Introduction
Gallbladder carcinoma (GBC) was the fifth most
common malignant tumor of the digestive system and the most common
malignant tumor of the biliary tract worldwide in 2015 (1,2). Early
diagnosis and radical surgical resection are the most effective and
preferred treatment approaches for patients with GBC, resulting in
improved long-term survival (3,4).
However, due to the atypical early symptoms, and frequently rapid
and asymptomatic progression, a large proportion of patients with
GBC are likely to be diagnosed with late stage cancer; thus,
resectable GBC accounts for only 15–47% of all cases (5,6). The
aforementioned features of GBC may account for the unsatisfactory
prognosis of patients for GBC. The median survival time of patients
with GBC is only ~6 months, with a 5-year survival rate of <5%
worldwide in 2019 (7). Eventually,
the majority of patients with GBC succumb to metastatic disease
(8).
Previous studies have reported that p53-upregulated
modulator of apoptosis (PUMA) serves important roles in inducing
apoptosis in both normal and tumor cells (9–11). In
vitro experiments have demonstrated that PUMA induced
esophageal and lung cancer cell apoptosis, and significantly
attenuated the viability and proliferative ability of head and neck
cancer cell lines; consistent with the in vitro results, the
inhibitory effect of PUMA on the tumor growth was confirmed in nude
mice in vivo (9–12). The aforementioned studies indicate
that PUMA serves a crucial inhibitory role in tumor development. In
addition, PUMA is associated with the progression, recurrence and
metastasis and prognosis of colon and prostate cancers (13,14).
However, the expression profile and potential
biological roles of PUMA in patients with GBC have not been fully
elucidated to date. Therefore, the current study aimed to determine
the expression levels of PUMA in GBC tissues and cell lines, and to
further investigate its effects in GBC pathogenesis.
Materials and methods
Clinical specimen collection
A total of 18 paired GBC and adjacent normal
gallbladder tissues were collected from patients who underwent
surgical treatment between December 2016 and December 2018 in the
Second Xiangya Hospital (Changsha, China), including 6 men and 12
women, with the mean age of 52 years (range, 42–68 years) in
Nevin's stage I–III (15). All
patients enrolled in the present study had not undergone any
treatment prior surgery. The fresh GBC and adjacent normal
gallbladder tissues were stored at −80°C in liquid nitrogen until
RNA extraction. Written informed consent for participation and
publication were obtained from all patients. Follow-up was
performed after surgery until December 2019, and no cases were lost
to the follow-up. This study was approved by the Research Ethics
Committee of the Second Xiangya Hospital (approval no. 179 in
2017).
Cell culture
The normal gallbladder cells were obtained by
primary culture of cells isolated from healthy gallbladder wall
tissue, which was obtained from six patients with gallbladder
polyps undergoing cholecystectomy, including three men and three
women, with the mean age of 50 years (range, 44–65 years). Written
informed consent for participation and publication were obtained
from all patients. The gallbladder wall tissue was transferred to
ice-cold Ca2+-free Hanks solution with 0.5 mM EGTA and
cut into 1-cm2 fragments under sterile conditions.
Subsequently, the tissue was placed on a 60-mm culture dish and
treated for 45 min at room temperature with 0.025% trypsin (Thermo
Fisher Scientific, Inc.) in PBS containing 0.02% EDTA-2Na. The
tissue was agitated every 15 min using a pipette to promote the
release of the cells. The gallbladder cells were collected by
centrifugation at 1,200 × g for 5 min and counted in a CC-108
microcell counter (TOA Corporation).
Human GBC cell lines GBC-SD, SGC-996, NOZ and EHGB-1
obtained from the Institute of Biochemistry and Cell Biology of the
Chinese Academy of Sciences. The GBC cells were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum supplemented
with 100 U/ml penicillin and 100 U/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
The TRIzol® Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract RNA from clinical
tissues and cell lines, which was subsequently treated with DNase I
(Invitrogen; Thermo Fisher Scientific, Inc.) for purification
according to the manufacturer's instructions. The miRcute miRNA
First-Strand cDNA Synthesis kit (Takara Bio, Inc.) was used to
perform reverse transcription according to the manufacturer's
instructions. qPCR was performed using the SYBR® Green
PCR detection kit (Takara, Bio, Inc.) in a 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions with the following
primers: PUMA forward, 5′-TGAAGAGCAAATGAGCCAAACG-3′ and reverse,
5′-CAGAGCACAGGATTCACAGTCT-3′; and GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The expression levels of GAPDH were
used to normalize the results and the 2−ΔΔCq method was
used to calculate the relative expression levels of target gene
(16).
Western blotting
Total protein was extracted from clinical tissues
and cell lines using RIPA lysis buffer (50 mM Tris pH 8.0, 120 mM
NaCl, 0.5% sodium deoxycholate, 0.5% NP-40, 0.1% SDS, 1 mM EDTA, 50
mM NaF, 1 mM Na2VO4, 1 mM PMSF and 2 µg/ml
aprotinin) on ice for 30 min. The protein concentration was
determined by BCA assay, and 50 µg protein/lane was separated by
10% SDS-PAGE at 120 V for 60 min and transferred to PVDF membranes.
The membranes were blocked with 5% non-fat milk in TBS (10 mM Tris
pH 7.4 and 150 mM NaCl) and 0.1% Tween-20 (TBST) at room
temperature for 60 min, followed by incubation with primary
antibodies against PUMA (1:1,000; cat. no. ab33906), E-cadherin
(1:10,000; cat. no. ab40772), vimentin (1:1,000; cat. no. ab92547),
Bax (1:1,000; cat. no. ab32503), Bcl-2 (1:2,000; cat. no. ab182858)
(all from Abcam) and β-actin (1:1,000; cat. no. A2228;
Sigma-Aldrich; Merck KGaA) at 4°C for 12 h. Subsequently, the
membranes were washed three times with TBST for 5 min and incubated
with HRP-conjugated goat anti-rabbit IgG (1:2,000; cat. no.
sc-2004) or anti-mouse IgG (1:2,000; cat. no. sc-2005) (both from
Santa Cruz Biotechnology, Inc.) secondary antibody at 37°C for 60
min. The protein bands were visualized with an enhanced
chemiluminescence system (Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. The Gel Doc 2000 imaging system
(Bio-Rad Laboratories, Inc.) was used for densitometric analysis of
the protein bands with ImageJ software (version 1.8.0.112; National
Institutes of Health) according to the manufacturer's instructions.
β-actin was used as the internal control.
Plasmid generation and cell
transfection
The PUMA sequence was synthesized and subcloned into
the LV-BBC3 vector (22944-1) (Shanghai GeneChem Co., Ltd.). Ectopic
expression of PUMA was achieved by the PUMA lentivirus
transfection, and an empty vector (KL8781-1) (Shanghai GeneChem
Co., Ltd.) used as a negative control. Prior to transfection, human
GBC cell lines were cultured in complete medium without antibiotics
for at least 24 h. When the GBC-SD and EHGB-1 cell confluence
reached 30–50%, the cells were washed with 1X PBS once and
transfected with the LV-BBC3 (22944-1) (5×108 TU/ml; 40
µl; MOI, 20) and empty (KL8781-1) (5×108 TU/ml; 40 µl;
MOI, 20) vector at 37°C for 72 h, in the presence of
Lipofectamine® 2000 (Invitrogen, Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. At
48 h post-transfection, the cells were harvested, and the
expression levels of PUMA were determined by RT-qPCR.
MTT assay
At 48 h post-transfection, the cells were seeded
into 96-well plates (2×104 cells/well). Following
culture for 4 h at 37°C, the supernatant was discarded, and 150 µl
DMSO was added to each well. After shaking for 10 min, 100 µl cell
suspension was placed into another 96-well plate with 100 µl DMSO
solution as a control. The Cell Proliferation Reagent Kit I (MTT;
Roche Applied Science) was used at 37°C for 4 h to determine the
cell viability. The absorbance values were determined at 450 nm
using a spectrophotometer (Omega Bio-Tek, Inc.).
Colony formation assay
At 48 h post-transfection, the cells were harvested,
trypsinized and counted. A total of 500 cells/well were placed into
a 6-well plate and cultured at 37°C. The culture medium was
refreshed every 3 days. Following 14-day culture, the cells were
washed with 1X PBS, fixed with 3.7% methanol at 37°C for 15 min and
stained with 0.1% crystal violet at 37°C for 10 min (Sigma-Aldrich;
Merck KGaA). Visible colonies ≥50 cells were manually counted using
an optical microscope (Olympus Corporation).
Transwell invasion assay
The invasive ability of the cells was evaluated
using an 8-µm pore Transwell chamber. The upper chamber was
pre-coated with Matrigel at 37°C overnight. At 48 h
post-transfection, the cells (1×105) were suspended in
serum-free DMEM and plated into the upper chamber. The lower
chamber was filled with DMEM containing 10% FBS. Following 48-h
incubation at 37°C with 5% CO2, the chamber was removed,
and the cells remaining in the upper chamber were cleaned with
cotton wool. The invasive cells were washed with 1X PBS, fixed with
100% methanol at 37°C for 30 min and stained with 0.1% crystal
violet at 37°C for 20 min. Finally, images of five random fields
were captured using an optical microscope (×400 magnification;
Olympus Corporation) for quantification analysis. Each experiment
was repeated three times.
Flow cytometry
The cells were inoculated in a 6-well plate
(1×105 cells/well) and divided into four groups: GBC-SD
control, GBC-SD PUMA overexpression, EHGB-1 control and EHGB-1 PUMA
overexpression. The cells were collected, washed and resuspended in
PBS. The Annexin V-FITC apoptosis detection kit (Beckman Coulter,
Inc., USA) was used to detect apoptotic cells with Annexin V-FITC
and PI double staining according to the manufacturer's
instructions. The analysis was performed using a BD FACSAria™ II
flow cytometer (Becton, Dickinson and Company), and the data were
analyzed using CellQuest Pro software (version 5.1; Becton,
Dickinson and Company).
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 17.0 software (SPSS, Inc.) was used for statistical analyses.
The differences were analyzed using paired-samples Student's t-test
for two paired groups, independent sample Student's t-test for two
independent groups and one-way ANOVA with the Bonferroni correction
for multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
PUMA is expressed at low levels in GBC
tissues and cell lines
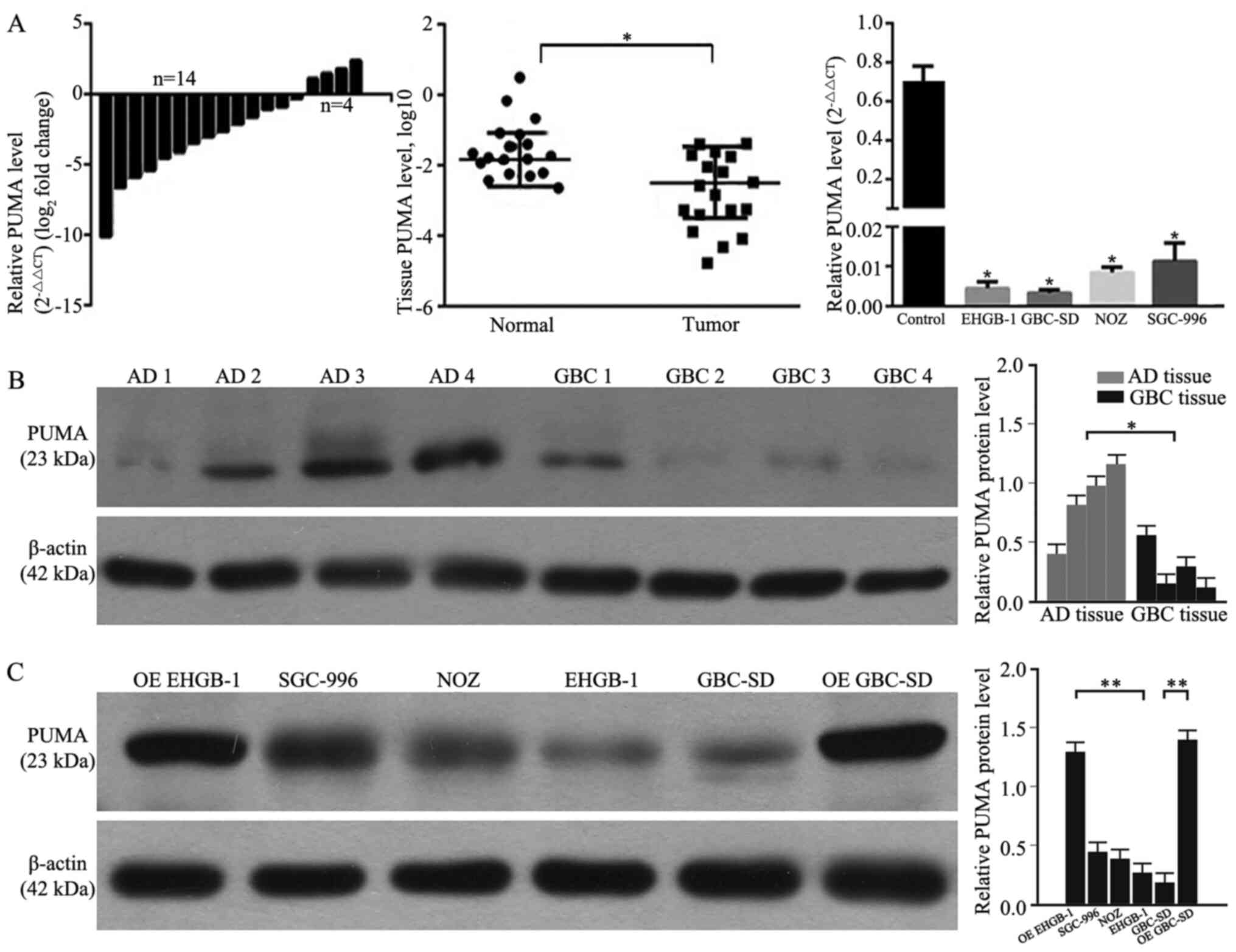
RT-qPCR and western blotting were performed to
determine the levels of PUMA expression in GBC and adjacent normal
gallbladder tissues, as well as in GBC cell lines. As demonstrated
in Fig. 1A and B, 77.8% (14/18) of
GBC tissues exhibited decreased mRNA expression levels of PUMA
compared with those in the adjacent tissues. Compared with those in
the adjacent normal gallbladder tissues, the expression levels of
PUMA were significantly lower in GBC tissues. Furthermore, the
expression levels of PUMA were also significantly lower in GBC cell
lines compared with those in primary normal gallbladder cells. As
PUMA expression levels were lower in GBC-SD and EHGB-1 cell lines
compared with those in the other two cell lines, the GBC-SD and
EHGB-1 cell lines were used in the subsequent experiments.
Following PUMA overexpression vector transfection, the levels of
PUMA protein expression were detected; as presented in Fig. 1C, the expression levels of PUMA
protein were significantly higher in PUMA-overexpressing EHGB-1 and
GBC-SD cells compared with those in the corresponding control
groups.
Overexpression of PUMA inhibits GBC
cell proliferation and invasion
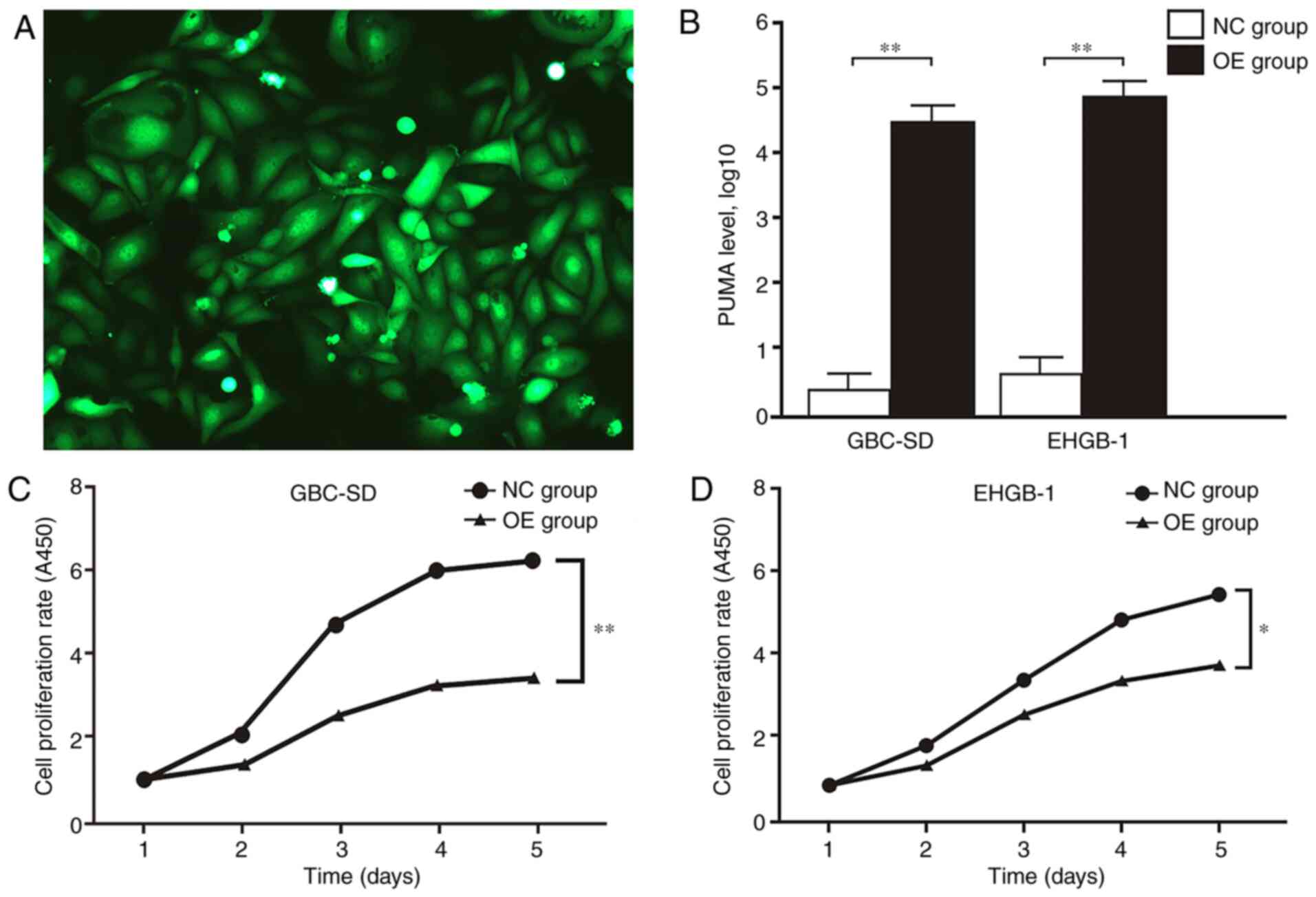
MTT and colony formation assays were performed to
verify the function of PUMA in GBC. The expression levels of PUMA
in GBC-SD and EHGB-1 cells were upregulated by transfection with
LV-BBC3 (22944-1) vector, and the empty (KL8781-1) vector was used
as a negative control (Fig. 2A). As
presented in Fig. 2B, PUMA mRNA
expression levels were significantly upregulated in GBC-SD and
EHGB-1 overexpression groups compared with those in the negative
control groups. The proliferation of GBC-SD and EHGB-1 cell lines
was significantly inhibited in the PUMA overexpression groups
compared with that in the control groups, and the inhibitory effect
appeared to become stronger with the increasing time
post-transfection (Fig. 2C and D).
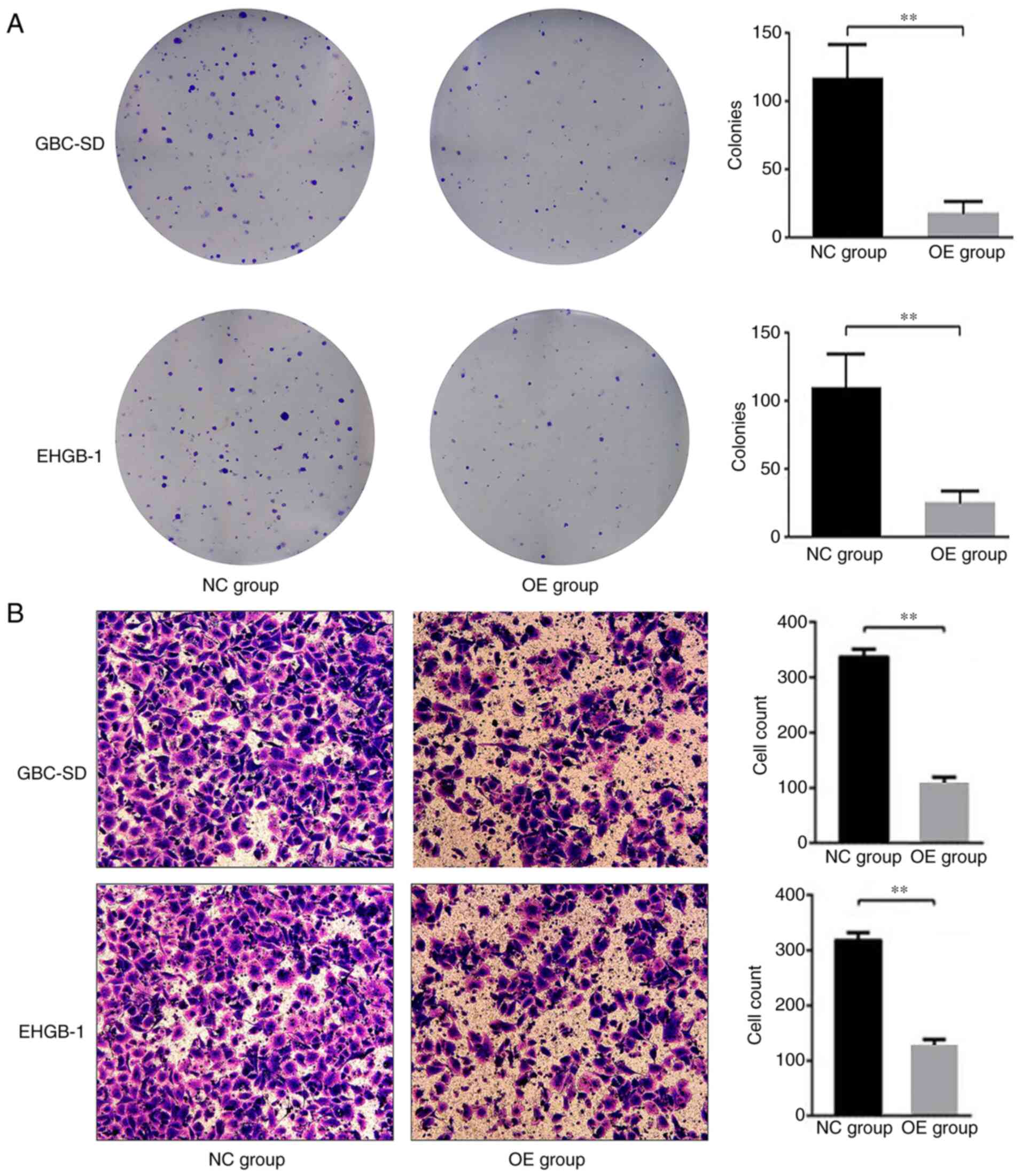
Furthermore, the colony formation ability of the GBC-SD and EHGH-1
cells was detected by colony formation assay; as demonstrated in
Fig. 3A, compared with that in the
negative control group, the colony formation ability was
significantly suppressed in the PUMA overexpression groups.
Transwell invasion assay was used to evaluate the
effects of PUMA on the cell invasive ability in vitro. The
results demonstrated that compared with those in the negative
control groups, the numbers of invasive cells were significantly
lower in the PUMA overexpression groups (Fig. 3B). These results suggested that PUMA
may act as a negative regulator of the invasive ability of GBC
cells.
PUMA inhibits the
epithelial-mesenchymal transition (EMT), and promotes the
upregulation of Bax and the downregulation Bcl-2
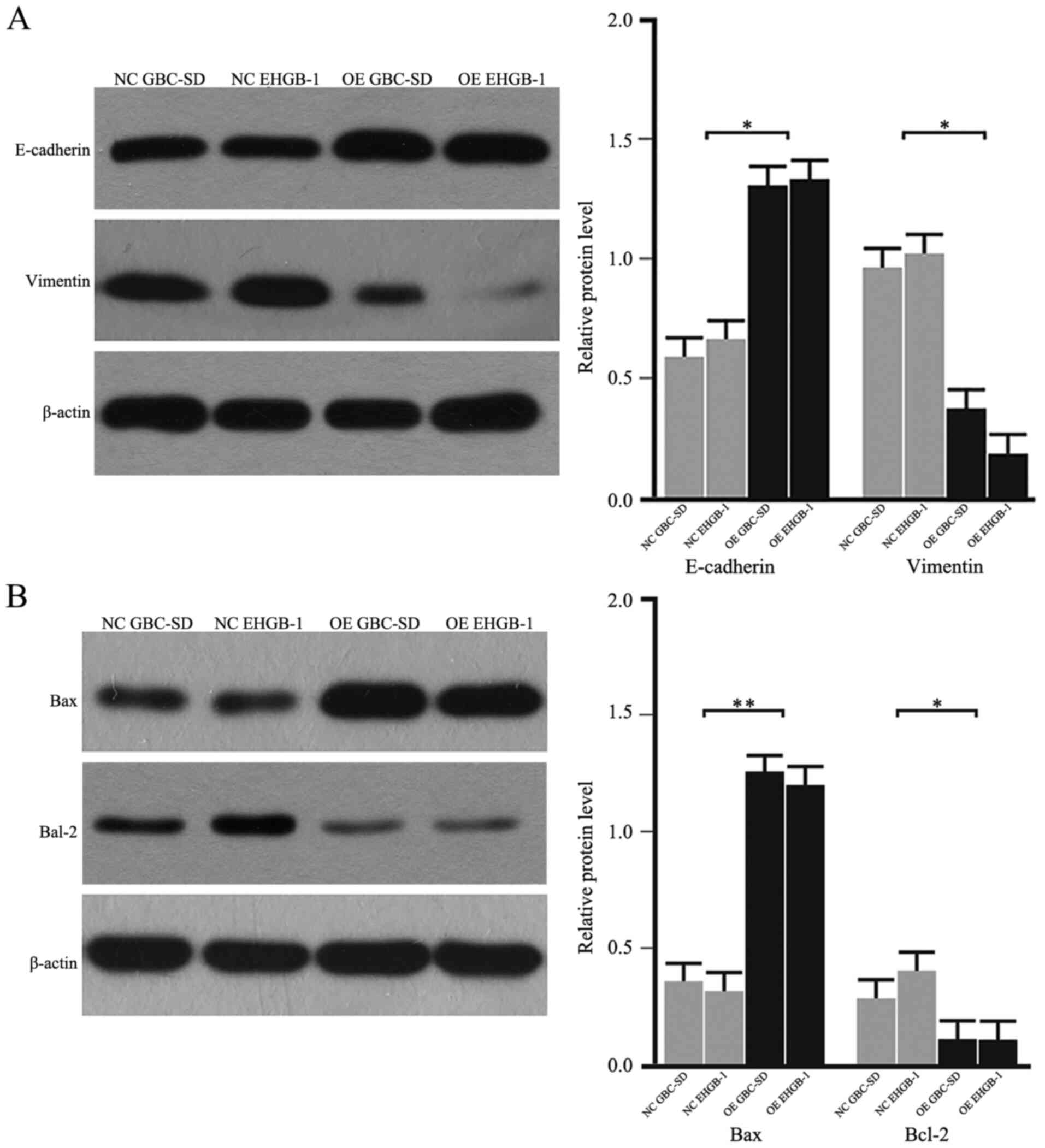
As the EMT process serves a critical role in cancer
cell migration and invasion, the expression levels of EMT-induced
makers were detected in GBC cells. As demonstrated in Fig. 4A, the protein levels of E-cadherin
were upregulated, whereas the levels of vimentin were downregulated
in the PUMA overexpression groups compared with those in the
negative control groups. In addition, the protein expression levels
of Bax were upregulated, and the levels of Bcl-2 were downregulated
in the PUMA overexpression groups compared with those in the
negative control groups (Fig. 4B).
These results suggested that PUMA may regulate the Bax/Bcl-2
signaling pathway and the EMT to inhibit the invasive ability of
GBC cells.
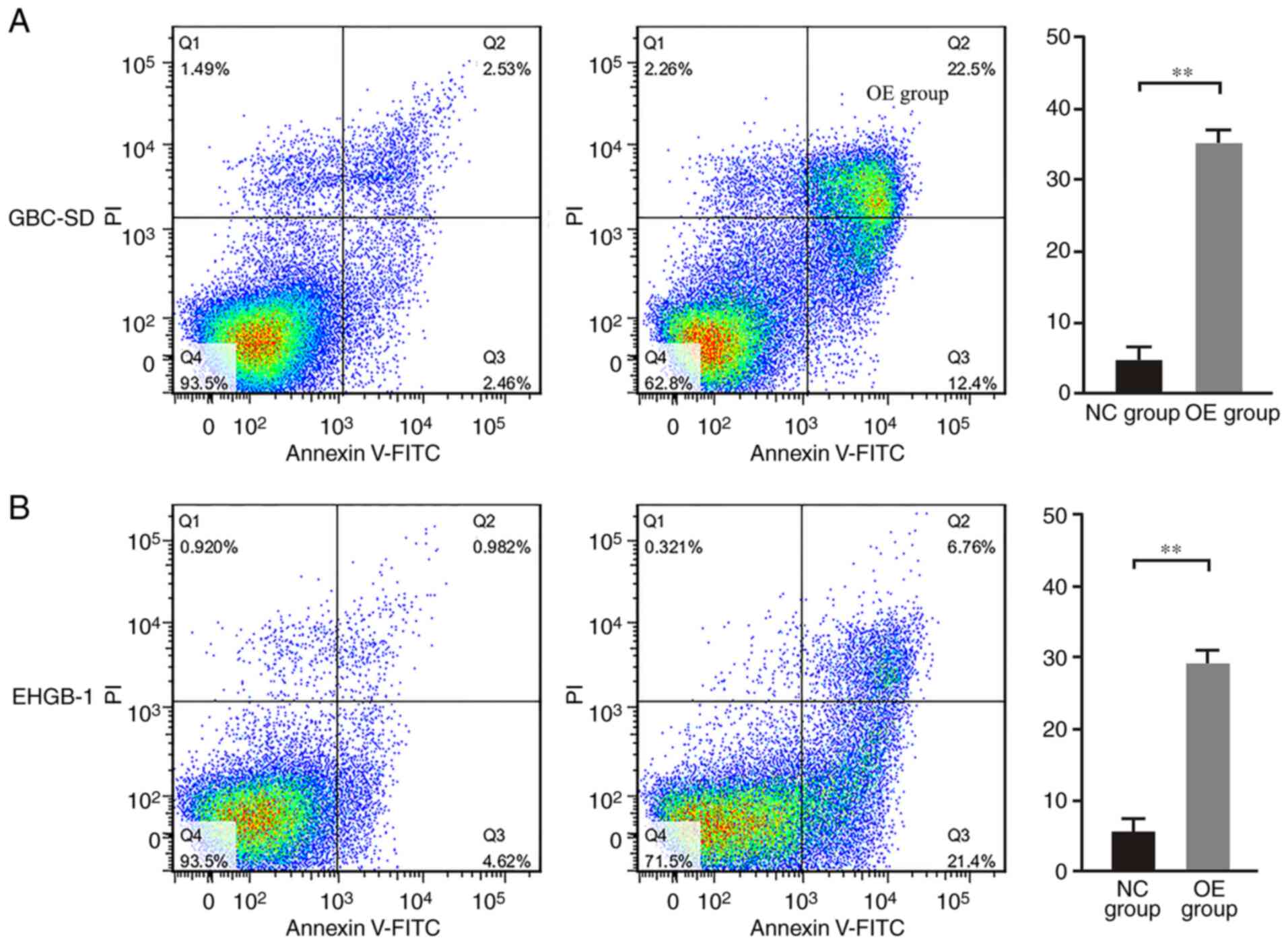
PUMA promotes apoptosis in GBC
cells
Following overexpression of PUMA, the apoptotic
rates of GBC-SD and EHGB-1 cells were detected by flow cytometry.
As demonstrated in Fig. 5, the
apoptotic rates were 5.0±1.7 and 5.4±1.8% in the GBC-SD and EHGB-1
control groups, respectively, but sharply increased to 35.0±4.5 and
28.6±3.9% in the GBC-SD and EHGB-1 PUMA overexpression groups,
respectively (both P<0.01).
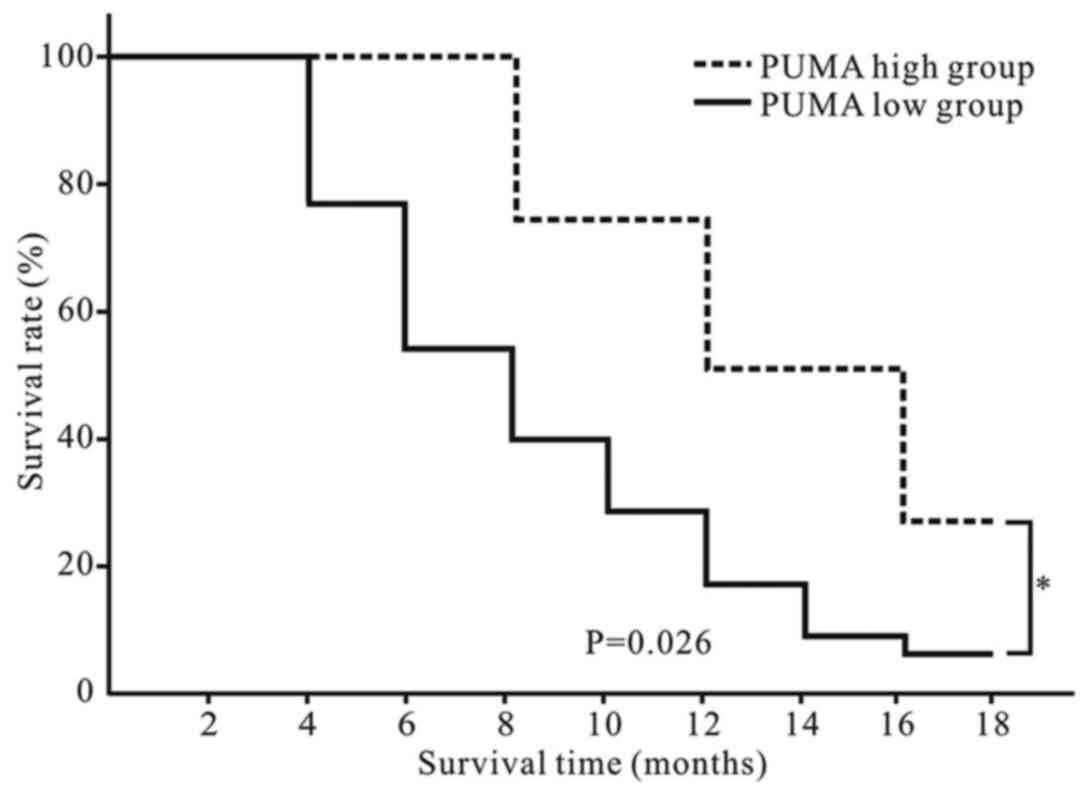
PUMA is a predictor of a favorable
prognosis in patients with GBC
Patients with GBC included in the present study were
followed up. According to the relative PUMA mRNA expression level
and the logarithm of 2−ΔΔCt value was zero as a cutoff
point, the patients were divided into PUMA high (n=4) and low
(n=14) groups. No deaths occurred in the two groups in first three
months after surgery. As demonstrated in Fig. 6, patients in the PUMA high group
exhibited a longer overall survival time compared with those in the
PUMA low group (P<0.05).
Discussion
Emerging evidence has suggested that alterations in
a number of tumor-suppressor genes and oncogenes are associated
with the oncogenesis and progression of GBC (17,18);
however, the specific molecular mechanism of GBC remains largely
unclear. Due to the poor prognosis of patients with GBC, it is
necessary to urgently identify the exact mechanisms of occurrence
and progression of GBC.
Previous studies have reported that PUMA induces
apoptosis in normal and tumor cells (19–22);
however, a limited number of studies are currently available on the
role of PUMA in GBC (23–25). Our previous study has demonstrated
the expression of PUMA and its pathological significance in GBC,
adjacent tissues, adenomatous gallbladder polyps and chronic
cholecystitis (23). Another study
has investigated the expression of PUMA and its clinicopathological
significance in benign and malignant lesions of the gallbladder,
concluding that PUMA is associated with the carcinogenesis,
progression, metastasis and high invasiveness of gallbladder
adenocarcinoma (24). Additionally,
the expression of WW domain-containing oxidoreductase and its
effects on inducing apoptosis by upregulating the expression of
PUMA in GBC cells have been demonstrated (25). However, in the three aforementioned
studies, the expression levels of PUMA in GBC cell lines have not
been reported. In the present study, the expression levels of PUMA
in GBC, adjacent tissues and GBC cell lines were investigated, and
a series of functional experiments were performed to study the
effects of PUMA on GBC for the first time.
The biological functions of the PUMA gene have been
introduced in detail in a previous study (23). The p53-dependent and non-dependent
apoptotic pathways serve key roles in PUMA-induced apoptosis;
previous studies have reported that the mechanism of PUMA-induced
apoptosis may include the following: i) PUMA and Bcl-2/Bcl2×l
combine to inhibit the Bax/Bak signaling pathway in normal or tumor
cells (9); ii) PUMA combines with
p53/Bcl2×l to release p53, which activates Bax to induce apoptosis
(9,20,21);
iii) PUMA directly binds to Bax/Bak to change its conformational,
and then Bax is activated to induce cell apoptosis (21,22).
However, further studies are needed to elucidate the precise
underlying mechanism of PUMA-induced apoptosis.
Our previous study has reported that PUMA is a
marker that may be associated with the clinical behavior of GBC and
may reflect the occurrence, development and prognosis of GBC in
patient tissues (23). In the
present study, the expression levels of PUMA were detected in GBC
tissues and cell lines, and its functional roles on the biological
behaviors of GBC cell lines were further investigated. The results
demonstrated that the expression levels of PUMA were significantly
lower in GBC tissues compared with those in the adjacent tissues,
and were low in GBC cell lines. Further experiments were performed
in vitro to demonstrate the functions of PUMA in the
biological behavior of GBC; the results demonstrated that following
overexpression, PUMA significantly inhibited the proliferative and
invasive abilities of GBC cells compared with those of the control
groups, which suggested that PUMA may serve as a tumor suppressor
in GBC.
The potential mechanisms of PUMA-inhibited invasion
were further investigated in the present study. The EMT is a
process during which epithelial cells trans-differentiate into
mesenchymal cells (26,27). The EMT endows tumor cells with the
traits of migration and invasion and induces cancer stem cell
properties (27,28). However, the effects of the EMT in
PUMA-induced GBC cell apoptosis remain unknown. The results of the
present study demonstrated that PUMA overexpression in GBC cell
lines increased the protein expression levels of E-cadherin and
decreased the levels of vimentin compared with those in the
negative control group. These results suggested that PUMA-induced
EMT reversal may account for the inhibitory effects of PUMA on GBC
cell invasion. However, further studies are needed to unravel the
precise signaling pathways involved in the PUMA-mediated inhibition
of the EMT.
The results of the present study also demonstrated
that the protein expression levels of Bax were upregulated, whereas
the levels of Bcl-2 were downregulated following PUMA
overexpression compared with those in the negative control groups.
The Bcl-2 family serves a vital role in apoptosis (29). As a member of the Bcl-2 family, Bax
promotes apoptosis; the protein expression levels of Bax are
associated with the regulation of apoptosis, since high levels of
the Bax protein induce apoptosis (30,31). As
a suppressor of apoptosis, Bcl-2 negatively regulates apoptosis by
regulating mitochondrial cytochrome c release (32). In addition, Bcl-2 binds to Bax to
form a Bax/Bcl-2 polymer to inhibit the apoptotic process (33). High expression levels of Bcl-2 also
maintain cell stability by inducing glutathione aggregation in the
cell nucleus to reduce caspase activity (34). The results of the present study
suggested that PUMA may inhibit GBC invasion at least partially by
regulating the expression of Bax and Bcl-2. However, further
studies are needed to clarify the exact mechanism of this
regulation process.
In the present study, a normal gallbladder
epithelial cell line was intended to be used as a control for the
in vitro experiments; however, careful review of the
available literature did not identify any normal gallbladder
epithelial cell lines. Primary culture of cells isolated from
normal gallbladder wall tissues were subsequently considered.
However, these cells could not be used as a control cell line due
to their short survival time.
The follow-up results in the present study
demonstrated that patients with GBC in the PUMA high group
exhibited a longer survival time compared with those in the PUMA
low group, which suggested that PUMA may be a new clinical marker
for the prognosis of GBC and may serve as a potential target for
the diagnosis and treatment of patients with GBC. However, a larger
sample and long-term studies are required to verify this
hypothesis.
In the present study, the expression levels of PUMA
and its inhibitory effects on GBC were only investigated by a
limited number of clinical specimen and cell experiments. Further
studies are required to confirm these results in a large sample and
in vivo animal experiments.
In conclusion, the results of the present study
demonstrated that PUMA inhibited the proliferative and invasive
capabilities of GBC cells through the Bax/Bcl-2 signaling pathway
and partially by regulating the EMT. These results suggested that
PUMA may serve as a potential tumor maker for the diagnosis and
treatment of GBC in clinical practice. In the last decade, studies
on circulating tumor cells (CTCs) have enriched the methods of
detecting tumor cells (35,36); in our future work, the potential
functions of PUMA in CTCs will be investigated.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China, Beijing (grant no. 81703767 to WC) and
the Hunan Natural Science Foundation of China, Changsha (grant no.
2019JJ50891 to WC).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and WC conceived and designed the study. ZL and
CY obtained the clinical samples and were the major contributors in
acquiring the data. WZ and LW performed the cell experiments. WC
and YX analyzed and interpreted the data, and drafted the
manuscript. QL and WC revised the manuscript. ZL and WC confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee of Second Xiangya Hospital (approval no. 179 in 2017;
Changsha, China). Informed consent was obtained from all included
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kanthan R, Senger JL, Ahmed S and Kanthan
SC: Gallbladder Cancer in the 21st Century. J Oncol.
2015:9674722015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiahong D, Jianming W and Jianping Z:
Guidelines for diagnosis and treatment of gallbladder cancer (2015
edition). J Clin Hepatol. 32:411–419. 2015.
|
|
3
|
Kakaei F, Beheshtirouy S, Nejatollahi SM,
Zarrintan S and Mafi MR: Surgical treatment of gallbladder
carcinoma: A critical review. Updates Surg. 67:339–351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cziupka K, Partecke LI, Mirow L, Heidecke
CD, Emde C, Hoffmann W, Siewert U, van den Berg N, von Bernstorff W
and Stier A: Outcomes and prognostic factors in gallbladder cancer:
A single-centre experience. Langenbecks Arch Surg. 397:899–907.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mekeel KL and Hemming AW: Surgical
management of gallbladder carcinoma: A review. J Gastrointest Surg.
11:1188–1193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang LF, Hou CS, Xu Z, Guo LM, Ling XF,
Wang LX and Xiu DR: Laparoscopic treatment for incidental
gallbladder cancer: A retrospective 10 years study from a single
institution. Zhonghua Wai Ke Za Zhi. 57:277–281. 2019.(In Chinese).
PubMed/NCBI
|
|
8
|
Butte JM, Matsuo K, Gönen M, D'Angelica
MI, Waugh E, Allen PJ, Fong Y, DeMatteo RP, Blumgart L, Endo I, et
al: Gallbladder cancer: Differences in presentation, surgical
treatment, and survival in patients treated at centers in three
countries. J Am Coll Surg. 212:50–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu J: PUMA kills stem cells to stall
cancer? Mol Cell Pharmacol. 1:112–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu J, Wang Z, Kinzler KW, Vogelstein B and
Zhang L: PUMA mediates the apoptotic response to p53 in colorectal
cancer cells. Proc Natl Acad Sci USA. 100:1931–1936. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He S, Ma X, Ye Y, Zhang M, Zhuang J, Song
Y and Xia W: HEATR1 modulates cell survival in non-small cell lung
cancer via activation of the p53/PUMA signaling pathway. Onco
Targets Ther. 12:4001–4011. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yee KS, Wilkinson S, James J, Ryan KM and
Vousden KH: PUMA- and Bax-induced autophagy contributes to
apoptosis. Cell Death Differ. 16:1135–1145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinicrope FA, Rego RL, Okumura K, Foster
NR, O'Connell MJ, Sargent DJ and Windschitl HE: Prognostic impact
of bim, puma, and noxa expression in human colon carcinomas. Clin
Cancer Res. 14:5810–5818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diallo JS, Aldejmah A, Mouhim AF, Péant B,
Fahmy MA, Koumakpayi IH, Sircar K, Bégin LR, Mes-Masson AM and Saad
F: NOXA and PUMA expression add to clinical markers in predicting
biochemical recurrence of prostate cancer patients in a survival
tree model. Clin Cancer Res. 13:7044–7052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nevin JE, Moran TJ, Kay S and King R:
Carcinoma of the gallbladder: Staging, treatment, and prognosis.
Cancer. 37:141–148. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma A, Kumar A, Kumari N, Krishnani N
and Rastogi N: Mutational frequency of KRAS, NRAS, IDH2, PIK3CA,
and EGFR in North Indian gallbladder cancer patients.
Ecancermedicalscience. 11:7572017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YW, Huh SH, Park YK, Yoon TY, Lee SM
and Hong SM: Expression of the c-erb-B2 and p53 protein in
gallbladder carcinomas. Oncol Rep. 8:1127–1132. 2001.PubMed/NCBI
|
|
19
|
Niizuma K, Endo H, Nito C, Myer DJ and
Chan PH: Potential role of PUMA in delayed death of hippocampal CA1
neurons after transient global cerebral ischemia. Stroke.
40:618–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun YL, Jiang WQ, Luo QY, Yang DJ, Cai YC,
Huang HQ and Sun J: A novel Bcl-2 inhibitor, BM-1197, induces
apoptosis in malignant lymphoma cells through the endogenous
apoptotic pathway. BMC Cancer. 20:12019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Letai A: Puma strikes Bax. J Cell Biol.
185:189–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erlacher M, Labi V, Manzl C, Böck G,
Tzankov A, Häcker G, Michalak E, Strasser A and Villunger A: Puma
cooperates with Bim, the rate-limiting BH3-only protein in cell
death during lymphocyte development, in apoptosis induction. J Exp
Med. 203:2939–2951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai W, Li Q, Yang Z, Miao X, Wen Y, Huang
S and Ouyang J: Expression of p53 upregulated modulator of
apoptosis (PUMA) and C-myb in gallbladder adenocarcinoma and their
pathological significance. Clin Transl Oncol. 15:818–824. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shu GS, Lv F, Yang ZL and Miao XY:
Immunohistochemical study of PUMA, c-Myb and p53 expression in the
benign and malignant lesions of gallbladder and their
clinicopathological significances. Int J Clin Oncol. 18:641–650.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei D, Zhang X, Zou H, Wang L, Fu B, Wu X,
Luo Z, Li X, Ge J, Li Y, et al: WW domain containing oxidoreductase
induces apoptosis in gallbladder-derived malignant cell by
upregulating expression of P73 and PUMA. Tumour Biol. 35:1539–1550.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and desease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ,
Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB and Fan J:
Macrophage-secreted IL-8 induces epithelial-mesenchymal transition
in hepatocellular carcinoma cells by activating the
JAK2/STAT3/Snail pathway. Int J Oncol. 46:587–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Babu PP, Suzuki G, Ono Y and Yoshida Y:
Attenuation of ischemia and/or reperfusion injury during myocardial
infarction using mild hypothermia in rats: An immunohistochemical
study of bcl-2, bax, Bak and TUNEL. Pathol Int. 54:896–903. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan XX, Ou JS, Li Y, Su JJ, Ou C, Yang C,
Yue HF and Ban KC: Dynamic expression of apoptosis related genes
during development of laboratory hepatocellular carcinoma and its
relation to apoptosis. World J Gastroenterol. 11:4740–4744. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin LJ: Neuronal cell death in nervous
system development, disease, and injury (Review). Int J Mol Med.
7:455–478. 2001.PubMed/NCBI
|
|
32
|
Cecconi F, Alvarez-Bolado G, Meyer BI,
Roth KA and Gruss P: Apaf1 (CED-4 homolog) regulates programmed
cell death in mammalian development. Cell. 94:727–737. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McDonnell TJ, Troncoso P, Brisbay SM,
Logothetis C, Chung LW, Hsieh JT, Tu SM and Campbell ML: Expression
of the protooncogene Bcl-2 in the prostate and its association with
emergence of androgen-independent prostate cancer. Cancer Res.
52:6940–6944. 1992.PubMed/NCBI
|
|
34
|
Roos A, Sato T, Maier H, van Kooten C and
Daha MR: Induction of renal cell apoptosis by antibodies and
compiement. Exp Nephrol. 9:65–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan ZC, Yan J, Liu GD, Tan XY, Weng XF, Wu
WZ, Zhou J and Wei XB: Real-time monitoring of rare circulating
hepatocelluar carcinoma cells in an orthotopic model by in vivo
flow cytometry assesses resection on metastasis. Cancer Res.
72:2683–2691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan J, Fan Z, Wu X, Xu M, Jiang J, Tan C,
Wu W, Wei X and Zhou J: Circulating tumor cells are correlated with
disease progression and treatment response in an orthotopic
hepatocellular carcinoma model. Cytometry A. 87:1020–1028. 2015.
View Article : Google Scholar : PubMed/NCBI
|