Introduction
Acute myeloid leukemia (AML) is a malignant clonal
disorder that originates from hematopoietic stem cells by
uncontrolled proliferation and suppressed differentiation of blast
cells in the myeloid lineage, which infiltrate the bone marrow,
blood and other tissues, including lymph node or spleen (1). AML is the most common type of acute
leukemia in adults, which accounts for 1.3% of new cancer cases
annually in the United states (2).
AML can occur in any age group; however, the most affected patients
are older adults, with a median age at diagnosis of 68 years
(2). It is estimated that 10–40% of
patients newly diagnosed with AML fail to achieve complete
remission (CR) with the standard induction chemotherapy treatment,
and these cases are defined as primary refractory cases (3). In addition, most patients who achieve
CR will eventually relapse because of unfavorable cytogenetics at
diagnosis and old age (4,5). Patients with relapsed/refractory (R/R)
AML have a poor prognosis, with a 3-year overall survival (OS) rate
of only 10%, thus the majority of patients die from recurrence
(5,6).
Currently, there is no standard treatment regimen
for patients with R/R AML, except for allogeneic hematopoietic stem
cell transplantation (allo-HSCT) (6,7). Most
R/R AML patients are old and have less than ideal performance
status or without a matched related donor, so only a minority of
patients are suitable for allo-HSCT (5). Thus, it remains critical to identify
early prognostic factors and select the appropriate conditioning
regimens for allo-HSCT for the effective treatment of patients with
AML.
Cladribine is a deoxyadenosine analogue that can be
rapidly phosphorylated into a triphosphate form, which resists
degradation by adenosine deaminase and increases cytotoxic levels
in the intracellular space, inhibits DNA synthesis and induces cell
apoptosis (8). Previous studies have
demonstrated that cladribine combination regimens are effective in
patients with R/R AML and may overcome abnormal chromosome
karyotypes with poor prognosis (8,9). In
addition, cladribine-based chemotherapy regimens sequential with
allo-HSCT are considered promising treatment strategies for
patients with R/R AML (10,11).
Conventional cytogenetic data has established
prognostic indications for patients with AML (2). Based on karyotypic analysis, recurring
translocations (t) or inversions (inv) or deletion (del), including
t(6;9), t(v.11q23. 3), t(9;22), inv(3), t(3;3), −5, del(5q), −7 and
−17 are associated with adverse risk of AML, designated by the
World Health Organization (2).
t(11;19)(q23;p13) is a rare recurrent cytogenetic abnormality in
patients with AML; however, its clinical and genetic
characteristics are not yet fully understood (12). Previous studies have reported that
the prognosis of patients with AML, with t(11;19)(q23;p13) is poor,
with a median OS time <1 year (13,14). It
has been reported that CLAM (cladribine, cytarabine, mitoxantrone)
and MEC (mitoxantrone, etoposide, and cytarabine) chemotherapy
regimens exhibit promising activity in patients with R/R AML
(15,16), thus, CLAM was selected as the regimen
in the present study. The present study reported two relapsed
patients with t(11;19)(q23;p13) AML who were successfully treated
with cladribine, cytarabine and etoposide (CLAE) chemotherapy
sequential with allo-HSCT.
Case reports
The present study was approved by the Institutional
Review Board of The Affiliated Huai'an No. 1 People's Hospital of
Nanjing Medical University (Huai'an, China; approval no.
YX-P-2020-004-01) and performed in accordance with the Declaration
of Helsinki (17). Written informed
consent was provided by both patients prior to the study start.
Case 1
On December 26, 2018, a 23-year-old man was admitted
to The Affiliated Huai'an No. 1 People's Hospital of Nanjing
Medical University due to stomachaches and experiencing weakness.
Peripheral blood analysis revealed the following: White blood cell
count of 70.85×109/l, hemoglobin level of 114 g/l and a
platelet count of 26×109/l. In addition, the bone marrow
(BM) smear revealed 42% of blast cells. Immunophenotype analysis
indicated that 33% of the blast cells were abnormal, and
demonstrated positive labeling for myeloperoxidase (MPO), cluster
of differentiation (CD)13, CD33, CD34, CD38 and CD117. The
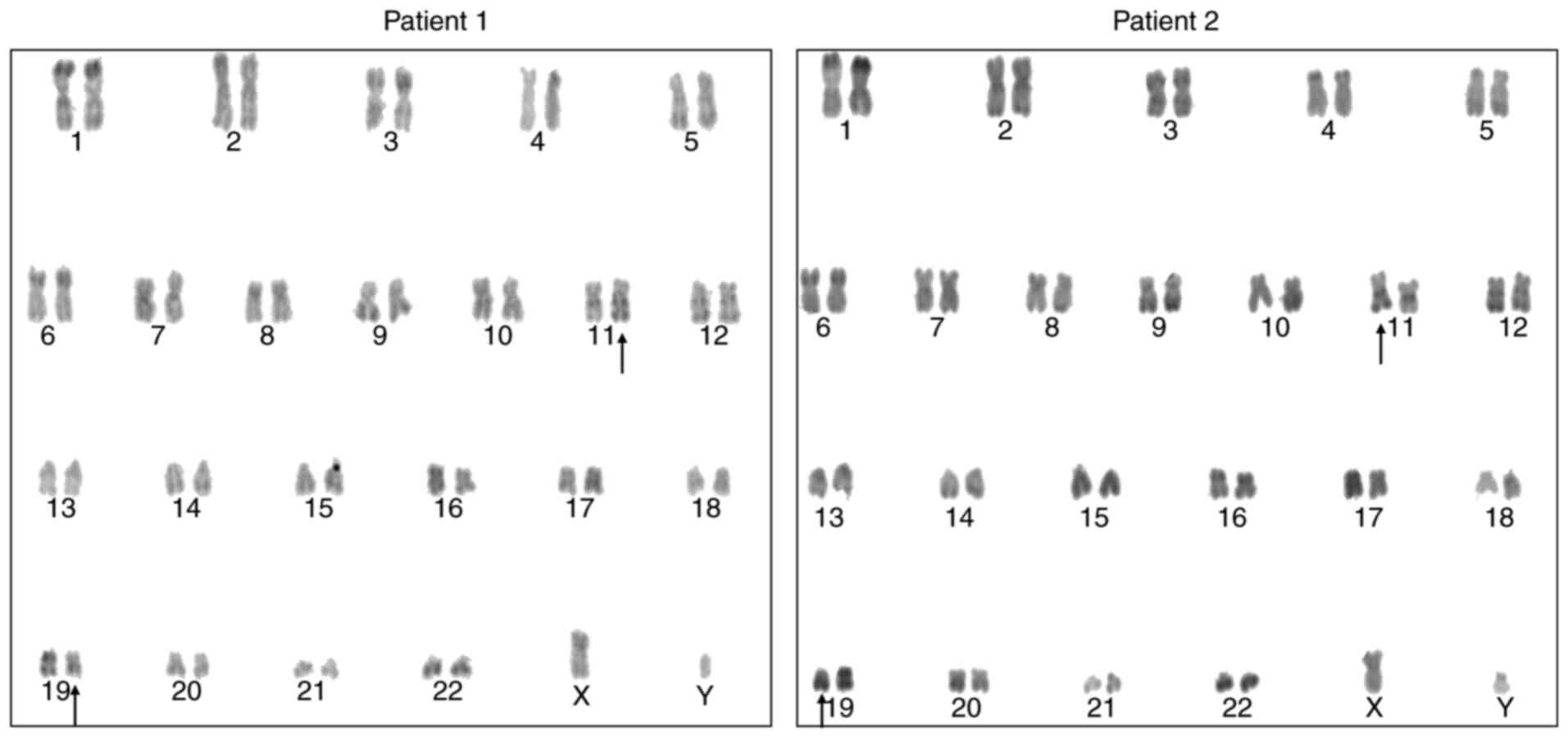
karyotype was 46,XY,t(11;19)(q23;p13)[17]/46,XY[3] (Fig. 1). Aberrant EVI1 and
NRAS expression levels were detected (mutation frequency,
8.32%) via reverse transcription (RT)-PCR and sanger sequencing
analyses, while fusion genes were detected via RT-PCR analysis.
Total RNA was extracted using the Omega whole-blood RNA extraction
kit (cat. no. R6616-02; Omega Bio-Tek, Inc.), according to the
manufacturer's protocol. Nested RT-PCR analysis was performed, and
the reaction system was used as previously described (18,19).
AML-associated mutated genes were detected using high-throughput
sequencing technology. Total DNA was extracted using the whole
blood DNA extraction kit (cat. no. D3392-02; Omega Bio-Tek, Inc.),
and genetic mutations were detected by the Kindstar Global Medical
Laboratory Center (http://www.kindstar.com.cn/kindstar/cn/platform.html),
using sanger sequencing as previously described (19,20).
Notably, MLL rearrangement was not detected. The patient was
diagnosed with AML (M4) according to the FAB (French American
British) classification system (21). The patient was treated with standard
‘3+7’ regimen with idarubicin and cytarabine (IA) as induction
therapy and achieved CR, with 1.5% blasts after one cycle. The
minimal residual disease (MRD) detection via flow cytometric (FCM)
analysis was negative (<0.01%) and was followed by three cycles
of consolidation (intermediate dose cytarabine with 2.0
g/m2 × 6 times). Following the third consolidation
chemotherapy, MRD detection was positive, with 6% abnormal cells
expressing CD13, CD33 and CD34 via FCM analysis, and the BM smear
indicated relapse (8.5% of blast cells; Fig. 2). The patient received one cycle of
homoharringtonine, daunorubicin and cytarabine reinduction
chemotherapy regimen; however, he failed to achieve CR. The BM
blasts were 11.5% and the MRD was 10.5%. The patient subsequently
received intense CLAE chemotherapy (cladribine 5
mg/m2/dx5d, cytarabine 1.5 g/m2/dx5d and
etoposide 100 mg/m2/dx3d), and underwent haploid
allo-HSCT (stem cells from his father) 3 days after CLAE
chemotherapy, with the conditioning regimen of Flu-Bu-ATG
(fludarabine 30 mg/m2/dx5d, busulfan 3.2 mg/kg/dx3d and
ATG 2.5 mg/kg/dx4d). Graft-versus-host diseases (GVHDs) were
prevented by cyclosporin A, methotrexate and mycophenolate mofetil
(Fig. 3). A total of
12.5×108/kg mononuclear cells and 2.75×106/kg
CD34+ cells from the donor were intravenously injected
into the patient, and no serious complications occurred during
allo-HSCT. The neutrophil and platelet engraftments were achieved
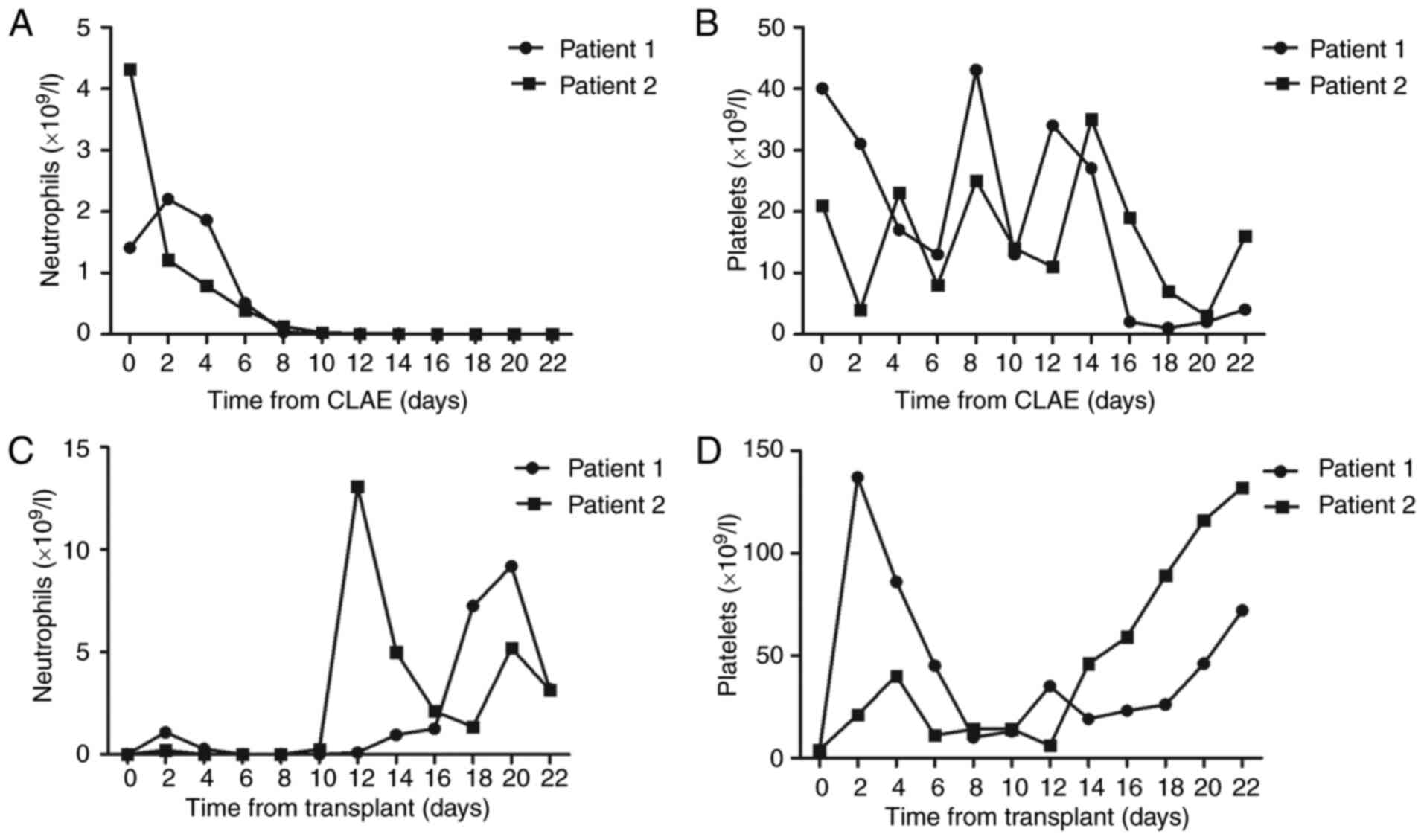
on days 14 and 12, respectively (Fig. 4C
and D). The MRD was 10−4 as detected via FCM
analysis, and the chromosome changed to normal karyotype 1 month
after transplantation. The donor chimerism rate was 100% following
multiplex PCR analysis of short tandem repeats at +14 days, +30
days and +60 days after transplantation. The decitabine regimen
(decitabine 15 mg/m2/dx5d) was implemented every 3
months for the prophylaxis of relapse, beginning at 2 months after
allo-HSCT. The patient received follow-up in the clinic weekly and
remained in a disease-free survival state based on the last
follow-up at 4.3 months after allo-HSCT.
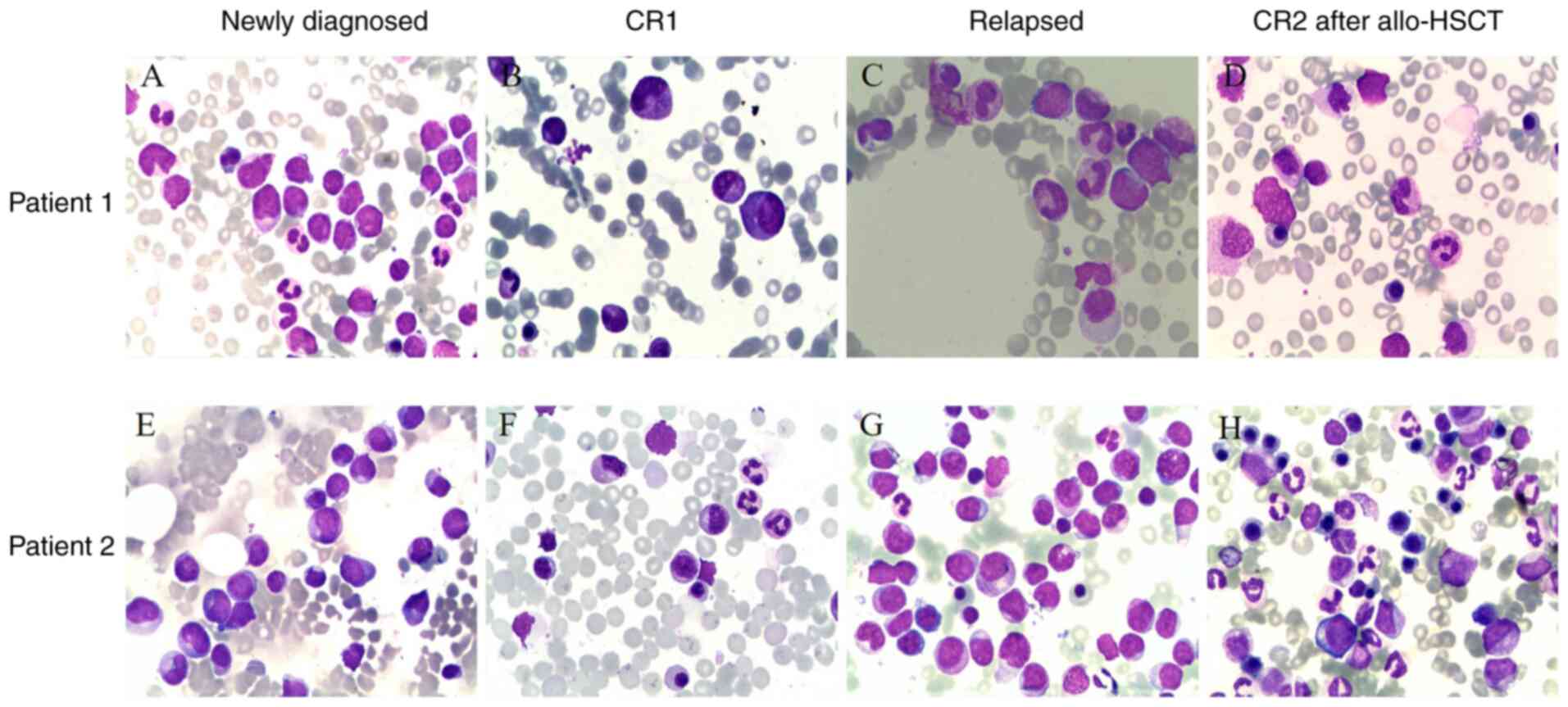 | Figure 2.Morphological features of two
patients with AML at different stages of the disease.
May-Grüwald-Giemsa-stained BM smear (magnification, ×1,000).
Patient 1: (A) Morphological features of BM at the newly diagnosed
stage, granulocytes increased and myeloblasts accounted for 42%.
The positive rate of myeloperoxidase staining was 76%. (B)
Morphological features following induction chemotherapy. (C)
Morphological features at the relapsed stage, myeloblasts accounted
for 9%. (D) Morphological features following allo-HSCT. Patient 2:
(E) Morphological features of BM at the newly diagnosed stage,
granulocytes increased and myeloblasts accounted for 28%. The
positive rate of myeloperoxidase staining was 92%. (F)
Morphological features following induction chemotherapy. (G)
Morphological features at the relapsed stage, myeloblasts accounted
for 58%. (H) Morphological features following allo-HSCT. AML, acute
myeloid leukemia; BM, bone marrow; allo-HSCT, allogeneic
hematopoietic stem cell transplantation; CR, complete
remission. |
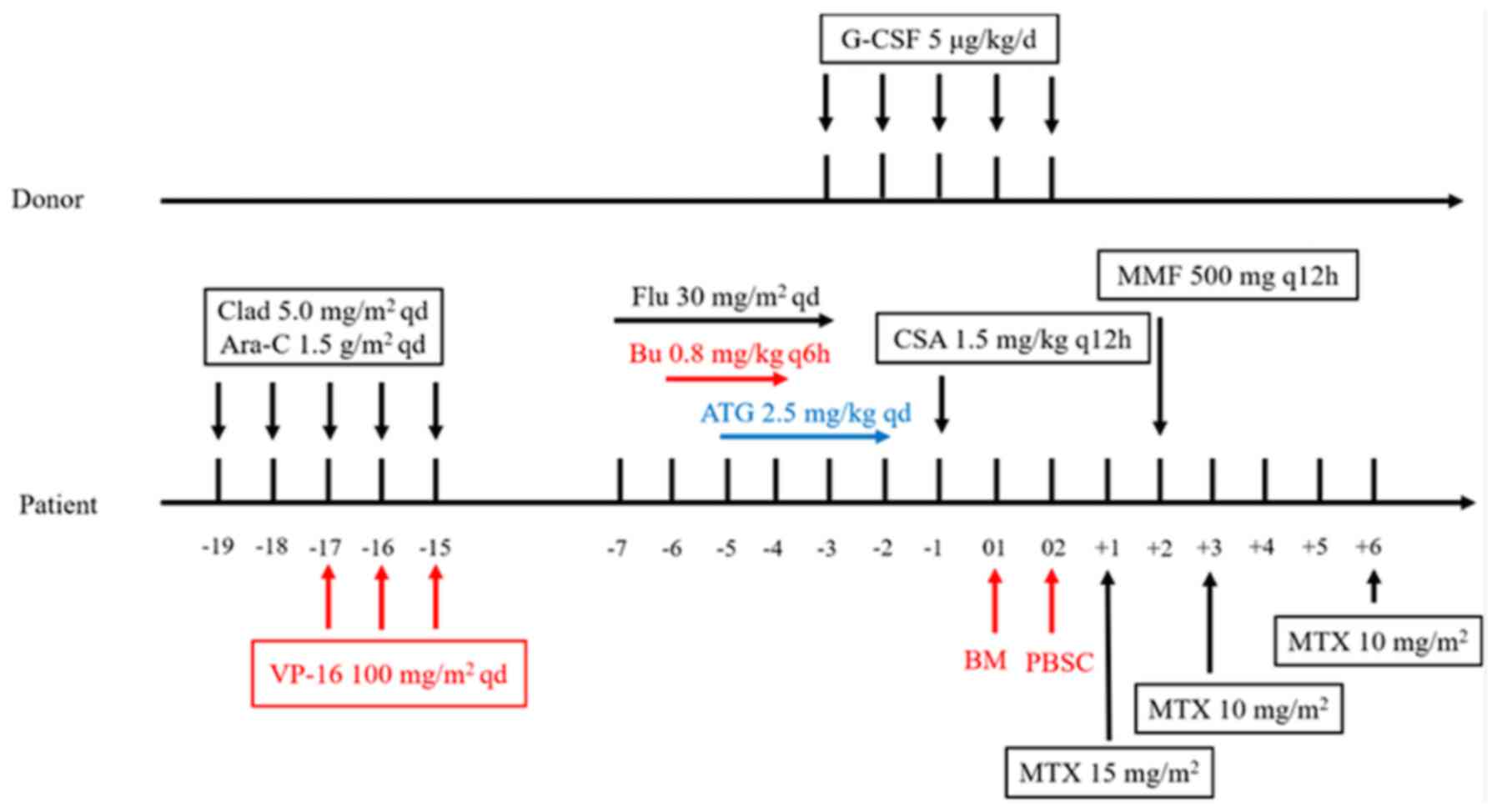 | Figure 3.Flowchart of regimen consisting of
cladribine, cytarabine and etoposide chemotherapy, and allogeneic
hematopoietic stem cell transplantation. Clad, cladribine; Ara-C,
cytarabine; VP-16, etoposide; Flu, fludarabine; Bu, busulfan; ATG,
anti-thymocyte globulin; G-CSF, granulocyte colony-stimulating
factor; CSA, cyclosporin A; BM, bone marrow; PBSC, peripheral blood
stem cell; MTX, methotrexate; MMF, mycophenolate mofetil. |
Case 2
On May 12, 2019, a 31-year-old man was admitted to
The Affiliated Huai'an No. 1 People's Hospital of Nanjing Medical
University due to fever and bleeding gums. Peripheral blood
analysis revealed the following: White blood cell count of
10.37×109/l, hemoglobin level of 114 g/l and a platelet
count of 8×109/l. In addition, the BM smear revealed 28%
of blasts cells. FCM analysis exhibited 39% of blast cells, and
demonstrated positive labeling for MPO, CD13, CD15, CD33, CD38,
CD56 and human leukocyte antigen-DR (HLA-DR). The karyotype was
46,XY,t(11;19)(q23;p13.1)[18]/46,XY[2] (Fig. 1). Aberrant EVI1 and
MLL/ELL rearrangement were detected using the same methods
performed for patient 1. The patient was diagnosed with AML (M2)
according to the FAB classification and was subsequently treated
with two cycles of IA regimen as induction, and achieved CR with
2.0% blasts. The MRD was negative (<0.01%) and was followed by
one cycle of consolidation with an intermediate dose of cytarabine
with 2.0 g/m2 × 6 times. The patient was re-admitted due
to severe crissum infection on October 10, 2019. The BM smear
revealed 58% of blast cells 6 months after consolidation,
suggesting disease relapse (Fig. 2).
The patient underwent matched unrelated allo-HSCT (stem cells from
a donor at the Chinese bone marrow bank) and simultaneously
received antibiotics to control the crissum infection. The patient
received intense CLAE chemotherapy (cladribine 5
mg/m2/dx5d, cytarabine 1.5 g/m2/dx5d and
etoposide 100 mg/m2/dx3d) and conditioning regimen,
followed by allo-HSCT. The BM smear revealed 23% of blasts cells
following CLAE, thus the total dose of busulfan in the conditioning
regimen was adjusted to 3.2 mg/kg/dx4d. The conditioning regimen
consisted of the following: Fludarabine 30 mg/m2/x5d,
busulfan 3.2 mg/kg/dx4d and ATG 2.5 mg/kg/dx4d. Cyclosporin A,
methotrexate and mycophenolate mofetil were used to prevent GVHDs.
A total of 8.0×108/kg mononuclear cells and
6.72×106/kg CD34+ cells were intravenously
infused into the patient. The patient suffered crissum abscess and
sepsis during transplantation. The neutrophil and platelet
engraftments were achieved on days 12 and 14, respectively
(Fig. 4C and D). MRD detection was
negative (<10−4), and the chromosome changed to
normal karyotype 1 month after transplantation. The donor chimerism
rate was 100% at +14 days, +30 days and +60 days following
transplantation. The decitabine regimen was implemented for the
prophylaxis of relapse. The patient received follow-up in the
clinic weekly and achieved CR based on the last follow-up at 3.5
months after allo-HSCT. The characteristics of both patients are
presented in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Case 1 | Case 2 |
|---|
| Age, year | 23 | 31 |
| Sex | Male | Male |
| WBC,
×109/l | 70.85 | 10.37 |
| PLT,
×109/l | 26 | 8 |
| Blasts in bone
marrow, % | 42 | 39 |
| FAB | AML-M4 | AML-M2 |
| Cytogenetics |
46,XY,t(11;19)(q23;p13)[17]/46,XY[3] |
46,XY,t(11;19)(q23;p13.1)[18]/46,XY[2] |
| Molecular
biology | EVI1, NRAS | EVI1, MLL/ELL |
| Risk
stratification | High risk | High risk |
| Induction
chemotherapy regimen | IA | IA |
| MRD after induction
chemotherapy | Negative | Negative |
| Disease status
before HSCT | Relapsed | Relapsed |
Discussion
In the present study, two relapsed patients with
t(11;19)(q23;p13) AML were successfully treated with CLAE regimens
sequential with allo-HSCT. Both patients achieved CR. The intense
chemotherapy prior to allo-HSCT decreased the leukemia burden and
inhibited the immune system of the recipient to promote the
implantation of HSCs. The results presented here confirm the
efficacy of intense CLAE chemotherapy sequential with allo-HSCT in
young relapsed patients with t(11;19)(q23;p13) AML. Hematological
toxicity and other side effects, including hemocytopenia, nausea
and vomiting, infection were presented during treatment.
Reciprocal chromosomal translocations can cause
genetic aberrations in pediatric and adult patients with AML
(22,23). t(11;19)(q23;p13) is a relatively rare
recurrent cytogenetic aberration that occurs in patients with AML
(12). MLL is involved in the
majority of 11q23 translocations of acute leukemias, whereby
rearrangement of MLL results in fusion of the MLL
gene with its partner gene (12).
However, heterogeneity rearrangements have been observed at this
chromosomal region (12,24). NRAS and KRAS are
frequently mutated in MLL-rearranged leukemia (25). The present study assessed two
patients with t(11;19)(q23;p13) AML, patient 1 (M4) had no
MLL rearrangement, while patient 2 (M2) had MLL/ELL
rearrangement, and both presented with early relapse (less than
half a year). Consistent with previous findings (12–14), the
results of the present study demonstrated that t(11;19)(q23;p13)
was associated with a poor prognosis and short OS time in patients
with AML, and indicated that it is necessary for these patients to
receive allo-HSCT in first CR.
The present study is not without limitations. First,
the MLL rearrangement in patient 2 was not detected via
fluorescence in situ hybridization as the sample size was
too small. Secondly, both patients had a relatively short follow-up
period, thus further studies are required with extended follow-up
periods and larger sample sizes.
Intensive chemotherapy is used to eliminate leukemia
cells, followed by allo-HSCT as consolidation therapy for patients
with R/R AML (26). This regimen has
been demonstrated to improve the long-term survival rate from 20 to
50%; however, reinduction chemotherapy-related side effects must be
acceptable to patients (27).
Previous studies have reported that FLAG-IDA (granulocyte
colony-stimulating factor, fludarabine, cytarabine, and
idarubicin), CLAG (cladribine, cytarabine, granulocyte
colony-stimulating factor), CLAM (cladribine, cytarabine,
mitoxantrone) and MEC (mitoxantrone, etoposide, and cytarabine)
chemotherapy regimens achieve high CR rates in patients with R/R
AML (28–31). However, CR rates in response to
common salvage regimens decrease (10-15%) in refractory or early
relapsed patients with AML (26).
Furthermore, common conditioning regimens followed by allo-HSCT in
patients with R/R AML exhibit disappointing results (32,33).
Thus, the concept of chemotherapy sequential with allo-HSCT was
developed (26). Recently, it has
been demonstrated that high-dose melphalan-based sequential
conditioning chemotherapy followed by allo-HSCT is feasible in
patients with R/R AML (26). Another
study reported the feasibility and efficacy of FLAG-IDA
chemotherapy sequential with Flu-Bu3 conditioning regimen in
patients with refractory AML (34).
Based on previous studies (28–31) and
patient characteristics, the present study selected the CLAE
regimen sequential with Flu-Bu conditioning regimen for allo-HSCT
in the assessed patients. Patient 1 received CLAE regimen
sequential with haploid transplantation, and exhibited granulocyte
deficiency at 21 after chemotherapy, the neutrophil and platelet
engraftments were achieved on days 14 and 12. Patient 2 received
CLAE regimen sequential with hematopoietic stem cell
transplantation from unrelated donors, the neutrophil and platelet
engraftments were achieved on days 12 and 14. No serious
transplant-related complications occurred in patient 1, while
patient 2 experienced crissum abscess and sepsis, which were
effectively controlled with antibiotics. Any grade of GVHDs were
absent in both cases. MRD was negative in both patients, and the
donor chimerism rate was 100%, while the karyotype of chromosomes
changed to normal karyotype. Decitabine was used to prevent disease
relapse following transplantation, and both patients remained alive
and disease-free based on the last follow-up.
In conclusion, the results of the present study
demonstrated the high antileukemic efficacy and acceptable toxicity
of CLAE regimen sequential with allo-HSCT for patients with
t(11;19)(q23;p13) AML. The results presented here suggest that
t(11;19)(q23;p13) is a poor prognostic factor in AML, and that the
CLAE regimen sequential with allo-HSCT may be an effective
treatment strategy for patients with t(11;19)(q23;p13) R/R AML.
However, prospective studies with larger sample sizes are required
to validate the effectiveness of this treatment regimen.
Acknowledgements
Not applicable.
Funding
The present study was supported by research grants
from the Science and Technology Fund of Huai'an City (grant no.
HAB201810) and the Science and Technology Fund of Jiangsu
Commission of Health (grant nos. H2018085 and H2019082).
Availability of data and materials
The data that support the findings of the present
study are available from Kindstar Global Medical Laboratory Center
(https://www.kindstar.com.cn/platform.html); however,
restrictions apply to the availability of these data, which were
used under license for the present study, and so are not publicly
available. Data are available from the authors upon reasonable
request and with permission from Kindstar Global Medical Laboratory
Center.
Authors' contributions
ST collected patient data and drafted the initial
manuscript. CW and LY conceived and designed the present study. ST
revised the manuscript along with LS, YD, YC, YG, YL, YHD, ZZ, BD
and ZH, and these individuals were responsible for the treatment of
the patients. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Affiliated Huai'an No. 1 People's Hospital of
Nanjing Medical University (Huai'an, China; approval no.
YX-P-2020-004-01) and performed in accordance with the Declaration
of Helsinki. Written informed consent was provided by both patients
prior to the study start.
Patient consent for publication
Both patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
R/R AML
|
relapsed/refractory acute myeloid
leukemia
|
|
CLAE
|
cladribine, cytarabine and
etoposide
|
|
allo-HSCT
|
allogeneic hematopoietic stem cell
transplantation
|
|
GVHD
|
graft-versus-host disease
|
|
CR
|
complete remission
|
|
MRD
|
minimal residual disease
|
|
OS
|
overall survival
|
References
|
1
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Short NJ, Rytting ME and Cortes JE: Acute
myeloid leukaemia. Lancet. 392:593–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thol F, Schlenk RF, Heuser M and Ganser A:
How I treat refractory and early relapsed acute myeloid leukemia.
Blood. 126:319–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurosawa S, Yamaguchi T, Miyawaki S,
Uchida N, Sakura T, Kanamori H, Usuki K, Yamashita T, Okoshi Y,
Shibayama H, et al: Prognostic factors and outcomes of adult
patients with acute myeloid leukemia after first relapse.
Haematologica. 95:1857–1864. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramos NR, Mo CC, Karp JE and Hourigan CS:
Current approaches in the treatment of relapsed and refractory
acute myeloid leukemia. J Clin Med. 4:665–695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bose P, Vachhani P and Cortes JE:
Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat
Options Oncol. 18:172017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yanada M, Mori J, Aoki J, Masuko M, Harada
K, Uchida N, Doki N, Fukuda T, Sakura T, Kanamori H, et al:
Allogeneic hematopoietic cell transplantation for patients with a
history of multiple relapses of acute myeloid leukemia. Ann
Hematol. 98:2179–2186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robak T and Wierzbowska A: Cladribine in
the treatment of acute myeloid leukemia. Leuk Res. 38:425–427.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fridle C, Medinger M, Wilk MC, Seipel K,
Passweg J, Manz MG and Pabst T: Cladribine, cytarabine and
idarubicin (CLA-Ida) salvage chemotherapy in relapsed acute myeloid
leukemia (AML). Leuk Lymphoma. 58:1068–1075. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao H, Li L, Pang Y, Wu Y, Jiang Z, Liu
Z, Wu J, Xiao Y, Huang F, Liu Q, et al: Sequential treatment
combining cladribine-based re-induction, myeloablative allogeneic
HSCT, and prophylactic donor lymphocyte infusion: A promising
treatment for refractory acute myeloid leukemia. Ann Hematol.
97:2479–2490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin M, Hu Y, Wu W, Luo Y, Tan Y, Yu J, Jin
A, Yang L, Huang H and Wei G: Decitabine plus CLAG chemotherapy as
a bridge to haploidentical transplantation in the setting of acute
myeloid leukemia relapse after HLA-matched sibling transplantation:
A case report. BMC Cancer. 19:2422019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto K, Kawamoto S, Kakiuchi S,
Yakushijin K, Matsuoka H and Minami H: Translocation t (11;19)
(q23; q13.1) without MLL rearrangement in acute myeloid leukemia:
Heterogeneity of the 11q23 Breakpoints. Acta Haematol. 134:76–79.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhatnagar B, Blachly JS, Kohlschmidt J,
Eisfeld AK, Volinia S, Nicolet D, Carroll AJ, Block AW, Kolitz JE,
Stone RM, et al: Clinical features and gene- and
microRNA-expression patterns in adult acute leukemia patients with
t (11;19) (q23; p13.1) and t (11;19) (q23; p13.3). Leukemia.
30:1586–1589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Kantarjian H, Pierce S, Faderl S,
O'Brien S, Qiao W, Abruzzo L, de Lima M, Kebriaei P, Jabbour E, et
al: Prognostic significance of 11q23 aberrations in adult acute
myeloid leukemia and the role of allogeneic stem cell
transplantation. Leukemia. 27:836–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wierzbowska A, Robak T, Pluta A,
Wawrzyniak E, Cebula B, Hołowiecki J, Kyrcz-Krzemień S, Grosicki S,
Giebel S, Skotnicki AB, et al: Cladribine combined with high doses
of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a
highly effective salvage regimen in patients with refractory and
relapsed acute myeloid leukemia of the poor risk: A final report of
the Polish Adult Leukemia Group. Eur J Haematol. 80:115–126. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Halpern AB, Othus M, Huebner EM, Buckley
SA, Pogosova-Agadjanyan EL, Orlowski KF, Scott BL, Becker PS,
Hendrie PC, Chen TL, et al: Mitoxantrone, etoposide and cytarabine
following epigenetic priming with decitabine in adults with
relapsed/refractory acute myeloid leukemia or other high-grade
myeloid neoplasms: A phase 1/2 study. Leukemia. 31:2560–2567. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
World Medical Association, . World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pallisgaard N, Hokland P, Riishøj DC,
Pedersen B and Jørgensen P: Multiplex reverse
transcription-polymerase chain reaction for simultaneous screening
of 29 translocations and chromosomal aberrations in acute leukemia.
Blood. 92:574–588. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang YL, Lin SR, Chen JS, Hsiao CC, Lin
KH, Sheen JM, Cheng CN, Wu KH, Lin SW, Yu SL, et al: Multiplex
reverse transcription-polymerase chain reaction as diagnostic
molecular screening of 4 common fusion chimeric genes in Taiwanese
children with acute lymphoblastic leukemia. J Pediatr Hematol
Oncol. 32:e323–e330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gou H, Zhou J, Ye Y, Hu X, Shang M, Zhang
J, Zhao Z, Peng W, Zhou Y, Zhou Y, et al: The prevalence and
clinical profles of FLT3-ITD, FLT3-TKD, NPM1, C-KIT, DNMT3A, and
CEBPA mutations in a cohort of patients with de novo acute myeloid
leukemia from southwest China. Tumour Biol. 37:7357–7370. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Creutzig U, van den Heuvel-Eibrink MM,
Gibson B, Dworzak MN, Adachi S, de Bont E, Harbott J, Hasle H,
Johnston D, Kinoshita A, et al: Diagnosis and management of acute
myeloid leukemia in children and adolescents: Recommendations from
an international expert panel. Blood. 120:3187–3205. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Limbergen H, Poppe B, Michaux L,
Herens C, Brown J, Noens L, Berneman Z, De Bock R, De Paepe A and
Speleman F: Identification of cytogenetic subclasses and recurring
chromosomal aberrations in AML and MDS with complex karyotypes
using M-FISH. Genes Chromosomes Cancer. 33:60–72. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meyer C, Hofmann J, Burmeister T, Gröger
D, Park TS, Emerenciano M, Pombo de Oliveira M, Renneville A,
Villarese P, Macintyre E, et al: The MLL recombinome of acute
leukemias in 2013. Leukemia. 27:2165–2176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reimer J, Knöß S, Labuhn M, Charpentier
EM, Göhring G, Schlegelberger B, Klusmann JH and Heckl D:
CRISPR-Cas9-induced t (11;19)/MLL-ENL translocations initiate
leukemia in human hematopoietic progenitor cells in vivo.
Haematologica. 102:1558–1566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Steckel NK, Groth C, Mikesch JH, Trenschel
R, Ottinger H, Kordelas L, Mueller-Tidow C, Schliemann C, Reicherts
C, Albring JC, et al: High-dose melphalan-based sequential
conditioning chemotherapy followed by allogeneic haematopoietic
stem cell transplantation in adult patients with relapsed or
refractory acute myeloid leukaemia. Br J Haematol. 180:840–853.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui L, Liu Y, Pang Y, Qian T, Quan L,
Cheng Z, Dai Y, Ye X, Pang Y, Shi J, et al: Emerging agents and
regimens for treatment of relapsed and refractory acute myeloid
leukemia. Cancer Gene Ther. 27:1–14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Westhus J, Noppeney R, Dührsen U and
Hanoun M: FLAG salvage therapy combined with idarubicin in
relapsed/refractory acute myeloid leukemia. Leuk Lymphoma.
60:1014–1022. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Xu J, Tian X, Lv T and Yuan G:
Analysis of efficacy and prognostic factors of CLAG treatment in
Chinese patients with refractory or relapsed acute myeloid
leukemia. Acta Haematol. 141:43–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bao Y, Zhao J and Li ZZ: Comparison of
clinical remission and survival between CLAG and FLAG induction
chemotherapy in patients with refractory or relapsed acute myeloid
leukemia: A prospective cohort study. Clin Transl Oncol.
20:870–880. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amadori S, Arcese W, Isacchi G, Meloni G,
Petti MC, Monarca B, Testi AM and Mandelli F: Mitoxantrone,
etoposide, and intermediate-dose cytarabine: An effective and
tolerable regimen for the treatment of refractory acute myeloid
leukemia. J Clin Oncol. 9:1210–1214. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ringden O, Labopin M, Ehninger G,
Niederwieser D, Olsson R, Basara N, Finke J, Schwerdtfeger R, Eder
M, Bunjes D, et al: Reduced intensity conditioning compared with
myeloablative conditioning using unrelated donor transplants in
patients with acute myeloid leukemia. J Clin Oncol. 27:4570–4577.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luger SM, Ringden O, Zhang MJ, Perez WS,
Bishop MR, Bornhauser M, Bredeson CN, Cairo MS, Copelan EA, Gale
RP, et al: Similar outcomes using myeloablative vs.
reduced-intensity allogeneic transplant preparative regimens for
AML or MDS. Bone Marrow Transplant. 47:203–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Devillier R, Wan M, Decroocq J,
Tian L, Fürst S, Wang LN, Vey N, Fan X, Blaise D and Hu J: Clinical
outcome of FLAG-IDA chemotherapy sequential with Flu-Bu3
conditioning regimen in patients with refractory AML: A parallel
study from Shanghai Institute of Hematology and Institut
Paoli-Calmettes. Bone Marrow Transplant. 54:458–464. 2019.
View Article : Google Scholar : PubMed/NCBI
|