Introduction
Approximately 70% of breast carcinomas express
estrogen receptor (ER) and progesterone receptor (PR), and these
tumors are subclassified as luminal A (Lum A) or B (Lum B)
according to the rate of proliferation (1,2).
Endocrine therapy has become one of the most important treatments
for breast cancer patients. Tamoxifen is a selective ER modulator
that acts as an ER antagonist in the breast (2). The majority of patients with
ER+ breast cancer undergo adjuvant endocrine therapy but
~20–30% of them eventually experience recurrence with distant
metastasis (3). Therefore, despite
improvements in treatment, therapy resistance remains a major
clinical problem (3,4). Numerous researchers around the world
are aiming to identify novel targets to improve treatment
efficiency for endocrine therapy-resistant patients.
Platelet-derived growth factor (PDGF) is a critical
regulator of cell proliferation, migration and angiogenesis in
various cells (4). The PDGF family
consists of five isoforms (PDGF-AA, -BB, -AB, -CC, and -DD) and
differentially binds to two receptor tyrosine kinases (RTKs),
PDGFRα and PDGFRβ (5). The different
receptors bind with the ligands with different affinities. PDGFRα
preferentially binds with PDGF-A, -B, and -C, whereas PDGFRβ binds
with PDGF-B and -D (6,7). Activated PDGFRα and β subsequently
trigger downstream signal transduction pathways, including
extracellular signal-regulated kinase 1/2 (ERK) and
phosphatidylinositol 3-kinase (PI3K)/AKT for promoting cell
proliferation, migration and survival (8). In particular, breast cancers with high
PDGFRα expression are associated with lymph node metastasis and
human epidermal growth factor receptor 2 (HER2) positivity
(9).
In the present study, the clinical significance of
PDGFB expression in ER+ breast cancer was investigated
and the pharmacological effects of two PDGFR inhibitors (sunitinib
and ponatinib) and/or tamoxifen in ER-α+ breast cancer
cells was also investigated.
Materials and methods
Reagents
Charcoal-stripped fetal bovine serum (FBS) was
purchased from Thermo Fisher Scientific Inc.. 4-hydroxytamoxifen
(4-OHT; the active metabolite of tamoxifen) was purchased from
Sigma-Aldrich (Merck KGaA). Anti-PDGFB (cat. no.sc-365805;
dilution, 1:1,000) and ER-α (cat. no. sc8002; dilution, 1:1,000)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
Anti-MMP-1 antibody (cat. no. ab137332; dilution, 1:1,000) was
purchased from Abcam. β-actin (cat. no. LF-PA0207; dilution,
1:2,000) antibody was purchased from Ab Frontier. Total (t)-,
phosphor (p)-ERK (cat. nos. 9102 (t); and 4370 (p); dilution,
1:1,000) and STAT3 (cat. nos. 4904 (t) and 9145 (p); dilution,
1:1,000) antibodies were purchased from Cell Signaling Technology,
Inc. Goat anti-rabbit (cat. no. sc-2004; dilution, 1:2,000) and
anti-mouse (cat. no. sc-2005; dilution, 1:2,000) IgG-HRP secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc.
Cell culture
Breast cancer ZR751, BT474 and T47D cell lines were
cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.),
supplemented with 10% fetal bovine serum (FBS; HyClone), 2 mM
glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin. Breast
cancer MCF-7 cells were grown in Dulbecco's modified Eagle's medium
(DMEM; Life Technologies; Thermo Fisher Scientific, Inc.) under the
same conditions. All the cells were maintained at 37°C in a
humidified incubator with 5% CO2. Cell culture medium
was collected to confirm the existence of mycoplasma. The absence
of mycoplasma was checked using the EZ-PCR Mycoplasma Test kit
(Biological Industries).
Analysis of public database
The prognostic value of PDGFB mRNA expression in
ER+ breast cancer was assessed according to DFS/DMFS
using the Kaplan-Meier plotter database (https://kmplot.com/analysis/index.php?p=service&cancer=breast)
(10). DFS (n=2061), DMFS (n=664)
and OS (n=548) were analyzed in patients with ER+ breast
cancer using Kaplan-Meier survival plots. Log-rank P-values and HRs
with 95% confidence intervals were determined on the webpage.
Western blotting
The cells were lyzed using PRO-PREP™ Protein
Extraction Solution (Intron Biotechnology, Inc.) and centrifuged
(16,100 × g for 15 min). The levels of protein expression were
assessed as previously described (11,12). In
brief, isolated proteins were dissolved in 5X sample buffer and
boiled for 5 min. An equal amount (30 µg/lane) of total protein was
electrophoresed in 10% SDS-PAGE gel. Separated proteins were
transferred onto PVDF membranes (GE Healthcare) and blocked with
10% skimmed milk in Tris-buffered saline with 0.01% Tween-20 (TBST)
buffer for 15 min at room temperature (RT). Blots were incubated
with anti-PDGFB, ER-α, PARP-1, pro-, cleaved-caspase-3 or β-actin
antibodies in 1% TBST buffer at 4°C overnight. Blots were washed
3–4 times in TBST and incubated with appropriate secondary
antibodies in TBST buffer for 1 h at RT. After 1 h, blots were
washed 3–4 times with TBST buffer. Protein expression bands were
visualized using the ECL™ Western Blotting Detection Reagent (GE
Healthcare).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extracted from human breast cancer cells
using TRIzol reagent (Thermo Fisher Scientific, Inc.) were used for
RT-qPCR analysis. In brief, 1 µg total RNA from each sample was
reverse transcribed (denaturation, 94°C for 30 sec; annealing 58°C
for 30 sec and extension 72°C for 45 sec) using a RevertAid First
Strand cDNA synthesis kit (Thermo Fisher Scientific, Inc.).
Alteration of gene expression was performed using SensiMix SYBR
kits (Bioline) and ABI PRISM 7900HT instrument (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Primer sequences were as follows:
human PDGFB (forward, 5′-CGAATGGTCACCCGAGTTTG-3′ and reverse,
5′-GAGATGCTGAGTGACCACTC-3′), human ER-α (forward,
5′-CGCTACTGTGCAGTGTGCAAT-3′ and reverse,
5′-CCTCACAGGACCAGACTCCATAA-3′) and GAPDH as an endogenous control
(forward, 5′-ATTGTTGCCATCAATGACCC-3′ and reverse,
5′-AGTAGAGGCAGGGATGT-3′). Thermocycling conditions were 95°C for 10
min, 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 15
sec. For data analysis, the raw threshold cycle
(CT) value was normalized to the housekeeping
gene for each sample to obtain ΔCT. Normalized
ΔCT was calibrated to control cell samples to
calculate ΔΔCT (13,14).
Colony-forming assays
MCF-7 and T47D breast cancer cells were plated onto
6-well tissue culture plates (2×103 cells/well) for the
colony formation assay. After 24 h, the cells were treated with 2
µM specific inhibitors (ponatinib and sunitinib; Selleck
Chemicals), followed by an additional incubation for 10 days.
Subsequently, the colonies were fixed in 10% ethanol for 5 min at
RT and stained with 0.01% crystal violet for 30 min at RT and
observed using a CK40 inverted microscope (magnification, ×20;
Olympus Corporation).
MTT assay
Breast cancer MCF-7 and T47D cell lines were plated
onto 96-well tissue culture plates (1×103 cells/well)
for the MTT assay. After 24 h, the cells were treated with
sunitinib or ponatinib at 0.3125–5.000 µM concentration at 37°C for
48 h. To analyze cell proliferation, equal volumes of serum-free
media and MTT solution were added into each well of 96-well tissue
culture plates. Following incubation at 37°C for 3 h, dimethyl
sulfoxide was added to completely dissolve the MTT formazan. The
optical density was read at 590 nm using a tunable microplate
reader (Spectra max 190; Molecular Devices, LLC).
Cell cycle analysis
Breast cancer MCF-7 cells (3×105 cells/60
mm dish) were seeded into each cell culture dish. Following
incubation for 24 h, cells were trypsinized and washed with
phosphate-buffered saline (PBS) twice. Following centrifugation
(524 × g for 5 min at RT), cells were resuspended in 1 ml PBS and
fixed in 70% ethanol for 20 min at RT. Fixed cells were centrifuged
at 524 ×g for 5 min and washed twice with PBS. The supernatant was
discarded and cell pellets were resuspended in 1 ml PBS with 100
µg/ml DNase-free RNase A (Biopure) and incubated for 30 min in a
37°C water bath. Next, 50 µg/ml propidium iodide (Sigma-Aldrich;
Merck KGaA) was added to the cell suspension and analyzed using the
FACSCalibur flow cytometer (Becton Dickinson and Company) (15).
Apoptosis assay
Apoptosis detection using Annexin V and 7-AAD was
performed according to the manufacturer's protocol (Biogems
Biotechnologies Inc.). As shown in Fig.
5, MCF-7 and T47D cell lines were cultured to 70% confluence,
and treated with 4-OHT and/or sunitinib or ponatinib at the
indicated concentration. After 48 h, cells were harvested and
washed twice with pre-cooled PBS and then resuspended in 1X Annexin
V binding buffer at a concentration of 1×106 cells/ml.
Next, 100 µl of this solution was mixed with 5 µl Annexin V and 5
µl 7-AAD for 15 min at room temperature in the dark. The mixed
solution was incubated at RT (25°C) in the dark for 15 min. Next,
400 µl Annexin V binding buffer was added to each tube. Analysis
was performed using the FACSCalibur flow cytometer and BD CellQuest
Pro software v.6 (Becton Dickinson and Company).
Statistical analysis
Statistical significance between two groups of data
was calculated using Student's t-test (two-tailed). One-way
analysis of variance and Dunnett's post hoc test were used for
comparisons among multiple groups. Statistical analysis was
performed using GraphPad Prism 8 software (GraphPad Software,
Inc.). Results are presented as the mean ± standard error of the
mean. All quoted P-values were two-tailed and P<0.05 was
considered to indicate a statistically significant difference.
Results
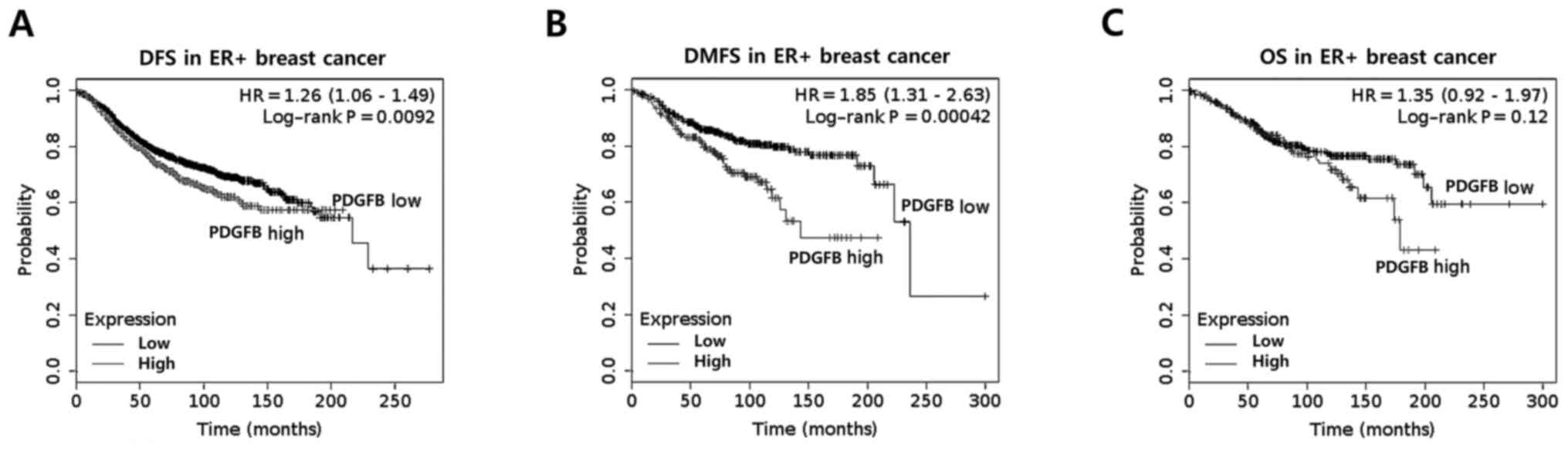
PDGFB expression is associated with
the poor prognosis of ER+ breast cancer
In a previous study, Paulsson et al (16) reported that high stromal PDGFRβ
expression was correlated with significantly shorter
recurrence-free and breast cancer-specific survival rates. In the
present study, the clinical significance of PDGFB expression in
ER+ breast cancer was analyzed using the Kaplan-Meier
method. It was identified that ER+ breast cancer with
high PDGFB expression had poorer disease-free survival (DFS) rates
(P=0.0092) and distant metastasis-free survival (DMFS) rates
(P=0.00042) than those with low PDGFB expression (Fig. 1A and B). However, overall survival
rates (OS; P=0.12) was not significantly different in
ER+ breast cancer (Fig.
1C). Based on these results, it was identified that the levels
of PDGFB expression have a significant impact on the survival rate
of patients with ER+ breast cancer.
Two PDGFR inhibitors induce G0-G1
phase cell cycle arrest and inhibit the growth of ER+
breast cancer cells
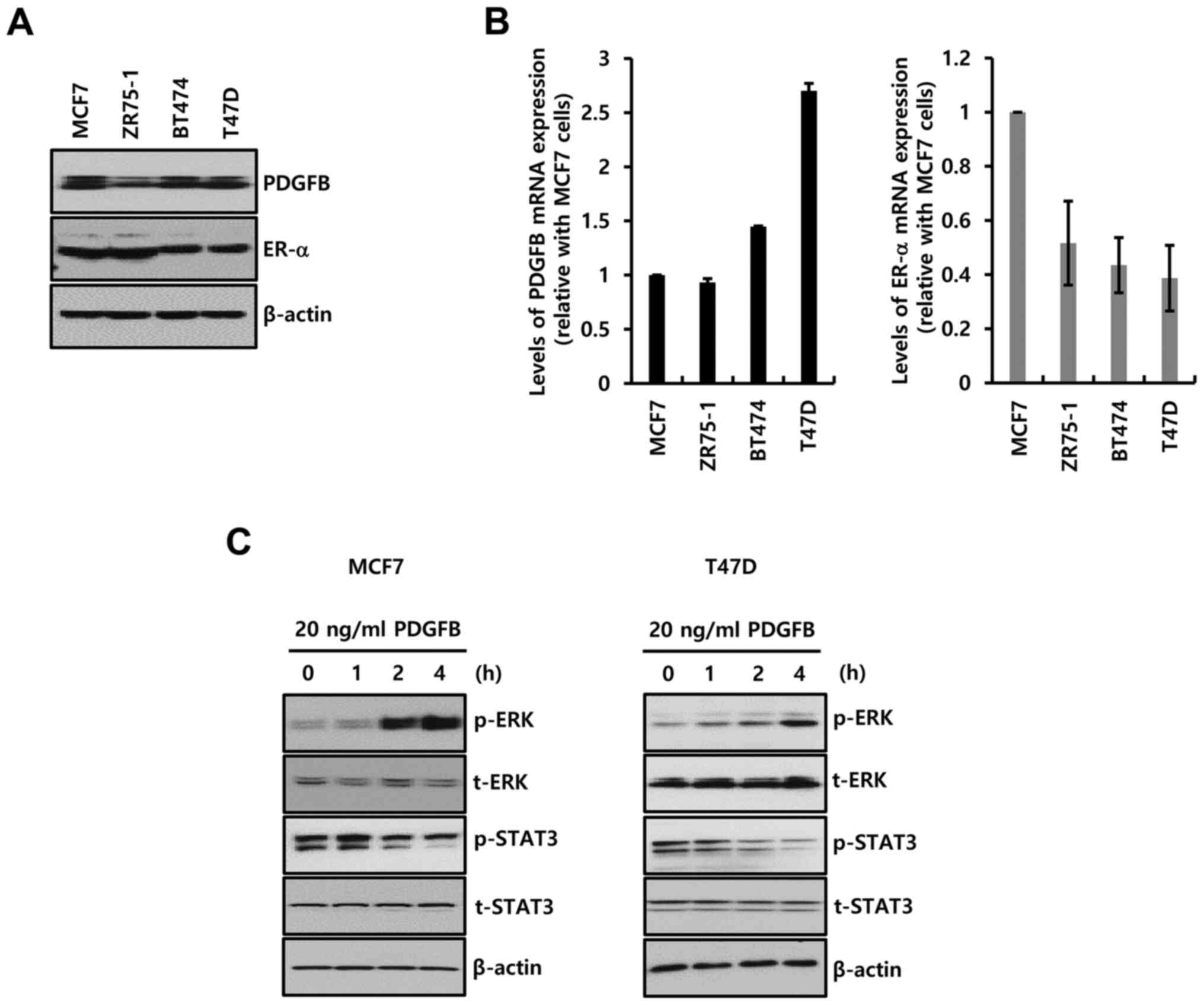
Four ER-α+ breast cancer cells were
selected to study the biological function of PDGFB. The
characteristics of these breast cancer cells are described in
Table I (17). The basal level of PDGFB protein
(Fig. 2A) and mRNA (Fig. 2B) expression was noted in all the
ER-α+ breast cancer cells.
 | Table I.Molecular subtypes of breast cancer
cell lines. |
Table I.
Molecular subtypes of breast cancer
cell lines.
| Cell lines | ER | PR | HER2 | Subtype | Tumor |
|---|
| MCF-7 | + | + | – | Luminal A | IDC |
| ZR75-1 | + | +/− | – | Luminal A | IDC |
| BT474 | + | + | + | Luminal B | IDC |
| T47D | + | + | – | Luminal A | IDC |
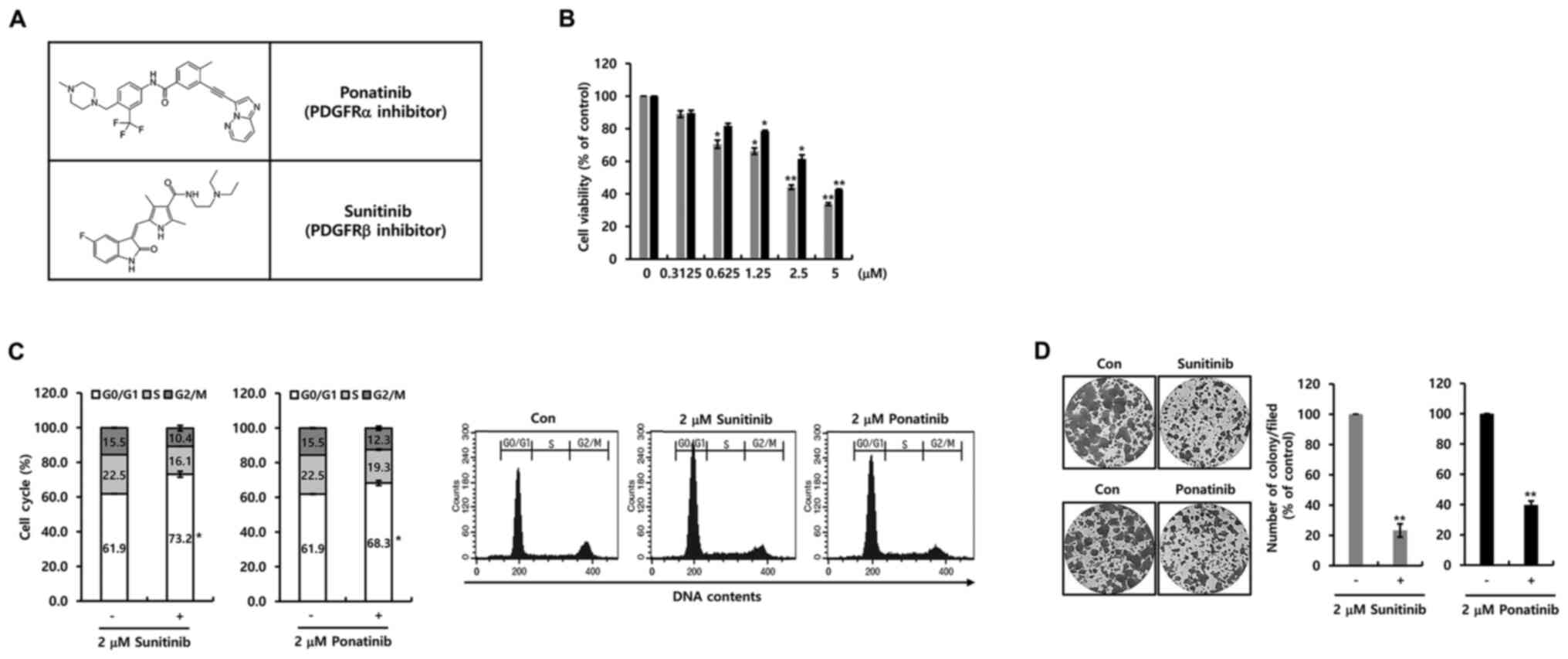
Next, the effect of two PDGFR inhibitors on cell
proliferation in breast cancer MCF-7 cells was investigated.
Fig. 3A demonstrates the structure
of ponatinib and sunitinib. As shown in Fig. 3B, cell viability was decreased by
ponatinib or sunitinib in a dose-dependent manner. The
IC50 value for sunitinib and ponatinib was 1.45 and 4.51
µM, respectively (Fig. 3B).
Furthermore, the influence of the two PDGFR inhibitors on the cell
cycle was investigated. Notable, the two inhibitors induced G0-G1
phase cell cycle arrest (Fig. 3C).
Additionally, cell proliferation was suppressed by 2 µM ponatinib
or sunitinib (Fig. 3D). Furthermore,
cell growth by specific PDGFR-α and PDGFR-β antibodies was
decreased (Fig. S1A). It was
evident that the two PDGFR inhibitors suppressed cell proliferation
through the G0/G1 phase cell cycle arrest.
Two PDGFR inhibitors suppress MMP-1
expression through the inhibition of STAT-3 and ERK pathway
Degradation and rearrangement of the extracellular
matrix by MMPs is a prerequisite for tumor invasion and metastasis
(18). Therefore, the effects of the
two PDGFR inhibitors on MMP-1 expression, which serves an important
role in cell migration and invasion, were investigated. Although
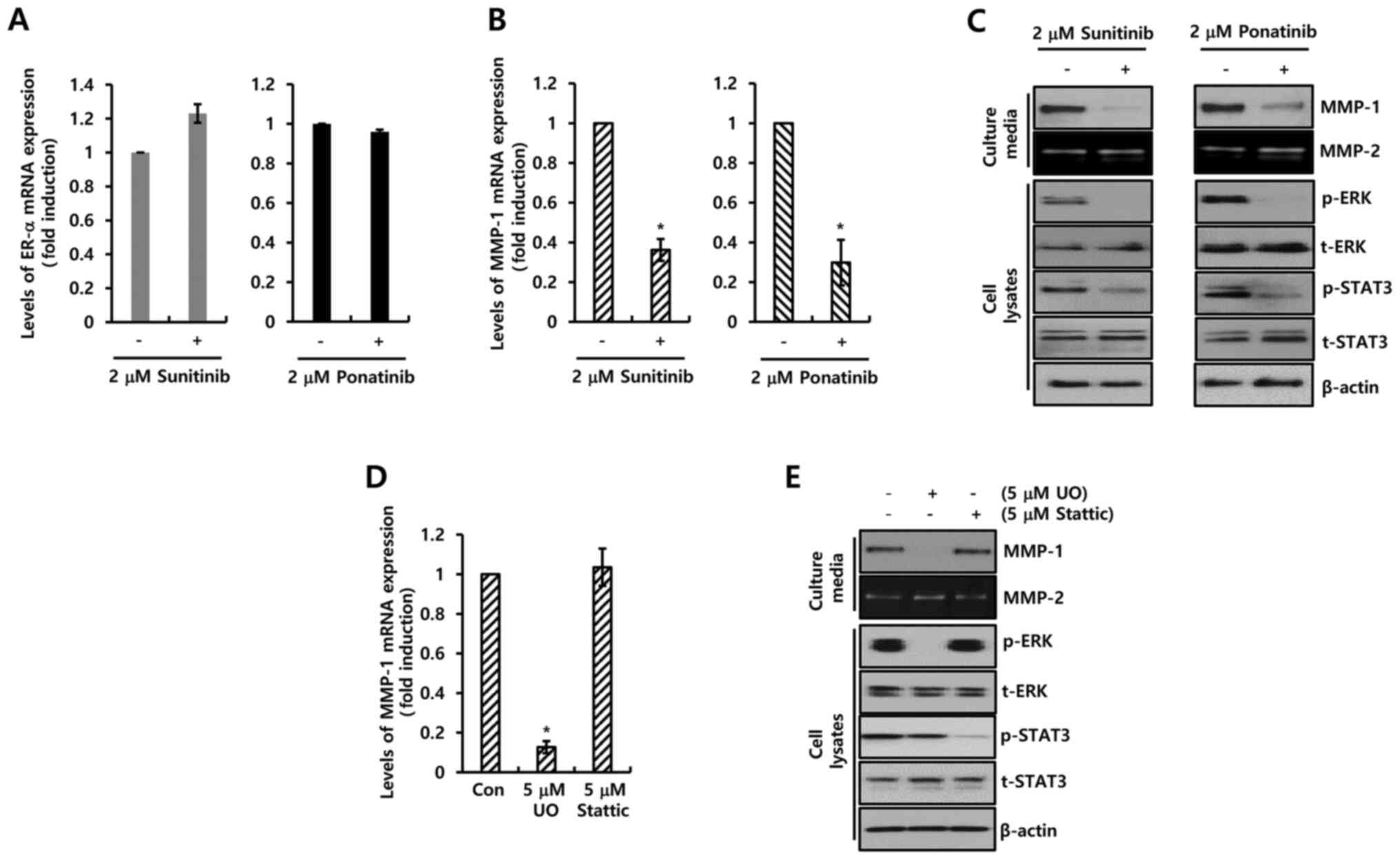
neither sunitinib nor ponatinib influenced the expression of ER-α
(Fig. 4A), the two PDGFR inhibitors
completely suppressed the expression of MMP-1 mRNA (Fig. 4B). Sunitinib (2 µM) decreased the
level of MMP-1 mRNA expression by 0.36±0.06-fold compared with the
control level, while ponatinib decreased the expression by
0.30±0.11-fold compared with the control level (Fig. 4B). Under similar conditions, the
expression level of MMP-1 protein was decreased by the two PDGFR
inhibitors (Fig. 4C). Furthermore,
the downstream signaling pathway of PDGFR was investigated. As
demonstrated in Fig. 4C, sunitinib
and ponatinib completely inhibited phosphorylation of STAT-3 and
ERK. Therefore, the MCF-7 cells were treated with specific
inhibitors (UO126, MEK inhibitor; Stattic, STAT-3 inhibitor) for 24
h. Levels of MMP-1 mRNA expression were significantly decreased by
UO126 treatment (Fig. 4D). In
addition, MMP-1 expression was analyzed using a specific PDGFR-β
antibody (Fig. S1B). As expected,
the basal level of MMP-1 expression was decreased by PDGFR-β
antibody treatment (Fig. S1B). The
results revealed that the two PDGFR inhibitors downregulated MMP-1
expression by inhibiting the MEK/ERK pathway.
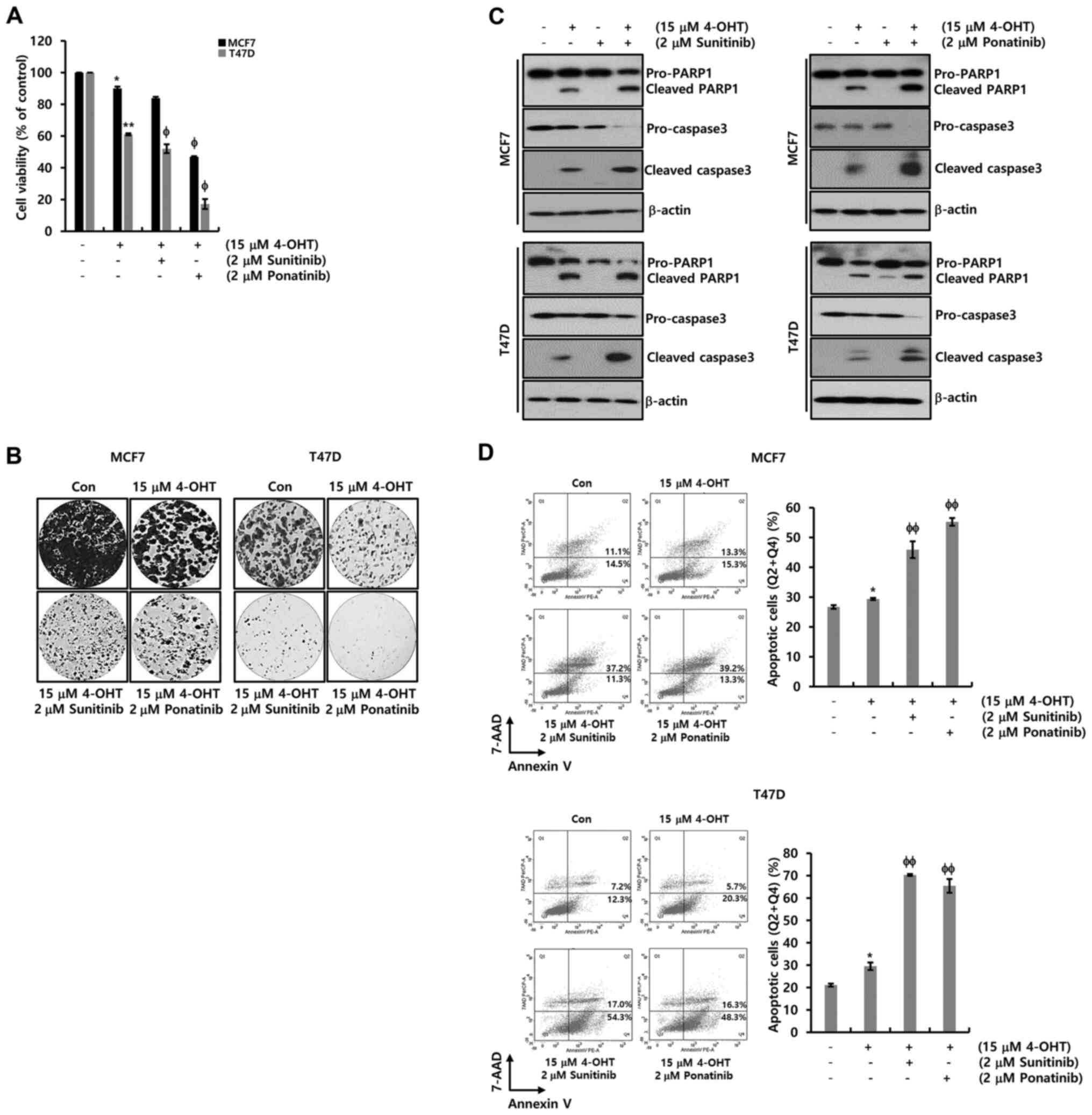
The combined effect of sunitinib or
ponatinib with tamoxifen
The combined effect of sunitinib or ponatinib with
tamoxifen on ER-α+ breast cancer cells was investigated.
Using conditioned media with charcoal-stripped FBS which removes
non-polar material such as growth factors, hormones and cytokines,
cells were treated with 4-OHT at the indicated concentration. As
shown in Fig. 5A, cell viability was
decreased by nearly half at a concentration of 15 µM 4-OHT. In
addition, when sunitinib or ponatinib were combined with 4-OHT,
cell death was accelerated in MCF-7 and T47D cells (Fig. 5A). These results were verified again
by the colony-forming assay (Fig.
5B). The colony size and number decreased significantly when
the drug was combined with sunitinib or ponatinib. Finally, the
expression levels of PARP-1 and procaspase-3 were measured for the
detection of apoptotic cell death in MCF-7 and T47D cells. The
cleaved forms of PARP-1 and caspase-3 increased in response to
treatment with 4-OHT, while a decrease in the levels of
pro-caspase-3 was observed (Fig.
5C). The cleaved forms of PARP-1 and caspase-3 were
significantly increased upon combined treatment of 4-OHT with
sunitinib or ponatinib while the levels of procaspase-3 were
decreased in MCF-7 and T47D cells (Fig.
5C). Furthermore, apoptotic cell death was analyzed using FACS.
As expected, when sunitinib or ponatinib were combined with 4-OHT,
apoptotic cell death (Q2, late apoptosis; Q4, early apoptosis) was
significantly increased in MCF-7 and T47D cells (Fig. 5D). Based on these results, it was
revealed that ER-α downregulation by EGFR ligands contributed
toward acquired tamoxifen resistance. Inhibition by the two PDGFR
inhibitors synergistically increased the pharmacological effects of
tamoxifen in ER-α+ breast cancer.
Discussion
At present, there is not yet a complete
understanding of the association between the expression of PDGFB
and the survival of patients with ER+ breast cancer. The
present study aimed to analyze the ER+ breast cancer
survival rate following the expression of PDGFB. In previous
studies, the prognosis of patients with low PDGF-BB improved the
progression-free survival and overall survival compared with that
of the others in numerous human tumors, including colorectal
cancer, pancreatic cancer, esophageal cancer and liver cancer
(19–22). Additionally, aberrant PDGFB
expression is associated with the vascular mechanism in 4T1 cancer
cells in vivo, but not with the direct proliferative
promotion of breast cancer cells (23). Consistent with these reports, the
survival rates of patients with ER+ breast cancer based
on PDGFB expression was analyzed using the Kaplan-Meier plotter.
Consequently, it was revealed that the expression of PDGFB is
directly associated with the DFS and DMFS in ER+ breast
cancer. Therefore, it was demonstrated that the downregulation of
PDGFB or the inhibition of the PDGFB/PDGFR signaling pathway may be
a novel strategy for ER+ breast cancer treatment.
PDGF-BB facilitates the stem-like characteristics of
OV6+ cancer stem-like cells (CSCs) by enhancing YAP
stability (24). PDGFB
downregulation by PDGFB shRNA suppresses cell proliferation and
invasion by blocking the PI3K/AKT pathway in esophageal squamous
cell carcinoma cells (21).
Autocrine PDGF/PDGFR signaling contributes toward the maintenance
of epithelial-mesenchymal transition (EMT) through the activation
of STAT1 in murine and human mammary carcinoma cell lines (25). Consistent with the published data,
the results of the present study demonstrated that the
phosphorylation of ERK and STAT3 is significantly decreased by the
two PDGFR inhibitors, ponatinib (PDGFR-α inhibitor) and sunitinib
(PDGFR-β inhibitor). MMP-1 expression, which serves an important
role in cell invasion and migration, was revealed to be completely
downregulated by the two inhibitors. In addition, these inhibitors
induced the G0/G1 phase arrest and suppressed cell proliferation in
ER+ breast cancer cells. Consequently, it was
demonstrated that accurate employment of sunitinib and ponatinib
will be of significance in curbing the growth and metastasis of
ER+ breast cancer.
Aberrant PDGFB induction decreases the sensitivity
to ionizing radiation in esophageal squamous cell carcinoma cells
and promotes the cis-platinum resistance of OV6+
CSCs in bladder cancer (21,24). PDGF-BB enhances c-myc expression and
decreases the melphalan sensitivity of multiple myeloma (26). In addition, Wang et al
(23) reported that metformin
greatly decreases PDGFB protein levels and improves the
chemosensitivity against cyclophosphamide in 4T1 cells in
vivo. PDGFB/PDGFRβ axis is involved in imparting resistance to
antiangiogenic therapy in renal cancer (27). Although sunitinib and ponatinib did
not affect apoptotic cell death and ER-α expression, it was evident
that combined treatment of tamoxifen with the inhibitors was more
effective than the treatment with tamoxifen alone in ER+
breast cancer cells. Additionally, the present study could have
aimed to verify the pharmacological effects of tamoxifen and/or
sunitinib or ponatinib through normal breast cells. However, only
the effectiveness of breast cancer cells was verified as no normal
breast cells were available. It is revealed that the blockage of
the PDGFB/PDGFR axis is one of the alternatives to overcome
tamoxifen resistance.
In conclusion, the clinical significance of PDGFB
expression and the pharmacological effect of PDGFR inhibitors was
investigated in ER+ breast cancer cells. It was
demonstrated that PDGFB is one of the factors that greatly affect
the survival rate in ER+ breast cancers. Ponatinib and
sunitinib induced cell cycle arrest and completely suppressed cell
proliferation. Furthermore, it was observed that the combined
therapy of tamoxifen with PDGFR inhibitors induced effectual cell
death than treatment with tamoxifen alone. Consequently, based on
these findings, it was suggested that the possibility of
combination treatment employing PDGFR inhibitors as an effective
treatment strategy for ER+ breast cancer in the
future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
2016R1D1A1B01010508) and was supported by the MSIT (Ministry of
Science and ICT), Korea, under the ICT Creative Consilience program
(grant no. IITP-2020-0-01821) supervised by the IITP (Institute for
Information & communications Technology Planning &
Evaluation).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SK and JEL contributed to the experiment design,
analyzed the results and wrote the manuscript. SK, DY, YJ, SYY, and
SAK performed the experiments and analyzed the results. SK, DY, YJ,
SYY, SAK and JEL confirmed the authenticity of the raw data. All
authors have read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osborne CK: Tamoxifen in the treatment of
breast cancer. N Engl J Med. 339:1609–1618. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fredriksson L, Li H and Eriksson U: The
PDGF family: Four gene products form five dimeric isoforms.
Cytokine Growth Factor Rev. 15:197–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostman A: PDGF receptors-mediators of
autocrine tumor growth and regulators of tumor vasculature and
stroma. Cytokine Growth Factor Rev. 15:275–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergsten E, Uutela M, Li X, Pietras K,
Ostman A, Heldin CH, Alitalo K and Eriksson U: PDGF-D is a
specific, protease-activated ligand for the PDGF beta-receptor. Nat
Cell Biol. 3:512–516. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kazlauskas A: PDGFs and their receptors.
Gene. 614:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heldin CH, Ostman A and Rönnstrand L:
Signal transduction via platelet-derived growth factor receptors.
Biochim Biophys Acta. 1378:F79–F113. 1998.PubMed/NCBI
|
|
9
|
Carvalho I, Milanezi F, Martins A, Reis RM
and Schmitt F: Overexpression of platelet-derived growth factor
receptor alpha in breast cancer is associated with tumour
progression. Breast Cancer Res. 7:R788–R795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Györffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim S, You D, Jeong Y, Yu J, Kim SW, Nam
SJ and Lee JE: Berberine down-regulates IL-8 expression through
inhibition of the EGFR/MEK/ERK pathway in triple-negative breast
cancer cells. Phytomedicine. 50:43–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeon M, Han J, Nam SJ, Lee JE and Kim S:
Elevated IL-1β expression induces invasiveness of triple negative
breast cancer cells and is suppressed by zerumbone. Chem Biol
Interact. 258:126–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosaka K, Yang Y, Seki T, Fischer C, Dubey
O, Fredlund E, Hartman J, Religa P, Morikawa H, Ishii Y, et al:
Pericyte-fibroblast transition promotes tumor growth and
metastasis. Proc Natl Acad Sci USA. 113:E5618–E5627. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim S, Lee J, Oh SJ, Nam SJ and Lee JE:
Differential effect of EGFR inhibitors on tamoxifen-resistant
breast cancer cells. Oncol Rep. 34:1613–1619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim S, Lee J, Jeon M, Nam SJ and Lee JE:
Elevated TGF-β1 and -β2 expression accelerates the epithelial to
mesenchymal transition in triple-negative breast cancer cells.
Cytokine. 75:151–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paulsson J, Sjöblom T, Micke P, Pontén F,
Landberg G, Heldin CH, Bergh J, Brennan DJ, Jirström K and Ostman
A: Prognostic significance of stromal platelet-derived growth
factor beta-receptor expression in human breast cancer. Am J
Pathol. 175:334–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai X, Cheng H, Bai Z and Li J: Breast
Cancer Cell Line Classification and Its Relevance with Breast Tumor
Subtyping. J Cancer. 8:3131–3141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCarty MF, Somcio RJ, Stoeltzing O, Wey
J, Fan F, Liu W, Bucana C and Ellis LM: Overexpression of PDGF-BB
decreases colorectal and pancreatic cancer growth by increasing
tumor pericyte content. J Clin Invest. 117:2114–2122. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura Y, Tanaka F, Yoshikawa Y, Mimori
K, Inoue H, Yanaga K and Mori M: PDGF-BB is a novel prognostic
factor in colorectal cancer. Ann Surg Oncol. 15:2129–2136. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Er P, Qian D, Zhang W, Zhang B, Wei H,
Zhang T, Chen X, Wang Y, Zhao J, Wang Q, et al: The expression of
PDGF-BB predicts curative effect in locally advanced esophageal
squamous cell carcinoma treated by radiotherapy. Aging (Albany NY).
12:6586–6599. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fingas CD, Bronk SF, Werneburg NW, Mott
JL, Guicciardi ME, Cazanave SC, Mertens JC, Sirica AE and Gores GJ:
Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling
in cholangiocarcinoma cells. Hepatology. 54:2076–2088. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang JC, Li GY, Wang B, Han SX, Sun X,
Jiang YN, Shen YW, Zhou C, Feng J, Lu SY, et al: Metformin inhibits
metastatic breast cancer progression and improves chemosensitivity
by inducing vessel normalization via PDGF-B downregulation. J Exp
Clin Cancer Res. 38:2352019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang KJ, Wang C, Dai LH, Yang J, Huang H,
Ma XJ, Zhou Z, Yang ZY, Xu WD, Hua MM, et al: Targeting an
Autocrine Regulatory Loop in Cancer Stem-like Cells Impairs the
Progression and Chemotherapy Resistance of Bladder Cancer. Clin
Cancer Res. 25:1070–1086. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jechlinger M, Sommer A, Moriggl R, Seither
P, Kraut N, Capodiecci P, Donovan M, Cordon-Cardo C, Beug H and
Grünert S: Autocrine PDGFR signaling promotes mammary cancer
metastasis. J Clin Invest. 116:1561–1570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Greco C, D'Agnano I, Vitelli G, Vona R,
Marino M, Mottolese M, Zuppi C, Capoluongo E and Ameglio F: c-MYC
deregulation is involved in melphalan resistance of multiple
myeloma: Role of PDGF-BB. Int J Immunopathol Pharmacol. 19:67–79.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cumpănas AA, Cimpean AM, Ferician O,
Ceausu RA, Sarb S, Barbos V, Dema A and Raica M: The Involvement of
PDGF-B/PDGFRβ Axis in the Resistance to Antiangiogenic and
Antivascular Therapy in Renal Cancer. Anticancer Res. 36:2291–2295.
2016.PubMed/NCBI
|