Introduction
Gastric cancer remains a major health problem
worldwide; the most recent worldwide epidemiological data suggest
that its incidence and mortality among the most common types of
solid tumour are at fifth and third place, respectively (1). Altogether, ~783,000 patients died due
to gastric cancer in 2018 (1). Even
though this type of cancer has been subject to a lot of research,
in the last decades its prognosis has not improved significantly.
The estimated median overall survival (OS) is usually 10–12 months
in metastatic patients with HER-2 negative disease (2). In this group of patients, palliative
chemotherapy represents the mainstay of treatment (3).
New targeted therapies have recently demonstrated
improvements in terms of patient outcomes. Trastuzumab (4) and Ramucirumab (5,6) have
shown significant benefits to survival and thus are widely used in
Western countries. Apatinib, which is currently under development,
is exhibiting signs of being a promising agent (7), whereas other drugs such as Regorafenib
are still under investigation or have yielded disappointing results
(such as in the case of Pertuzumab) (8,9).
Another therapeutic approach that should be
considered is surgery. The real benefit of surgical resection of
the primary tumour in patients with metastatic disease remains
unclear. The only available randomized trial (REGATTA) has not
revealed any survival benefit of gastrectomy followed by
chemotherapy compared with chemotherapy alone in 175 patients with
advanced gastric cancer with a single non-curable site of disease
confined to either the liver, peritoneum or para-aortic lymph nodes
(10). Conversely, previous
retrospective data (11–14) and meta-analyses (15) have suggested that surgery of the
primary tumour significantly prolongs survival, increasing the
median OS time to 14.9 months in patients with palliative
gastrectomy, regardless of metastatic site. An even greater
survival advantage has been observed in highly selected patients
with synchronous distant metastases who had undergone both
gastrectomy and metastasectomy, with a median OS time of 21.9
months (16). The resection of sites
of metastatic involvement with a different likelihood of obtaining
radical surgery (hepatic, peritoneal or distant lymph nodes) has
been associated with an increase in survival from 1.3 to 5 years
(17). Prolonged survival has been
also achieved in patients who have been randomized to maximal
cytoreductive surgery combined with regional heated intraperitoneal
and systemic chemotherapy, as shown by the GYMMSA trial (18).
However, the aforementioned retrospective data
cannot be considered conclusive due to selection bias. In fact,
only patients with good performance status, a more limited disease
and a tumour biology favouring slow tumour growth and selective
metastatic spread (19,20) could have been scheduled for surgery.
Furthermore, therapies administered before and after surgery may
represent a confounding factor. Results have shown that palliative
chemotherapy combined with gastrectomy may determine a survival
benefit compared with palliative gastrectomy alone (15).
Notably, albeit scarce data concerning locoregional
treatment of patients with gastric cancer have been published, only
a few retrospectively collected case series for patients with
isolated lung metastases due to gastric cancer can be found in
published literature (21,22). About 15% of patients with advanced
disease present metastases to lungs, and regardless of the
treatment received (either chemotherapy or best supportive care),
they seem to have an improved OS compared with other types of
metastatic spread (23).
In other gastrointestinal malignancies, such as in
colorectal cancer, there is published evidence that supports
locoregional management of lung metastases (24). However, there are no published
prospective randomized studies on this matter (the PULMICC trial is
now ongoing) (25). Nonetheless,
despite a lack of proper clinical trials, surgical resection of
lung metastases is performed currently and safely in everyday
clinical practice. The 5-year survival rates of patients undergoing
this procedure are 30–50%, which are comparable to those observed
in patients who undergo liver resection (26). A few prognostic indicators that may
affect the outcome have been identified, including number of
metastases, disease-free interval between the primary tumour and
the lung recurrence, and hilar/mediastinal lymph node involvement
(24–26).
There is a lack of similar evidence in metastatic
gastric cancer, mostly due to the relatively small amount of
available data and the worse prognosis of this disease compared
with colorectal cancer. In addition, lung metastases are frequently
removed at the same time as liver metastases, according to a
population-based review (23), thus
decreasing the number of patients that would ultimately be
candidates to receive radical surgery.
The present study aimed to determine the role of
several prognostic factors, highlighting the differences among
patients according to metastatic site, tumour histology and
treatment received (either systemic or local). Wider knowledge of
mechanisms involved in metastatic gastric cancer development and
metastatic spread, in light of the new molecular classification
(27), may strengthen the rationale
behind lung metastasectomy and ensure a more tailored and
evidence-based approach for these patients.
Materials and methods
Patient selection and main
stratification factors
A total of 184 patients with histologically
confirmed metastatic gastric or gastroesophageal junction
adenocarcinoma were considered eligible for analysis. Patients
should have received ≥1 line of chemotherapy for metastatic disease
with doublet or triplet chemotherapy (either combinations with
cisplatin or oxaliplatin, or combinations with 5-fluorouracil or
capecitabine were admitted). Patients who received prior surgery
for locally advanced disease and who received adjuvant chemotherapy
were admitted into the analysis if >6 months had elapsed between
the end of adjuvant chemotherapy and the first radiological sign of
disease relapse.
In addition to sex, age at diagnosis, performance
status at the start of palliative chemotherapy and previous
adjuvant chemotherapy, the main stratification factors were disease
histotype by Lauren classification (28,29)
(intestinal, diffuse, signet ring cell or not otherwise specified),
primary sites of metastatic involvement (lung only, liver only,
other sites with the addition of peritoneal involvement or other
sites of metastatic spread without peritoneal involvement),
palliative surgery for the primary tumour (yes or no) and timing of
metastatic involvement (synchronous or metachronous). The impact of
age at the start of first-line treatment on survival outcomes was
assessed by using two different clinically chosen cut-off values,
<75 or ≥75 years, and ≤40 or >40 years. Whether patients had
received second-line treatment or not was used as a stratification
factor only for OS. Consecutive patients treated at Azienda
Ospedaliera Universitaria Ospedali Riuniti Ancona (Ancona, Italy)
between January 1999 and June 2017 were included in the present
study.
Statistical analysis
The aim of the analysis was to assess whether one or
more of the aforementioned stratification factors may have an
impact on patient prognosis. Survival outcomes and response to
first-line treatment were retrospectively collected for all
patients included in the analysis. P<0.05 was considered to
indicate a statistically significant difference.
OS time was calculated as the time interval between
the start of first-line chemotherapy and the time of death due to
any cause or last follow-up visit. Progression-free survival (PFS)
time was calculated as the time interval between the start of
first-line chemotherapy and the time of death or of the first
radiological or clinically meaningful sign of disease progression
(whichever came first). Survival analysis was calculated using the
Kaplan-Meier method and differences among stratifying factors were
assessed by log-rank test. Multivariate analysis was conducted by
Cox-proportional hazards regression.
Response rates were defined according to the
Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 (30). Patients, as per standard clinical
practice, had received chest-abdomen CT scans once every 3 months
in order to evaluate response to treatment. Disease control rate
(DCR) was defined as the sum of patients who had stable disease
(SD), partial response (PR) or complete response (CR). For patients
with non-measurable disease according to RECIST, only survival
outcomes were assessed. The association among categorical variables
was assessed by Fishers exact test for binomial categorical
variables and by χ2 test in all other instances. The
present study was reviewed by a biomedical statistician. All
statistical analyses were performed using MedCalc Statistical
Software version 19.2.1 (MedCalc Software bvba) and R software
(version 3.6.2; http://www.r-project.org).
Results
Treatment outcomes in the whole
patient population
A total of 184 patients were eligible for analysis.
The main stratifying factors of the whole population are shown in
Table I. In the whole cohort of
patients, median OS time was 8.32 months (95% CI, 7.016–9.410),
while mean OS time was 19.33 months (95% CI, 13.39–25.26).
Similarly, median PFS time was 4.16 months (95% CI, 3.24–5.08),
while mean PFS time was 7.35 months (95% CI, 5.22–9.49) (data not
shown).
 | Table I.Baseline tumour and patient
characteristics (n=184). |
Table I.
Baseline tumour and patient
characteristics (n=184).
| Characteristic | Value | Percentage, % |
|---|
| Median age (range),
years | 63 (25–83) |
|
| Sex, n |
|
|
|
Male | 119 | 65 |
|
Female | 65 | 35 |
| ECOG PS, n |
|
|
| 0 | 110 | 60 |
| 1 | 74 | 40 |
| Resection of
primary tumour, n |
|
|
|
Yes | 95 | 52 |
| No | 89 | 48 |
| Adjuvant
chemotherapy, n |
|
|
|
Yes | 41 | 22 |
| No | 143 | 78 |
| Neoadjuvant
chemotherapy, n |
|
|
|
Yes | 23 | 13 |
| No | 161 | 87 |
| Histological
subtype, n |
|
|
|
Intestinal | 38 | 21 |
|
Diffuse | 35 | 19 |
| Signet
ring cells, n | 38 | 21 |
|
Other | 2 | 1 |
|
Unknown | 71 | 38 |
| HER-2 status,
n |
|
|
|
Positive | 9 | 5 |
|
Negative | 39 | 21 |
| Not
assessed | 136 | 74 |
| Second-line
chemotherapy, n |
|
|
|
Yes | 102 | 55 |
| No | 82 | 45 |
| Timing of
metastases presentation, n |
|
|
|
Synchronous | 107 | 58 |
|
Metachronous | 77 | 42 |
| Site of metastatic
involvement, n |
|
|
| Lung
only | 7 | 4 |
| Liver
only | 41 | 22 |
| Lymph
nodes only | 12 | 6 |
|
Peritoneal only | 68 | 37 |
| Bone
only | 4 | 2 |
| Other
sites with peritoneal involvement | 92 | 50 |
| Other
sites without peritoneal involvement | 44 | 24 |
| Locoregional
treatment, n |
|
|
|
Yes | 20 | 11 |
| No | 164 | 89 |
| Ethnicity, n |
|
|
| White
Caucasian | 183 | 99 |
|
Hispanic | 1 | 1 |
| Age ≥75 years old,
n |
|
|
|
Yes | 29 | 16 |
| No | 155 | 84 |
| Age ≤40 years old,
n |
|
|
|
Yes | 14 | 8 |
| No | 170 | 92 |
| Response to first
line chemotherapy |
|
|
|
Complete response | 10 | 6 |
| Partial
response | 42 | 24 |
| Stable
disease | 37 | 21 |
|
Progressive disease | 86 | 49 |
| Not
evaluable | 9 | 5 |
A total of 10 (6%) patients achieved CR, 42 (24%)
achieved PR, 37 (21%) achieved SD and 86 (49%) progressed during
first-line chemotherapy. A total of 9 (5%) patients were not
assessed for response by RECIST due to a lack of target lesions (as
they were affected by bone or peritoneal metastases) (Table I). A total of 7/184 (4%) patients had
only lung metastases, 41/184 (22%) had only liver metastases,
12/184 (6%) patients had only metastases in distant lymph nodes
(either abdominal or thorax lymph nodes), 68/184 (37%) had only
peritoneal involvement and 52/184 (28%) patients had metastases in
multiple organs. The remaining 4/184 (2%) had only bone metastases
(Table I).
Treatment outcomes stratified by
metastatic site
A statistically significant association between
different sites of metastatic involvement and both OS (P=0.003) and
PFS (P=0.0018) was observed. When comparing lung metastases only
vs. other sites of metastatic involvement, significantly improved
OS was observed in the former group [median OS, 154 months vs. 7.93
months, respectively; hazard ratio (HR), 0.27; 95% CI, 0.14–0.50;
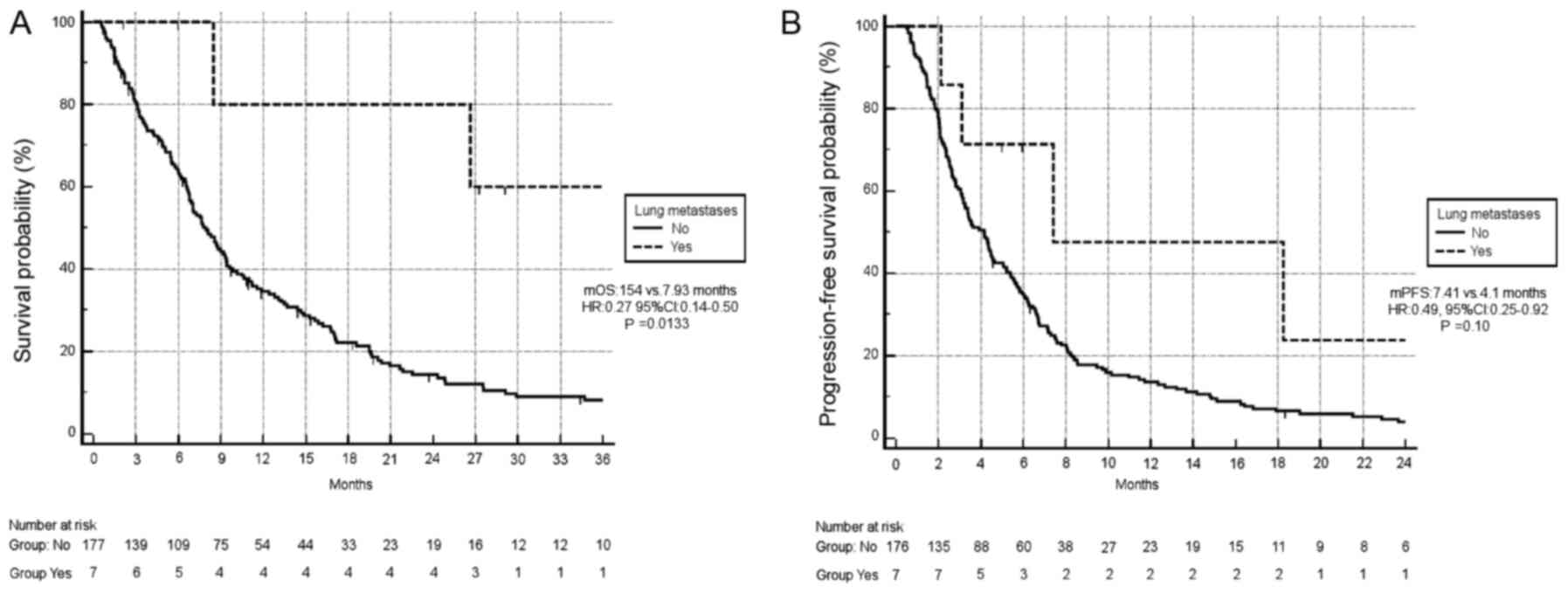
P=0.0133; Fig. 1A]. Similarly, there
was a trend towards improved PFS in patients with lung metastases
compared with other sites of metastatic involvement (median PFS,
7.4 months vs. 4.1 months, respectively; HR, 0.49; 95% CI,
0.25–0.92; P=0.10; Fig. 1B).
Similarly, response rates in patients with only lung metastases
(7/184) were significantly improved compared with those in patients
with other sites of metastatic involvement (6/7, 85% vs. 46/177,
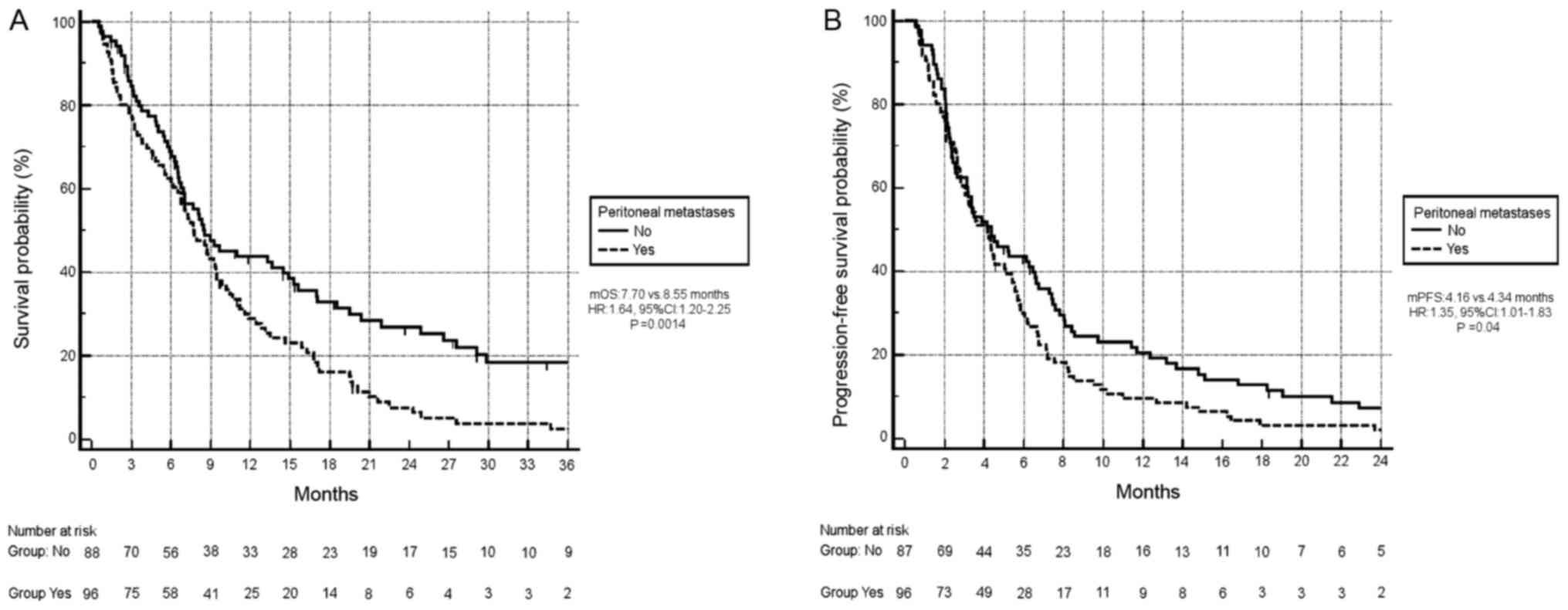
26%; P=0.0022; data not shown). Conversely, peritoneal metastases
had an unfavourable impact on survival: Median OS time for patients
with peritoneal metastases (96/184, 52%) was 7.70 months vs. 8.55
months, respectively (HR, 1.64; 95% CI, 1.20–2.25; P=0.0014;
Fig. 2A). In addition, patients with
peritoneal metastases had a significantly worse median PFS time
(median PFS, 4.16 months vs. 4.34 months, respectively; HR, 1.35;
95% CI, 1.01–1.83; P=0.04; Fig.
2B).
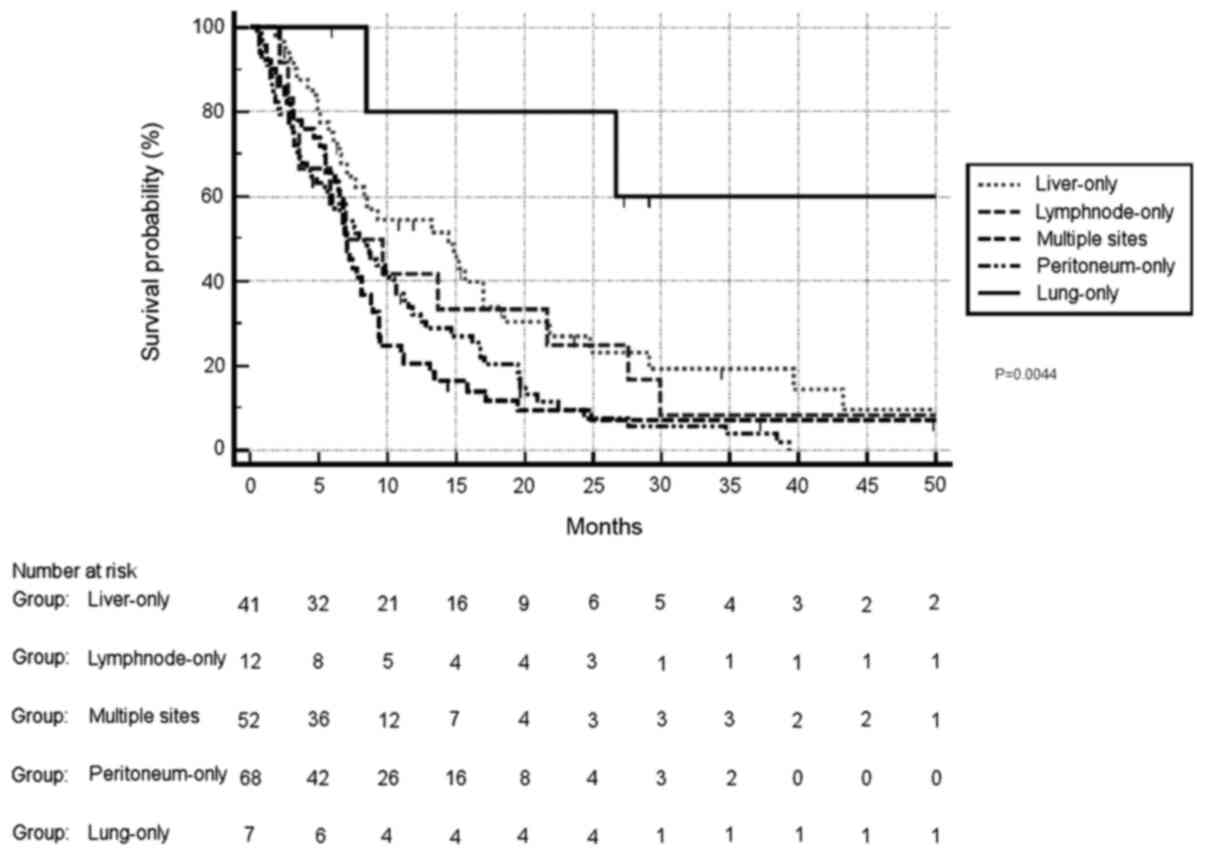
When comparing the OS of patients stratified by
single-organ involvement, lung metastases were associated with
significantly improved prognosis (HR, 0.39 and 95% CI, 0.20–0.77
for lung vs. liver; HR, 0.33 and 95% CI, 0.14–0.75 for lung vs.
lymph nodes; HR, 0.23 and 95% CI, 0.11–0.44 for lung vs.
peritoneum; and HR, 0.22 and 95% CI, 0.12–0.45 for lung vs.
multiple sites). On the other hand, liver metastases were not
associated with differences in survival compared with lymph node
metastases (HR, 0.84 and 95% CI, 0.45–1.57), while survival was
significantly improved compared with either peritoneal metastases
(HR, 0.58 and 95% CI, 0.39–0.88) or multiple metastases (HR, 0.56
and 95% CI, 0.36–0.87). Lymph node metastases were not associated
with differences in OS compared with peritoneal metastases (HR,
0.66 and 95% CI, 0.38–1.28) or multiple sites of metastatic
involvement (HR, 0.66 and 95% CI, 0.35–1.26). Finally, peritoneal
metastases were not associated with differences in OS compared with
multiple sites of metastatic involvement (HR, 0.95 and 95% CI,
0.62–1.46) (Fig. 3). Overall,
different sites of metastatic involvement were associated with
significantly different survival outcomes (P=0.0044).
Treatment outcomes stratified by other
factors, including histology, other additional treatments,
second-line chemotherapy and age
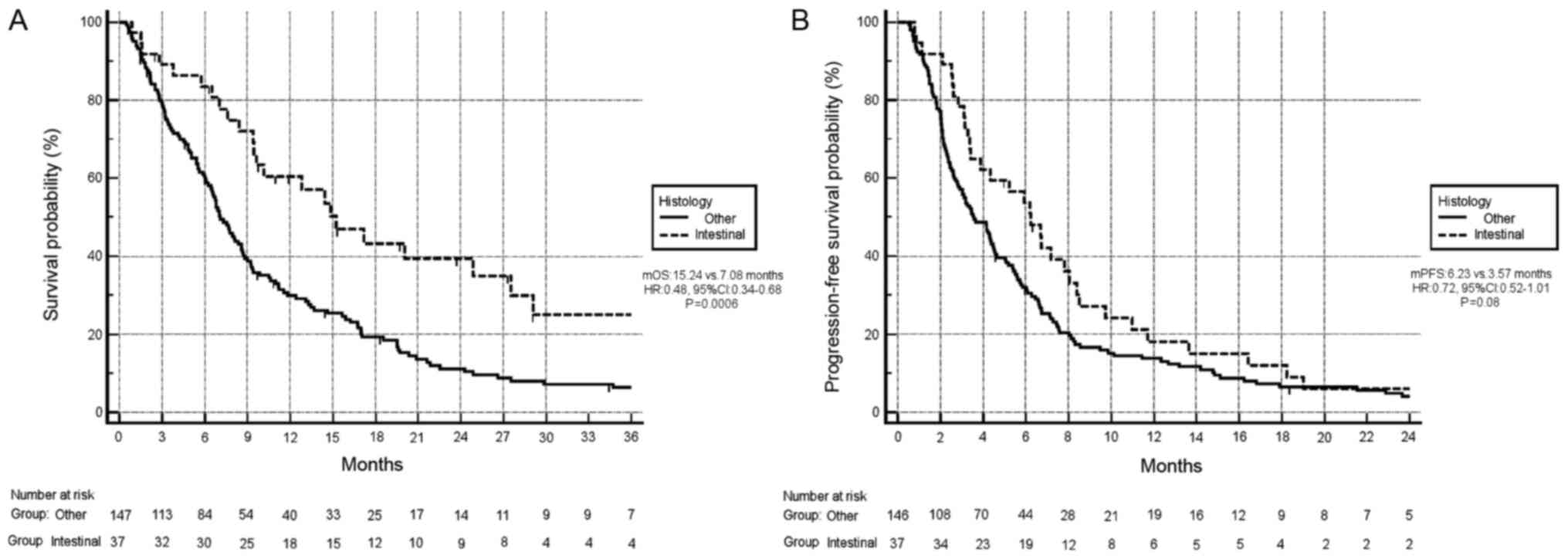
Survival analysis exhibited improved OS in patients
with the intestinal subtype compared with other histological
subtypes. In particular, the median OS time of patients with the
intestinal subtype compared with other histotypes was 15.24 months
vs 7.08 months, respectively (HR, 0.48; 95% CI, 0.34–0.68;
P=0.0006; Fig. 4A). Additionally,
there was a trend towards improved PFS in patients with the
intestinal subtype compared with other subtypes (median PFS, 6.23
months vs. 3.57 months, respectively; HR, 0.72; 95% CI, 0.52–1.01;
P=0.08; Fig. 4B). In terms of
response rates, 19/37 (51%) patients achieved PR or CR in the
intestinal subtype group vs. 23/146 (16%) in the remaining group,
and this difference was statistically significant (P=0.000017)
(data not shown).
A total of 20/184 (11%) patients received
locoregional treatment in addition to chemotherapy: 5 patients
received liver metastasectomy, 1 patient received stereotactic
radiosurgery for an isolated brain metastasis and underwent surgery
for a single skin metastasis, 2 patients underwent peritonectomy
and hyperthermic intraperitoneal chemotherapy, 2 patients underwent
bilateral palliative oophorectomy for ovarian metastases, 1 patient
underwent abdominal lymphnodal dissection for para-aortic
lymphnodal involvement, 2 patients underwent lung resection (and
one of them was subsequently submitted to liver surgery for an
isolated liver metastasis), 2 patients received stereotactic
radiosurgery for lung metastases, 1 patient received radiotherapy
in mediastinal lymph nodes and the remaining 4 patients all
received radiotherapy for bone metastases.
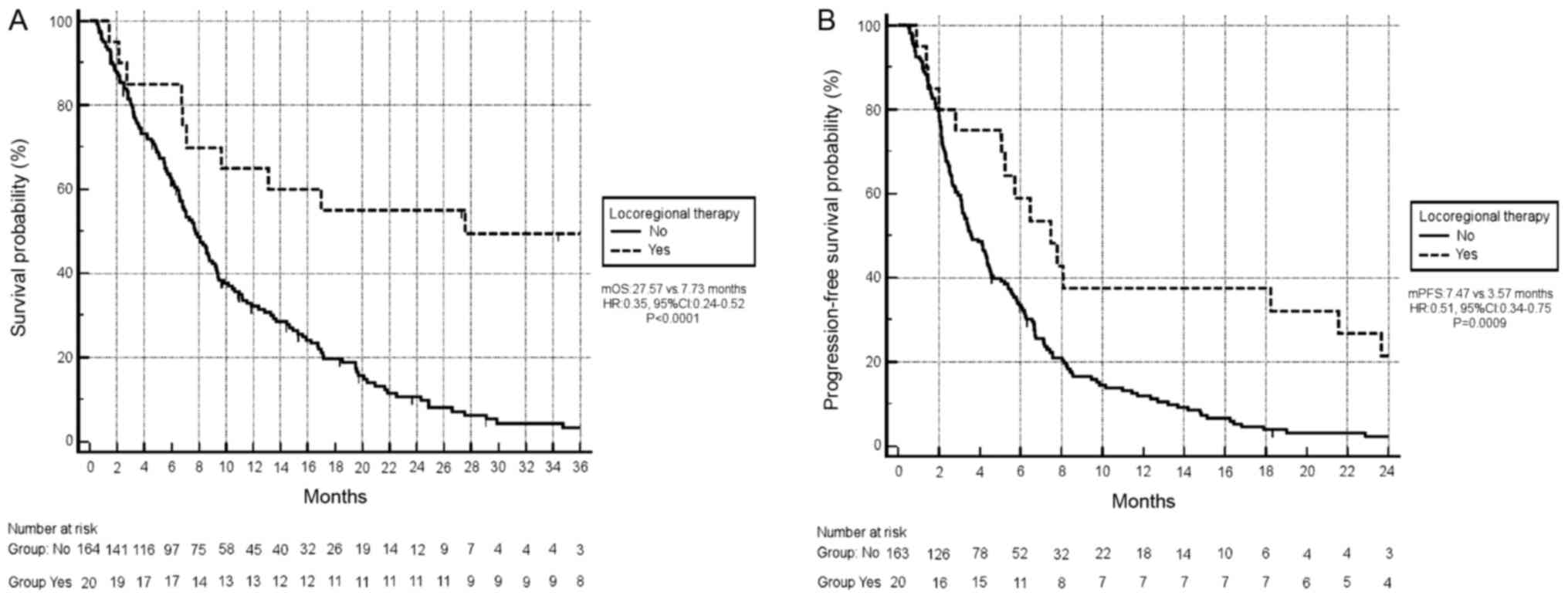
Survival analysis revealed that locoregional
treatment of metastatic sites (either by surgery or radiotherapy)
was significantly associated with an improved OS (mOS 27.57 months
vs. 7.73 months, respectively; HR, 0.35; 95% CI, 0.24–0.52;
P<0.0001; Fig. 5A; OS curve in
patients who also received locoregional treatment of metastatic
sites vs. only chemotherapy). Locoregional treatment of metastatic
sites was also associated with significantly longer PFS (median PFS
7.47 months vs. 3.57 months, respectively; HR, 0.51; 95% CI,
0.34–0.75; P=0.0009; Fig. 5B; PFS
curve in patients who also received locoregional treatment of
metastatic sites vs. only chemotherapy).
A total of 29/184 (16%) patients were ≥75 years old.
OS was not significantly different compared with younger patients
(median OS 7.08 months vs. 8.32 months, respectively; HR, 0.98; 95%
CI, 0.62–1.53; P=0.93). First-line PFS was also not significantly
different (median PFS 4.36 months vs. 4.16 months, respectively;
HR, 0.89; 95% CI, 0.59–1.35; P=0.61). A total of 14/184 (8%)
patients were ≤40 years old. OS was not significantly different
compared with older patients (median OS 11.83 months vs. 8.13
months, respectively; HR, 0.78; 95% CI, 0.46–1.34; P=0.38).
First-line PFS was also not significantly different (median PFS
2.82 months vs. 4.29 months, respectively; HR, 0.76; 95% CI,
0.44–1.29; P=0.31) (data not shown).
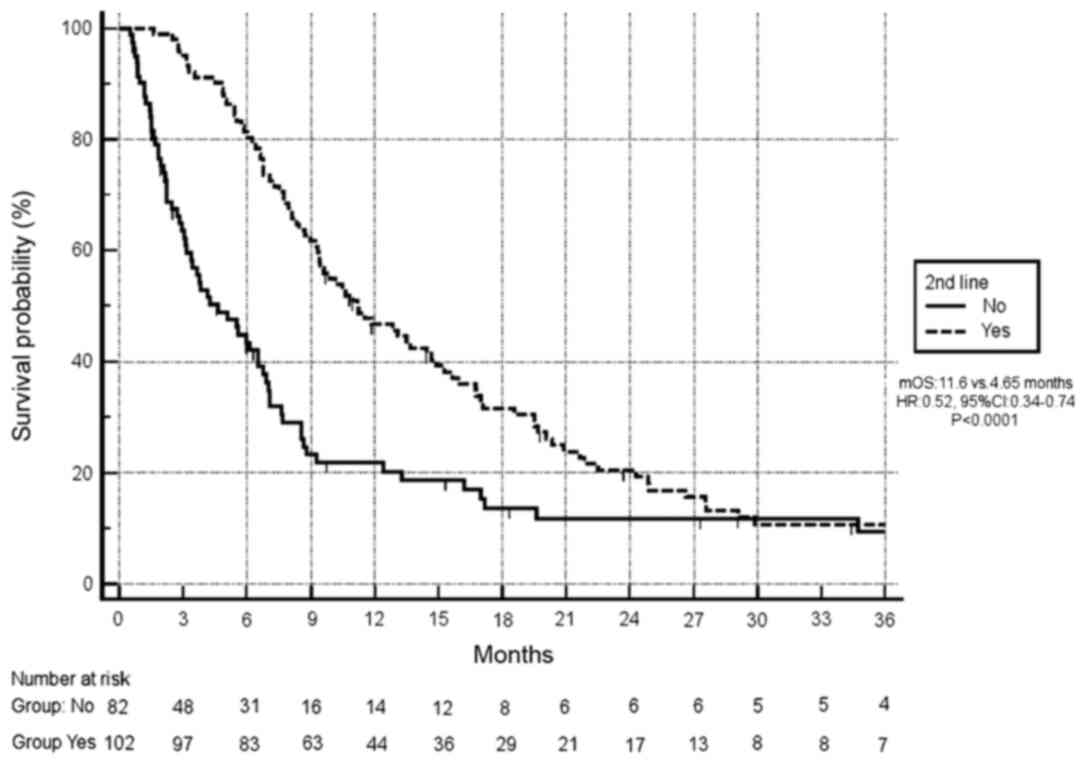
Finally, 82/184 (45%) patients received second-line
therapy after first-line disease progression. A significantly
improved OS was observed in this group of patients compared with
those without second-line treatment (11.6 months vs. 4.65 months,
respectively; HR, 0.52; 95% CI, 0.37–0.74; P<0.0001; Fig. 6).
Results of multivariate analysis
Results of the multivariate analysis are shown in
Table II. The only factors that
remained significantly associated with differences in OS were
locoregional treatment (HR, 0.35; 95% CI, 0.19–0.64; P=0.007),
second-line treatment (HR, 0.50; 95% CI, 0.36–0.69; P<0.0001)
and intestinal histology (HR, 0.58; 95% CI, 0.37–0.90; P=0.0152).
The only factor that was significantly associated with differences
in PFS was locoregional treatment of metastases (HR, 0.45; 95% CI,
0.27–0.72; P=0.0034).
 | Table II.Summary of results of multivariate
analysis. |
Table II.
Summary of results of multivariate
analysis.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Stratifying
factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Intestinal
histology (yes vs. no) | 0.81 | 0.55–1.19 | 0.277 | 0.58 | 0.37–0.90 | 0.015 |
| Lung metastases
(yes vs. no) | 0.63 | 0.25–1.59 | 0.328 | 0.49 | 0.15–1.60 | 0.241 |
| Peritoneal
metastases (yes vs. no) | 1.21 | 0.88–1.64 | 0.210 | 1.35 | 0.97–1.89 | 0.073 |
| Second-line
treatment (yes vs. no) | / | / | / | 0.50 | 0.36–0.69 | <0.001 |
| Locoregional
treatment (yes vs. no) | 0.45 | 0.27–0.77 | 0.003 | 0.35 | 0.19–0.64 | <0.001 |
Discussion
The present analysis was focused on assessing the
role of a series of prognostic factors in patients with metastatic
gastric cancer. The analysed population was comprised almost
entirely of white-Caucasian patients: Since ethnicity is an
important factor in gastric cancer, this should be considered when
assessing the current results. Tumour histology has been previously
described as a strong prognostic factor. A previous study of 248
patients with metastatic gastric cancer stratified by histology
demonstrated that patients with the intestinal subtype of gastric
cancer had improved PFS and OS compared with patients with the
diffuse subtype (31). Additionally,
a recent meta-analysis including 61,468 patients confirmed that the
diffuse type of gastric cancer is a poor prognostic factor
regardless of the treatment received (32).
Another factor that was associated with improved OS
in the present study was whether patients had received second-line
treatment. In a recently published paper (33), stratification by several clinical
factors, such as performance status, lactate dehydrogenase levels,
neutrophil/lymphocytes ratio and first-line PFS time, was able to
identify subsets of patients with second-line OS estimates >7
months compared with patients with poor prognostic features.
The present survival analysis suggested that
different sites of metastatic involvement may be associated with
differences in OS: Lung metastases seemed to have the best survival
outcomes, while peritoneal metastases were associated with the
worst survival outcomes. However, this was disproved by the
multivariate analysis; when the site of metastatic involvement was
assessed together with tumour histology, second-line treatment and
locoregional therapies, it lost its independent prognostic impact.
To the best of our knowledge, this is the first time that this has
been proven in patients with gastric cancer.
Regarding other gastrointestinal malignancies, there
are several studies that have focused on the improved survival of
patients affected by lung metastases, stratified by surgical
resection. For example, Brandi et al (34) analysed a retrospective series of 151
patients with metastatic colorectal cancer who received liver or
lung metastases resection. A total of 20/151 (13%) patients had
lung involvement only, but no statistically significant differences
in survival outcomes were observed in patients with lung metastases
compared with liver metastases; the only factors that influenced
survival in the multivariate analysis were adjuvant chemotherapy
and disease-free interval (34).
Gonzalez et al (26) reported
survival outcomes of patients who underwent lung metastasectomy for
metachronous lung metastases in patients with colorectal cancer
previously treated with surgery for liver metastases. Their results
revealed a median disease-free survival time after pulmonary
metastasectomy of 13 months and that patients who benefited the
most from surgery were those with single lung metastases, compared
with those having >1 site of lung involvement (26).
Regarding gastric cancer, the published data are
even scarcer. Iijima et al (21) have analysed the role of lung
metastasectomy in patients who have lung metastases as the only
site of involvement for gastric cancer. In the 10 patients who were
eligible for the analysis, the 3-year OS rate was 30% (21). In another study by Yoshida et
al (22), 10 patients with
metastatic gastric cancer were submitted to surgery for solitary
lung metastases, with relatively favourable results (75% 4-year
survival rate), as in the present analysis. In a review by Aurello
et al (35), 10 published
papers including a total of 44 patients with metastatic gastric
cancer were described. The median disease-free interval of the
resected patients was 35 months and among them, 38 patients had
single lung metastases, whereas 6 patients presented with >1
lesion (28). Median OS time after
resection was 45 months, with a median disease-free survival time
of 9 months (35).
Finally, in a large registry based observational
retrospective analysis by Shiono et al (36), comprising 3,831 patients who
underwent surgical resection for lung metastases arising from
different types of cancer, 51 patients had primary gastric cancer.
In these patients, survival outcomes were less favourable than in
the present analysis, with a 5-year OS rate after resection of 28%,
a median survival time of 29 months and median time to recurrence
after lung resection of 6 months (36). Even if these results seem to be less
optimistic than the present data, it should be considered that
these survival rates are better than what is currently achieved
just through the use of standard palliative chemotherapy (usually
with a median OS time of 12–16 months (2,37). In
addition, in the population of patients with disease-free intervals
>12 months, a statistically significant impact on improved OS
was observed, with the 5-year OS rate rising up to 31% (36).
In the present study, different types of lung
metastatic involvement were analysed. In particular, the shortest
survival times were observed for patients with diffuse lung
involvement, a rarely described phenomenon called Bards syndrome
(caused by diffuse lymphangitic involvement of the whole lung with
severe respiratory impairment and imminent risk of death) (38). Similarly to the aforementioned
studies, improved survival was observed for patients submitted to
surgery with the presence of solitary lung metastases, with
sufficiently long (>12 months) relapse-free survival times
following the resection of the primary tumour. Additionally, the
present study observed a particularly favourable prognosis in one
patient who, although not submitted to surgery, received high dose
stereotactic radiotherapy of a solitary lesion.
Locoregional treatments in patients with metastatic
gastric cancer, although not recommended for the majority of
patients, should be at least offered in selected cases with
favourable prognostic features, such as a single site of metastatic
involvement, positive probability of achieving a R0 resection and
sufficiently prolonged observation time following surgery for the
primary tumour (17). A recently
published meta-analysis by Gadde et al (17), focusing solely on patients with
metastatic gastric cancer amenable to surgery regardless of their
site of involvement, revealed significant survival advantages in
patients who received surgery, and this improvement in survival was
significantly higher when looking at the 1-year time point (with a
decreased impact over the course of the following years of
observation).
In conclusion, the present data contributes to the
body of evidence on patients with oligometastatic gastric cancer,
suggesting that, in a few selected cases (those with a relatively
favourable first-line PFS) locoregional treatment of isolated
metastatic sites should be offered, as it allows for increased
survival that would otherwise not be achieved with any other type
of medical treatment.
Acknowledgements
The authors would like to thank Dr Andrew Davies
Burd for English revision of this manuscript.
Funding
The present study was funded by the Università
Politecnica delle Marche (Ancona, Italy).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
RG, LC, MDP, EG and RBe conducted the present study.
EG, LC, MDP, RBi, TM, MGB and AM collected the clinical data. RG,
AL and EM performed the statistical analyses. RG, TM, MGB, AM, EG,
LC, AB, EM, RBe, MS and RBi participated in the study design and
concept. All authors oversaw the drafting of the manuscript, and
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Regional
Ethical Committee of Marche, Azienda Ospedaliero-Universitaria
Ospedali Riuniti of Ancona (Ancona, Italy). All patients provided
written informed consent to the research and to all the
diagnostic-therapeutic procedures.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
DCR
|
disease control rate
|
|
SD
|
stable disease
|
|
PR
|
partial response
|
|
CR
|
complete response
|
References
|
1
|
Bray F, Ferlay J, Soerjomataran I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: A
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: REGARD Trial Investigators. Ramucirumab monotherapy for
previously treated advanced gastric or gastro-oesophageal junction
adenocarcinoma (REGARD): An international, randomised, multicentre,
placebo-controlled, phase 3 trial. Lancet. 383:31–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pavlakis N, Sjoquist KM, Martin AJ,
Tsobanis E, Yip S, Kang YK, Bang YJ, Alcindor T, OCallaghan CJ,
Burnell MJ, et al: Regorafenib for the treatment of advanced
gastric cancer (INTEGRATE): A multinational placebo-controlled
phase II Trial. J Clin Oncol. 34:2728–2735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang YK, Rha SY, Tassone P, Barriuso J, Yu
R, Szado T, Garg A and Bang YJ: A phase IIa dose-finding and safety
study of first-line pertuzumab in combination with trastuzumab,
capecitabine and cisplatin in patients with HER2-positive advanced
gastric cancer. Br J Cancer. 111:660–666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujitani K, Yang HK, Mizusawa J, Kim YW,
Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, et
al: Gastrectomy plus chemotherapy versus chemotherapy alone for
advanced gastric cancer with a single non-curable factor (REGATTA):
A phase 3, randomised controlled trial. Lancet Oncol. 17:309–318.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samarasam I, Chandran BS, Sitaram V,
Perakath B, Nair A and Mathew G: Palliative gastrectomy in advanced
gastric cancer: Is it worthwhile? ANZ J Surg. 76:60–63. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hartgrink HH, Putter H, Klein Kranenbarg
E, Bonenkamp JJ and van de Velde CJ; Dutch Gastric Cancer Group, :
Value of palliative resection in gastric cancer. Br J Surg.
89:1438–1443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Medina-Franco H, Contreras-Saldívar A,
Ramos-De La Medina A, Palacios-Sanchez P, Cortés-González R and
Ugarte JA: Surgery for stage IV gastric cancer. Am J Surg.
187:543–546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fornaro L, Fanotto V, Musettini G, Uccello
M, Rimassa L, Vivaldi C, Fontanella C, Leone F, Giampieri R, Rosati
G, et al: Selecting patients for gastrectomy in metastatic
esophago-gastric cancer: Clinics and pathology are not enough.
Future Oncol. 13:2265–2275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun J, Song Y, Wang Z, Chen X, Gao P, Xu
Y, Zhou B and Xu H: Clinical significance of palliative gastrectomy
on the survival of patients with incurable advanced gastric cancer:
A systematic review and meta-analysis. BMC Cancer. 13:5772013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohri Y, Tanaka K, Ohi M, Saigusa S,
Yasuda H, Toiyama Y, Araki T, Inoue Y and Kusunoki M:
Identification of prognostic factors and surgical indications for
metastatic gastric cancer. BMC Cancer. 14:4092014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gadde R, Tamariz L, Hanna M, Avisar E,
Livingstone A, Franceschi D and Yakoub D: Metastatic gastric cancer
(MGC) patients: Can we improve survival by metastasectomy? A
systematic review and meta-analysis. J Surg Oncol. 112:38–45. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rudloff U, Langan RC, Mullinax JE, Beane
JD, Steinberg SM, Beresnev T, Webb C, Walker M, Toomey MA, Schrump
D, et al: Impact of maximal cytoreductive surgery plus regional
heated intraperitoneal chemotherapy (HIPEC) on outcome of patients
with peritoneal carcinomatosis of gastric origin: Results of the
GYMSSA trial. J Surg Oncol. 110:275–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scartozzi M, Loretelli C, Galizia E,
Mandolesi A, Pistelli M, Bittoni A, Giampieri R, Faloppi L,
Bianconi M, Del Prete M, et al: Role of vascular endothelial growth
factor (VEGF) and VEGF-R genotyping in guiding the metastatic
process in pT4a resected gastric cancer patients. PLoS One.
7:e381922012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scartozzi M, Giampieri R, Loretelli C,
Bittoni A, Mandolesi A, Faloppi L, Bianconi M, Del Prete M,
Andrikou K, Bearzi I and Cascinu S: Tumor angiogenesis genotyping
and efficacy of first-line chemotherapy in metastatic gastric
cancer patients. Pharmacogenomics. 14:1991–1998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iijima Y, Akiyama H, Atari M, Fukuhara M,
Nakajima Y, Kinosita H and Hidetaka U: Pulmonary resection for
metastatic gastric cancer. Ann Thorac Cardiovasc Surg. 22:230–236.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshida Y, Imakiire T, Yoneda S, Obuchi T,
Inada K and Iwasaki A: Ten cases of resected solitary pulmonary
metastases arising from gastric cancer. Asian Cardiovasc Thorac
Ann. 22:578–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riihimäki M, Hemminki A, Sundquist K,
Sundquist J and Hemminki K: Metastatic spread in patients with
gastric cancer. Oncotarget. 7:52307–52316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Raemdonck D: Pulmonary metastasectomy:
Common practice but is it also best practice? Futur Oncol.
11:11–14. 2015. View Article : Google Scholar
|
|
25
|
Treasure T, Fallowfield L and Lees B:
Pulmonary metastasectomy in colorectal cancer: The PulMiCC trial. J
Thorac Oncol. 5 (6 Suppl 2):S203–S206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gonzalez M and Gervaz P: Risk factors for
survival after lung metastasectomy in colorectal cancer patients:
Systematic review and meta-analysis. Future Oncol. 11 (Suppl
2):S31–S33. 2015. View Article : Google Scholar
|
|
27
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YC, Fang WL, Wang RF, Liu CA, Yang
MH, Lo SS, Wu CW, Li AF, Shyr YM and Huang KH: Clinicopathological
variation of lauren classification in gastric cancer. Pathol Oncol
Res. 22:197–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bittoni A, Scartozzi M, Giampieri R,
Faloppi L, Bianconi M, Mandolesi A, Del Prete M, Pistelli M,
Cecchini L, Bearzi I and Cascinu S: Clinical evidence for three
distinct gastric cancer subtypes: Time for a new approach. PLoS
One. 8:e785442013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Petrelli F, Berenato R, Turati L, Mennitto
A, Steccanella F, Caporale M, Dellera P, de Braud F, Pezzica E, Di
Bartolomeao M, et al: Prognostic value of diffuse versus intestinal
histotype in patients with gastric cancer: A systematic review and
meta-analysis. J Gastrointest Oncol. 8:148–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fanotto V, Cordio S, Pasquini G,
Fontanella C, Rimassa L, Leone F, Rosati G, Santini D, Giampieri R,
Di Donato S, et al: Prognostic factors in 868 advanced gastric
cancer patients treated with second-line chemotherapy in the real
world. Gastric Cancer. 20:825–833. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brandi G, Derenzini E, Falcone A, Masi G,
Loupakis F, Pietrabissa A, Pinna AD, Ercolani G, Pantaleo MA, Di
Girolamo S, et al: Adjuvant systemic chemotherapy after putative
curative resection of colorectal liver and lung metastases. Clin
Colorectal Cancer. 12:188–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aurello P, Petrucciani N, Giulitti D,
Campanella L, DAngelo F and Ramacciato G: Pulmonary metastases from
gastric cancer: Is there any indication for lung metastasectomy? A
systematic review. Med Oncol. 33:92016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shiono S, Sato T, Horio H, Chida M,
Matsuguma H, Ozeki Y, Nakajima J and Okumura S; Metastatic Lung
Tumor Study Group of Japan, : Outcomes and prognostic factors of
survival after pulmonary resection for metastatic gastric cancer.
Eur J Cardiothorac Surg. 43:e13–e16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bittoni A, Del Prete M, Scartozzi M,
Pistelli M, Giampieri R, Faloppi L, Bianconi M and Cascinu S: Three
vs. two drugs first-line chemotherapy regimen in advanced gastric
cancer patients: A retrospective analysis. Springerplus. 4:7432015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zieliński M, Ochman M, Głowacki J and
Kozielski J: Pulmonary lesions in the course of gastric cancer-two
cases of Bards syndrome. Pneumonol Alergol Pol. 84:33–37. 2016.
View Article : Google Scholar : PubMed/NCBI
|