Introduction
Uterine cervical cancer is the fourth most common
cancer and the fourth leading cause of cancer death in women
worldwide. As a result, it is considered to be one of the most
significant public health concerns (1). Despite advances in screening,
prevention, and diagnosis, some patients are diagnosed with a
locally advanced stage, including International Federation of
Gynecology and Obstetrics (FIGO) cancer stages IIIA, IIIB, and IVA.
The established standard of care for locally advanced uterine
cervical cancer is concurrent platinum-based chemoradiotherapy
(CCRT) (2–4). However, these patients experience a
higher rate of recurrence and poorer survival rates compared with
patients diagnosed at an early stage of the disease. In addition,
the 5-year survival of patients with locally advanced uterine
cervical cancer is <60% (5,6).
Neoadjuvant chemotherapy (NAC) has been shown to be an effective
strategy to reduce tumor size in patients with locally advanced
cervical cancer and facilitate a hysterectomy, thereby resulting in
a better prognosis (7). However, NAC
failure compels physicians to switch to radiation therapy, which
can result in a worse prognosis due to a delay in the initiation of
the core therapy (8–10). Therefore, there is an urgent need to
discover a biomarker that can predict the efficacy of NAC in
patients with locally advanced cervical cancer to select the right
candidates for NAC (10–14).
The anticancer agent cisplatin exhibits
antineoplastic activity by inducing DNA damage, particularly
intrastrand DNA crosslinks, leading to apoptosis (15). Therefore, one of the underlying
mechanisms of cisplatin resistance is the inactivation of apoptotic
pathways (16). Protein arginine
methyltransferase (PRMT) is an enzyme that catalyzes the
methylation of histone and non-histone proteins and transfers
methyl groups from S-adenosylmethionine to arginine (17). PRMT1 has been shown to methylate
apoptosis signal-regulated kinase 1 (ASK1) and inhibit its activity
resulting in chemotherapy resistance (18,19). In
other carcinomas (esophageal cancer and colorectal cancer),
previous reports suggest a relationship between PRMT1 and
chemotherapy resistance (20,21).
However, the effects of PRMT1 on the response to cisplatin in
cervical cancer cell lines and patients with cervical cancer are
unknown.
In the present study, we aimed to examine the
association between the tumor expression of PRMT1 and the efficacy
of NAC in locally advanced cervical cancer and determine the use of
PRMT1 expression as a predictive biomarker.
Materials and methods
Patients and tissue samples
Fifty-three patients with locally advanced cervical
cancer (FIGO stages IIIB) were evaluated. All patients were under
70 years of age and first cared for at Osaka City University
Hospital (Osaka, Japan) between April 1995 and March 2010. Tumor
tissue specimens were acquired by a punch biopsy prior to NAC.
Based on the effect on NAC, patients were divided into two groups:
Patients who responded to NAC underwent a hysterectomy and received
radiation therapy (NAC + OP + R group; n=28), and patients who were
not successfully treated with NAC and received radiation therapy
only (NAC + R group; n=25). Balloon-occlusive arterial infusion
chemotherapy for NAC was given to all patients. Cisplatin
(Bristol-Myers Squibb) was injected into the artery through a
catheter for more than 30 min. Cisplatin was given three times at
doses of 50, 75, or 100 mg/m2, depending on the
patient's age and renal function (22). All patients were treated with
cisplatin every 4 weeks. After the completion of three courses of
NAC with cisplatin, the effect of NAC was evaluated. If NAC was
effective, the patient underwent surgery 1 month after the
administration of NAC. If NAC was ineffective, radiotherapy was
initiated soon after the evaluation. We evaluated the effect of NAC
by pelvic examination and computed tomography or magnetic resonance
imaging. We considered NAC successful if the stage was downgraded
to stage I or II, and surgery was able to be performed. Informed
consent was acquired from all patients prior to the tumor biopsy.
This study was approved by the Institutional Review Board of Osaka
City University Hospital (IRB no. 4282).
Immunohistochemical staining
PRMT1 protein expression was evaluated using 4-µm
sections generated from paraffin-embedded tissue samples using a
rabbit polyclonal antibody against PRMT1 (cat. no. ab-70724; Abcam)
and Dako LSAB2 Peroxidase kit (cat. no. K0675; Agilent
Technologies). Following routine deparaffinization and rehydration,
sections were immersed in 3% hydrogen peroxide for 10 min at room
temperature to inhibit endogenous peroxidase activity. For
heat-mediated antigen retrieval, sections were incubated in 10 mM
citrate buffer (pH 6.0) in an autoclave at 110°C for 20 min. After
washing with phosphate-buffered saline (PBS), tumor tissue sections
were incubated at 4°C overnight in a 1:500 dilution of the
anti-PRMT1 antibody. Next, the sections were washed in PBS for 15
min, incubated with biotinylated goat anti-mouse and anti-rabbit
immunoglobulin G secondary antibodies included in the Dako LSAB2
Peroxidase kit (cat. no. K0675; Agilent Technologies) for 10 min,
and then incubated with a streptavidin-peroxidase complex solution
and 3,3′-diaminobenzidine as a colorimetric agent for color
development. Lastly, the tissue sections were counterstained using
hematoxylin. The primary antibodies were excluded, and the
specificity control was prepared in the same way.
PRMT1 expression was quantitatively analyzed using
the weighted score method described by Sinicrope et al
(23). The average percent of
stained tumor cells was scored on a scale of 0 to 4 as follows: 0,
≤5%; 1, 5–25%; 2, 25–50%; 3, 50–75%; and 4, >75%. The staining
intensity was categorized into three classes: 1+, weak; 2+,
moderate; and 3+, intense. The score for the percentage of stained
tumor cells was multiplied by the score for staining intensity, and
the weighted scores were calculated for each tissue sample.
Cell culture
The human cervical cancer cell line Ca Ski (cat. no.
IFO50007; Japanese Collection of Research Biosources Cell Bank) was
cultured in RMPI medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin and maintained at 37°C in a
moist atmosphere containing 5% CO2.
RNA interference
A small interfering RNA (siRNA) targeting PRMT1
(5′-GCAACUCCAUGUUUCAUAAtt, 5′-UUAUGAAACAUGGAGUUGCgg) and negative
control sequence (cat. no. sc-37007) were obtained from Santa Cruz
Biotechnology. Cells seeded into 6-well plates were transfected
with siRNA (Invitrogen; Thermo Fisher Scientific, Inc.) overnight
using lipofectamine RNAiMax following the description of the
manufacturer. The medium was changed, and the cells were taken to
the laboratory 24 h after transfection.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from Ca Ski cells using the
RNeasy Mini kit (Qiagen GmbH) following the instructions of the
manufacturer. High capacity cDNA reverse transcription kits
(Applied Biosystems; Thermo Fisher Scientific, Inc.) were used to
reverse transcribe the RNA. A TaqMan Gene expression assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and an Applied
Biosystems 7500 Fast Real-Time PCR system were used to perform PCR.
PRMT1 mRNA levels were normalized to glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) mRNA in the same sample. The TaqMan probes
were Hs04193290_g1 for PRMT1 and Hs99999905_m1 for GAPDH. The
2(-Delta Delta C(T)) method was used to analyze the relative
changes in gene expression from real-time quantitative PCR
experiments (24).
Chemosensitivity assay
The sensitivity of Ca Ski cells to cisplatin was
evaluated with a Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies). In the first step, cells were transfected with
negative control or PRMT1-specific siRNA as described above and
seeded at a density of 2×103 cells/well in 96-well
tissue culture plates. After 24 h, the medium was removed, and
vehicle or cisplatin (0 to 7.5 µM) was added to the cells for 48 h.
Then, 10 µl/well CCK-8 was added, and the plates were incubated for
2 h. The absorbance at 450 nm was then measured with a microplate
reader (Corona Electric, Co., Ltd.). Dose-response curves for the
percentage of viable cells relative to untreated cells were
generated.
Statistical analysis
All statistical analyses were performed using EZR
(Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Data are shown as the mean ± standard deviation in the Tables and
as the mean ± standard error in the Figures. A prognostic analysis
was performed using Kaplan-Meier plots and the log-rank test. The
Mann-Whitney U test was used to compare the weighted scores.
Significant differences between group means were compared using
Student's t-test, and the χ2 test was used to identify
relationships between group classification variables. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Fifty-three patients diagnosed with locally advanced
cervical cancer were classified into the two following groups based
on the treatment effect: The NAC effective group (NAC + OP + R
group; n=28) and the NAC ineffective group (NAC + R group; n=25).
Table I shows the patient
clinicopathological characteristics. We found no statistically
significant difference between the two groups.
 | Table I.Characteristics of patients in the
NAC+OP+R and NAC+R groups. |
Table I.
Characteristics of patients in the
NAC+OP+R and NAC+R groups.
| Variables | NAC+OP+R | NAC+R | P-value |
|---|
| No. of patients | 28 | 25 |
|
| Age, years |
|
| 0.269a |
| Mean ±
SD | 48.5±13.4 | 52.3±11.5 |
|
|
Range | 24–69 | 36–68 |
|
| Histology, n |
|
| 0.555b |
| SCC | 24 | 21 |
|
| A | 4 | 3 |
|
| AS | 0 | 1 |
|
| Tumor size, mm |
|
| 0.456a |
| Mean ±
SD | 48.4±17.2 | 51.8±12.3 |
|
PRMT1 expression in uterine cervical
cancer tissues
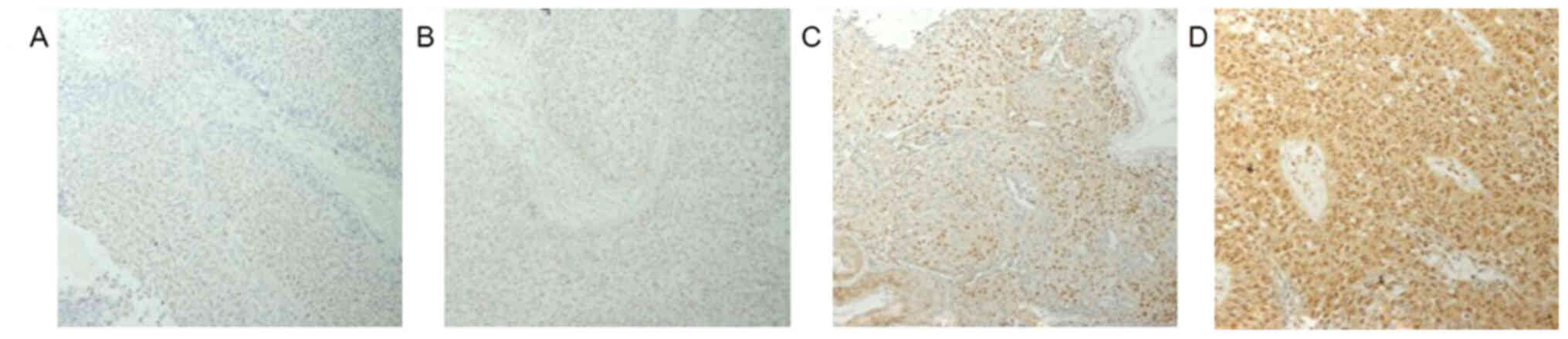
PRMT1 expression was found in both the nuclei and
cytoplasm of the tumor cells (Fig.
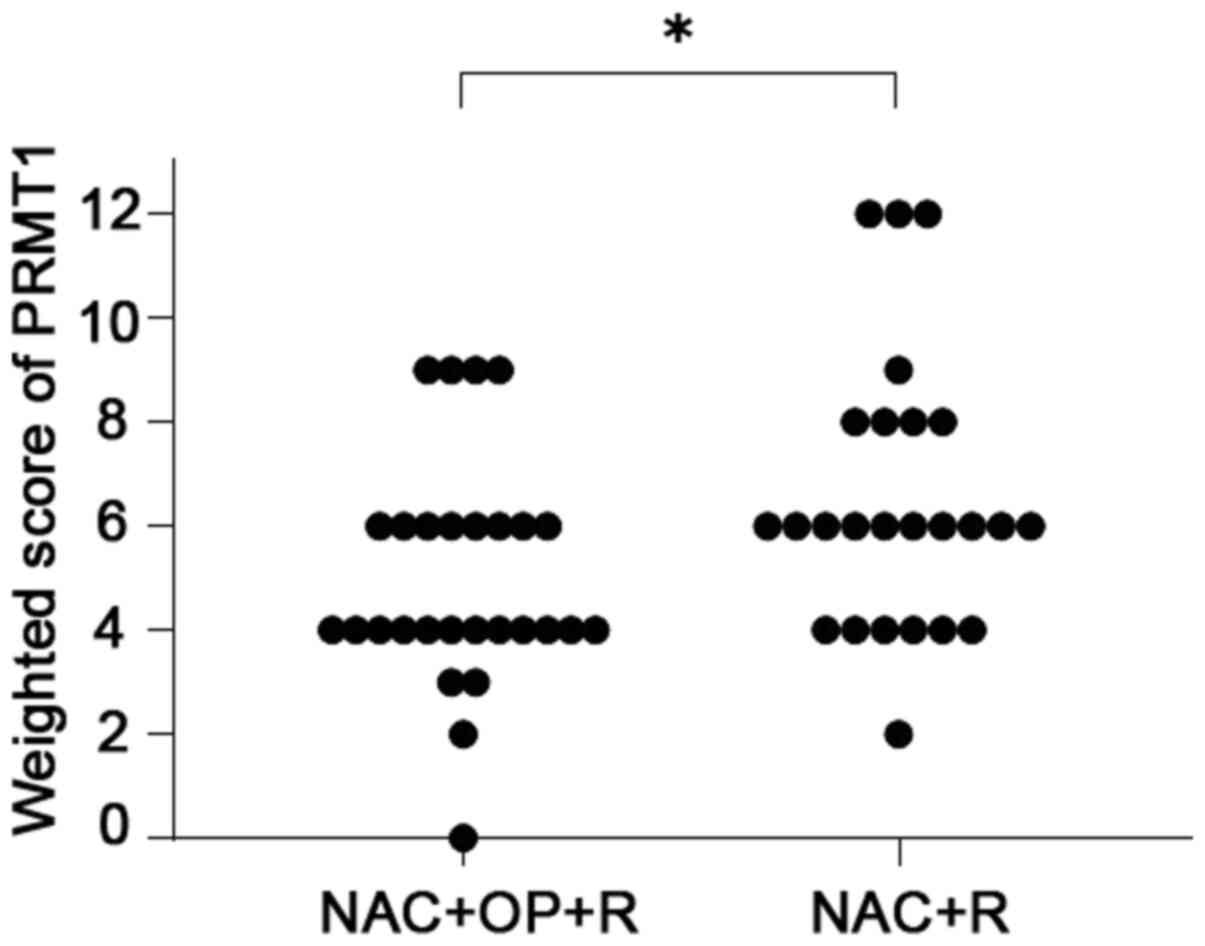
1). The weighted scores of PRMT1 tissue staining are shown in
Table II. The weighted score of the
NAC ineffective group was significantly higher than that of the NAC
effective group (P=0.030) (Fig. 2).
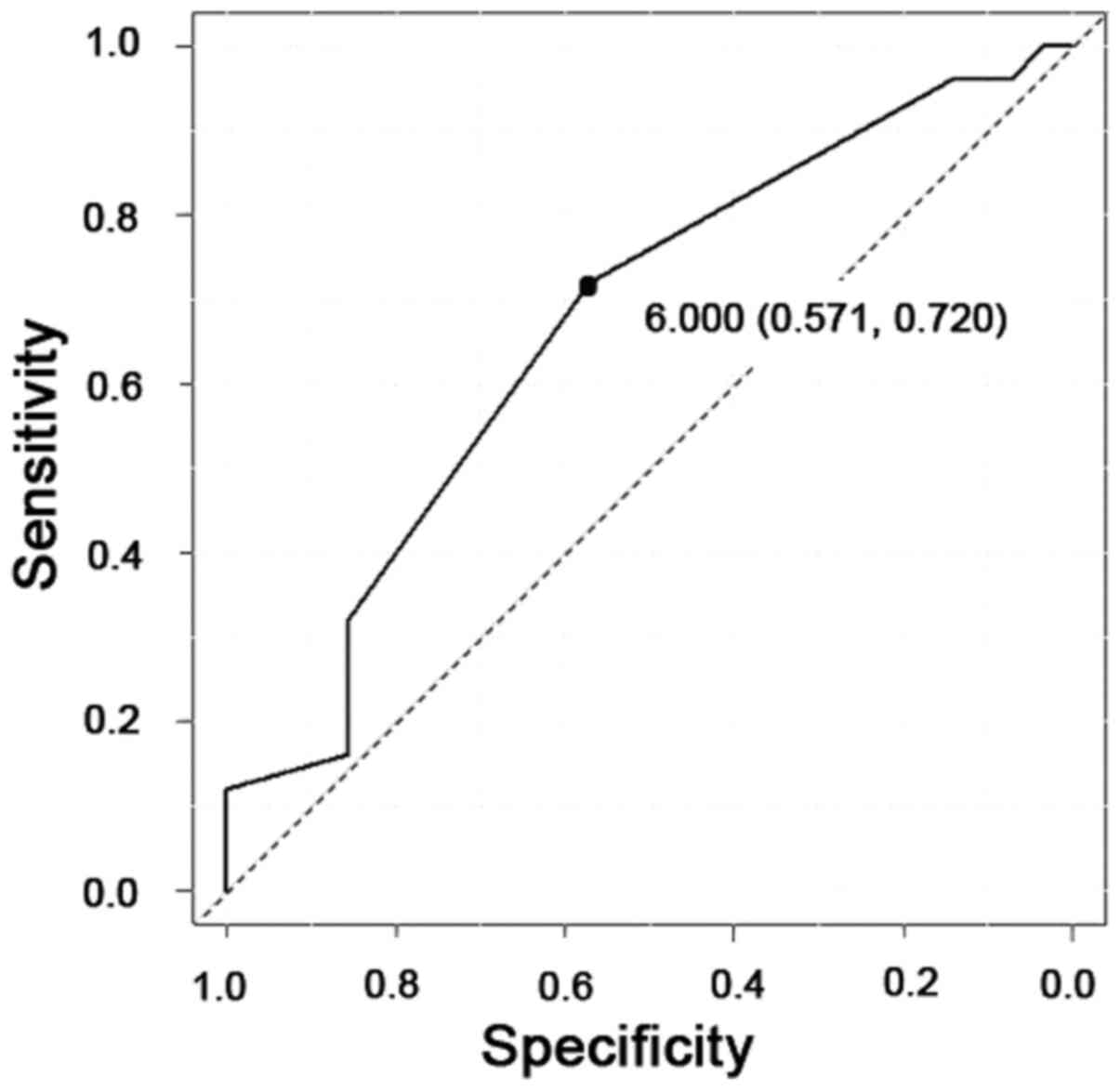
To predict the efficacy of NAC, receiver operator characteristic
(ROC) curves were generated, and the cut-off value of the PRMT1
score was examined. When a score of 6 was used as the cut-off
value, the specificity was 57%, and the sensitivity was 72%
(Fig. 3). Therefore, we classified
the cases into two different groups. One was the low expression
group with a score of 0 to 4, and the other was the high expression
group with a score of 6–12. We found no statistically significant
difference between the two groups (Table II).
 | Table II.Characteristics of the patients in the
low and high PRMT1 expression groups. |
Table II.
Characteristics of the patients in the
low and high PRMT1 expression groups.
|
| PRMT1 expression |
|
|---|
|
|
|
|
|---|
| Variables | Score ≤4 | Score ≥6 | P-value |
|---|
| No. of patients | 23 | 30 |
|
| Age, years |
|
| 0.820a |
| Mean ±
SD | 50.7±14.0 | 49.9±11.6 |
|
|
Range | 24–69 | 37–68 |
|
| Histology, n |
|
| 0.514b |
| SCC | 19 | 26 |
|
| A | 4 | 3 |
|
| AS | 0 | 1 |
|
| Tumor size, mm |
|
| 0.133a |
| Mean ±
SD | 45.9±14.5 | 52.8±15.2 |
|
NAC effectiveness correlates with
PRMT1 expression
In a group of 23 patients with low PRMT1 expression,
16 (69.6%) were in the NAC effective group, and 7 (30.4%) were in
the NAC ineffective group. In the high PRMT1 expression group, 12
patients (40.0%) were in the NAC effective group, and 18 (60.0%)
were in the NAC ineffective group. In this way, we found that the
low PRMT1 expression group was more responsive to NAC than the high
PRMT1 expression group (P=0.033, Table
III).
 | Table III.Numbers of patients with low and high
PRMT1 expression in the NAC+OP+R and NAC+R groups. |
Table III.
Numbers of patients with low and high
PRMT1 expression in the NAC+OP+R and NAC+R groups.
| Expression | NAC+OP+R, n
(%) | NAC+R, n (%) | P-value |
|---|
| Low (score ≤4) | 16 (69.6) | 7 (30.4) | 0.033a |
| High (score
≥6) | 12 (40.0) | 18 (60.0) |
|
Survival
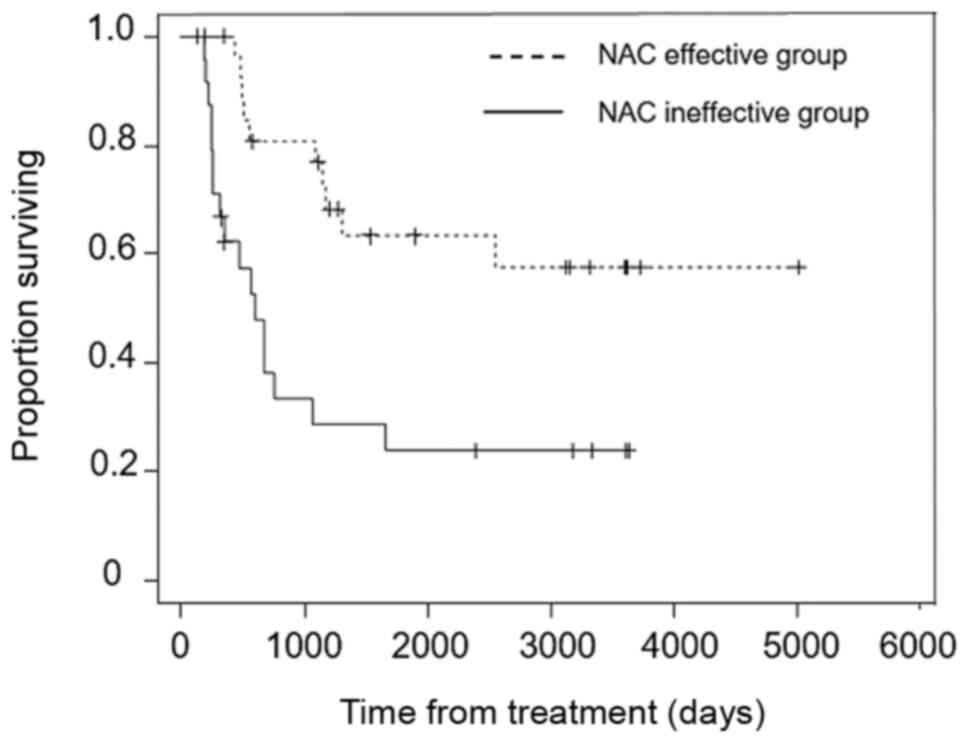
Overall survival was significantly longer in the NAC
effective group than the NAC ineffective group (P<0.001)
(Fig. 4) and in the low PRMT1
expression group than the high PRMT1 expression group (P=0.012)
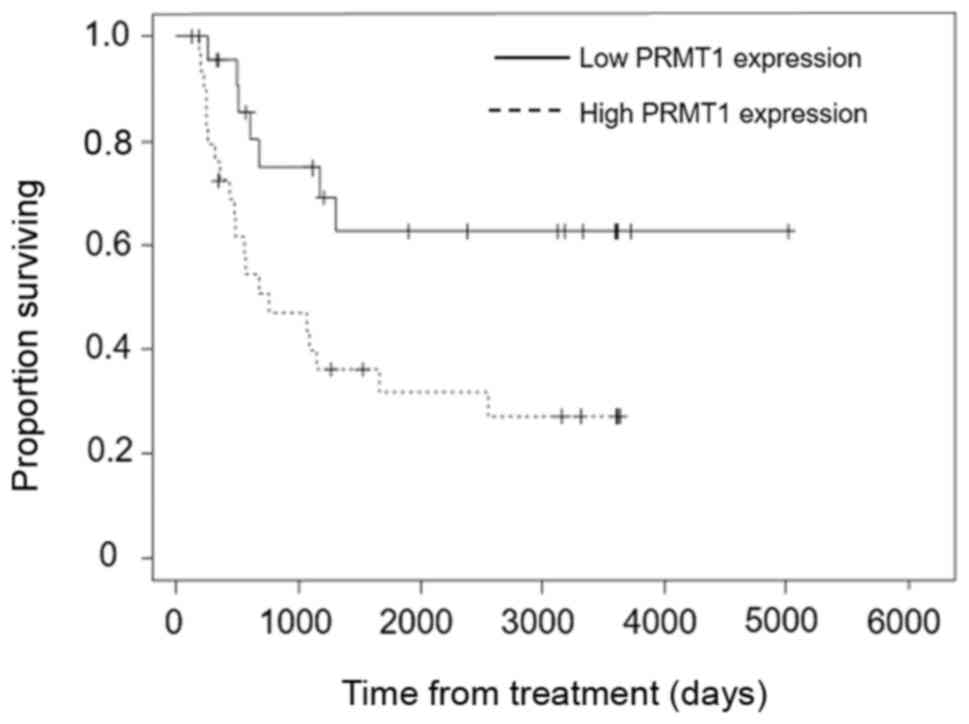
(Fig. 5).
PRMT1 knockdown enhances the
sensitivity of a uterine cervical cancer cell line to cisplatin
treatment
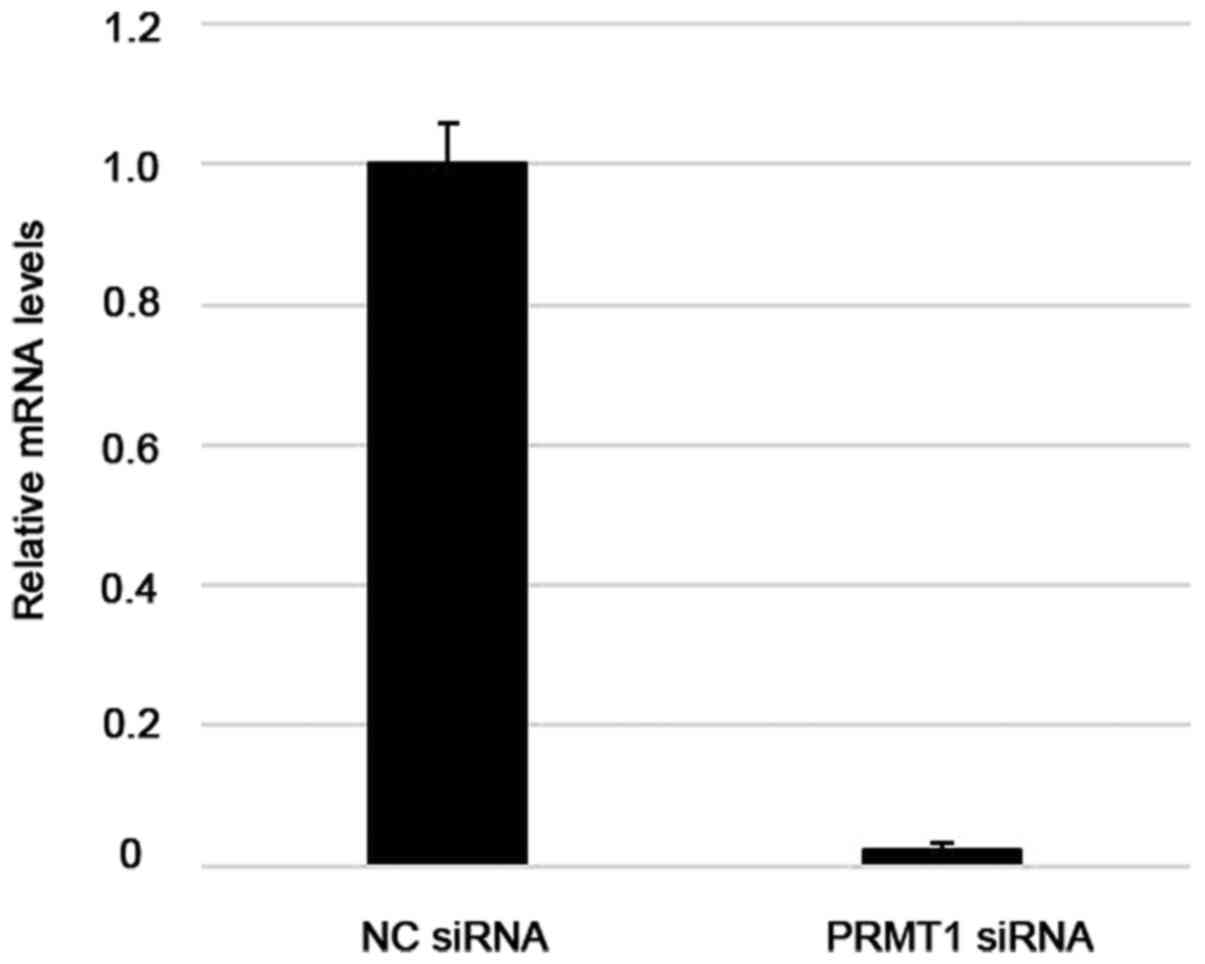
RT-qPCR analysis of Ca Ski cells showed that
transfection with specifically targeted siRNAs efficiently
suppressed PRMT1 expression, whereas non-targeted control siRNAs
did not (Fig. 6). Differences in the
sensitivity of cells transfected with PRMT1-specific siRNA to
cisplatin were significantly greater than those of control cells
(P<0.05) (Fig. 7).
Discussion
The standard of care for patients with locally
advanced cervical cancer is CCRT. However, the prognosis of these
patients remains poor. Successful NAC can shrink the tumor and
enable patients to undergo a hysterectomy, which can improve their
prognosis (7). It is important to
select the right candidate for NAC because NAC failure compels the
physician to switch to radiation therapy, which can delay the
initiation of the core therapy and result in a worse prognosis
(8–10). Thus, the identification of biomarkers
that can predict the effect of NAC in patients with locally
advanced cervical cancer is essential for the effective treatment
of these patients.
The methylation of various protein substrates is
catalyzed by PRMTs, many of which are linked to the development,
progression, and aggressiveness of various cancers (17). PRMTs are categorized as Type I or
Type II according to the characteristics of the modification of
their substrate. Both types catalyze the production of
monomethylarginine as an intermediate, but type I enzymes mediate
the production of asymmetric dimethylarginine, whereas type II
enzymes produce symmetric dimethylarginine. PRMT1 is a Type I
arginine methyltransferase. PRMT1 catalyzes the methylation of ASK1
and leads to the negative regulation of downstream signaling
events, resulting in stress-induced stimulation of ASK1 and
apoptotic cell death (25).
Anticancer agents, such as cisplatin, exhibit their
antineoplastic activity by introducing DNA damage, particularly
intrastrand DNA crosslinks, leading to apoptosis (15). Therefore, the inactivation of
apoptotic pathways is associated with cisplatin resistance. The
PRMT1-induced inactivation of apoptotic pathways via ASK1
inhibition can contribute to the development of cisplatin
resistance.
This study found a significant association between
PRMT1 expression and the efficacy of NAC in patients with locally
advanced cervical cancer; patients with low PRMT1 expression were
more likely to benefit from NAC and undergo surgery after NAC.
Consistent with this, the low PRMT1 expression and NAC effective
groups had longer overall survival periods than the high PRMT1
expression and NAC ineffective groups, respectively. We also
observed that downregulating PRMT1 expression increased the
cisplatin sensitivity of cultured cervical cancer cells, indicating
that PRMT1 is a cisplatin-resistance factor. To the best of our
knowledge, this study is the first to report a clearly defined
relationship between PRMT1 expression and the effectiveness of NAC
in locally advanced cervical cancer. However, the efficacy of NAC
cannot be determined based on PRMT1 expression alone yet. As far as
the standard treatment for locally advanced cervical cancer
patients is CRT, we should choose NAC in just clinical trial
settings. Moreover, one limitation of this study was that it
included only 53 patients. Therefore, further investigation with
more cases is needed to confirm our findings.
In summary, PRMT1 expression has the potential to be
a predictive marker of the efficacy of NAC in patients with locally
advanced cervical cancer. This finding can contribute to
improvements in the prognosis of these patients.
Acknowledgements
The authors would like to thank Dr Melissa Crawford
for editing a draft of this manuscript.
Funding
The present study was supported by The Osaka Medical
Research Foundation for Intractable Diseases (grant no.
26-2-47).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF and TS designed the current study. MS, HM, YI,
SN, YA and MY performed the experiment and collected the data. MS,
TF, TY and TS analyzed the data. MS and TF wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Osaka City University Hospital (IRB no. 4282) prior
to initiation of the study. Written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2011. View Article : Google Scholar
|
|
2
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Japan Society of Gynecologic Oncology
(eds), . Formulation Committee of the Treatment Guidelines for
Cervical Cancer, 2011. Kanehara & Co.; Tokyo: 2011, (In
Japanese).
|
|
4
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology. Cervical Cancer
Version II. 2013.simplehttps://www2.tri-kobe.org/nccn/guideline/gynecological/english/cervical.pdf
|
|
5
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: An update of
radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Kawamura N, Ogita S, Kamino T, Nakamura K and Yamada R:
Balloon-occluded arterial infusion chemotherapy, simple total
hysterectomy and radiotherapy as a useful combination-therapy for
advanced cancer of the uterine cervix. Oncol Rep. 7:141–144.
2000.PubMed/NCBI
|
|
8
|
Souhami L, Gil RA, Allan SE, Canary PC,
Araújo CM, Pinto LH and Silveira TR: A randomized trial of
chemotherapy followed by pelvic radiation therapy in stage IIIB
carcinoma of the cervix. J Clin Oncol. 9:970–977. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tattersall MH, Lorvidhaya V, Vootiprux V,
Cheirsilpa A, Wong F, Azhar T, Lee HP, Kang SB, Manalo A, Yen MS,
et al: Randomized trial of epirubicin and cisplatin chemotherapy
followed by pelvic radiation in locally advanced cervical cancer.
Cervical cancer study group of the asian oceanian clinical oncology
association. J Clin Oncol. 13:444–451. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Ogita S, Kaminou T, Nakamura K and Yamada R: Tumor marker and MR
imaging criteria for evaluating the efficacy of cyclic
balloon-occluded arterial infusion for advanced cancer of the
uterine cervix. Oncol Rep. 7:827–830. 2000.PubMed/NCBI
|
|
11
|
Ishiko O, Sumi T, Yoshida H, Ogita S and
Yamada R: Expression of apoptosis regulatory proteins in advanced
cancer of the uterine cervix after cyclic balloon-occluded arterial
infusion chemotherapy. Int J Oncol. 18:1151–1155. 2001.PubMed/NCBI
|
|
12
|
Okamoto E, Sumi T, Misugi F, Nobeyama H,
Hattori K, Yoshida H, Matsumoto Y, Yasui T, Honda K and Ishiko O:
Expression of apoptosis-related proteins in advanced uterine
cervical cancer after balloon-occluded arterial infusion
chemotherapy as an indicator of the efficiency of this therapy. Int
J Mol Med. 15:41–47. 2005.PubMed/NCBI
|
|
13
|
Nobeyama H, Sumi T, Misugi F, Okamoto E,
Hattori K, Matsumoto Y, Yasui T, Honda K, Iwai K and Ishiko O:
Association of HPV infection with prognosis after neoadjuvant
chemotherapy in advanced uterine cervical cancer. Int J Mol Med.
14:101–105. 2004.PubMed/NCBI
|
|
14
|
Benedetti Panici P, Bellati F, Manci N,
Pernice M, Plotti F, Di Donato V, Calcagno M, Zullo MA, Muzii L and
Angioli R: Neoadjuvant chemotherapy followed by radical surgery in
patients affected by FIGO stage IVA cervical cancer. Ann Surg
Oncol. 14:2643–2648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Shi S, He W, Padilla MT, Zhang L,
Wang X, Zhang B and Lin Y: Retaining MKP1 expression and
attenuating JNK-mediated apoptosis by RIP1 for cisplatin resistance
through miR-940 inhibition. Oncotarget. 5:1304–1314. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bedford MT and Richard S: Arginine
methylation an emerging regulator of protein function. Mol Cell.
18:263–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baldwin RM, Morettin A and Côté J: Role of
PRMTs in cancer: Could minor isoforms be leaving a mark? World J
Biol Chem. 5:115–129. 2014.PubMed/NCBI
|
|
19
|
Hirata Y, Katagiri K, Nagaoka K, Morishita
T, Kudoh Y, Hatta T, Naguro I, Kano K, Udagawa T, Natsume T, et al:
TRIM48 promotes ASK1 activation and cell death through
ubiquitination-dependent degradation of the ASK1-negative regulator
PRMT1. Cell Rep. 21:2447–2457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao HW, Hsu JM, Xia W, Wang HL, Wang YN,
Chang WC, Arold ST, Chou CK, Tsou PH, Yamaguchi H, et al:
PRMT1-mediated methylation of the EGF receptor regulates signaling
and cetuximab response. J Clin Invest. 125:4529–4543. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Lu Q, Li C, Wang X, Jiang L, Huang
L, Wang C and Chen H: PRMT1 regulates the tumour-initiating
properties of esophageal squamous cell carcinoma through histone H4
arginine methylation coupled with transcriptional activation. Cell
Death Dis. 10:3592019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsuji K, Yamada R, Kawabata M, Mitsuzane
K, Sato M, Iwahashi M, Kitayama S and Nakano R: Effect of balloon
occluded arterial infusion of anticancer drugs on the prognosis of
cervical cancer treated with radiation therapy. Int J Radiat Oncol
Biol Phys. 32:1337–1345. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: Bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho JH, Lee MK, Yoon KW, Lee J, Cho SG and
Choi EJ: Arginine methylation-dependent regulation of ASK1
signaling by PRMT1. Cell Death Differ. 19:859–870. 2012. View Article : Google Scholar : PubMed/NCBI
|