Introduction
The human body can regulate the acid-base balance
during normal metabolism. Under normal conditions, the body's pH
will remain 7.35–7.45 over a long period of time. This balance is
important for the maintenance of physiological processes in the
body, including cell proliferation and apoptosis, the regulation of
various enzyme activities, and assembly and depolymerization of the
cytoskeleton, and it is closely associated with the precise
regulation of pH (1,2). In recent years, with continued in-depth
research, scholars have found that the acidic microenvironment of
tumors participates in the regulation of various abnormal
biological behaviors of tumor cells. The imbalance of intracellular
and extracellular pH is an important factor in tumorigenesis and
continued tumor development. Studies have reported that with the
decrease in pH (acidification), the cells will undergo apoptosis
and necrosis, and the acidic extracellular pH accelerates cell
metabolism and enhances the abilities of tumor cell migration,
invasion and metastasis. On the one hand, tumor cell invasion and
metastasis largely depend on a series of proteases, such as
metalloproteinases, thiol proteases, serine proteases and acid
proteases, which can degrade a series of tissue barriers, and their
activity can be enhanced in an acidic microenvironment, creating
conditions favoring cancer cell invasion (3,4). On the
other hand, the driving force for the migration and invasion of
tumor cells is derived from the plate-like pseudopodia, which are
primarily composed of actin and can protrude into the extracellular
matrix (ECM) to assemble a microfilament mesh that drives
cytoskeletal formation; therefore, the acidic extracellular
microenvironment of tumor cell ]may be an important factor in the
early events of malignant transformation.
NHE family and NHE1
The formation of the extracellular acid-base milieu
is mainly dependent on the efflux of excess intracellular hydrogen
ions. A number of channels and genes are known to be involved in
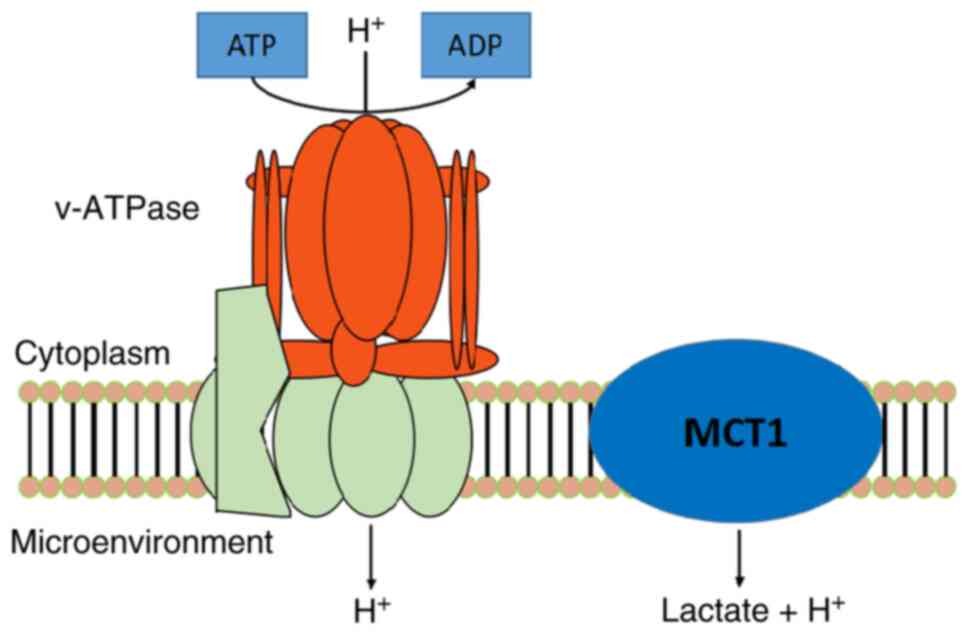
intra- and extracellular pH regulation, such as the
Na+/H+ exchanger (NHE),
Na+-dependent and Na+-independent
Cl−/HCO3- exchange protein, monocarboxylate
transporter 1 (MCT1) and V-ATPase (5–7)
(Fig. 1). The NHE1 subtype of the
NHE family has been confirmed as an important hydrogen ion
exchanger on the cell membrane, and it plays an important role in
the regulatory mechanism of the pH balance inside and outside the
cell.
The NHE family is a membrane-incorporated protein
family that is widely expressed on eukaryotic cells, and it
regulates the intra- and extracellular pH balance mainly through
the 1:1 exchange of intracellular H+ with extracellular
Na+ (8). Structurally,
there are 12 α-helices at the N-terminus of the NHE, which play an
important role in ion exchange. The C-terminus is a cytoplasmic
regulatory domain, which is modified by various cell surface
receptor-mediated extracellular signals that combine to regulate
NHE transport activity (9). Nine
subtypes of the NHE family have been identified and named NHE1-9
(10,11) according to the order of discovery,
with each subtype exhibiting unique tissue distribution and
functional characteristics. NHE1 is widely distributed in the
plasma membrane of all tissues; NHE2 is mainly distributed in the
kidney, intestine and adrenal gland; NHE3 is mainly distributed on
the surface of the epithelial cell basement membrane (12) and is highly expressed in the small
intestine and kidney; NHE4 is mainly expressed, albeit at low
levels, in gastric epithelial cells and in cells of the small
intestine and kidney (13); NHE5 is
mainly expressed in the brain; and NHE6-NHE9 are distributed in
intracellular compartments (14),
namely the Golgi complex and Golgi postendosomal compartments in
human cells.
The function of NHE1 is the most extensively
investigated among all the subtypes. It is known that NHE1 consists
of 815 amino acids, including a hydrophobic N-terminus consisting
of 500 amino acids and a hydrophilic C-terminus consisting of 315
amino acids. The hydrophobic N-terminus mediates the transport of
ions. Na+/K+ ATPase produces a Na+
chemical gradient, driving one H+ from the inside to the
outside of the cell in exchange for one extracellular
Na+ that enters the cell (15). The Na+/K+
ATPase C-terminus participates in the regulation of NHE1 activity
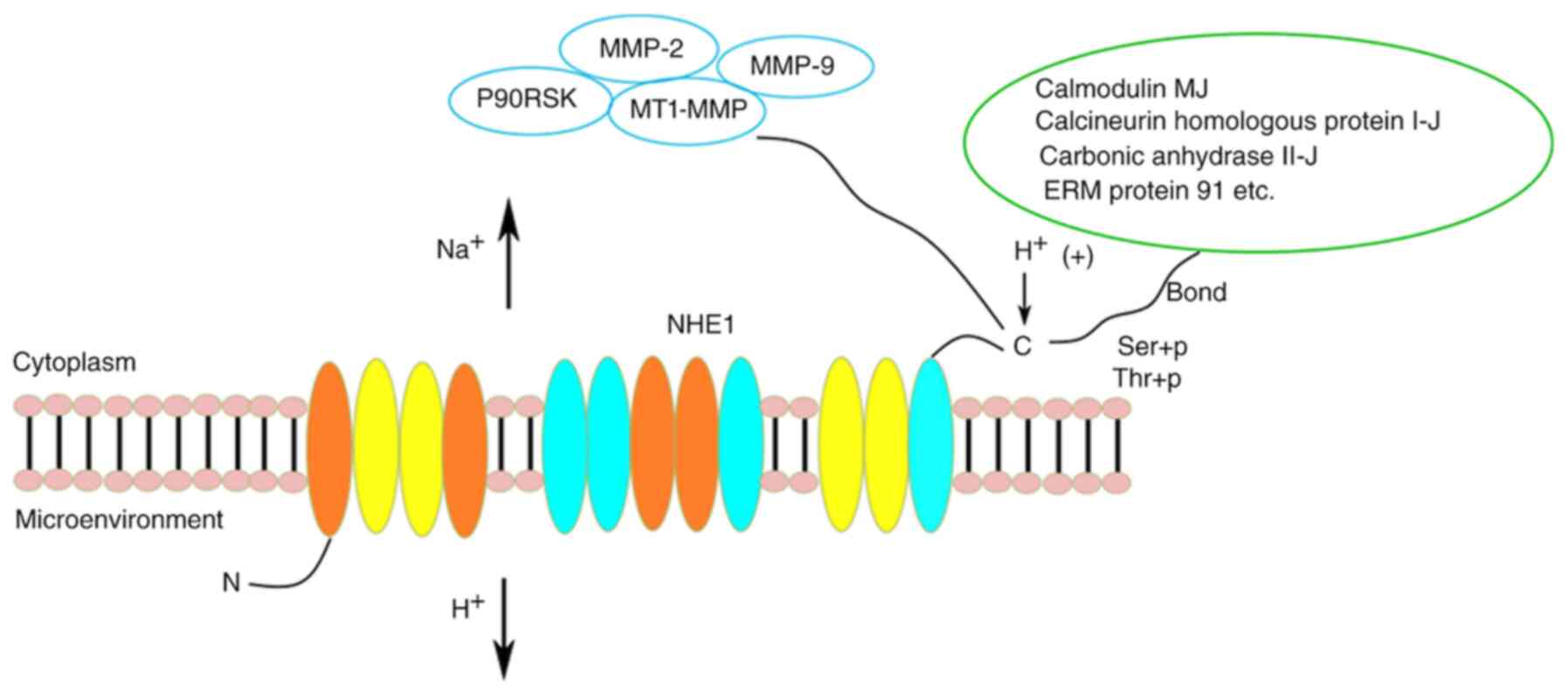
and function, mainly by mediating the following three functions: i)
The intracellular H+ level, which enhances the
conformational change of the proton-binding site on the C-terminus,
increases NHE1 activity and pumps H+ into the
extracellular space; ii) extracellular stimulation can
phosphorylate some of the serine/threonine residues in the
C-terminus, thereby activating NHE1 activity (16); and iii) C-terminus-specific protein
binding sites that activate NHE1 upon binding of specific proteins,
such as calmodulin MJ, calcineurin homologous protein I-J, carbonic
anhydrase II-J and ERM protein 91 (Fig.
2).
Current domestic and foreign studies have revealed
that NHE1 participates not only in the maintenance of the pH
balance inside and outside the cell and the prevention of
intracellular acidification, but also in several processes such as
cell migration, proliferation control, adhesion and apoptosis, as
well as expression and function changes (such as the occurrence and
development of inflammation, cardiovascular disease and diabetes)
that are closely associated with a number of diseases (17). Recent research reported that
replication of subgroup J avian leukosis virus depends on a
functional cellular receptor, the chicken
Na+/H+ exchanger type 1, and editing the gene
for a virus receptor may confer resistance to this virus and its
associated diseases (18). More
importantly, it has been reported that NHE is widely expressed in
different cancer tissues, where both its activity and expression
levels are apparently increased during processes closely associated
with invasive growth and distant metastasis of tumor cells. NHE is
a key transporter involved in the pH-dependent activation of
cathepsin B, matrix metallopeptidase (MMP)-9, MMP-2 and membrane
type 1 (MT1)-MMP (5,19). In MV3 cells, NHE1 overexpression
causes rearrangement of F-actin at the cell cortex, which is
associated with an increase in cortical stiffness. This
rearrangement in F-actin likely relies on the pH-sensitive and,
thus, NHE1-dependent interaction between cortactin and cofilin.
Despite their higher cortical stiffness, NHE1-overexpressing cells
are considerably more invasive in a collagen type I substrate,
which is most likely due to increases in MMP-3 secretion and
activity (20). In hypoxic cancer
cells, NHE1 localizes to invasive podocytes, where it promotes the
formation of invasive pseudopods by expressing and activating p90
ribosomal S6 kinase (p90RSK) (21).
However, NHE1 can also affect invasive function by controlling the
proteolytic activity of the cell cycle. In addition, activated NHE1
can also increase the expression of MMP-9 and MMP-2 and fuse with
pseudopods via lysosomes and vesicles, where it targets the
transport of proteases and promotes the hydrolysis of ECM proteins
(22–26).
To date, there is no systematized literature to
clarify the regulatory mechanism of NHE1 in various tumors or the
progress of NHE1-targeted therapy. Therefore, the present study was
undertaken to review the research advances on NHE1 expression and
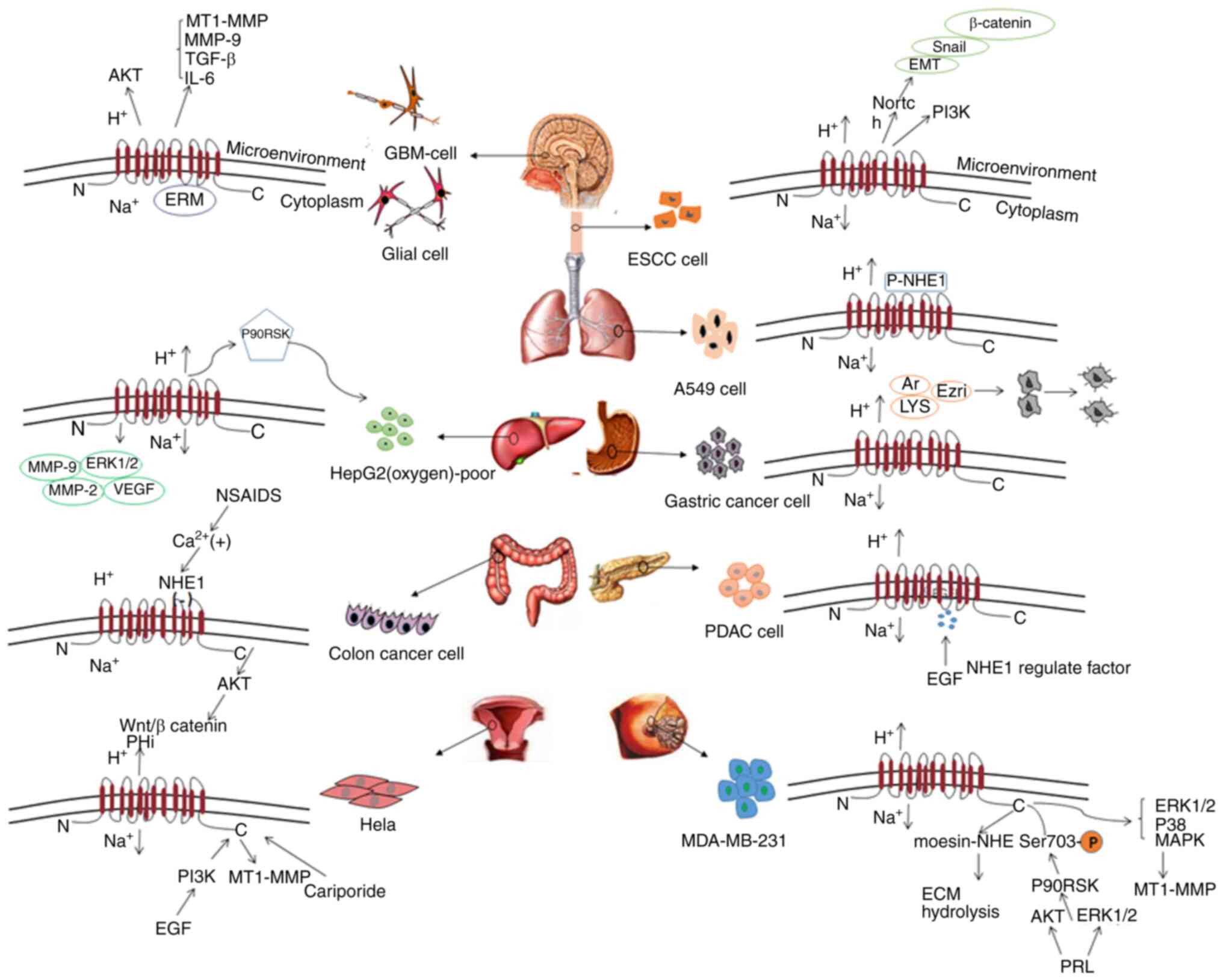
regulatory mechanisms in various tumors (Fig. 3), with the aim of providing research
directions for developing novel antitumor drugs.
NHE1 and gastric cancer
The incidence of gastric cancer China is high, and
most cases are diagnosed at an advanced stage. Therefore, it is
particularly important to seek prognostic indicators and
therapeutic targets for gastric cancer. It is a well-known fact
that cells undergoing progressive acidification can undergo
apoptosis and necrosis. Tumor cells rely on NHE1 to maintain a
neutral or even alkaline intracellular environment to prevent
apoptosis and necrosis. Previous studies have confirmed that NHE1
is highly expressed in gastric mucous cells, which are integrated
between the parietal cells and the surface mucous cells of the
basolateral membrane. In both types of cells, intracellular
acidification and hypertonicity can increase NHE1 activity, while
carbonic anhydrase II is highly expressed on the surface of gastric
mucous cells and cervical mucous cells (Fig. 4) expressing NHE1; therefore, it may
be inferred that NHE1 is closely associated with gastric acid
secretion (27,28). It has been confirmed that, in rabbit
gastric parietal cells, NHE1 and NHE4 are involved in the
regulation of the acid secretion phase, thereby regulating the
volume of gastric acid secretion (29). Niu et al found that the NHE1
mRNA and protein expression levels were upregulated in gastric
cancer tissues. In addition, downregulation of NHE1 gene expression
induced by transfected genes led to intracellular acidification and
the induction of apoptosis (30),
which enabled the treatment of gastric cancer in experimental
studies. The aforementioned results suggest that NHE1 may be
involved in the initiation process of gastric cancer (31). In addition, Liu et al found
that the carboxy-terminal proximal membrane region of NHE1 contains
a large number of lysine and arginine residue sequences, which can
interact with the coding gene of ezrin, which belongs to the ERM
family and is involved in mediating cell-cell interactions and the
formation of a cell surface adhesion complex that promotes
cell-cell and cell-ECM adhesion. If the surface of the tumor cells
expresses a large number of ezrin proteins, the cell surface
adhesion complex is diminished, and the adhesion between tumor
cells decreases or disappears, thereby promoting tumor metastasis.
The clinical data reported by Xie et al also demonstrated
that, compared with normal gastric mucosal tissue, the mRNA and
protein expression of NHE1 and ezrin were significantly increased
in gastric cancer tissues (32).
This increased expression was positively correlated with gastric
cancer stage, depth of invasion and extent of lymph node metastasis
(3), suggesting that NHE1 and ezrin
may be important factors for gastric cancer metastasis and that
NHE1 may be of value as a new target for the treatment of gastric
cancer.
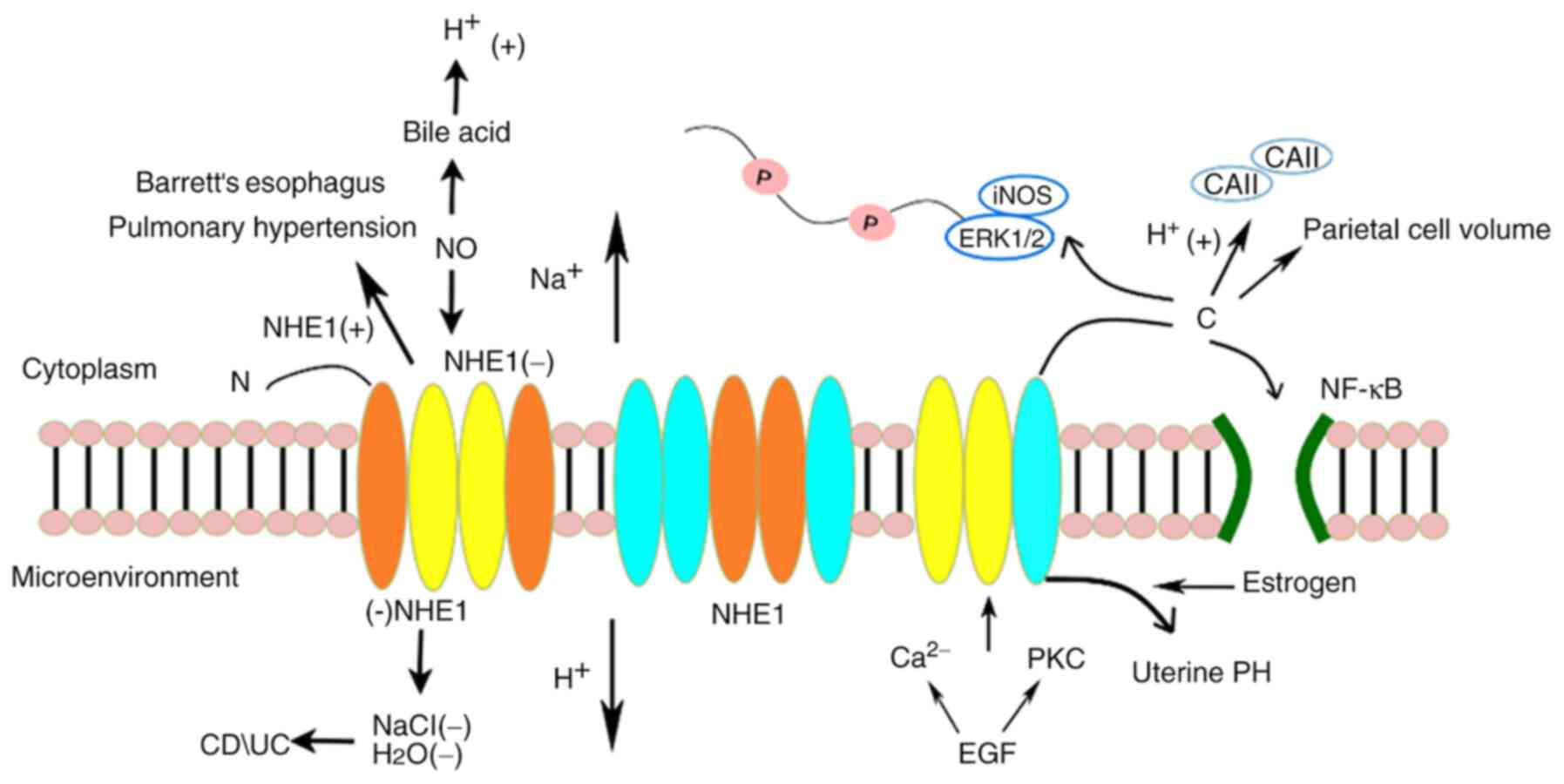 | Figure 4.Effect and regulatory mechanisms of
NHE1 in diseases other than cancer. NHE1 mediates the systemic
inflammatory response and multiple organ damage and involves
regulatory pathways such as NF-κB activation, neutrophil
infiltration, increased expression of iNOS, and phosphorylation of
ERK1/2. NHE1 is also involved in the regulation of ulcerative
colitis, Crohn's disease, Barrett's esophagus, pulmonary
hypertension, gastric acid secretion, etc. NHE1,
Na+/H+ exchanger isoform 1; NF-κB, nuclear
factor-κB; iNOS, inducible nitric oxide synthase; ERK,
extracellular signal-regulated kinase; PKC, protein kinase C; EGF,
epidermal growth factor. |
NHE1 and liver cancer
Liver cancer is the fifth most prevalent malignant
tumor worldwide, and no definitive indicator is currently available
for estimating the prognosis or as targeted therapy of liver
cancer. NHE1 mediates systemic inflammatory response and the
subsequent damage of multiple organs, including the heart, liver
and kidney (33), through regulatory
pathways that mediate NF-κB activation (Fig. 3), neutrophil infiltration, increased
iNOS expression and ERK1/2 phosphorylation. It was previously
demonstrated that the expression of NHE1 was significantly
increased in hepatocellular carcinoma (HCC) tissues and cells
(4), and this expression was closely
associated with tumor size (34),
extensive vascular invasion and advanced tumor stage (pTNM). In
addition, the survival time of patients with high levels of NHE1
expression was significantly shortened. Other reports have
confirmed that some inflammatory factors, such as TNF-α and IL-6,
can upregulate the expression of NHE1 in hepatocytes, thereby
affecting the functional activity of HCC cells (35) and suggesting that NHE1 may be
important for the diagnosis and treatment of patients with HCC.
During the process of invasion and metastasis of HCC cells, NHE1
plays a key role in upregulating the expression and activity of
MMP-2, MMP-9, ERK1/2 and VEGF (36–38). In
HepG2 cells, hypoxia promotes the expression of NHE1, and
extracellular acidification promotes the production of invasive
pseudopods by inducing p90RSK (21).
The formation of invasive pseudopods contributes to ECM degradation
and promotes invasion and migration of cancer cells. In addition,
NHE1 can also affect tumor cell apoptosis, as it has been reported
that the NHE1 inhibitor EIPA downregulates Bcl-2 and upregulates
Bax expression in HepG2 cells, leading to tumor cell apoptosis
(35). As common ion transporters,
NHEs are regulated by numerous substances. For example, IL6 and
B[a]P can enhance the activity of NHE1, while Rg3 can inhibit the
activity of NHEs (35,39,40). Kim
et al demonstrated that curcumin treatment or glucose
restriction (GR) slightly inhibited NHE1, while the combined
treatment with curcumin and GR further enhanced the inhibitory
effect on NHE1 and reduced pHi (41). Therefore, based on the aforementioned
description of IL6, B[a]P and Rg3 in HCC, it was suggested that
NHEs may represent a potential therapeutic target in HCC. However,
further studies are required to identify the potential underlying
mechanisms (42).
NHE1 and pancreatic cancer
Pancreatic cancer is a common malignant tumor of the
digestive tract. In recent years, its incidence and mortality have
continued to increase, severely affecting the health and quality of
life of the patients. Pancreatic ductal carcinoma (PDAC) alone
accounts for ~90% of pancreatic cancer cases, and new diagnostic
and therapeutic options are urgently needed. However, little
research has been conducted on the association between NHE and PDAC
in recent years. NHE1 and NHE4 are distributed on the basolateral
side of pancreatic acinar cells, and on the basal side of
pancreatic ducts (43,44), while NHE2 and NHE3 are mainly
distributed in the luminal membrane. In terms of physiological
function, NHE1 has been shown to be a major regulator of pHi in
pancreatic acinar cells that are stimulated at rest by muscarinic
agonists (45). In a study on
pancreatic diseases, the human pancreatic gene expression database
revealed that the promoter of NHE1 transfer is associated with
epidermal growth factor receptor (EGFR) in PDAC, which may drive
three-dimensional growth and early invasion of the basal membrane
and EGF stimulation via digestion of the ECM through invasive
pseudopods to subsequently regulate pancreatic cancer development.
EGF promotes the formation of an EGFR and NHE1 complex via the
scaffold protein Na+/H+ exchange regulator 1,
and controls the extent and duration of EGFR-mediated transduction
of oncogenic signals in a negative regulatory state (46), in which NHE1 is specific for cell
proliferation or invasion. The type of dimeric subtype complex that
is separated by the transient signaling of the
EGFR/Na+/H+ exchange regulator 1/NHE1 complex
differs by lipid raft membrane domains. Laminin γ2 (LAMC2) was
found to be responsible for generating the extracellular acidic
conditions that mediate invasion of pancreatic cancer cells by
activating AKT/NHE1 signaling. LAMC2 is a characteristic prognostic
and therapeutic factor in PDCA (47).
NHE1 and esophageal cancer
Previous studies have demonstrated that NHE1
induction may be the basis of the pathogenesis of gastroesophageal
reflux disease and may play an important role in the protection of
esophageal epithelial cells from acid. Some studies have found that
EGF can activate NHE1 through Ca2+/calmodulin and
protein kinase C pathways to protect the esophageal epithelium from
acid (48,49). Barrett's esophagus is a precancerous
lesion associated with an increased risk of esophageal
adenocarcinoma and is a current hot research topic (50). Goldman et al found that the
activity of NHE1 in Barrett's epithelium (Fig. 3) was significantly increased to
accommodate cellular acidification, while bile acid-induced
cellular acidification involved NO-mediated NHE inhibition, which
resulted in increased intracellular acidification and DNA damage
that may lead to mutations and cancer progression (51). Clinical data from a study of patients
with esophageal cancer revealed that, compared with that in normal
esophageal mucosa, the expression of NHE1 in primary esophageal
squamous cell carcinoma (ESCC) was significantly increased,
particularly in patients with highly differentiated tumors. In the
low NHE1 expression group, the 5-year survival rate of patients was
significantly better compared with that of the high NHE1 expression
group, suggesting that NHE1 may be associated with poor prognosis
in patients with ESCC. In vitro cell experiments also
confirmed that NHE1 was highly expressed in primary ESCC cells and
significantly promoted their proliferation, migration and invasion,
whereas NHE1 inhibition led to increased apoptosis. The mechanism
may be associated with NHE1 downregulation of PI3K-AKT and Notch
signaling (52,53). Epithelial-to-mesenchymal transition
(EMT)-related genes and proteins, including Snail, β-catenin,
NF-κB, AKT and p21, are involved in the progression and metastasis
of cancer and have prognostic value. It has been confirmed that
NHE1 inhibits the Notch signaling pathway, whereas the upregulation
of Snail, β-catenin and other EMT markers leads to EMT in
esophageal cancer cells (54,55),
thereby increasing cell migration and invasion. In general, NHE1
exerts a tumor-promoting effect on ESCC and represents a prognostic
indicator for patients with ESCC and a promising new target for
ESCC treatment.
NHE1 and breast cancer
NHE1 is widely distributed on the plasma membrane of
all tissues, promoting the formation of the acidic tumor
microenvironment. Extracellular acidification promotes the
formation of new blood vessels in metastatic breast cancer
(56), thus promoting tumor invasive
ability. It has been demonstrated that the expression and activity
of NHE were increased in breast microinvasive foci and adjacent
ductal cells, which are associated with local infiltration and
distant metastasis of cancer cells (57). In MDA-MB-231 breast cancer cells,
NHE1 stimulated the expression of MT1-MMP by activating the ERK1/2
and p38 MAPK signaling pathways (23,58,59),
thereby mediating the invasion and metastasis of MDA-MB-231 cells.
In addition, it has been found that cytoskeletal agonist-binding
proteins are novel upstream regulators of the subcellular
distribution and activity of moesin-NHE1 (60), which can promote invasive pseudopod
maturation and mediate NHE1 regulation of tumor invasion.
Specifically, the invasive pseudopod acts as a site for regulatory
protease activity to induce ECM proteolysis under the stimulation
of the acidic microenvironment produced by NHE1 (61). Elena Pedraz-Cuesta et al also
found that the PRL/PRLR-NHE1 signaling axis is involved in breast
cancer cell invasion, PRL-induced NHE1 activation and the resulting
NHE1-dependent invasion, thus promoting the invasive behavior of
human breast cancer cells. Among the signaling pathways involved in
cancer cell invasion, PRL-mediated activation of AKT and ERK1/2
activates p90RSK, which leads to the phosphorylation of NHE1
Ser703, thereby increasing NHE1 activity, including NHE1-dependent
cell migration (62). As a pHi
regulatory protein, a previous study examined the role of
negatively charged amino acids of extracellular loop 3 (EL3) in the
activity of the NHE protein, and demonstrated that amino acids E217
and D226 form part of a negatively charged coordination sphere,
which facilitates cation transport in the NHE1 protein (63). NHE7, a unique protein member of the
NHE family, dynamically shuttles across the Golgi network between
endosomes and the plasma membrane to regulate the intraluminal pH
in these organelles. NHE7 overexpression in MDA-MB-231 breast
cancer cells enhances the tumor cell coverage area, cell-cell
adhesion and cell invasion, which is independent of tumor growth
and tumor sphericity (64),
according to the first report (63)
indicating that NHE participates in tumor regulation at the
organelle level. In studies on antitumor therapy, it was confirmed
that the inhibition of NHE1 expression and activity may act
synergistically with paclitaxel to induce cell apoptosis, and it
has been confirmed that PKA and p38 are upstream regulatory points
of NHE1 in the induction of paclitaxel-dependent apoptosis. It has
been suggested that NHE1 may provide a new approach to improving
the efficacy of paclitaxel chemotherapy. Studies have also found
that pyrazinoylguanidine-type NHE1 inhibitors potently inhibit the
growth and survival of cancer cell spheroids, and this effect is
unrelated to NHE1 inhibition (65).
NHE1 and lung cancer
With the obvious increase in the incidence of lung
cancer on a global scale, lung cancer is currently the most common
cause of cancer-related mortality worldwide, as well as in China.
However, the mechanism of NHE1 regulation in lung cancer remains
unclear. Recent studies have demonstrated that NHE1 is expressed in
all parts of the human airway, and regional differences in NHE1
mRNA levels suggest that differential expression may be associated
with differences in airway absorption, electrolyte secretion and
acid load (66,67). For example, in pulmonary artery
smooth muscle cells under chronic hypoxia, NHE1 expression and
activity are significantly increased (68), which may be associated with the
mechanism of the development of pulmonary hypertension (Fig. 3). There is also evidence that NHE1 is
involved in the regulation of malignant cytological behaviors, such
as growth, proliferation and invasion of lung cancer cells
(69). The investigators (70) transfected pNHE1 (NHE1 antisense
expression vector) into A549 human lung cancer cells to induce the
inhibition of NHE1 mRNA expression and related activity, which
resulted in significantly decreased pHi, suppressed cell
proliferation and increased apoptosis rate of tumor cells. In
addition, in a study on the effect of the NHE1 antisense gene
sequence on cell proliferation and apoptosis in drug-resistant
small cell lung cancer (SCLC) (71),
it was observed that the NHE1 antisense gene could induce
acidification and apoptosis in H446/CDDP drug-resistant human SCLC
cells, which suggests a potential new treatment for
multidrug-resistant SCLC.
NHE1 and brain tumors
Malignant gliomas are the most common primary brain
tumors, and options for their treatment are currently limited. The
majority of the patients with clinically diagnosed glioblastoma
(GBM) have a survival time of <1 year. Studies by Pizzonia et
al have demonstrated the expression of NHE1 throughout the
human brain (72), including the
hippocampus, where it interacts with ERM proteins to promote glial
cell survival, apoptosis resistance, migration and invasion by
regulating cell proliferation (73),
pHi, extracellular and tumor microenvironment pH. It has been
reported in the literature that NHE1 activation may inhibit cell
apoptosis by activating the AKT signaling pathway (74). Tumor cell migration and invasion may
be associated with NHE1 regulation of cell volume, cytoskeletal
stability (75–77) and the plasma membrane. Therefore,
NHE1 has great potential for cancer treatment, including for GBM
(78). Studies by Zhu et al
found that NHE1 is highly expressed in glioma-associated microglia,
and NHE1 in glial cells regulates microglia-derived factors
(79), such as MT1-MMP, MMP-9, TGF-β
and IL-6, thus promoting the proliferation and viability of glial
cells. In addition, lactic acidosis is a common characteristic of
ischemic brain tissue. Some scholars have found that NHE1 can
regulate the pHi of glial cells, particularly under pathologically
low pH conditions (73), enhancing
the NHE1 regulatory role. Inhibition of NHE1 during oxygen
deprivation contributes to the maintenance of the energy state of
glial cells. In addition to NHE1, NHE5 is also highly expressed in
brain tissue (80), and it may play
a role in neuronal tissue. Recently, Kurata et al
demonstrated NHE activity in brain tumors using C6 glioma cells
which expressed both NHE1 and NHE5. NHE5 knockdown C6 glioma cells
exhibited downregulation of MET and EGFR, resulting in loss of
invasion and proliferation ability, while NHE1 knockdown did not
exert the same effects (81).
NHE1 and colorectal cancer
The NHE family proteins are widely expressed in the
intestine, but they exhibit certain differences in localization.
For example, NHE1 is evenly distributed throughout the intestine.
NHE2 is mainly distributed in the small intestine, distal colon and
proximal colon. NHE3 is expressed, in descending order of
prevalence, in the ileum, jejunum and colon (82). It is known that, in ulcerative
colitis and Crohn's disease (Fig.
3), inhibition of NHE1 transcription can reduce NaCl and water
uptake in the colonic lumen (83),
resulting in diarrhea. In addition, NHE2 and NHE3 also appear to be
involved in the absorption of Na+ in the intestine
(84). In a study of colon cancer,
the acidic tumor microenvironment and the Wnt/β-catenin activation
pathway were identified as two key elements associated with the
development and progression of cancer. It has been reported in the
literature that T84 human colon cancer cells contain three isoforms
of NHE1, NHE2 and NHE4 (85). A
study of rat colon cancer cells also strongly supported the
hypothesis that AKT activity may be associated with the NHE1
regulation of pHi and the Wnt/β-catenin signaling pathway (86). In colorectal cancer cells, β1
integrin-mediated adhesion to ECM proteins, such as collagen type I
and adhesin, triggers an early and transient pHi alkalinization,
from 6.7 to 7.2. The effect is caused by NHE1 activation and is
modulated by the activity of the voltage-dependent K+
channel KV11.1 (87). With respect
to antitumor therapy, cisplatin is considered as a broad-spectrum
antitumor drug. Some scholars have suggested that inhibition of
NHE1 at the plasma membrane may promote the activation of acid
sphingomyelinase and increase membrane fluidity. The aforementioned
process plays an important role in the cytotoxicity mediated by
cisplatin (88), suggesting that
NHE1 is a new potential target for cisplatin antitumor therapy. In
colorectal cancer, non-steroidal anti-inflammatory drugs (NSAIDs)
appear to be promising for chemoprevention. It has been reported
that certain NSAIDs, such as sulindac and celecoxib (89), prevent colorectal cancer through a
mechanism that may be associated with the downregulation of NHE1,
the increase in intracellular Ca2+ and the activation of
the downstream calpain 9 signaling pathway.
NHE1 and cervical cancer
Studies of the correlation between cervical cancer
and NHE are still in the early stages. It has been demonstrated
that the NHE1-specific inhibitor cariporide can inhibit the
migration and invasion of HeLa cervical cancer cells in
vitro (90). Further mechanistic
studies (90) have reported that
NHE1 mediates the metastasis of HeLa cells by regulating the
expression and localization of MT1-MMP. An experiment by Chiang
et al demonstrated that EGF is involved in the regulation of
NHE1 expression via the PI3K signaling pathway in cervical cancer
(91), and NHE1 can also interact
with the actin-related protein ezrin to reconstitute the
cytoskeleton and stimulate the migration and invasion of cervical
cancer cells. It was also reported that Andrographolide regulates
the expression of apoptotic proteins to induce cells apoptosis, and
concentration-dependently decreases pHi by decreasing the activity
of NHE1/V-ATPase and the expression of NHE1 in HeLa cells; thus,
Andrographolide may represent a promising novel agent in the
treatment of cervical cancer in the clinical setting (92). In addition, NHE has also been found
to be involved in the regulation of uterine fluid pH (Fig. 3) under the influence of estrogen
(93). Studies by Ismail et
al have confirmed that enhancement of NHE1, NHE2 and NHE4
protein and mRNA expression in the cervix can help restore
cervical-vaginal fluid pH and prevent cervical and vaginal
complications associated with menopause (94).
Outlook
NHE is widely expressed in human multisystem tumors.
Recently, there have been several studies on the role and
regulatory mechanism of NHE1 in tumors, but studies on subtypes
other than NHE are rare. As an important regulator in tumors, NHE1
is associated with the regulation of tumor drug resistance,
invasion, metastasis, apoptosis resistance and other malignant
biological behaviors, contributing to poor prognosis. With
continued in-depth research, NHE1 may be used as a new target for
the development of novel anticancer drugs, in the hope of improving
the treatment of tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by research grants
from the National Natural Science Foundation of China (grant nos.
81770610 and 81970541).
Availability of data and materials
Not applicable.
Authors' contributions
YH and JL drafted the initial manuscript. ZJ, XY,
WS, YH, QD and QL performed the literature review. JX primarily
revised and finalized the manuscript. RX revised the manuscript for
clarity and style. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reshkin SJ, Bellizzi A, Caldeira S,
Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V
and Tommasino M: Na+/H+ exchanger-dependent
intracellular alkalinization is an early event in malignant
transformation and plays an essential role in the development of
subsequent transformation-associated phenotypes. FASEB J.
14:2185–2197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Izumi H, Torigoe T, Ishiguchi H, Uramoto
H, Yoshida Y, Tanabe M, Ise T, Murakami T, Yoshida T, Nomoto M and
Kohno K: Cellular pH regulators: Potentially promising molecular
targets for cancer chemotherapy. Cancer Treat Rev. 29:541–549.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li L, Wang YY, Zhao ZS and Ma J: Ezrin is
associated with gastric cancer progression and prognosis. Pathol
Oncol Res. 17:909–915. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang X, Wang D, Dong W, Song Z and Dou K:
Expression and modulation of Na(+) /H(+) exchanger 1 gene in
hepatocellular carcinoma: A potential therapeutic target. J
Gastroenterol Hepatol. 26:364–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brisson L, Reshkin SJ, Gore J and Roger S:
pH regulators in invadosomal functioning: Proton delivery for
matrix tasting. Eur J Cell Biol. 91:847–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGuire C, Cotter K, Stransky L and Forgac
M: Regulation of V-ATPase assembly and function of V-ATPases in
tumor cell invasiveness. Biochim Biophys Acta. 1857:1213–1218.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Payen VL, Mina E, Van Hée VF, Porporato PE
and Sonveaux P: Monocarboxylate transporters in cancer. Mol Metab.
33:48–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dibrov P and Fliegel L: Comparative
molecular analysis of Na+/H+ exchangers: A
unified model for Na+/H+ antiport? FEBS Lett.
424:1–5. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Putney LK, Denker SP and Barber DL: The
changing face of the Na+/H+ exchanger, NHE1:
Structure, regulation, and cellular actions. Annu Rev Pharmacol
Toxicol. 42:527–552. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malo ME and Fliegel L: Physiological role
and regulation of the Na+/H+ exchanger. Can J
Physiol Pharmacol. 84:1081–1095. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Landau M, Herz K, Padan E and Ben-Tal N:
Model structure of the Na+/H+ exchanger 1
(NHE1): Functional and clinical implications. J Biol Chem.
282:37854–37863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noel J, Roux D and Pouyssegur J:
Differential localization of Na+/H+ exchanger
isoforms (NHE1 and NHE3) in polarized epithelial cell lines. J Cell
Sci. 109:929–939. 1996.PubMed/NCBI
|
|
13
|
Orlowski J, Kandasamy RA and Shull GE:
Molecular cloning of putative members of the Na/H exchanger gene
family. cDNA cloning, deduced amino acid sequence, and mRNA tissue
expression of the rat Na/H exchanger NHE-1 and two structurally
related proteins. J Biol Chem. 267:9331–9339. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura N, Tanaka S, Teko Y, Mitsui K and
Kanazawa H: Four Na+/H+ exchanger isoforms
are distributed to Golgi and post-Golgi compartments and are
involved in organelle pH regulation. J Biol Chem. 280:1561–1572.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pedersen SF, O'Donnell ME, Anderson SE and
Cala PM: Physiology and pathophysiology of
Na+/H+ exchange and Na+
-K+ −2Cl- cotransport in the heart, brain, and blood. Am
J Physiol Regul Integr Comp Physiol. 291:R1–R25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denker SP, Huang DC, Orlowski J, Furthmayr
H and Barber DL: Direct binding of the Na-H exchanger NHE1 to ERM
proteins regulates the cortical cytoskeleton and cell shape
independently of H(+) translocation. Mol Cell. 6:1425–1436. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loo SY, Chang MK, Chua CS, Kumar AP,
Pervaiz S and Clement MV: NHE-1: A promising target for novel
anti-cancer therapeutics. Curr Pharm Des. 18:1372–1382. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koslová A, Trefil P, Mucksová J, Reinišová
M, Plachý J, Kalina J, Kučerová D, Geryk J, Krchlíková V, Lejčková
B and Hejnar J: Precise CRISPR/Cas9 editing of the NHE1 gene
renders chickens resistant to the J subgroup of avian leukosis
virus. Proc Natl Acad Sci USA. 117:2108–2112. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reshkin SJ, Cardone RA and Harguindey S:
Na+-H+ exchanger, pH regulation and cancer.
Recent Pat Anticancer Drug Discov. 8:85–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keurhorst D, Liashkovich I, Frontzek F,
Nitzlaff S, Hofschröer V, Dreier R and Stock C: MMP3 activity
rather than cortical stiffness determines NHE1-dependent
invasiveness of melanoma cells. Cancer Cell Int. 19:2852019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lucien F, Brochu-Gaudreau K, Arsenault D,
Harper K and Dubois CM: Hypoxia-induced invadopodia formation
involves activation of NHE-1 by the p90 ribosomal S6 kinase
(p90RSK). PLoS One. 6:e288512011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kato Y, Lambert CA, Colige AC, Mineur P,
Noël A, Frankenne F, Foidart JM, Baba M, Hata R, Miyazaki K and
Tsukuda M: Acidic extracellular pH induces matrix
metalloproteinase-9 expression in mouse metastatic melanoma cells
through the phospholipase D-mitogen-activated protein kinase
signaling. J Biol Chem. 280:10938–10944. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin Y, Chang G, Wang J, Jin W, Wang L, Li
H, Ma L, Li Q and Pang T: NHE1 mediates MDA-MB-231 cells invasion
through the regulation of MT1-MMP. Exp Cell Res. 317:2031–2040.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Poincloux R, Lizarraga F and Chavrier P:
Matrix invasion by tumour cells: A focus on MT1-MMP trafficking to
invadopodia. J Cell Sci. 122:3015–3024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caldieri G and Buccione R: Aiming for
invadopodia: Organizing polarized delivery at sites of invasion.
Trends Cell Biol. 20:64–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cougoule C, Carreno S, Castandet J,
Labrousse A, Astarie-Dequeker C, Poincloux R, Le Cabec V and
Maridonneau-Parini I: Activation of the lysosome-associated p61Hck
isoform triggers the biogenesis of podosomes. Traffic. 6:682–694.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stuart-Tilley A, Sardet C, Pouyssegur J,
Schwartz MA, Brown D and Alper SL: Immunolocalization of anion
exchanger AE2 and cation exchanger NHE-1 in distinct adjacent cells
of gastric mucosa. Am J Physiol. 266:C559–C568. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rossmann H, Sonnentag T, Heinzmann A,
Seidler B, Bachmann O, Vieillard-Baron D, Gregor M and Seidler U:
Differential expression and regulation of Na(+)/H(+) exchanger
isoforms in rabbit parietal and mucous cells. Am J Physiol
Gastrointest Liver Physiol. 281:G447–G458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bachmann O, Heinzmann A, Mack A, Manns MP
and Seidler U: Mechanisms of secretion-associated shrinkage and
volume recovery in cultured rabbit parietal cells. Am J Physiol
Gastrointest Liver Physiol. 292:G711–G717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niu YY, Yu PW, Tang B, Shi Y and Hao YX:
Expression of Na+-H+ exchanger 1 in human
gastric carcinoma tissue and its clinical significance. Zhonghua
Wei Chang Wai Ke Za Zhi. 13:604–607. 2010.(In Chinese). PubMed/NCBI
|
|
31
|
Liu HF, Teng XC, Zheng JC, Chen G and Wang
XW: Effect of NHE1 antisense gene transfection on the biological
behavior of SGC-7901 human gastric carcinoma cells. World J
Gastroenterol. 14:2162–2167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie R, Wang H, Jin H, Wen G, Tuo B and Xu
J: NHE1 is upregulated in gastric cancer and regulates gastric
cancer cell proliferation, migration and invasion. Oncol Rep.
37:1451–1460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu D and Qi J: Mechanisms of the
beneficial effect of NHE1 inhibitor in traumatic hemorrhage:
Inhibition of inflammatory pathways. Resuscitation. 83:774–781.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang X, Wang D, Dong W, Song Z and Dou K:
Over-expression of Na+/H+ exchanger 1 and its
clinicopathologic significance in hepatocellular carcinoma. Med
Oncol. 27:1109–1113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu J, Ji B, Wen G, Yang Y, Jin H, Liu X,
Xie R, Song W, Song P, Dong H and Tuo B:
Na+/H+ exchanger 1,
Na+/Ca2+ exchanger 1 and calmodulin complex
regulates interleukin 6-mediated cellular behavior of human
hepatocellular carcinoma. Carcinogenesis. 37:290–300. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang X, Wang D, Dong W, Song Z and Dou K:
Inhibition of Na(+)/H(+) exchanger 1 by 5-(N-ethyl-N-isopropyl)
amiloride reduces hypoxia-induced hepatocellular carcinoma invasion
and motility. Cancer Lett. 295:198–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang X, Wang D, Dong W, Song Z and Dou K:
Suppression of Na+/H + exchanger 1 by RNA
interference or amiloride inhibits human hepatoma cell line
SMMC-7721 cell invasion. Med Oncol. 28:385–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu L, Fukumura D and Jain RK: Acidic
extracellular pH induces vascular endothelial growth factor (VEGF)
in human glioblastoma cells via ERK1/2 MAPK signaling pathway:
Mechanism of low pH-induced VEGF. J Biol Chem. 277:11368–11374.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hardonnière K, Saunier E, Lemarié A,
Fernier M, Gallais I, Héliès-Toussaint C, Mograbi B, Antonio S,
Bénit P, Rustin P, et al: The environmental carcinogen
benzo[a]pyrene induces a Warburg-like metabolic reprogramming
dependent on NHE1 and associated with cell survival. Sci Rep.
6:307762016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang J, Tian L, Khan MN, Zhang L, Chen Q,
Zhao Y, Yan Q, Fu L and Liu J: Ginsenoside Rg3 sensitizes hypoxic
lung cancer cells to cisplatin via blocking of NF-κB mediated
epithelial-mesenchymal transition and stemness. Cancer Lett.
415:73–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim SW, Cha MJ, Lee SK, Song BW, Jin X,
Lee JM, Park JH and Lee JD: Curcumin treatment in combination with
glucose restriction inhibits intracellular alkalinization and tumor
growth in hepatoma cells. Int J Mol Sci. 20:23752019. View Article : Google Scholar
|
|
42
|
Li T and Tuo B: Pathophysiology of hepatic
Na+/H+ exchange (Review). Exp Ther Med.
20:1220–1229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roussa E, Alper SL and Thévenod F:
Immunolocalization of anion exchanger AE2, Na(+)/H(+) exchangers
NHE1 and NHE4, and vacuolar type H(+)-ATPase in rat pancreas. J
Histochem Cytochem. 49:463–474. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee MG, Ahn W, Choi JY, Luo X, Seo JT,
Schultheis PJ, Shull GE, Kim KH and Muallem S: Na(+)-dependent
transporters mediate HCO(3)(−) salvage across the luminal membrane
of the main pancreatic duct. J Clin Invest. 105:1651–1658. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brown DA, Melvin JE and Yule DI: Critical
role for NHE1 in intracellular pH regulation in pancreatic acinar
cells. Am J Physiol Gastrointest Liver Physiol. 285:G804–G812.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cardone RA, Greco MR, Zeeberg K,
Zaccagnino A, Saccomano M, Bellizzi A, Bruns P, Menga M, Pilarsky
C, Schwab A, et al: A novel NHE1-centered signaling cassette drives
epidermal growth factor receptor-dependent pancreatic tumor
metastasis and is a target for combination therapy. Neoplasia.
17:155–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang H, Cai J, Du S, Wei W and Shen X:
LAMC2 modulates the acidity of microenvironments to promote
invasion and migration of pancreatic cancer cells via regulating
AKT-dependent NHE1 activity. Exp Cell Res. 391:1119842020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Siddique I and Khan I: Regulation of Na/H
exchanger-1 in gastroesophageal reflux disease: Possible
interaction of histamine receptor. Dig Dis Sci. 48:1832–1838. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fujiwara Y, Higuchi K, Takashima T,
Hamaguchi M, Hayakawa T, Tominaga K, Watanabe T, Oshitani N,
Shimada Y and Arakawa T: Roles of epidermal growth factor and
Na+/H+ exchanger-1 in esophageal epithelial
defense against acid-induced injury. Am J Physiol Gastrointest
Liver Physiol. 290:G665–G673. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rastogi A, Puli S, El-Serag HB, Bansal A,
Wani S and Sharma P: Incidence of esophageal adenocarcinoma in
patients with Barrett's esophagus and high-grade dysplasia: A
meta-analysis. Gastrointest Endosc. 67:394–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Goldman A, Shahidullah M, Goldman D,
Khailova L, Watts G, Delamere N and Dvorak K: A novel mechanism of
acid and bile acid-induced DNA damage involving
Na+/H+ exchanger: Implication for Barrett's
oesophagus. Gut. 59:1606–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guan B, Hoque A and Xu X: Amiloride and
guggulsterone suppression of esophageal cancer cell growth in vitro
and in nude mouse xenografts. Front Biol (Beijing). 9:75–81. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ariyoshi Y, Shiozaki A, Ichikawa D,
Shimizu H, Kosuga T, Konishi H, Komatsu S, Fujiwara H, Okamoto K,
Kishimoto M, et al: Na+/H+ exchanger 1 has
tumor suppressive activity and prognostic value in esophageal
squamous cell carcinoma. Oncotarget. 8:2209–2223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim YS, Yi BR, Kim NH and Choi KC: Role of
the epithelial-mesenchymal transition and its effects on embryonic
stem cells. Exp Mol Med. 46:e1082014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bourguignon LY, Singleton PA, Diedrich F,
Stern R and Gilad E: CD44 interaction with
Na+-H+ exchanger (NHE1) creates acidic
microenvironments leading to hyaluronidase-2 and cathepsin B
activation and breast tumor cell invasion. J Biol Chem.
279:26991–27007. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gatenby RA, Smallbone K, Maini PK, Rose F,
Averill J, Nagle RB, Worrall L and Gillies RJ: Cellular adaptations
to hypoxia and acidosis during somatic evolution of breast cancer.
Br J Cancer. 97:646–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cardone RA, Casavola V and Reshkin SJ: The
role of disturbed pH dynamics and the Na+/H+
exchanger in metastasis. Nat Rev Cancer. 5:786–795. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Itoh Y and Seiki M: MT1-MMP: A potent
modifier of pericellular microenvironment. J Cell Physiol. 206:1–8.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Beaty BT, Wang Y, Bravo-Cordero JJ, Sharma
VP, Miskolci V, Hodgson L and Condeelis J: Talin regulates
moesin-NHE-1 recruitment to invadopodia and promotes mammary tumor
metastasis. J Cell Biol. 205:737–751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Greco MR, Antelmi E, Busco G, Guerra L,
Rubino R, Casavola V, Reshkin SJ and Cardone RA: Protease activity
at invadopodial focal digestive areas is dependent on NHE1-driven
acidic pHe. Oncol Rep. 31:940–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pedraz-Cuesta E, Fredsted J, Jensen HH,
Bornebusch A, Nejsum LN, Kragelund BB and Pedersen SF: Prolactin
signaling stimulates invasion via Na(+)/H(+) exchanger NHE1 in T47D
human breast cancer cells. Mol Endocrinol. 30:693–708. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li X, Quan S, Corsiatto T and Fliegel L:
Acidic residues of extracellular loop 3 of the
Na+/H+ exchanger type 1 are important in
cation transport. Mol Cell Biochem. 468:13–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Onishi I, Lin PJ, Numata Y, Austin P,
Cipollone J, Roberge M, Roskelley CD and Numata M: Organellar
(Na+, K+)/H+ exchanger NHE7
regulates cell adhesion, invasion and anchorage-independent growth
of breast cancer MDA-MB-231 cells. Oncol Rep. 27:311–317.
2012.PubMed/NCBI
|
|
65
|
Rolver MG, Elingaard-Larsen LO, Andersen
AP, Counillon L and Pedersen SF: Pyrazine ring-based
Na+/H+ exchanger (NHE) inhibitors potently
inhibit cancer cell growth in 3D culture, independent of NHE1. Sci
Rep. 10:58002020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dudeja PK, Hafez N, Tyagi S, Gailey CA,
Toofanfard M, Alrefai WA, Nazir TM, Ramaswamy K and Al-Bazzaz FJ:
Expression of the Na+/H+ and Cl-/HCO-3
exchanger isoforms in proximal and distal human airways. Am J
Physiol. 276:L971–L978. 1999.PubMed/NCBI
|
|
67
|
Al-Bazzaz FJ, Hafez N, Tyagi S, Gailey CA,
Toofanfard M, Alrefai WA, Nazir TM, Ramaswamy K and Dudeja PK:
Detection of Cl-HCO3- and Na+-H+
exchangers in human airways epithelium. JOP. 2 (Suppl 4):S285–S290.
2001.
|
|
68
|
Rios EJ, Fallon M, Wang J and Shimoda LA:
Chronic hypoxia elevates intracellular pH and activates
Na+/H+ exchange in pulmonary arterial smooth
muscle cells. Am J Physiol Lung Cell Mol Physiol. 289:L867–L874.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang GZ, Huang GJ, Li WL, Wu GM and Qian
GS: Effect of co-inhibition of MCT1 gene and NHE1 gene on
proliferation and growth of human lung adenocarcinoma cells. Ai
Zheng. 21:719–723. 2002.(In Chinese). PubMed/NCBI
|
|
70
|
Wu GM, Qian GS, Huang GJ, Li SP, Yao W and
Lu JY: The effect of Na(+)/H(+) exchanger-1 (NHE-1) antisense
expression vector on NHE-1 gene expression in human lung
adenocarcinoma cells and its biological significance. Zhonghua Jie
He He Hu Xi Za Zhi. 27:191–194. 2004.(In Chinese). PubMed/NCBI
|
|
71
|
Li S, Bao P, Li Z, Ouyang H, Wu C and Qian
G: Inhibition of proliferation and apoptosis induced by a
Na+/H+ exchanger-1 (NHE-1) antisense gene on
drug-resistant human small cell lung cancer cells. Oncol Rep.
21:1243–1249. 2009.PubMed/NCBI
|
|
72
|
Pizzonia JH, Ransom BR and Pappas CA:
Characterization of Na+/H+ exchange activity
in cultured rat hippocampal astrocytes. J Neurosci Res. 44:191–198.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cong D, Zhu W, Kuo JS, Hu S and Sun D: Ion
transporters in brain tumors. Curr Med Chem. 22:1171–1181. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wu KL, Khan S, Lakhe-Reddy S, Jarad G,
Mukherjee A, Obejero-Paz CA, Konieczkowski M, Sedor JR and
Schelling JR: The NHE1 Na+/H+ exchanger
recruits ezrin/radixin/moesin proteins to regulate Akt-dependent
cell survival. J Biol Chem. 279:26280–26286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
McHardy LM, Sinotte R, Troussard A,
Sheldon C, Church J, Williams DE, Andersen RJ, Dedhar S, Roberge M
and Roskelley CD: The tumor invasion inhibitor dihydromotuporamine
C activates RHO, remodels stress fibers and focal adhesions, and
stimulates sodium-proton exchange. Cancer Res. 64:1468–1474. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Paradiso A, Cardone RA, Bellizzi A,
Bagorda A, Guerra L, Tommasino M, Casavola V and Reshkin SJ: The
Na+-H+ exchanger-1 induces cytoskeletal
changes involving reciprocal RhoA and Rac1 signaling, resulting in
motility and invasion in MDA-MB-435 cells. Breast Cancer Res.
6:R616–R628. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
77
|
Reshkin SJ, Bellizzi A, Albarani V, Guerra
L, Tommasino M, Paradiso A and Casavola V: Phosphoinositide
3-kinase is involved in the tumor-specific activation of human
breast cancer cell Na(+)/H(+) exchange, motility, and invasion
induced by serum deprivation. J Biol Chem. 275:5361–5369. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Charles NA, Holland EC, Gilbertson R,
Glass R and Kettenmann H: The brain tumor microenvironment. Glia.
59:1169–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhu W, Carney KE, Pigott VM, Falgoust LM,
Clark PA, Kuo JS and Sun D: Glioma-mediated microglial activation
promotes glioma proliferation and migration: Roles of
Na+/H+ exchanger isoform 1. Carcinogenesis.
37:839–851. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Attaphitaya S, Park K and Melvin JE:
Molecular cloning and functional expression of a rat
Na+/H+ exchanger (NHE5) highly expressed in
brain. J Biol Chem. 274:4383–4388. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kurata T, Rajendran V, Fan S, Ohta T,
Numata M and Fushida S: NHE5 regulates growth factor signaling,
integrin trafficking, and degradation in glioma cells. Clin Exp
Metastasis. 36:527–538. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Dudeja PK, Rao DD, Syed I, Joshi V, Dahdal
RY, Gardner C, Risk MC, Schmidt L, Bavishi D, Kim KE, et al:
Intestinal distribution of human Na+/H+
exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am J Physiol.
271:G483–G493. 1996.PubMed/NCBI
|
|
83
|
Khan I, Siddique I, Al-Awadi FM and Mohan
K: Role of Na+/H+ exchanger isoform-1 in
human inflammatory bowel disease. Can J Gastroenterol. 17:31–36.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zachos NC, Tse M and Donowitz M: Molecular
physiology of intestinal Na+/H+ exchange.
Annu Rev Physiol. 67:411–443. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Beltran AR, Ramirez MA, Carraro-Lacroix
LR, Hiraki Y, Reboucas NA and Malnic G: NHE1, NHE2, and NHE4
contribute to regulation of cell pH in T84 colon cancer cells.
Pflugers Arch. 455:799–810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Serafino A, Moroni N, Psaila R, Zonfrillo
M, Andreola F, Wannenes F, Mercuri L, Rasi G and Pierimarchi P:
Anti-proliferative effect of atrial natriuretic peptide on
colorectal cancer cells: Evidence for an Akt-mediated cross-talk
between NHE-1 activity and Wnt/β-catenin signaling. Biochim Biophys
Acta. 1822:1004–1018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Iorio J, Duranti C, Lottini T, Lastraioli
E, Bagni G, Becchetti A and Arcangeli A: KV11.1 potassium channel
and the Na+/H+ antiporter NHE1 modulate
adhesion-dependent intracellular pH in colorectal cancer cells.
Front Pharmacol. 11:8482020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rebillard A, Tekpli X, Meurette O, Sergent
O, LeMoigne-Muller G, Vernhet L, Gorria M, Chevanne M, Christmann
M, Kaina B, et al: Cisplatin-induced apoptosis involves membrane
fluidification via inhibition of NHE1 in human colon cancer cells.
Cancer Res. 67:7865–7874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Vaish V and Sanyal SN: Role of Sulindac
and Celecoxib in chemoprevention of colorectal cancer via intrinsic
pathway of apoptosis: Exploring NHE-1, intracellular calcium
homeostasis and Calpain 9. Biomed Pharmacother. 66:116–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lin Y, Wang J, Jin W, Wang L, Li H, Ma L,
Li Q and Pang T: NHE1 mediates migration and invasion of HeLa cells
via regulating the expression and localization of MT1-MMP. Cell
Biochem Funct. 30:41–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chiang Y, Chou CY, Hsu KF, Huang YF and
Shen MR: EGF upregulates Na+/H+ exchanger
NHE1 by post-translational regulation that is important for
cervical cancer cell invasiveness. J Cell Physiol. 214:810–819.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Loh SH, Tsai YT, Huang SF, Yu TC, Kuo PC,
Chao SC, Chou MF, Tsai CS and Lee SP: Effects of andrographolide on
intracellular pH regulation, cellular migration, and apoptosis in
human cervical cancer cells†. Cancers (Basel). 12:3872020.
View Article : Google Scholar
|
|
93
|
Gholami K, Muniandy S and Salleh N:
Differential expression of Na+/H+-exchanger
(NHE-1, 2, and 4) proteins and mRNA in rodent's uterus under sex
steroid effect and at different phases of the oestrous cycle.
Biomed Res Int. 2013:8401212013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ismail N, Giribabu N, Muniandy S and
Salleh N: Enhanced expression of sodium hydrogen exchanger (NHE)-1,
2 and 4 in the cervix of ovariectomised rats by phytoestrogen
genistein. Int J Med Sci. 12:468–477. 2015. View Article : Google Scholar : PubMed/NCBI
|