethics Committee of the Medical University of Gdańsk
(NKBBN/427/2014), and the requirement for patient consent was
waived by the committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HER2
|
human epidermal growth factor receptor
2
|
|
CNI
|
copy number increase
|
|
CEP17
|
chromosome 17 centromere enumeration
probe
|
|
IHC
|
immunochemistry
|
|
FISH
|
fluorescence in situ
hybridization
|
|
MLPA
|
multiplex ligation-dependent probe
amplification
|
|
CAP/ASCP/ASCO
|
College of American Pathologists,
American Society for Clinical Pathology and the American Society of
Clinical Oncology
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jomrich G and Schoppmann SF: Targeted
therapy in gastric cancer. Eur Surg. 48:278–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartley AN, Washington MK, Colasacco C,
Ventura CB, Ismaila N, Benson AB III, Carrato A, Gulley ML, Jain D,
Kakar S, et al: HER2 testing and clinical decisions making in
gastroesophageal adenocarcinoma: Guideline from the College of
American Pahologists, American Society for Clinical Pathology, and
the American Society of Clinical Oncology. J Clin Oncol.
35:446–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boku N: HER2-positive gastric cancer.
Gastric Cancer. 17:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abrahao-Machado LF and Scapulatempo-Neto
C: HER2 testing in gastric cancer: An update. World J
Gastroenterol. 22:4619–4625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rüschoff J, Dietel M, Barreton G, Arbogast
S, Walch A, Monges G, Chenard M-P, Panault-LLorca F, Nagelmeier I,
Schlake W, et al: HER2 diagnostics in gastric cancer-guideline
validation and development of standardized immunohistochemical
testing. Virchows Arch. 457:299–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baretton G, Kreipe HH, Schirmacher P,
Gaiser T, Hofheinz R, Berghäuser KH, Koch W, Künzel C, Morris S and
Rüschoff J; Nicht-interventionelle Untersuchung (NIU) HER2 Study
Group, : HER2 testing in gastric cancer diagnosis: Insights on
variables influencing HER2-positivity from a large, multicenter,
observational study in Germany. Virchows Arch. 474:551–560. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gunn S, Yeh IT, Lytvak I, Tirtorahardjo B,
Dzidic N, Zadeh S, Kim J, McCaskill C, Lim L, Gorre M and Mohammed
M: Clinical array-based karyotyping of breast cancer with equivocal
HER2 status resolves gene copy number and reveals chromosome 17
complexity. BMC Cancer. 10:396–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanna WM, Rüschoff J, Bilous M, Coudry RA,
Dowsett M, Osamura RY, Penault-Llorca F, van de Vijver M and Viale
G: HER2 in situ hybridization in breast cancer: Clinical
implications of polysomy 17 and genetic heterogeneity. Mod Pathol.
27:4–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim A, Shin HC, Bae YK, Kim MK, Kang SH,
Lee SJ and Lee EH: Multiplication of chromosome 17 centromere is
associated with prognosis in patients with invasive breast cancer
exhibiting normal HER2 and TOP2A status. J Breast Cancer. 15:24–33.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee K, Jang MH, Chung YR, Lee Y, Kang E,
Kim S-W, Kim YJ, Kim JH, Kim IA and Park SY: Prognostic
significance of centromere 17 copy number gain in breast cancer
depends on breast cancer subtype. Hum Pathol. 61:111–120. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ciesielski M, Kruszewski WJ, Śmiałek U,
Walczak J, Szajewski M, Szefel J, Wydra J and Kawecki K: The HER2
gene and HER2 protein status and chromosome 17 polysomy in gastric
cancer cells in own material. Appl Immunohistochem Mol Morphol.
23:113–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene F and Trotti A: American Joint Committee on Cancer, American
Cancer Society, AJCC Cancer Staging Manual. 7th edition. Springer;
New York, NY: 2010
|
|
15
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Degiuli M, De Manzoni G, Di Leo A, D'Ugo
D, Galasso E, Marrelli D, Petrioli R, Połom K, Roviello F, Santullo
F and Morino M: Gastric cancer: Current status of lymph node
dissection. World J Gastroenterol. 22:2875–2893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomez-Martin C, Plaza JC, Pazo-Cid R,
Salud A, Pons F, Fonseca P, Leon A, Alsina M, Visa L, Rivera F, et
al: Level of HER2 gene amplification predicts response and overall
survival in HER2-positive advanced gastric cancer treated with
trastuzumab. J Clin Oncol. 31:4445–4452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McLean MH and El-Omar EM: Genetics of
gastric cancer. Nat Rev Gastroenterol Hepatol. 11:664–674. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshida H, Yamamoto N, Taniguchi H, Oda I,
Katai H, Kushima R and Tsuda H: Comparison of HER2 status between
surgically resected specimens and matched biopsy specimens of
gastric intestinal-type adenocarcinoma. Virchows Arch. 465:145–154.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grabsch H, Sivakumar S, Gray S, Gabbert HE
and Müller W: HER2 expression in gastric cancer: Rare,
heterogeneous and of no prognostic value-conclusions from 924 cases
of two independent series. Cell Oncol. 32:57–65. 2010.PubMed/NCBI
|
|
21
|
Van Cutsem E, Bang YJ, Feng-yi F, Xu JM,
Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, et
al: HER2 screening data from TOGA: Targeting HER2 in gastric and
gastroesophageal junction cancer. Gastric Cancer. 18:476–484. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang T, Amemiya Y, Henry P, Seth A, Hanna
W and Hsieh ET: Multiplex ligation-dependent probe amplification
can clarify HER2 status in gastric cancer with ‘polysomy 17’. J
Cancer. 6:403–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeh IT, Martin MA, Robetorye RS, Bolla AR,
McCaskill C, Shah RK, Gorre ME, Mohammed MS and Gunn SR: Clinical
validation of an array CGH test for HER2 status in breast cancer
reveals that polysomy 17 is a rare event. Mod Pathol. 22:1169–1175.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sanguedolce F and Bufo P: HER2 assessment
by silver in situ hybridization: Where are we now? Expert Rev Mol
Diagn. 15:385–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koudelakova V, Trojanec R, Vrbkova J,
Donevska S, Bouchalova K, Kolar Z, Varanasi L and Hajduch M:
Frequency of chromosome 17 polysomy in relation to CEP17 copy
number in a large breast cancer cohort. Genes Chromosomes Cancer.
55:409–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marchiò C, Lambros MB, Gugliotta P, Di
Cantogno LV, Botta C, Pasini B, Tan DS, Mackay A, Fenwick K, Tamber
N, et al: Does chromosome 17 centromere copy number predict
polysomy in breast cancer? A fluorescence in situ hybridization and
microarray-based CGH analysis. J Pathol. 219:16–24. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moelans CB, de Weger RA and van Diest PJ:
Absence of chromosome 17 polysomy in breast cancer: Analysis by
CEP17 chromogenic in situ hybridization and multiplex
ligation-dependent probe amplification. Breast Cancer Res Treat.
120:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varga Z, Tubbs RR, Wang Z, Sun Y, Noske A,
Kradolfer D, Bosshard G, Jochum W, Moch H and Öhlschlegel C:
Co-amplification of the HER2 gene and chromosome 17 centromere: A
potential diagnostic pitfall in HER2 testing in breast cancer.
Breast Cancer Res Treat. 132:925–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu FP, Wang K, Xu J, Chen J, Zhang YF, Wu
HM, Zhang MH, Long XX, Luo XL, Zhang KP, et al: Impact of repeat
HER2 testing after initial equivocal HER2 FISH results using 2013
ASCO/CAP guidelines. Breast Cancer Res Treat. 166:757–764. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Onchi H, Hirose K, Yamaguchi A, Noriki S
and Fukuda M: Prognostic value of numerical aberrations of
chromosome 17 in differentiated gastric cancer: Evaluation by
multivariate regression analysis. Oncol Rep. 7:1317–1322.
2000.PubMed/NCBI
|
|
31
|
Krishnamurti U, Hammers JL, Atem FD,
Storto PD and Silverman JF: Poor prognostic significance of
unamplified chromosome 17 polysomy in invasive breast carcinoma.
Mod Pathol. 22:1044–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orsaria M, Khelifa S, Buza N, Kamath A and
Hui P: Chromosome 17 polysomy: Correlation with histological
parameters and HER2NEU gene amplification. J Clin Pathol.
66:1070–1075. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hofmann M, Stoss O, Gaiser T, Kneitz H,
Heinmöller P, Gutjahr T, Kaufmann M, Henkel T and Rüschoff J:
Central HER2 IHC and FISH analysis in a trastuzumab (Herceptin)
phase II monotherapy study: Assessment of test sensitivity and
impact of chromosome 17 polysomy. J Clin Pathol. 61:89–94. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaufman PA, Broadwater G, Lezon-Geyda K,
Dressler LG, Berry D, Friedman P, Winer EP, Hudis C, Ellis MJ,
Seidman AD and Harris LN: Correlation of HER2 and chromosome 17
(ch17) copy number with trastuzumab (T) efficacy in CALGB 9840,
paclitaxel (P) with or without T in HER2+ and
HER2− metastatic breast cancer (MBC). J Clin Oncol.
25:10092007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reinholz MM, Bruzek AK, Visscher DW,
Lingle WL, Schroeder MJ, Perez EA and Jenkins RB: Breast cancer and
aneusomy 17: Implications for carcinogenesis and therapeutic
response. Lancet Oncol. 10:267–277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Macdonald JS, Smalley SR, Benedetti J,
Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA,
Gunderson LL, Jessup JM and Martenson JA: Chemoradiotherapy after
surgery compared with surgery alone for adenocarcinoma of the
stomach or gastroesophageal junction. N Engl J Med. 345:725–730.
2001. View Article : Google Scholar : PubMed/NCBI
|