Introduction
Pituitary adenomas are benign neuroendocrine tumors
(1,2)
that originate from adenohypophyseal cells, and they account for
10–20% of intracranial neoplasms, in epidemiological data from the
United States between 2005 and 2009 (3–5).
Pituitary adenomas can be divided into functional and
non-functional adenomas according to clinical and biochemical
characteristics, like GH-secreting adenoma characterized acromegaly
caused by growth hormone abnormal rise (6). Non-functional pituitary adenomas
(NFPAs) are the most common and account for 43% of pituitary
adenomas, in epidemiological data from Iceland between 1955 and
2012 (7,8). NFPA is often characterized by a lack of
symptoms associated with excessive hormone production, like
acromegaly and Cushing's disease (6). Due to the mass effect on surrounding
structures, NFPA may cause headaches, visual defects, and/or
hypopituitarism (7,9). Surgical resection is the primary
treatment for NFPA, although patients are often left with tumor
residue, as the tumor can invade the cavernous sinus or area
surrounding the internal carotid artery (10,11). In
total, 12–58% of patients with NFPA with macroadenoma may
experience regrowth within 5 years (12–15).
Radiotherapy is often recommended for patients with tumor residue,
although its long-term complications, such as visual defects and
hypopituitarism, are still of concern (16,17).
Therefore, surgery is still the best option for patients with tumor
recurrence. Serum hormone monitoring is an approach used to detect
functional pituitary adenoma (18);
however, early intervention is difficult to achieve due to the
absence of an effective evaluation approach for NFPA. Therefore,
research on the molecular mechanisms underlying tumor recurrence
and effective prognosis prediction methods is important.
Studies have shown that protein-coding genes (PCGs)
are involved in the activation of pathways or key proteins and play
vital roles in the biological processes of pituitary adenomas. For
example, Uraki et al (19)
showed that reducing the expression of MSH6 and MSH2 can directly
promote the growth of pituitary tumors through the ATR-Chk1
pathway. Long et al (20)
suggested that collagen α VI chain interacts with P4HA3 to inhibit
pituitary adenoma cell proliferation and invasion by inhibiting the
PI3K-Akt pathway. The low expression of TGF-β RII may be related to
the development and invasion of NFPAs (21), and Zhu et al (22) confirmed that the expression of TGF-β1
and Wnt inhibitory factor 1 (WIF1) in recurrent tumors is higher
compared with that in primary tumors, suggesting that these PCGs
may be related to cell proliferation and recurrence. Compared with
non-invasive NFPAs, the expression levels of WIF1 and secreted
frizzled-related protein 4 are reduced in invasive NFPAs; thus,
WIF1 may be a potential biomarker for the aggressiveness of NFPAs
(23).
Long non-coding RNAs (lncRNAs) are a type of RNA
molecule with a transcript >200 nucleotides in length, and they
play an important role in regulating gene expression through
epigenetic or posttranscriptional mechanisms; however, they do not
encode proteins (24–26). The differential expression and
dysregulation of lncRNAs is considered to be involved in
carcinogenesis and cancer progression, recurrence and metastasis
(24). However, the role of lncRNAs
in NFPA recurrence and the regulation of cellular processes remains
unknown. A study have shown that LINC00858 plays a tumor-promoting
role in colon cancer by upregulating hepatocyte nuclear factor 4-α
and downregulating WNK2 (27). Xu
et al (28) showed that the
overexpression of lncRNA PAXIP1-AS1 can upregulate KIF14, thereby
enhancing human umbilical vein endothelial cell migration, invasion
and angiogenesis in gliomas. Moreover, several studies found that
identifying novel lncRNA-mRNA networks using microarray analyses
could contribute to exploring the potential molecular mechanisms
and prognosis of tumors (29–31). The
aforementioned studies indicate that the dysregulation of lncRNAs
and lncRNA-mRNA interactions may affect the prognosis of NFPAs.
The present study aims to screen out the critical
PCGs and lncRNAs, which play an essential role in NFPAs recurrence.
We obtained differentially expressed PCGs and lncRNAs by performing
differential expression analyses of recurrence within 1 year after
surgery (fast recurrence group) and after 5 years (slow recurrence
group). Protein-protein interaction (PPI) networks and coregulatory
networks between lncRNAs and mRNAs were also identified. The hub
lncRNA-mRNA modules related to NFPA recurrence were further screen
and the enrichment of the differentially expressed genes (DEGs) was
assessed in different pathways by Gene Set Enrichment Analysis
(GSEA). In addition, the ability of the hub and module genes
[nucleolar protein 6 (NOL6), cyclin dependent kinase 15 (CDK15),
Moloney leukemia virus 10 (MOV10), SAMM50 sorting and assembly
machinery component (SAMM50), collagen type XXIV α 1 chain
(COL24A1), epoxide hydrolase 1 (EPHX1) and decapping mRNA 1A
(DCP1A)] to predict recurrence and progression-free survival (PFS)
time was evaluated in patients with NFPA. These results may help us
explore the mechanisms underlying NFPA recurrence, and may also
provide future effective biomarkers and therapeutic targets.
Materials and methods
Patients and samples
Patients diagnosed for NFPA (n=73) who underwent
surgical resection at Beijing Tiantian Hospital (Beijing, China)
from October 2007 to July 2014 were included in the study. The
study inclusion criteria were: i) Patients older than 18 years; ii)
MRI/CT showed a sellar region lesion; iii) pathological diagnosis
was pituitary adenoma with no hormonal excess and iv) sufficient
pre- and postoperative clinical and radiologic data. The exclusion
criteria were: i) Patients with functioning adenoma, including
tumor which secreted ACTH, prolactin, growth hormone and/or TSH and
lead to the corresponding clinical syndromes of hormone excess and
ii) history of pituitary surgery or radiotherapy. The patients
included 34 men and 39 women, with a median age of 52 years (age
range, 25–73 years). Out-patient clinic follow-up was conducted,
the minimum follow-up time was 4 months, and the median follow-up
time was 60 months (range, 4–98 months). The clinicopathological
characteristics of all patients are shown in Table I. All tumor samples were immediately
placed into a sample tube, frozen in liquid nitrogen and stored.
Among them, 6 cases of recurrence within 1 year were randomly
selected as the fast recurrence group and 6 cases of recurrence
after 5 years were selected as the slow recurrence group. The
postoperative recurrence of NFPA refers to the increase in the
maximum tumor diameter by >2 mm from the day of surgery to the
end of follow-up as measured from any direction on magnetic
resonance imaging. The histological subtype of tumors was defined
according to the World Health Organization 2017 classification of
endocrine tumors (32). The Medical
Ethics Committee of Beijing Tiantan Hospital (Beijing, China)
approved the study.
 | Table I.Summary of non-functioning pituitary
adenoma clinical characteristics. |
Table I.
Summary of non-functioning pituitary
adenoma clinical characteristics.
| Characteristic | Value, n |
|---|
| Sex |
|
|
Female | 39 |
|
Male | 34 |
| Age, years |
|
|
≤52 | 41 |
|
>52 | 32 |
| Invasion |
|
|
Yes | 47 |
| No | 26 |
| Histological
type |
|
| GA | 41 |
| SA | 29 |
| NC | 3 |
| Tumor size
classification |
|
|
Macroadenoma | 53 |
| Giant
adenoma | 20 |
| Headache |
|
|
Yes | 35 |
| No | 38 |
| Vision and visual
field disorders |
|
|
Yes | 53 |
| No | 20 |
| Recurrence |
|
|
Yes | 27 |
| No | 46 |
Total RNA extraction and RNA
microarray
The Phenol-free mirVana™ miRNA Isolation kit (cat.
no. AM1561; Ambion; Thermo Fisher Scientific, Inc.) was used to
extract and purify total RNA to generate fluorescently labeled cRNA
targets (4×180 K), following the manufacturer's protocol. The
labeled cRNA targets were then hybridized to a glass slide. After
hybridization, the slides were scanned using an Agilent microarray
scanner (Agilent Technologies, Inc.). After extracting data using
Feature Extraction software 10.7 (Agilent Technologies), the
Quantum algorithm was used to normalize the raw data using the
limma software package of the R program (http://www.R-project.org). The analysis was performed
by version 3.6.1. (http://www.rstudio.com/).
Identification of differentially
expressed lncRNAs and mRNAs
A differential gene expression analysis was
performed within 1 year after the initial postoperative NFPA (n=6)
and 5 years later (n=6), and a significance analysis of microarrays
(SAM) was performed to identify the differentially expressed PCGs
and lncRNAs (DEGLs) between the two groups (33). The Biobase, multtest and siggenes
packages were downloaded from Bioconductor (http://www.bioconductor.org/). Subsequently, the
available data were analyzed using R (www.r-project.org), and DEGLs with fold-changes of
>2 and <-2, and P<0.05, were selected for further
research.
Construction of a PPI network and
lncRNA-mRNA coexpression network
Cytoscape software (version 3.2.3) was used to
construct, visualize and analyze the PPI network (34). The latest version of the validated
human PPI dataset was downloaded from both the Human Protein
Reference Database (HPRD) (www.hprd.org/;
release 9) and BioGRID (www.thebiogrid.org/; release 3.4.140) (35,36).
These two datasets contain 18,595 unique proteins and 174,552
interactions, and were used as parent PPIs in the present study;
their reliability has been effectively verified, and they have been
used extensively in disease research involving human PPI networks.
The non-redundant interactions in Homo sapiens from these
two data sets were manually integrated (37).
First, a PPI subnetwork was generated by mapping all
the DEGs and extracting them from the PPI network. To improve
reliability, network reconstruction was limited to the first
interacting protein neighbors of these DEGs. Second, the
DEG-adjacent protein axis was detected, and a DEG-central PPI
network was constructed. Third, after mapping all DEGs to the PPI
network to detect internal interactions between the DEGs, Cytoscape
was used to select nodes with all edges to create a subnetwork. The
single-node and self-interactions of proteins in these subnets were
deleted. Pearson's correlation test was used to calculate the
coexpression relationships between lncRNAs and PCGs, and the
coexpression relationships with a P<0.05 and a Pearson
coefficient absolute value of >0.9 were selected. Finally, an
lncRNA-mRNA network related to NFPA composed of differential genes
was obtained. The PPI-lncRNA network was visualized using
Cytoscape, and Molecular Complex Detection (MCODE) (38) was used to identify important modules
in the PPI network. The key modules and hub genes were further
analyzed and visualized using the MCODE plugin in Cytoscape. The
screening criteria for module genes were as follows: Degree
cut-off, 2; node score cut-off, 0.2; k score, 2; and maximum depth,
100.
Functional enrichment analysis
The ClueGO (39)
plugin of Cytoscape was used to perform Gene Ontology (GO) and
Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses
of the DEGs and the biological functions of the lncRNAs. Functional
annotations with P<0.05 were considered significant. In
addition, GSEA was used to identify the relevant pathways of the
selected genes. GSEA was performed using GSEA (4.1.0) software
(40). The gene set used in the
study was downloaded from the Molecular Signatures Database
(http://software.broadinstitute.org/gsea/msigdb/index.jsp,
MSigDB version 4.0, released June 7, 2013). The Molecular
Signatures Database contains various types of gene sets. The online
pathway database includes 1,320 canonical pathways derived from
pathway databases such as BioCarta, KEGG, Pathway Interaction
Database and Reactome (40).
Validation and efficacy evaluation of
the hub genes by survival analysis
Among the hub genes, genes of interest that have not
been studied with regard to NFPA were further validated in the two
groups. The PFS analysis of the hub genes and module genes was
performed using Kaplan-Meier curves in the R program. P<0.05 was
considered to indicate a statistically significant difference.
Validation of gene expression using
reverse transcription-quantitative (RT-q)PCR
RT-qPCR was performed using another set of NFPA
samples to verify the credibility of the bioinformatics analyses.
According to the inclusion and exclusion criteria, five cases of
NFPAs with recurrence within 1 year and four cases of NFPAs with
recurrence after 5 years were randomly selected from the patients
who underwent surgical resection in Beijing Tiantan Hospital
between August 2009 and November 2014 as the validation set. The
total RNA of validated samples was extracted and purified as
aforementioned. Reverse transcription into cDNA was performed using
a High Capacity cDNA Reverse Transcription kit (cat. no. 0049472;
Thermo Fisher Scientific, Inc.) following the manufacturer's
instructions. Next, a Power SYBR™ Green PCR Master Mix (cat. no.
4367659; Thermo Fisher Scientific, Inc.) was used for qPCR with a
total reaction volume of 20 µl. Amplification was performed as
follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec
and 60°C for 60 sec, and a final extension at 72°C for 5 min. GAPDH
was used as an internal control gene. All primers were synthesized
by Sangon Biotech Co., Ltd. The level of mRNAs was determined using
QuantStudio 3 and 5 systems (Applied Biosystems; Thermo Fisher
Scientific, Inc.). For relative quantitation, expression levels
were calculated expression levels were calculated using the
2−ΔΔCq method (41). The
sequences of the primers are as follows: GAPDH forward,
5′-ACCCACTCCTCCACCTTTGA-3′ and reverse, 5′-CCACCCTGTTGCTGTAGCCA-3′;
NOL6 forward, 5′-ATTCGGGAAGCTGTGGTCTG-3′ and reverse,
5′-ATGTCAGCATGGAGTGCCAA-3′; and LL21NC02-21A1.1 forward,
5′-CTGCCCGATCTCACCTCTTC-3′ and reverse,
5′-TCAGGGAAGGACTCCAGGTT-3′.
Statistical analysis
Data are presented as the mean ± standard deviation,
unless otherwise shown. All experiments were performed in
triplicate. The differentially expressed PCGs and lncRNAs were
identified using an unpaired t-test. The difference in mRNA and
lncRNA expression level between the two groups was assessed by the
Mann-Whitney U test with GraphPad Prism 7 software (GraphPad
Software). Kaplan-Meier analysis of PFS was conducted using the
log-rank or the Renyi test (when there was survival curve crossover
between the observed groups) using the R packages ‘survival’ and
‘survMisc’ in R (3.5.1). P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of DEGLs between the
fast recurrence and slow recurrence groups
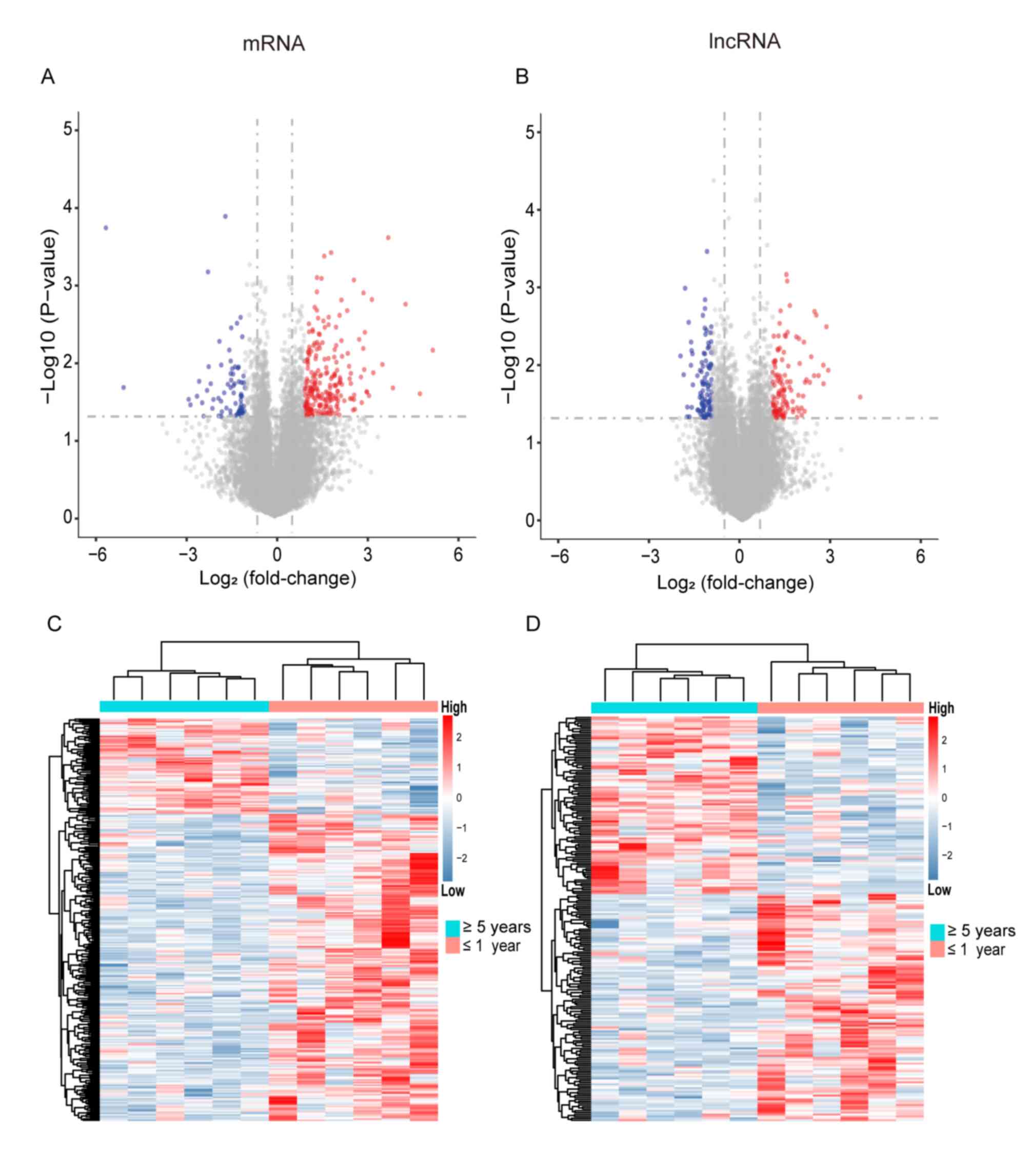
Through microarray sequencing of 73 NFPA samples,
18,827 PCGs and 19,740 lncRNAs with expression values >0 were
identified. The differences in PCGs and lncRNAs between 6 cases of
NFPA recurrence within 1 year after surgery and 6 cases of NFPA
recurrence 5 years after surgery were analyzed using the SAM test.
By selecting the threshold |fold-change| >2 or adjusted
P<0.05, a total of 299 differentially expressed PCGs (228
upregulated and 71 downregulated PCGs) and 214 differentially
expressed lncRNAs (120 upregulated and 94 downregulated lncRNAs)
were identified (Fig. 1A and B). The
expression heat map further validated the results, and Fig. 1C and D shows the differential PCGs
and lncRNAs with different expression trends for recurrence.
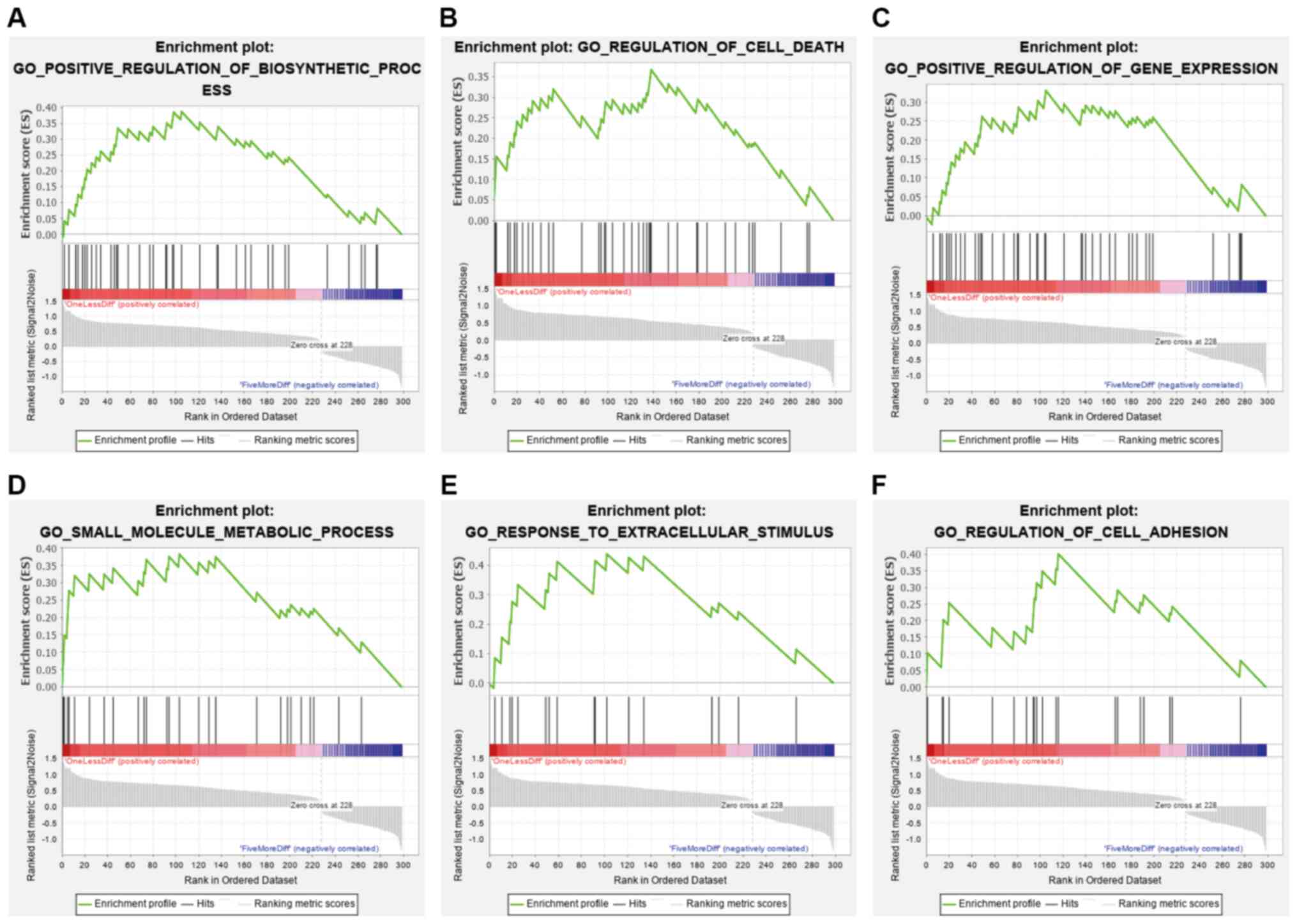
Pathway enrichment of the DEGs by GSEA
classifies the fast recurrence and slow recurrence groups
GSEA demonstrated that 30 different pathways related
to 299 differentially expressed PCGs were downregulated or
upregulated according to the recurrence rate. Several enriched
terms are presented in Fig. 2 and
Table SI, such as ‘regulation of
cell death’, ‘regulation of cell adhesion’, ‘positive regulation of
biosynthetic process’, ‘positive regulation of gene expression’,
‘small molecule metabolic process’ and ‘response to extracellular
stimulus’. The results indicated that changes in these pathways
lead to the recurrence and progression of NFPAs.
Dysregulated lncRNA-mRNA interaction
network establishment and module analysis
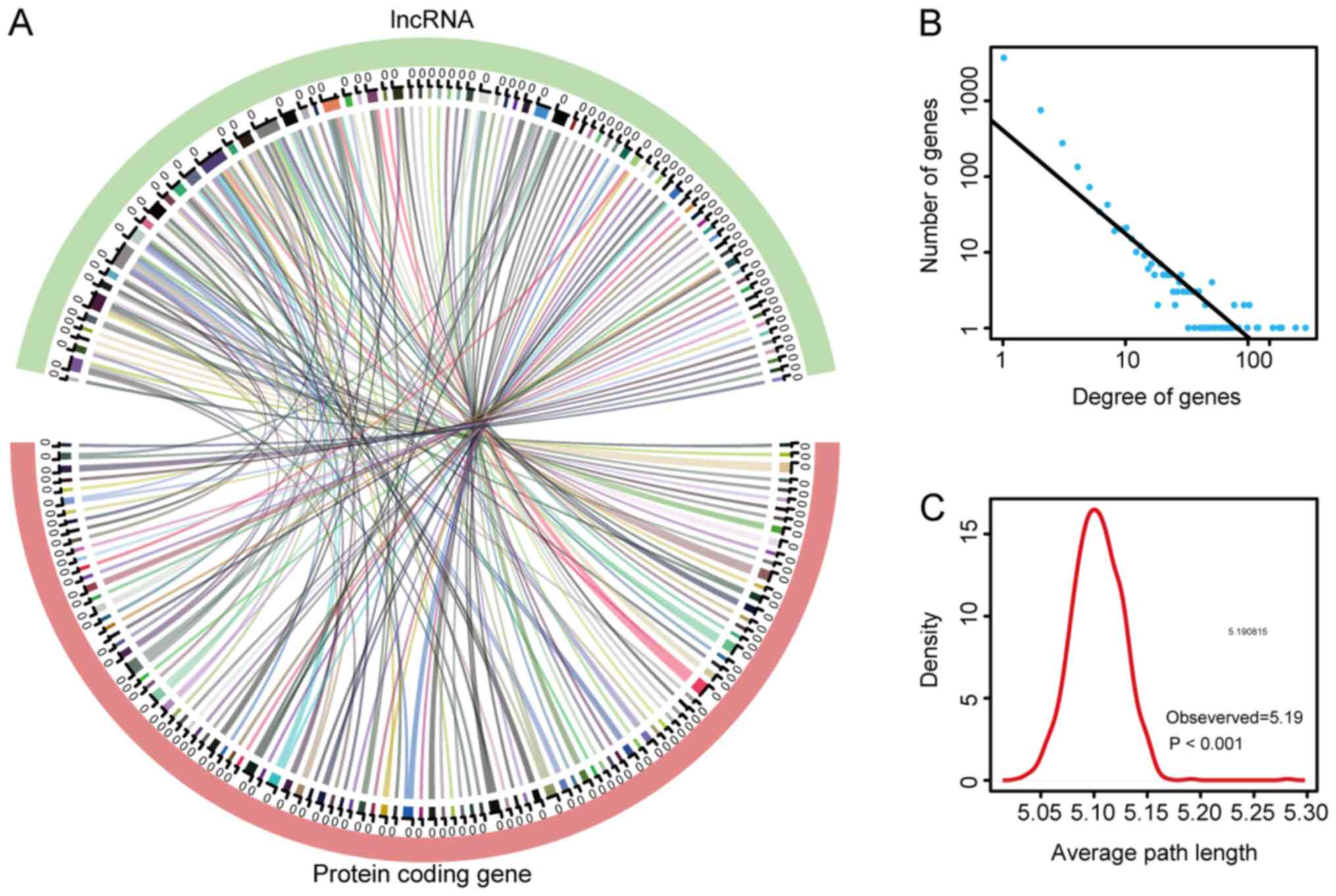
Based on Pearson's correlation test, a differential
coexpression network of lncRNAs and mRNAs was constructed,
selecting genes with P<0.05 and a Pearson's coefficient absolute
value of >0.9 (lncRNA/mRNA quantity, 78/104; Fig. 3A). This network was transferred to
the differential PCG PPI parent network. Subsequently, the
lncRNA-mRNA interaction network was obtained by combining these two
networks (Table SII). The
lncRNA-mRNA network for the DEGLs contained a total of 4,490 nodes
and 6,933 interactions. Fig. 3B
shows that the degrees of the genes followed a power-law
distribution, further illustrating that the network was similar to
most biological networks, and the network is scale-free. The
average path length of the network was also calculated, and it
indicated that the characteristic path length of the network was
much longer compared with the path length of the random network
(1,000 times longer; P<0.001; Fig.
3C), which implied that the network had reduced global
efficiency.
Numerous studies have shown that PCGs and lncRNAs
usually function by participating in functional modules (42–44).
Through cluster analysis of the PPI network using the MCODE plugin
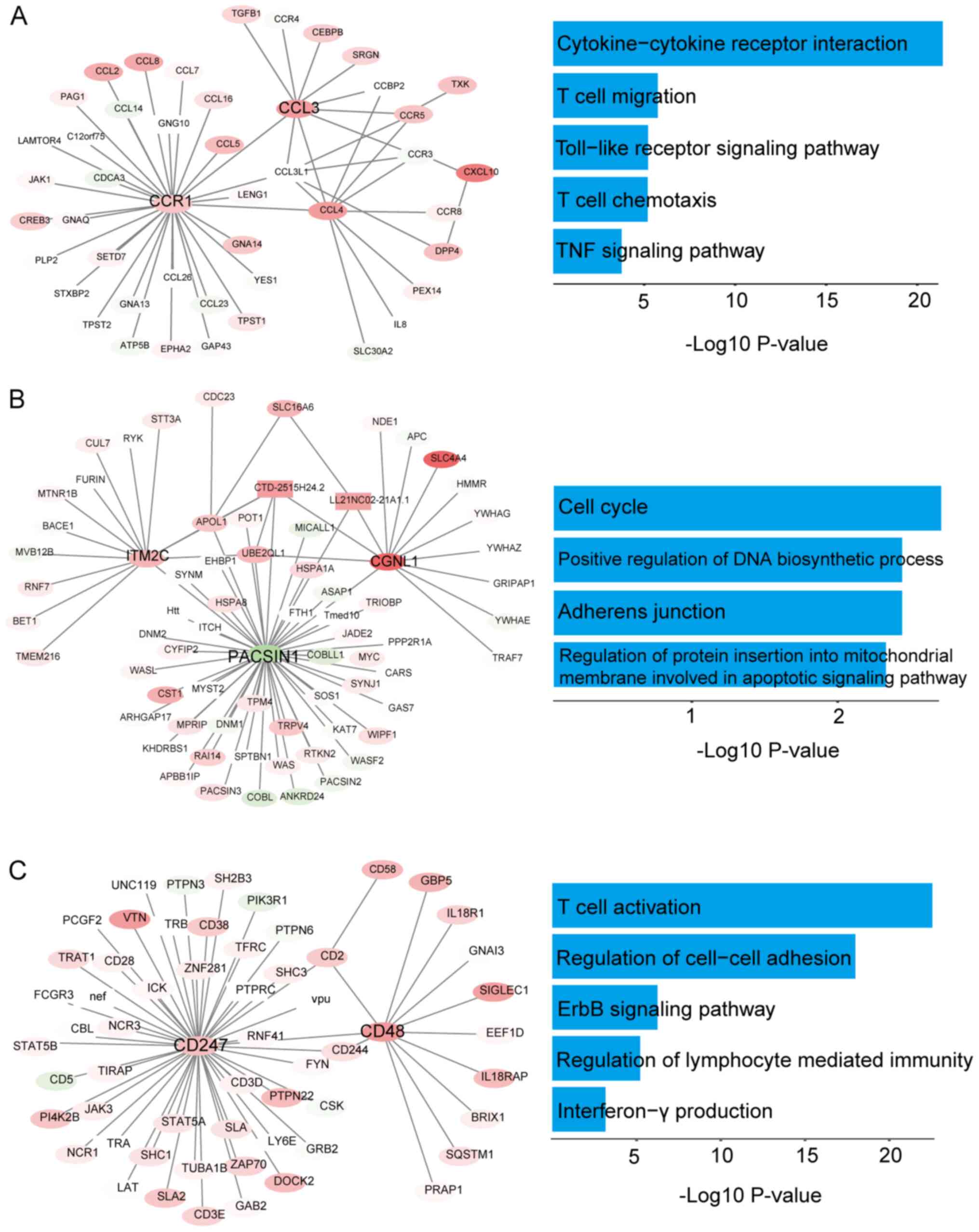
of Cytoscape, eight important modules according to the degree of
importance were obtained. Module 1 contained 46 nodes and 54 edges
(Fig. 4A), module 2 contained 71
nodes and 79 edges (Fig. 4B), module
3 contained 59 nodes and 61 edges (Fig.
4C), module 4 contained 59 nodes and 65 edges (Fig. S1A), module 5 contained 55 nodes and
55 edges (Fig. S1B), module 6
contained 44 nodes and 44 edges (Fig.
S2A), module 7 contained 83 nodes and 88 edges (Fig. S2B), and module 8 contained 214 nodes
and 218 edges (Fig. S2C).
ClueGO was used to perform an enrichment analysis of
the genes in these modules. As shown in Figs. 4, S1
and S2, the GO analysis indicated
that the genes in modules 1–8 were mainly concentrated in the
categories ‘T cell migration’, ‘T cell chemotaxis’ and ‘T cell
activation’, ‘regulation of cell-cell adhesion’ (Fig. 4A-C) and ‘regulation of cytokine
production involved in the immune response’ (Fig. S1A). In addition, the KEGG analysis
showed that enrichment of these module genes mainly occurred in the
categories ‘cell cycle’, ‘adherens junction’, ‘TNF signaling
pathway’ (Fig. 4A-C), ‘VEGF
signaling pathway’ and ‘TGF-β signaling pathway’ (Fig. S1A and B). The expression of 21 hub
genes and 10 module lncRNAs from the aforementioned eight modules
[CCR1, CCL3, CCL4, PACSIN1, CGNL1, TRIM69, STAB2, ATP8A1, CD48,
NOL6, ZFP36, KLF4, CD247, REEP6, SQRDL, KCNJ6, ANXA2, SPRY2, KCNS3,
ITM2C, THBS2, CTD-2515H24.2, LL21NC02-21A1.1, LOC200772,
RP11-402C9.1, LINC01203, RP11-479G22.8, RP11-615I2.1, RP1-249I4.2,
RP11-116N8.2, and RP11-288L9.4) showed significant differences in
the different recurrence time groups [|Log2
(fold-change)| ≥1, P<0.05; Figs.
5 and S3]. These results
indicated that lncRNAs may regulate the downstream pathways in NFPA
through gene modules and thus play an important role in tumor
recurrence.
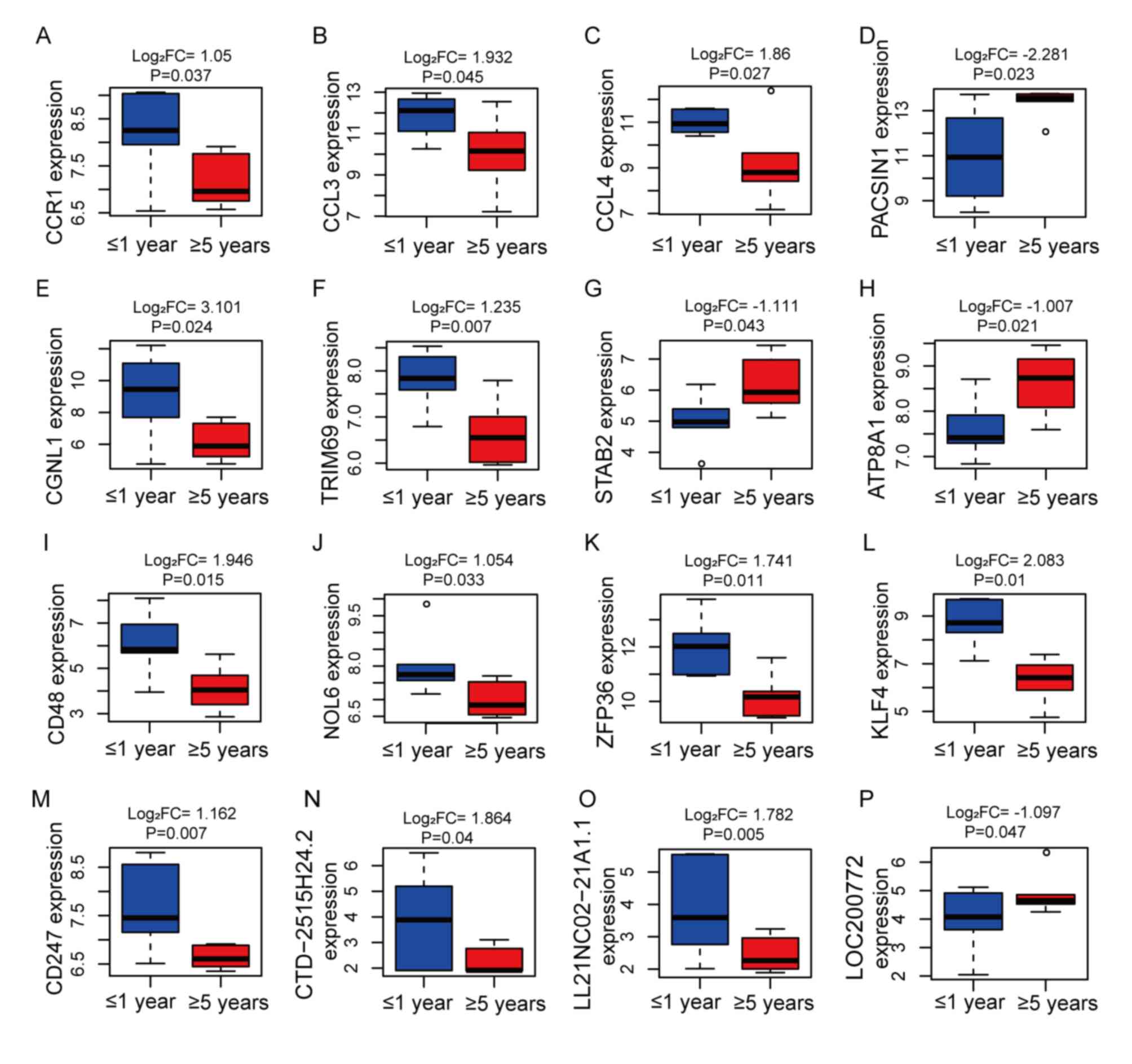 | Figure 5.Analysis of 16 hub genes and module
gene expression levels in NFPA recurrence at ≥5 years (red boxes)
or ≤1 year (blue boxes), including (A) CCR1, (B) CCL3, (C) CCL4,
(D) PACSIN1, (E) CGNL1, (F) TRIM69, (G) STAB2, (H) ATP8A1, (I)
CD48, (J) NOL6, (K) ZFP36, (L) KLF4, (M) CD247, (N) CTD-2515H24.2,
(O) LL21NC02-21A1.1 and (P) LOC200772. FC, fold-change; NFPA,
non-functioning pituitary adenoma. |
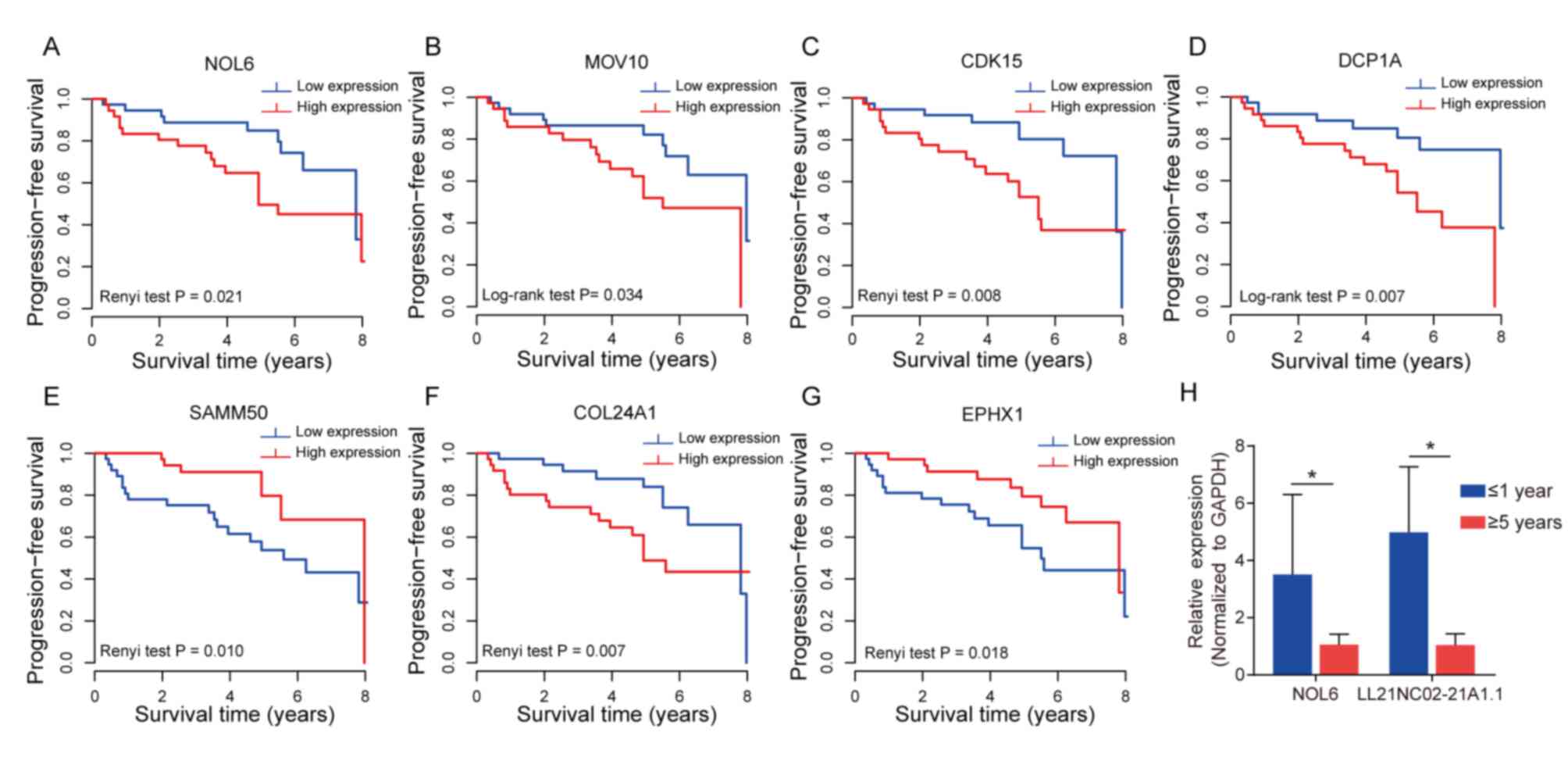
Evaluation of the hub and module genes
for predicting the recurrence and PFS of patients with NFPA
Next, the predictive ability of the module genes for
the recurrence process was evaluated. Kaplan-Meier analysis of the
central or module genes, NOL6, CDK15, MOV10, SAMM50, COL24A1, EPHX1
and DCP1A, showed that the patients could be divided into two
groups with different risk in the recurrence, according to the
median value of each gene expression as the cut-off value. Compared
with patients at low risk, the PFS time of patients at high risk
was significantly shorter (P<0.05; Fig. 6A-G).
RT-qPCR was conducted to confirm the reliability of
the expression profiles generated using the microarray and DEG
analyses. Among the prognostic hub and module genes aforementioned,
NOL6 and LL21NC02-21A1.1 were randomly selected for verification
(P<0.05; Fig. 6H). As expected,
the RT-qPCR results basically matched those of the microarray
analyses. These results indicated that the bioinformatics analysis
of the microarray data reliably identified critical candidate genes
involved in NFPA recurrence.
Discussion
NFPAs are pituitary adenomas without clinical
evidence of hormonal hypersecretion, and they have a prevalence of
7 to 41.34 cases/100,000 and an annual incidence of 0.65 to 2.34
cases/100,000, in epidemiological data from global between 1955 and
2014 (7,45,46).
Transsphenoidal surgery is the recommended first-line treatment
(47). However, compared with that
in functioning pituitary adenoma, monitoring of the tumor
recurrence of NFPA through specific serum hormone alterations is
difficult. Therefore, the present study aimed to develop a new
predictive signature that could be used as a prognostic prediction
model to identify early recurrence. The main purpose of the study
was to divide patients into high-risk or low-risk groups so that
the most effective and timely treatment can be performed for
NFPA.
Numerous studies have focused on the factors of
tumor recurrence of NFPA to improve the prognosis of patients
postoperatively. Age is recognized as an important independent
factor influencing the prognosis of NFPA, and a younger age
indicates a greater chance of tumor recurrence (12,48).
Ki-67 is another commonly used pathological prognostic evaluation
index (49), although a single
indicator used in prognostic assessment has certain limitations in
accurately evaluating the prognosis of each patient. A previous
study tried to establish a statistical model that combined clinical
features (age and tumor volume) and molecular markers (p16, WIF1
and TGF-β) to evaluate the recurrence probability of patients with
NFPA postoperatively (50).
Moreover, compared with a previous study (51), the current study added a temporal
component to the prognostic assessment and independently assessed
the prognosis of patients at different time points.
In recent years, lncRNAs have been reported in
various tumors and serve as promising new molecular markers for
tumor biological behavior, tumor diagnosis and prognostic
evaluation (52,53). For example, lncRNA H19 is decreased
in pituitary adenomas, and its overexpression could markedly
inhibit the growth of pituitary tumor cells and be used as a drug
resistance marker (54). Xing et
al (55) identified
differentially expressed mRNAs and lncRNAs in NFPA and normal
pituitary tissue samples, and constructed an mRNA-lncRNA
coexpression network. However, the research failed to illustrate
the regulatory mechanisms of the key genes or lncRNAs and their
influence on patient prognosis. The current study focused on
identifying molecular markers of NFPA recurrence.
First, the DEGLs based on NFPA recurrence at <1
year and >5 years were obtained. According to GSEA, these DEGs
were enriched in the ‘regulation of cell death’ and ‘cell
adhesion’. The present results are consistent with those of
previous studies, which have shown that intercellular adhesion and
adhesion molecules play a crucial role in tumor recurrence and
proliferation (56,57).
Second, a total of eight modules were identified via
cluster analysis using the PPI network based on the DEGLs. GO and
KEGG enrichment analyses illustrated that these module genes were
mainly involved in different GO functions and pathways. For module
1, the related GO functions were ‘T cell migration’ and
‘chemotaxis’, which implied that the process of recurrence may be
associated with the immune-related tumor microenvironment. Similar
to the current study, Marques et al (58) found that a low CD8:CD4 ratio is
associated with a higher proliferative index (Ki-67) in pituitary
adenoma. In addition, the present KEGG analysis of other modules
found that these genes were involved in the ‘cell cycle’, ‘TNF
signaling pathway’, ‘VEGF signaling pathway’ and ‘TGF-β signaling
pathway’. These pathways might participate and regulate the
proliferation and recurrence processes that occur in NFPAs.
Third, the current analysis obtained hub genes and
module lncRNAs with significant differential expression (CCR1,
CCL3, CCL4, PACSIN1, CGNL1, TRIM69, STAB2, ATP8A1, CD48, NOL6,
ZFP36, KLF4, CD247, REEP6, SQRDL, KCNJ6, ANXA2, SPRY2, KCNS3,
ITM2C, THBS2, CTD-2515H24.2, LL21NC02-21A1.1, LOC200772,
RP11-402C9.1, LINC01203, RP11-479G22.8, RP11-615I2.1, RP1-249I4.2,
RP11-116N8.2 and RP11-288L9.4). As an example, ANXA2 is a
pleiotropic calcium-dependent phospholipid-binding protein that is
abnormally expressed in a variety of cancer types (59), including prostate cancer (60) and liver cancer (61). Liu et al (62) performed a meta-analysis and indicated
that ANXA2-overexpression might be related to poor outcomes in
patients with malignant tumors, which is consistent with the
present findings. In addition, the current study reported that
lncRNAs could be used as a prognostic signature. However, the
functions and regulatory mechanisms of lncRNAs in NFPA have not yet
been reported.
Finally, the present study also assessed the
predictive ability of the module genes (such as NOL6, CDK15, MOV10,
SAMM50, COL24A1, EPHX1 and DCP1A) for the recurrence process. In
addition, the expression levels of NOL6 and LL21NC02-21A1.1 were
validated, which were randomly selected from among the hub and
module genes, using RT-qPCR. The results confirmed the accuracy of
the bioinformatics analyses.
NOL6 encodes a nucleolar RNA-associated protein that
is associated with the early stage of ribosome biosynthesis
(63). Dong et al (64) found that NOL6 is highly expressed in
human prostate cancer and that knockdown of NOL6 inhibits the
proliferation and mitosis, and increases the apoptosis of human
prostatic carcinoma cells (PC-3). In the current study, NOL6 was
found to be upregulated in NFPAs that became recurrent within 1
year compared with those recurring after >5 years, suggesting
that NOL6 could be a critical gene in prognostic development and a
potential target for NFPA treatment. MOV10 belongs to the RNA
helicase superfamily of proteins and could regulate mRNA stability
and translation (65). Nakano et
al (66) demonstrated that the
mRNA and protein levels of MOV10 in cancer cells, such as human
leukemia and human cervical carcinoma cells, were higher compared
with those in normal cells. In addition, MOV10 has been revealed to
promote the angiogenesis of glioma by binding circ-DICER1 (51). These studies indicated that MOV10
could be critical in tumorigenesis. DCP1A is a protein-coding gene
for mRNA-decapping enzyme 1a, and several studies have revealed
that DCP1A is upregulated in tumor tissues, such as malignant
melanoma, colorectal carcinoma and gastric cancer (67–69). In
addition, Tang et al (67)
and Wu et al (68) reported
that the high expression of DCP1A in colorectal carcinoma is
correlated with poor prognosis, which is consistent with the
current results, thus indicating that the other present PCGs and
lncRNAs could also be prognostic indicators for NFPA.
A few limitations of the current study need to be
acknowledged. First, the molecular mechanisms underlying the action
of these PCGs and lncRNAs in NFPA are still unclear, and further
studies might provide important information to understand their
functional roles. Second, sequencing data for NFPA are limited;
thus, it was not possible to verify the results in an independent
validation set. Third, the limited number of samples used for
RT-qPCR testing make it necessary to perform larger scale
experiments in the future. Finally, the application of the present
signature in clinical practice should be tested prospectively.
Despite these limitations, the current study verified a certain
association between PCG and lncRNA signatures and regression, which
is a potentially powerful prognostic marker of NFPA.
In conclusion, to the best of our knowledge, the
present study is first to integrate PCGs and lncRNAs to predict
tumor recurrence in patients with NFPA. The current study may
provide novel insights into prognostic evaluation and help patients
benefit from early intervention.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81771489) and The
Beijing Municipal Science & Technology Commission (grant no.
Z171100000117002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
WX, YZ and CL conceived the study. QF and YL
contributed to collecting and analyzing the clinical data of the
patients. JG performed the experiments, analyzed the data and wrote
the manuscript. WX and YZ confirmed the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committees of Beijing Tiantan Hospital (Beijing, China; approval
no. KY2013-015-02). All subjects provided written informed consent,
and the study was performed in full compliance with all principles
of the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NFPA
|
non-functioning pituitary adenoma
|
|
PCGs
|
protein-coding genes
|
|
lncRNAs
|
long non-coding RNAs
|
|
PFS
|
progression-free survival
|
|
DEGs
|
differentially expressed genes
|
|
DEGLs
|
differentially expressed PCGs and
lncRNAs
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
References
|
1
|
Ostrom QT, Gittleman H, Fulop J, Liu M,
Blanda R, Kromer C, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS Statistical Report: Primary brain and central nervous system
tumors diagnosed in the United States in 2008–2012. Neuro-oncology.
17 (Suppl 4):iv1–iv62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernandez A, Karavitaki N and Wass JA:
Prevalence of pituitary adenomas: A community-based,
cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol
(Oxf). 72:377–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dolecek TA, Propp JM, Stroup NE and
Kruchko C: CBTRUS statistical report: Primary brain and central
nervous system tumors diagnosed in the United States in 2005–2009.
Neuro Oncol. 14 (Suppl 5):v1–v49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asa SL and Ezzat S: The cytogenesis and
pathogenesis of pituitary adenomas. Endocr Rev. 19:798–827. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daly AF, Rixhon M, Adam C, Dempegioti A,
Tichomirowa MA and Beckers A: High prevalence of pituitary
adenomas: A cross-sectional study in the province of Liege,
Belgium. J Clin Endocrinol Metab. 91:4769–4775. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dekkers OM, Pereira AM and Romijn JA:
Treatment and follow-up of clinically nonfunctioning pituitary
macroadenomas. J Clin Endocrinol Metabol. 93:3717–3726. 2008.
View Article : Google Scholar
|
|
7
|
Ntali G and Wass JA: Epidemiology,
clinical presentation and diagnosis of non-functioning pituitary
adenomas. Pituitary. 21:111–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agustsson TT, Baldvinsdottir T, Jonasson
JG, Olafsdottir E, Steinthorsdottir V, Sigurdsson G, Thorsson AV,
Carroll PV, Korbonits M and Benediktsson R: The epidemiology of
pituitary adenomas in Iceland, 1955–2012: A nationwide
population-based study. Eur J Endocrinol. 173:655–664. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, White WL, Spetzler RF and Xu B: A
prospective study of nonfunctioning pituitary adenomas:
Presentation, management, and clinical outcome. J Neurooncol.
102:129–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shomali ME and Katznelson L: Medical
therapy of gonadotropin-producing and nonfunctioning pituitary
adenomas. Pituitary. 5:89–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meij BP, Lopes MB, Ellegala DB, Alden TD
and Laws ER Jr: The long-term significance of microscopic dural
invasion in 354 patients with pituitary adenomas treated with
transsphenoidal surgery. J Neurosurg. 96:195–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brochier S, Galland F, Kujas M, Parker F,
Gaillard S, Raftopoulos C, Young J, Alexopoulou O, Maiter D and
Chanson P: Factors predicting relapse of nonfunctioning pituitary
macroadenomas after neurosurgery: A study of 142 patients. Eur J
Endocrinol. 163:193–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrante E, Ferraroni M, Castrignanò T,
Menicatti L, Anagni M, Reimondo G, Del Monte P, Bernasconi D, Loli
P, Faustini-Fustini M, et al: Non-functioning pituitary adenoma
database: A useful resource to improve the clinical management of
pituitary tumors. Eur J Endocrinol. 155:823–829. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greenman Y, Ouaknine G, Veshchev I, Reider
G II, Segev Y and Stern N: Postoperative surveillance of clinically
nonfunctioning pituitary macroadenomas: Markers of tumour
quiescence and regrowth. Clin Endocrinol (Oxf). 58:763–769. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dekkers OM, Pereira AM, Roelfsema F,
Voormolen JH, Neelis KJ, Schroijen MA, Smit JW and Romijn JA:
Observation alone after transsphenoidal surgery for nonfunctioning
pituitary macroadenoma. J Clin Endocrinol Metab. 91:1796–1801.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brada M and Jankowska P: Radiotherapy for
pituitary adenomas. Endocrinol Metab Clin North Am. 37263–275.
(xi)2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pollock BE, Cochran J, Natt N, Brown PD,
Erickson D, Link MJ, Garces YI, Foote RL, Stafford SL and Schomberg
PJ: Gamma knife radiosurgery for patients with nonfunctioning
pituitary adenomas: Results from a 15-year experience. Int J Radiat
Oncol Biol Phys. 70:1325–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mehta GU and Lonser RR: Management of
hormone-secreting pituitary adenomas. Neuro Oncol. 19:762–773.
2017.PubMed/NCBI
|
|
19
|
Uraki S, Ariyasu H, Doi A, Kawai S,
Takeshima K, Morita S, Fukai J, Fujita K, Furuta H, Nishi M, et al:
Reduced expression of mismatch repair genes MSH6/MSH2 directly
promotes pituitary tumor growth via the ATR-Chk1 pathway. J Clin
Endocrinol Metab. 103:1171–1179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Long R, Liu Z, Li J and Yu H: COL6A6
interacted with P4HA3 to suppress the growth and metastasis of
pituitary adenoma via blocking PI3K-Akt pathway. Aging (Albany NY).
11:8845–8859. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gu YH and Feng YG: Down-regulation of
TGF-β RII expression is correlated with tumor growth and invasion
in non-functioning pituitary adenomas. J Clin Neurosci. 47:264–268.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu H, Yao X, Wu L, Li C, Bai J, Gao H, Ji
H and Zhang Y: Association of TGF-β1 and WIF1 expression with 36
paired primary/recurrent nonfunctioning pituitary adenomas: A
high-throughput tissue microarrays immunohistochemical study. World
Neurosurg. 119:e23–e31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song W, Qian L, Jing G, Jie F, Xiaosong S,
Chunhui L, Yangfang L, Guilin L, Gao H and Yazhuo Z: Aberrant
expression of the sFRP and WIF1 genes in invasive non-functioning
pituitary adenomas. Mol Cell Endocrinol. 474:168–175. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu D, Su C, Jiang M, Shen Y, Shi A, Zhao
F, Chen R, Shen Z, Bao J and Tang W: Fenofibrate inhibited
pancreatic cancer cells proliferation via activation of p53
mediated by upregulation of LncRNA MEG3. Biochem Biophys Res
Commun. 471:290–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang W, Shi S, Jiang J, Li X, Lu H and
Ren F: lncRNA MEG3 inhibits cell epithelial-mesenchymal transition
by sponging miR-421 targeting E-cadherin in breast cancer. Biomed
Pharmacother. 91:312–319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu T, Wu K and Zhang L, Zheng S, Wang X,
Zuo H, Wu X, Tao G, Jiang B and Zhang L: Long non-coding RNA
LINC00858 exerts a tumor-promoting role in colon cancer via
HNF4alpha and WNK2 regulation. Cell Oncol (Dordr). 43:297–310.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu H, Zhao G, Zhang Y, Jiang H, Wang W,
Zhao D, Yu H and Qi L: Long non-coding RNA PAXIP1-AS1 facilitates
cell invasion and angiogenesis of glioma by recruiting
transcription factor ETS1 to upregulate KIF14 expression. J Exp
Clin Cancer Res. 38:4862019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou S, Wang L, Yang Q, Liu H, Meng Q,
Jiang L, Wang S and Jiang W: Systematical analysis of lncRNA-mRNA
competing endogenous RNA network in breast cancer subtypes. Breast
Cancer Res Treat. 169:267–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Q, Cheng Y, Liang T, He Y, Ren C, Sun
L and Zhang G: Comprehensive analysis of lncRNA-mRNA co-expression
patterns identifies immune-associated lncRNA biomarkers in ovarian
cancer malignant progression. Sci Rep. 5:176832015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang ZL, Zhao LJ, Chai L, Zhou SH, Wang
F, Wei Y, Xu YP and Zhao P: Seven LncRNA-mRNA based risk score
predicts the survival of head and neck squamous cell carcinoma. Sci
Rep. 7:3092017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lopes MBS: The 2017 World Health
Organization classification of tumors of the pituitary gland: A
summary. Acta Neuropathol. 134:521–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tusher VG, Tibshirani R and Chu G:
Significance analysis of microarrays applied to the ionizing
radiation response. Proc Natl Acad Sci USA. 98:5116–5121. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goel R, Muthusamy B, Pandey A and Prasad
TS: Human protein reference database and human proteinpedia as
discovery resources for molecular biotechnology. Mol Biotechnol.
48:87–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chatr-Aryamontri A, Oughtred R, Boucher L,
Rust J, Chang C, Kolas NK, O'Donnell L, Oster S, Theesfeld C,
Sellam A, et al: The BioGRID interaction database: 2017 update.
Nucleic Acids Res. 45:D369–D379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Du ZP, Wu BL, Wu X, Lin XH, Qiu XY, Zhan
XF, Wang SH, Shen JH, Zheng CP, Wu ZY, et al: A systematic analysis
of human lipocalin family and its expression in esophageal
carcinoma. Sci Rep. 5:120102015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A Cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin X, Jiang T, Bai J, Li J, Wang T, Xiao
J, Tian Y, Jin X, Shao T, Xu J, et al: Characterization of
transcriptome transition associates long noncoding RNAs with glioma
progression. Mol Ther Nucleic Acids. 13:620–632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yin X, Wang P, Yang T, Li G, Teng X, Huang
W and Yu H: Identification of key modules and genes associated with
breast cancer prognosis using WGCNA and ceRNA network analysis.
Aging (Albany NY). 12:2020.
|
|
44
|
Kang Z, Guo L, Zhu Z and Qu R:
Identification of prognostic factors for intrahepatic
cholangiocarcinoma using long non-coding RNAs-associated ceRNA
network. Cancer Cell Int. 20:3152020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tjörnstrand A, Gunnarsson K, Evert M,
Holmberg E, Ragnarsson O, Rosén T and Filipsson Nyström H: The
incidence rate of pituitary adenomas in western Sweden for the
period 2001–2011. Eur J Endocrinol. 171:519–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Olsson DS, Nilsson AG, Bryngelsson IL,
Trimpou P, Johannsson G and Andersson E: Excess mortality in women
and young adults with nonfunctioning pituitary adenoma: A Swedish
Nationwide Study. J Clin Endocrinol Metabol. 100:2651–2658. 2015.
View Article : Google Scholar
|
|
47
|
Freda PU, Beckers AM, Katznelson L,
Molitch ME, Montori VM, Post KD and Vance ML; Endocrine Society, :
Pituitary incidentaloma: An endocrine society clinical practice
guideline. J Clin Endocrinol Metab. 96:894–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tampourlou M, Ntali G, Ahmed S, Arlt W,
Ayuk J, Byrne JV, Chavda S, Cudlip S, Gittoes N, Grossman A, et al:
Outcome of nonfunctioning pituitary adenomas that regrow after
primary treatment: A study from two large UK centers. J Clin
Endocrinol Metab. 102:1889–1897. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hasanov R, Aydogan BI, Kiremitci S, Erden
E and Gullu S: The Prognostic roles of the Ki-67 proliferation
index, P53 expression, mitotic index, and radiological tumor
invasion in pituitary adenomas. Endocr Pathol. 30:49–55. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cheng S, Wu J, Li C, Li Y, Liu C, Li G, Li
W, Hu S, Ying X and Zhang Y: Predicting the regrowth of clinically
non-functioning pituitary adenoma with a statistical model. J
Transl Med. 17:1642019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guo J, Wang Z, Miao Y, Shen Y, Li M, Gong
L, Wang H, He Y, Gao H, Liu Q, et al: A two-circRNA signature
predicts tumour recurrence in clinical non-functioning pituitary
adenoma. Oncol Rep. 41:113–124. 2019.PubMed/NCBI
|
|
52
|
Chen X, Dai M, Zhu H, Li J, Huang Z, Liu
X, Huang Y, Chen J and Dai S: Evaluation on the diagnostic and
prognostic values of long non-coding RNA BLACAT1 in common types of
human cancer. Mol Cancer. 16:1602017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu ZR, Yan L, Liu YT, Cao L, Guo YH, Zhang
Y, Yao H, Cai L, Shang HB, Rui WW, et al: Inhibition of mTORC1 by
lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary
tumours. Nat Commun. 9:46242018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Y, Liu YT, Tang H, Xie WQ, Yao H, Gu
WT, Zheng YZ, Shang HB, Wang Y, Wei YX, et al: Exosome-transmitted
lncRNA H19 inhibits the growth of pituitary adenoma. J Clin
Endocrinol Metab. 104:6345–6356. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xing W, Qi Z, Huang C, Zhang N, Zhang W,
Li Y, Qiu M, Fang Q and Hui G: Genome-wide identification of
lncRNAs and mRNAs differentially expressed in non-functioning
pituitary adenoma and construction of an lncRNA-mRNA co-expression
network. Biol Open. 8:bio0371272019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen L, Liu D, Yi X, Qi L, Tian X, Sun B,
Dong Q, Han Z, Li Q, Song T, et al: The novel miR-1269b-regulated
protein SVEP1 induces hepatocellular carcinoma proliferation and
metastasis likely through the PI3K/Akt pathway. Cell Death Dis.
11:3202020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ichikawa T, Okugawa Y, Toiyama Y, Tanaka
K, Yin C, Kitajima T, Kondo S, Shimura T, Ohi M, Araki T and
Kusunoki M: Clinical significance and biological role of L1 cell
adhesion molecule in gastric cancer. Br J Cancer. 121:1058–1068.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Marques P, Barry S, Carlsen E, Collier D,
Ronaldson A, Awad S, Dorward N, Grieve J, Mendoza N, Muquit S, et
al: Chemokines modulate the tumour microenvironment in pituitary
neuroendocrine tumours. Acta Neuropathol Commun. 7:1722019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ma S, Lu CC, Yang LY, Wang JJ, Wang BS,
Cai HQ, Hao JJ, Xu X, Cai Y, Zhang Y and Wang MR: ANXA2 promotes
esophageal cancer progression by activating MYC-HIF1A-VEGF axis. J
Exp Clin Cancer Res. 37:1832018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tan SH, Young D, Chen Y, Kuo HC,
Srinivasan A, Dobi A, Petrovics G, Cullen J, Mcleod DG, Rosner IL,
et al: Prognostic features of Annexin A2 expression in prostate
cancer. Pathology. 2020.(Online ahead of print).
|
|
61
|
Zhuang C, Wang P, Sun T, Zheng L and Ming
L: Expression levels and prognostic values of annexins in liver
cancer. Oncol Lett. 18:6657–6669. 2019.PubMed/NCBI
|
|
62
|
Liu X, Ma D, Jing X, Wang B, Yang W and
Qiu W: Overexpression of ANXA2 predicts adverse outcomes of
patients with malignant tumors: A systematic review and
meta-analysis. Med Oncol. 32:3922015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Utama B, Kennedy D, Ru K and Mattick JS:
Isolation and characterization of a new nucleolar protein, Nrap,
that is conserved from yeast to humans. Genes Cells. 7:115–132.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dong D, Song M, Wu X and Wang W: NOL6, a
new founding oncogene in human prostate cancer and targeted by
miR-590-3p. Cytotechnology. 72:469–478. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang W, Snyder N, Worth AJ, Blair IA and
Witze ES: Regulation of lipid synthesis by the RNA helicase Mov10
controls Wnt5a production. Oncogenesis. 4:e1542015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nakano M, Kakiuchi Y, Shimada Y, Ohyama M,
Ogiwara Y, Sasaki-Higashiyama N, Yano N, Ikeda F, Yamada E,
Iwamatsu A, et al: MOV10 as a novel telomerase-associated protein.
Biochem Biophys Res Commun. 388:328–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tang Y, Xie C, Zhang Y, Qin Y and Zhang W:
Overexpression of mRNA-decapping enzyme 1a predicts
disease-specific survival in malignant melanoma. Melanoma Res.
28:30–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu C, Liu W, Ruan T, Zhu X, Tao K and
Zhang W: Overexpression of mRNA-decapping enzyme 1a affects
survival rate in colorectal carcinoma. Oncol Lett. 16:1095–1100.
2018.PubMed/NCBI
|
|
69
|
Shi C, Liu T, Chi J, Luo H, Wu Z, Xiong B,
Liu S and Zeng Y: LINC00339 promotes gastric cancer progression by
elevating DCP1A expression via inhibiting miR-377-3p. J Cell
Physiol. 234:23667–23674. 2019. View Article : Google Scholar : PubMed/NCBI
|