Introduction
Histiocytic neoplasms are a type of tumor that arise
from the monocyte-macrophage system, including dendritic cells
(DCs), macrophages and sometimes histiocytes (1,2). DC
tumors are associated with certain types of antigen-presenting
cells (2). Follicular DC sarcoma
(FDCS) is extremely rare, so its epidemiology is unclear. There is
a wide patient age range, with an adult predominance (median
patient age, 50 years) and an almost equal sex distribution
(2,3).
FDCS consists of a neoplastic proliferation of
spindled to ovoid cells exhibiting morphological and
immunophenotypic features of FDCs (1). Misdiagnosis occurs frequently, due to
the fact that it has the same micromorphology as other common
neoplasms, such as other types of sarcoma and lymphoma (2,3). In ~35%
of cases, FDCS presents in the lymph nodes (commonly in cervical
nodes), while ~65% of cases present with extranodal disease
(1,4,5). A
number of extranodal sites can be affected, such as tonsils,
gastrointestinal tract, soft tissue, mediastinum, retroperitoneum,
omentum and lungs (2,3). Surgical excision is the main treatment,
sometimes combined with adjuvant radiotherapy or chemotherapy
(3). The 2-year survival rates for
early, locally advanced and distant metastatic diseases are 82, 80
and 42%, respectively (1,2). Some patients may die from refractory
paraneoplastic pemphigus (2).
Inflammatory pseudotumour-like FDCs or fibroblastic DCs often
affect the liver or spleen in female patients (1,2).
Histologically, the neoplastic-spindled cells are dispersed within
a prominent lymphoplasmacytic infiltrate (1). In the present study, two cases of
extranodal FDCS were reported, which affected the tonsils and soft
tissue of the chest wall were collected. Additionally, an analysis
of 102 cases of FDCS from the literature was performed to further
explore the biological behavior of extranodal FDCS.
Case report
Samples
The present study retrospectively reviewed all cases
of extranodal FDCS in the past 5 years, including excision surgery
and biopsy, a total of two cases were collected from the
pathological archives of the Affiliated Hospital of Chengde Medical
College (Chengde, China) between January 2013 and January 2020. Two
experienced pathologists reviewed all the slides of the two cases
independently, identifying the diagnosis. Case 1 was further
reviewed and confirmed by the pathologists of the Department of
Pathology of the China-Japan Friendship Hospital (Beijing, China).
The case history was consulted to collect the corresponding
clinical data. Additionally, two experienced radiologists were
asked to confirm the preoperative imaging test. The two patients
were recommended routine re-testing monthly and were followed up
until now (followed for 4 and 5 months, respectively), and all the
information was recorded.
Histological examination
Tissue specimens were collected for 10% buffered
formalin fixation, grossing, routine dehydration, embedding into
paraffin and sectioning into 4-µm-thick sections for hematoxylin
and eosin staining (10% buffered formalin for 12 h at room
temperature; hematoxylin for 5–15 min at room temperature; water
for 1-3secat room temperature; 1% acid alcohol for 1–3 sec at room
temperature; and eosin for 1–3 min, at room temperature).
Microscopic and immunohistochemical phenotypes were observed to
ensure the accuracy of the diagnosis, according to the diagnostic
criteria of the 2017 World Health Organization (WHO) Classification
of Tumors of Haematopoietic and Lymphoid Tissues (5).
Immunohistochemistry (IHC)
IHC was performed on paraffin blocks, using the
Leica automatic immunostaining device (Leica Microsystems, Inc.).
The 4-µm-thick sections were fixed using 10% buffered formalin for
12 h at room temperature. All monoclonal antibodies used were
purchased from OriGene Technologies, Inc., and are listed in
Table I. The known positive tissue
was used as the positive control, and PBS as the negative control.
The scoring method based on both the intensity (0, no staining; 1,
weak staining; 2, medium staining; and 3, strong staining) and
proportion of positive cells (0, 0%; 1, 1–25%; 2, 26–75%; and 3,
76–100%). The final staining scores were calculated by multiplying
the staining intensity score by the extent of staining score. A
final staining score of ≥3 was considered positive, and others were
classified as negative.
 | Table I.Antibodies and results of
immunostaining. |
Table I.
Antibodies and results of
immunostaining.
|
|
|
| Results |
|---|
|
|
|
|
|
|---|
| Antibodies | Clone no. | Catalogue
numbers | Case 1 | Case 2 |
|---|
| CD21 | EP64 | ZA-0525 | +/− | + |
| D2-40 | D2-40 | ZM-0465 | + | / |
| CD23 | OTI2B6 | TA801531 | +/− | / |
| Vimentin | EP21 | ZA-0511 | + | / |
| CD117 | EP10 | ZM-0371 | Partly + | / |
| EGFR | EP22 | ZA-0505 | Partly + | / |
| ALK | OTI1H7 | ZM-0248 | Partly + | – |
| HMB45 | HMB45 | ZM-0187 | Partly + | / |
| Ki-67 | EP5 | ZA-0502 | 10% | / |
| CK | AE1/AE3 | ZM-0069 | – | / |
| SMA | UMAB237 | ZM-0003 | – | / |
| Actin | HHF35 | ZM-0001 | – | / |
| P53 | EP9 | ZM-0408 | – | / |
| Desmin | EP15 | ZA-0610 | – | / |
| MelanA | A103 | TA807226 | – | / |
| CD1a | EP80 | ZA-0544 | – | – |
| CD34 | EP88 | ZA-0550 | – | / |
| EMA | UMAB57 | ZM-0095 | / | + |
| CD68 | PG-M1 | ZM-0464 | / | +/− |
| S-100 | 15E2E2+4C4.9 | ZM-0224 | / | – |
| Lyso | / | / | / | – |
| LCA |
2B11&PD7/26 | ZM-0183 | / | – |
| CD20 | EP7 | ZA-0293 | / | – |
| CD3 | EP4 | ZA-0503 | / | – |
| CD30 | EP154 | ZA-0591 | / | – |
| CD35 | EP197 | ZA-0638 | / | + |
Case description
Case 1
A 63-year-old male patient visited the doctor
presenting with a chest wall neoplasm without any complications in
September 2019. He had no weight loss, pain, fever or other feeling
of discomfort. Physical examination revealed a mass lesion
measuring 5×4 cm under the skin of the chest wall. No palpable
lymphadenopathy was found. The patient was suggested to undergo
surgery to resect the mass. Grossly, the resected tissue with skin
measured 6×5×1.5 cm. A white nodule measuring ~4.5×4 cm was found,
with a complete capsule and firm quality. Some gray regions were
observed on the cut surfaces, without necrosis and hemorrhage.
Microscopically, the neoplasm was composed of ovoid to
spindle-shaped tumor cells, arranged into whorls arrays. Collagen
fiber and multinucleated cells were observed in the background,
with areas showing clustering. Parts of the tumor were accompanied
with hemosiderin. Necrosis and hemorrhage were not observed.
Mitotic figures were rare (Ki-67 immunoproliferative index of ~10%;
Fig. 1D). A total of 500–2,000 tumor
cells were counted manually to evaluate the Ki-67
immunoproliferative index. The tumor was lightly infiltrated by
small lymphocytes with lymphoid follicle formation (Fig. 1A).
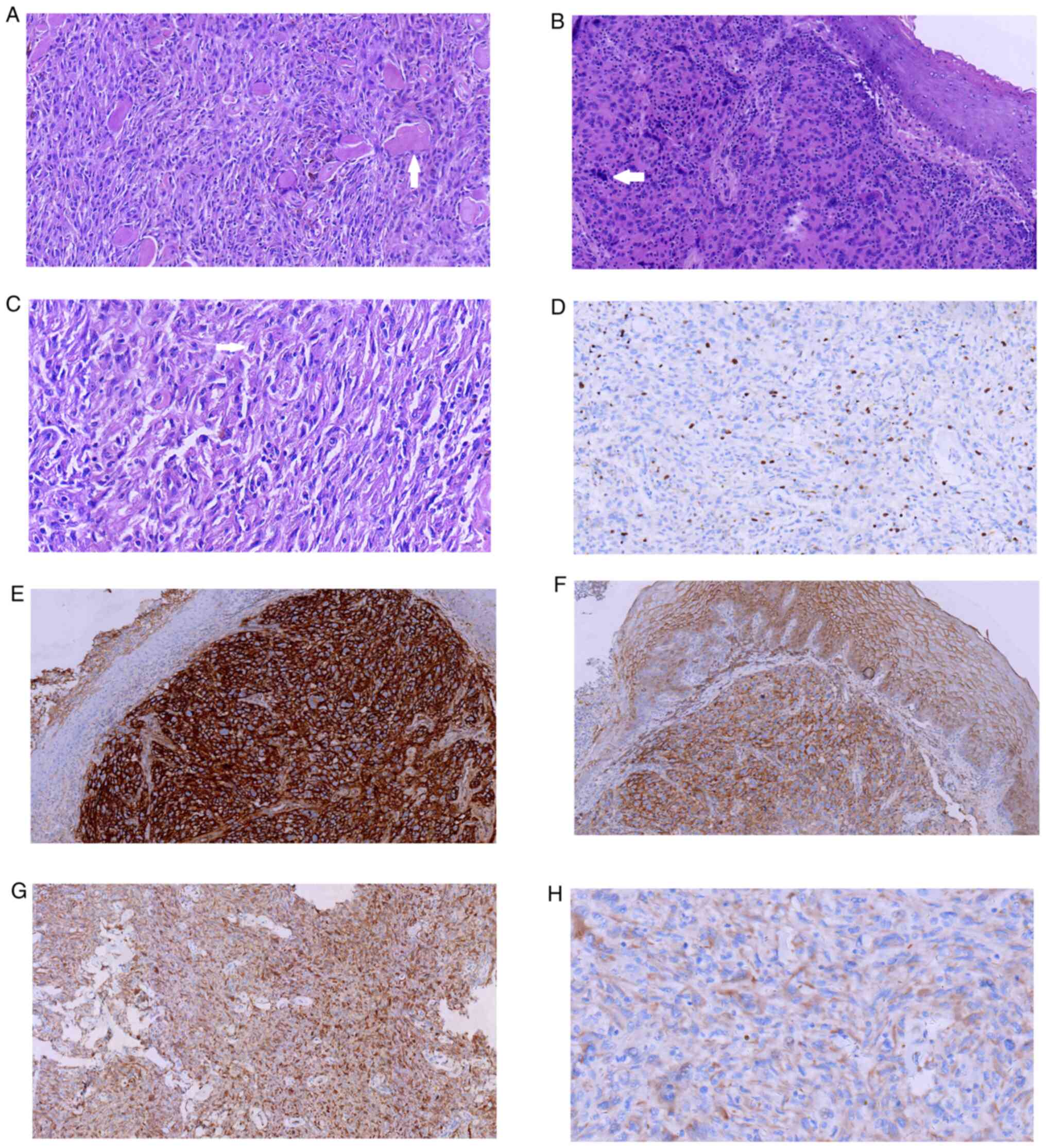 | Figure 1.Pathological features of extranodal
follicular dendritic cell sarcoma. (A) Neoplasm cells are arranged
in a fascicular pattern and storiform structure, with collagen
fibers seen in the background (HE; magnification, ×20). Arrow
denotes collagen fibers seen in the background. (B) Tumor cells
under the squamous cells (H&E; magnification, ×20). Arrow
denotes significant cytological atypia. (C) The shape of tumor
cells was ovoid-round (H&E; magnification, ×40). Arrow denotes
ovoid-shape of the tumor cells. (D) Ki-67 expression in case 1
(IHC; magnification, ×20). (E) CD21 expression in case 2 (IHC;
magnification, ×10). (F) EMA expression in case 2 (IHC;
magnification, ×10). (G) D2-40 expression in case 1 (IHC;
magnification, ×10). (H) CD35 expression in case 2 (IHC;
magnification, ×20). HE, hematoxylin and eosin; IHC,
immunohistochemistry. |
IHC revealed that the tumor cells were positive for
D2-40 (Fig. 1G), Vimentin, CD21,
CD23, CD117, EGFR, ALK and HMB45, and negative for cytokeratin
(CK), actin smooth muscle (SMA), Actin, P53, Desmin, MelanA, CD1a
and CD34 (Fig. 1D and G).
The diagnosis of FDCS was made based on the
morphological and immunological characteristics. In view of its
rarity, the patient was suggested to further consult the
pathologists of the Department of Pathology of the China-Japan
Friendship Hospital, and the diagnosis was determined again. The
postoperative recovery of the patient was rapid without
complications. During the 5-month post-operative follow-up period,
no recurrence, metastasis or other signs of discomfort were
observed.
Case 2
A 70-year old male patient checked into the Oncology
Department of the Affiliated Hospital of Chengde Medical College,
with an abnormal sensation in the throat for 3 months. The patient
had no dyscatabrosis, fever or dyspnea. Upon ultrasound
examination, a suspicious mass was revealed at the right tonsil.
The cervical lymph node was not enlarged. A right tonsillectomy was
performed under general anesthesia. Grossly, fragmentary tissue
measuring 2×2×0.5 cm was observed. Microscopically, the biggest
part of the tonsil was covered by mature squamous cells, while an
ulcer was found on the surface. Under the squamous cells, an ovoid
to round-shaped tumor formed solid or nested patterns. Some tumor
cells exhibited prolonged nuclei and obvious nucleoli, as well as
an eosinophilic cytoplasm. Significant cytological atypia, focal
coagulative necrosis and hemorrhage were identified. Lymphocytes,
eosinophils and plasmocytes were also observed under light
microscopy (Fig. 1B and C).
Immunohistochemically, the tumor cells were positive for CD21,
epithelial membrane antigen (EMA) and CD35 (Fig. 1E, F and H, respectively), weakly
positive for CD68, and negative for CD20, CD3, CD30, S-100, Lyso,
CD1a, ALK and leukocyte common antigen (LCA). The patient underwent
postoperative radiotherapy as well. During the 4-month follow-up
period after undergoing tonsillectomy, no local recurrence or
distant metastasis was observed.
Literature review
Literature review
The literature was reviewed by searching the key
words ‘extranodal follicular dendritic cell’ on PubMed (www.ncbi.nlm.nih.gov/pubmed), and the Chinese
literature was searched on the China National Knowledge
Infrastructure (http://www.cnki.net/) between 2008
and 2019. All the cases of FDCS, except those in lymph nodes, were
collected. As much of the literature as possible was consulted to
ensure each case was reported only once and discern the renewed
information about the cases.
Statistical analysis
SPSS 17.0 (SPSS, Inc.) was used for statistical
analysis. The Life Tables method was used to calculate the overall
and tumor-free survival rates. The association between
clinicopathological parameters and prognosis were calculated using
Kaplan-Meier estimation and the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Clinical features of extranodal
FDCS
A total of 102 cases of extranodal FDCS were
retrieved from the literature (Table
II) (1–55). The clinicopathological features,
management and clinical outcomes were extracted and recorded. Of
the 102 cases, 55 were male and 47 female (male to female ratio,
1.17:1) and the age range was 16–79 years at the time of diagnosis
(mean age, 48.87 years; median age, 47.5 years). The tumors were
located in different regions, including the abdominal cavity
(n=46), tonsil (n=20), oropharynx (n=15), mediastinum (n=12), neck
soft tissue (n=2), pelvic cavity (n=2), bone (n=1), lower limbs
(n=1), intracranium (n=1), esophagus (n=1) and thyroid (n=1). The
tumor longitudinal diameter measured 1–20 cm, with a mean diameter
of 6.63 cm, an abdominal cavity diameter of 8.33 cm and a diameter
of the outside of the abdominal cavity of 4.74 cm. A total of 8
cases were coexistent with other diseases, including 1 with
Castleman disease, 3 with paraneoplastic pemphigus, 2 with chronic
hepatitis B virus infection and 2 with carcinoma.
 | Table II.Clinical characteristics of 102
patients with extranodal FDCS from the literature. |
Table II.
Clinical characteristics of 102
patients with extranodal FDCS from the literature.
| Case no. | Age, years | Sex | Size, cm | Mitotic Counts (/10
HPF) | Site | Necrosis | Initial
diagnosis | EBER | Treatment | Follow-up,
months |
Recurrence/metastasis, months | Status | (Refs.) |
|---|
| 1 | 62 | M | NA | NA | Mediastinal | Yes | FDCS | NA | Surg+ChT | 24 | No | STD | (1) |
| 2 | 46 | M | NA | NA | Mediastinal | Yes | FDCS | NA | Surg+RT | 12 | No | NED | (1) |
| 3 | 31 | M | NA | NA | Mediastina | No | FDCS | NA | Surg | 10 | No | NED | (1) |
| 4 | 43 | M | 5 | Obvious | Mouth, tongue | No | Paraneoplastic
pemphigus | NA | NA | NA | NA | NA | (2) |
| 5 | 36 | F | 3 | NA | Tonsil | NA | Non-specific
inflammation | – | Surg | 15 | Recurrence, 6 | AWD | (3) |
| 6 | 59 | F | 4.5 | NA | Tonsil | NA | Benign tumor | – | NA | 24 | Recurrence, 17 | STD | (3) |
| 7 | 64 | F | 6.0 | NA | Oropharyngeal | NA | Squamous
Carcinoma | – | Surg+ChT | 7 | No | STD | (3) |
| 8 | 59 | M | 4.6 | NA | Right tonsil | NA | Lymphoma | NA | Surg+RT | 44 | No | NED | (4) |
| 9 | 31 | F | 3.5 | NA | Liver | NA | FDCS | + | Surg | 10 | No | NED | (5) |
| 10 | 48 | M | 10.0 | NA | Liver | NA | FDCS | + | Surg | 2 | No | NED | (5) |
| 11 | 54 | F | 3.5 | NA | Spleen | NA | FDCS | NA | Surg | 10 | No | NED | (6) |
| 12 | 79 | M | 6.0 | NA | Spleen | NA | FDCS | NA | Surg | 18 | No | NED | (6) |
| 13 | 46 | M | 12.0 | <10 | Abdominal | Yes | FDCS | NA | Surg | 12 | No | NED | (7) |
| 14 | 60 | F | 2.0 | Obvious | Stomach | NA | FDCS | NA | Surg | 8 | Recurrence, 8 | STD | (8) |
| 15 | 47 | M | 4.5 | Few | Hepatogastric
Ligament | NA | FDCS | NA | Surg | 3 | No | NED | (9) |
| 16 | 46 | F | 8.6 | 12 |
Retroperitoneal | Yes | FDCS | NA | Surg+ChT+RT | 36 | Metastasis, 36 | AWD | (10) |
| 17 | 67 | F | 4.0 | NA | Liver | NA | FDCS | + | Surg | 36 | No | NED | (11) |
| 18 | 50 | M | 3.1 | NA | Liver | Yes | FDCS | + | Surg | 6 | Metastasis, 6 | AWD | (12) |
| 19 | 39 | M | 18.0 | Rare |
Intraperitoneal | Yes | FDCS | – | Surg | 1 | No | STD | (13) |
| 20 | 16 | F | 8.0 | Rare | R Posterior
Mediastinum | Yes | FDCS | – | Surg | 24 | No | NED | (14) |
| 21 | 60 | M | 5.0 | <1 | Tonsil | NA | Granuloma | NA | Surg+RT | 86 | No | NED | (15) |
| 22 | 35 | F | 5.0 | 10 | Parapharyn-geal
Space | NA | Nasopharyngeal
carcinoma | NA | Surg | 12 | Recurrence, 2 | AWD | (15) |
| 23 | 63 | M | 4.0 | 1 | Infratemporal
fossa | NA | PNET | – | Surg+RT+ChT | 72 | No | NED | (15) |
| 24 | 30 | F | 5.0 | 9 | Pyform Sinus | NA | FDCS | – | Surg | 25 | Metastasis, 25 | AWD | (15) |
| 25 | 23 | M | 8.0 | 3 | Mediastinum | NA | Malignant nerve
sheath tumor | – | Surg+RT+ChT | 45 | Metastasis, 45 | AWD | (15) |
| 26 | 45 | M | 14.5 | <1 | Liver | NA | FDCS | + | Surg | 27 | No | NED | (15) |
| 27 | 36 | F | 15.0 | 7 | Mesentery | NA | Malignant GIST | – | Surg | 27 | Metastasis, 4 | AWD | (15) |
| 28 | 28 | F | 6.0 | 3 | Parapharyn-Geal
Space | NA | FDCS | – | Surg+RT+ChT | 22 | Metastasis, 14 | AWD | (15) |
| 29 | 55 | M | 2.0 | 9 | Tonsil | NA | FDCS | NA | Surg+RT | 21 | Recurrence, 18 | AWD | (15) |
| 30 | 63 | F | 4.0 | Obvious | Urinary
bladder | Yes | Infiltrating
Urothelial Carcinoma | – | Surg+RT | 24 | Metastasis, 24 | AWD | (16) |
| 31 | 66 | F | 2.3 | NA | Liver | NA | FDCS | + | Surg+RT+ChT | 12 | Metastasis, 12 | AWD | (17) |
| 32 | 65 | M | 1.0 | 16 | Tonsil | NA | FDCS | NA | Surg+RT | 24 | No | NED | (18) |
| 33 | 19 | F | 4.0 | >10f | Small
intestine | Yes | FDCS | – | Surg+ChT | 8 | No | NED | (19) |
| 34 | 72 | F | 4.3 | Obvious | Middle
Mediastinum | NA | FDCS | NA | RT | 12 | No | STD | (20) |
| 35 | 51 | F | 9.1 | Obvious | Middle
Mediastinum | NA | FDCS | NA | Surg+RT | 10 | Metastasis, 10 | STD | (20) |
| 36 | 53 | F | 10.3 | NA | Anterior
Mediastinum | NA | FDCS | NA | Surg | 18 | No | NED | (20) |
| 37 | 72 | M | 3.5 | NA | Tonsil | NA | FDCS | NA | Surg+ChT | 12 | No | STD | (21) |
| 38 | 43 | M | 20.0 | NA | Mesentery | Yes | FDCS | NA | Surg | 18 | No | NED | (22) |
| 39 | 22 | M | 2.0 | NA | Parapharyngeal
Space | NA | FDCS | – | Surg+RT | 26 | No | NED | (23) |
| 40 | 74 | M | 3.3 | 10-13 | Small intestine
Mesentery | Yes | FDCS | NA | Surg | 12 | No | STD | (24) |
| 41 | 34 | F | 9.0 | Obvious | Small intestine
Mesentery | NA | FDCS | NA | Surg+RT | 96 | No | NED | (24) |
| 42 | 31 | M | 4.7 | 0-1 | L Parapharynx | No | FDCS | NA | Surg+RT | NA | NA | NA | (25) |
| 43 | 73 | M | NA | 30 | Urinary
bladder | No | FDCS | NA | Surg+ChT | 1.5 | Recurrence,
1.5 | AWD | (26) |
| 44 | 64 | F | 19.0 | NA | Spleen | NA | FDCS | NA | Surg | 36 | No | NED | (27) |
| 45 | 63 | M | NA | NA | L Tonsil | NA | Paraganglioma | NA | Surg+RT | 52 | Recurrence, 52 | AWD | (28) |
| 46 | 26 | M | NA | NA | Nasopharynx | NA | Poorly
Differentiated Carcinoma | NA | Surg+RT+ChT | 44 | Recurrence, 44 | AWD | (28) |
| 47 | 64 | M | NA | NA | Hypopharynx | NA | Poorly
Differentiated Carcinoma | NA | Surg+RT | 39 | Recurrence, 39 | AWD | (28) |
| 48 | 28 | M | NA | NA | R Tonsil | NA | FDCS | NA | Surg+RT+ChT | NA | NA | NA | (28) |
| 49 | 66 | M | NA | NA | R Tonsil | NA | FDCS | NA | Surg+RT | 31 | No | NED | (28) |
| 50 | 68 | F | NA | NA | L Tonsil | NA | FDCS | NA | Surg | 19 | Recurrence, 19 | AWD | (28) |
| 51 | 65 | F | NA | NA | L Tonsil | NA | FDCS | NA | Surg+ChT | 47 | Recurrence, 47 | AWD | (28) |
| 52 | 40 | M | NA | NA | L Tonsil | NA | FDCS | NA | Surg+ChT | NA | NA | NA | (28) |
| 53 | 51 | F | NA | NA | L Tonsil | NA | FDCS | NA | Surg+ChT | NA | NA | NA | (28) |
| 54 | 38 | M | NA | NA | R Tonsil | NA | FDCS | NA | Surg+RT+ChT | 45 | Recurrence, 45 | AWD | (28) |
| 55 | 24 | M | NA | NA | Bone | NA | FDCS | – | ChT | 108 | No | NED | (29) |
| 56 | 24 | F | NA | NA | Pelvic, abdominal
Cavity | NA | FDCS | – | Surg+ChT | 5 | No | STD | (29) |
| 57 | 61 | F | NA | Obvious | Intracranial | Yes | FDCS | NA | Surg+ChT | 12 | No | STD | (30) |
| 58 | 67 | F | 1.5 | 15 | Esophagus | NA | FDCS | – | Surg+RT+ChT | 26 | Metastasis, 24 | STD | (31) |
| 59 | 42 | M | 5.0 | NA | Neck | NA | FDCS | NA | Surg | 12 | No | NED | (32) |
| 60 | 24 | F | 6.5 | 8 | Ileocecal
region | NA | FDCS | – | Surg | 12 | No | NED | (33) |
| 61 | 27 | M | 2.8 | Rare | Tonsil | NA | FDCS | NA | Surg+RT | 6 | No | NED | (34) |
| 62 | 67 | F | 4.5 | Obvious | Pancreas | Yes | FDCS | – | Surg | NA | NA | NA | (35) |
| 63 | 63 | F | 13.4 | 3 | R Liver | Yes | FDCS | + | Surg | 48 | No | NED | (36) |
| 64 | 46 | F | 11.0 | NA | Posterior
Mediastinal | NA | Fibrosing
Mediastinitis | – | Surg+ChT+RT | 27 | Metastasis, 24 | STD | (37) |
| 65 | 44 | F | NA | NA | Thyroid | NA | FDCS | NA | Surg+RT | NA | NA | NA | (38) |
| 66 | 59 | F | 4.7 | NA | Soft palate | NA | FDCS | NA | Surg+RT | 12 | No | NED | (39) |
| 67 | 78 | F | 3.9 | Rare | Colonic | NA | FDCS | + | Surg | 5 | No | NED | (40) |
| 68 | 22 | M | NA | 10 | Mesentery | NA | High-Risk GIST | NA | Surg+ChT | NA | NA | NA | (41) |
| 69 | 28 | M | NA | NA | mesentery | NA | GIST | NA | NA | 60 | Metastasis, 48 | AWD | (42) |
| 70 | 63 | M | 12.0 | NA |
Retroperitoneal | NA | GIST | NA | Surg+ChT+RT | 60 | Metastasis, 48 | AWD | (42) |
| 71 | 59 | F | NA | Obvious | Thigh | Yes | FDCS | NA | NA | NA | NA | NA | (43) |
| 72 | 70 | M | 14.0 | NA | Pancreas
Spleen | Yes | FDCS | + | NA | NA | NA | NA | (44) |
| 73 | 37 | M | 11.0 | NA | Mediastinum | Yes | FDCS | NA | Surg+ChT | 6 | No | NED | (45) |
| 74 | 39 | M | 6.0 | NA | Mediastinum | NA | FDCS | NA | Surg+RT | 96 | No | NED | (46) |
| 75 | 61 | M | 10.0 | Rare | Spleen | Yes | FDCS | + | NA | 12 | No | NED | (47) |
| 76 | 19 | F | 6.0 | NA | Liver | NA | FDCS | + | Surg | 12 | No | NED | (48) |
| 77 | 60 | M | 4.5 | NA | Neck | NA | FDCS | NA | Surg | NA | NA | NA | (49) |
| 78 | 53 | M | 1.0 | NA | Tonsil | NA | FDCS | NA | Surg | NA | NA | NA | (49) |
| 79 | 43 | F | 2.0 | NA | Tonsil | NA | FDCS | NA | Surg | 51 | No | NED | (49) |
| 80 | 42 | M | 12.0 | NA | Omentum | NA | FDCS | NA | Surg+ChT | 10 | No | STD | (49) |
| 81 | 45 | M | 7.5 | NA | Posterior
Mediastinum | NA | FDCS | NA | Surg | 36 | No | NED | (49) |
| 82 | 60 | M | 2.2 | NA | Spleen | NA | FDCS | NA | Surg | 5 | No | NED | (49) |
| 83 | 46 | F | 6.0 | NA | Liver | NA | FDCS | NA | Surg | 2 | No | NED | (49) |
| 84 | 71 | M | 5.5 | NA | Spleen | NA | FDCS | + | Surg | 26 | No | NED | (50) |
| 85 | 32 | M | 3.0 | NA | Liver | NA | FDCS | + | Surg | 19 | No | NED | (50) |
| 86 | 69 | F | 9.0 | NA | Spleen | Yes | FDCS | + | Surg | 1 | No | NED | (50) |
| 87 | 59 | F | 15.0 | >10 | Spleen | NA | FDCS | + | Surg | 48 | No | NED | (51) |
| 88 | 71 | F | 4.5 | >10 | Spleen | NA | FDCS | + | Surg | 24 | No | NED | (51) |
| 89 | 77 | M | 4.6 | >10 | Spleen | NA | FDCS | + | Surg | 12 | No | NED | (51) |
| 90 | 45 | F | 1.8 | >10 | Liver | NA | FDCS | + | Surg | 5 | No | NED | (51) |
| 91 | 30 | F | 1.0 | >10 | Tonsil | NA | FDCS | – | Surg | 3 | No | NED | (51) |
| 92 | 62 | M | 12.0 | >3 | Mesentery | Yes | FDCS | – | Surg+ChT | 2 | No | NED | (52) |
| 93 | 26 | M | NA | NA | Spleen | NA | FDCS | – | Surg | NA | NA | NA | (53) |
| 94 | 57 | M | NA | NA |
Retroperitoneal | NA | FDCS | – | Surg | NA | NA | NA | (53) |
| 95 | 24 | M | NA | NA | Pelvis | NA | FDCS | – | Surg | 10 | No | NED | (53) |
| 96 | 70 | M | 3.9 | 10 | Pharyngeal | NA | FDCS |
| Surg | NA | NA | NA | (54) |
| 97 | 40 | F | 2.0 | 2 | Pharyngeal | NA | Malignant Fibrous
Histiocytoma | NA | Surg | NA | NA | NA | (54) |
| 98 | 38 | M | 5.0 | 20 | Pharyngeal | NA | FDCS | NA | Surg | NA | NA | NA | (54) |
| 99 | 45 | M | 2.0 | NA | Nasal cavity | NA | FDCS | + | Surg | NA | NA | NA | (55) |
| 100 | 59 | F | 19.0 | NA | Small
intestine | NA | FDCS | NA | Surg+ChT | NA | NA | NA | (55) |
| 101 | 37 | F | 3.5 | >30 | Tonsil | NA | FDCS | NA | Surg+ChT | 28 | No | STD | (55) |
| 102 | 31 | F | 10.5 | NA | Mesojejunum | NA | FDCS | NA | Surg | NA | NA | NA | (55) |
Pathological features of extranodal
FDCS
Most of the cases from the literature were well
circumscribed, with grey white-red cutting surfaces. Focal
coagulative necrosis (n=20) and hemorrhage (n=14) were observed in
some cases. Mitotic figures were rarely observed [range,
rare-30/high power field (HPF)]. Generally, extranodal FDCS is
positive for specific markers for DCs, such as CD23, D2-40, CD35
and CD21. One case of urinary bladder FDCS was negative for BRAF.
EBV-encoded RNA (EBER) was detected using in situ
hybridization (ISH) in 53 cases and it was positive in 20 and
negative in 23 cases, most located in the liver and spleen. A total
of 19 cases were misdiagnosed to other tumors or inflammatory
lesions (Table II).
Management and clinical outcomes of
extranodal FDCS
The data of the management of the extranodal FDCS
cases were available for 95 cases. A total of 50 cases underwent
surgery to resect the neoplasm. A total of 1 case received
chemotherapy procedures, while 44 cases came to the clinic for
adjuvant treatment (17 cases for radiation treatment, 16 for
chemotherapy and 11 for both). Complete follow-up information was
accessible in 82 cases. The follow-up duration was 1–108 months,
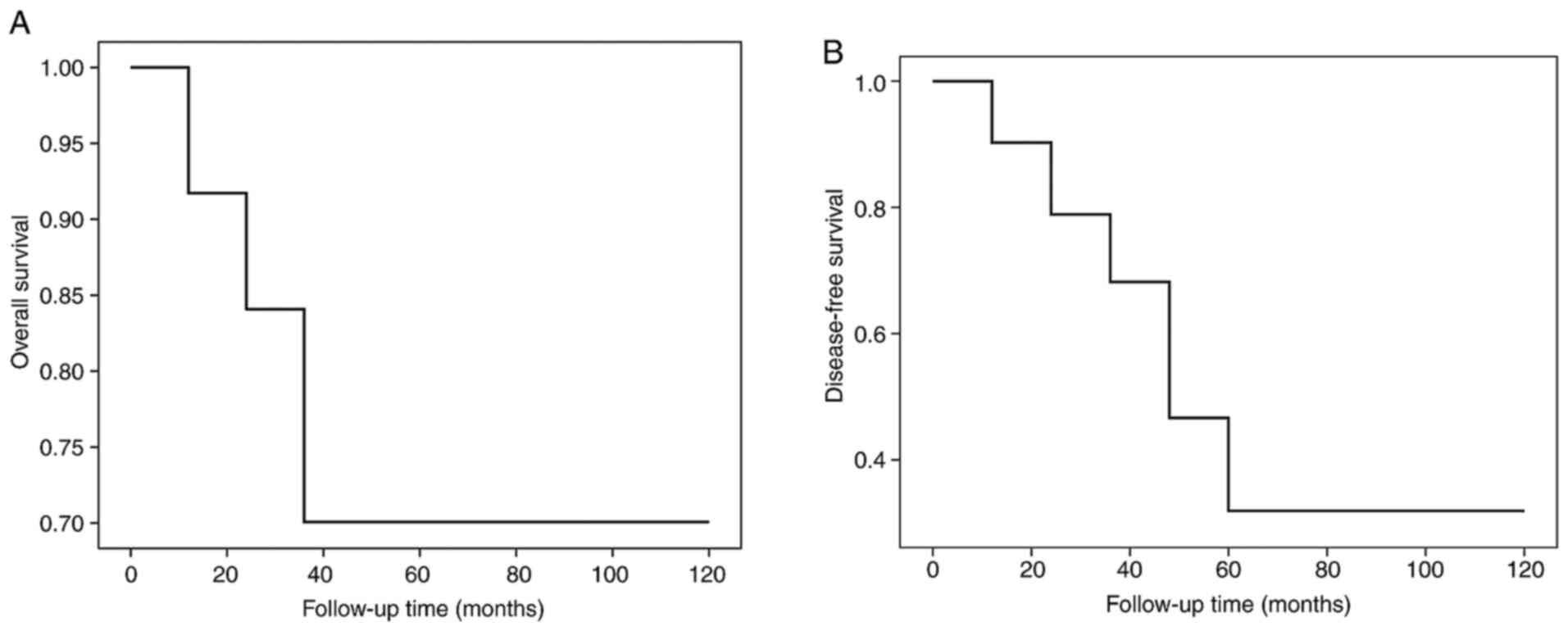
with an average of 24.31±22.98 months (Table II). The 2- and 5-year total survival
rates were 70 and 70%, respectively. The 2- and 5-year tumor-free
total survival rates were 68 and 32%, respectively (Table III and Fig. 2). The rates of recurrence, metastasis
and mortality were 14.63 (12/82), 17.07 (14/82) and 18.29% (15/82),
respectively (Table III). The
median survival time was 108 months.
 | Table III.Survival rates. |
Table III.
Survival rates.
| Characteristic | Total, n | OS rate, %
2-year | 5-year | P-value | DFS rate, %
2-year | 5-year | P-value | Recurrence rate, %
(n/total) | Metastasis rate, %
(n/total) | Mortality rate, %
(n/total) |
|---|
| Available
total | 82 | 70 | 70 |
| 68 | 32 |
| 14.63 (12/82) | 17.07 (14/82) | 18.29 (15/82) |
| Sex | 82 |
|
|
|
|
|
|
|
|
|
|
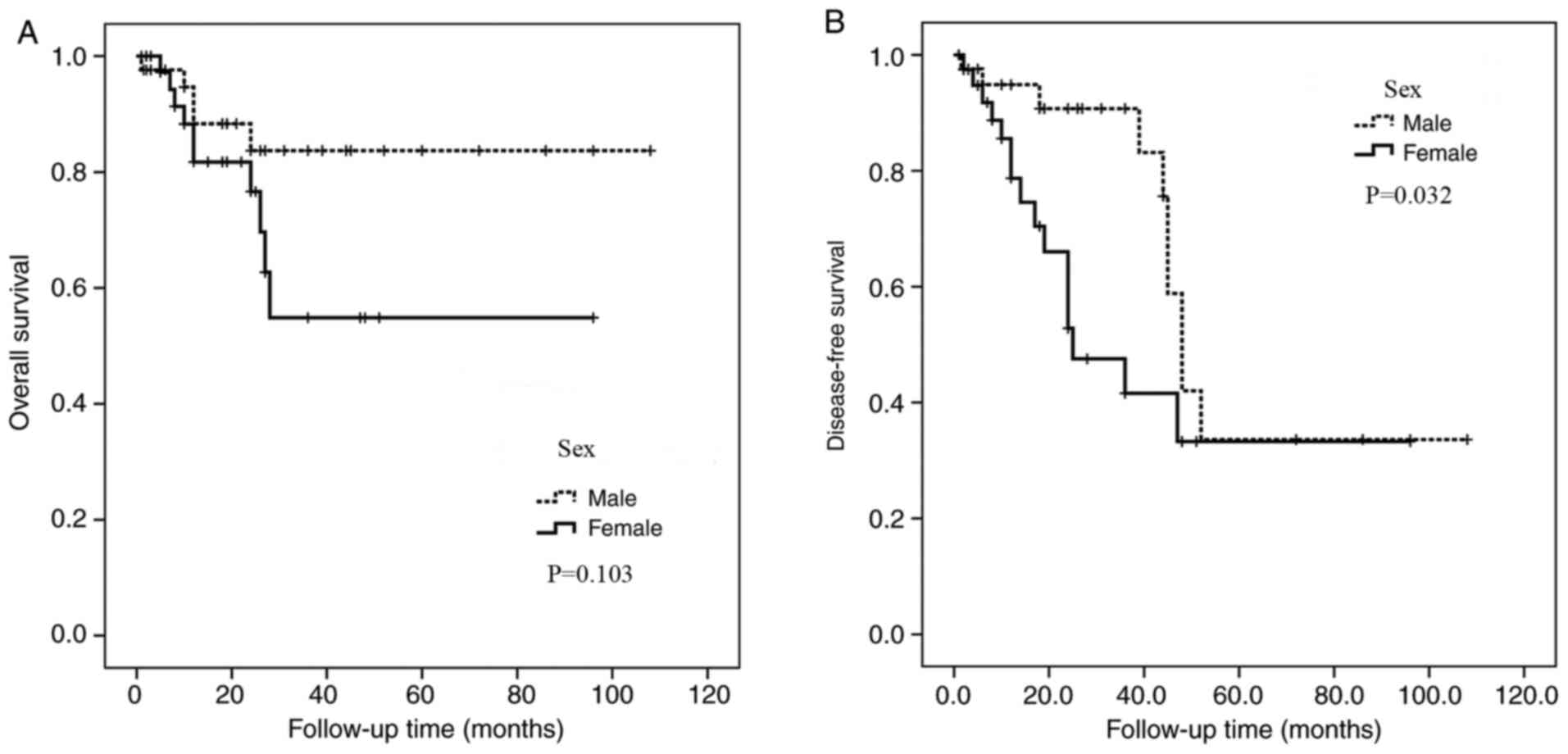
Male | 42 | 82 | 82 | 0.103 | 91 | 35 | 0.032 | 14.29 (6/42) | 9.52 (4/42) | 11.90 (5/42) |
|
Female | 40 | 57 | 57 |
| 47 | 34 |
| 15.00 (6/40) | 25.00 (10/40) | 25.00 (10/40) |
| Age, years | 82 |
|
|
|
|
|
|
|
|
|
|
<50 | 41 | 78 | 78 | 0.274 | 74 | 33 | 0.623 | 9.76 (4/41) | 17.07 (7/41) | 12.20 (5/41) |
|
≥50 | 41 | 64 | 64 |
| 63 | 30 |
| 19.51 (8/41) | 17.07 (7/41) | 24.39 (10/41) |
| Size, cm | 66 |
|
|
|
|
|
|
|
|
|
|
<4 | 20 | 36 | / | 0.119 | 45 | / | 0.235 | 15.00 (3/20) | 15.00 (3/20) | 25.00 (5/20) |
| ≥4 | 46 | 87 | 77 |
| 69 | 47 |
| 4.35 (2/46) | 21.74 (10/46) | 15.22 (7/46) |
| Treatment | 77 |
|
|
|
|
|
|
|
|
|
|
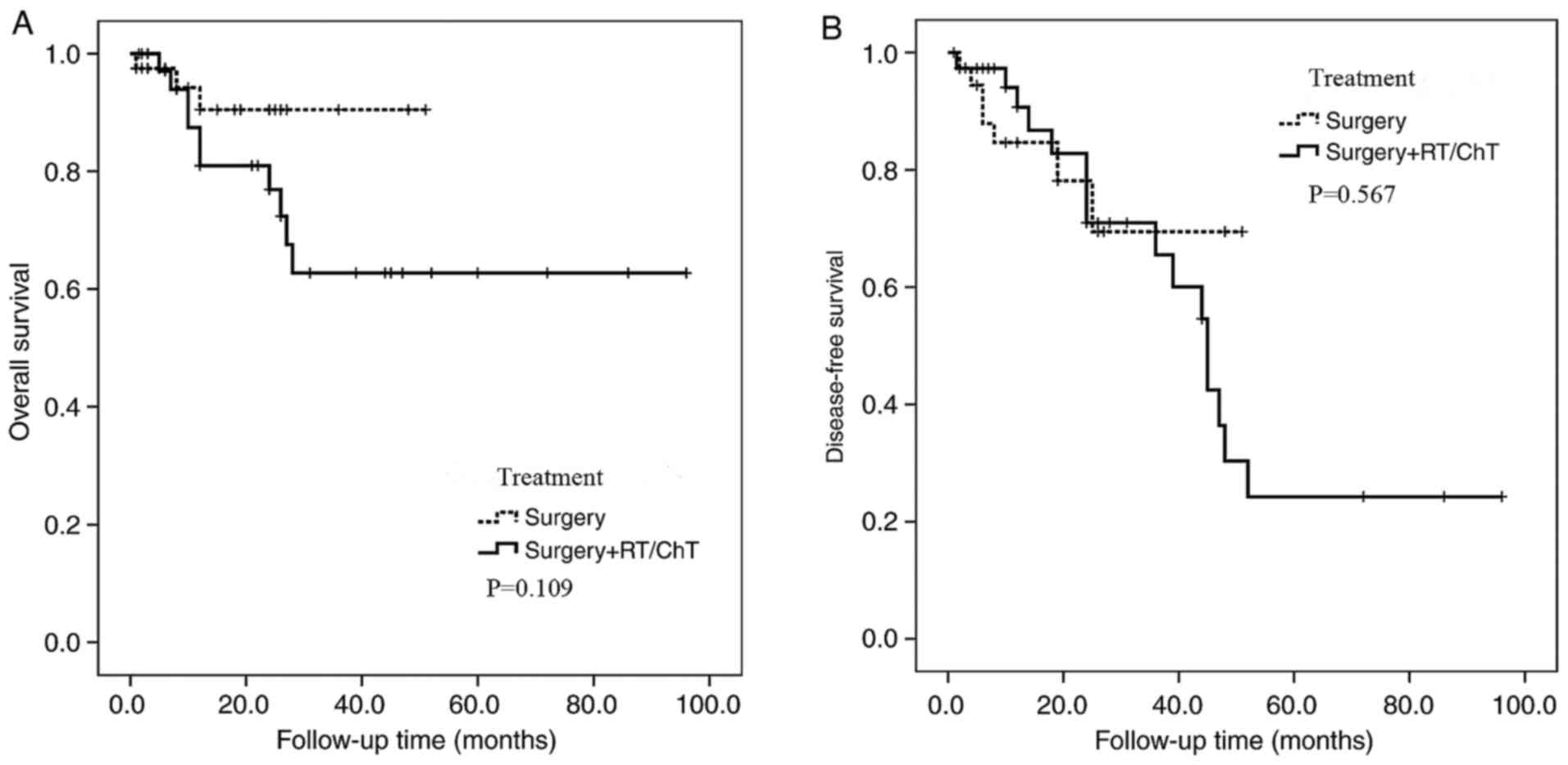
Surgery | 40 | 89 | / | 0.109 | 80 | / | 0.567 | 10.00 (4/40) | 7.50 (3/40) | 7.50 (3/40) |
| Surgery
+ RT/ChT | 37 | 63 | 63 |
| 70 | 24 |
| 18.91 (7/37) | 24.32 (9/37) | 27.30 (10/37) |
Prognostic factors of extranodal
FDCS
The Kaplan-Meier method was used to analyze the
association between clinicopathological features and prognosis
(Table III and Figs. 3–6).
Upon this analysis, it was found that sex was associated with
disease-free survival (P=0.032; Table
III), with female patients having a poorer prognosis than male
patients. Kaplan-Meier estimation exhibited no other statistically
significant differences between disease-free survival rates or
overall survival rates and age, tumor size or treatment (Table III).
Discussion
FDCS is an extremely rare tumor that affects the
lymphoid tissues and mostly presents in the lymph nodes, while the
extranodal type of the disease accounts for only one-third of cases
(5). Due to the limited number of
reported cases, a proportion of FDCSs, particularly extranodal
FDCSs, has been difficult to recognize, especially on purely
morphological grounds (11). FDCS
has been proven to derive from DCs or macrophages, making it
similar to diffuse large B-cell lymphoma or anaplastic large cell
lymphoma, and therefore complicating its diagnosis (6,9).
Additionally, the diagnosis of extranodal FDCS is even more
challenging (2,5,10).
Currently, to the best of our knowledge, the clinical
manifestations and prognosis-associated factors of extranodal FDCS
have not been statistically described. The present study presented
two cases of extranodal FDCS affecting the tonsil and soft tissue
of the chest wall, respectively. Additionally, 102 cases of
extranodal FDCS from the literature were analyzed (1–55).
The existence of FDC tumors (FDCTs) was first
described by Lennert in 1978 (56),
but it was Monda et al (57)
who in 1986 recognized and characterized this type of tumor. As
antigen-presenting cells, DCs can be found in various sites and
participate in multiple types of activations (20,22).
Langerhans cells are specialized dendritic cells in mucosal sites
and skin that upon activation become specialized for antigen
presentation to T cells, and then migrate to the lymph node through
lymphatics (14,15). In contrast to other types of
myeloid-derived DCs (such as Langerhans cells, interdigitating DCs
and dermal/interstitial DCs), FDCs seem to stem from bone marrow
stromal cells, with myofibroblasts as a characteristic (35,42).
FDCs are located in primary and secondary follicles, trapping and
presenting antigens to B cells, and storing immune complexes for
long periods of time on the cell surface (58,59). The
cause of FDCT remains unknown; potential risk factors may be
Epstein Barr Virus (EBV) infection or Castleman disease (2,26), which
may be found concurrently with FDCS or may precede the latter by
several years (27). EBV is
suspected to carry a viral oncogene-latent membrane protein 1 that
may encourage transformation, often detected in the spleen and
liver (30,34).
Among the 102 cases from the literature, EBER was
positive mostly in the liver and spleen, except for one case in the
colon. A number of cases appeared to be associated with autoimmune
diseases, such as paraneoplastic pemphigus and myasthenia gravis.
It has been suggested that FDCS encourages aberrant immune system
activation, given that patients often demonstrate immature T cells
(8,15).
The epidemiology of FDCS is unclear. A wide age
range has been reported, but FDCS was most common in adults
(50). Similarly, the mean age of
the patients in the present study was 48.87 years (range, 16–79
years), and the median age was 47.5 at initial presentation. The
sex distribution was similar, and the overall male to female ratio
was 1.17:1. In the present study, FDCS was slightly more common in
males, which was inconsistent with the results of Shaw et al
(8). The mean diameter of the tumors
was 6.63 cm (range, 1–20 cm). Tumor size was closely associated
with the primary site and was larger in the inner abdomen compared
with in other sites. Most FDCS present as lymphadenopathy, but a
number of extranodal regions, such as the soft tissue, tonsil,
stomach and intestines, were found to be the primary sites.
Systemic symptoms were uncommon. Sometimes patients complained of a
slow-growing, painless lump, while others visited the doctor
presenting with abdominal pain, which was usually due to an
abdominal tumor; rarely patients had paraneoplastic pemphigus (such
as 2 cases in the present literature review).
The gross observation and histopathology are
manifold. Overall, the cut surface of most extranodal FDCS had a
yellowish white appearance, was circumscribed and caused extrinsic
compression in some cases. Microscopically, the neoplastic cells
were arranged in a fascicular pattern and had a storiform
structure, with an ovoid-round shape. Similar to meningioma, a
whorl pattern was observed in certain areas. At high power, tumor
cells with a slightly eosinophilic cytoplasm, distinct elongated
nuclei and cell membrane were observed. Lymphocytes were dispersed
characteristically in the background. The mitotic rate was
0–10/HPF, with a higher rate of >30/HPF in pleomorphic cases,
with easily seen coagulative necrosis and pathological
karyokinesis. FDCS is classified into two types, the classic FDCS
and inflammatory pseudotumor (IPT)-like FDCS (35,48).
IPT-like FDCS is rarer than the classic type and typically presents
as a renal and hepatic lump (35,51).
Histologically, lymphoplasmacytic spindle cells infiltrate the
tumor, mainly including plasma cells, lymphocytes and a small
number of neutrophils, sometimes with a lymphoid follicle formation
(51). In the 2 cases of the present
study, tumor cells were ovoid-to-spindle-shaped, forming solid or
nested patterns or whorls arrays. The tumor was lightly infiltrated
by small lymphocytes and multinucleate cells. IHC and ISH are
essential for the diagnose of FDCS. No single marker is able to
identify all DC subsets; as described in the WHO classification
(58,60), important markers include D2-40, CD23,
CD21 and CD35 (3). In the two cases
of the present study, the tumor cells were both positive for
CD21.
The diagnosis of extranodal FDCS depends mainly on
pathology; therefore, due to its infrequency and non-specific
histopathological features, misdiagnosis occurs frequently. The
most common reason for misdiagnosis is failure to consider FDCS at
the initial pathological evaluation. By reviewing the
aforementioned literature, it was found that some cases were
misdiagnosed as non-specific inflammation, benign tumor, carcinoma,
lymphoma, granuloma, pancreatic neuroendocrine tumour and
gastrointestinal stromal tumour (GIST). In the study by Hu et
al (3), the misdiagnosis rate
was 57%, higher than that in the present study. Carcinoma and FDCS
cells are all ovoid cells with avesicular nuclei; however,
carcinomas are positive for CK and negative for CD21, CD23 and CD35
(21,53). Similarly to FDCS, GISTs exhibit
fascicles, storiform arrays and whorls patterns, and are negative
for CK, but immune histochemical markers are positive for Dog-1,
CD34 and CD117, and negative for specific markers for FDCS, which
may be used to distinguish GIST from FDCS (5,19).
The limited cytogenetic data exhibit complex
karyotypes. A targeted next-generation sequencing study indicated
frequent function loss alterations in tumor suppressor genes,
negative regulation of NF-κB and cell-cycle progression involvement
(60).
The use of the genomic sequencing approach enhanced
the understanding of genomic features of FDCS in the thyroid
(38). Extensive mutations were
detected, including VEGFR1, CLTCL1 and TP53 mutations and
hepatoma-derived growth factor related protein 3 (HDGFRP3) and Src
homology 2 domain containing family member 4 (SHC4) (10,19).
SHC4 is associated with the EGFR signaling pathway, from which it
was deduced that this pathway may serve a role in the
etiopathogenesis of FDCS (25). The
BRAF V600E mutation was also reported in 0–19% of cases (61,62).
The treatment of FDCS has not been standardized, as
there is no worldwide consensus due to the rarity of the reported
cases and limited prospective research on prognosis. The shortage
of medical molecular genetics hindered the development of targeted
treatments. In most cases, patients with FDCS receive surgery and
adjuvant radiotherapy or chemotherapy (33,52).
Radical dissection is an important treatment of regional lumps,
particularly tumors appearing to have clear boarders (63). Postoperative radiotherapy is
recommended, with total doses of 6,000-7,000 cGy in the head and
neck region (3,8). With regards to chemotherapy, the
options targeting non-Hodgkin's lymphoma are most commonly used
(64). However, it remains
controversial whether it is beneficial to administer radiotherapy
or chemotherapy post-surgically. In the present study, only 50
cases underwent surgery, and combination therapy was administered
post-operatively to 45 cases; however, a comparison of prognosis
between the surgery only and the adjuvant treatment groups did not
yield any significant results (P>0.05).
FDCS is a type of low-intermediate grade malignant
tumor. Due to a shortage of cases, the prognosis and predictive
factors are not definite. Saygin et al (65) reported 2-year survival rates for
early stage, local infiltration and distant metastasis stage of 82,
80 and 42%, respectively. By reviewing the data of 42 FDCS cases in
the tonsils, Lu et al (4)
revealed that the 3-year overall survival rate was 86.5%, a little
higher than the 5- and 8-year rates (both 77.8%). By reviewing 32
subjects of mediastinal FDCS, Wu et al (1) identified that the 1-year total and
tumor-free survival rates were 80.4 and 76.9%, respectively, the
3-year total and tumor-free survival rates were 68.5 and 51.7%,
respectively, and the 5-year rates were 58.8 and 32.3%,
respectively.
According to the investigation of WHO, prognostic
analysis of extranodal FDCS is scarce. In the present study, the
follow-up duration was 1–108 months, with an average of 24.31±22.98
months, and the 2- and 5-year total survival rates were both 70%.
The 2- and 5-year disease-free total survival rates were 68 and
32%, respectively. Domínguez-Malagón et al (66) demonstrated that FDCS originating from
the pharyngeal region had low recurrence (25%), metastasis (25%)
and mortality rates (5%), similar to those of Duan et al
(67) (23, 21 and 3%, respectively).
In the present study, the rates of recurrence, metastasis and
mortality were 14.63 (12/82), 17.07 (14/82) and 18.29% (15/82),
respectively. These different results may be due to the limitation
of the tumor sites. FDCS in the parapharyngeal space exhibited
poorer outcomes, while intra-abdominal tumors are more likely to
recur (40,42). However, in the present study, the
follow-up data available for analysis were scarce, the follow-up
time was short and the survival curves were founded on a small
number of cases, affecting the availability and effectiveness of
the present study. The prognostic factors of extranodal FDCS remain
unclear, and may include tumor diameter, necrosis and mitotic count
(65). Lu et al (4) reported that a large tumor size resulted
in a poor prognosis, and Hu et al (3) detected that combined treatment improved
survival rates. The current study revealed that sex was a
significant prognostic factor. However, Kaplan-Meier estimation
exhibited no other statistically significant differences between
disease-free survival rates or overall survival rates and age,
tumor size or treatment. Due to the scarcity of the follow-up data
available for analysis, the current data are insufficient, and more
data and further analyses are urgently required.
In conclusion, two rare cases of primary extranodal
FDCS were presented, and 102 cases from the literature were
reviewed. The present study described the known biological behavior
of extranodal FDCS. The confirmation of pathology of extranodal
FDCS is challenging, leading to further delays in diagnosis.
Surgical resection remains essential for definitive treatment.
Further research into the pathogenesis and therapy of FDCS is
required to improve the outcomes of this rare disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by Baoding Science
and Technology Project (grant no. 18ZF097).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XZ collected and analyzed the data, GZ made
substantial contributions to the acquisition of data, analysis and
interpretation of data and DS made substantial contributions to
conception and design. All authors wrote the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Chengde Medical College (Chengde, China;
approval no. LL049). Written informed consent was obtained from the
patients for the storage of samples and data, follow-up contact,
and further use of samples and data for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu YL, Wu F, Xu CP, Chen GL, Zhang Y, Chen
W, Yan XC and Duan GJ: Mediastinal follicular dendritic cell
sarcoma: A rare, potentially under-recognized, and often
misdiagnosed disease. Diagn Pathol. 14:52019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su Z, Liu G, Liu J, Fang T, Zeng Y, Zhang
H, Yang S, Wang Y, Zhang J, Wei J, et al: Paraneoplastic pemphigus
associated with follicular dendritic cell sarcoma: Report of a case
and review of literature. Int J Clin Exp Pathol. 8:11983–11994.
2015.PubMed/NCBI
|
|
3
|
Hu T, Wang X, Yu C, Yan J, Zhang X, Li L,
Li X, Zhang L, Wu J, Ma W, et al: Follicular dendritic cell sarcoma
of the pharyngeal region. Oncol Lett. 5:1467–1476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu ZJ, Li J, Zhou SH, Dai LB, Yan SX, Wu
TT and Bao YY: Follicular dendritic cell sarcoma of the right
tonsil: A case report and literature review. Oncol Lett. 9:575–582.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang BX, Chen ZH, Liu Y, Zeng YJ and Li
YC: Inflammatory pseudotumor-like follicular dendritic cell
sarcoma: A brief report of two cases. World J Gastrointest Oncol.
11:1231–1239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ge R, Liu C, Yin X, Chen J, Zhou X, Huang
C, Yu W and Shen X: Clinicopathologic characteristics of
inflammatory pseudotumor-like follicular dendritic cell sarcoma.
Int J Clin Exp Pathol. 7:2421–2429. 2014.PubMed/NCBI
|
|
7
|
Testori A, Meroni S, Colombo P, Fiori S,
Voulaz E and Alloisio M: Follicular dendritic cell sarcoma with
atypical features surrounding undescended testis: Description of a
rare case. World J Surg Oncol. 13:692015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaw D, Cuison R and Ito H: Follicular
dendritic cell sarcoma of the stomach: Case report and review of
the literature. Curr Oncol. 21:e775–e778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan WX, Yu YX, Zhang P, Liu XK and Li Y:
Follicular dendritic cell sarcoma detected in hepatogastric
ligament: A case report and review of the literature. World J Clin
Cases. 7:116–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan T, Yang Q, Zhang H, Li J and Zhang X:
A 46-year-old Chinese woman presenting with retroperitoneal
follicular dendritic cell sarcoma: A case report. J Med Case Rep.
8:1132014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng S and Gao J: Inflammatory
pseudotumor-like follicular dendritic cell sarcoma: A rare
presentation of a hepatic mass. Int J Clin Exp Pathol.
12:3149–3155. 2019.PubMed/NCBI
|
|
12
|
Chin KM, Ho WY, Lim KHT, Chung YFA and Lee
SY: Follicular dendritic cell sarcoma of the liver with
metachronous small bowel and splenic metastases: A case report and
literature review. Hepatobiliary Surg Nutr. 6:179–189. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akel R, Fakhri G, Salem R, Boulos F, Habib
K and Tfayli A: Paraneoplastic pemphigus as a first manifestation
of an intra-abdominal follicular dendritic cell sarcoma: Rare case
and review of the literature. Case Rep Oncol. 11:353–359. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyoshi R, Sonobe M, Miyamoto E and Date
H: Completely resected follicular dendritic cell sarcoma of the
posterior mediastinum: A report of a case. Surg Case Rep. 2:282016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Shi YH, Guo ZJ, Qiu T, Guo L, Yang
HY, Zhang X, Zhao XM and Su Q: Clinicopathological features and
prognosis assessment of extranodal follicular dendritic cell
sarcoma. World J Gastroenterol. 16:2504–2519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan GJ, Wu YL, Sun H, Lang L, Chen ZW and
Yan XC: Primary follicular dendritic cell sarcoma of the urinary
bladder: The first case report and potential diagnostic pitfalls.
Diagn Pathol. 12:352017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen HM, Shen YL and Liu M: Primary
hepatic follicular dendritic cell sarcoma: A case report. World J
Clin Cases. 7:785–791. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eun YG, Kim SW and Kwon KH: Follicular
dendritic cell sarcoma of the tonsil. Yonsei Med J. 51:602–604.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang YC, Chau IY, Yeh YC and Chau GY:
Small intestine follicular dendritic cell sarcoma with liver
metastasis: A case report. Medicine (Baltimore). 96:e72612017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu J, Dong D, Jiang Z and Hu H:
Clinicopathological characteristics of mediastinal follicular
dendritic cell sarcoma: Report of three cases. J Cardiothorac Surg.
11:562016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kara T, Serinsoz E, Arpaci RB and
Vayisoglu Y: Follicular dendritic cell sarcoma of the tonsil. BMJ
Case Rep. 2013:bcr20120074402013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Jin K, Yu X, Teng X, Zhou H, Wang Y,
Teng L and Cao F: Extranodal follicular dendritic cell sarcoma in
mesentery: A case report. Oncol Lett. 2:649–652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Hussain T, Saleem M, Velagapudi SB and
Dababo MA: Follicular dendritic cell sarcoma of parapharyngeal
space: A Case report and review of the literature. Head Neck
Pathol. 9:135–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xin Z and Kong D: Clinicopathologic
profile of extranodal follicular dendritic cell sarcoma in the
mesentery of small intestine: A study of two cases with literature
review. Int J Clin Exp Pathol. 11:2372–2376. 2018.PubMed/NCBI
|
|
25
|
Pyo JS, Kang G, Do SI, Chae SW, Kim K, Lee
SH, Choi YL, Choi JH, Sohn JH and Kim DH: Extranodal follicular
dendritic cell sarcoma with rapid growth in parapharynx: A case
report. Korean J Pathol. 46:306–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun J, Wang C, Wang D, Wu J, Wang L, Zhao
L and Teng L: Follicular dendritic cell sarcoma (FDCS) of urinary
bladder with coexisting urothelial carcinoma-a case report. BMC
Urol. 19:832019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Xu D, Qiao Z, Shen L, Dai H and Ji
Y: Follicular dendritic cell sarcoma of the spleen: A case report
and review of the literature. Oncol Lett. 12:2062–2064. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amirtham U, Manohar V, Kamath MP,
Srinivasamurthy PC, Chennagiriyappa LK, Shenoy AM, Renuka PK and
Kumar RV: Clinicopathological Profile and outcomes of follicular
dendritic cell sarcoma of the head and neck Region-A study of 10
cases with literature review. J Clin Diagn Res. 10:XC08–XC11.
2016.PubMed/NCBI
|
|
29
|
Ma Y, Sun J, Yang C, Yuan D and Liu J:
Follicular dendritic cell sarcoma: Two rare cases and a brief
review of the literature. Onco Targets Ther. 8:1823–1830. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haranhalli N, Ammar AE, Weidenheim KM,
Rosenblum MK and Altschul DJ: Hemorrhagic intracranial follicular
dendritic cell sarcoma: A case report. Surg Neurol Int. 8:2482017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren W, Sun Q, Wu PY, Huang B, Yang J, Yan
J and Liu BR: Profiles of genomic alterations in primary esophageal
follicular dendritic cell sarcoma: A case report. Medicine
(Baltimore). 97:e134132018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chidananda-Murthy G, Babu P, Chandran J
and Raja G: Unusual presentation of follicular dendritic cell
sarcoma as a cystic Neck Swelling. Case Rep Oncol Med.
2018:40382502018.PubMed/NCBI
|
|
33
|
Sarkar R, Sharma S, Roy N, Shankar A and
Basu S: Follicular dendritic cell sarcoma of Ileoceacal Region in a
young woman: A rare case report with review of literature. Oman Med
J. 28:e0552013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mondal SK, Bera H, Bhattacharya B and
Dewan K: Follicular dendritic cell sarcoma of the tonsil. Natl J
Maxillofac Surg. 3:62–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang W, He W and Li Z: Extranodal
follicular dendritic cell sarcoma originating in the pancreas: A
case report. Medicine (Baltimore). 95:e33772016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ang WW, Bundele MM and Shelat VG:
Follicular dendritic cell sarcoma: Rare presentation of incidental
large hepatic mass. Ann Hepatobiliary Pancreat Surg. 23:74–76.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cingam SR, Al Shaarani M, Takalkar A and
Peddi P: Follicular dendritic sarcoma masquerading as fibrosing
mediastinitis. BMJ Case Rep. 2017:bcr20162188892017. View Article : Google Scholar
|
|
38
|
Davila JI, Starr JS, Attia S, Wang C,
Knudson RA, Necela BM, Sarangi V, Sun Z, Ren Y, Casler JD, et al:
Comprehensive genomic profiling of a rare thyroid follicular
dendritic cell sarcoma. Rare Tumors. 9:68342017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang L, Cheng H, Li J, Bian D, Chen O, Jin
C and Zhao M: Extranodal follicular dendritic cell sarcoma of the
soft palate: A case report. Int J Clin Exp Pathol. 7:8962–8966.
2014.PubMed/NCBI
|
|
40
|
Pan ST, Cheng CY, Lee NS, Liang PI and
Chuang SS: Follicular dendritic cell sarcoma of the inflammatory
pseudotumor-like variant presenting as a colonic polyp. Korean J
Pathol. 48:140–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pai VD, Desai S, Desouza A and Saklani AP:
Extranodal follicular dendritic cell sarcoma: A frequently
misdiagnosed entity. J Postgrad Med. 61:55–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gupta AM, Goel M, Sahay A, Janjal SP and
Patkar S: Role of adjuvant chemotherapy in extranodal follicular
dendritic cell sarcoma. ACG Case Rep J. 6:1–4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xie Q, Chen L, Fu K, Harter J, Young KH,
Sunkara J, Novak D, Villanueva-Siles E and Ratech H: Podoplanin
(d2-40): A new immunohistochemical marker for reactive follicular
dendritic cells and follicular dendritic cell sarcomas. Int J Clin
Exp Pathol. 1:276–284. 2008.PubMed/NCBI
|
|
44
|
Mograbi M, Stump MS, Luyimbazi DT,
Shakhatreh MH and Grider DJ: Pancreatic inflammatory
pseudotumor-like follicular dendritic cell tumor. Case Rep Pathol.
2019:26481232019.PubMed/NCBI
|
|
45
|
Bushan K: Follicular dendritic cell
sarcoma mediastinum-a case report. Indian J Surg Oncol. 5:290–292.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Prakasan AM, Prabhu AJ, Velarasan K,
Backianathan S and Ram TS: Paraneoplastic pemphigus associated with
follicular dendritic cell tumor in the mediastinum. Case Rep
Dermatol Med. 2016:69015392016.PubMed/NCBI
|
|
47
|
Vardas K, Manganas D, Papadimitriou G,
Kalatzis V, Kyriakopoulos G, Chantziara M, Exarhos D and
Drakopoulos S: Splenic inflammatory pseudotumor-like follicular
dendritic cell tumor. Case Rep Oncol. 7:410–416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang X, Zhu C, Hu Y and Qin X: Hepatic
inflammatory pseudotumour-like follicular dendritic cell tumor: A
case report. Mol Clin Oncol. 6:547–549. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xuexue S, Nan L, Shan Z, Tingting X, Ruxue
X, Dichen L and Zhenzhong F: Clinicopathologic analysis of 10 cases
follicular dendritic cell sarcoma. J Clin Exp Pathol. 34:566–568.
2018.
|
|
50
|
Min G, Shi-Hao Z, Jing C and Fang W:
Clinicopathological features of inflammatory pseudotumor-like
follicular dendritic cell sarcoma. J Diag Pathol. 26:432–435.
2019.
|
|
51
|
Qi D, Xiaoqi Y, Yuqing C and Jianchen F:
Follicular dendritic cell sarcoma: 6 cases report and literature
review. Zhejiang Practical Med. 23:274–277. 2018.
|
|
52
|
Xiaofei C, Juan W, Qingxin X, Fangfang G,
Zhandong Z, Shuke Z, Yanyan L, Jianbo Z and Ling M:
Clinicopathological analysis of colon follicular dentritic cell
sarcomawith metastasis. J Basic Clin Oncol. 30:209–212. 2017.
|
|
53
|
Yong-Ta H and Xiang-Lan M:
Clinicopathologic features of follicular dendritic cell sarcoma and
its relation with EB virus infection. Chin J N Clin Med.
10:110–113. 2017.
|
|
54
|
Lian-Hua Z, Hualiang X, Li L and Juan D:
Extranodal follicular dendritic cell sarcoma of the pharyngeal
region: A report of 3 cases with review of literature. J Clin Exp
Pathol. 31:673–676. 2015.
|
|
55
|
Chen LS and Yang YH: Follicular Dendritic
Cell Sarcoma: A Report of 5 Cases and Literature Review. J Hubei
Univ Nationalities. Med Ed. 31:24–27+30+93. 2014.
|
|
56
|
Lennert K: Malignant lymphomas other than
Hodgkin's disease, histology, Cytology, Ultra structure,
Immunology. Springer; Berlin: pp. 59–64. 1978
|
|
57
|
Monda L, Warnke R and Rosai J: A primary
lymph node malignancy with features suggestive of dendritic
reticulum cell differentiation: A report of 4 cases. Am J Pathol.
122:562–572. 1986.PubMed/NCBI
|
|
58
|
Wang HT, Xu HY, Zhang R, Liu ZG and Zhang
GJ: Interdigitating dendritic cell sarcoma located in the groin: A
case report and literature review. J Int Med Res. 46:4791–4799.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hirji SA, Senturk JC, Hornick J, Sonoda T
and Bleday R: A rare case of interdigitating dendritic cell sarcoma
of the rectum: Review of histopathology and management strategy.
BMJ Case Rep. 2017:bcr20172217542017. View Article : Google Scholar
|
|
60
|
Griffin GK, Sholl LM, Lindeman NI,
Fletcher CD and Hornick JL: Targeted genomic sequencing of
follicular dendritic cell sarcoma reveals recurrent alterations in
NF-kB regulatory genes. Mod Pathol. 29:67–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Go H, Jeon YK, Huh J, Choi SJ, Choi YD,
Cha HJ, Kim HJ, Park G, Min S and Kim JE: Frequent detection of
BRAF(V600E) mutations in histiocytic and dendritic cell neoplasms.
Histopathology. 65:261–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Greisman HA, Lu Z, Tsai AG, Greiner TC, Yi
HS and Lieber MR: IgH partner breakpoint sequences provide evidence
that AID initiates t(11;14) and t(8;14) chromosomal breaks in
mantle cell and Burkitt lymphomas. Blood. 120:2864–2867. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
De Pas T, Spitaleri G, Pruneri G,
Curigliano G, Noberasco C, Luini A, Andreoni B, Testori A and de
Braud F: Dendritic cell sarcoma: An analytic overview of the
literature and presentation of original five cases. Crit Rev Oncol
Hematol. 65:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tisch M, Hengstermann F, Kraft K, von
Hinüber G and Maier H: Follicular dendritic cell sarcoma of the
tonsil: Report of a rare case. Ear Nose Throat J. 82:507–509. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Saygin C, Uzunaslan D, Ozguroglu M,
Senocak M and Tuzuner N: Dendritic cell sarcoma: A pooled analysis
including 462 cases with presentation of our case series. Crit Rev
Oncol Hematol. 88:253–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Domínguez-Malagón H, Cano-Valdez AM,
Mosqueda-Taylor A and Hes O: Follicular dendritic cell sarcoma of
the pharyngeal region: Histologic, cytologic, immunohistochemical,
and ultrastructural study of three cases. Ann Diagn Pathol.
8:325–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Duan GJ, Wu F, Zhu J, Guo DY, Zhang R,
Shen LL, Wang SH, Li Q, Xiao HL, Mou JH and Yan XC: Extranodal
follicular dendritic cell sarcoma of the pharyngeal region: A
potential diagnostic pitfall, with literature review. Am J Clin
Pathol. 133:49–58. 2010. View Article : Google Scholar : PubMed/NCBI
|