Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer, accounting for 3% of adult malignancies (1). Although the morbidity of RCC continues
to increase in most countries, mortality has remained the same in
numerous highly developed countries during the last decades
(2). RCC can occur in both men and
women, but the ratio of male to female incidence is ~1.5:1, and the
morbidity was 4.5 times higher in developed countries than in
developing countries in 2008 (2).
Early detection of RCC is difficult, as this disease lacks early
clinical manifestations (3). By the
time RCC is diagnosed, 20–30% of patients are already in the
advanced stages of the disease and have developed distant
metastasis, which results in a poor prognosis, with a 5-year
survival rate of <10% (3).
According to the 2016 World Health Organization
classification (4), the most common
histotypes in RCC are clear cell RCC (ccRCC), papillary RCC (pRCC)
and chromophobe RCC (chRCC), which account for 75–80, 10–15 and
6–11% of RCC cases, respectively (5). The histotype of RCC is an important
predictor of tumor prognosis, but it cannot be fully determined by
imaging examinations such as abdominal ultrasound or computed
tomography. The application of biopsies is controversial since they
require experienced cytopathologists and can lead to complications,
such as bleeding and tumor spreading along the biopsy route
(6). Therefore, a biomarker with
good sensitivity and specificity is urgently required, and further
studies on the molecular mechanisms of RCC are required to improve
the early diagnosis and treatment of RCC.
Neutrophil gelatinase-associated lipocalin (NGAL),
also known as lipocalin 2 (LCN2), belongs to the apolipoprotein
superfamily. NGAL is a 25-kDa secreted glycoprotein that was first
discovered by Kjeldsen et al (7) in 1993 while studying matrix
metalloproteinase-9 (MMP-9) in the specific granules of
neutrophils. NGAL has received much attention for its biological
role as an early warning sign of acute and chronic kidney diseases,
and it is regarded as an optional biomarker of renal tubular injury
(8). In the last two decades,
studies on NGAL in cardiovascular and cerebrovascular diseases, as
well as diabetes, inflammatory diseases and cancer, have been
performed, and as a tumor biomarker, NGAL has attracted much
attention (9,10). Previous studies on NGAL have found
that the mRNA expression levels of NGAL differ in different types
of cancer, and its role may vary (11,12).
Additionally, NGAL is expressed in several types of RCC (13) and it may be involved in the
development, proliferation, invasion and metastasis of RCC. The
purpose of the present review was to discuss the advances in the
understanding of the role of NGAL in RCC.
Literature search
Data and references from relevant articles were
identified by searches of the electronic database PubMed
(https://pubmed.ncbi.nlm.nih.gov/) using
the search terms ‘neutrophil gelatinase-associated lipocalin’,
‘NGAL’, ‘lipocalin 2’, ‘Lcn2’, ‘renal cell carcinoma’, ‘carcinoma’,
‘renal cell’ and ‘RCC’. The last search was run in March 2020.
Studies on animal models were excluded. The eligibility criteria
included the following: i) Studies on NGAL expression in RCC; ii)
Studies that evaluated NGAL as a prognostic or diagnostic marker in
RCC; iii) Studies on the functions of NGAL in RCC cell lines in
vitro; and iv) Studies identified from relevant references from
eligible studies, such as studies on the structure of NGAL or the
association between NGAL and breast cancer, colorectal cancer
(14), pancreatic cancer (15) or renal cancer. Eligible studies were
screened by two authors, and no disagreement existed between
authors.
Review of NGAL in RCC
Structure and biological function of
NGAL
The human NGAL gene is a monocistronic gene located
on chromosome 9q34, with a total length of 5,869 bp, including a
3,696 bp coding region (7 exons and 6 introns), a 1,695 bp
5′-terminal non-transcribed region and a 178 bp 3′-terminal
non-transcribed region (11). The
cDNA sequence of the NGAL gene was first identified by cloning in
1994 and consists of a coding region and a 5′-terminal untranslated
region. The peptide chain encoded by the NGAL gene contains 198
amino acid residues, including a leading sequence (containing 20
amino acid residues) and the mature peptide (containing 178 amino
acid residues) (11). The molecular
weight of NGAL is 22.6 kDa, which increases to 25 kDa after
glycosylation (16,17). NGAL belongs to the apolipoprotein
superfamily and has a highly conserved tertiary calyx-shaped
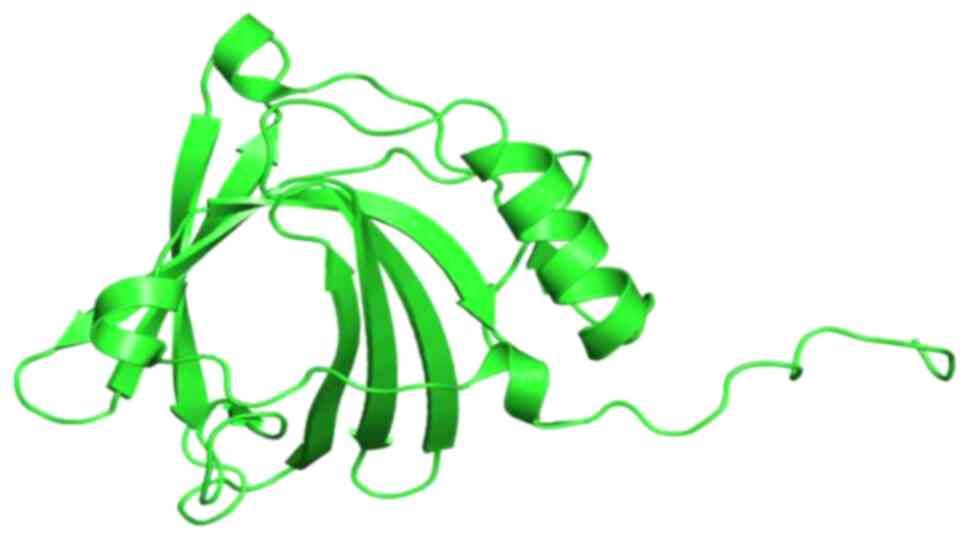
structure, composed of 8 anti-parallel β-strands, a C-terminal
α-helix and an N-terminal 310-helix (Fig. 1). In humans, NGAL can exist in at
least three forms: A monomer of 25 kDa, a homodimer of 45 kDa and a
heterodimer of 135 kDa (disulfide bound with MMP-9) (18).
Vaidya et al (19) found that under normal physiological
conditions, NGAL can be synthesized during a narrow window of
granulocyte maturation in the bone marrow, and that NGAL exists in
peroxidase-negative neutrophil granules. Additionally, NGAL is
expressed at a low level in a variety of cell types, including fat
cells, cartilage cells, endometrial carcinoma cells, epithelial
cells, endothelial cells, fibroblasts, liver cells, keratinocytes,
macrophages, mesangial cells, microglia, lung cells, spleen cells,
thymic cells and vascular smooth muscle cells (9). In addition, it is expressed in small
amounts in the epithelium of normal human organs, such as lung,
kidney, uterus and breast, as well as in the gastrointestinal tract
(20). Furthermore, NGAL can be
detected in different biological fluids, including blood and urine
(21), bile (22), bronchoalveolar lavage fluid (23), hydrothorax (24), ascites (25) and cerebrospinal fluid (26).
The calyx-like structure of NGAL enables it to bind
to low molecular weight siderophores to form a
NGAL-siderophore-iron complex, which can induce the differentiation
of precursor cells into epithelial cells, promote the maturation of
primitive renal epithelial cells, scavenge free radicals and
decrease the damage caused by oxidative stress to promote the
repair of renal injury (27). In
addition, NGAL can be coupled with relevant receptors on the cell
surface, such as the megalin-cubilin multiscavenger complex found
on the brushborder surface of renal tubular epithelial cells, and
induce the cell to phagocytose siderophores (28). NGAL can strongly bind to
enterobactin, and its iron-binding activity provides a highly
effective antibacterial effect, which competitively inhibits the
iron intake of bacteria, blocks their access to iron, which is an
important nutrient for bacteria, and ultimately inhibits their
growth (29). Furthermore, NGAL
serves a role in the inflammatory response in vivo,
transporting a number of lipophilic molecules that mediate
inflammation, such as leukotriene B, platelet activating factor and
lipopolysaccharide (30). Another
important function of NGAL is to bind to MMP-9 to form
heterodimers, preventing the endogenous degradation of MMP-9 and
thus maintaining its ability to degrade a large number of
structural molecules, such as collagen, fibronectin and laminin
(31).
There is evidence indicating that NGAL can be
produced by renal tubules and that its concentration is
significantly increased after renal injury (13). The diagnostic and prognostic value of
NGAL in acute kidney injury (AKI) has been conclusively proven in a
series of clinical studies (11).
Currently, NGAL is considered the biochemical gold standard for the
diagnosis of AKI. When AKI occurs, NGAL concentrations increase in
both urine and blood, with the increase in urine being more marked
than that in blood (32). It has
been confirmed that NGAL levels are positively correlated with the
degree of AKI, and NGAL concentrations in urine and blood can be
used as an early, sensitive and highly specific biological marker
for the diagnosis of AKI (32–34).
Sequence analysis indicated that the cDNA of NGAL
and its mouse homolog 24p3 have 71.3% homology, and in regards to
the coding sequence, the homology is 74.2% (35). In mice, 24p3 has been proven to be an
oncogene, suggesting that NGAL may also be a novel oncogene in
humans (36). Increasing attention
has been paid to the role of NGAL in the development and
progression of cancer. Some progress has been made in uncovering
the association between NGAL and various malignancies, such as
breast cancer (37), colorectal
cancer (14), pancreatic cancer
(15), renal cancer (38) and liver cancer (10).
NGAL expression in RCC tissues
Preliminary studies have revealed that NGAL protein
expression in the kidney is very limited, but NGAL protein can be
detected in renal proximal tubular epithelial cells (20), whereas studies on NGAL protein
expression in RCC have not yielded consistent results (Table I). Notably, according to
immunohistochemistry (IHC) staining intensities, Friedl et
al (20) found that 10/12 cases
of RCC, which is considered to originate from proximal convoluted
tubules, had very low NGAL expression, while moderate NGAL
expression was found in 2 cases. This was supplemented by further
research by Barresi et al (13) in 2010, where NGAL expression was
examined by an intensity-distribution (ID) score (≥4 was defined as
high NGAL expression) in 30 renal tumors. The results revealed that
NGAL was weakly expressed in collecting duct epithelial cells and
urothelial cells of the renal pelvis, as well as in normal
para-carcinoma renal tubules, whereas variable levels of NGAL were
detected in 28/30 patients with RCC (1 patient with ccRCC and 1
with a sarcomatoid tumor were excluded, with negative NGAL
staining) (13). Moreover, different
expression patterns were found in NGAL-positive ccRCC (13). In addition to the staining in the
cytoplasm of tumor cells, most ccRCCs had distinct membrane
staining, which may be due to the fact that NGAL has not yet been
internalized as a result of binding to membrane receptors (13). In another study, Zhang et al
(38) found that 57/84 patients with
pRCC had positive NGAL expression (ID score >2 was identified as
high NGAL expression) in the cytoplasm of cancer cells, while only
14/105 patients with ccRCC had cells that expressed NGAL. However,
Perrin et al (39) studied 74
RCC samples by IHC and found that NGAL was not expressed in renal
tumor cells, but was expressed in neutrophils infiltrating ccRCC
tissues (39). The aforementioned
studies indicate that there are no unified results on NGAL
expression and its subcellular localization in RCC, which may be
associated to its differential functions in tumor cells.
 | Table I.Studies of the gene or protein
expression levels of NGAL in RCC tissues. |
Table I.
Studies of the gene or protein
expression levels of NGAL in RCC tissues.
| First author,
year | Type of cancer | NGAL measuring
method | No. of
patients | No. of
controls | Main
outcome(s) | Refs. |
|---|
| Friedl et
al, 1999 | RCC | IHC | 12 | – | 10/12 cases with
RCC had very low NGAL expression, while 2 had moderate levels. | (20) |
| Barresi et
al, 2010 | Renal tumor | IHC | 30 | 30 | NGAL
immunoexpression was found in 28/30 cases. | (13) |
| Perrin et
al, 2011 | ccRCC | IHC | 74 | – | NGAL was observed
in neutrophils infiltrating ccRCC rather than tumor cells. | (39) |
| Zhang et al,
2015 | RCC | IHC | 189 | – | NGAL was found in
14/105 ccRCC and 57/84 pRCC cases. | (38) |
| Rehwald et
al, 2020 | RCC | Immunofluorescence
staining/quantitative PCR | 41 | 41 | There was a
significant increase in NGAL protein expression in tumor tissues
but no significant changes were observed in NGAL mRNA
expression. | (41) |
| Liu et al,
2018 | ccRCC | TCGA and GEO
database analysis | 533 samples in
TCGA; 11 GEO datasets | Paired
paracancerous tissues | Lower gene
expression levels of NGAL in ccRCC samples were observed compared
with in paired paracancerous tissues. | (40) |
The aforementioned studies were conducted by IHC in
tumor tissues, but control groups were rarely included. By
contrast, Liu et al (40)
analyzed 12 ccRCC datasets [1 dataset from The Cancer Genome Atlas
(TCGA) and 11 datasets from the Gene Expression Omnibus (GEO)
database] and found that, compared with in the paired tissues
adjacent to the carcinomas, the gene expression levels of NGAL in
the ccRCC group were decreased. Rehwald et al (41) suggested that NGAL gene expression may
be inconsistent with NGAL protein expression, since significant
increases in NGAL protein expression in ccRCC samples were
detected, compared with in corresponding adjacent healthy tissues,
but no significant changes in mRNA levels were identified by
quantitative PCR and immunofluorescence staining when comparing
tumor tissues with adjacent healthy tissues (41). Further studies are required to
elucidate the differences in gene and protein expression levels of
NGAL between RCC and normal tissues.
NGAL as a diagnostic and prognostic
marker in RCC
Due to the insidious onset of renal cancer and the
lack of early clinical symptoms, a non-invasive screening method is
urgently required to improve the early diagnosis and treatment of
renal cancer. Therefore, searching for a biomarker with excellent
sensitivity and specificity that can be found in the blood or urine
of patients with kidney cancer has become a research hotspot. NGAL
has attracted considerable attention as a tumor biomarker. Previous
studies have revealed that elevated NGAL expression may help
predict disease-free survival (DFS) in patients with colorectal
cancer (42), but the prognostic
utility and diagnostic accuracy of NGAL in RCC remain uncertain
(10).
NGAL is expressed in tissues, serum and urine of
patients with RCC. Studies on whether NGAL can be used as a
diagnostic and prognostic biomarker for RCC are currently underway
(Table II). By analyzing NGAL
expression in renal tumor tissues, Barresi et al (13) revealed that high NGAL expression is
associated with the pRCC and chRCC histotypes and is significantly
associated with the Fuhrman grading of ccRCC and pRCC (43). There was no significant association
between NGAL expression and patient age, sex, serum iron levels,
tumor size or tumor stage (13).
Zhang et al (38) studied the
association between NGAL expression and the prognosis of patients
with ccRCC and pRCC, and found that high NGAL expression was
associated with decreased overall survival (OS) and DFS in patients
with pRCC, but it was not associated with OS and DFS in patients
with ccRCC. Survival analysis of 533 patients with ccRCC in the
TCGA database revealed that high NGAL expression was associated
with a decreased survival rate compared with low NGAL expression
(40,41). Although high NGAL expression in tumor
tissues is associated with the type of tissue, it is impossible to
distinguish different histotypes of RCC based on NGAL expression,
but it may help to predict the OS and DFS of patients with RCC.
 | Table II.Studies of NGAL as a biomarker for
the diagnosis and prognosis of RCC. |
Table II.
Studies of NGAL as a biomarker for
the diagnosis and prognosis of RCC.
| First author,
year | Type of cancer | Sample | NGAL measuring
method | Main
outcome(s) | Refs. |
|---|
| Barresi et
al, 2010 | Renal tumor | Tissue | IHC | 1. Increase in NGAL
expression was parallel to the histological grade of the tumors in
ccRCC and pRCC. | (13) |
|
|
|
|
| 2. High NGAL
expression was significantly associated with pRCC and chRCC
histotype. |
|
| Zhang et al,
2015 | RCC | Tissue | IHC | 1. NGAL expression
was a significant predictor of decreased OS and DFS in pRCC, but
not in ccRCC. | (38) |
|
|
|
|
| 2. In pRCC, NGAL
expression was significantly associated with Fuhrman grade, tumor
size, TNM stage and presence of lymph node metastases. |
|
| Perrin et
al, 2011 | ccRCC | Tissue/serum | IHC/ELISA | 1. By IHC, NGAL was
not expressed in renal tumor cells but was expressed in neutrophils
infiltrating ccRCC tissue. | (39) |
|
|
|
|
| 2. High NGAL
concentrations in serum (>150 ng/ml) were associated with
shorter PFS. |
|
|
|
|
|
| 3. High
concentrations of NGAL-MMP-9 complex (>15 ng/ml) in serum were
associated with short PFS and poor OS. |
|
| Morrissey et
al, 2011 | RCC | Urine | ELISA | 1. Levels of uNGAL
were not significantly associated with tumor size or stage. | (47) |
| Di Carlo, 2013 | 16 ccRCC, 4
oncocytoma | Serum/urine | ELISA | 1. Values of sNGAL
and uNGAL in patients with ccRCC were not increased compared with
the mean values of healthy subjects. | (48 |
| Shalabi et
al, 2013 | RCC | Urine | ELISA | 1. uNGAL is not
suitable as a specific biomarker for RCC. | (6) |
| Saint et al,
2017 | Kidney tumors | Urine | ELISA | 1. uNGAL was
associated with tumor stage, and Furhman grade. | (49) |
|
|
|
|
| 2. uNGAL excretion
was associated with ccRCC PFS and disease-specific survival. |
|
| Porta et al,
2010 | Advanced RCC | Serum | ELISA | 1. sNGAL can
predict a longer PFS in patients with kidney cancer treated with
sunitinib malate. | (50) |
| Liu et al,
2018 | ccRCC | TCGA database | Data analysis | 1. NGAL
significantly predicted the clinical outcome of patients with
ccRCC. | (40) |
| Rehwald et
al, 2020 | ccRCC | TCGA
database/tissue | Data
analysis/Immunofluorescence staining | 1. There was a
significantly decreased patient survival probability associated
with higher NGAL expression. | (41) |
|
|
|
|
| 2. A significant
increase in NGAL protein expression was observed in ccRCC samples,
which was associated with tumor grade and tumor stage. |
|
With current technological limitations, the only
clinical test of NGAL in biofluids is used to diagnose AKI, as its
levels are associated with the severity of kidney injury in adults
and neonates (44–46). Whether the NGAL level in biofluids
can be used as a diagnostic and prognostic indicator for patients
with kidney cancer is under investigation. Previous studies have
revealed that elevated levels of urinary and serum NGAL in patients
with RCC are independent of histotype, stage and grade (47,48).
NGAL was not a sensitive or a specific urinary biomarker for RCC.
Although the concentration of NGAL in urine (uNGAL) in 67 patients
with renal cancer undergoing nephrectomy (0.52 ng/mg creatinine;
range, 0.28–0.73 ng/mg creatinine) was statistically different from
55 patients undergoing non-nephrectomy (typically orthopedic)
surgery (0.15 ng/mg creatinine; range, 0.04–0.31 ng/mg creatinine),
only 8 patients had an uNGAL level with no overlap with the control
group before nephrectomy. In addition, there was no significant
association between uNGAL and tumor size with stage (47). In another study, Saint et al
(49) revealed that high NGAL
expression was associated with increased tumor stage and Furhman
grade in patients with renal cancer and that high uNGAL excretion
was associated with poor progression-free survival (PFS) and
disease-specific survival (DSS) in patients with ccRCC. Although
uNGAL may not be suitable as a specific biomarker for RCC (6,47,48),
elevated levels of NGAL in serum (sNGAL) are associated with
decreased PFS in patients with RCC (39,50).
Although high concentrations of NGAL in biofluids do not help to
diagnose RCC, these are negatively associated with the prognosis of
patients with RCC. Measuring the levels of NGAL may be helpful for
selecting the appropriate treatment for patients with advanced RCC.
Patients with advanced RCC were treated with sunitinib, a small
molecule tyrosine kinase inhibitor with targets including vascular
endothelial growth factor, platelet-derived growth factor
receptor-α and β, and stem cell factor receptor, which has become
one of the two main first-line treatments available for RCC
(51). With 177 ng/ml being the
cut-off value, low sNGAL predicted a longer PFS than high sNGAL and
was superior to the best available clinical factor, the Motzer
score (50). Furthermore, the
expression levels of NGAL in RCC cells can be used as a predictive
biomarker for sensitivity to sunitinib before targeted therapy
(51).
Mechanism of NGAL in RCC
Since the synthesis of NGAL is induced by
cancer-promoting factors, it is considered to serve a key role in
the development and progression of human tumors (52). Previous studies have highlighted how
NGAL is involved in the development, proliferation and invasion of
cancer. In fact, elevated levels of this protein have been detected
in the serum or urine of patients with different types of tumor,
such as colon, breast, brain, thyroid, esophageal and bladder
cancer (10). However, depending on
the type of tumor, NGAL may have distinct roles in promoting or
preventing cancer (10). Bolignano
et al (52) observed that
when NGAL acts as an intracellular iron carrier and protects MMP-9
from degradation, it has a significant cancer-promoting effect, as
identified in human tumors including breast, stomach, esophageal,
rectal, thyroid and brain tumors. By contrast, Zhang et al
(53) reported that NGAL inhibited
the production of the cancer-promoting factor hypoxia-inducible
factor 1 (HIF-1), phosphorylation of focal adhesion kinase and
synthesis of vascular endothelial growth factor (VEGF), and had an
antitumor and anti-metastatic effect on colon, ovarian and
pancreatic tumors.
In a recent study of RCC, Rehwald et al
(41) revealed that iron-loaded NGAL
had tumor-promoting effects in RCC cell lines, while iron-free NGAL
had the opposite effect. In another study, Yu et al
(51) reported that NGAL promoted
RCC cell proliferation by enhancing the activation of the Ras-GTP,
Erk1/2 and STAT1a signaling pathways. Currently, a number of
hypotheses have been proposed regarding the possibility of NGAL
promoting tumor progression, most of which involve the binding of
NGAL to MMP-9 or the involvement of NGAL in iron uptake (41,54).
Based on the aforementioned literature, it is likely that NGAL may
serve a role in the development, progression, invasion and
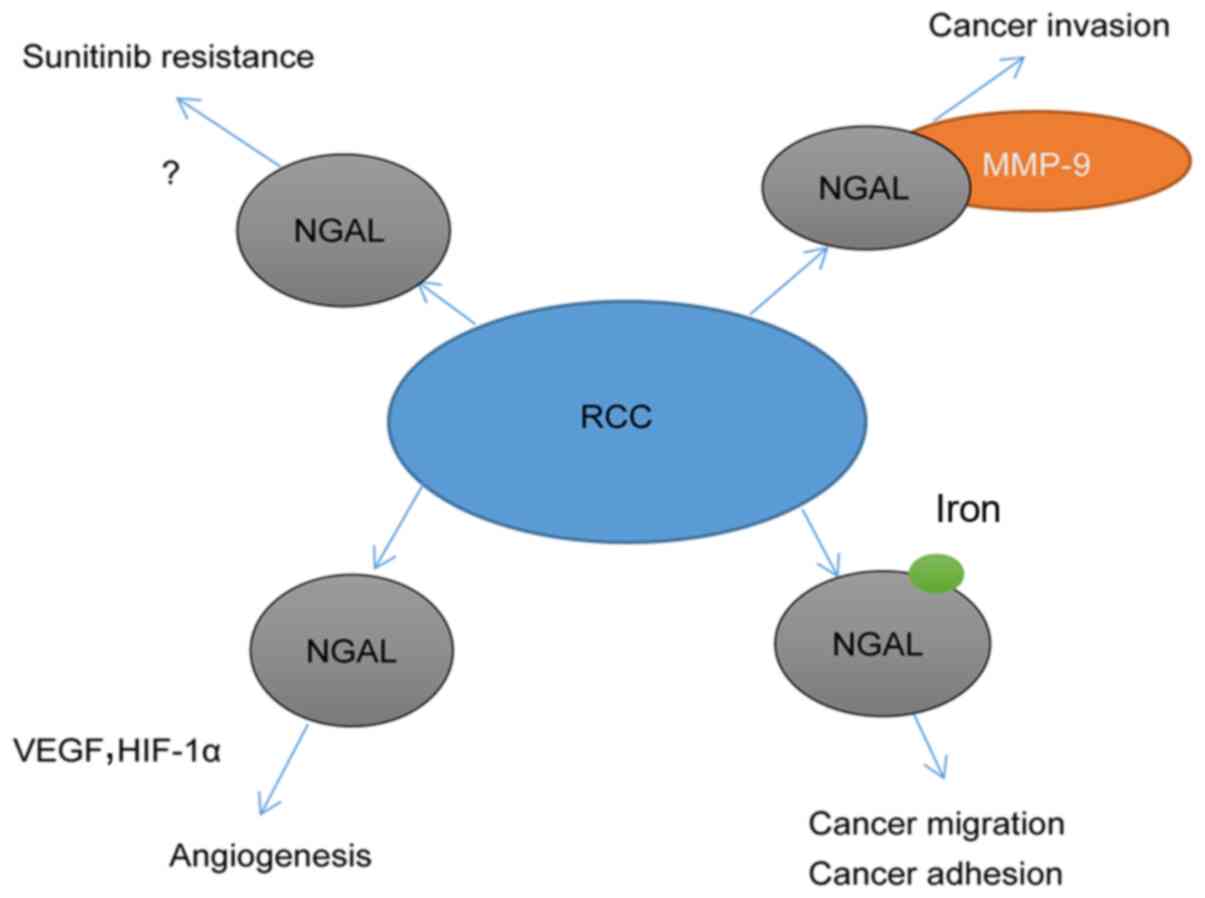
metastasis of RCC through the aforementioned pathways (Fig. 2).
MMP-9 is a gelatinase that degrades a wide range of
substrates, including collagen, fibronectin and laminin, to promote
tumor invasion and metastasis (11).
In addition, numerous experimental evidence supports that MMP-9 is
directly involved in angiogenesis (55,56).
NGAL can regulate the activity of MMP-9 by binding to pro-MMP-9 and
forming a ternary complex to decrease the degradation of MMP-9
(57). In a ccRCC cohort, high
levels of the MMP-9/NGAL complex in patient serum were associated
with shorter PFS and lower OS than low levels of the complex
(39), suggesting that NGAL may
promote invasion and metastasis of cancer by binding with MMP-9 in
ccRCC.
Due to the high proliferation rate of tumor cells,
large amounts of iron are required to maintain their enhanced
metabolic conversion (58). The
unique iron transport capacity of NGAL is crucial to its
tumor-promoting ability. Several studies have explored the
mechanisms by which NGAL promotes human cancer, emphasizing how
NGAL promotes iron uptake in the extracellular space of malignant
cells to sustain tumor cell proliferation. In breast cancer, for
example, blocking the release of iron-loaded NGAL by inhibiting
tumor-associated macrophages significantly decreased tumor growth
(59). Rehwald et al
(41) found a significant increase
in NGAL expression, especially iron-loaded NGAL, in tumor tissues
of patients with RCC compared with that of adjacent healthy
tissues. Iron-loaded NGAL accounts for ~2% of NGAL in healthy
tissues and ~20% of NGAL in tumor tissues (41). Similarly, in a study with RCC cell
lines, compared with iron-free NGAL and mutated NGAL (which was
unable to bind to iron), iron-loaded NGAL significantly enhanced
the migration and adhesion of RCC cells (786-O, RCC4, A498 and
CAKI1), but cell proliferation remained unchanged after stimulation
with the aforementioned three molecules (41). These results indicate that although
iron-loaded NGAL cannot promote the proliferation of RCC cells, it
may be able to promote the invasion and metastasis of RCC by
enhancing the migration and adhesion of RCC cells.
In addition to the aforementioned hypotheses, NGAL
is considered to be a cytokine that promotes angiogenesis in tumors
(60). Overexpression of NGAL in a
ductal adenocarcinoma cell line (PANC1) characterized by low
endogenous NGAL expression significantly enhanced tumor invasion,
adhesion and growth, and promoted VEGF and HIF-1α expression, which
serve an important role in cancer angiogenesis (11). However, Ferreira et al
(61) argued that in the presence of
ferrous iron, NGAL is involved in iron absorption, which inhibits
HIF-1α. In addition, NGAL has similar features to NF-κB, such as
the potential to protect thyroid cancer cells from apoptosis
induced by growth factor deprivation (11). However, to the best of our knowledge,
the aforementioned findings have not been verified in RCC.
Discussion
To the best of our knowledge, the present review was
the first to evaluate the possible role of NGAL in RCC. Previous
studies on NGAL have found that NGAL expression varies among
different types of cancer, and its role may be different. In breast
cancer (62) or thyroid cancer
(63), overexpression of NGAL
enhances cancer cell motility and invasiveness, while in pancreatic
cancer (64), overexpression of NGAL
decreases tumor volume, along with local and distant metastasis.
Therefore, the present review aimed to address the role of NGAL in
RCC.
NGAL can be detected in most RCCs. Studies of the
TCGA and GEO databases revealed that NGAL gene expression in RCC
tissues was decreased compared with that in normal tissues
(40,41), while RCC samples had variable levels
of NGAL protein expression. There was no significant association
between NGAL expression and patient age, sex, serum iron level,
tumor type, tumor size or tumor stage (13), but it may be useful for predicting
patient prognosis. In addition, different studies have not obtained
consistent results on NGAL expression in RCC and on whether NGAL is
located in the cytoplasm or the cell membrane, whether it is
secreted by neutrophils, RCC infiltrating cells or cancer cells,
and why the gene and protein expression levels of NGAL are
inconsistent, which requires further investigation.
NGAL has attracted considerable attention as a tumor
biomarker. Whether NGAL expression in biological fluids can be used
as a diagnostic and prognostic biomarker in patients with RCC is
currently being investigated. Based on the existing studies,
although uNGAL may be associated with tumor stage and grade, as
well as PFS and DSS in patients with RCC (49), it may not be suitable as a specific
diagnostic biomarker for RCC. By contrast, sNGAL may be more
promising. Increased sNGAL was associated with decreased PFS in
patients with RCC (39,50). Additionally, sNGAL may be used to
select patients with RCC who are suitable for sunitinib treatment
(51). However, whether uNGAL or
sNGAL may be utilized for the diagnosis and prognosis of RCC remain
controversial, as there are numerous problems to be solved. For
example, no studies have confirmed that uNGAL or sNGAL originates
from RCC tissues. In addition, the cut-off value for NGAL
concentration in serum or urine used to distinguish between healthy
individuals and patients with RCC is not always clear. In the
future, the detection of NGAL in blood and urine of patients with
RCC should be further studied.
Research on the role of NGAL is helpful for the
treatment of patients with RCC. NGAL is likely to promote invasion
and metastasis of RCC cells by binding to MMP-9, and it may also
promote cancer development by binding to iron or inducing
angiogenesis via VEGF and HIF-1 (11). Experiments in vitro have
proven that iron-loaded NGAL can contribute to the migration and
adhesion of RCC cells, but it has no effect on the proliferation of
tumor cells (41). Blocking the
binding of NGAL to MMP-9 or iron may inhibit the invasion and
metastasis of RCC. Cancers characterized by high NGAL expression,
such as chRCC and pRCC, may be better candidates for treatment with
anticancer agents that function as iron chelators than cancers with
low NGAL expression (65). In
addition, a previous study has revealed that patients with RCC
develop resistance to sunitinib by upregulating NGAL expression to
activate the angiogenesis pathway (51). Therefore, we speculate that the
inhibition of NGAL may decrease resistance to sunitinib.
In conclusion, although NGAL in biofluids cannot be
used as a diagnostic marker for early screening of RCC, NGAL
expression in serum, urine or tumor tissues may be used to predict
the prognosis of patients with RCC. The prognostic value of NGAL in
patients with RCC requires to be further investigated. Further
research on NGAL may be helpful to decrease sunitinib resistance
and identify new treatment strategies for RCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MZ designed the study, and KC drafted the manuscript
and revised the manuscript together with WH. HN reviewed the
manuscript and gave final approval of the version to be published.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Linehan WM, Srinivasan R and Schmidt LS:
The genetic basis of kidney cancer: A metabolic disease. Nat Rev
Urol. 7:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Znaor A, Lortet-Tieulent J, Laversanne M,
Jemal A and Bray F: International variations and trends in renal
cell carcinoma incidence and mortality. Eur Urol. 67:519–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L and
Merseburger AS: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Signoretti S, Chen YB, Reuter VE and
Flaifel A: Renal cell carcinoma in the era of precision medicine:
From molecular pathology to tissue-based biomarkers. J Clin Oncol.
36:3553–3559. 2018. View Article : Google Scholar
|
|
6
|
Shalabi A, Abassi Z, Awad H, Halachmi S,
Moskovitz B, Kluger Y and Nativ O: Urinary NGAL and KIM-1:
Potential association with histopathologic features in patients
with renal cell carcinoma. World J Urol. 31:1541–1545. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kjeldsen LS, Johnsen AH, Sengeløv H and
Borregaard N: Isolation and primary structure of NGAL, a novel
protein associated with human neutrophil gelatinase. J Biol Chem.
268:10425–10432. 1993.PubMed/NCBI
|
|
8
|
Castillo-Rodriguez E, Fernandez-Prado R,
Martin-Cleary C, Pizarro-Sánchez MS, Sanchez-Niño MD, Sanz AB,
Fernandez-Fernandez B and Ortiz A: Kidney injury marker 1 and
neutrophil gelatinase-associated lipocalin in chronic kidney
disease. Nephron. 136:263–267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makris K, Rizos D, Kafkas N and Haliassos
A: Neurophil gelatinase-associated lipocalin as a new biomarker in
laboratory medicine. Clin Chem Lab Med. 50:1519–1532. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roli L, Pecoraro V and Trenti T: Can NGAL
be employed as prognostic and diagnostic biomarker in human
cancers? A systematic review of current evidence. Int J Biol
Markers. 32:e53–e61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lippi G, Meschi T, Nouvenne A, Mattiuzzi C
and Borghi L: Neutrophil gelatinase-associated lipocalin in cancer.
Adv Clin Chem. 64:179–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monisha J, Roy NK, Padmavathi G, Banik K,
Bordoloi D, Khwairakpam AD, Arfuso F, Chinnathambi A, Alahmadi TA,
Alharbi SA, et al: NGAL is downregulated in oral squamous cell
carcinoma and leads to increased survival, proliferation, migration
and chemoresistance. Cancers (Basel). 10:2282018. View Article : Google Scholar
|
|
13
|
Barresi V, Ieni A, Bolignano D, Magno C,
Buemi M and Barresi G: Neutrophil gelatinase-associated lipocalin
immunoexpression in renal tumors: Correlation with histotype and
histological grade. Oncol Rep. 24:305–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marti J and Fuster J: Prognostic value of
serum neutrophil gelatinase-associated lipocalin in metastatic and
non-metastatic colorectal cancer: Reply. World J Surg. 37:27292013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu B, Jin DY, Lou WH and Wang DS:
Lipocalin-2 is associated with a good prognosis and reversing
epithelial-to-mesenchymal transition in pancreatic cancer. World J
Surg. 37:1892–1900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bundgaard JR, Sengeløv H, Borregaard N and
Kjeldsen L: Molecular cloning and expression of a cDNA encoding
NGAL: A lipocalin expressed in human neutrophils. Biochem Biophys
Res Commun. 202:1468–1475. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cowland JB and Borregaard N: Molecular
characterization and pattern of tissue expression of the gene for
neutrophil gelatinase-associated lipocalin from humans. Genomics.
45:17–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chakraborty S, Kaur S, Guha S and Batra
SK: The multifaceted roles of neutrophil gelatinase associated
lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta.
1826:129–169. 2012.PubMed/NCBI
|
|
19
|
Vaidya VS, Ferguson MA and Bonventre JV:
Biomarkers of acute kidney injury. Ann Rev Pharmacol Toxicol.
48:463–493. 2008. View Article : Google Scholar
|
|
20
|
Friedl A, Stoesz SP, Buckley P and Gould
MN: Neutrophil gelatinase-associated lipocalin in normal and
neoplastic human tissues. Cell type-specific pattern of expression.
Histochem J. 31:433–441. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lippi G and Plebani M: Neutrophil
gelatinase-associated lipocalin (NGAL): The laboratory perspective.
Clin Chem Lab Med. 0:1–5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zabron AA, Horneffer-van der Sluis VM,
Wadsworth CA, Laird F, Gierula M, Thillainayagam AV, Vlavianos P,
Westaby D, Taylor-Robinson SD, Edwards RJ and Khan SA: Elevated
levels of neutrophil gelatinase-associated lipocalin in bile from
patients with malignant pancreatobiliary disease. Am J
Gastroenterol. 106:1711–1717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Capoluongo E, Vento G, Lulli P, Di Stasio
E, Porzio S, Vendettuoli V, Tana M, Tirone C, Romagnoli C, Zuppi C
and Ameglio F: Epithelial lining fluid
neutrophil-gelatinase-associated lipocalin levels in premature
newborns with bronchopulmonary dysplasia and patency of ductus
arteriosus. Int J Immunopathol Pharmacol. 21:173–179. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kotyza J, Bunatova K, Pesek M and Puzman
P: Pleural injury and pleurisy-induced progelatinase B/proMMP-9 is
associated with markers of neutrophil degranulation. Scand J Clin
Lab Invest. 66:487–496. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lippi G, Caleffi A, Pipitone S, Elia G,
Ngah A, Aloe R, Avanzini P and Ferrari C: Assessment of neutrophil
gelatinase-associated lipocalin and lactate dehydrogenase in
peritoneal fluids for the screening of bacterial peritonitis. Clin
Chim Acta. 418:59–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lippi G, Avanzini P, Calzetti C, Caleffi
A, Pipitone S, Musa R, Aloe R and Ferrari C: The role of neutrophil
gelatinase-associated lipocalin (NGAL) in cerebrospinal fluids for
screening of acute bacterial meningitis. Clin Lab. 60:377–381.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmidt-Otta KM, Mori K, Kalandadzea A, Li
JY, Paragasa N, Nicholas T, Devarajan P and Barasch J: Neutrophil
gelatinase-associated lipocalin-mediated iron traffic in kidney
epithelia. Curr Opin Nephrol Hypertens. 15:442–449. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soni SS, Cruz D, Bobek I, Chionh CY,
Nalesso F, Lentini P, de Cal M, Corradi V, Virzi G and Ronco C:
NGAL: A biomarker of acute kidney injury and other systemic
conditions. Int Urol Nephrol. 42:141–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gwira JA, Wei F, Ishibe S, Ueland JM,
Barasch J and Cantley LG: Expression of neutrophil
gelatinase-associated lipocalin regulates epithelial morphogenesis
in vitro. J Biol Chem. 280:7875–7882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goetz DH, Willie ST, Armen RS, Bratt T,
Borregaard N and Strong RK: Ligand preference inferred from the
structure of neutrophil gelatinase associated lipocalin.
Biochemistry. 39:1935–1941. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan L, Borregaard N, Kjeldsen L and Moses
MA: The high molecular weight urinary matrix metalloproteinase
(MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil
gelatinase-associated lipocalin (NGAL). Modulation of MMP-9
activity by NGAL. J Biol Chem. 276:37258–37265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Antonucci E, Lippi G, Ticinesi A, Pigna F,
Guida L, Morelli I, Nouvenne A, Borghi L and Meschi T: Neutrophil
gelatinase-associated lipocalin (NGAL): A promising biomarker for
the early diagnosis of acute kidney injury (AKI). Acta Biomed.
85:289–294. 2014.PubMed/NCBI
|
|
33
|
Rosner MH: Urinary biomarkers for the
detection of renal injury. Adv Clin Chem. 49:73–97. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cervellin G and di Somma S: Neutrophil
gelatinase-associated lipocalin (NGAL): The clinician's
perspective. Clin Chem Lab Med. 50:1489–1493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kjeldsen L, Cowland JB and Borregaard N:
Human neutrophil gelatinase-associated lipocalin and homologous
proteins in rat and mouse. Biochim Biophys Acta. 1482:272–283.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garay-Rojas E, Harper M, Hraba-Renevey S
and Kress M: An apparent autocrine mechanism amplifies the
dexamethasone- and retinoic acid-induced expression of mouse
lipocalin-encoding gene 24p3. Gene. 170:173–180. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wenners AS, Mehta K, Loibl S, Park H,
Mueller B, Arnold N, Hamann S, Weimer J, Ataseven B, Darb-Esfahani
S, et al: Neutrophil gelatinase-associated lipocalin (NGAL)
predicts response to neoadjuvant chemotherapy and clinical outcome
in primary human breast cancer. PLoS One. 7:e458262012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang M, Zhao X, Deng Y, Tang B, Sun Q,
Zhang Q, Chen W, Yao D, Yang J, Cao L and Guo H: Neutrophil
gelatinase associated lipocalin is an independent predictor of poor
prognosis in cases of papillary renal cell carcinoma. J Urol.
194:647–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perrin C, Patard JJ, Jouan F, Collet N,
Théoleyre S, Edeline J, Zerrouki S, Laguerre B, Bellaud-Roturaud
MA, Rioux-Leclercq N and Vigneau C: The neutrophil
gelatinase-associated lipocalin, or LCN 2, marker of aggressiveness
in clear cell renal cell carcinoma. Prog Urol. 21:851–858. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu F, Li N, Yang W, Wang R, Yu J and Wang
X: The expression analysis of NGAL and NGALR in clear cell renal
cell carcinoma. Gene. 676:269–278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rehwald C, Schnetz M, Urbschat A, Mertens
C, Meier JK, Bauer R, Baer P, Winslow S, Roos FC and Zwicker K: The
iron load of lipocalin-2 (LCN-2) defines its pro-tumour function in
clear-cell renal cell carcinoma. Br J Cancer. 122:421–433. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barresi V, Reggiani-Bonetti L, Di Gregorio
C, Vitarelli E, De Leon MP and Barresi G: Neutrophil
gelatinase-associated lipocalin (NGAL) and matrix
metalloproteinase-9 (MMP-9) prognostic value in stage I colorectal
carcinoma. Pathol Res Pract. 207:479–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lang H, Lindner V, de Fromont M, Molinié
V, Letourneux H, Meyer N, Martin M and Jacqmin D: Multicenter
determination of optimal interobserver agreement using the fuhrman
grading system for renal cell carcinoma: Assessment of 241 patients
with > 15-year follow-up. Cancer. 103:625–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haase-Fielitz A, Haase M and Devarajan P:
Neutrophil gelatinase-associated lipocalin as a biomarker of acute
kidney injury: A critical evaluation of current status. Ann Clin
Biochem. 51:335–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Devarajan P: Neutrophil
gelatinase-associated lipocalin (NGAL): A new marker of kidney
disease. Scand J Clin Lab Invest Suppl. 241:89–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Candido S, Abrams SL, Steelman LS,
Lertpiriyapong K, Fitzgerald TL, Martelli AM, Cocco L, Montalto G,
Cervello M, Polesel J, et al: Roles of NGAL and MMP-9 in the tumor
microenvironment and sensitivity to targeted therapy. Biochim
Biophys Acta. 1863:438–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Morrissey JJ, London AN, Lambert MC and
Kharasch ED: Sensitivity and specificity of urinary neutrophil
gelatinase-associated lipocalin and kidney injury molecule-1 for
the diagnosis of renal cell carcinoma. Am J Nephrol. 34:391–398.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
DI Carlo A: Evaluation of neutrophil
gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9
(MMP-9) and their complex MMP-9/NGAL in sera and urine of patients
with kidney tumors. Oncol Lett. 5:1677–1681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saint F, Rose-Robert F, Herpe YE, de Sousa
P, Sevestre H, Choukhroun G and Amant C: ARCHITECT®
urine-neutrophil gelatinase-associated lipocalin (uNGAL) essay: New
prognostic marker for clear cell renal cell carcinoma (ccRCC).
Annals Oncol. 28:vii92017. View Article : Google Scholar
|
|
50
|
Porta C, Paglino C, De Amici M, Quaglini
S, Sacchi L, Imarisio I and Canipari C: Predictive value of
baseline serum vascular endothelial growth factor and neutrophil
gelatinase-associated lipocalin in advanced kidney cancer patients
receiving sunitinib. Kidney Int. 77:809–815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu DS, Wu CL, Ping SY, Huang YL and Shen
KH: NGAL can alternately mediate sunitinib resistance in renal cell
carcinoma. J Urol. 192:559–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bolignano D, Donato V, Lacquaniti A, Fazio
MR, Bono C, Coppolino G and Buemi M: Neutrophil
gelatinase-associated lipocalin (NGAL) in human neoplasias: A new
protein enters the scene. Cancer Lett. 288:10–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang XF, Zhang Y, Zhang XH, Zhou SM, Yang
GG, Wang OC, Guo GL, Yang GY and Hu XQ: Clinical significance of
neutrophil gelatinase-associated lipocalin(NGAL) expression in
primary rectal cancer. BMC Cancer. 9:1342009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bouchet S and Bauvois B: Neutrophil
gelatinase-associated lipocalin (NGAL), pro-matrix
metalloproteinase-9 (pro-MMP-9) and their complex pro-MMP-9/NGAL in
leukaemias. Cancers (Basel). 6:796–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Monier F, Surla A, Guillot M and Morel F:
Gelatinase isoforms in urine from bladder cancer patients. Clin
Chim Acta. 299:11–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hawinkels LJ, Zuidwijk K, Verspaget HW, de
Jonge-Muller ES, van Duijn W, Ferreira V, Fontijn RD, David G,
Hommes DW, Lamers CB and Sier CF: VEGF release by MMP-9 mediated
heparan sulphate cleavage induces colorectal cancer angiogenesis.
Eur J Cancer. 44:1904–1913. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Van den Steen PE, Van Aelst I, Hvidberg V,
Piccard H, Fiten P, Jacobsen C, Moestrup SK, Fry S, Royle L,
Wormald MR, et al: The hemopexin and O-glycosylated domains tune
gelatinase B/MMP-9 bioavailability via inhibition and binding to
cargo receptors. J Biol Chem. 281:18626–18637. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mertens C, Mora J, Ören B, Grein S,
Winslow S, Scholich K, Weigert A, Malmström P, Forsare C, Fernö M,
et al: Macrophage-derived lipocalin-2 transports iron in the tumor
microenvironment. Oncoimmunology. 7:e14087512018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yoshiji H, Harris SR and Thorgeirsson UP:
Thorgeirsson: Vascular endothelial growth factor is essential for
initial but not continued in vivo growth of human breast carcinoma
cells. Cancer Res. 57:3924–3928. 1997.PubMed/NCBI
|
|
61
|
Ferreira AC, Da Mesquita S, Sousa JC,
Correia-Neves M, Sousa N, Palha JA and Marques F: From the
periphery to the brain: Lipocalin-2, a friend or foe? Prog
Neurobiol. 131:120–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang J, Bielenberg DR, Rodig SJ, Doiron R,
Clifton MC, Kung AL, Strong RK, Zurakowski D and Moses MA:
Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci
USA. 106:3913–3918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Iannetti A, Pacifico F, Acquaviva R,
Lavorgna A, Crescenzi E, Vascotto C, Tell G, Salzano AM, Scaloni A,
Vuttariello E, et al: The neutrophil gelatinase-associated
lipocalin (NGAL), a NF-kappaB-regulated gene, is a survival factor
for thyroid neoplastic cells. Proc Natl Acad Sci USA.
105:14058–14063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tong Z, Kunnumakkara AB, Wang H, Matsuo Y,
Diagaradjane P, Harikumar KB, Ramachandran V, Sung B, Chakraborty
A, Bresalier RS, et al: Neutrophil gelatinase-associated lipocalin:
A novel suppressor of invasion and angiogenesis in pancreatic
cancer. Cancer Res. 68:6100–6108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Le NT and Richardson DR: The role of iron
in cell cycle progression and the proliferation of neoplastic
cells. Biochim Biophys Acta. 1603:31–46. 2002.PubMed/NCBI
|