Introduction
Cancer is the second largest disease after
cardiovascular disease in terms of morbidity and mortality, and has
become the second leading cause of death in humans (1). Additionally, cancer has been reported
to affect other systems in the body; for example, it was discovered
that rats implanted with Yoshida ascites sarcoma rapidly developed
anemia (2). In addition, the
metastasis of colorectal cancer to the liver exacerbated skeletal
muscle wasting by regulating differential gene expression
signatures (3). On the other hand,
abnormalities in the whole body have been discovered to affect the
progression of cancer. For example, a previous study identified a
strong association between the prevalence of autism and the
incidence of breast cancer in situ (4). Diabetic patients with insulin
resistance were identified to exhibit changes in gene expression
levels that increased the risk of colorectal cancer (5). Therefore, the state of the cancer may
affect the reproductive system and the quality of the oocytes.
Successful embryo and fetal development are
dependent on the quality of the oocytes, which is regulated by
various factors (6), for instance,
the appropriate metabolism and sufficient ATP production were
discovered to be associated with the quality of oocytes and the
preimplantation embryo development (6). Alves et al (7) reported that short-term supplementation
with a high-fat diet altered the dynamics of follicles, but did not
affect the proportion of morphologically normal oocytes. Meanwhile,
Yu et al (8) identified that
increased levels of reactive oxygen species were present in the
ovary with increased age, and the subsequent oxidative stress led
to a decrease in the number and quality of oocytes. Mihalas et
al (9) suggested that proteasome
dysfunction may be the primary reason for the decline in the
quality of age-related oocytes. Additionally, it was reported that
melatonin enhanced DNA repair, thereby preventing the quality of
oocytes from decreasing during meiosis (10). In addition, clinical medicines can
affect the quantity and quality of oocytes; a previous study
demonstrated that common first-line anti-tuberculosis drugs,
including rifampicin, isoniazid, pyrazinamide, streptomycin and
ethambutol, decreased the number of oocytes in females and
increased their degeneration, as well as increasing the rate and
changing the distribution pattern of cytoplasmic organelles
(11).
With changes to modern lifestyle habits and the
deterioration of the environment, the age of cancer onset is
decreasing (12,13); however, it is currently unknown
whether the reproductive system of female patients with
non-reproductive system cancers is affected. In the present study,
the effects of cancer on the quantity and quality of oocytes were
studied using sarcoma-180 (S-180) tumor-bearing mice, which may
provide novel insights into the reproductive ability following
cancer.
Materials and methods
Cell culture
The mouse S-180 cell line was purchased from the
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. Cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (HyClone;
Cytiva) and maintained at 37°C in a humidified atmosphere
containing 5% CO2.
Animal studies and tissue
collection
The experimental procedures in the present study
were approved by the Committee on Laboratory Animal Care and Use of
Guangdong Pharmaceutical University (Guangzhou, China). In total,
42 C57BL/6J female mice (age, 5–8 weeks; weight, 13±2.0 g) were
purchased from Hunan SJA Laboratory Animal Co., Ltd. The mice were
housed in a temperature-controlled room (23±2°C) at a relative
humidity of 50±10% with a 12-h light/dark cycle and free access to
a standard diet and water. The general conditions of the mice were
checked every day. The mice were randomly divided into 2 groups:
The tumor-bearing group and the control group, each containing 21
mice. The S-180 cells were washed with pre-chilled PBS (HyClone;
Cytiva) and the density of the cells within the fluid was
subsequently adjusted to 1×107 cells/ml prior to 0.2 ml
injected into the right armpit of the mice in the tumor-bearing
group to establish the S-180 tumor-bearing model. The control group
was subcutaneously inoculated with an equal volume of PBS buffer
solution at the same location. The mice were checked every day for
~10 days, and the tumors could be seen or felt under the skin of
the right armpit of the tumor-bearing group. The allowed maximum
diameter of the tumors was 1.5 cm, and the allowed maximum tumor
volume was 2,000 mm3. The formula used to calculate
tumor volume was V (mm3)=π/6 ab2 with a>b
(14). The average tumor volume of
the tumor-bearing group was 1,740±490 mm3. The images of
tumors are shown in Fig. S1.
Inducing superovulation in mice
On the 10th day after cell injection, each mouse was
intraperitoneally injected with 5 IU pregnant horse serum
gonadotropin (PMSG; Shanghai Gaochuang Chemical Technology Co.,
Ltd.) and after 48 h, the mice were intraperitoneally injected with
5 IU human chorionic gonadotropin (HCG; Shanghai Gaochuang Chemical
Technology Co., Ltd.). After injection of HCG for 12 h, the mice
were sacrificed by cervical dislocation to collect the bilateral
ovaries. The quality of the ovaries was assessed using an
electronic scale (precision 0.1 mg; Beijing Taize Jiaye Technology
Development Co., Ltd.). The bilateral oviducts were separated and
placed in PBS at room temperature. The ampulla of the oviduct was
punctured with a 26G needle, and the oocytes were removed with a
pipette and transferred into M2 culture medium containing
hyaluronidase (Shanghai Yuanye Biotechnology Co., Ltd.) and the
cumulus cells were subsequently removed. The morphology of the
oocytes could be clearly observed when the granular cells alongside
the oocytes had disappeared. Briefly, the oocytes were transferred
into the M2 culture using the pipette and washed 3 times in the
nutrient solution. The total number of oocytes was counted, and the
morphological characteristics of the oocytes under different
magnification were observed using a Leica DMi8 light microscope
(Leica Microsystems, Inc.) under ×100, ×200 and ×400
magnification.
Serum profile assays
The mice were adequately anesthetized with ether for
blood collection, and the pinch reflex was monitored to ensure full
anesthesia. The anesthesia method was approved by the Committee on
Laboratory Animal Care and Use of Guangdong Pharmaceutical
University. The whole blood was collected from the orbit, and after
collecting 1 ml blood, the mice were sacrificed by cervical
dislocation. The blood glucose levels were detected using a blood
glucose detection kit (cat. no. 561510) purchased from Shanghai
Rongsheng Biology Pharmaceutical Co., Ltd., according to the
manufacturer's protocol. Serum was obtained from the blood by
centrifugation at 1,160 × g at 4°C for 15 min. The concentrations
of serum total cholesterol (TC), triglyceride (TG), insulin and
lipopolysaccharide (LPS) were analyzed using the TC detection kit
(cat. no. A111-1-1), TG detection kit (cat. no. A110-1-1), insulin
detection kit (cat. no. Z26018441) and LPS detection kit (cat. no.
Z24018439), respectively, according to the manufacturers'
protocols. The detection kits for analyzing TG and TC levels were
purchased from Nanjing Jiancheng Bioengineering Institute. The
ELISA kits for analyzing insulin and LPS levels were purchased from
Wuhan Huamei Biological Engineering Co., Ltd.
Hematoxylin and eosin (H&E)
staining
The ovarian tissues were fixed in 4%
paraformaldehyde overnight at 4°C, dehydrated with an ascending
alcohol series and embedded into paraffin. Paraffin-embedded
tissues were subsequently sliced into 4-µm-thick sections and
stained with hematoxylin (Sigma-Aldrich; Merck KGaA) for 3 min and
eosin (Sigma-Aldrich; Merck KGaA) for 20 sec, both at room
temperature. The stained sections were visualized using an
automated quantitative pathology imaging system (PerkinElmer,
Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the native ovarian
tissue of the mice using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed
into cDNA using a PrimeScript RT Reagent kit (Takara Bio, Inc.)
according to the manufacturer's protocol. The primers were designed
using the National Center for Biotechnology Information (NCBI)
online primer design software (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome)
according to the gene sequence published on the NCBI Gene webpage,
and synthesized by Sangon Biotech Co., Ltd. The specific primer
sequences are presented in Table I.
RT-qPCR was subsequently performed using a SYBR Premix Ex Taq kit
(Takara Bio, Inc.) and a PikoReal Real-Time PCR system (Thermo
Fisher Scientific, Inc.). The following thermocycling conditions
were used for qPCR: 95°C for 30 sec, followed by 40 cycles of 95°C
for 5 sec, 60°C for 20 sec and 65°C for 15 sec. The data were
analyzed using the 2−∆∆Cq method (15), and GAPDH was used as the internal
loading control.
 | Table I.Primer sequences of genes for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences of genes for reverse
transcription-quantitative PCR.
| Genes | Sequences |
|---|
| MARF1 | F:
5′-TCAGAACTCCAGCTCCGAACAGG-3′ |
|
| R:
5′-GGATGGCAGGCATGAAGGAACAG-3′ |
| SENP7 | F:
5′-TTGTTTATCCTCCACCACCTAC-3′ |
|
| R:
5′-CTCTGAGCCACTGATAGATCTG-3′ |
| AANAT | F:
5′-CTTTATCTCTGTCTCCGGTACC-3′ |
|
| R:
5′-CTGAGTAAGTCTCTCCTTGTCC-3′ |
| CDC25B | F:
5′-GGACCACGAATGTGCCGCTTCA-3′ |
|
| R:
5′-AGTTCCCTCCTGCTCCGTTCCC-3′ |
| GPR3 | F:
5′-CTCACCAGAGATGAGCTTGATG-3′ |
|
| R:
5′-TAAGTGAGAGCATTGTACAGGG-3′ |
| GAPDH | F:
5′-GCATCCACTGGTGCTGCC-3′ |
|
| R:
5′-TCATCATACTTGGCAGGTTTC-3′ |
Statistical analysis
Statistical analysis was performed using SPSS 24.0
software (IBM Corp.) and all data are expressed as the mean ± SD.
Unpaired Student's t-test was used to determine the statistical
differences between two groups. The differences in the abnormal
rate of oocytes between the different groups were analyzed using a
χ2 test. Each experiment was repeated ≥3 times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of S-180 tumors on the serum
profile
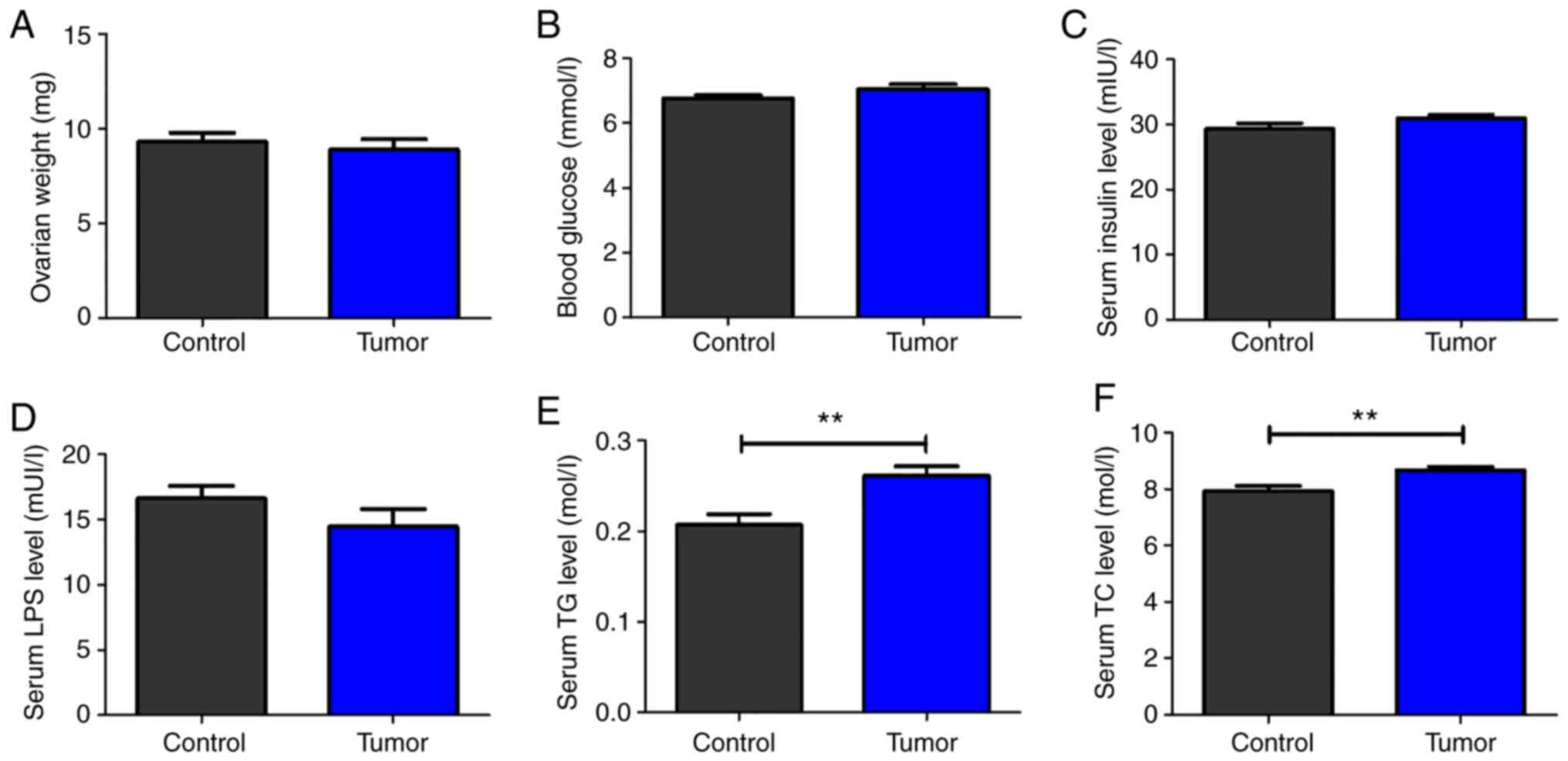
No significant difference was identified in the
weight of the ovaries between the tumor-bearing and the control
groups (Fig. 1A). Similarly, no
significant differences were observed in the levels of blood
glucose, serum insulin and LPS between the two groups (Fig. 1B-D). However, the serum TG and TC
levels were significantly increased in the tumor-bearing group
compared with in the control group (Fig.
1E and F).
Effects of S-180 tumors on the
morphology of the ovaries and oocytes
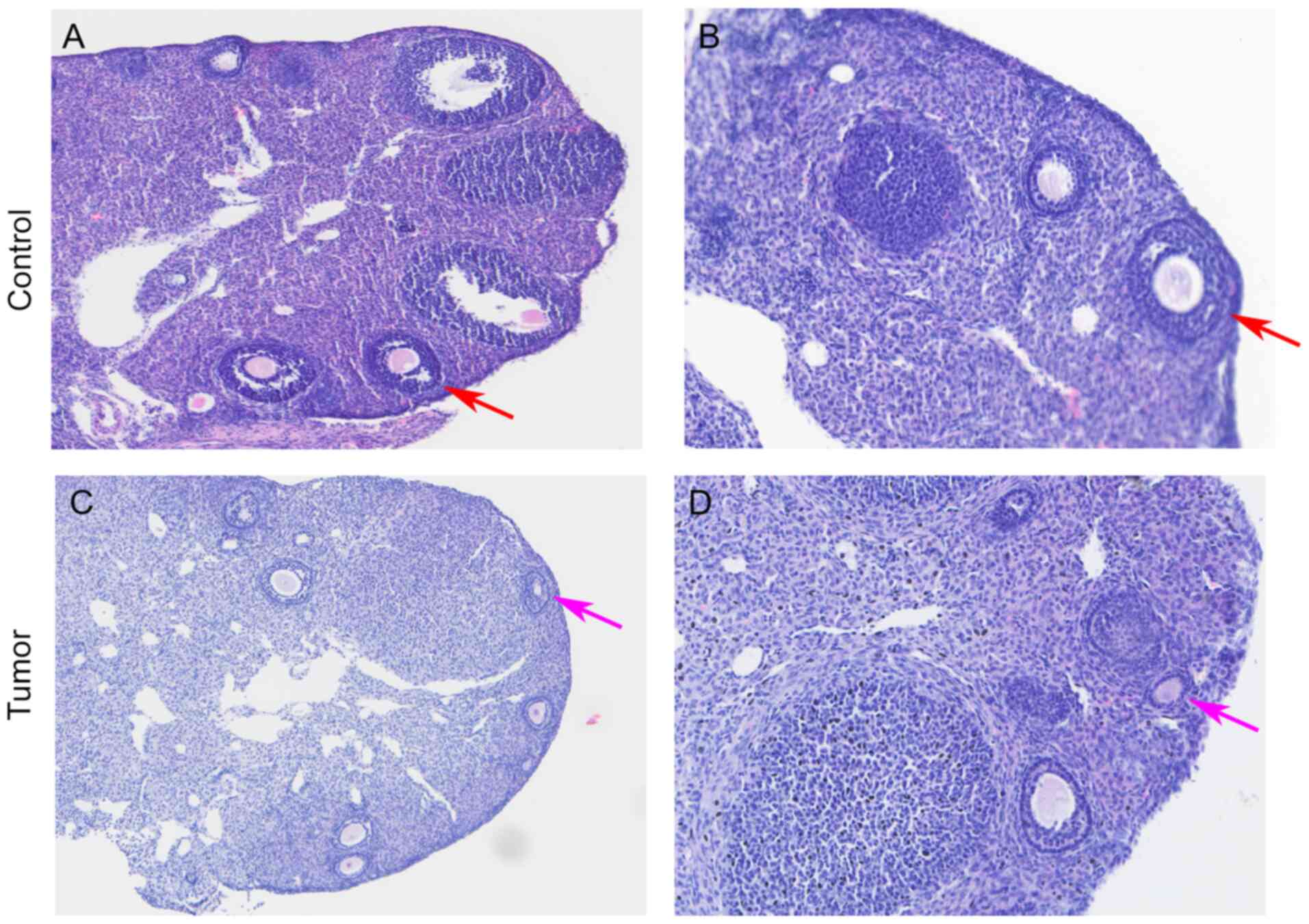
The results of the H&E staining revealed that
there were more mature oocytes ready to be expelled at the edge of
the ovary in the control group, which were accompanied by a large
number of granulosa cells around the follicles (Fig. 2A and B). In the tumor-bearing group,
there were fewer surrounding granulosa cells and the majority of
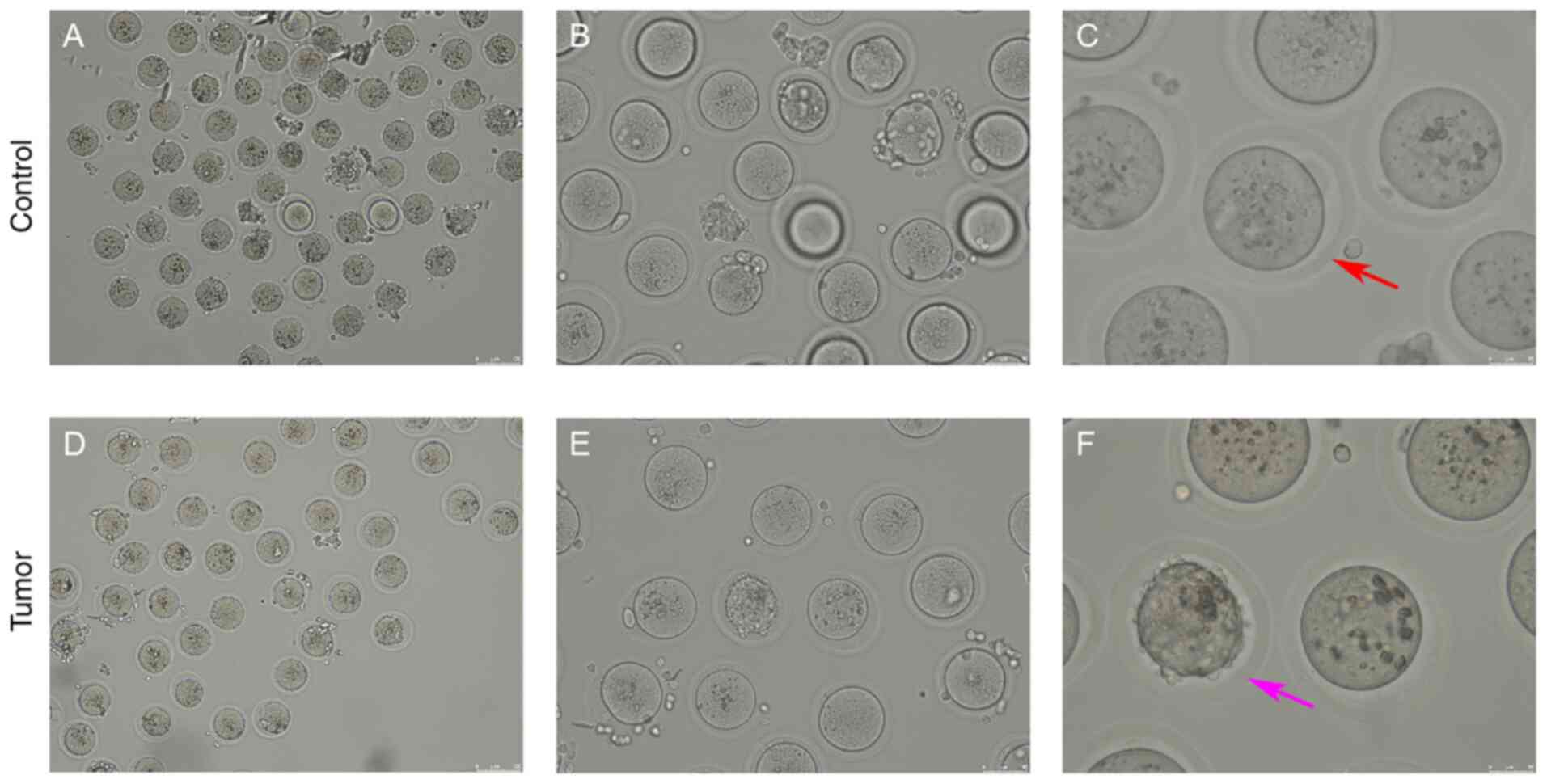
the follicles were in the primary phase (Fig. 2C and D). Each oocyte was separated by
a glass needle under the microscope, and the morphology of the
oocytes was observed. A qualified mature oocyte was suitable in
size (80–100 µm), with a complete membrane and nucleus, and a full
cytoplasm; the first polar body had also been expelled (Fig. 3). An abnormal oocyte was found to be
either enlarged or shrunk in size, with a broken cell membrane, a
dissolved nucleus and a broken or retained polar body (Fig. 3). The number of normal and abnormal
oocytes was counted; the results revealed that there were 39
abnormal oocytes out of a total of 93 oocytes in the control group,
which was higher than that of a previous study (16), while there were 36 abnormal oocytes
out of a total of 63 oocytes in the tumor-bearing group (Table II). The abnormal rate of oocytes in
the tumor-bearing group (57.10%) was increased compared with that
in the control group (41.90%) (Table
II).
 | Table II.Number of oocytes and abnormal
rate. |
Table II.
Number of oocytes and abnormal
rate.
| Groups | Total oocytes | Abnormal oocytes | χ2 | P-value |
|---|
| Control group | 93 | 39 | 3.479 | 0.062 |
| Tumor-bearing
group | 63 | 36 |
|
|
S-180 tumors regulate the expression
levels of reproduction-associated genes
The expression levels of reproduction-associated
genes were further analyzed in the ovarian tissue of the mice. The
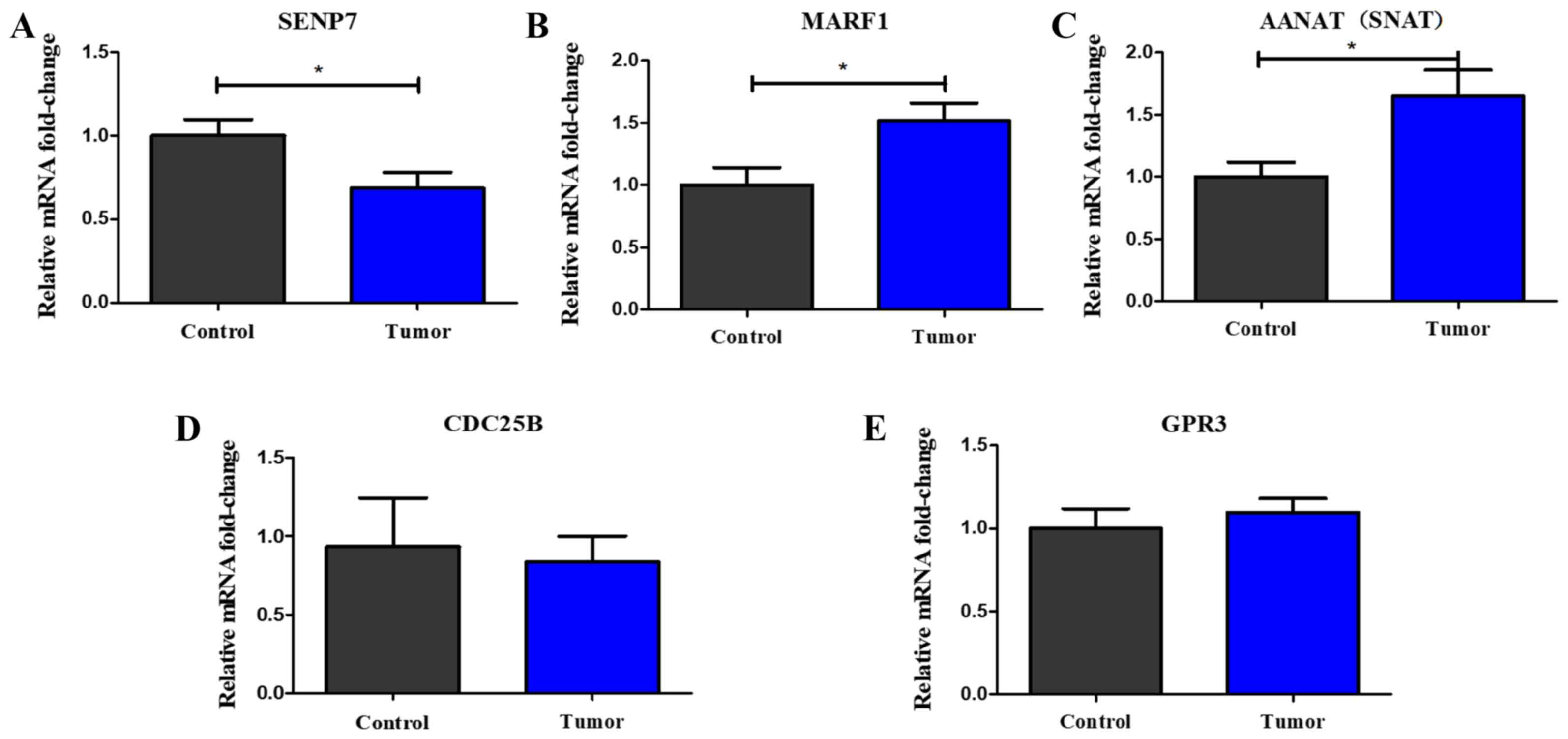
results of the RT-qPCR analysis revealed that the expression levels
of SUMO-specific protease 7 (SENP7) were significantly
downregulated in the tumor-bearing group compared with those in the
control group (Fig. 4A), while the
expression levels of meiosis arrest female 1 (MARF1) and
aralkylamine N-acetyltransferase (AANAT) were significantly
upregulated in the tumor-bearing group compared with those in the
control group (Fig. 4B and C). There
were no significant differences identified in the expression levels
of cell division cycle 25B (CDC25B) and glycine-rich protein 3
(GRP3) between the two groups (Fig. 4D
and E).
Discussion
In the present study, the effects of S-180 tumor on
the quantity and quality of oocytes were investigated using
tumor-bearing mice. The results revealed that the serum TG and TC
levels were significantly increased in the tumor-bearing group
compared with in the control group, alongside the number of
abnormal oocytes. The abnormal rate of oocytes of the tumor-bearing
group (57.10%) was increased compared with that of the control
group (41.90%), although the number of abnormal oocytes in the
control group was higher than that in a previous report (39
abnormal oocytes out of 93 oocytes) (16), which may be associated with the batch
deference of mice. It was previously reported that the growth and
survival of tumor cells depended on lipid metabolism, as the lipids
could provide the energy for the proliferation of tumor cells
(17). Moreover, Wang et al
(18) reported that the levels of
TG, TC and low-density lipoprotein were negatively associated with
the number of normal fertilized oocytes, and that the TG levels
were negatively associated with the number of oocytes and cleavage
embryos. The results of the present study identified that the serum
TG and TC levels were significantly increased in the tumor-bearing
group, which may explain how the S-180 tumor may affect the
quantity and quality of the oocytes. However, whether serum TG and
TC levels are associated with the progression of cancer and the
oocyte quality in patients with cancer requires further
investigation.
The expression levels of reproduction-associated
genes were further analyzed in the ovaries of mice using RT-qPCR.
The results demonstrated that the expression levels of SENP7 were
significantly downregulated in the tumor-bearing group, while the
expression levels of MARF1 and AANAT were significantly upregulated
in the tumor-bearing group compared with in the control group.
SUMOylation can regulate numerous different cellular processes, and
SENP7 is a de-SUMOylation enzyme (19). It was reported that SENP7 was
essential for chromatin relaxation in response to DNA damage, and
the de-SUMOylation by SENP7 provided a permissive chromatin
environment for DNA repair (20).
Since SENP7 is essential for heterochromatin integrity and DNA
repair, its roles in the mammalian reproductive system were
studied. Huang et al (21)
reported that the deficiency of SENP7 in oocytes arrested the
meiosis process at the prophase I and metaphase I stages, resulting
in a substantial decrease in the number of mature oocytes in mice.
In addition, the maternal-zygotic transition was defective in
SENP7-depleted embryos, which led to very little blastocyst
production (21). These findings
suggested that SENP7 may be essential in the epigenetic programming
of the oocyte and embryonic development. The present results
demonstrated that the expression levels of SENP7 were significantly
downregulated in the tumor-bearing group compared with in the
control group, which was consistent with the findings of Huang
et al (21), suggesting that
S-180 tumors may affect the quantity and quality of oocytes by
regulating the expression levels of SENP7.
MARF1 is essential for the control of meiosis and
the maintenance of genomic integrity during the development of
oocytes, and mutations in MARF1 were discovered to upregulate a
cohort of transcripts and increase retrotransposon expression,
leading to defective cytoplasmic maturation, meiotic arrest and
female infertility (22–24). AANAT is expressed in oocytes at all
stages of follicular development and it synthesizes serotonin to
melatonin enzymatically, together with acetylserotonin
O-methyltransferase (25). The
synthesis of melatonin in oocytes is essential for the maturation
of oocytes and the development of the embryo through its ability to
protect the oocytes from oxidative damage (25,26). In
the present study, the expression levels of MARF1 and AANAT were
significantly upregulated in the tumor-bearing group compared with
in the control group. These effects may be due to the S-180 tumors
creating a high stress environment that affected the function of
the ovaries in the mice, which in turn upregulated the expression
levels of several reproduction-associated genes, such as MARF1 and
AANAT, as a protective mechanism. However, these mechanisms require
further study.
CDC25B is an essential regulator for meiotic
resumption in mouse oocytes (27),
and GPR3 potentiates meiotic competence by increasing cyclic AMP
levels (28). The present results
identified no significant differences in the expression levels of
CDC25B and GRP3 between the tumor-bearing and control groups.
In conclusion, the findings of the present study
revealed that the number of oocytes in the tumor-bearing group and
the control group of mice was similar, while the number of abnormal
oocytes was increased in the tumor-bearing group. The serum levels
of TG and TC were significantly elevated in the tumor-bearing group
compared with in the control group. In addition, the expression
levels of SENP7 were downregulated, while the expression levels of
MARF1 and AANAT were upregulated in the ovaries of the
tumor-bearing group compared with the control group. To the best of
our knowledge, the present study was the first to investigate the
possible effects of non-reproductive system tumors on the female
reproductive system, in addition to suggesting the possible
mechanisms of the effects of S-180 tumors on oocytes in mice. These
results may provide the foundations to use for the clinical
reference of female patients with cancer when determining their
fertility; however, the specific mechanisms remain unclear, and
changes in the expression levels of other reproduction-associated
genes require to be verified.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81803912), the
Scientific Research Project of the Administration of Traditional
Chinese Medicine of Guangdong Province (grant no. 20182079), the
Characteristic Innovation Project (Natural Science) of the
Education Department of Guangdong Province and the ‘Innovation
Strong School Project’ of Guangdong Pharmaceutical University
(grant no. 2017KTSCX102), the Science and Technology Project of
Yue-Xiu District of Guangzhou (grant no. 2018-WS-011) and the
Innovation and Entrepreneurship Training Program for College
Students of Guangdong Province (grant no. S201910573021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY designed and conceived the study, wrote the first
draft of the manuscript and critically revised the manuscript. ZC
established the mouse model and performed the reverse
transcription-quantitative PCR. ZC, SW and XL performed the serum
analysis and the H&E staining. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The experimental procedures in the present study
were approved by the Committee on Laboratory Animal Care and Use of
Guangdong Pharmaceutical University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional, and national cancer incidence, mortality, years
of life lost, years lived with disability, and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the global burden of disease study. JAMA Oncol.
3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhanushali AA, Raghunathan R, Kalraiya RD
and Mehta NG: Cancer-related anemia in a rat model:
Alpha2-macroglobulin from Yoshida sarcoma shortens erythrocyte
survival. Eur J Haematol. 68:42–48. 2015. View Article : Google Scholar
|
|
3
|
Huot JR, Novinger LJ, Pin F, Narasimhan A,
Zimmers TA, O'Connell TM and Bonetto A: Formation of colorectal
liver metastases induces musculoskeletal and metabolic
abnormalities consistent with exacerbated cachexia. JCI Insight.
5:1366872020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kao HT, Buka SL, Kelsey KT, Gruber DF and
Porton B: The correlation between rates of cancer and autism: An
exploratory ecological investigation. PLoS One. 5:e93722010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia Z, Su YR, Petersen P, Qi L, Kim AE,
Figueiredo JC, Lin Y, Nan H, Sakoda LC, Albanes D, et al:
Functional informed genome-wide interaction analysis of body mass
index, diabetes and colorectal cancer risk. Cancer Med.
9:3563–3573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dunning KR and Robker RL: Promoting lipid
utilization with l-carnitine to improve oocyte quality. Anim Reprod
Sci. 134:69–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alves JPM, Fernandes CCL, Rossetto R,
Silva CPD, Galvão ITOM, Bertolini M and Rondina D: Impact of short
nutrient stimuli with different energy source on follicle dynamics
and quality of oocyte from hormonally stimulated goats. Reprod
Domest Anim. 54:1206–1216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu S, Zhao Y, Feng Y, Zhang H, Li L, Shen
W, Zhao M and Min L: β-carotene improves oocyte development and
maturation under oxidative stress in vitro. in vitro Cell Dev Biol
Anim. 55:548–558. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mihalas BP, Bromfield EG, Sutherland JM,
De Iuliis GN, McLaughlin EA, Aitken RJ and Nixon B: Oxidative
damage in naturally aged mouse oocytes is exacerbated by
dysregulation of proteasomal activity. J Biol Chem.
293:18944–18964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leem J, Bai GY, Kim JS and Oh JS:
Melatonin protects mouse oocytes from DNA damage by enhancing
nonhomologous end-joining repair. J Pineal Res. 67:e126032019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rao A, Nayak G, Kumari S, Kalthur SG,
Mutalik SP, Mutalik S, Adiga SK and Kalthur G: Exposure to first
line anti-tuberculosis drugs in prepubertal age reduces the quality
and functional competence of spermatozoa and oocytes in Swiss
albino mice. Environ Toxicol Pharmacol. 73:1032922020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel SG and Ahnen DJ: Colorectal cancer
in the young. Curr Gastroenterol Rep. 20:152018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore K and Brewer MA: Endometrial cancer:
Is this a new disease? Am Soc Clin Oncol Educ Book. 37:435–442.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raab M, Kappel S, Krämer A, Sanhaji M,
Matthess Y, Kurunci-Csacsko E, Calzada-Wack J, Rathkolb B, Rozman
J, Adler T, et al: Toxicity modelling of Plk1-targeted therapies in
genetically engineered mice and cultured primary mammalian cells.
Nat Commun. 2:3952011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He YT, Yang LL, Zhao Y, Shen W, Yin S and
Sun QY: Fenoxaprop-ethyl affects mouse oocyte quality and the
underlying mechanisms. Pest Manag Sci. 75:844–851. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brantley KD, Riis AH, Erichsen R,
Thorlacius-Ussing O, Møller HJ and Lash TL: The association of
serum lipid levels with colorectal cancer recurrence. Cancer
Epidemiol. 66:1017252020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Wang J, Jiang Y and Jiang W:
Association between blood lipid level and embryo quality during in
vitro fertilization. Medicine (Baltimore). 99:e196652020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiang JW, Xiao Y, Gan Y, Chen H, Liu Y,
Wang L, Nie Q, Liu F, Gong X, Fu JL, et al: Glucose oxidase- and
UVA-induced changes in the expression patterns of seven
de-sumoylation enzymes (SENPs) are associated with cataract
development. Curr Mol Med. 19:48–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garvin AJ, Densham RM, Blair-Reid SA,
Pratt KM, Stone HR, Weekes D, Lawrence KJ and Morris JR: The
deSUMOylase SENP7 promotes chromatin relaxation for homologous
recombination DNA repair. EMBO Rep. 14:975–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang CJ, Wu D, Jiao XF, Khan FA, Xiong
CL, Liu XM, Yang J, Yin TL and Huo LJ: Maternal SENP7 programs
meiosis architecture and embryo survival in mouse. Biochim Biophys
Acta Mol Cell Res. 1864:1195–1206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su YQ, Sugiura K, Sun F, Pendola JK, Cox
GA, Handel MA, Schimenti JC and Eppig JJ: MARF1 regulates essential
oogenic processes in mice. Science. 335:1496–1499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao GY, Li MZ, Wang H, Shi LY and Su YQ:
Interference with the C-terminal structure of MARF1 causes
defective oocyte meiotic division and female infertility in mice. J
Biomed Res. 32:58–67. 2018.PubMed/NCBI
|
|
24
|
Yao Q, Cao G, Li M, Wu B, Zhang X, Zhang
T, Guo J, Yin H, Shi L, Chen J, et al: Ribonuclease activity of
MARF1 controls oocyte RNA homeostasis and genome integrity in mice.
Proc Natl Acad Sci USA. 115:11250–11255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakaguchi K, Itoh MT, Takahashi N, Tarumi
W and Ishizuka B: The rat oocyte synthesises melatonin. Reprod
Fertil Dev. 25:674–682. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tamura H, Jozaki M, Tanabe M, Shirafuta Y,
Mihara Y, Shinagawa M, Tamura I, Maekawa R, Sato S, Taketani T, et
al: Importance of melatonin in assisted reproductive technology and
ovarian aging. Int J Mol Sci. 21:11352020. View Article : Google Scholar
|
|
27
|
Kang H, Hwang SC, Park YS and Oh JS:
Cdc25B phosphatase participates in maintaining metaphase II arrest
in mouse oocytes. Mol Cells. 35:514–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Firmani LD, Uliasz TF and Mehlmann LM: The
switch from cAMP-independent to cAMP-dependent arrest of meiotic
prophase is associated with coordinated GPR3 and CDK1 expression in
mouse oocytes. Dev Biol. 434:196–205. 2018. View Article : Google Scholar : PubMed/NCBI
|