Introduction
Colorectal carcinoma (CRC) is one of the most common
malignant tumors, with the incidence of CRC being 15–25/100,000
population in China in 2011 (1,2). Given
that there are no specific symptoms in the early stage of CRC,
patients are diagnosed when the tumor is in the advanced stage
(3). Despite surgical treatment, the
5-year overall survival (OS) rate is still <50% (3). Thus, one of the most effective ways to
improve the survival rate is to identify molecular markers that can
reflect the invasion, metastasis and prognosis of CRC, and provide
targeted adjuvant treatment to patients (4,5).
Recently, it has been demonstrated that small
non-coding microRNAs (miRNAs/miRs) play an important role in
tumor-related gene regulation (6,7). miRNAs
are highly conserved, single-stranded non-coding small RNA
molecules (8,9). As endogenous regulators, they regulate
the expression of target genes by binding to the 3′-untranslated
region (UTR) of target mRNAs at the post-transcriptional level
(10). Increasing evidence suggests
that abnormal expression of miRNAs is associated with the
occurrence, invasion and metastasis of human malignant tumors,
indicating that miRNAs are involved in the regulation of
tumorigenesis, invasion and metastasis (11–13). For
example, miR-92b-3p contributes to CRC invasion by inhibiting FBXW7
(9). In addition, increased levels
of miR-15a-5p predict poor disease-free survival and OS of patients
with CRC (14).
The present study screened the miRNAs that are
dysregulated in the tissues and serum samples of patients with CRC
via microarray assay. The present study aimed to investigate miRNAs
in CRC and target genes that are associated with CRC.
Materials and methods
Patient samples
A total of 50 patients with CRC (male/female ratio,
24/26; mean age ± SD, 53.6±15.8 years; age range, 34–73 years) and
21 healthy individuals (male/female ratio, 11/10; mean age ± SD,
52.8±17.4 years; age range, 36–72 years) were recruited in the
present study between January 2019 and December 2019. Due to
financial constraints, the present study could not recruit equal
numbers of healthy controls and patients with CRC. Among the
patients with CRC, 46 patients received radical surgery, while 4
patients did not receive surgery. CRC tissues and adjacent
non-cancer tissues (≥5 cm) were collected. The patient
characteristics are present in Table
I. All patients had complete clinical and pathological data,
and the inclusion criteria were as follows: i) CRC was diagnosed
via histopathology at the initial diagnosis and treatment; ii) no
radiotherapy or chemotherapy was received prior to treatment; iii)
no significant abnormalities of heart, liver or kidney functions
were observed and iv) no other tumor or serious diseases were
observed. Peripheral blood (5 ml) was collected from all patients
with CRC on an empty stomach following surgery, and 5 ml of
peripheral blood was collected from the healthy individuals. The
samples were centrifuged at 3,500 × g for 15 min at 4°C and the
supernatant was stored at −80°C until subsequent experimentation.
The present study was approved by the Medical Ethics Committee of
Hongqi Hospital Affiliated to Mudanjiang Medical University
(Mudanjiang, China; approval no. MDJHQ-20180925), and written
informed consent was provided by all participants prior to the
study start.
 | Table I.Clinicopathological characteristics of
patients with colorectal cancer (n=50). |
Table I.
Clinicopathological characteristics of
patients with colorectal cancer (n=50).
| Characteristic | Number of patients,
n |
|---|
| Sex |
|
| Male | 24 |
|
Female | 26 |
| Age, years |
|
| ≥60 | 32 |
|
<60 | 18 |
| Location |
|
| LSCC | 9 |
| RSCC | 17 |
|
Rectum | 24 |
| Type |
|
|
Protuberant | 19 |
|
Ulcerative | 23 |
|
Invasive | 8 |
| Differentiation |
|
| Poor | 16 |
|
Poor/Moderate | 14 |
|
Moderate | 12 |
| Well | 8 |
| TNM |
|
| I/II | 21 |
|
III/IV | 29 |
| Lymph node
metastasis |
|
| No | 23 |
| Yes | 27 |
RNA isolation
Total RNA was extracted from the tissues and serum
samples using RNAVzol (Vigorous Biotechnology Beijing Co., Ltd.),
according to the manufacturer's protocol. The concentration and
purity of the RNA samples were determined by measuring the optical
density (OD) 260/OD 280.
Microarray assay
To compare the miRNA transcriptome between CRC
tissues/serum samples and the respective controls, seven different
samples were taken as one mixture. For each group, three different
mixtures were included in each group for miRNA profiling using an
Agilent miRNA array (Agilent Technologies, Inc.). Each miRNA was
detected using probes (Agilent Technologies, Inc.) and repeated 30
times. The array also contained 2,164 Agilent control probes. The
miRNA samples were Cy3 labeled using the Agilent miRNA Complete
Labeling and Hyb kit (cat. no. 5190-0456; Agilent Technologies,
Inc.), according to the manufacturer's instructions. The
differentially expressed miRNAs were obtained using a combine
threshold of fold change >1.5 and t-test P<0.05 for
transcriptome comparisons.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from the serum and tissue
samples using RNAVzol LS or RNAVzol (Vigorous Biotechnology Beijing
Co., Ltd.) according to the manufacturer's protocol. The
concentration and the purity of RNA samples was determined by
measuring the optical density (OD) 260/OD280. A total of 1 µg RNA
was reverse transcribed using Moloney Murine Leukemia Virus (MMLV)
reverse transcription enzyme (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with specific primers. The RT system (10 µl) was
composed as follows: 3.5 µl of DEPC water, 2.0 µl of 5X reverse
transcription buffer, 1.0 µl of 10 mmol/l dNTPs, 0.5 µl of MMLV
reverse transcriptase (all Applied Biosystems; Thermo Fisher
Scientific, Inc.), 1.0 µl of miRNA reverse transcription primer and
2.0 µl of RNA sample. The reaction conditions were as follows: 16°C
for 30 min, 42°C for 30 min, 85°C for 5 min and hold at 4°C. To
quantify the relative mRNA levels, qPCR was performed using SYBR
Green Supermix (Bio-Rad Laboratories, Inc.) in an iCycleriQ
real-time PCR detection system. The reaction conditions were as
follows: 95°C for 5 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. Relative miRNA expression was calculated using
the 2−∆∆Cq method (15)
and normalized to the internal reference gene U6. The primers used
in the present study were as follows: miR-325-3p-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAATAAC-3′; U6-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATG-3′;
miR-325-3p forward, 5′-GCGCAACTATCCTCCAGG-3′; U6 forward,
5′-GCGCGTCGTGAAGCGTTC-3′; universal reverse primer for miR-325-3p
and U6, 5′-GTGCAGGGTCCGAGGT-3′.
Cell culture
The human CRC cell line, HT-29, was purchased from
the American Type Culture Collection and authenticated via STR
profiling. The cells were maintained in Ham's F-12 nutrient medium
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS; HyClone; Cytiva), 100 U/ml penicillin
(Invitrogen; Thermo Fisher Scientific, Inc.) and 0.1 mg/ml
streptomycin (HyClone; Cytiva), at 37°C with 5% CO2.
The human CRC cell lines, SW480 and HCT116, the
normal colon cell line, FHC, and 293T cells were purchased from the
Chinese Academy of Sciences Cell Bank of Type Culture Collection.
All cells were maintained in RPMI-1640 medium (HyClone; Cytiva)
supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin, at 37°C with 5% CO2.
Transient transfection
miR-325-3p mimic (5′-AACTATCCTCCAGGAGTTATTT-3′) or
inhibitor (5′-AAATAACTCCTGGAGGATAGTT-3′) and the respective
negative controls (NCs; NC mimic, 5′-TTCTCCGAACGTGTCACGT-3′; NC
inhibitor, 5′-TTCTCCGAACGTTGTCACGT-3′) (all from Shanghai
GenePharma Co., Ltd.) are chemically modified analogs, which can be
transfected into cells without using a vector. Transfection was
performed using HiPerFect Transfection Reagent (Qiagen GmbH), as
previously described (16). Briefly,
HT-29 cells were seeded in a 6-well plate at a density of
106 cells/well. Subsequently, the cells were transfected
with miR-325-3p mimic, inhibitor or NC for 48 h using HiPerFect
Transfection Reagent according to the manufacturer's instructions.
A total of 12 µl HiPerFect Transfection Reagent was mixed with 100
µl cell culture in serum-free DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.). Meanwhile, 10 µl miR-325-3p mimic, inhibitor or
NC was mixed with serum-free DMEM. Then, the two mixtures were
mixed and incubated at room temperature for 15 min. After that, the
mixture was added in the 6-well plate at a final concentration of
20 nM. Following transfection for 48 h, the cells were collected
for subsequent experiments.
Cell viability analysis
To examine cell viability, HT-29 cells were seeded
in 96-well plates at a density of 1.0×104 cells/well.
miR-325-3p mimics, inhibitors or NC were transfected into cells and
the viability of transfected cells was measured at 24, 48 and 72 h
after seeding of cells. MTT assay was performed as previously
described (17).
Cell migration and invasion
Cell migration assays were performed using Boyden
chambers (8-µm pore filter; Corning Inc.). For the cell invasion
assay, the filter surfaces were precoated with Matrigel (BD
Biosciences) at 37°C for 2 h. Briefly, transfected HT-29 cells were
seeded at a density of 105 cells/well in the upper
chamber for 24 h in RPMI-1640 medium without FBS. RPMI-1640 medium
(600 µl) with 20% FBS was plated in the lower chamber. After 48 h
of incubation at 37°C, non-migratory and non-invading cells were
removed with cotton swabs. The migratory or invasive cells located
on the lower side of the chamber were fixed in methanol for 30 min
at 37°C and stained with 0.5% crystal violet for 1 h at 37°C.
Stained cells were counted in 5 random fields using fluorescence
microscopy (magnification, ×40). All experiments were performed in
triplicate.
Dual-luciferase reporter assay
Based on the TargetScan database (http://www.targetscan.org/mamm_31/), a conserved
binding site was identified in the 3′-UTR of cytokeratin 18 (CK18).
The 3′-UTR of CK18 containing the predicted binding site was cloned
into the pmirGLO luciferase reporter vector (Promega Corporation).
Subsequently, the plasmid and/or miR-325-3p mimic was transfected
into HT-29 cells using Vigofect transfection reagent (Vigorous
Biotechnology Beijing Co., Ltd.), according to the manufacturer's
protocol. 293T cells were seeded in a 6-well plate at a density of
106 cells/well. Subsequently, the cells were transfected
with miR-325-3p mimic (5′-AACTATCCTCCAGGAGTTATTT-3′) or NC
(5′-TTCTCCGAACGTGTCACGT-3′) (both Shanghai GenePharma Co., Ltd.)
for 48 h using Vigofect transfection reagent (Vigorous
Biotechnology Beijing Co., Ltd.) according to the manufacturer's
instructions. Briefly, 10 µl Vigofect transfection reagent was
mixed with 100 µl cell culture in serum-free DMEM. Meanwhile, 10 µl
miR-325-3p mimic or NC and pmirGLO-CK18-3′-UTR plasmid was mixed
with the aforementioned mixture. Then, the two mixtures were mixed
and incubated at room temperature for 10 min. Subsequently, the
mixture was added in the 6-well plate at a final concentration of
20 nM. Following transfection for 48 h, the luciferase activity was
detected using a Dual Luciferase Reporter Assay System (Promega
Corporation). Firefly luciferase activity was normalized to
Renilla luciferase activity.
Western blotting
Total protein isolated from CRC samples or HT-29
cells was extracted using RIPA buffer (Beijing Solarbio Science
& Technology Co., Ltd.). A bicinchoninic protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.) was used to determine the
protein concentration. A total of 20 µg protein/lane was separated
by 10% SDS-PAGE and subsequently transferred onto PVDF membranes
(EMD Millipore). Membranes were blocked with 5% skimmed milk at
room temperature for 2 h. The membranes were incubated with primary
antibodies against: CK18 (1:1,000; cat. no. ab133263; Abcam), α-SMA
(1:1,000; cat. no. 19245; Cell Signaling Technology, Inc.),
vimentin (1:1,000; cat. no. 5741; Cell Signaling Technology, Inc.)
and GAPDH (1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.)
overnight at 4°C. Membranes were washed with PBST three times, and
subsequently incubated with HRP-conjugated anti-rabbit secondary
antibodies (1:5,000; cat. no. ZB-2301; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.) for 2 h at room temperature.
Protein bands were visualized using the ECL Plus detection system
(EMD Millipore), according to the manufacturer's instructions.
GAPDH was used as the internal control.
TGF-β treatment
HT-29 cells were treated with 10 ng/µl TGF-β
(Sigma-Aldrich; Merck KGaA) for 24, 48 and 72 h at 37°C. To
determine the effect of miR-325-3p on epithelial-to-mesenchymal
transition (EMT), HT-29 cells were treated with or without TGF-β
for 24 h at 37°C. Subsequently, the cells were transfected with or
without miR-325-3p inhibitors for 48 h, as aforementioned. The
cells were collected for further analysis.
Statistical analysis
Statistical analysis was performed using SPPS 20.0
software (IBM Corp.). Data are presented as the mean ± standard
deviation. Two-tailed unpaired Student's t-test or paired Student's
t-test (for tumor vs. adjacent non-cancer tissues) was used to
compare differences between two groups. One-way ANOVA followed by
Tukey's post hoc test were used to compare difference between
multiple groups. A receiver operating characteristic (ROC) curve
was used to analyze the area under the curve (AUC) in the diagnosis
of patients with CRC. P<0.05 was considered to indicate a
statistically significant difference.
Results
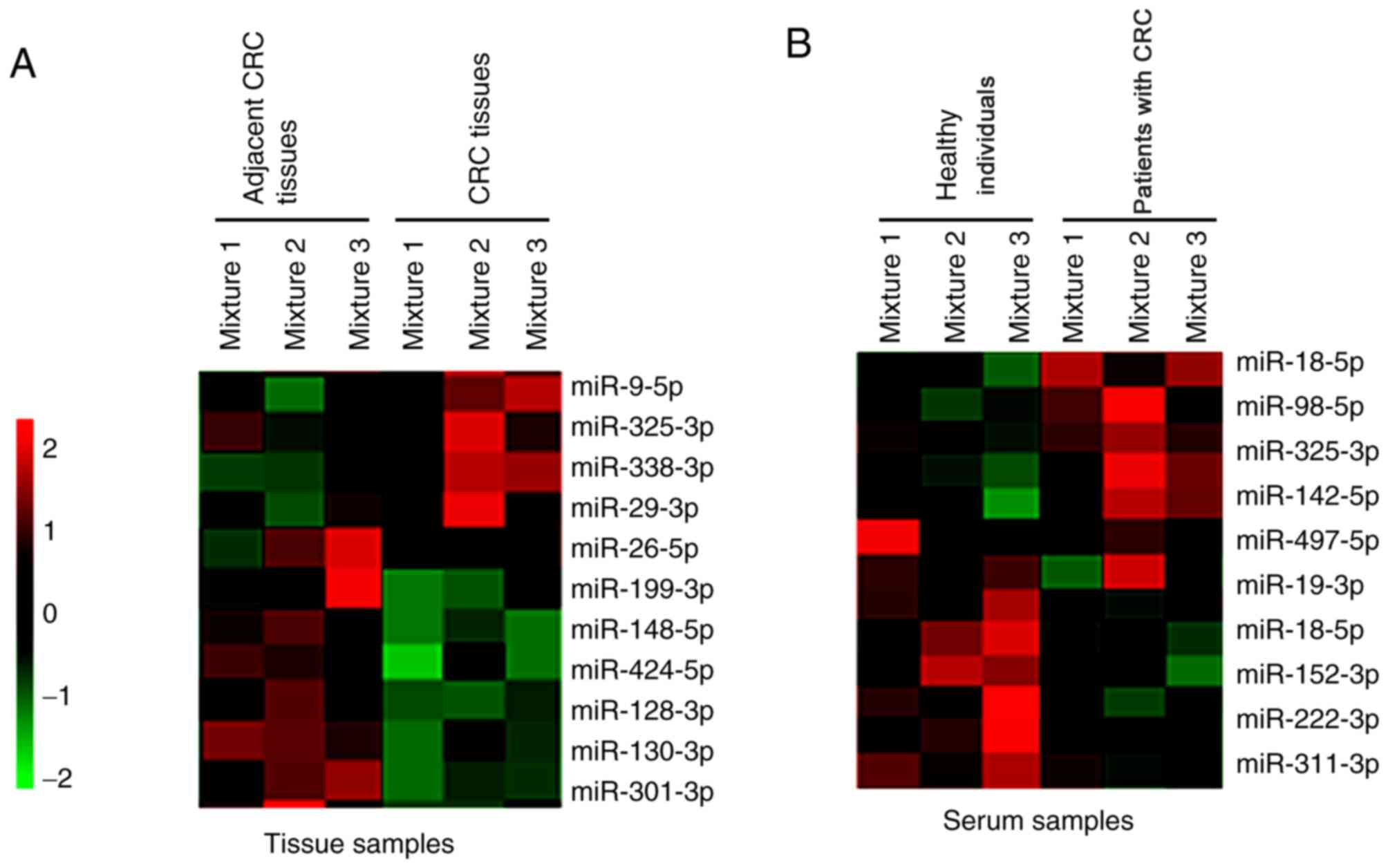
Microarray assay
Tissue and serum samples were collected from
patients with CRC and healthy individuals. A microarray assay was
performed, and the results demonstrated that different miRNAs were
dysregulated in the tissue and serum samples of patients with CRC
and the healthy individuals. Notably, miR-325-3p expression was
significantly upregulated in both the tissues and serum samples of
patients with CRC compared with the healthy individuals (Fig. 1A and B).
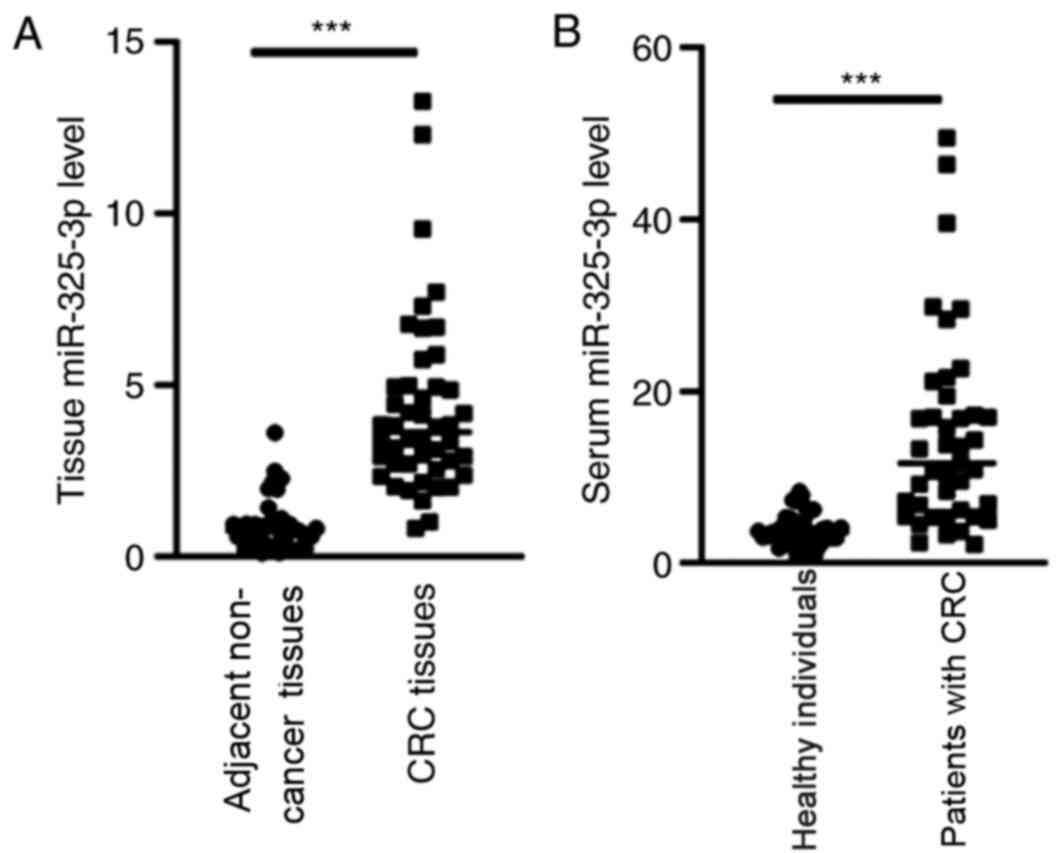
miR-325-3p expression is elevated in
patients with CRC
Based on the microarray data, RT-qPCR analysis was
performed to detect miR-325-3p expression in patients with CRC. The
results demonstrated that miR-325-3p expression was significantly
upregulated in the tissues and serum samples of patients with CRC
compared with the healthy individuals (Fig. 2A and B; P<0.001).
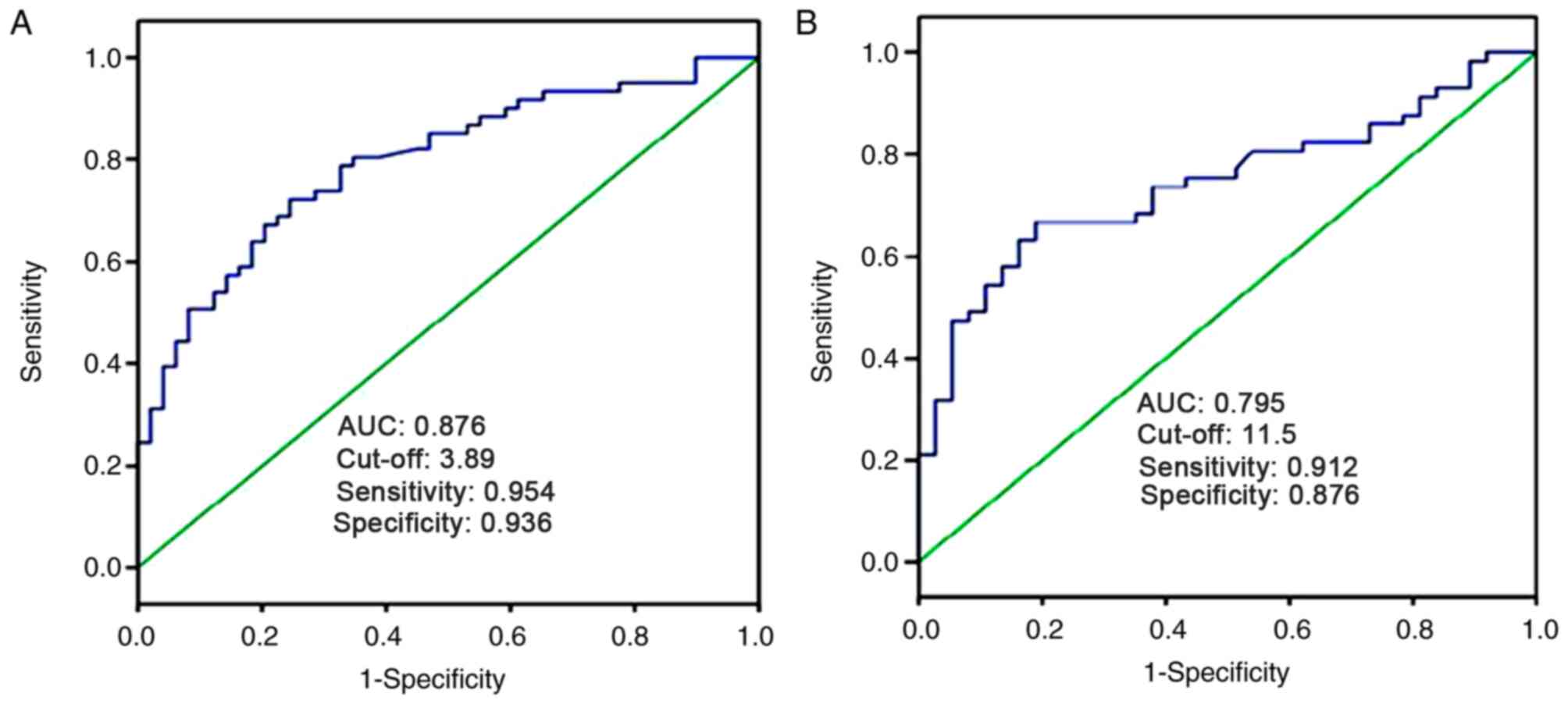
Diagnostic value of miR-325-3p in
patients with CRC
The ROC curve analysis was performed to determine
the diagnostic value of tissue and serum miR-325-3p levels in
patients with CRC. As presented in Fig.
3A, tissue miR-325-3p expression could be used to differentiate
patients with CRC from healthy individuals, with an AUC value of
0.876. When the cut-off value was 3.89, the sensitivity was 95.4%
and the specificity was 93.6%. Furthermore, the AUC value of serum
miR-325-3p expression was 0.795 in screening patients with CRC from
healthy individuals. When the cut-off value was 11.5, the
sensitivity was 91.2% and the specificity was 87.6% (Fig. 3B). Taken together, these results
suggest that miR-325-3p is a useful biomarker in identifying
patients with CRC from healthy individuals.
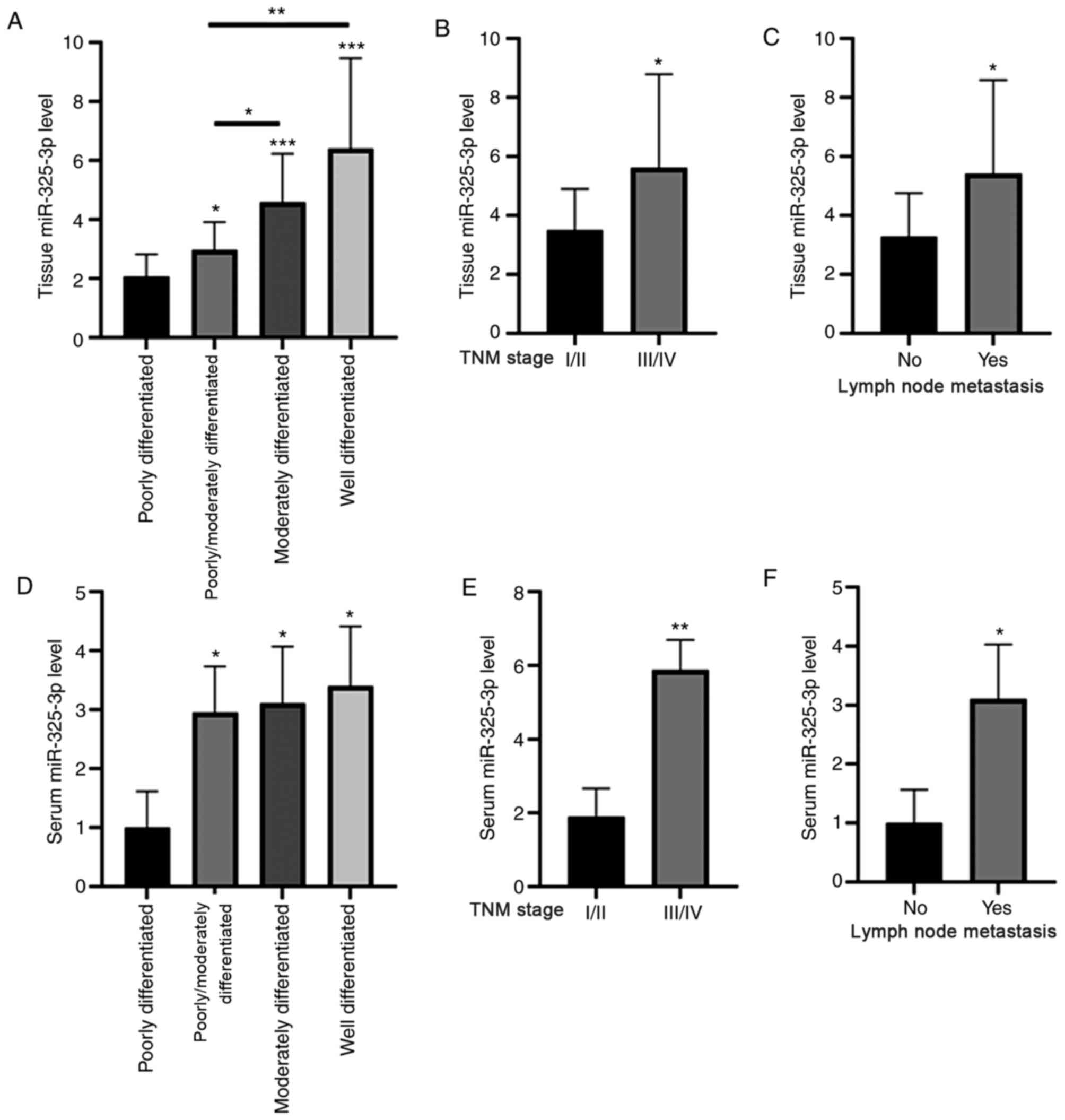
Patients with CRC have higher serum
miR-325-3p expression
The association between miR-325-3p expression and
the clinicopathological characteristics of patients with CRC was
assessed. As presented in Fig. 4A and
D, tissue and serum miR-325-3p levels were significantly
upregulated in patients with well differentiated CRC than those
with poorly differentiated CRC. In addition, tissue and serum
miR-325-3p levels were significantly upregulated in patients at TNM
stages III and IV compared with those at stages I and II (Fig. 4B and E). Furthermore, tissue and
serum miR-325-3p levels were significantly upregulated in patients
with lymph node metastasis (Fig. 4C and
F).
miR-325-3p promotes the migration and
invasion of CRC cells
miR-325-3p expression was detected in the CRC cell
lines, SW480, HCT116 and HT-29, as well as the normal colon cell
line, FHC. The results demonstrated that miR-325-3p was highly
expressed in HT-29 cells (Fig. 5A),
thus, this cell line was used for subsequent experimentation. HT-29
cells were transfected with miR-325-3p mimics or inhibitors and
RT-qPCR analysis was performed to assess transfection efficiency.
The results demonstrated that transfection with miR-325-3p mimic
significantly enhanced miR-325-3p expression, the effects of which
were reversed following transfection with miR-325-3p inhibitor
(Fig. 5B). The effect of miR-325-3p
on the viability, migration and invasion of CRC cells was
subsequently assessed. As presented in Fig. 5C, overexpression of miR-325-3p
increased the cell viability in a time-dependent manner. In
addition, the cell migratory and invasive abilities were
significantly enhanced following transfection with miR-325-3p mimic
(Fig. 5D). Conversely, miR-325-3p
knockdown decreased HT-29 cell viability at 24, 48 and 72 h
(Fig. 5E). Furthermore, the
migratory and invasive abilities of HT-29 cells decreased following
transfection with miR-325-3p inhibitors (Fig. 5F). Collectively, these results
suggest that miR-325-3p acts as an oncogene in CRC.
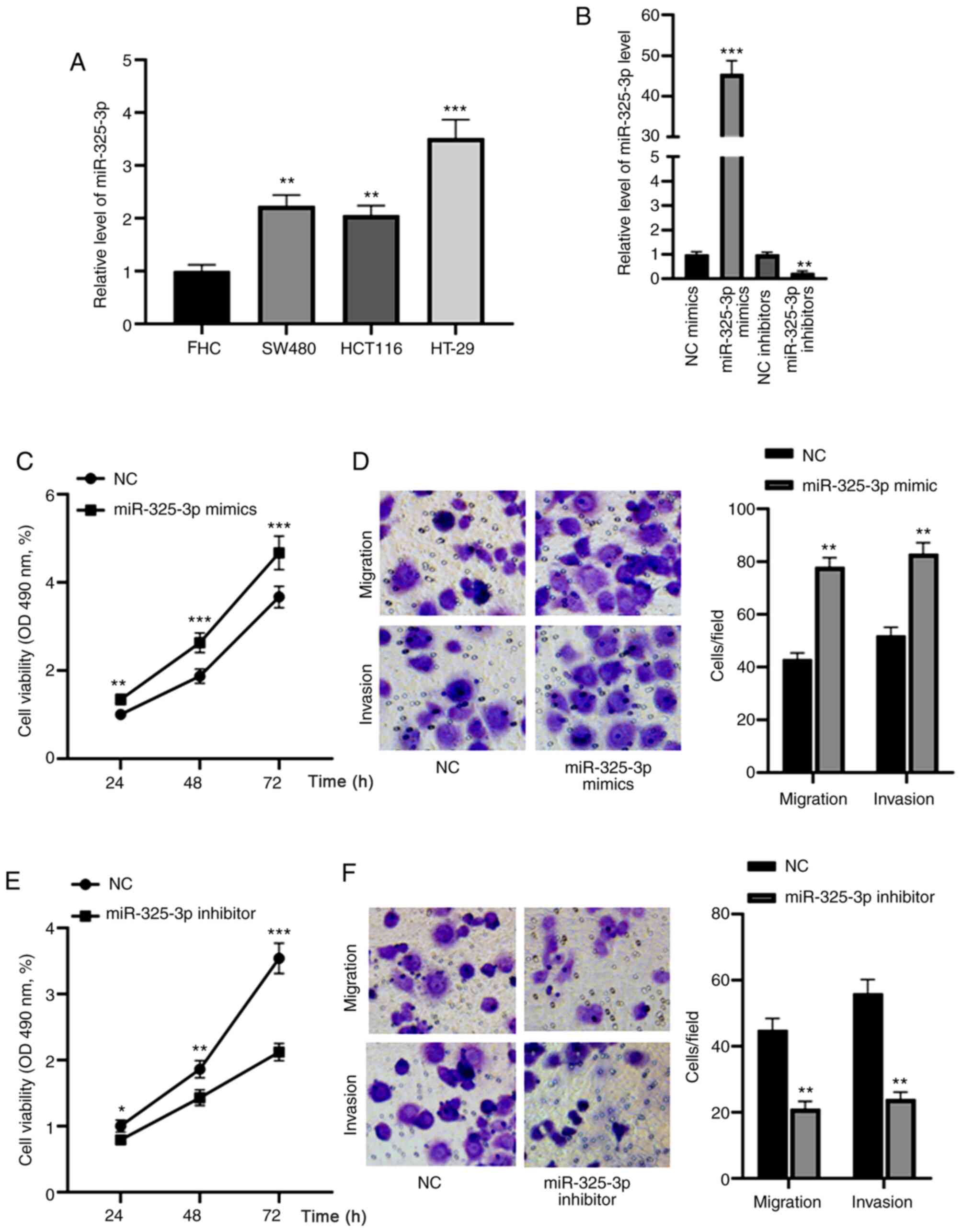 | Figure 5.miR-325-3p promotes the migration and
invasion of CRC cells. (A) RT-qPCR analysis was performed to detect
miR-325-3p expression in the CRC cell lines, SW480, HCT116 and
HT-29, as well as the normal colon cell line, FHC. (B) RT-qPCR
analysis was performed to assess the transfection efficiency of
miR-325-3p mimics or inhibitors into HT-29 cells. (C) The results
of the MTT assay demonstrated that overexpression of miR-325-3p
increased cell viability in a time-dependent manner. (D) Cell
migratory and invasive abilities were significantly enhanced
following transfection with miR-325-3p mimic. (E) miR-325-3p
knockdown decreased HT-29 cell viability at 24, 48 and 72 h. (F)
The migratory and invasive abilities of HT-29 cells significantly
decreased following transfection with miR-325-3p inhibitors.
*P<0.05; **P<0.01; ***P<0.001. miR, microRNA; CRC,
colorectal carcinoma; RT-qPCR, reverse transcription-quantitative
PCR; NC, negative control; OD, optical density. |
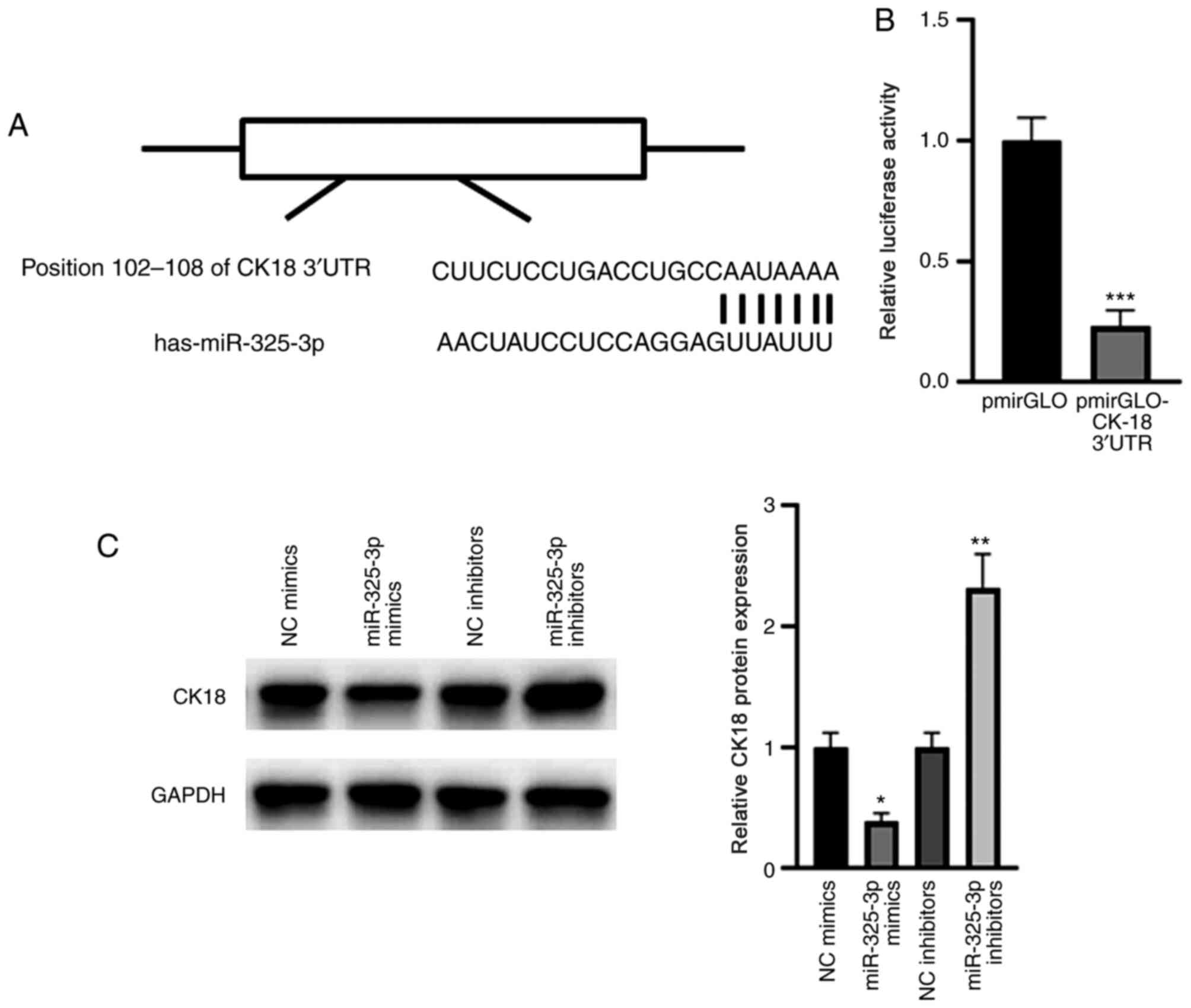
CK18 is a target gene of
miR-325-3p
Notably, a conserved binding of miR-325-3p was
identified in the 3′-UTR of CK18 (Fig.
6A). The results of the dual-luciferase reporter assay
demonstrated that miR-325-3p significantly suppressed the relative
luciferase activity of the pmirGLO-CK18-3′-UTR compared with that
of the blank pmirGLO plasmid (Fig.
6B). Western blot analysis demonstrated that overexpression of
miR-325-3p significantly suppressed CK18 expression; however,
miR-325-3p knockdown significantly elevated CK18 expression
(Fig. 6C). Taken together, these
results suggest that CK18 is a target gene of miR-325-3p.
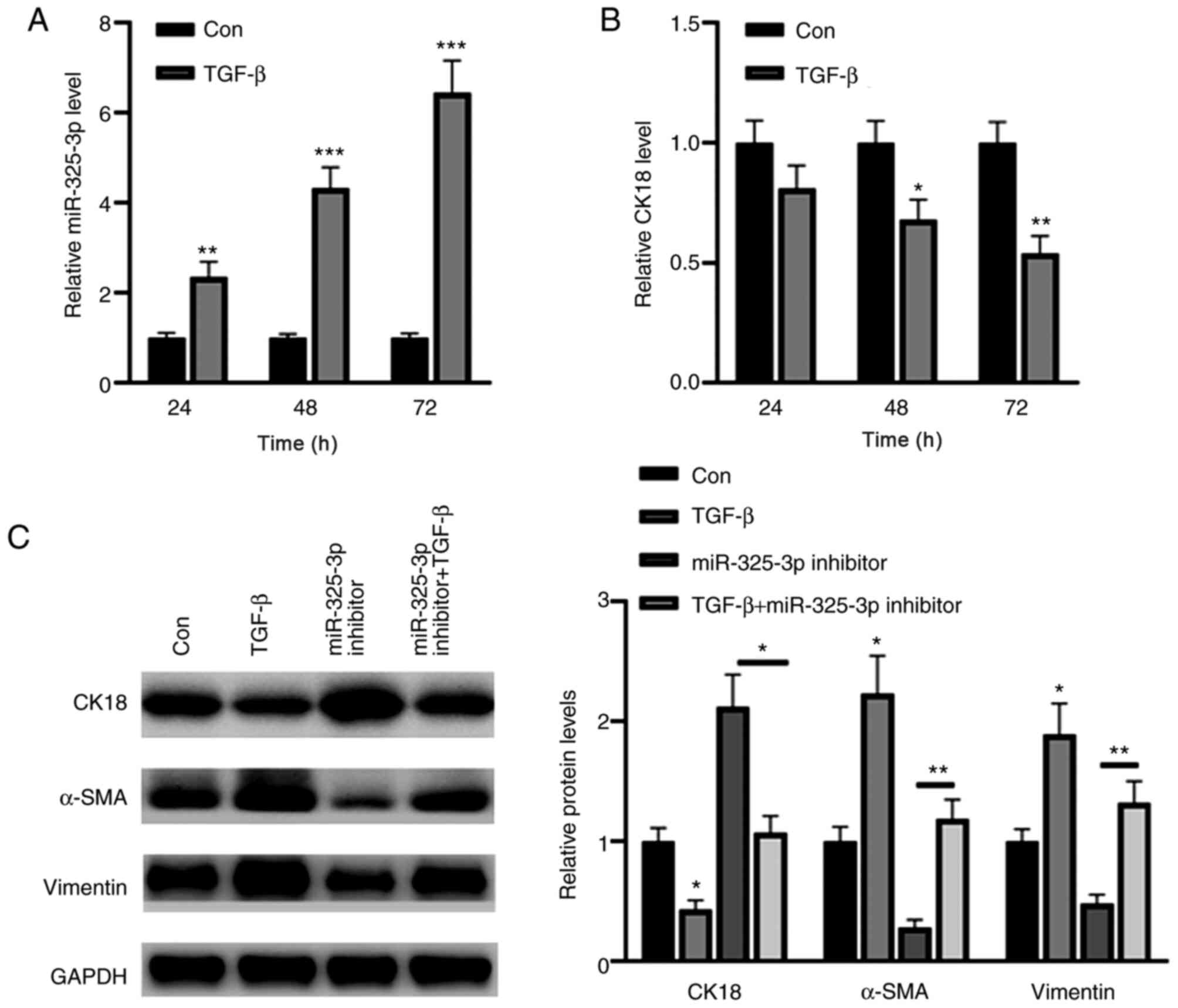
miR-325-3p knockdown partially
reverses transforming growth factor (TGF)-β-induced CK18
downregulation
The results demonstrated that treatment with TGF-β
upregulated miR-325-3p expression in a time-dependent manner
(Fig. 7A). Conversely, TGF-β
significantly decreased CK18 expression at 48 and 72 h (Fig. 7B). Western blot analysis demonstrated
that TGF-β1 significantly decreased the expression of the
epithelial marker, CK18, and increased the expression levels of the
mesenchymal markers, α-SMA and vimentin (Fig. 7C). Notably, these effects were
reversed following inhibition of miR-325-3p (Fig. 7C). Collectively, these results
suggest that miR-325-3p is a key regulator in TGF-β-induced CK18
downregulation.
Discussion
With the development of the economy, the incidence
rate of CRC continues to increase and has become one of the most
notable threats to human health (9).
Despite advancements in endoscopic diagnosis and surgical
techniques for CRC, the 5-year survival rate was slightly >10%
in patients with stage IV disease (18,19). The
differential expression of several miRNAs in tumor tissues and
normal tissues suggests that miRNAs may be used as molecular
markers for tumor diagnosis (7,8). As
non-invasive markers, circulating miRNAs are expected to be
sensitive and act as specific markers for tumor diagnosis and
prognosis evaluation (20).
The present study screened miRNAs that are
differentially expressed in the tissues and serum of patients with
CRC. The results demonstrated that miR-325-3p tissue and serum
levels were significantly upregulated in patients with CRC compared
with healthy individuals. In addition, ROC curve analyses
demonstrated that both tissue and serum miR-325-3p levels may be
used as indicators for the diagnosis of CRC, respectively.
Furthermore, high miR-325-3p expression was significantly
associated with tumor differentiation, TNM stage and lymph node
metastasis. Taken together, these results suggest that miR-325-3p
expression can be used as an indicator to monitor the progression
of patients with CRC.
EMT is an important mechanism that affects the
invasion and metastasis of CRC (21). Several growth factors, such as TGF-β
and epidermal growth factor, mediate EMT (22,23).
Notably, the results of the present study demonstrated that TGF-β
induced miR-325-3p expression. In addition, CK18 was identified as
a target gene of miR-325-3p, suggesting that miR-325-3p is
associated with the degree of EMT in CRC. EMT is widely involved in
the invasion and metastasis of colon cancer due to the loss of
epithelial characteristics into mesenchymal cells (24,25). The
results of the present study demonstrated that high miR-325-3p
expression significantly decreased the expression of the epithelial
marker, CK18, but increased the expression levels of the
mesenchymal markers, vimentin and α-SMA. It is suggested that HT-29
cells gradually lose the characteristics of epithelial cells and
attain the characteristics of mesenchymal cells, which are more
likely to detach from the tumor and transfer to other areas of the
body (26). Collectively, the
results of the present study suggest that upregulated miR-325-3p
expression in the tissue and serum samples of patients with CRC is
closely associated with the progression of CRC.
The present study is not without limitations. First,
the sample size was relatively small. Secondly, due to time
constraints, the present study was unable to investigate the
predictive value of serum miR-325-3p in patients with CRC.
In conclusion, elevated miR-325-3p expression is an
important factor that affects EMT, and is likely to act as a
molecular marker and potential therapeutic target in the
progression of CRC. However, further studies are required to
validate the results presented here.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Hongqi Hospital Affiliated to Mudanjiang Medical University (grant
no. 20190158).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
CS performed the experiments, analyzed the data and
drafted the initial manuscript. XW, XZ, JA and YQ helped perform
reverse transcription-quantitative PCR and western blot analyses.
AC designed the experiments and analyzed the data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Hongqi Hospital Affiliated to Mudanjiang
Medical University (Mudanjiang, China; approval no. MDJHQ-20180925)
and written informed consent was provided by all participants prior
to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu L, Zhang Y, Wang H, Zhang G, Ding Y and
Zhao L: Tumor suppressor miR-1 restrains epithelial-mesenchymal
transition and metastasis of colorectal carcinoma via the MAPK and
PI3K/AKT pathway. J Transl Med. 12:2442014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Chen Z and Li J: The current
status of treatment for colorectal cancer in China: A systematic
review. Medicine (Baltimore). 96:e82422017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Y, Han S, Lei K, Chang X, Wang K, Li Z
and Liu J: Anti-Warburg effect of rosmarinic acid via miR-155 in
colorectal carcinoma cells. Eur J Cancer Prev. 25:481–489. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Wang R and Wang M: miR-331-3p
suppresses cell invasion and migration in colorectal carcinoma by
directly targeting NRP2. Oncol Lett. 18:6501–6508. 2019.PubMed/NCBI
|
|
5
|
Zhao DW, Li MM, Han JP, Wang Y, Jiang LX
and Chang HL: miR-30c exerts tumor suppressive functions in
colorectal carcinoma by directly targeting BCL9. Eur Rev Med
Pharmacol Sci. 23:3335–3343. 2019.PubMed/NCBI
|
|
6
|
Ding T, Cui P, Zhou Y, Chen C, Zhao J,
Wang H, Guo M, He Z and Xu L: Antisense oligonucleotides against
miR-21 inhibit the growth and metastasis of colorectal carcinoma
via the DUSP8 pathway. Mol Ther Nucleic Acids. 13:244–255. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fasihi A, M Soltani B, Atashi A and Nasiri
S: Introduction of hsa-miR-103a and hsa-miR-1827 and hsa-miR-137 as
new regulators of Wnt signaling pathway and their relation to
colorectal carcinoma. J Cell Biochem. 119:5104–5117. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghanbari R, Mosakhani N, Asadi J, Nouraee
N, Mowla SJ, Yazdani Y, Mohamadkhani A, Poustchi H, Knuutila S and
Malekzadeh R: Downregulation of plasma MiR-142-3p and MiR-26a-5p in
patients with colorectal carcinoma. Iran J Cancer Prev.
8:e23292015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong L, Ren M, Lv Z, Yang Y and Wang Z:
MiR-92b-3p promotes colorectal carcinoma cell proliferation,
invasion, and migration by inhibiting FBXW7 in vitro and in vivo.
DNA Cell Biol. 37:501–511. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo H, Chen Y, Hu X, Qian G, Ge S and
Zhang J: The regulation of Toll-like receptor 2 by miR-143
suppresses the invasion and migration of a subset of human
colorectal carcinoma cells. Mol Cancer. 12:772013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iacona JR and Lutz CS: MiR-146a-5p:
Expression, regulation, and functions in cancer. Wiley Interdiscip
Rev RNA. 10:e15332019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang G, Xiong G, Cao Z, Zheng S, You L,
Zhang T and Zhao Y: MiR-497 expression, function and clinical
application in cancer. Oncotarget. 7:55900–55911. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kulda V, Pesta M, Topolcan O, Liska V,
Treska V, Sutnar A, Rupert K, Ludvikova M, Babuska V, Holubec L Jr
and Cerny R: Relevance of miR-21 and miR-143 expression in tissue
samples of colorectal carcinoma and its liver metastases. Cancer
Genet Cytogenet. 200:154–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kontos CK, Tsiakanikas P, Avgeris M,
Papadopoulos IN and Scorilas A: MiR-15a-5p, a novel prognostic
biomarker, predicting recurrent colorectal adenocarcinoma. Mol
Diagn Ther. 21:453–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo J, Fang W, Sun L, Lu Y, Dou L, Huang
X, Tang W, Yu L and Li J: Ultraconserved element uc.372 drives
hepatic lipid accumulation by suppressing miR-195/miR4668
maturation. Nat Commun. 9:6122018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang F, Wang H, Jiang Z, Hu A, Chu L, Sun
Y and Han J: MicroRNA19a mediates gastric carcinoma cell
proliferation through the activation of nuclear factor-κB. Mol Med
Rep. 12:5780–5786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Dai Y, Zhang X, Jin D, Li X and
Zhang Y: Increased miR-449a expression in colorectal carcinoma
tissues is inversely correlated with serum carcinoembryonic
antigen. Oncol Lett. 7:568–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brenner H, Werner S and Chen H:
Multitarget stool DNA testing for colorectal-cancer screening. N
Engl J Med. 371:184–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Igder S, Mohammadiasl J and Mokarram P:
Altered miR-21, miRNA-148a expression in relation to KRAS mutation
status as indicator of adenoma-carcinoma transitional pattern in
colorectal adenoma and carcinoma lesions. Biochem Genet.
57:767–780. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pal I, Rajesh Y, Banik P, Dey G, Dey KK,
Bharti R, Naskar D, Chakraborty S, Ghosh SK, Das SK, et al:
Prevention of epithelial to mesenchymal transition in colorectal
carcinoma by regulation of the E-cadherin-beta-catenin-vinculin
axis. Cancer Lett. 452:254–263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mylavarapu S, Kumar H, Kumari S, Sravanthi
LS, Jain M, Basu A, Biswas M, Mylavarapu SVS, Das A and Roy M:
Activation of epithelial-mesenchymal transition and altered
β-catenin signaling in a novel Indian colorectal carcinoma cell
line. Front Oncol. 9:542019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niknami Z, Muhammadnejad A, Ebrahimi A,
Harsani Z and Shirkoohi R: Significance of E-cadherin and Vimentin
as epithelial-mesenchymal transition markers in colorectal
carcinoma prognosis. EXCLI J. 19:917–926. 2020.PubMed/NCBI
|
|
24
|
Wang JJ, Chong QY, Sun XB, You ML, Pandey
V, Chen YJ, Zhuang QS, Liu DX, Ma L, Wu ZS, et al: Autocrine hGH
stimulates oncogenicity, epithelial-mesenchymal transition and
cancer stem cell-like behavior in human colorectal carcinoma.
Oncotarget. 8:103900–103918. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang P, Gao XY, Yang SQ, Sun ZX, Dian LL,
Qasim M, Phyo AT, Liang ZS and Sun YF: Jatrorrhizine inhibits
colorectal carcinoma proliferation and metastasis through
Wnt/β-catenin signaling pathway and epithelial-mesenchymal
transition. Drug Des Devel Ther. 13:2235–2247. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
You S, Guan Y and Li W:
Epithelialmesenchymal transition in colorectal carcinoma cells is
mediated by DEK/IMP3. Mol Med Rep. 17:1065–1070. 2018.PubMed/NCBI
|