Introduction
Pancreatic adenocarcinoma (PAAD) is an aggressive
type of malignancy, characterized by rapid progression and dismal
prognosis. In most patients, PAAD is unresectable after diagnosis
(1). According to National
Institutes of Health statistics, regardless of tumor stage, the
5-year survival rate of patients with PAAD is only 10% based on
cases of PAAD-associated mortality between 2010 and 2016 (2). PAAD exhibits a limited response to
traditional therapy approaches; however, a number of novel
therapies, including immunotherapy, have been tested in the
clinical trial phase (3).
Unfortunately, the immunosuppressive tumor microenvironment (TME)
limits the efficacy of immunotherapy (4). Therefore, the discovery of novel
biomarkers that characterize TMEs that would be suitable candidates
for immunotherapy is urgently required for the prognostic
assessment of patients with PAAD (5).
UDP-GlcNAc:βGal
β-1,3-N-acetylglucosaminyltransferase 3 (B3GNT3) is a type II
transmembrane protein on the Golgi membrane that acts as the
catalytic center in the synthesis of poly-N-acetyllactosamine
chains and generation of the backbone components of dimeric sialyl
Lewis A (6). Additionally, this gene
serves vital roles in L-selectin ligand synthesis, which is
involved in lymphocyte homing and trafficking. Due to its
biological characteristic, B3GNT3 has been considered to be
involved in the tumorigenesis of non-Hodgkin's lymphoma (7,8). B3GNT3
expression is tissue-selective. It is aberrantly expressed in the
pancreas and distributed throughout the gastrointestinal tract,
liver, placenta, kidney, trachea, neutrophils and lymphocytes
(9,10). However, to the best of our knowledge,
the role of B3GNT3 in the tumorigenesis of PAAD has not been fully
revealed.
Currently, the molecular function of B3GNT3
is controversial. Ho et al (11) reported that B3GNT3 is an independent
predictor for a good prognosis of neuroblastoma via the suppression
of extend core (T-antigen) oligosaccharide formation. However,
other researchers have revealed that B3GNT3 has a negative role in
cancer. For example, Zhang et al (12) claimed that increased expression
levels of B3GNT3 were associated with pelvic lymph node metastasis
and poor prognosis in patients with early-stage cervical cancer.
Furthermore, Gao et al (13)
demonstrated that, among patients who suffer from non-small cell
lung cancer, patients with high B3GNT3 expression have worse
disease-free survival (DFS) time and overall survival (OS) time.
According to another study, B3GNT3 is essential for the epidermal
growth factor-induced communication of receptor programmed cell
death protein-1 (PD-1) and programmed death-ligand 1 in
triple-negative breast cancer (14).
Therefore, the downregulation of B3GNT3 may enhance cytotoxic T
cell-mediated anti-neoplastic effects. Overall, B3GNT3 may have
tumor-promoting and tumor-suppressive effects.
In the present study, The Cancer Genome Atlas (TCGA)
was used to analyze the immunohistochemical (IHC) results of
samples from a single center to evaluate the prognostic value of
B3GNT3 expression in PAAD. Gene Set Enrichment Analysis (GSEA) was
used to gain further insights into the biological pathways involved
in the pathogenesis of PAAD. Furthermore, the association between
B3GNT3 and tumor-infiltrating immune cells in the TME was examined
to identify a probable immunotherapy target in PAAD.
Materials and methods
Data source and clinical
information
The gene expression profiles of PAAD (171 cases;
workflow type, HTSeq-Counts), and the relevant clinical information
and pathologic characteristics were downloaded from TCGA
(https://portal.gdc.cancer.gov; Project
ID, TCGA-PAAD; Table I).
 | Table I.Clinical information from TCGA
database (project ID, TCGA-PAAD). |
Table I.
Clinical information from TCGA
database (project ID, TCGA-PAAD).
| Clinical
characteristics | Total, n
(n=171) | Percentage |
|---|
| Sex |
|
|
|
Male | 93 | 54.4 |
|
Female | 78 | 45.6 |
| Age, years |
|
|
|
≤60 | 57 | 33.3 |
|
>60 | 114 | 66.7 |
| T stage |
|
|
| T1 | 7 |
4.1 |
| T2 | 21 | 12.3 |
|
T3,4 | 141 | 82.5 |
| N stage |
|
|
| N1 | 47 | 27.5 |
| N0 | 119 | 69.6 |
| M stage |
|
|
| M1 | 77 | 45 |
| M0 | 4 | 2.3 |
| Grade |
|
|
| G1 | 28 | 16.4 |
|
G2/3/4 | 141 | 82.5 |
| Histology |
|
|
|
PDAC | 140 | 81.9 |
|
Others | 30 | 17.5 |
| MSIa |
|
|
|
MSI-L | 9 | 5.3 |
|
MSS | 137 | 80.1 |
IHC analysis
To assess the protein expression patterns of B3GNT3
and CD68, IHC staining was performed on 177 paraffin-embedded PAAD
samples (collected from the Department of Pathology, Changhai
Hospital, Navy Medical University, Shanghai, China). The inclusion
criteria were as follows: i) Histological type of adenocarcinoma;
and ii) treatment by surgery. Among 177 cases, 115 were male and 62
were female. The age range was 32–86 years with a mean age of
60.98±10.75 years and a median age of 62 years. All data were
collected between July 2018 and December 2019. The IHC procedures
abided by established protocols (12); however, 16 tissue dots on the
microarrays were missed during the procedure. In brief, fresh
tissues were fixed in 10% formalin for 48 h at room temperature and
then dehydrated in ethanol, cleared in xylene for transparence and
embedded in molten paraffin. The paraffin-embedded tissue block was
sectioned into 4-µm thick slices using a microtome (Leica
Microsystems, Inc.). Paraffin sections were heated, dewaxed in
xylene and hydrated in different concentrations of ethanol (100,
95, 85 and 70%) and washed in PBS buffer (15). The sections were submerged in a
high-pressure cooker filled with EDTA antigenic retrieval buffer
and heated at 110°C for 5–10 min. Endogenous peroxidase activity
was inhibited by incubation with 3% hydrogen peroxide for 25 min,
followed by incubation with goat serum working fluid (undiluted;
cat. no. ZLI-9056; OriGene Technologies, Inc.) to block the
non-specific protein binding for 30 min at 25°C. Subsequently, the
specimens were coated with polyclonal antibodies against B3GNT3
(dilution, 1:100; cat. no. 18098-1-AP; ProteinTech Group, Inc.) and
CD68 (dilution, 1:800; cat. no. 76437; Cell Signaling Technology,
Inc.) (16), and incubated overnight
at 4°C. PBS replaced the primary antibodies as a negative control.
After three washes with PBS with 0.2% Tween-20, the tissue slices
were incubated with a biotinylated anti-rabbit/mouse secondary
antibody working fluid (undiluted; cat. no. PV8000-1; OriGene
Technologies, Inc.) at room temperature (25°C) for 30 min. For
visual staining, 3,3-Diaminobenzidine (cat. no. ZLI-9017; OriGene
Technologies, Inc.) was dripped on the sections. The tissue
sections were then washed with running water, counterstained with
10% Mayer's hematoxylin at room temperature for 1–3 min, dehydrated
in anhydrous ethanol and sealed with a coverslip.
The IHC staining results were observed using a
confocal microscope (Olympus Corporation) and scored independently
by two pathologists blinded to the clinical characteristics in a
semi-quantitative manner. The tissue specimens were scored based on
the proportion of positive cancer cells and staining strength. A
positive reaction was defined as a cell exhibiting brown staining
in the cytoplasm. For B3GNT3 expression scoring, the
staining index was determined as the staining intensity score (0,
negative; 1, weak; 2, moderate; 3, strong) multiplied by the score
for the positive area (0, <5%; 1, 5–25%; 2, 25–50%; 3, 50–75%;
4, >75%). Therefore, the final sores were 0, 1, 2, 3, 4, 6, 8, 9
and 12. For statistical analysis, the scores of 0–1 were considered
negative; 2–4 were considered weak; 6 and 8 were considered
moderate; and 9 and 12 were considered strong. For immune cells,
the proportion of positive cells was counted and the samples were
classified into four groups: 0, <5%; 1, 5–25%; 2, 25–75%; and 3,
>75%.
GSEA
GSEA was performed to investigate the difference in
survival between the high and low B3GNT3 expression groups using
GSEA software (http://www.broadinstitute.org/gsea; version 4.1.0 for
Windows). Gene set permutations were conducted 1,000 times for each
analysis. The expression degree of B3GNT3 was used as a phenotype
marker. The nominal P-value and normalized enrichment score were
used to rank the enrichment pathways in each phenotype.
Tumor Immune Estimation Resource
(TIMER) database analysis
The tumor immune cell infiltration characterization
of PAAD was estimated using data provided by TIMER web portal
(http://cistrome.dfci.harvard.edu/TIMER/). The
correlations among B3GNT3 and different immune cells and associated
gene markers were explored. The related module generated scatter
plots of the expression of a pair of user-defined genes in
pancreatic cancer, as well as the Spearman's correlation analysis
and the estimated statistical significance.
Tumor-Immune System Interaction
Database (TISIDB) analysis
The TISIDB web portal (http://cis.hku.hk/TISIDB) comprises 988 identified
immune-associated oncogenes and antitumor genes, high-throughput
screening techniques, exome and RNA sequencing data, and a variety
of resources for immunological data collected from other public
databases. It facilitates the analysis of the interaction of
certain genes with immunocytes, immunomodulators and cytokines. In
the present study, this database was utilized to investigate the
associations between B3GNT3 expression and lymphocytes and
immunomodulators (15,17).
Statistical analysis
Clinical data and B3GNT3 expression information were
collected from TCGA database and analyzed using R studio
(https://cran.r-project.org/; version
1.2.1335) and SPSS (version 23.0.0; IBM Corp). All data are
presented as the mean ± SD. The association between
clinicopathologic features and B3GNT3 expression was analyzed using
an unpaired t-test. A univariate Cox proportional hazards model was
used to evaluate risk factors associated with the survival of
patients with pancreatic cancer. Subsequently, clinical parameters
with P<0.05 were used in a multivariate Cox proportional hazards
model to assess prognostic factors. The OS time associated with
B3GNT3 expression was examined by Kaplan-Meier analysis to analyze
the diversity between the high- and low-expression groups. The
cut-off value of B3GNT3 expression was determined by its median
value. The log-rank P-value was also computed. Clinical
information, such as survival-associated IHC results, was analyzed
using Cox regression analysis. The correlation analyses of
B3GNT3 and immune infiltration were based on Pearson's
correlation analysis (after tests of normal distribution) and
Spearman's regression analysis. While gene set permutations were
conducted 1,000 times for each analysis, other analyses were
repeated only once. All statistical tests were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
B3GNT3 expression is associated with
survival in PAAD
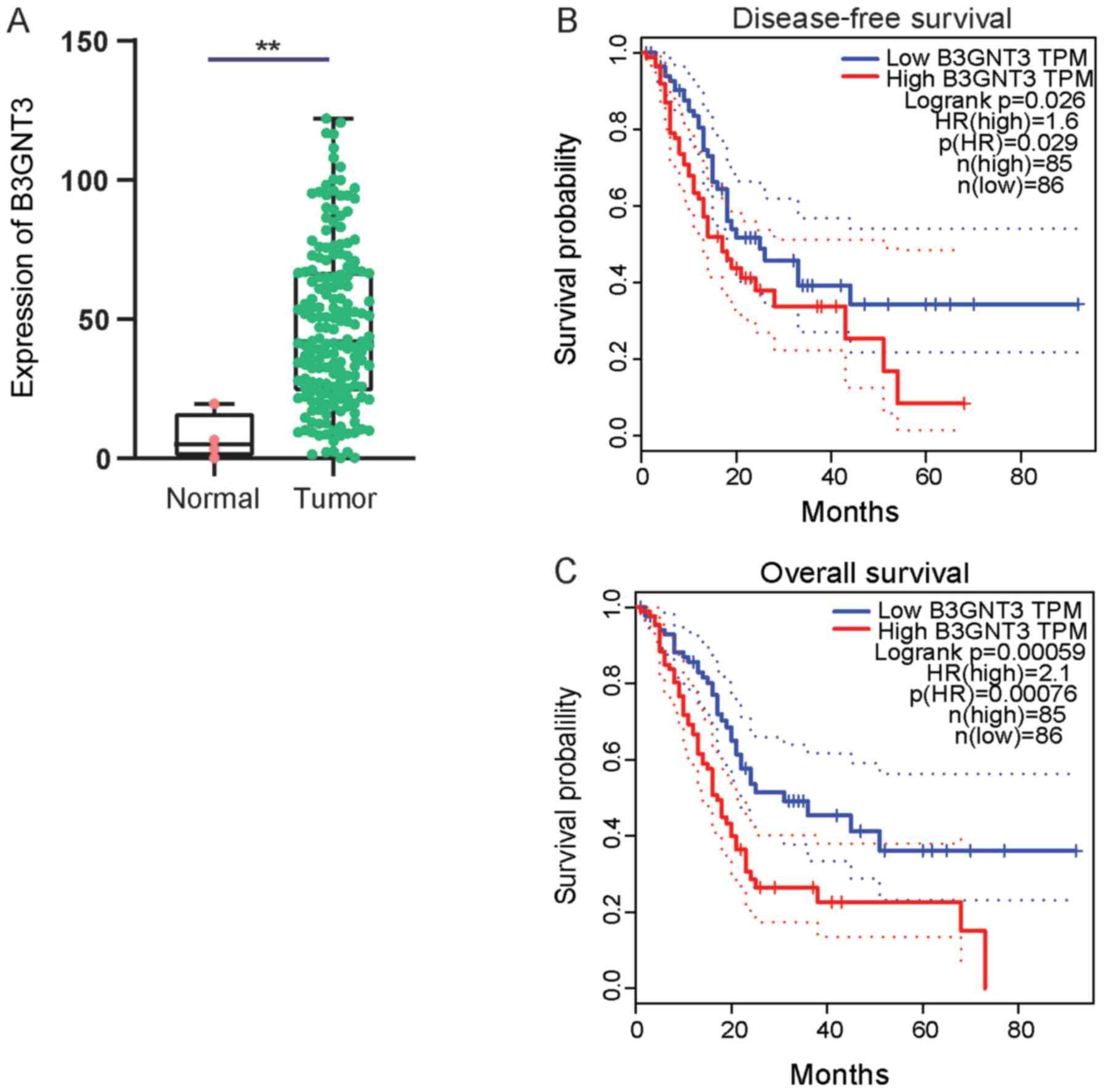
First, B3GNT3 expression in pancreatic cancer was
investigated using TCGA data. A cohort analysis revealed that
B3GNT3 expression was significantly higher in pancreatic ductal
adenocarcinoma tissues than in normal tissues at the mRNA level
(Fig. 1A). Kaplan Meier survival
curves were generated to analyze the association between B3G NT3
expression and the prognosis of the pancreatic cancer cohort using
follow-up information. Patients in the high B3GNT3 expression group
had a shorter OS time and DFS time [OS: Hazard ratio (HR)=2.1,
P=0.00076; DFS: HR=1.6, P=0.029; Fig. 1B
and C].
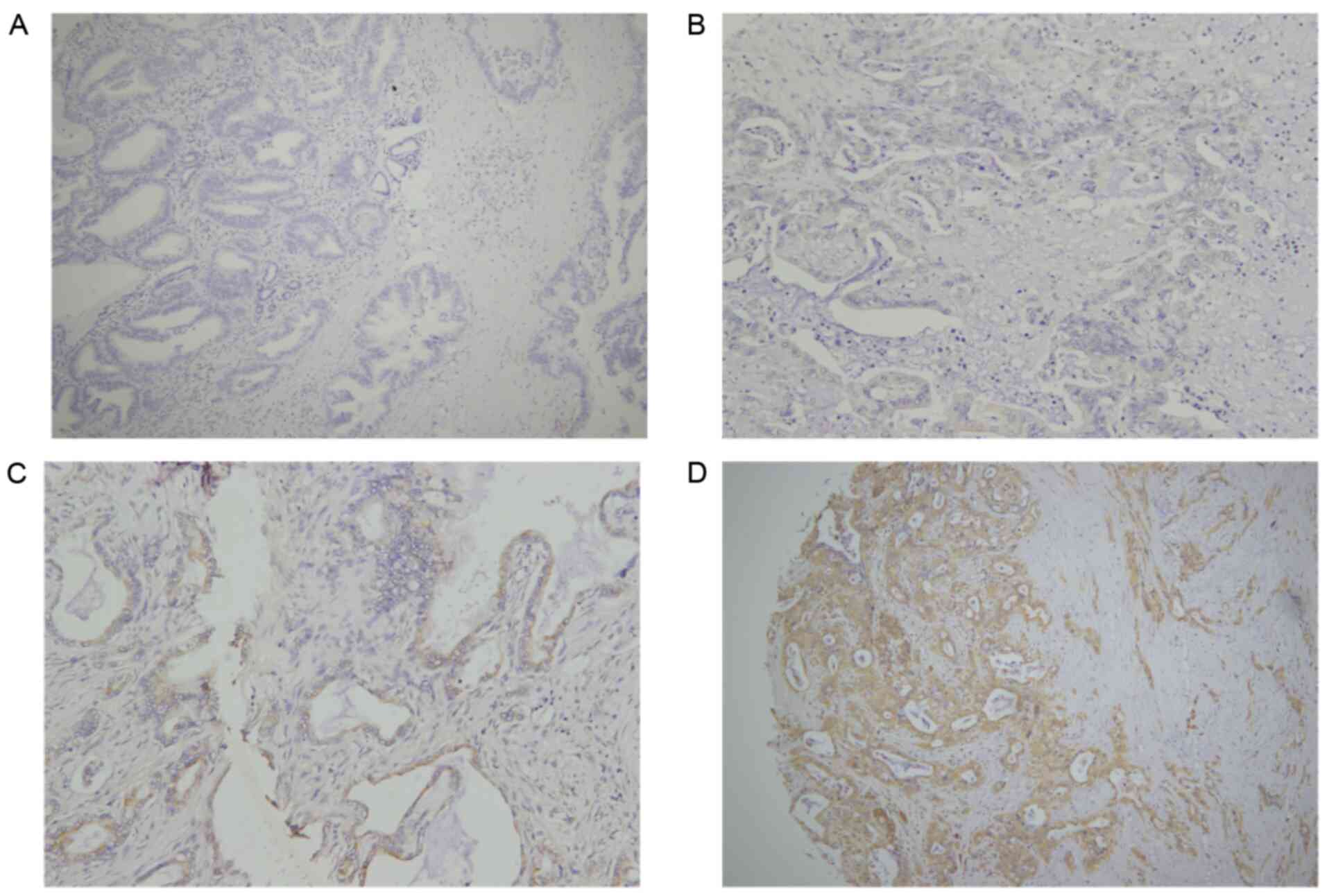
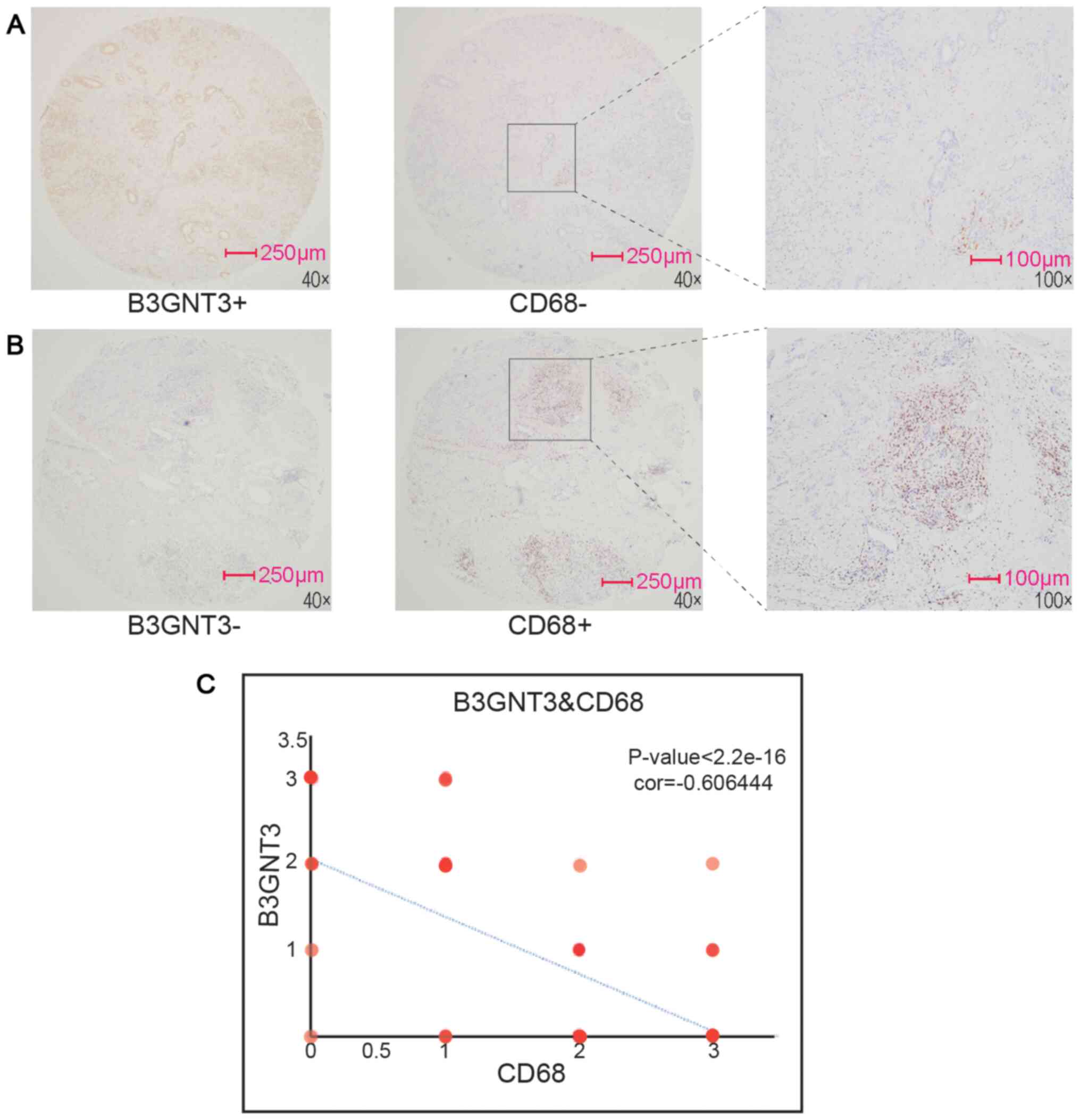
IHC was performed to estimate B3GNT3 protein
expression in a retrospective cohort of 177 pancreatic ductal
adenocarcinoma samples, among which 16 cases were censored.
Immunoreactivity to the B3GNT3 antibody was detected primarily in
the cytoplasm (Fig. 2). The IHC
staining of B3GNT3 was positive in 137 cases, among which 41 cases
(30%) were stained weakly for B3GNT3, 62 (45%) were stained
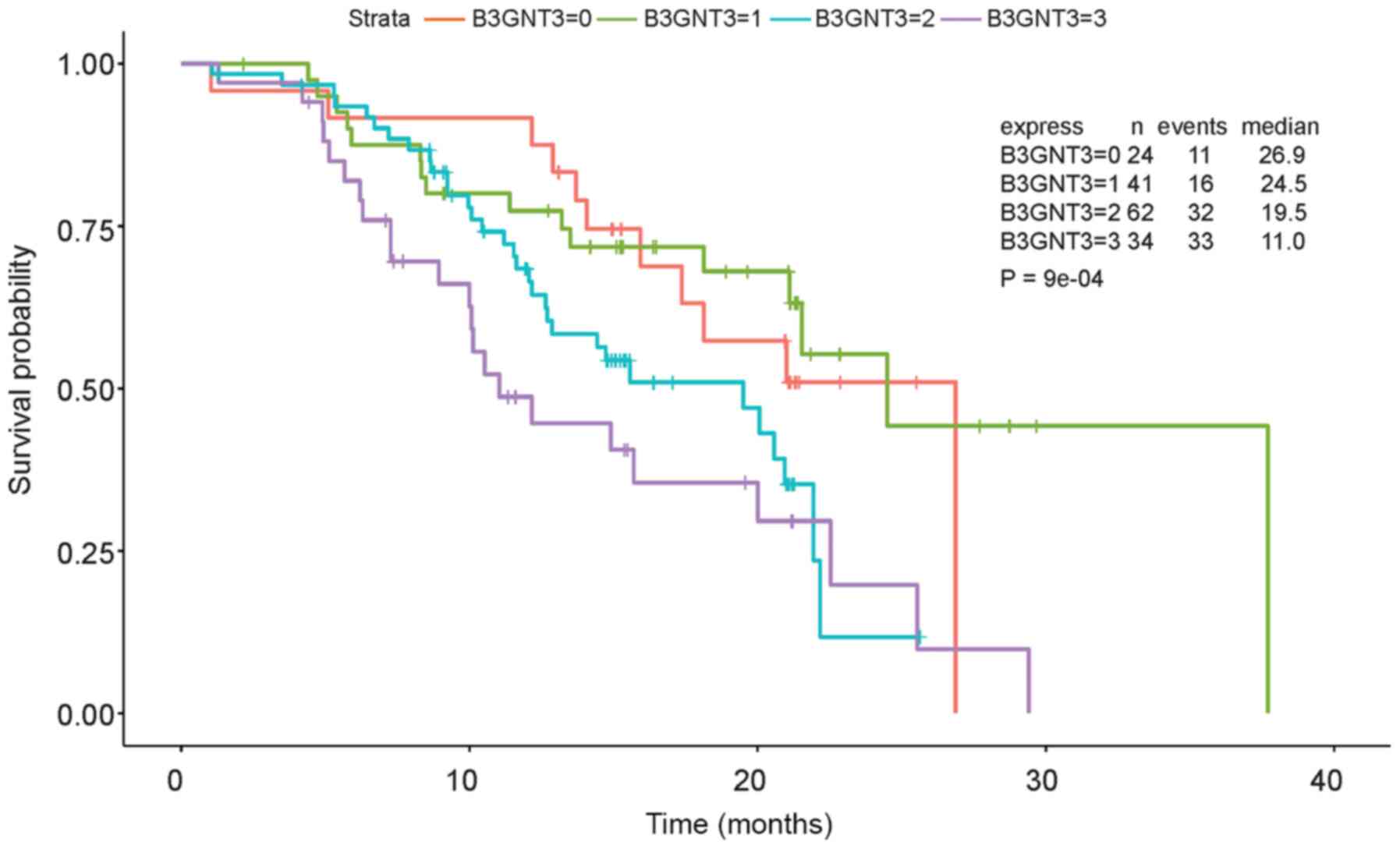
moderately and 34 (25%) were stained strongly. Furthermore, Cox
regression analysis indicated that the group with the highest
expression levels of B3GNT3 had worst outcomes (Fig. 3).
B3GNT3 expression is associated with
clinicopathologic factors
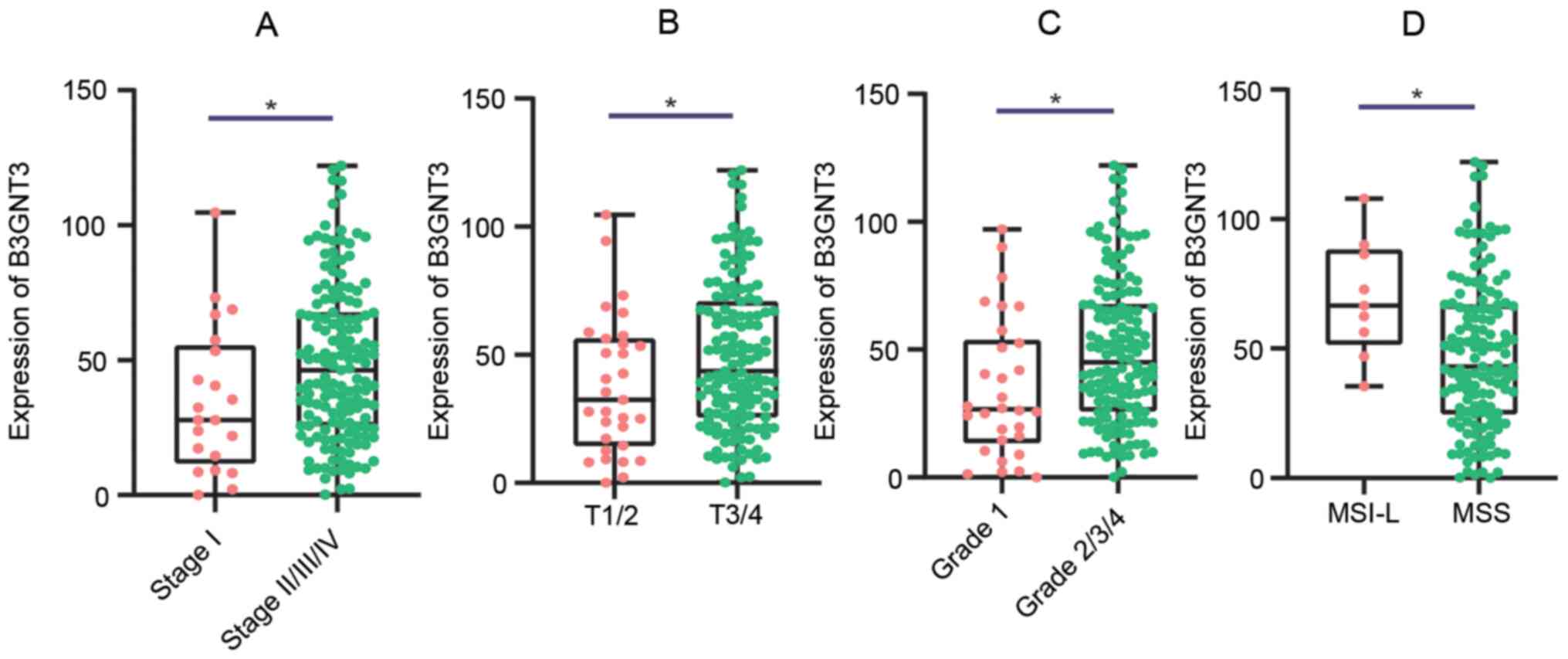
The association between B3GNT3 expression and
the clinicopathological characteristics of patients with PAAD was
analyzed using data from TCGA. High B3GNT3 expression in PAAD was
significantly associated with intraepithelial neoplasia,
tumor/topography (18), stage and
microsatellite instability (Fig. 4).
The univariate analysis revealed that high expression levels of
B3GNT3 were associated with poor OS time in PAAD. Other
clinicopathologic variables associated with poor survival included
high grade, advanced stage, high levels of tumor/topography and
microsatellite instability (Table
II).
 | Table II.Associations between overall survival
and clinicopathologic characteristics in TCGA patients analyzed
using Cox regression analysis. |
Table II.
Associations between overall survival
and clinicopathologic characteristics in TCGA patients analyzed
using Cox regression analysis.
| Clinicopathologic
variable | OR | P-value | 95% CI |
|---|
| Grade (1 vs. grade
2/3/4) | 2.160 | 0.019 | 1.137-4.104 |
| Stage (I vs.
II/III/IV) | 2.283 | 0.038 | 1.046-4.980 |
| Topography (T1/2
vs. T3/4) | 2.021 | 0.030 | 1.071-3.815 |
| MSI (MSI-L vs.
MSS/MSI-H) | 0.450 | 0.046 | 0.206-0.985 |
| B3GNT3 | 2.025 | 0.016 | 1.325-3.093 |
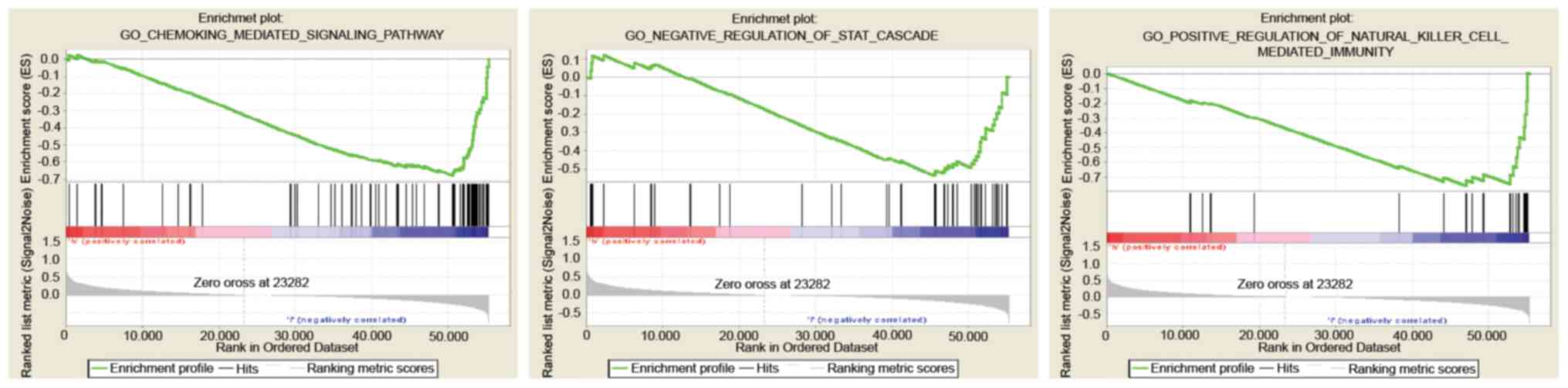
GSEA identification of a
B3GNT3-related signaling pathway
GS EA between the low- and high-expression B3GNT3
datasets was performed to identify differentially activated
signaling pathways in pancreatic cancer (Fig. 5; Table
III).
 | Table III.Gene sets enriched in phenotype
low. |
Table III.
Gene sets enriched in phenotype
low.
| Gene set | NES | NOM P-value | FDR q-val |
|---|
| GO: CHEMOKINE
MEDIATED SIGNALING PATHWAY | −1.89 | 0.006 | 0.16 |
| GO: POSITIVE
REGULATION OF NATURAL KILLER CELL-MEDIATED IMMUNITY | −1.76 | 0.005 | 0.14 |
| GO: NEGATIVE
REGULATION OF STAT CASCADE | −1.73 | 0.002 | 0.14 |
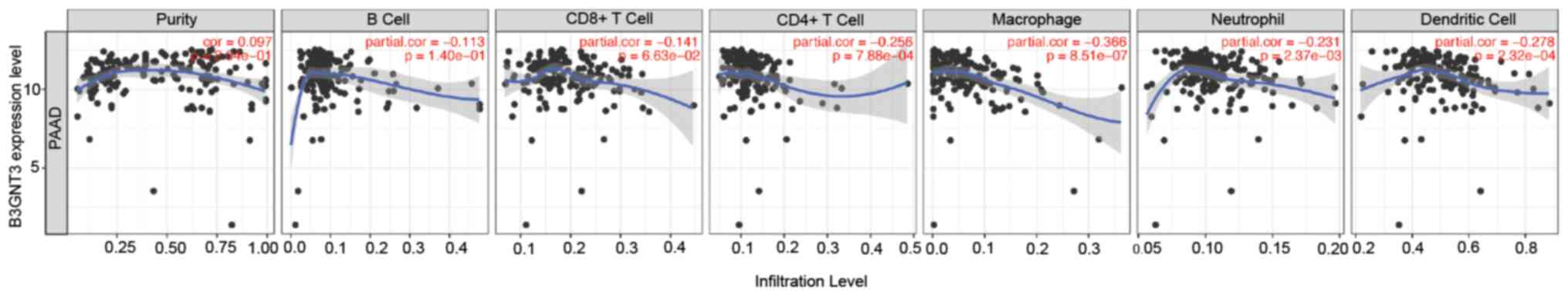
B3GNT3 expression is associated with
immune infiltration levels in PAAD
The association between B3GNT3 expression and immune
infiltration levels in pancreatic cancer was investigated by
assessing the correlations between B3GNT3 expression and tumor
immune infiltration levels using TIMER. B3GNT3 expression and
immune infiltration levels, including CD4+ T cells,
neutrophils, macrophages and dendritic cells, were negatively
correlated in pancreatic cancer (Fig.
6), especially macrophages (|cor|=0.366). To support this
result, CD68, a maker of macrophages, was assessed by
immunohistochemical staining. Spearman's regression analysis was
performed to evaluate the correlation of each molecule with
B3GNT3 expression. The results revealed that the
infiltration of macrophages exhibited a negative correlation with
B3GNT3 expression (Fig.
7).
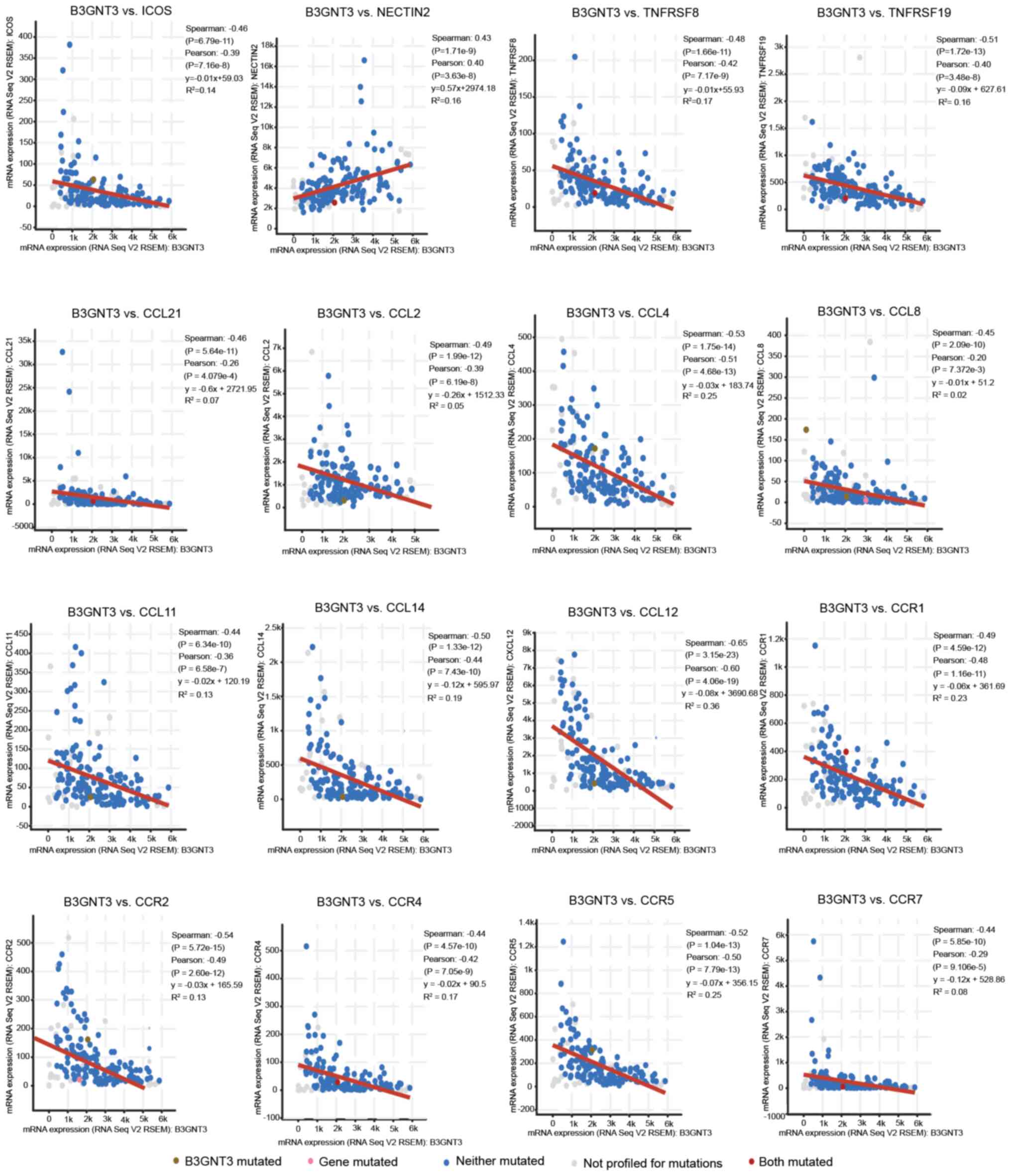
B3GNT3 expression is associated with
immune signatures
The correlation between B3GNT3 expression and
various immune signatures was examined by TISIDB analysis. The
present study focused on tumor-infiltrating immunocytes, immune
inhibitory or stimulatory genes (including immune checkpoint gene
sets) and cytokine-related genes. In Spearman correlation analysis
with filtering for P<0.05 and |±rho|>0.4, B3GNT3 expression
was correlated with a set of immune markers in infiltrating immune
cells of pancreatic cancer, such as inducible co-stimulator (ICOS)
and NECTIN2 (CD112).
The expression levels of immune checkpoint proteins,
such as ICOS, were correlated with B3GNT3 expression.
Furthermore, a positive correlation was observed between
B3GNT3 expression and poliovirus receptor-related 2
(NECTIN2) expression. Nevertheless, members of the tumor
necrosis factor receptor superfamily (TNFRSF), TNFRSF8 (CD30) and
TNFRSF19, exhibited negative correlations with B3GNT3 expression in
pancreatic cancer. Specifically, the expression levels of chemokine
(C-C motif) ligand (CCL)-2, CCL4, CCL8, CCL14, CCL11, CCL21 and CXC
motif chemokine ligand 12, and associated chemokine receptors,
including C-C motif chemokine receptor (CCR)1, CCR2, CCR4, CCR5 and
CCR7, were significantly correlated with B3GNT3 expression
(P<0.001; Fig. 8). Overall, these
results demonstrated the correlation between B3GNT3 and the immune
infiltrating cells in PAAD, which suggests that B3GNT3 has an
important immune escape role in the TME and can be used as a target
for immunotherapy.
Discussion
Studies have demonstrated that B3GNT3 is involved in
non-Hodgkin lymphoma tumorigenesis (7) and the determination of malignant
behaviors (12,13). In the present study, high expression
levels of B3GNT3 in PAAD were positively associated with poor
prognosis and advanced clinicopathological features (e.g., high
grade and clinical staging) according to biomolecular informatics
analysis of high-throughput RNA profiling sequencing data in TCGA.
Univariate analysis revealed that high B3GNT3 expression was
associated with poor OS time for PAAD. Furthermore, B3GNT3
expression was negatively associated with OS time in clinical PAAD
samples from our center.
To further investigate the role of B3GNT3 in
pancreatic cancer, IHC analysis was performed to investigate immune
cell infiltration. B3GNT3 expression was associated with macrophage
infiltration. Similarly, Cerhan et al (7) reported that B3GNT3 is associated with
tumor immunity and inflammation and serves an important role in
lymphocyte migration and transport, leading to the survival and
metastasis of non-Hodgkin's lymphoma tumor cells. Furthermore, the
correlation between B3GNT3 expression and the immune-related genes
suggests the potential regulatory function of B3GNT3 in tumor
immunity in PAAD. Additionally, the present results revealed that
B3GNT3 may inhibit regulatory T cell (Treg) migration, since
the expression levels of B3GNT3 were negatively correlated with the
infiltrating levels of Tregs (ICOS and TNF receptor superfamily
member). ICOS is a standard T cell co-stimulating molecule that
promotes T cell activation via the activation of PI3K signaling
(19). High expression levels of
ICOS predict a favorable survival outcome in esophageal squamous
cell carcinoma (20), gallbladder
cancer (21) and hepatocellular
carcinoma (22). In addition, a
positive correlation was observed between B3GNT3 expression
and poliovirus receptor-related 2 (NECTIN2) expression, the
inhibitory effect of which is mediated by PVR related
immunoglobulin domain containing (23). It has been reported that nectin-2
expression is associated with disease progression and poor
prognosis in patients with pancreatic ductal adenocarcinoma
(24). Nectin-2 expression is
upregulated in breast and ovarian carcinoma and could be a
promising target for antibody therapy (25). It has been reported that nectin-2
expression is associated with disease progression and poor
prognosis in patients with pancreatic ductal adenocarcinoma
(24). GSEA analysis revealed that
B3GNT3 might contribute to the chemokine-mediated activity of the
PI3K signaling pathway, and indicated the negative correlation
between B3GNT3 expression and immunostimulator expression.
CCL2 is required for tumor-associated macrophages to induce immune
evasion (26), to promote cancer
cell progression (27) and invasion
(28). Furthermore, CCL4 is
associated with a T cell-inflamed phenotype in primary and
metastatic pancreatic cancer (29).
Therefore, B3GNT3, as a potential unfavorable prognostic
maker, might contribute to the low infiltration of immune cells and
immunostimulators. For the GSEA analysis, B3GNT3 might
downregulate the frequency, rate or extent of natural killer cell-
and chemokine-mediated immunity. Additionally, STAT is implicated
in a wide range of human cancer types, including pancreatic cancer
(30–32). Therefore, B3GNT3 could
increase the malignant behavior of PAAD via the regulation of the
STAT cascade signaling pathway (33).
In a follow-up study, the investigation of effective
immune inhibitors, such as myeloid-derived suppressor cells,
tumor-associated macrophages and Tregs, particularly in the early
stage of cancer, is required to develop novel immunotherapeutic
agents. The present study demonstrated that B3GNT3 might be
a potential target for prognostic prediction and immune therapy in
patients with pancreatic carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81972282).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and MX conceived and designed the experiments,
and KK, LX and YZ carried out the experiments. HJ and LX analyzed
the data. KK and YZ wrote the manuscript. All the authors discussed
and suggested the experiments. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All the human tissues used in the present study were
microarrays of clinical samples from the study of national Natural
Science Foundation of China (‘The Malignant Biological Behavior and
Mechanism of Pancreatic Ducted Adenocarcinoma Mediated via a Novel
Spliceosome MDA5’; approval no. 81972282). The present study has
passed the ethics review by the Committee on Ethics of Medicine,
Navy Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mizrahi J, Surana R, Valle J and Shroff R:
Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Institute, ; Bethesda M:
SEER cancer stat facts: Pancreatic cancer. Journal. simplehttps://seer.cancer.gov/statfacts/html/pancreas.htmlDecember
17–2020PubMed/NCBI
|
|
3
|
Ngo P, Shanshal M and Rojan A:
Immunotherapy in pancreatic cancer and the importance of tumour
testing. BMJ Case Rep. 13:e2357742020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang S, Li Y, Xing C, Ding C, Zhang H,
Chen L, You L, Dai M and Zhao Y: Tumor microenvironment in
chemoresistance, metastasis and immunotherapy of pancreatic cancer.
Am J Cancer Res. 10:1937–1953. 2020.PubMed/NCBI
|
|
5
|
Christenson E, Jaffee E and Azad N:
Current and emerging therapies for patients with advanced
pancreatic ductal adenocarcinoma: A bright future. Lancet Oncol.
21:e135–e145. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hennet T, Dinter A, Kuhnert P, Mattu TS,
Rudd PM and Berger EG: Genomic cloning and expression of three
murine UDP-galactose: Beta-N-acetylglucosamine
beta1,3-galactosyltransferase genes. J Biol Chem. 273:58–65. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cerhan JR, Ansell SM, Fredericksen ZS, Kay
NE, Liebow M, Call TG, Dogan A, Cunningham JM, Wang AH, Liu-Mares
W, et al: Genetic variation in 1253 immune and inflammation genes
and risk of non-Hodgkin lymphoma. Blood. 110:4455–4463. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeh JC, Hiraoka N, Petryniak B, Nakayama
J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB and Fukuda
M: Novel sulfated lymphocyte homing receptors and their control by
a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell.
105:957–969. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shiraishi N, Natsume A, Togayachi A, Endo
T, Akashima T, Yamada Y, Imai N, Nakagawa S, Koizumi S, Sekine S,
et al: Identification and characterization of three novel beta
1,3-N-acetylglucosaminyltransferases structurally related to the
beta 1,3-galactosyltransferase family. J Biol Chem. 276:3498–3507.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haider S, Wang J, Nagano A, Desai A,
Arumugam P, Dumartin L, Fitzgibbon J, Hagemann T, Marshall JF,
Kocher HM, et al: A multi-gene signature predicts outcome in
patients with pancreatic ductal adenocarcinoma. Genome Med.
6:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ho WL, Che MI, Chou CH, Chang HH, Jeng YM,
Hsu WM, Lin KH and Huang MC: B3GNT3 expression suppresses cell
migration and invasion and predicts favorable outcomes in
neuroblastoma. Cancer Sci. 104:1600–1608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Hou T, Niu C, Song L and Zhang Y:
B3GNT3 expression is a novel marker correlated with pelvic lymph
node metastasis and poor clinical outcome in early-stage cervical
cancer. PLoS One. 10:e01443602015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao L, Zhang H, Zhang B, Zhu J, Chen C and
Liu W: B3GNT3 overexpression is associated with unfavourable
survival in non-small cell lung cancer. J Clin Pathol. 71:642–647.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li CW, Lim SO, Chung EM, Kim YS, Park AH,
Yao J, Cha JH, Xia W, Chan LC, Kim T, et al: Eradication of
triple-negative breast cancer cells by targeting glycosylated
PD-L1. Cancer Cell. 33:187–201 e110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wester K, Wahlund E, Sundstrom C, Ranefall
P, Bengtsson E, Russell PJ, Ow KT, Malmström PU and Busch C:
Paraffin section storage and immunohistochemistry. Effects of time,
temperature, fixation, and retrieval protocol with emphasis on p53
protein and MIB1 antigen. Appl Immunohistochem Mol Morphol.
8:61–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lucca LE, Lerner BA, Park C, DeBartolo D,
Harnett B, Kumar VP, Ponath G, Raddassi K, Huttner A, Hafler DA and
Pitt D: Differential expression of the T-cell inhibitor TIGIT in
glioblastoma and MS. Neurol Neuroimmunol Neuroinflamm. 7:e7122020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ru B, Wong CN, Tong Y, Zhong JY, Zhong
SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, et al: TISIDB: An
integrated repository portal for tumor-immune system interactions.
Bioinformatics. 35:4200–4202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Roessel S, Kasumova G, Verheij J,
Najarian RM, Maggino L, de Pastena M, Malleo G, Marchegiani G,
Salvia R, Ng SC, et al: International validation of the eighth
edition of the american joint committee on cancer (AJCC) TNM
staging system in patients with resected pancreatic cancer. JAMA
Surg. 153:e1836172018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Fu T, Suh WK, Tsavachidou D, Wen
S, Gao J, Tang DN, He Q, Sun J and Sharma P: CD4 T cells require
ICOS-mediated PI3K signaling to increase T-Bet expression in the
setting of anti-CTLA-4 therapy. Cancer Immunol Res. 2:167–176.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong MH, Shin SJ, Shin SK, Kim DJ, Zo JI,
Shim YM, Lee SE, Cho BC, Park SY, Choi YL and Kim HR: High CD3 and
ICOS and low TIM-3 expression predict favourable survival in
resected oesophageal squamous cell carcinoma. Sci Rep. 9:201972019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, He M, Shi W, Sha H, Feng J, Wang S
and Wang Y: Inducible costimulator (ICOS) enhances the cytolytic
activity of cytokine-induced killer cells against gallbladder
cancer in vitro and in vivo. Cancer Invest. 27:244–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Liu J, Chen Y, Tang W, Bo K, Sun Y
and Chen J: Investigation of ICOS, CD28 and CD80 polymorphisms with
the risk of hepatocellular carcinoma: A case-control study in
eastern Chinese population. Biosci Rep. 39:BSR201818242019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whelan S, Ophir E, Kotturi MF, Levy O,
Ganguly S, Leung L, Vaknin I, Kumar S, Dassa L, Hansen K, et al:
PVRIG and PVRL2 are induced in cancer and inhibit CD8+
T-cell function. Cancer Immunol Res. 7:257–268. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang S, Yang Z, Li D, Miao X, Yang L, Zou
Q and Yuan Y: The clinical and pathological significance of
nectin-2 and DDX3 expression in pancreatic ductal adenocarcinomas.
Dis Markers. 2015:3795682015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oshima T, Sato S, Kato J, Ito Y, Watanabe
T, Tsuji I, Hori A, Kurokawa T and Kokubo T: Nectin-2 is a
potential target for antibody therapy of breast and ovarian
cancers. Molecular Cancer. 12:602013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Zhang Q, Xu M, Wang L, Chen X,
Feng Y, Li Y, Zhang X, Cui W and Jia X: CCL2-CCR2 axis recruits
tumor associated macrophages to induce immune evasion through PD-1
signaling in esophageal carcinogenesis. Mol Cancer. 19:412020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Q, Song J, Pan Y, Shi D, Yang C, Wang
S and Xiong B: Wnt5a/CaMKII/ERK/CCL2 axis is required for
tumor-associated macrophages to promote colorectal cancer
progression. Int J Biol Sci. 16:1023–1034. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He M, Yu W, Chang C, Miyamoto H, Liu X,
Jiang K and Yeh S: Estrogen receptor α promotes lung cancer cell
invasion via increase of and cross-talk with infiltrated
macrophages through the CCL2/CCR2/MMP9 and CXCL12/CXCR4 signaling
pathways. Mol Oncol. 14:1779–1799. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Romero JM, Grunwald B, Jang GH, Bavi PP,
Jhaveri A, Masoomian M, Fischer SE, Zhang A, Denroche RE, Lungu IM,
et al: A four-chemokine signature is associated with a
T-cell-inflamed phenotype in primary and metastatic pancreatic
cancer. Clin Cancer Res. 26:1997–2010. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ko HJ and Kim YJ: Signal transducer and
activator of transcription proteins: Regulators of myeloid-derived
suppressor cell-mediated immunosuppression in cancer. Arch Pharm
Res. 39:1597–1608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim BH, Yi EH and Ye SK: Signal transducer
and activator of transcription 3 as a therapeutic target for cancer
and the tumor microenvironment. Arch Pharm Res. 39:1085–1099. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao
AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL and Xie K: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scholz A, Heinze S, Detjen KM, Peters M,
Welzel M, Hauff P, Schirner M, Wiedenmann B and Rosewicz S:
Activated signal transducer and activator of transcription 3
(STAT3) supports the malignant phenotype of human pancreatic
cancer. Gastroenterology. 125:891–905. 2003. View Article : Google Scholar : PubMed/NCBI
|