Introduction
Hepatocellular carcinoma (HCC) is one of the most
common solid tumors worldwide, and the third most common cause of
cancer-related death (1). For liver
resection (Hx) in patients with HCC, the perioperative
complications and mortality have improved in recent years from 30%
to <5% in high-volume centers (2,3);
however, it is crucial to manage patients with various
comorbidities. Hx is still associated with a 40% morbidity rate,
with post-hepatectomy liver failure (PHLF) being the most lethal
complication (4). Furthermore, the
recurrence rate after curative Hx for HCC is still higher compared
with that for other digestive organ cancers, suggesting that it is
also important to assess the risk factors for recurrence following
curative Hx.
The prognostic nutritional index (PNI) was first
established in 1980 in order to predict perioperative risk for
gastrointestinal surgeries (5).
However, the calculation method was highly complicated as it used a
number of parameters such as serum albumin (Alb), triceps skinfold,
transferrin and delayed hypersensitivity skin testing. A simpler
modified PNI using serum Alb and total lymphocyte count (TLC) alone
was reported 4 years later by Onodera et al (6), and the modified PNI has been widely
used for perioperative risk assessment. In addition to
perioperative risk prediction, the PNI has also been reported to be
associated with long-term prognosis of various types of cancer
after curative treatment, such as lung (7), ovarian (8), cervical (9), gastric (10) and colorectal (11) cancer.
In Hx for HCC, the preoperative PNI is associated
with liver function markers such as the albumin-bilirubin grade and
predicts short-term outcomes within the Milan criteria (12). A previous study has been reported
that the preoperative PNI predicts long-term prognosis after Hx in
only early Barcelona clinic liver cancer stage HCC (13). To the best of our knowledge, there
have been no reports on the use of the preoperative PNI for
predicting both short- and long-term outcomes in early and advanced
stages of HCC.
The present study aimed to compare three types of
immune parameters, namely the PNI, neutrophil-to-lymphocyte ratio
(NLR) and aspartate aminotransferase (AST)-to-lymphocyte ratio
(ALRI), as predictors of short- and long-term outcomes following
Hx. Furthermore, the aim of the present study was to determine the
prognostic significance of the preoperative PNI for short- and
long-term outcomes after Hx for patients with all stages of
HCC.
Materials and methods
Patients
Among 229 patients who underwent Hx at Tokushima
University Hospital (Tokushima, Japan) between January 2006 and
December 2014, 162 patients were enrolled in the present study. The
inclusion criteria were as follows: i) Primary Hx; ii) no other
treatments before Hx; and iii) availability of the follow-up data.
The study was approved by Tokushima University Hospital Ethics
Committee, and the experiments were carried out in accordance with
the approved guidelines (Tokushima Clinical Trial Management System
Number, 3215). All patients involved in the study signed the
informed consent form and agreed to participate.
Preoperative immune parameters
Blood samples were collected prior to Hx. The PNI
was calculated as the sum of Alb level and 0.005× lymphocyte count.
The NLR was calculated by dividing neutrophil count by lymphocyte
count. The ALRI was calculated by dividing AST by lymphocyte count.
The cutoff value of the PNI, NLR and ALRI was 45, 2.3 and 30.8,
respectively, calculated by a receiver operating characteristic
curve for predicting recurrence after Hx (Fig. S1).
Assessment of fat mass (FM) and
skeletal muscle mass (SMM)
Preoperative FM and SMM was determined from CT using
Synapse Vincent® (Version 4, Fujifilm Corporation).
Visceral FM (cm2), subcutaneous FM (cm2) and
SMM/height (cm2/m2) were automatically
calculated.
Short-term outcomes after Hx
The short-term outcomes were defined as follows: i)
Operative factors (surgical procedures, surgery time, blood loss
and the presence or absence of transfusion); and ii) postoperative
factors (the presence or absence of complications and hospital stay
duration) according to our previous studies (14,15). The
‘50–50 criteria’ introduced by Balzan et al (16) was used as to determine postoperative
liver failure. Postoperative complications of grade III or IV in
the Clavien-Dindo classification (14,15) were
recorded in the present study.
Follow-up after Hx
The mean follow-up period was 2.48 years (0.02-7.96
years). Monthly follow-up was conducted by ultrasonography and
assessment of the tumor markers α-fetoprotein (AFP),
des-gamma-carboxy prothrombin (DCP) and AFP-L3. Dynamic CT scan and
gadoliniumethoxybenzyl-diethylene-triaminepentaacetic acid-enhanced
MRI were conducted at 3 and 6 months post-surgery. Recurrence was
defined as the appearance of new lesions with radiological features
typical of HCC confirmed by at least two imaging methods. Overall
survival (OS) was defined as the time between Hx and death from any
cause, and disease-free survival (DFS) was defined as the time
between Hx and recurrence.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation. Statistical analysis was performed using SPSS
Version 21.0 statistical software (IBM Corp.). Associations between
the PNI and patient clinicopathological characteristics were
analyzed using the χ2 and Mann-Whitney U tests. Survival
curves were drawn using the Kaplan-Meier method and compared with
the log-rank test. Univariate analysis of differences between two
groups was also determined by log-rank tests. Multivariate analysis
was performed based on the Cox proportional hazards regression
model. The factors included for analyses were patient age (>70
years vs. 70 years), sex (male vs. female), hepatitis B antigen
(absent vs. present), hepatitis C virus antibody (absent vs.
present), AFP (>200 ng/ml vs. ≥200 ng/ml), DCP (<400 mAU/ml
vs. ≥400 mAU/ml), tumor number (single vs. multiple), tumor size
(<3 cm vs. ≥3 cm), tumor differentiation (well- and moderately
vs. poorly differentiated), portal invasion (absent vs. present),
stage (I and II vs. III and IV) and PNI (high vs. low). P<0.05
was considered to indicate a statistically significant
difference.
Results
Comparison of immune parameters among
the PNI, NLR and ALRI
Table I presents the
comparison in of short-term (postoperative factors; frequency of
postoperative complications and hospital stays) and long-term (OS
and DFS) outcomes among patients divided into groups based on the
PNI, NLR or ALRI. Postoperative factors alone are presented as
short-term outcomes for simplicity. The PNI alone was associated
with short-term outcome, and both the PNI and NLR were reliable
parameters for predicting the long-term outcomes (Table I).
 | Table I.Comparison of immune parameters in
short- and long-term outcomes. |
Table I.
Comparison of immune parameters in
short- and long-term outcomes.
|
| Incidence of
complications | Hospital stay | 3-year OS | 3-year DFS |
|---|
|
|
|
|
|
|
|---|
| Parameter | % | P-value | Days | P-value | % | P-value | % | P-value |
|---|
| PNI |
| 0.12 |
| <0.01a |
| 0.06 |
| 0.03a |
|
<45.0 | 8.2 |
| 31±34 |
| 68.4 |
| 38.2 |
|
|
≥45.0 | 2.7 |
| 17±9 |
| 79.1 |
| 53.7 |
|
| NLR |
| 0.98 |
| 0.13 |
| 0.01a |
| 0.04a |
|
<2.3 | 5.7 |
| 21±16 |
| 83.9 |
| 49.5 |
|
|
≥2.3 | 5.6 |
| 27±33 |
| 65.7 |
| 40.5 |
|
| ALRI |
| 0.87 |
| 0.67 |
| 0.25 |
| 0.25 |
|
<30.8 | 6.5 |
| 23±34 |
| 77.8 |
| 51.6 |
|
|
≥30.8 | 5.8 |
| 25±18 |
| 71.5 |
| 41.5 |
|
Associations between the preoperative
PNI and clinicopathological characteristics
Associations between the preoperative PNI values and
clinicopathological characteristics are presented in Table II. A low PNI was significantly
associated with an older age, female sex and impaired liver
function, such as high AST and indocyanine green retention test
values. Among the tumor factors, a low PNI only exhibited a
tendency towards the presence of microscopic portal vein invasion.
In addition, a low PNI was significantly associated with a low SMM
(sarcopenia).
 | Table II.Associations between the preoperative
PNI and patient clinicopathological characteristics. |
Table II.
Associations between the preoperative
PNI and patient clinicopathological characteristics.
|
| Preoperative
PNI |
|
|---|
|
|
|
|
|---|
| Factor | <45 (n=86) | ≥45 (n=76) | P-value |
|---|
| Age, years, mean ±
SD | 70±10 | 60±10 |
<0.01a |
| Sex |
|
| 0.03 |
|
Male | 57 | 62 |
|
|
Female | 29 | 14 |
|
| Hepatitis B
antigen |
|
| 0.66 |
|
Absent | 67 | 57 |
|
|
Present | 19 | 19 |
|
| Hepatitis C virus
antibody |
|
| 0.34 |
|
Absent | 48 | 48 |
|
|
Present | 38 | 28 |
|
| Aspartate
aminotransferase, IU/I, mean ± SD | 60±36 | 42±24 |
<0.01a |
| Prothrombin, %,
mean ± SD | 100±17 | 103±22 | 0.31 |
| Total bilirubin,
mg/dl, mean ± SD | 0.9±0.4 | 0.8±0.3 | 0.19 |
| Indocyanine green
retention test, %, mean ± SD | 16±10 | 11±8 |
<0.01a |
| Platelet count,
×104, mean ± SD | 21±32 | 20±14 | 0.79 |
| Tumor size, cm,
mean ± SD | 4.9±4.5 | 4.1±2.7 | 0.18 |
| Tumor number |
|
| 0.46 |
|
Single | 60 | 57 |
|
|
Multiple | 26 | 19 |
|
| Portal vein
invasion |
|
| 0.06 |
|
Absent | 62 | 65 |
|
|
Present | 24 | 11 |
|
| Intrahepatic
metastasis |
|
| 0.65 |
|
Absent | 79 | 70 |
|
|
Present | 7 | 6 |
|
| Tumor
differentiation |
|
| 0.47 |
| Well-
or moderately differentiated | 17 | 19 |
|
| Poorly
differentiated | 69 | 57 |
|
| Stage |
|
| 0.73 |
| I and
II | 57 | 53 |
|
| III and
IV | 29 | 23 |
|
| AFP, ng/ml, mean ±
SD | 4,385±22,223 | 1,350±5,256 | 0.25 |
| DCP, mAU/ml, mean ±
SD | 9,359±32,458 | 3,172±13,090 | 0.22 |
| BMI, mean ± SD | 22±3 | 23±3 | 0.43 |
| Visceral FM,
cm2, mean ± SD | 76±47 | 87±47 | 0.11 |
| Subcutaneous FM,
cm2, mean ± SD | 108±77 | 108±64 | 0.97 |
| SMM/height,
cm2/m2, mean ± SD | 51±13 | 56±15 | 0.03a |
Preoperative PNI and short-term
outcomes
No significant differences were observed in the
operative procedures between the low and high PNI groups. Blood
loss was significantly higher in the low PNI group compared with
that in the high PNI group. The low PNI group had significantly
longer hospital stays after Hx compared with those in the high PNI
group (Table III).
 | Table III.Associations between the preoperative
PNI and short-term outcomes. |
Table III.
Associations between the preoperative
PNI and short-term outcomes.
| A, Operative
factors |
|---|
|
|---|
|
| Preoperative
PNI |
|
|---|
|
|
|
|
|---|
| Factor | <45 (n=86) | ≥45 (n=76) | P-value |
|---|
| Procedure |
|
| 0.65 |
|
Hr0 | 29 | 17 |
|
| S | 4 | 25 |
|
| 1 | 22 | 18 |
|
| 2 | 10 | 15 |
|
| 3 | 1 | 1 |
|
| Surgery time, min,
mean ± SD | 328±93 | 318±85 | 0.48 |
| Blood loss, m, mean
± SD | 394±433 | 253±224 |
<0.01a |
| Transfusion |
|
| 0.48 |
|
Absent | 75 | 63 |
|
|
Present | 11 | 12 |
|
|
| B, Postoperative
factors |
|
|
| Preoperative
PNI |
|
|
|
|
|
| Factor | <45
(n=86) | ≥45
(n=76) | P-value |
|
| Complications of CD
grade III or IV |
|
| 0.12 |
|
Absent | 79 | 74 |
|
|
Present | 7 | 2 |
|
| Liver failure | 3 | 0 | 0.10 |
| Intra-abdominal
abscess | 3 | 1 | 0.37 |
| Resistant
ascites | 1 | 1 | 0.92 |
| Hospital stay,
days, mean ± SD | 31±34 | 17±9 |
<0.01a |
Preoperative PNI and long-term
outcomes
Patients in the low PNI group exhibited a tendency
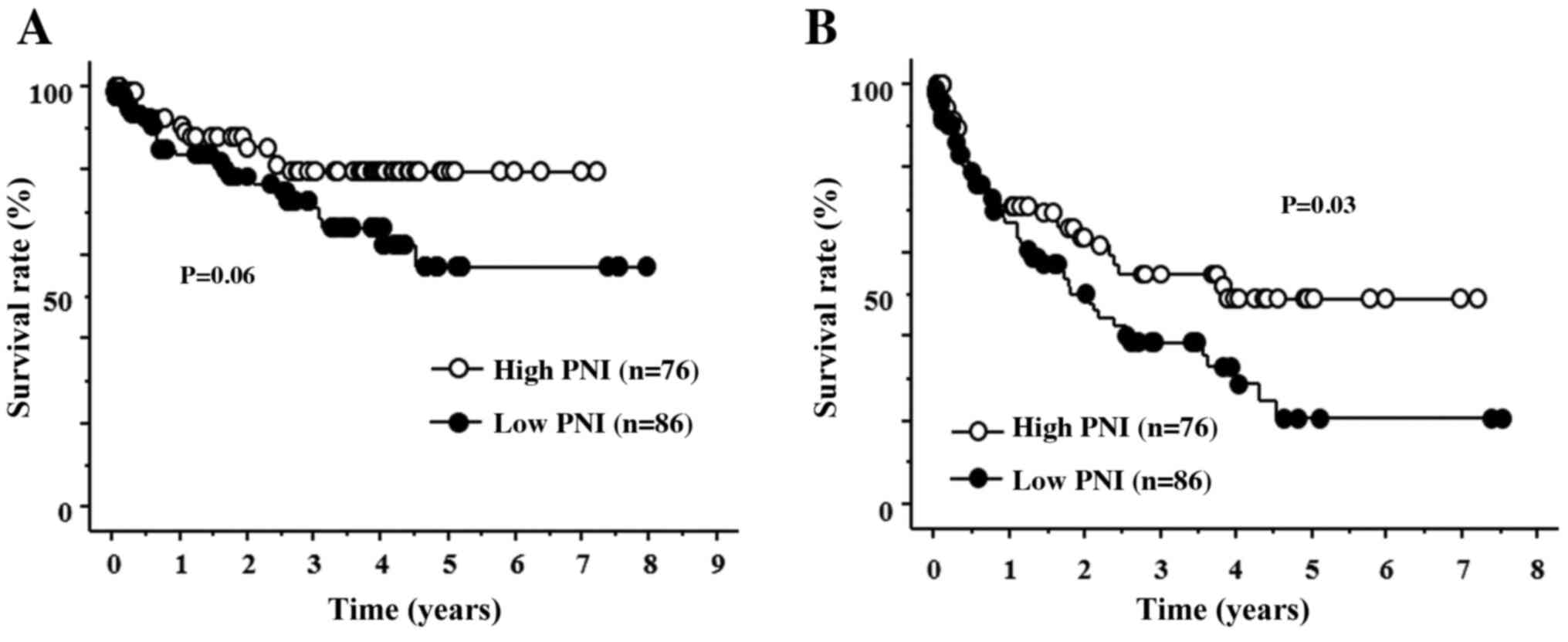
towards a lower OS rate (Fig. 1A).
In addition, patients in the low PNI group had a significantly
poorer DFS compared with that in the high PNI group (Fig. 1B). In the univariate analysis of DFS,
high AFP, high DCP, multiple tumors, the presence of microvascular
invasion, advanced stage and low PNI were identified as indicators
of a poor prognosis. The results of the multivariate analysis
revealed that high AFP, multiple tumors and a low PNI were
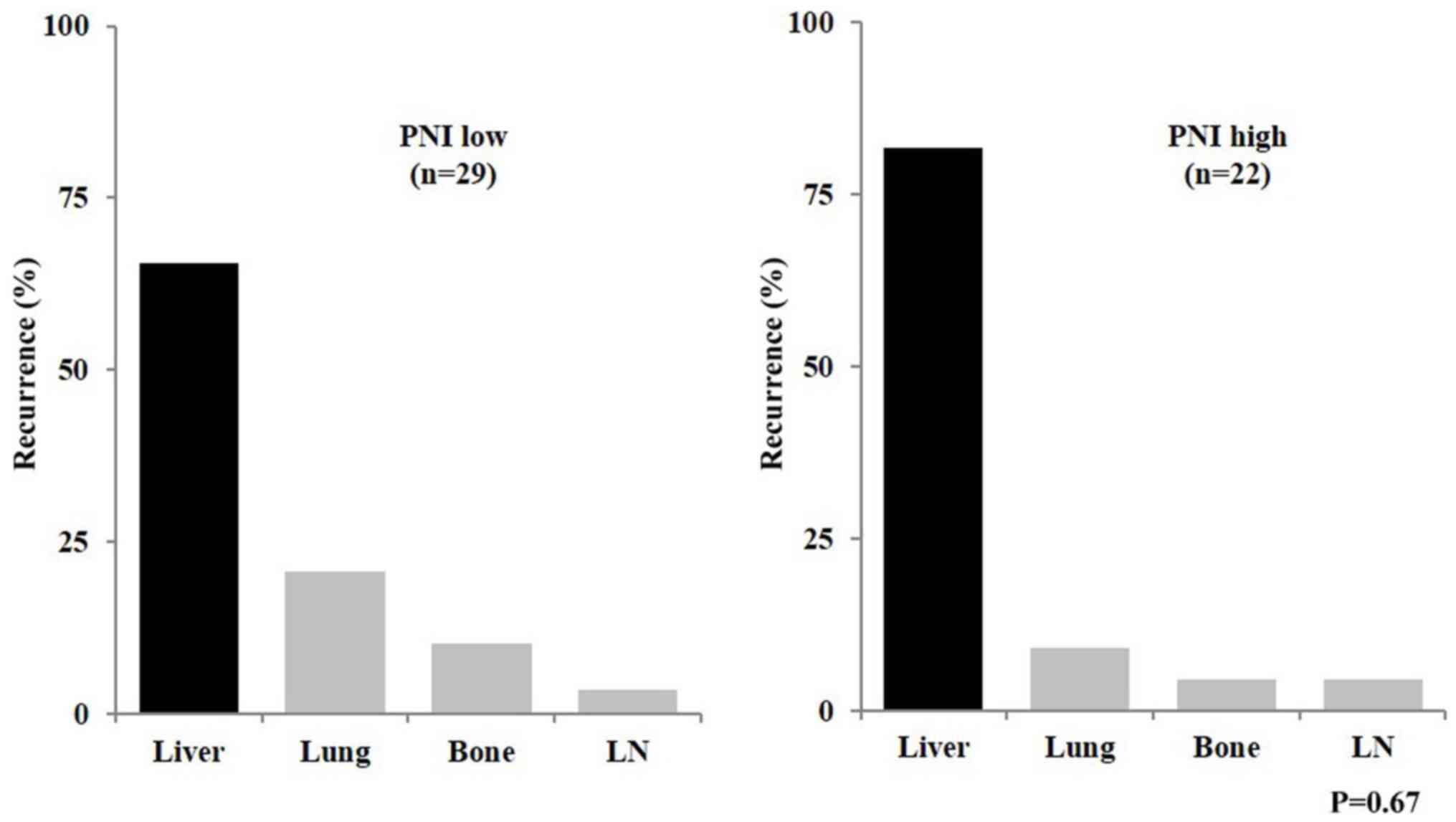
independent prognostic factors for DFS (Table IV). Regarding the recurrence
patterns, no significant differences were observed between the low
and high PNI groups (Fig. 2).
 | Table IV.Univariate and multivariate analysis
of disease-free survival. |
Table IV.
Univariate and multivariate analysis
of disease-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | P-value | Hazard ratio | 95% CI | P-value |
|---|
| AFP ≥200 ng/ml |
<0.01a | 1.77 | 1.00-3.13 | 0.04a |
| DCP ≥400
mAU/ml | 0.04a | 1.04 | 0.59-1.84 | 0.89 |
| Multiple
tumors |
<0.01a | 2.09 | 1.25-3.51 |
<0.01a |
| Portal vein
invasion present |
<0.01a | 1.39 | 0.79-2.44 | 0.25 |
| PNI <45 | 0.03a | 1.65 | 1.00-2.71 | 0.04a |
Discussion
In the present study, a number of immune parameters
including the PNI, NLR and ALRI were compared in terms of short-
and long-term outcomes after Hx in patients with HCC. In addition
to the prediction of outcomes following Hx, the PNI was
significantly associated with a low SMM. Previous studies have
reported similar benefits of the PNI, especially in the prediction
of long-term outcomes after Hx. For example, the preoperative
(13,17) and postoperative (18) PNIs have been reported to be
prognostic factors for OS, especially in early-stage HCC.
Furthermore, a meta-analysis has also demonstrated that
preoperative PNI is a prognostic marker for long-term survival
after not only Hx, but also TACE or non-surgical treatment
(19). By contrast, a limited number
of studies on the prediction of short-term outcomes after Hx are
currently available. One previous report focused on the prognostic
ability of the PNI for postoperative complications after Hx within
the Milan criteria (12). Therefore,
the novelty of the present study compared with the previous ones
was that the prognostic ability of the PNI was analyzed in both
short- and long-term outcomes after Hx, that the analysis included
all stages of HCC, and that the PNI was compared with other immune
parameters including the NLR and ALRI.
Previous studies have reported the use of the NLR as
a prognostic factor in HCC after curative treatment. The usefulness
of the NLR and platelet-to-lymphocyte ratio after transarterial
chemoembolization has been demonstrated (20). Another study has reported that the
NLR predicted disease progression following intra-arterial therapy
of HCC (21). In the present study,
the NLR was a predictor of long-term outcomes after Hx. The
molecular mechanism of the increase in the NLR involves a number of
factors and remains poorly understood. However, an association
between the accumulation of tumor-associated macrophages in HCC and
high NLR values has been observed in patients with HCC who
underwent Hx and living donor liver transplantation (22). A high NLR is also associated with
high levels of infiltration of tumor-associated macrophages and
inflammatory cytokine production in the tumor, such as
interleukin-6, interleukin-8 and interleukin-17, which promote
systemic neutrophilia (23,24).
In the present study, a low PNI was significantly
associated with a longer hospital stays and a poor DFS. In
addition, patients with a low PNI exhibited a poor OS. By contrast,
the NLR was a good prognostic factor predicting long-term outcomes
including both OS and DFS. There were no significant associations
between the NLR and short-term outcomes. This suggests that the
preoperative PNI may be a prognostic factor for evaluating both
short- and long-term outcomes. However, there were insufficient
data in the present study to conclude that the preoperative PNI was
the most significant prognostic factor for evaluating both short-
and long-term outcomes among the PNI, NLR and ALRI.
The PNI value, which is a combination of Alb and the
total lymphocyte count, may be used to evaluate the immunological
and nutritional aspects of patients undergoing surgery. A previous
study has reported that the PNI was established as an indirect
measure of a patient's nutritional status, suggesting that the PNI
may be associated with postoperative complications (12). The level of Alb and the lymphocyte
count have been reported to be closely associated with the
induction of the inflammatory response (25). Therefore, the PNI may not only
reflect the nutritional status, but also systemic inflammation. In
the patient cohort included in the present study, Alb level was
also a good prognostic factor for both OS and DFS (data not shown).
However, no statistically significant differences were observed
between Alb levels and the short-term outcomes (data not shown).
The PNI appeared to be a better prognostic factor compared with Alb
level alone.
Recently, the lymphocyte-to-C-reactive protein ratio
(LCR) was reported as a useful marker for predicting surgical and
oncological outcomes (26). The
present study also investigated the association between the
preoperative LCR and prognosis in the study cohort; a low LCR
exhibited a tendency towards an association with OS (P=0.08), but
not with DFS. There was no significant association between the LCR
and short-term outcomes (data not shown).
A low PNI was not associated with tumor
characteristics such as differentiation, stage or tumor markers in
the present study. A low PNI was associated with female sex, older
age, a poor liver function and low skeletal muscle levels. The same
tendency was observed in previous studies (13,17). We
therefore speculate that the PNI may reflect the immune-nutritional
condition rather than tumor malignancy. Tumor-infiltrating
lymphocytes (TILs) are a specific histological feature of human
cancers, reflecting an individual's immunological tumor response
(27). Previous studies have
reported that TILs may be associated with peripheral blood cells,
such as the TLC and absolute neutrophil count (28,29). In
esophageal cancer, the PNI and TIL score have been reported to be
associated with clinical outcomes (30). Considering the relationship between
the PNI and TILs, nutritional status and systemic immune competence
may affect patient prognosis through local immune response.
For patients with a low PNI, it is essential to
improve their outcomes through perioperative nutritional
interventions, e.g. the administration of branched-chain amino
acid-enriched nutrient support (31,32). In
the present study, the PNI was significantly associated with
sarcopenia; thus, further nutritional intervention may be necessary
for patients with a low PNI.
In conclusion, the results of the present study
demonstrated that a low PNI and sarcopenia reflected a poor
nutritional status in patients with HCC. Furthermore, the
preoperative PNI was a reliable prognostic factor for evaluating
both short- and long-term outcomes after Hx for patients with HCC.
Therefore, nutritional intervention may be beneficial for patients
with HCC with a low preoperative PNI.
Supplementary Material
Supporting Data
Acknowledgements
The results of the present study were reported at
the Clinical Congress of the American College of Surgeons, October
22–26, 2017, in San Diego, CA, USA.
Funding
This study was partly supported by the Research
Program on Hepatitis from the Japan Agency for Medical Research and
Development (grant nos. JP19fk0210048 and JP20fk0210048).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS participated in the study design, performed the
research and data analysis, and wrote the manuscript. SI
participated in the study design and data analysis. YM, TI and MS
participated in the study design. SY and MS participated in data
analysis. MS also critically revised the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by Tokushima University
Hospital Ethics Committee (Tokushima, Japan), and all experiments
were carried out in accordance with the approved guidelines
(Tokushima Clinical Trial Management System Number; 3215). All
patients involved in the study signed the informed consent form and
agreed to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PNI
|
prognostic nutritional index
|
|
Hx
|
liver resection
|
|
HCC
|
hepatocellular carcinoma
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
ALRI
|
aspartate
aminotransferase-to-lymphocyte ratio
|
|
AFP
|
α-fetoprotein
|
|
DCP
|
des-gamma-carboxy prothrombin
|
References
|
1
|
Villanueva A: Hepatocellular Carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harimoto N, Shirabe K, Yamashita YI,
Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y, Nishie A and
Yamanaka T: Sarcopenia as a predictor of prognosis in patients
following hepatectomy for hepatocellular carcinoma. Br J Surg.
100:1523–1530. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Voron T, Tselikas L, Pietrasz D, Pigneur
F, Laurent A, Compagnon P, Salloum C, Luciani A and Azoulay D:
Sarcopenia impacts on short- and long-term results of hepatectomy
for hepatocellular carcinoma. Ann Surg. 261:1173–1183. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okuda Y, Taura K, Yoshino K, Ikeno Y,
Nishio T, Yamamoto G, Tanabe K, Koyama Y, Hatano E, Tanaka S and
Uemoto S: Usefulness of Mac-2 binding protein glycosylation isomer
for prediction of posthepatectomy liver failure in patients with
hepatocellular carcinoma. Ann Surg. 265:1201–1208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buzby GP, Mullen JL, Matthews DC, Hobbs CL
and Rosato EF: Prognostic nutritional index in gastrointestinal
surgery. Am J Surg. 139:160–167. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
7
|
Okada S, Shimada J, Kato D, Tsunezuka H,
Teramukai S and Inoue M: Clinical significance of prognostic
nutritional index after surgical treatment in lung cancer. Ann
Thorac Surg. 104:296–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miao Y, Li S, Yan Q, Li B and Feng Y:
Prognostic significance of preoperative prognostic nutritional
index in epithelial ovarian cancer patients treated with
platinum-based chemotherapy. Oncol Res Treat. 39:712–719. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haraga J, Nakamura K, Omichi C, Nishida T,
Haruma T, Kusumoto T, Seki N, Masuyama H, Katayama N, Kanazawa S
and Hiramatsu Y: Pretreatment prognostic nutritional index is a
significant predictor of prognosis in patients with cervical cancer
treated with concurrent chemoradiotherapy. Mol Clin Oncol.
5:567–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han WX, Chen ZM, Wei ZJ and Xu AM:
Preoperative pre-albumin predicts prognosis of patients after
gastrectomy for adenocarcinoma of esophagogastric junction. World J
Surg Oncol. 14:2792016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Gao P, Chen X, Song Y, Shi J, Zhao
J, Sun J, Xu Y and Wang Z: Prognostic significance of preoperative
prognostic nutritional index in colorectal cancer: Results from a
retrospective cohort study and a meta-analysis. Oncotarget.
7:58543–58552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ke M, Xu T, Li N, Ren Y, Shi A, Lv Y and
He H: Prognostic nutritional index predicts short-term outcomes
after liver resection for hepatocellular carcinoma within the Milan
criteria. Oncotarget. 7:81611–81620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan AW, Chan SL, Wong GL, Wong VW, Chong
CC, Lai PB, Chan HL and To KF: Prognostic nutritional index (pni)
predicts tumor recurrence of very early/early stage hepatocellular
carcinoma after surgical resection. Ann Surg Oncol. 22:4138–4148.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamada S, Shimada M, Morine Y, Imura S,
Ikemoto T, Arakawa Y, Saito Y, Yoshikawa M and Miyazaki K:
Significance of frailty in prognosis after hepatectomy for elderly
patients with hepatocellular carcinoma. Ann Surg Oncol. Jun
19–2020.(Epub ahead of print).
|
|
15
|
Yamada S, Morine Y, Imura S, Ikemoto T,
Arakawa Y, Saito Y, Yoshikawa M, Miyazaki K and Shimada M:
Prognostic prediction of apparent diffusion coefficient obtained by
diffusion-weighted MRI in mass-forming intrahepatic
cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 27:388–395. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balzan S, Belghiti J, Farges O, Ogata S,
Sauvanet A, Delefosse D and Durand F: The ‘50–50 criteria’ on
postoperative day 5: An accurate predictor of liver failure and
death after hepatectomy. Ann Surg. 242:824–829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji F, Liang Y, Fu S, Chen D, Cai X, Li S,
Peng B, Liang L and Hua Y: Prognostic value of combined
preoperative prognostic nutritional index and body mass index in
HCC after hepatectomy. HPB (Oxford). 19:695–705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng W, Li C, Wen TF, Yan LN, Li B, Wang
WT, Yang JY and Xu MQ: Postoperative prognostic nutritional index
change is an independent predictor of survival in patients with
small hepatocellular carcinoma. Am J Surg. 212:122–127. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Man Z, Pang Q, Zhou L, Wang Y, Hu X, Yang
S, Jin H and Liu H: Prognostic significance of preoperative
prognostic nutritional index in hepatocellular carcinoma: A
meta-analysis. HPB (Oxford). 20:888–895. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He CB and Lin XJ: Inflammation scores
predict the survival of patients with hepatocellular carcinoma who
were treated with transarterial chemoembolization and recombinant
human type-5 adenovirus H101. PLoS One. 12:e01747692017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taussig MD, Irene Koran ME, Mouli SK,
Ahmad A, Geevarghese S, Baker JC, Lipnik AJ, Banovac F and Brown
DB: Neutrophil to lymphocyte ratio predicts disease progression
following intra-arterial therapy of hepatocellular carcinoma. HPB
(Oxford). 19:458–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shirabe K, Mano Y, Muto J, Matono R,
Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T,
Taketomi A, et al: Role of tumor-associated macrophages in the
progression of hepatocellular carcinoma. Surg Today. 42:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harimoto N, Shirabe K, Nakagawara H,
Toshima T, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T
and Maehara Y: Prognostic factors affecting survival at recurrence
of hepatocellular carcinoma after living-donor liver
transplantation: With special reference to neutrophil/lymphocyte
ratio. Transplantation. 96:1008–1012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng W, Li C, Wen TF, Yan LN, Li B, Wang
WT, Yang JY and Xu MQ: Neutrophil to lymphocyte ratio changes
predict small hepatocellular carcinoma survival. J Surg Res.
192:402–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koike Y, Miki C, Okugawa Y, Yokoe T,
Toiyama Y, Tanaka K, Inoue Y and Kusunoki M: Preoperative
C-reactive protein as a prognostic and therapeutic marker for
colorectal cancer. J Surg Oncol. 98:540–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okugawa Y, Toiyama Y, Yamamoto A,
Shigemori T, Ide S, Kitajima T, Fujikawa H, Yasuda H, Hiro J,
Yoshiyama S, et al: Lymphocyte-C-reactive protein ratio as
promising new marker for predicting surgical and oncological
outcomes in colorectal cancer. Ann Surg. Aug;2019.(Epub ahead of
print).
|
|
27
|
Ohtani H, Mori K, Nakajima M and Hamaichi
U: Defining lymphocyte-predominant breast cancer by the proportion
of lymphocyte-rich stroma and its significance in routine
histopathological diagnosis. Pathol Int. 65:644–651. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee YY, Choi CH, Sung CO, Do IG, Hub SJ,
Kim HJ, Kim TJ, Lee JW, Bae DS and Kim BG: Clinical significance of
changes in peripheral lymphocyte count after surgery in early
cervical cancer. Gynecol Oncol. 127:107–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoon CI, Park S, Cha YJ, Lee HS, Bae SJ,
Cha C, Lee DY, Ahn SG and Jeong QJ: Associations between absolute
neutrophil count and lymphocyte-predominant breast cancer. Breast.
50:141–148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okadome K, Baba Y, Yagi T, Kiyozumi Y,
Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M and Baba
H: Prognostic nutritional index, tumor-infiltrating lymphocytes,
and prognosis in patients with esophageal cancer. Ann Surg.
271:693–700. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okabayashi T, Nishimori I, Sugimoto T,
Maeda H, Dabanaka K, Onishi S, Kobayashi M and Hanazaki K: Effects
of branched-chain amino acids-enriched nutrient support for
patients undergoing liver resection for hepatocellular carcinoma. J
Gastroenterol Hepatol. 23:1869–1873. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okabayashi T, Nishimori I, Nishioka A,
Yamashita K, Sugimoto T, Dabanaka K, Maeda H, Kohsaki T, Ogawa Y,
Kobayashi M, et al: Long-term effects of multimodal treatment for
patients with resectable carcinoma of the pancreas. Oncol Rep.
20:651–656. 2008.PubMed/NCBI
|