Introduction
Lung cancer is the most common type of tumor and is
has the highest mortality rate worldwide (1). The incidence rate of lung cancer was
11.6% and mortality rate was 18.4% worldwide in 2018 (1). The majority of patients with lung
cancer (>80%) are diagnosed with non-small cell lung cancer
(NSCLC) (2). There are two main
subtypes of NSCLC: Lung adenocarcinoma (LUAD) and lung squamous
cell carcinoma (LUSC) (3,4). Different subtypes of NSCLC are related
to different molecular biological characteristics (5,6). LUAD,
for example, arising from the distal airway, is often associated
with the mutation of the KRAS gene. By contrast, LUSC, occurring in
the proximal airway, is often associated with the deletion of
chromosome 3p (7,8). To date, limited information is
available on the molecular variations between the two subtypes,
which are considered to be the reason for the different responses
to treatment. Patients with LUSC survive longer compared with those
with LUAD following treatment with ipilimumab (9), while patients with LUAD most likely
benefit from the use of gefitinib, a drug targeting epithelial
growth factor receptor (EGFR) kinase mutation (10). These differences in tumor biology and
drug response suggest the importance of accurately distinguishing
LUAD from LUSC. Recently, it has been found that mRNAs can
distinguish LUSC from LUAD (11);
thus, the determination of differential gene expression may aid in
distinguishing between the two subtypes.
Exosomes are extracellular vesicles secreted by
cells; they exist in urine, plasma, lavage fluid, serous cavity
effusion, cerebrospinal fluid and other body fluids (12,13).
They have a lipid bilayer with a diameter of 30–150 nm, and their
contents include DNA, RNA, protein and lipids (14,15).
Exosomes are released through cells into the circulation and body
fluids. Studies have demonstrated that exosomes have different
protein and RNA contents in healthy subjects and patients with
cancer, which provides the basis for their use as diagnostic
markers (16). However, it remains
unknown whether the aforementioned molecular diagnostic markers are
carried by exosomes in patients with NSCLC, and whether they can be
used to distinguish between LUSC and LUAD.
The purpose of the present study was to analyze the
differentially expressed genes (DEGs) in LUSC and LUAD from The
Cancer Genome Atlas (TCGA) database. mRNAs were identified as
potential molecular diagnostic markers to distinguish LUSC and LUAD
using bioinformatics analyses, and these molecular markers were
verified using exosomes from the serum of patients with NSCLC.
Materials and methods
Microarray datasets
Gene expression profile analysis data were obtained
from TCGA database (https://portal.gdc.cancer.gov/). The data of LUSC and
LUAD tissues were used in the present study. The microarray data
included 504 cases of LUSC and 522 cases of LUAD. EdgeR-3.30.0
software was used to analyze the DEGs (17). The corrected P-value was obtained for
the false discovery rate (FDR) using the Benjamini and Hochberg
(BH) method. mRNAs with FDR <0.01, fold change >2 and median
of trans per million (TPM) >5 were defined as having
statistically significant differential expression. According to the
National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov), genes corresponding to
these mRNAs were identified.
Bioinformatics analysis
The present study used the Database for Annotation,
Visualization and Integrated Discovery (DAVID) bioinformatics web
server (https://david.ncif crf.gov/tools.jsp/) as a tool to
explore the potential function of differentially expressed DEGs by
performing Gene Ontology (GO; www.geneontology.org/) and Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathway-enrichment analysis. GO functional
enrichment analysis of DEGs was performed using the GO online
database (http://www.geneontology.org). KEGG
database (http://www.genome.jp/kegg) was used
to analyze the functions involved in the pathways. The P-value
indicates the importance of pathways and related genes. According
to the order of the P-value, the terms/pathways with P<0.01 were
selected for the network. As the number of terms/pathway-related
genes was countless, the data were filtered to produce the network,
with 30 terms/pathways maintained for each network. According to
the specific standard: Due to the large number of genes screened
out, 30 genes with the most obvious changes were chosen and GO
analysis and KEGG analysis performed. if there were too many genes
(>30) with P<0.01, the threshold P-value was further reduced
(P<0.001 or P<0.0001 and so on). The final filtered
terms/pathways included ~30 genes, or <30 genes.
Clinical patients
A total of 16 patients with LUSC and 54 patients
with LUAD were recruited from the Department of Thoracic Surgery,
Fourth Hospital of Hebei Medical University (Shijiazhuang, China)
between October 2017 and October 2018. There were 38 males and 32
females (mean age ± SD, 65±9.8 years; age range, 54–73 years). The
inclusion criteria were as follows: i) All patients were diagnosed
with LUSC or LUAD by histopathological or cytological examination.
The exclusion criteria were as follows: i) Patients with multiple
types of cancer; ii) patients with the tumor site originating from
the lung; iii) patients with pulmonary metastasis; iv) patients
with hemolysis, hyperlipidemia and other abnormal blood diseases;
v) and patients who had received radiotherapy or chemotherapy. The
samples were collected from the Department of Thoracic Surgery of
the Fourth Hospital of Hebei Medical University. The study was
approved by the Ethics Committee of the Fourth Hospital of Hebei
Medical University. Informed consent was signed by all patients or
their families.
Blood collection and separation of
plasma exosomes
A total of 5 ml venous blood samples were collected
from all subjects, centrifuged within 4 h at 3,000 × g for 15 min
at 4°C, and the serum on the upper layer was collected. Hemolysis
was avoided during the whole process, if hemolysis was found,
samples were collected again. The samples were labeled and stored
at −80°C. The separation of exosomes was performed by
ultracentrifugation, as previously described (18). The serum samples were thawed on ice
and centrifuged at 4°C for 30 min at 10,000 × g. The samples were
then centrifuged at 100,000 × g at 4°C for 120 min. The precipitate
was then resuspended in 2–3 ml 1X PBS and filtered using a 0.22-µm
aperture filter. The mixture was centrifuged again for 120 min at
100,000 × g at 4°C.
Transmission electron microscopy
(TEM)
A 10 µl exosomes solution was placed on a copper
mesh and incubated at room temperature for 1 min. After washing
with sterile distilled water, the exosome was contrasted by uranyl
acetate solution for 1 min. The sample was then dried for 2 min
under incandescent light. The copper mesh was observed and
photographed under a transmission electron microscope (H-7650;
Hitachi Ltd.).
Nanoparticle tracking analysis
(NTA)
The measurement of exosome size and particle
concentration was performed according to the manufacturer's
protocol using a ZetaView PMX 110 (Particle Metrix GmbH). Izon
Control Suite software v.3.3.2.2000 (Izon Science Ltd.) was used to
analyze the data.
Western blot analysis
Total exosome protein was extracted from the exosome
samples using RIPA lysis buffer (Invitrogen; Thermo Fisher
Scientific, Inc.) with PMSF protease inhibitor (Invitrogen; Thermo
Fisher Scientific, Inc.). The protein quality of samples were
calculated by the BSA standard protein solutions curve with
PierceTM BCA Protein Assay Kit (cat. no. 23,225; Thermo Fisher
Scientific Inc.). The samples (30 µg per lane) were separated via
SDS-PAGE (12% gel) for the western blot analysis. The proteins were
transferred to PVDF membranes and blocked with evaporated skimmed
milk for 1 h at 37°C and incubated overnight at 4°C with primary
antibodies. The membranes were then incubated for 2 h at 37°C with
sheep anti-rabbit secondary antibody conjugated with HRP (1:10,000;
cat. no. RS0002; ImmunoWay Biotechnology) or sheep anti-mouse
secondary antibody conjugated with HRP (1:10,000; cat. no. RS0001;
ImmunoWay Biotechnology). The protein bands were exposed using ECL
blotting detection reagents (cat. no. P0018; Beyotime Institute of
Biotechnology). The primary antibodies were as follows: Anti-CD63
[1:1,000; cat. no. sc-5275 (M), Santa Cruz Biotechnology, Inc.],
anti-ALG-2 interacting protein X (1:1,000; ALIX; cat. no. sc-53540;
Santa Cruz Biotechnology, Inc.), anti-calnexin (1:1,000; cat. no.
10427-2-AP; ProteinTech Group, Inc.) and anti-tumor susceptibility
gene 101 protein (1:1,000; TSG101; cat. no. sc-136111; Santa Cruz
Biotechnology, Inc.) were used for western blot analysis. Calnexin
was the exosome-negative marker.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Following the suspension of exosomes with 1 ml PBS,
total RNA was extracted from the exosomes using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The RNA
concentration was measured using a Nanodrop 2000 UV–Vis
spectrophotometer (Nanodrop Technologies; Thermo Fisher Scientific,
Inc.). A total of 1 µg total RNA was reverse transcribed into cDNA
according to the manufacturer's protocol by using the PrimeScript™
RT reagent kit (cat. no. RR037A; Takara Bio Inc.). qPCR was then
performed using Premix Ex Taq™ reagent (cat. no. RR390A; Takara
Bio, Inc.). The thermocycling conditions were as follows:
Predenaturation at 95°C for 5 min, denaturation at 95°C for 15 sec,
annealing at 58°C for 30 sec, extension at 72°C for 30 sec, a total
of 40 cycles; extension at 72°C for 10 min. GAPDH was used as an
internal control. The relative expression levels of DEGs in serum
samples were evaluated using the 2−ΔΔCq method (19). The primer sequences are listed in
Table I. All experiments were
repeated three times. RT-qPCR analysis is highly sensitive, and its
accuracy can be affected by RNA quantity, transcription efficiency,
amplification efficiency and experimental procedures between
samples (20). To avoid bias,
normalization of gene expression is an essential step (20). The most common practice is to compare
a target gene expression with an internal reference gene (21). Housekeeping genes, such as β-actin
(ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and
solute carrier family 25 member 6 (SLC25A6) (22–24) have
been used extensively for RT-qPCR analysis. However, under any
given experimental condition, the expression of these commonly used
reference genes may vary substantially (25,26). In
order to ensure the accuracy of the results, β-actin (ACTB) and
solute carrier family 25 member 6 (SLC25A6) were used as internal
references in the present study.
 | Table I.Sequences of the primers. |
Table I.
Sequences of the primers.
| Gene | Sequence |
|---|
| TP63 |
|
|
Forward |
5′-CTCCAACACCGACTACCCAG-3′ |
|
Reverse |
5′-GCGGATAACAGCTCCCTGAG-3′ |
| KRT5 |
|
|
Forward |
5′-GGGCGAGGAATGCAGACTC-3′ |
|
Reverse |
5′-ACTGCCATATCCAGAGGAAACA-3′ |
| CEACAM6 |
|
|
Forward |
5′-TCTTGTGAATGAAGAAGCAACCG-3′ |
|
Reverse |
5′-CACAGCATCCTTGTCCTCCA-3′ |
| SFTPB |
|
|
Forward |
5′-GCTGGACAGGGAAAAGTGC-3′ |
|
Reverse |
5′-TGGATACACTGGAGAGGGCT-3′ |
| ACTB |
|
|
Forward |
5′-CCTCGCCTTTGCCGATCC-3′ |
|
Reverse |
5′-CATGCCCACCATCACGC-3′ |
| SLC25A6 |
|
|
Forward |
5′-GGCCTACTTCGGCGTGTAC-3′ |
|
Reverse |
5′-CGAAGGGGTAGGACACCACG-3′ |
Statistical analysis
All data are analyzed using SPSS (version 22.0; IBM
Corp.) and GraphPad Prism (version 6.0; GraphPad Software, Inc.)
software. EdgeRv.3.30.0 software was used to analyze the DEGs
(17). The P-value was corrected for
FDR using the Benjamini and Hochberg (BH) method (27). mRNAs with FDR <0.01, fold change
>2 and a median TPM >5 were defined as having a statistically
significant differential expression. Fisher's exact test was used
to calculate the significance (P-value) of the GO and KEGG
enrichment analyses. The means of the expression of four DEGs in
the LUSC and LUAD group were compared using an independent t-test.
The association between gene expression and clinical biological
parameters was analyzed using the Chi-square test or Fisher's test.
The logistic regression model of generalized linear models (R
programming language) was used to model the ΔCq data of 70 cases,
and the single gene and four gene combinations were analyzed,
respectively. A receiver operating characteristic (ROC) curve was
drawn for each model and the area under the curve (AUC) of the
model was calculated to evaluate the performance. P<0.05 was
considered to indicate a statistically significant difference.
Results
DEGs of LUSC and LUAD
In order to determine the specific mRNA profiles of
serum exosomes in patients with LUSC and LUAD, 1,026 patients with
NSCLC, including 504 LUSCs and 522 LUADs from TCGA database were
analyzed using bioinformatics. The purpose of this study was to
analyze the DEGs of LUSC and LUAD, and explore these genes in order
to distinguish between LUSC and LUAD. Therefore, samples from
healthy individuals or adjacent non-cancerous samples were not
used. Following the data analysis, a total of 1,619 genes were
identified as differentially expressed in patients with LUSC and
LUAD. Considering that the markers in tissues are diluted by the
RNA from other tissue sources when they are detected in blood, the
markers with a high expression were selected. In the present study,
17 DEGs with a median TPM >1,000 were selected from the LUSC and
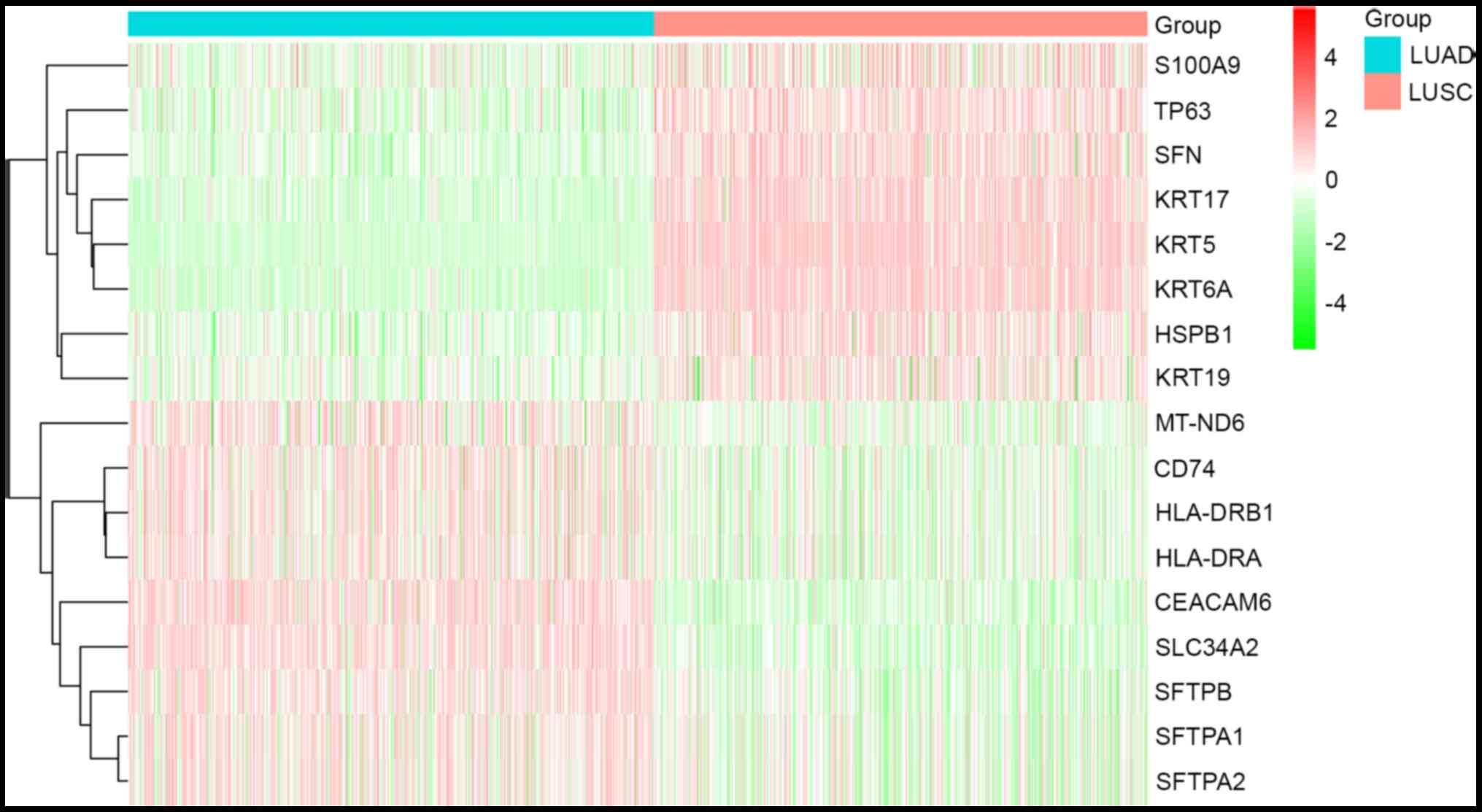
LUAD group. Furthermore, hierarchical cluster analysis was applied
to the 17 DEGs (Fig. 1). Among
these, eight of the 17 DEGs were upregulated in the LUSC and
downregulated in the LUAD group; nine of the 17 DEGs were
upregulated in the LUAD and downregulated in the LUSC group
(Table II) (28–43).
 | Table II.Differentially expressed genes
screened using The Cancer Genome Atlas database. |
Table II.
Differentially expressed genes
screened using The Cancer Genome Atlas database.
|
|
|
|
| Regulation |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| First author/s,
year | Gene Name |
log2FC | Chromosome | LUSC | LUAD | Reported
functioning in lung cancer (PMID) | Refs. |
|---|
| Liu et al,
2018 | S100
calcium-binding protein A9 | 1.94 | chr1 | Up | Down | Activates the MAPK
pathway by phosphorylating P38 and ERK1/2 (30519313) | (28) |
| Stewart et
al, 2019 | Tumor protein
p63 | 6.98 | chr3 | Up | Down | Upregulates
glutathione metabolism and promotes tumorigenesis (31395880) | (29) |
| Kim et al,
2018 | Stratifin | 2.21 | chr1 | Up | Down | Enhances receptor
tyrosine kinases stabilization through abnormal ubiquitin-specific
protease 8 regulation (29880877) | (30) |
| Wang et al,
2019 | Keratin 17 | 6.38 | chr17 | Up | Down | Promotes
proliferation and invasion, and indicates poor prognosis
(31496806) | (31) |
| Khayyata et
al, 2009 | Keratin 5 | 11.17 | chr3 | Up | Down | Overexpression is
the unique characteristic of SCC(19170169) | (32) |
| Yang et al,
2020 | Keratin 6A | 10.28 | chr12 | Up | Down | Promotes
epithelial-mesenchymal transition and cancer stem cell
transformation (32329414) | (33) |
| Konda et al,
2017 | Heat shock protein
beta-1 | 1.37 | chr7 | Up | Down | Is synthetic lethal
to cells with oncogenic activation of MET Proto-Oncogene Receptor
Tyrosine Kinase, EGFR and BRAF (28182330) | (34) |
| Ohtsuka et
al, 2016 | Keratin 19 | 1.28 | chr17 | Up | Down | Binds to HER2 and
active HER2-Erk pathway (28008968) | (35) |
|
| Mitochondrially
encoded NADH dehydrogenase 6 | −1.18 | chrMT | Down | Up | Unclear |
|
| Song et al,
2020 | CD74 antigen | −1.20 | chr18 | Down | Up | Involved in Yes1
associated transcriptional regulator-induced multidrug resistance
(31692299) | (36) |
| Tokumoto et
al, 1998 | Major
histocompatibility complex, class II, DR beta 1 | −1.20 | chr6 | Down | Up | Differences in HLA
status influence the susceptibility and resistance to lung cancer.
(9808426) | (37) |
| Martins et
al, 2007 | Major
histocompatibility complex, class II, DR alpha | −.1.19 | chr6 | Down | Up | MAPK activity in
melanoma is associated with high levels of expression of major
histocompatibility complex class II DR α (17304627) | (38) |
| Hong et al,
2015 | Carcinoembryonic
antigen cell adhesion molecule 6 | −4.28 | chr19 | Down | Up | Overexpression is
associated with paclitaxel resistance in lung adenocarcinoma cells,
and anti-CEACAM6 monoclonal antibody treatment enhances
chemosensitivity in vitro (26204223) | (39) |
| Wang et al,
2015 | Solute carrier
family 34 member 2 | −4.01 | chr4 | Down | Up | Act as a new tumor
suppressor gene in non-small cell lung cancer and the relative
mechanism might be related to the changes of PI3K-Akt-mtor and
Ras-Raf-MEK-ERK signal pathways (26156586) | (40) |
| Lee et al,
2017 | Surfactant protein
B | −3.17 | chr2 | Down | Up | Suppresses lung
cancer progression by inhibiting secretory phospholipase A2
activity and arachidonic acid production (28743125) | (41) |
| Hasegawa et
al, 2017 | Surfactant protein
A1 | −2.64 | chr10 | Down | Up | Downregulates EGFR
which does not suppress EGF-induced phosphorylation of EGFR or cell
proliferation. (28972165) | (42) |
| Wang et al,
2009 | Surfactant protein
A2 | −2.55 | chr10 | Down | Up | Interferes with
protein trafficking and causes familial idiopathic pulmonary
fibrosis and lung cancer (19100526) | (43) |
Biological function and signaling
pathway enrichment analysis of DEGs
The DEGs of LUAD and LUSC were analyzed using the
DAVID database (https://david.ncifcrf.gov/tools.jsp/). DEGs in LUAD
were mainly enriched in multiple biological processes, such as the
‘regulation of complement activation’, ‘signaling pattern
recognition receptor activity’, ‘pattern recognition receptor
signaling pathway’ and the ‘negative regulation of
epithelial-to-mesenchymal transition’, ‘BMP receptor binding’ and
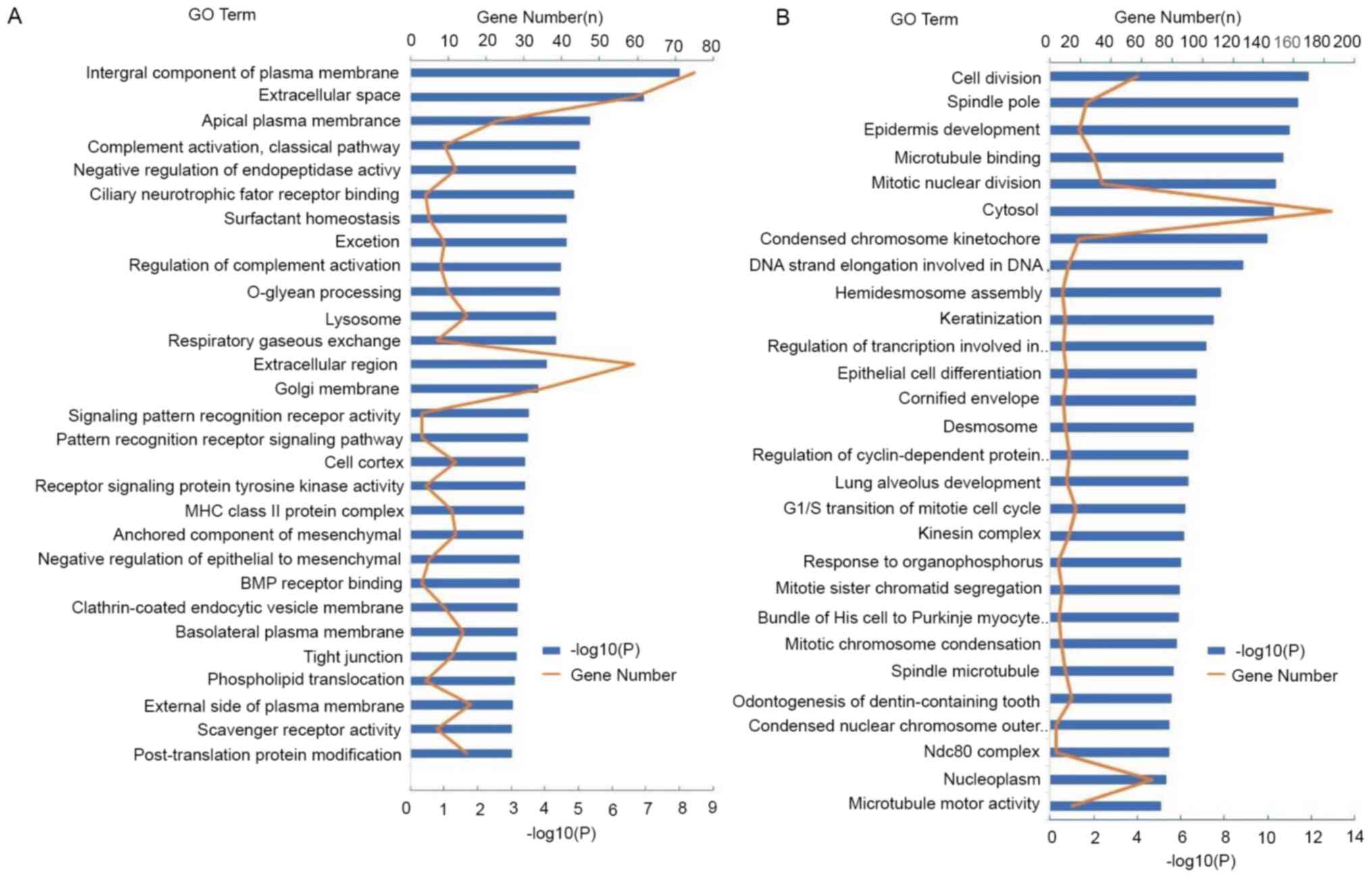
‘tight junction’ (Fig. 2A). However,
DEGs in LUSC were mainly enriched in multiple biological processes,
such as ‘cell division’, ‘mitotic nuclear division’, the
‘G1/S transition of mitotic cell cycle’, ‘mitotic sister
chromatid segregation’, ‘spindle pole’ and ‘spindle microtubule’
(Fig. 2B). Complement activation is
an important early event in the inflammatory response. However, DEG
enrichment in LUAD was higher compared with that of DEGs in LUSC in
the complement activation pathway, indicating that there was a more
prominent association with the inflammatory response.
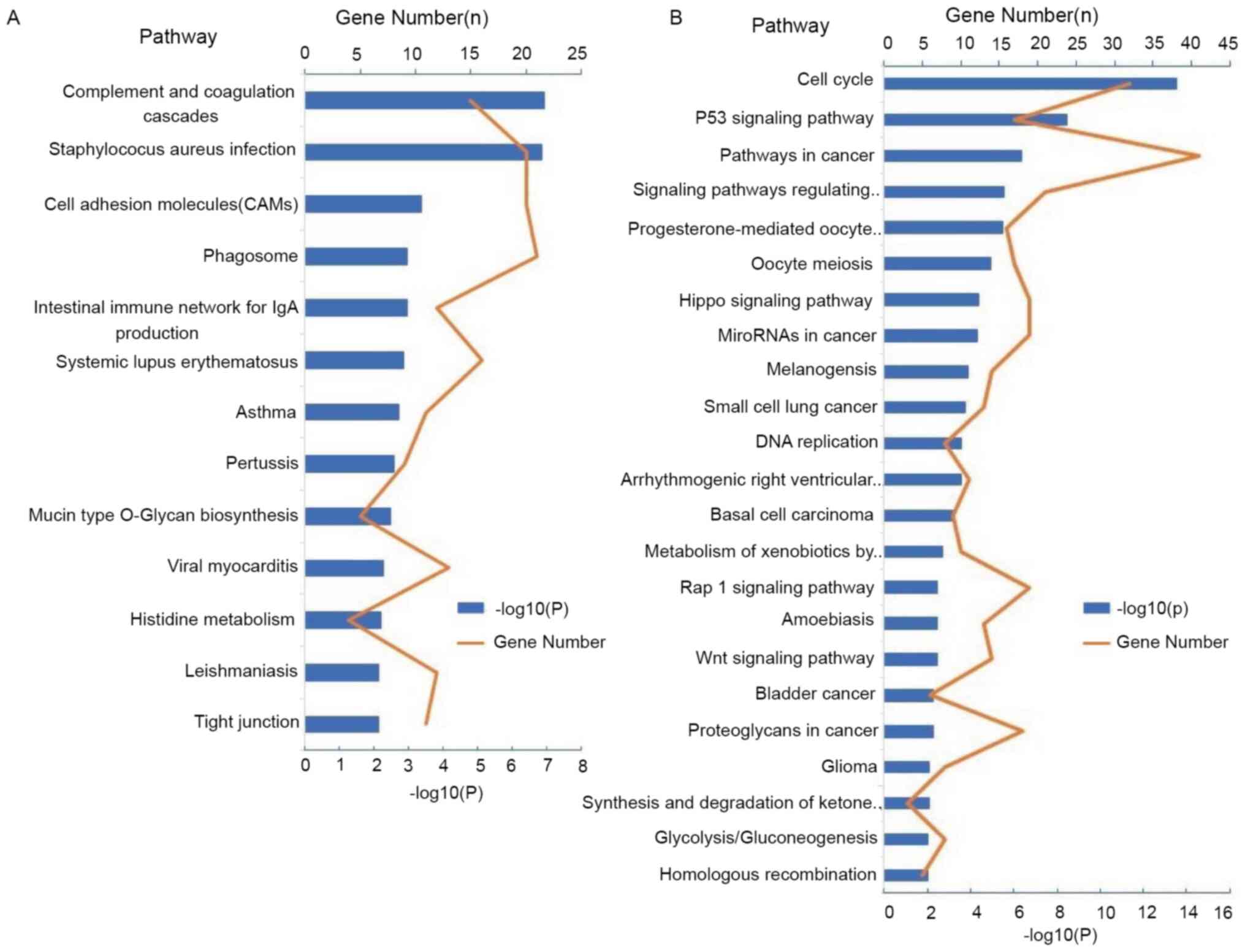
To investigate the important ways of DEGs, KEGG
pathway analysis was performed. According to the expression of
DEGs, three signaling pathways with the highest enrichment in LUAD
were ‘complement and coagulation cascades’, ‘Staphylococcus aureus
infection’, ‘cell adhesion molecules’ and ‘tight junction’
(Fig. 3A). However, three signaling
pathways with the highest enrichment in LUSC were the cell cycle,
p53 signaling pathway and pathways in cancer (Fig. 3B).
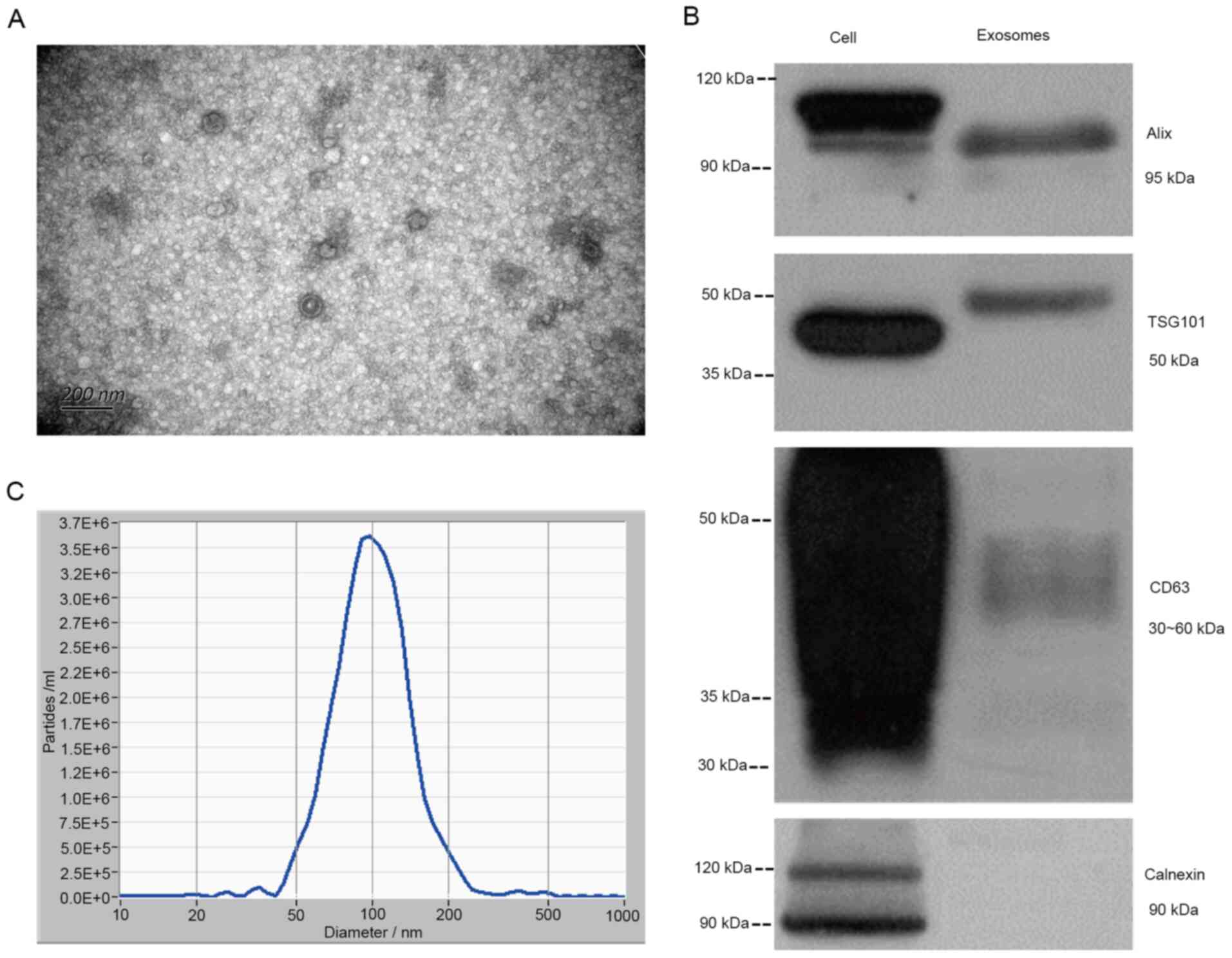
Identification of exosomes
The characteristics of exosomes were identified by
TEM, western blot analysis and NTA, and the plasma exosomes of one
patient with lung cancer (Fig. 4A)
were observed under a transmission electron microscope. It was
observed that the structure of plasma exosomes included a lipid
bilayer membrane and had a size <200 nm. In addition, Alix,
TSG101 and CD63, protein markers of plasma-derived exosomes, were
observed in exosomes and the whole cell extract, whereas calnexin
was the exosome-negative marker. There were two bands of Alix in
the whole cell lysate, with the top band indicating non-specific
staining. As the protein modification level of TSG101 in different
types of samples differs, the whole cell lysate band was smaller
compared with that of the plasma exosome, which is a normal
phenomenon (36). Due to the
different glycosylation levels of CD63, the bands were diffuse with
a fragment size of 30–60 kDa (44).
Therefore, the protein bands of CD63 in exosomes were shallower,
but were also distributed in this region (30–60 kDa). Due to the
antibody used, calnexin presented two bands with the lower band
corresponding to calnexin (band size, 90 kDa) and the upper 120 kDa
band being non-specific (Fig. 4B).
Furthermore, NTA revealed that the diameter of the exosomes was
25–235 nm, enriched at 99.2 nm and the concentration was
~4.2×107 particles/ml (Fig.
4C). In addition, Alix, TSG101 and CD63, protein markers of
plasma-derived exosomes, were observed in exosomes and the whole
cell extract, whereas Calnexin was the exosome-negative marker.
There were two bands of Alix in the whole cell lysate, with the top
band indicating non-specific staining. As the protein modification
level of TSG101 in different types of samples differs, the whole
cell lysate band was smaller compared with that of the plasma
exosome, which is a normal phenomenon (36). Due to the different glycosylation
levels of CD63, the bands were diffuse with a fragment size of
30–60 kDa (44). Therefore, the
protein bands of CD63 in exosomes were shallower, but were also
distributed in this region (30–60 kDa). Due to the antibody used,
calnexin presented two bands with the lower band corresponding to
calnexin (band size, 90 kDa) and the upper 120 kDa band being
non-specific (Fig. 4C).
Exosomal tumor protein P63 (TP63),
keratin 5 (KRT5), CEA cell adhesion molecule 6 (CEACAM6) and
surfactant protein B (SFTPB) mRNA as biomarkers for distinguishing
LUAD from LUSC
To confirm the feasibility of these genes in
differentiating LUAD from LUSC, TP63, KRT5, CEACAM6 and SFTPB were
selected for verification. A total of four differentially expressed
exosomal mRNAs were selected for validation with serum samples from
70 patients with NSCLC (54 patients with LUAD and 16 patients with
LUSC) using RT-qPCR. In order to ensure the accuracy of the
results, ACTB and SLC25A6 were used as internal references in the
present study. As shown in Fig. 5A and
B, exosomal TP63 and KRT5 mRNA levels were significantly
upregulated in patients with LUSC (P<0.01) compared with those
in patients with LUAD. However, exosomal CEACAM6 and SFTPB mRNA
levels were significantly upregulated in patients with LUAD
compared with those in patients with LUSC, with ACTB as the
internal control (P<0.01 and P<0.05, respectively; Fig. 5C and D). Similarly, as shown in
Fig. 5E and F, exosomal TP63 and
KRT5 mRNA levels were significantly upregulated in patients with
LUSC (P<0.01) compared with those in patients with LUAD.
However, exosomal CEACAM6 and SFTPB mRNA levels were significantly
upregulated in patients with LUAD compared with those in patients
with LUSC, with SLC25A6 as an internal control (P<0.01 and
P<0.05, respectively; Fig. 5G and
H).
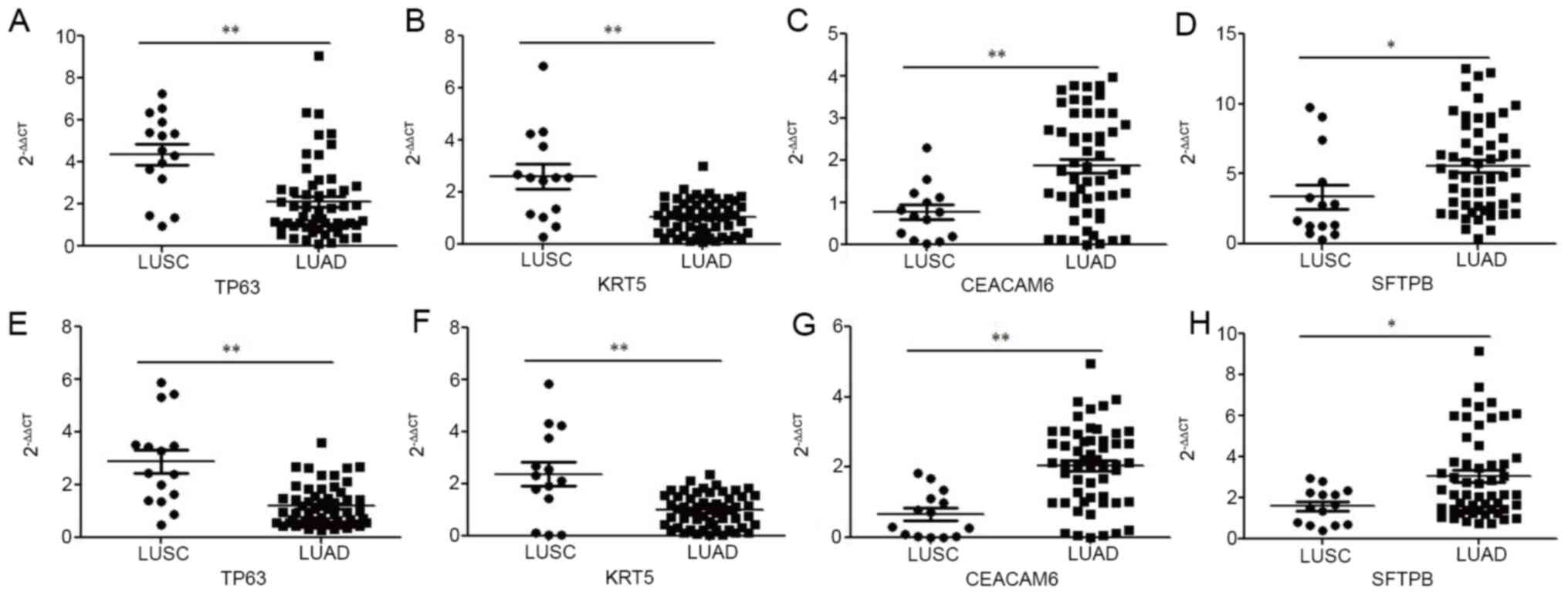 | Figure 5.Relative expression of TP63, KRT5,
CEACAM6 and SFTPB from exosomes isolated from two types of
non-small cell lung cancer measured by RT-qPCR. (A) TP63 based on
ACTB; (B) KRT5 based on ACTB; (C) CEACAM6 based on ACTB; (D) SFTPB
based on ACTB; (E) TP63 based on SLC25A6; (F) KRT5 based on
SLC25A6; (G) CEACAM6 based on SLC25A6; (H) SFTPB based on SLC25A6.
*P<0.05; **P<0.01; TP63, tumor protein P63; KRT5, keratin 5;
CEACAM6, CEA cell adhesion molecule 6; SFTPB, surfactant protein B;
ACTB, β-actin; SLC25A6, solute carrier family 25 member 6; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; LUSC,
lung squamous cell carcinoma; LUAD, lung adenocarcinoma. |
ROC curves were drawn to evaluate the clinical
diagnostic value of four DEGs (TP63, KRT5, CEACAM6 and SFTPB) for
patients with LUAD and LUSC. ROC curves were calculated using ACTB
and SLC25A6 for modeling. The AUC of exosomal TP63 was 0.682 (95%
CI, 0.526–0.837), and the sensitivity and specificity were 74.1 and
62.5%, respectively, based on ACTB (Fig.
6A). The AUC of exosomal KRT5 was 0.683 (95% CI, 0.525–0.842),
and the sensitivity and specificity were 79.6 and 62.5%,
respectively, based on ACTB (Fig.
6B). The AUC of exosomal CEACAM6 was 0.681 (95% CI,
0.547–0.814), and the sensitivity and specificity were 87.5 and
53.7%, respectively, based on ACTB (Fig.
6C). The AUC of exosomal SFTPB was 0.686 (95% CI, 0.533–0.838),
and the sensitivity and specificity were 43.7 and 90.7%,
respectively, based on ACTB (Fig.
6D). In addition, the combination of TP63, KRT5, CEACAM6 and
SFTPB yielded an AUC of 0.757 (95% CI, 0.625–0.889) with a
sensitivity of 75.0% and specificity of 72.2%, indicating a greater
efficacy compared with that obtained by any one marker alone, based
on ACTB (Fig. 6E). Similarly, the
AUC of exosomal TP63 was 0.716 (95% CI, 0.560–0.872), and the
sensitivity and specificity were 61.1 and 75.0%, respectively,
based on SLC25A6 (Fig. 6F). The AUC
of exosomal KRT5 was 0.715 (95% CI, 0.586–0.844), and the
sensitivity and specificity were 81.3 and 66.7%, respectively,
based on SLC25A6 (Fig. 6G). The AUC
of exosomal CEACAM6 was 0.730 (95% CI, 0.592–0.869), and the
sensitivity and specificity were 81.3% and 66.7%, respectively,
based on SLC25A6 (Fig. 6H). The AUC
of exosomal SFTPB was 0.701 (95% CI, 0.548–0.855), and the
sensitivity and specificity were 50.0 and 87.0%, respectively,
based on SLC25A6 (Fig. 6I). In
addition, the combination of TP63, KRT5, CEACAM6 and SFTPB yielded
an AUC of 0.822 (95% CI, 0.699–0.945) with a sensitivity of 62.5%
and specificity of 88.89%, indicating a greater efficacy compared
with that obtained by any one marker alone, based on SLC25A6
(Fig. 6J).
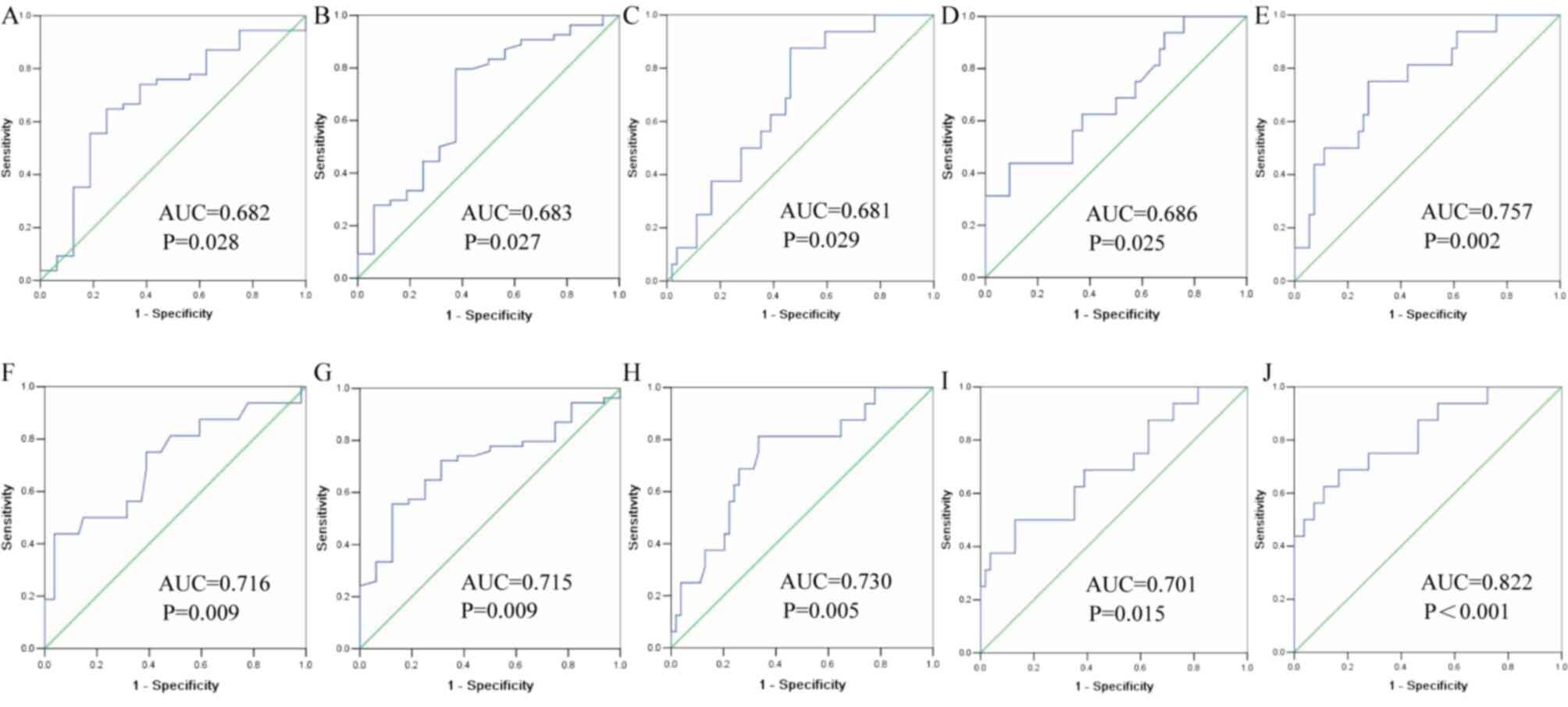 | Figure 6.Receiving operator characteristic
curve of four exosomal DEGs in distinguishing between LADC and
LSCC. (A) TP63 based on ACTB; (B) KRT5 based on ACTB; (C) CEACAM6
based on ACTB; (D) SFTPB based on ACTB; (E) four DEGs based on
ACTB; (F) TP63 based on SLC25A6; (G) KRT5 based on SLC25A6; (H)
CEACAM6 based on SLC25A6; (I) SFTPB based on SLC25A6; (J) four DEGs
based on SLC25A6. LUAD, lung adenocarcinoma; LUSC, lung squamous
cell carcinoma; AUC, area under the curve; DEGs, differentially
expressed genes; TP63, tumor protein P63; KRT5, keratin 5; CEACAM6,
CEA cell adhesion molecule 6; SFTPB, surfactant protein B; ACTB,
β-actin; SLC25A6, solute carrier family 25 member 6. |
Although two internal reference genes were used, the
statistical results were basically consistent (Figs. 5 and 6), and the combined use of the four genes
improved the ability to distinguish LUAD from LUSC. These
aforementioned results demonstrated that exosomal TP63, KRT5,
CEACAM6 and SFTPB mRNA were potential clinical diagnostic markers
for distinguishing LUAD from LUSC.
Discussion
Recently, a number of studies have demonstrated that
exosomes can carry biomarkers, and have diagnostic and prognostic
value (45–47). Although certain studies have
demonstrated that mRNAs in peripheral blood can be used as
biomarkers for the clinical diagnosis of LUAD and LUSC (48,49), to
the best of our knowledge, there is currently no relevant research
available on the mRNAs from exosomes. In the present study,
exosomes were isolated from patient serum and the diagnostic value
of exosomes as biomarkers to distinguish LUAD from LUSC was
investigated. Due to the endocytic origin of exosomes, the
composition of exosomes reflects the composition of parental cells;
exosomes represent potential substitutes for primitive cells
(50). It is well known that
exosomes are stable and are relatively difficult to degrade, and
can be used to identify original cells (51). The formation of exosomes is
associated with cell secretion and involves a variety of proteins,
such as Rab proteins (Rab27A/B), heat shock protein 70, TSG101,
Alix and tetraspanins (CD9, CD63, CD81 and CD82); these proteins
are considered to be the exosome markers for identifying true
exosomes (50,52). As exosomes can be easily obtained and
identified from most body fluids, they may become a promising
biomarker for lung cancer (53). In
the present study, Alix, TSG101 and CD63 were selected as markers
for identifying true exosomes. The results revealed that the
exosome markers were positive, indicating that exosomes were
successfully extracted.
It has been shown that multiple genes can be used as
potential biomarkers for the diagnosis and prognosis of lung cancer
(54,55). However, to the best of our knowledge,
there are currently limited studies available on the effective
molecular diagnostic markers of LUAD and LUSC. Based on TCGA
database, the present study identified eight DEGs that were
overexpressed in patients with LUSC compared with those with LUAD;
and nine DEGs that were downregulated in patients with LUSC
compared with those with LUAD.
In addition, KEGG and GO analyses were performed on
these DEGs. The results demonstrated that LUAD was associated with
the GO term ‘BMP receptor binding’. Bone morphogenetic proteins
(BMPs) belong to the transforming growth factor β superfamily
(56). Deng et al (57) observed that the mRNA level of BMP5
was higher in LUAD tissue compared with that in LUSC tissue. In the
present study, ‘Tight junction’ was obviously enriched in LUAD, in
both GO and KEGG analyses. Tight junctions belong to epithelial
cells when forming intercellular junction complexes (58). Claudins are major components of tight
junctions (59), and Claudin-2 is
highly expressed in LUAD tissues and increases cell proliferation
(60). In the present study, LUSC
was associated with the GO terms ‘Spindle pole’ and ‘Spindle
microtubule’. Abnormal spindle-like microcephaly-associated protein
(ASPM) is a type of microtubule-associated centrosome protein,
which plays an important role in cell development (61). ASPM is mainly expressed in the
ventricular zone of posterior fossa, and participates in the
functional regulation of spindle tissue and cytokinesis (62). Yuan et al (63) demonstrated that ASPM promoted the
progression of LUSC by targeting CDK4. The present study
demonstrated that the p53 signaling pathway was upregulated in
LUSC. The key role of p53 mutation in malignant transformation,
histological progress, invasion and metastasis of lung cancer has
been confirmed in lung cancer models in vitro and in
vivo (64,65). Smoking is closely associated with p53
mutation (66), which may explain
the universality of p53 alterations in LUSC. These results suggest
that DEGs affect the development of LUSC and LUAD via different
pathways. Therefore, the screened DEGs can be used as markers to
distinguish LUSC from LUAD.
Considering the expression level and relatively high
specificity of exosome-derived DEGs in peripheral blood, TP63,
KRT5, CEACAM6 and SFTPB were selected for follow-up experiments.
TP63 and KRT5 levels were more concentrated in LUSC cases. It is
well known that TP63 is a biomarker of squamous cell carcinoma
(67,68). The results of the present study
revealed that TP63 expression in LUSC was significantly higher
compared with that in LUAD. It has been demonstrated that TP63 can
regulate cell proliferation and plays a key role in squamous cell
carcinoma (69,70). The results indicated that cell
cycle-related molecular pathways play an important role in the
development of squamous cell carcinoma. KRT5 protein is mainly
expressed in basal keratinocytes of the epidermis and its
overexpression is a unique feature of squamous cell carcinoma
(32). Similar results were also
obtained in a recent study (71).
The aforementioned results provide a theoretical basis for the use
of TP63 and KRT5 as clinical biomarkers for the diagnosis of LUSC.
The expression of CEACAM6 and SFTPB from exosomes was higher in
LUAD compared with in LUSC. It has been demonstrated that CEACAM6
is expressed at low levels in laryngeal squamous cell carcinoma
cell lines (72), whereas it is
expressed at high levels in prostate cancer and gastric
adenocarcinoma (73,74). Hong et al (39) found CEACAM6 protein expression in
85.7% of 70 LUAD tissues, whereas all LUSC tissues stained negative
for CEACAM6, which is consistent with the findings of previous
studies. Borczuk et al (75)
demonstrated that SFTPB, a member of lung-specific transcription
pathways, played a role in the development of LUAD. These findings
correspond with the results of the present study. At least in part,
it was confirmed that the four DEGs selected herein may be used as
markers to distinguish LUSC from LUAD.
In clinical practice, certain patients are highly
suspected as having malignant tumors or have been confirmed to have
malignant tumors; however, this cannot be further classified. In
clinical practice, it is difficult to obtain tumor tissues, or only
a small number of pathological specimens are obtained, which is
insufficient for pathological diagnosis as the whole pathological
tissue includes necrotic tissue that cannot be stained by
immunohistochemistry. In addition, the lipid bilayer of exosomes
allows for stable cargo, which are relatively difficult to degrade
(76). If biomarkers for
distinguishing pathological types in peripheral blood were detected
accurately, this method would improve the efficiency and accuracy
of diagnosis, and may provide clinical guidance for these
patients.
In conclusion, it is evident that TP63, KRT5,
CEACAM6 and SFTPB derived from exosomes may be used as potential
blood biomarkers for distinguishing LUAD from LUSC. The combination
of multiple biomarkers may improve the specificity and sensitivity
of the diagnosis of different lung cancer subtypes. Due to the
imbalance in LUAD and LUSC sample numbers, the statistical results
may be deviated. The authors aim to further expand the sample size
and perform a multicenter study in order to investigate the
potential use of these biomarkers in the diagnosis of LUAD and
LUSC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
BC and JL contributed to the experimental design. BC
and PW performed some of the experiments. BC and LG made
contributions to acquisition and analysis of data. BC analyzed the
data and drafted the initial manuscript. BC and JL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Fourth Hospital of Hebei Medical University.
Informed consent was signed by all patients or their families. If
the patient died or had other incapacity, a family member signed
the consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Otsmane A, Kacimi G, Adane S, Cherbal F
and Aouichat Bouguerra S: Clinico-epidemiological profile and redox
imbalance of lung cancer patients in Algeria. J Med Life.
11:210–217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang H, Lin Y and Liang Y: Treatment of
lung carcinosarcoma and other rare histologic subtypes of non-small
cell lung cancer. Curr Treat Options Oncol. 18:542017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim SL, Jia Z, Lu Y, Zhang H, Ng CT, Bay
BH, Shen HM and Ong CN: Metabolic signatures of four major
histological types of lung cancer cells. Metabolomics. 14:1182018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Björkqvist AM, Husgafvel-Pursiainen K,
Anttila S, Karjalainen A, Tammilehto L, Mattson K, Vainio H and
Knuutila S: DNA gains in 3q occur frequently in squamous cell
carcinoma of the lung, but not in adenocarcinoma. Genes Chromosomes
Cancer. 22:79–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cooper WA, Lam DC, O'toole SA and Minna
JD: Molecular biology of lung cancer. J Thorac Dis. 5 (Suppl
5):S479–S490. 2013.PubMed/NCBI
|
|
7
|
Davidson MR, Gazdar AF and Clarke BE: The
pivotal role of pathology in the management of lung cancer. J
Thorac Dis. 5 (Suppl 5):S463–S478. 2013.PubMed/NCBI
|
|
8
|
Langer CJ, Besse B, Gualberto A, Brambilla
E and Soria JC: The evolving role of histology in the management of
advanced non-small-cell lung cancer. J Clin Oncol. 28:5311–5320.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomasini P, Khobta N, Greillier L and
Barlesi F: Ipilimumab: Its potential in non-small cell lung cancer.
Ther Adv Med Oncol. 4:43–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao J, Lu X, Chen X, Zou Y, Liu A, Li W,
He B, He S and Chen Q: Eight potential biomarkers for
distinguishing between lung adenocarcinoma and squamous cell
carcinoma. Oncotarget. 8:71759–71771. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu F, Vermesh O, Mani V, Ge TJ, Madsen
SJ, Sabour A, Hsu EC, Gowrishankar G, Kanada M, Jokerst JV, et al:
The exosome total isolation chip. ACS Nano. 11:10712–10723. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fitts CA, Ji N, Li Y and Tan C: Exploiting
exosomes in cancer liquid biopsies and drug delivery. Adv Healthc
Mater. 8:e18012682019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carretero-González A, Otero I,
Carril-Ajuria L, de Velasco G and Manso L: Exosomes: Definition,
role in tumor development and clinical implications. Cancer
Microenviron. 11:13–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chuo ST, Chien JC and Lai CP: Imaging
extracellular vesicles: Current and emerging methods. J Biomed Sci.
25:912018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Revenfeld ALS Bæk R, Nielsen MH,
Stensballe A, Varming K and Jørgensen M: Diagnostic and prognostic
potential of extracellular vesicles in peripheral blood. Clin Ther.
36:830–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang N, Song X, Liu L, Niu L, Wang X, Song
X and Xie L: Circulating exosomes contain protein biomarkers of
metastatic non-small-cell lung cancer. Cancer Sci. 109:1701–1709.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bustin SA: Quantification of mRNA using
real-time reverse transcription PCR (RT-PCR): Trends and problems.
J Mol Endocrinol. 29:23–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lourenço AP, Mackert A, Cristino ADS and
Simões ZLP: Validation of reference genes for gene expression
studies in the honey bee, Apis mellifera, by quantitative
real-time RT-PCR. Apidologie. 39:372–385. 2008. View Article : Google Scholar
|
|
23
|
Macabelli CH, Ferreira RM, Gimenes LU, de
Carvalho NA, Soares JG, Ayres H, Ferraz ML, Watanabe YF, Watanabe
OY, Sangalli JR, et al: Reference gene selection for gene
expression analysis of oocytes collected from dairy cattle and
buffaloes during winter and summer. PLoS One. 9:e932872014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong ZZ, Tang CH, Zhang Z, Zhou W, Zhao R,
Wang L, Xu JC, Wu YY, Wu J, Zhang X, et al: Simultaneous detection
of exosomal membrane protein and RNA by highly sensitive aptamer
assisted multiplex-PCR. ACS Appl Bio Mater. 3:2560–2567. 2020.
View Article : Google Scholar
|
|
25
|
Pan H, Yang XW, Bidne K, Hellmich RL,
Siegfried BD and Zhou XG: Selection of reference genes for RT-qPCR
analysis in the monarch butterfly, Danaus plexippus (L.), a
migrating bio-indicator. PLoS One. 10:e01294822015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, An S, Li Z, Wu F, Yang Q, Liu Y,
Cao J, Zhang H, Zhang Q and Liu X: Identification and validation of
reference genes for normalization of gene expression analysis using
qRT-PCR in Helicoverpa armigera (Lepidoptera: Noctuidae).
Gene. 555:393–402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J Royal Stat Soc B. 57:289–300. 1995.
|
|
28
|
Liu P, Wang H, Liang Y, Hu A, Xing R,
Jiang L, Yi L and Dong J: LINC00852 promotes lung adenocarcinoma
spinal metastasis by targeting S100A9. J Cancer. 9:4139–4149. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stewart PA, Welsh EA, Slebos RJC, Fang B,
Izumi V, Chambers M, Zhang G, Cen L, Pettersson F, Zhang Y, et al:
Proteogenomic landscape of squamous cell lung cancer. Nat Commun.
10:35782019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim Y, Shiba-Ishii A, Nakagawa T, Iemura
SI, Natsume T, Nakano N, Matsuoka R, Sakashita S, Lee S, Kawaguchi
A, et al: Stratifin regulates stabilization of receptor tyrosine
kinases via interaction with ubiquitin-specific protease 8 in lung
adenocarcinoma. Oncogene. 37:5387–5402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Yang MQ, Lei L, Fei LR, Zheng YW,
Huang WJ, Li ZH, Liu CC and Xu HT: Overexpression of KRT17 promotes
proliferation and invasion of non-small cell lung cancer and
indicates poor prognosis. Cancer Manag Res. 11:7485–7497. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khayyata S, Yun S, Pasha T, Jian B,
McGrath C, Yu G, Gupta P and Baloch Z: Value of P63 and CK5/6 in
distinguishing squamous cell carcinoma from adenocarcinoma in lung
fine-needle aspiration specimens. Diagn Cytopathol. 37:178–183.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang B, Zhang W, Zhang M, Wang X, Peng S
and Zhang R: KRT6A promotes EMT and cancer stem cell transformation
in lung adenocarcinoma. Technol Cancer Res Treat.
19:15330338209212482020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Konda JD, Olivero M, Musiani D, Lamba S
and Di Renzo MF: Heat-shock protein 27 (HSP27, HSPB1) is synthetic
lethal to cells with oncogenic activation of MET, EGFR and BRAF.
Mol Oncol. 11:599–611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohtsuka T, Sakaguchi M, Yamamoto H, Tomida
S, Takata K, Shien K, Hashida S, Miyata-Takata T, Watanabe M,
Suzawa K, et al: Interaction of cytokeratin 19 head domain and HER2
in the cytoplasm leads to activation of HER2-Erk pathway. Sci Rep.
6:395572016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song Y, Sun Y, Lei Y, Yang K and Tang R:
YAP1 promotes multidrug resistance of small cell lung cancer by
CD74-related signaling pathways. Cancer Med. 9:259–268. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tokumoto H: Analysis of HLA-DRB1-related
alleles in Japanese patients with lung cancer-relationship to
genetic susceptibility and resistance to lung cancer. J Cancer Res
Clin Oncol. 124:511–516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martins I, Deshayes F, Baton F, Forget A,
Ciechomska I, Sylla K, Aoudjit F, Charron D, Al-Daccak R and
Alcaide-Loridan C: Pathologic expression of MHC class II is driven
by mitogen- activated protein kinases. Eur J Immunol. 37:788–797.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hong KP, Shin MH, Yoon S, Ji GY, Moon YR,
Lee OJ, Choi SY, Lee YM, Koo JH, Lee HC, et al: Therapeutic effect
of anti CEACAM6 monoclonal antibody against lung adenocarcinoma by
enhancing anoikis sensitivity. Biomaterials. 67:32–41. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Yang W, Pu Q, Yang Y, Ye S, Ma Q,
Ren J, Cao Z, Zhong G, Zhang X, et al: The effects and mechanisms
of SLC34A2 in tumorigenesis and progression of human non-small cell
lung cancer. J Biomed Sci. 22:522015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee S, Kim D, Kang J, Kim E, Kim W, Youn H
and Youn B: Surfactant protein B suppresses lung cancer progression
by inhibiting secretory phospholipase A2 activity and arachidonic
acid production. Cell Physiol Biochem. 42:1684–1700. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hasegawa Y, Takahashi M, Ariki S, Saito A,
Uehara Y, Takamiya R, Kuronuma K, Chiba H, Sakuma Y, Takahashi H
and Kuroki Y: Surfactant protein A down-regulates epidermal growth
factor receptor by mechanisms different from those of surfactant
protein D. J Biol Chem. 292:18565–18576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Kuan PJ, Xing C, Cronkhite JT,
Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV and Garcia
CK: Genetic defects in surfactant protein A2 are associated with
pulmonary fibrosis and lung cancer. Am J Hum Genet. 84:52–59. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Min L, Zhu S, Chen L, Liu X, Wei R, Zhao
L, Yang Y, Zhang Z, Kong G, Li P and Zhang S: Evaluation of
circulating small extracellular vesicles derived miRNAs as
biomarkers of early colon cancer: A comparison with plasma total
miRNAs. J Extracell Vesicles. 8:16436702019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Y, Yin Z, Fan J, Zhang S and Yang W:
The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal
Transduct Target Ther. 4:472019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li C, Li C, Zhi C, Liang W, Wang X, Chen
X, Lv T, Shen Q, Song Y, Lin D and Liu H: Clinical significance of
PD-L1 expression in serum-derived exosomes in NSCLC patients. J
Transl Med. 17:3552019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen R, Xu X, Qian Z, Zhang C, Niu Y, Wang
Z, Sun J, Zhang X and Yu Y: The biological functions and clinical
applications of exosomes in lung cancer. Cell Mol Life Sci.
76:4613–4633. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fu L, Wang H, Wei D, Wang B, Zhang C, Zhu
T, Ma Z, Li Z, Wu Y and Yu G: The value of CEP55 gene as a
diagnostic biomarker and independent prognostic factor in LUAD and
LUSC. PLoS One. 15:e02332832020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang C, Tan S, Liu WR, Lei Q, Qiao W, Wu
Y, Liu X, Cheng W, Wei YQ, Peng Y and Li W: RNA-Seq profiling of
circular RNA in human lung adenocarcinoma and squamous cell
carcinoma. Mol Cancer. 18:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kowal J, Tkach M and Théry C: Biogenesis
and secretion of exosomes. Curr Opin Cell Biol. 29:116–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Molina-Vila MA, Mayo-de-Las-Casas C,
Giménez-Capitán A, Jordana-Ariza N, Garzón M, Balada A, Villatoro
S, Teixidó C, García-Peláez B, Aguado C, et al: Liquid biopsy in
non-small cell lung cancer. Front Med (Lausanne).
3:692016.PubMed/NCBI
|
|
52
|
Yoshioka Y, Konishi Y, Kosaka N, Katsuda
T, Kato T and Ochiya T: Comparative marker analysis of
extracellular vesicles in different human cancer types. J Extracell
Vesicles. Jun 18–2013.(Epub ahead of print). do:
10.3402/jev.v2i0.20424 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vlassov AV, Magdaleno S, Setterquist R and
Conrad R: Exosomes: Current knowledge of their composition,
biological functions, and diagnostic and therapeutic potentials.
Biochim Biophys Acta. 1820:940–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang K, Chen R, Feng Z, Zhu YM, Sun XX,
Huang W and Chen ZN: Identification of differentially expressed
genes in non-small cell lung cancer. Aging (Albany NY).
11:11170–11185. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mehta A, Sriramanakoppa NN, Agarwal P,
Viswakarma G, Vasudevan S, Panigrahi M, Kumar D, Saifi M, Chowdhary
I, Doval DC and Suryavanshi M: Predictive biomarkers in nonsmall
cell carcinoma and their clinico-pathological association. South
Asian J Cancer. 8:250–254. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Miyazono K, Kamiya Y and Morikawa M: Bone
morphogenetic protein receptors and signal transduction. J Biochem.
147:35–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Deng T, Lin D, Zhang M, Zhao Q, Li W,
Zhong B, Deng Y and Fu X: Differential expression of bone
morphogenetic protein 5 in human lung squamous cell carcinoma and
adenocarcinoma. Acta Biochim Biophys Sin (Shanghai). 47:557–563.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Powell DW: Barrier function of epithelia.
Am J Physiol. 241:G275–G288. 1981.PubMed/NCBI
|
|
59
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hichino A, Okamoto M, Taga S, Akizuki R,
Endo S, Matsunaga T and Ikari A: Down-regulation of Claudin-2
expression and proliferation by epigenetic inhibitors in human lung
adenocarcinoma A549 cells. J Biol Chem. 292:2411–2421. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pai VC, Hsu CC, Chan TS, Liao WY, Chuu CP,
Chen WY, Li CR, Lin CY, Huang SP, Chen LT and Tsai KK: ASPM
promotes prostate cancer stemness and progression by augmenting
Wnt-Dvl-3-β-catenin signaling. Oncogene. 38:1340–1353. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gai M, Bianchi FT, Vagnoni C, Vernì F,
Bonaccorsi S, Pasquero S, Berto GE, Sgrò F, Chiotto AA, Annaratone
L, et al: ASPM and CITK regulate spindle orientation by affecting
the dynamics of astral microtubules. EMBO Rep. 18:18702017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yuan YJ, Sun Y, Gao R, Yin ZZ, Yuan ZY and
Xu LM: Abnormal spindle-like microcephaly-associated protein (ASPM)
contributes to the progression of lung squamous cell carcinoma
(LSCC) by regulating CDK4. J Cancer. 11:5413–5423. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kemp CJ, Donehower LA, Bradley A and
Balmain A: Reduction of p53 gene dosage does not increase
initiation or promotion but enhances malignant progression of
chemically induced skin tumors. Cell. 74:813–822. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jackson EL, Olive KP, Tuveson DA, Bronson
R, Crowley D, Brown M and Jacks T: The differential effects of
mutant p53 alleles on advanced murine lung cancer. Cancer Res.
65:10280–10288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gibbons DL, Byers LA and Kurie JM:
Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 12:3–13.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang Z, Zhuan B, Yan Y, Jiang S and Wang
T: Integrated analyses of copy number variations and gene
differential expression in lung squamous-cell carcinoma. Biol Res.
48:472015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang S, Li M, Ji H and Fang Z: Landscape
of transcriptional deregulation in lung cancer. BMC Genomics.
19:4352018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cheng H, Yang X, Si H, Saleh AD, Xiao W,
Coupar J, Gollin SM, Ferris RL, Issaeva N, Yarbrough WG, et al:
Genomic and transcriptomic characterization links cell lines with
aggressive head and neck cancers. Cell Rep. 25:1332–1343.e5. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yoshida M, Yokota E, Sakuma T, Yamatsuji
T, Takigawa N, Ushijima T, Yamamoto T, Fukazawa T and Naomoto Y:
Development of an integrated CRISPRi targeting ΔNp63 for treatment
of squamous cell carcinoma. Oncotarget. 9:29220–29232. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li X, Shi G, Chu Q, Jiang W, Liu Y, Zhang
S, Zhang Z, Wei Z, He F, Guo Z and Qi L: A qualitative
transcriptional signature for the histological reclassification of
lung squamous cell carcinomas and adenocarcinomas. BMC Genomics.
20:8812019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bednarek K, Kostrzewska-Poczekaj M,
Szaumkessel M, Kiwerska K, Paczkowska J, Byzia E, Ustaszewski A,
Janiszewska J, Bartochowska A, Grenman R, et al: Downregulation of
CEACAM6 gene expression in laryngeal squamous cell carcinoma is an
effect of DNA hypermethylation and correlates with disease
progression. Am J Cancer Res. 8:1249–1261. 2018.PubMed/NCBI
|
|
73
|
Duxbury MS, Matros E, Clancy T, Bailey G,
Doff M, Zinner MJ, Ashley SW, Maitra A, Redston M and Whang EE:
CEACAM6 is a novel biomarker in pancreatic adenocarcinoma and PanIN
lesions. Ann Surg. 241:491–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zang M, Zhang Y, Zhang B, Hu L, Li J, Fan
Z, Wang H, Su L, Zhu Z, Li C, et al: CEACAM6 promotes tumor
angiogenesis and vasculogenic mimicry in gastric cancer via FAK
signaling. Biochim Biophys Acta. 1852:1020–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Borczuk AC, Gorenstein L, Walter KL,
Assaad AA, Wang L and Powell CA: Non-small-cell lung cancer
molecular signatures recapitulate lung developmental pathways. Am J
Pathol. 163:1949–1960. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cui S, Cheng Z, Qin W and Jiang L:
Exosomes as a liquid biopsy for lung cancer. Lung Cancer.
116:46–54. 2018. View Article : Google Scholar : PubMed/NCBI
|