Introduction
Multiple myeloma (MM) is a cancer of the plasma
cells, which presents as anemia, kidney function damage, bone
disease and extramedullary plasmacytoma (1,2). Novel
drugs have improved the outcome of patients with MM; however,
almost all patients with MM relapse and become resistant to drugs
(3,4). Thus, it is of great importance to
develop novel drugs that can overcome resistance. Tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) is a member of the
TNF superfamily and can specifically induce apoptosis of myeloma
cells. Furthermore, it has no cytotoxic effects on hematopoietic
stem cells and normal tissue cells (5). Beijing Sunbio Biotech Co., Ltd. has
developed a recombinant version of TRAIL termed circularly permuted
TRAIL (CPT). It has been shown that CPT exhibits potent antitumor
activity in vivo and in vitro, but no effects on
normal human cells (6). Compared
with wild-type TRAIL, CPT exhibits improved stability and antitumor
activity without significant toxic effects. CPT is a novel
anti-myeloma drug prototype which has entered phase III clinical
trials. In the previous phase II trials, CPT was shown to be
effective as a treatment for MM, but there were some patients who
exhibited resistance to CPT (7,8). Thus,
there is an urgent need to find novel drugs that can improve
sensitivity of myeloma cells to CPT. Cyclopamine is the major
Hedgehog signal pathway inhibitor and can induce apoptosis in
certain types of cancer cells. Studies have shown that it exhibits
synergistic therapeutic effects when combined with other anticancer
drugs (9–12).
In the present study, two myeloma cell lines,
RPMI-8266 and SKO-007, were used to evaluate the synergistic
effects of cyclopamine and CPT on the proliferation and apoptosis
of MM cells. It was shown that SKO-007 cells were resistant to both
cyclopamine and CPT. However, the combination of cyclopamine and
CPT exhibited a synergistic effect of inhibition of proliferation
and induction of apoptosis in myeloma cells.
Materials and methods
Cells and reagents
The human myeloma cell lines RPMI-8226 and SKO-007
were obtained from Beijing Sunbio Biotech, Co., Ltd, and cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
glutamine (2 mmol/l), penicillin (100 IU/ml) and streptomycin (100
Ag/ml), in a cell incubator containing 5% CO2 at 37°C.
CPT was obtained from Beijing Sunbio Biotech, Co., Ltd.,
cyclopamine was purchased from Selleck Chemicals and the Cell
Counting Kit-8 (CCK-8) assay kit was purchased from Sigma-Aldrich;
Merck KGaA. An Annexin V-FITC apoptosis detection kit, Hoechst
33342 and verapamil were purchased from Sigma-Aldrich; Merck KGaA.
Propidium iodide (PI) was purchased from BD Biosciences. Other
reagents were purchased from Beijing Chemical Reagents Company,
unless otherwise stated.
Cell growth inhibition assay
To determine the effects of cyclopamine and CPT on
the proliferation of myeloma cells, cells (2×104
cells/well) in the logarithmic growth phase were seeded in a
96-well microtiter plate in a total volume of 100 µl. Cells were
incubated in an incubator at 37°C with 5% CO2 for 24 h.
The following day, medium containing increasing concentrations of
cyclopamine and CPT were added. At different time intervals, the
cell viability was measured using a CCK-8 kit assay according to
the manufacturer's protocol. The proliferation inhibition rate was
calculated using the following formula: Inhibition rate (%) = A490
(drug)/A490 (control) ×100%.
Assessment of apoptosis
Cells in the logarithmic growth phase were
trypsinized and a cell suspension of 1×105 cells/ml was
prepared. The combination of 10 µmol/l cyclopamine combined and 100
ng/ml CPT was selected because the maximum concentration of
cyclopamine that we can get was 10 µmol/l and 100 ng/ml CPT had a
better inhibition effect at lower concentration. Different
treatment times for the two drugs were selected because cyclopamine
had relatively weak anti-myeloma effect and need more time to
induce apoptosis compared to CPT according to the growth inhibition
assay results. A total of 1×105 cells were seeded in
6-well plates in a total volume of 3 ml and incubated for 24 h. The
following day, media containing 10 µmol/l cyclopamine was added for
24 h, and then media containing 100 ng/ml CPT was added for 96 h.
All cells were harvested and cell apoptosis was determined using
flow cytometry following Annexin V-FITC and PI staining, which was
performed according to the manufacturer's protocols. The
percent-specific cell apoptosis was calculated as follows:
Non-apoptotic cells, Annexin V-negative and PI-negative; early
apoptotic cells, Annexin V-positive and PI-negative; and necrotic
cells or late apoptotic cells, Annexin V-positive and
PI-positive.
Side population (SP) analysis
Cells in the logarithmic growth phase were
trypsinized to prepare a suspension of cells (1×105/ml).
The cells were treated with CPT for 48 h, since the SP cell ratio
had more significant changes at this time point according to
previous result. Cells were seeded in 6-well plates in a total
volume of 3 ml media and incubated in a 5% CO2 incubator
at 37°C for 24 h. The following day, medium containing 10 µmol/l
cyclopamine was added and cells were incubated for a further 72 h,
and then medium containing 100 ng/ml CPT was added for 48 h.
Subsequently, all cells were harvested and washed with PBS, and
suspended at a density of 1×106 cells/ml in culture
medium containing 2% FBS. SKO-007 cells were incubated with Hoechst
33342 dye at a final concentration of 5 µg/ml with or without
verapamil (100 µmol/l;) at 37°C for 90 min with intermittent
shaking every 15 min. Cells preincubated with 100 µmol/l verapamil
were used as the control group. PI (2 µg/ml) was added to label and
exclude dead cells. SP cells that were not stained using Hoechst
33342 were counted using BD FACSAria SORP cytometer (BD
Biosciences) and the data were analyzed with BD FACSDiva v.8.0.1
software (BD Biosciences).
Gene expression analysis
Cells in the logarithmic growth phase were
trypsinized to prepare a cell suspension (1×105
cells/ml). Cells were seeded in 6-well plates in a total volume of
3 ml media and incubated in a 5% CO2 incubator at 37°C
for 24 h. The following day, medium containing 10 µmol/l
cyclopamine was added for 48 h, and then medium containing 100
ng/ml CPT was added for 24 h. All cells were harvested, and
TRIzol® (Invitrogen; Thermo Fisher Scientific Inc.) was
used to isolate total RNA. A total amount of 2 µg RNA per sample
was used for cDNA synthesis with a WCGENE® miRNA cDNA
kit according to the manufacturer's protocol (Wcgene Biotech, Co.,
Ltd.). The gene expression levels of the genes of interest were
quantified using WCGENE® miRNA qPCR mix (Wcgene Biotech,
Co., Ltd.) according to the manufacturer's protocol. The sequences
of the primers used in the PCR array analysis are listed in
Table SI. β-actin and GAPDH were
used as the endogenous loading controls. The relative gene
expression levels of target genes were calculated using the
2−ΔΔCq method (13).
Statistical analysis
Data are presented as the mean ± standard deviation
of three repeats. Statistical analysis was performed using a
one-way ANOVA test with post hoc contrasts by SNK test. Jin's
formula: q=Ea + Eb/(Ea + Eb-Ea × Eb), was used to evaluate whether
two drugs had a synergistic effect. Ea + Eb represents the
inhibition ratio of the combination group and Ea and Eb represented
respectively the inhibition ratios of simple drug a and simple
group b. If the calculated q value was between 0.85 and 1.15, the
effect of combination of two drugs was the simple summation of
respective effects; however, if q>1.15, the two drugs had a
synergistic effect; whereas if q<0.85, the two drugs had an
antagonistic effect. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cyclopamine enhances CPT-induced
decrease in the proliferation of MM cells
Following treatment with cyclopamine, the
proliferation of RPMI-8226 cells was weakly decreased. At lower
doses, the inhibition of proliferation was very low and high doses
of cyclopamine increased inhibition. The maximum concentration of
cyclopamine that could be prepared was 10 µmol/l. The inhibition
rate of 10 µmol/l cyclopamine was significantly higher compared
with that of 5 nmol/l cyclopamine (P<0.01), when treated for the
same amount of time. However, there were no significant differences
between treatment with concentrations ≤1 µmol/l (P>0.05). The
inhibition rate was <40%, when treated with a very high
concentration of cyclopamine, which was close to the maximum
concentration, thus, it was hypothesized that RPMI-8226 cells were
resistant to cyclopamine (Table I).
Similarly, the inhibitory effect of cyclopamine on SKO-007 cells
was also weak. The inhibitory rate of 10 µM cyclopamine was also
significantly higher compared with that of 5 nmol/l cyclopamine
(P<0.05). The maximum inhibitory rate was <40%, and SKO-007
cells were also resistant to cyclopamine (Table I).
 | Table I.Inhibitory effects of cyclopamine on
RPMI-8226 and SKO-007 cell proliferation. |
Table I.
Inhibitory effects of cyclopamine on
RPMI-8226 and SKO-007 cell proliferation.
| A, RPMI-8226 cell
proliferation inhibition rate, % |
|---|
|
|---|
|
| Cyclopamine,
nmol/l |
|---|
|
|
|
|---|
| Treatment times,
h | 5 | 20 | 100 | 1,000 | 10,000 |
|---|
| 24 | 3.00±7.11 | −2.39±7.27 | 2.70±4.41 | 5.53±5.03 |
12.39±4.55b |
| 72 | −2.36±11.80 | −0.16±18.86 | 10.39±17.99 | 7.09±11.20 |
20.71±7.01b |
| 120 | 2.12±17.02 | 8.12±17.31 |
10.43±7.53a | 4.53±17.06 |
34.52±12.09b |
|
| B, SKO-007 cell
proliferation inhibition rate, % |
|
| Cyclopamine,
nmol/l |
|
|
|
| Treatment times,
h | 5 | 20 | 100 | 1,000 | 10,000 |
|
| 24 | 1.96±6.85 | −2.27±7.17 | 2.80±3.65 | 3.46±4.89 |
5.00±3.19a |
| 72 | −6.77±9.25 | −5.32±16.78 | 2.40±14.79 | 5.53±11.50 |
18.86±5.89a |
| 120 | 0.36±17.39 | 6.34±17.94 | 6.68±11.55 | 7.96±18.97 |
36.67±8.40b |
CPT significantly inhibited the proliferation of
RPMI8226 cells in both a time- and dose-dependent manner
(P<0.05). After 72 h of treatment, the inhibitory rate of
treatment with a low dose was >50%, and ~80%. with a high dose.
Therefore, RPMI-8226 cells were sensitive to CPT. However, the
proliferation of SKO-007 cells was not effectively inhibited by
CPT. When treated with 10 µg/ml CPT, the inhibitory rate was
20.49±3.4%, 29.82±13.07% and 11.68±23.26% at 24, 72 and 120 h
post-treatment, respectively. The inhibitory rate was <30% when
treated with a very high concentration, thus SKO-007 cells were
considered resistant to CPT (Table
II).
 | Table II.Inhibitory effects of CPT on RPMI-8226
and SKO-007 cells proliferation. |
Table II.
Inhibitory effects of CPT on RPMI-8226
and SKO-007 cells proliferation.
| A, RPMI-8226 cell
proliferation inhibitory effect, % |
|---|
|
|---|
|
| CPT, ng/ml |
|---|
|
|
|
|---|
| Treatment times,
h | 5 | 20 | 100 | 500 | 2,000 | 10,000 |
|---|
| 24 | 6.06±5.49 |
13.22±5.49a |
25.70±4.50b |
33.49±9.32b | / | / |
| 48 | 11.73±7.11 |
32.29±8.18b |
44.89±8.91b |
50.47±6.45b | / | / |
| 72 |
29.32±7.22b |
54.91±7.19b |
71.70±7.66b |
78.44±3.00b | / | / |
|
| B, SKO-007 cell
proliferation inhibitory effect, % |
|
|
| CPT,
ng/ml |
|
|
|
| Treatment times,
h | 5 | 20 | 100 | 500 | 2,000 | 10,000 |
|
| 24 | 12.05±6.53 | 4.77±9.63 | 3.01±3.20 | 7.85±5.70 |
15.39±7.79b |
20.49±3.74b |
| 72 | 17.75±21.25 | 18.58±21.46 | 20.68±18.12 | 19.14±13.57 |
27.00±17.83a |
29.82±13.07a |
| 120 | −2.50±14.24 | −7.48±14.57 | −2.39±12.50 | 3.88±19.28 | 10.05±22.50 | 11.68±23.26 |
To determine whether cyclopamine can increase the
sensitivity of myeloma cells to CPT, SKO-007 cells were selected
for subsequent experiments, since they were considered as resistant
to cyclopamine and CPT. The effects of a combination of cyclopamine
and CPT on the proliferation of SKO-007 cells were evaluated using
10 µmol/l cyclopamine, and 10 and 100 ng/ml CPT after 96 and 120 h
post-treatment. This showed that a combination of cyclopamine and
CPT could significantly inhibit cell proliferation, and the
inhibitory rate of 10 and 100 ng/ml CPT combined with 10 µmol/l
cyclopamine was 19.65±13.71% and 35.82±10.91% 96 h post-treatment,
respectively (P<0.05). Moreover, the Q value showed that
cyclopamine combined with CPT could synergistically inhibit the
proliferation of SKO-007 cells (1.40 and 2.55, respectively). A
total of 120 h post-treatment, the inhibitory rate of 10 and 100
ng/ml CPT combined with 10 µmol/l cyclopamine was 53.80±7.85% and
62.62±5.08%, respectively (P<0.05) and also showed synergistic
inhibition on proliferation (Q value, 1.57 and 2.09, respectively;
Table III).
 | Table III.Combination effects of cyclopamine
and CPT on SKO-007 cells proliferation. |
Table III.
Combination effects of cyclopamine
and CPT on SKO-007 cells proliferation.
| Treatment times,
h | Cyclopamine,
µmol/l | CPT, ng/ml | Inhibitory rate,
% | Q-value |
|---|
| 96 | 10 | 0 | 11.67±13.67 |
|
|
| 0 | 10 | 2.66±10.26 |
|
|
| 0 | 100 | 2.72±16.00 |
|
|
| 10 | 10 |
19.65±13.71a,c | 1.40 |
|
| 10 | 100 |
35.82±10.91a–d | 2.55 |
| 120 | 10 | 0 |
28.91±15.20a,b |
|
|
| 0 | 10 |
7.41±9.08b |
|
|
| 0 | 100 |
1.41±7.35b |
|
|
| 10 | 10 |
53.80±7.85a–d | 1.57 |
|
| 10 | 100 |
62.62±5.08a–e | 2.09 |
Cyclopamine promotes CPT-induced
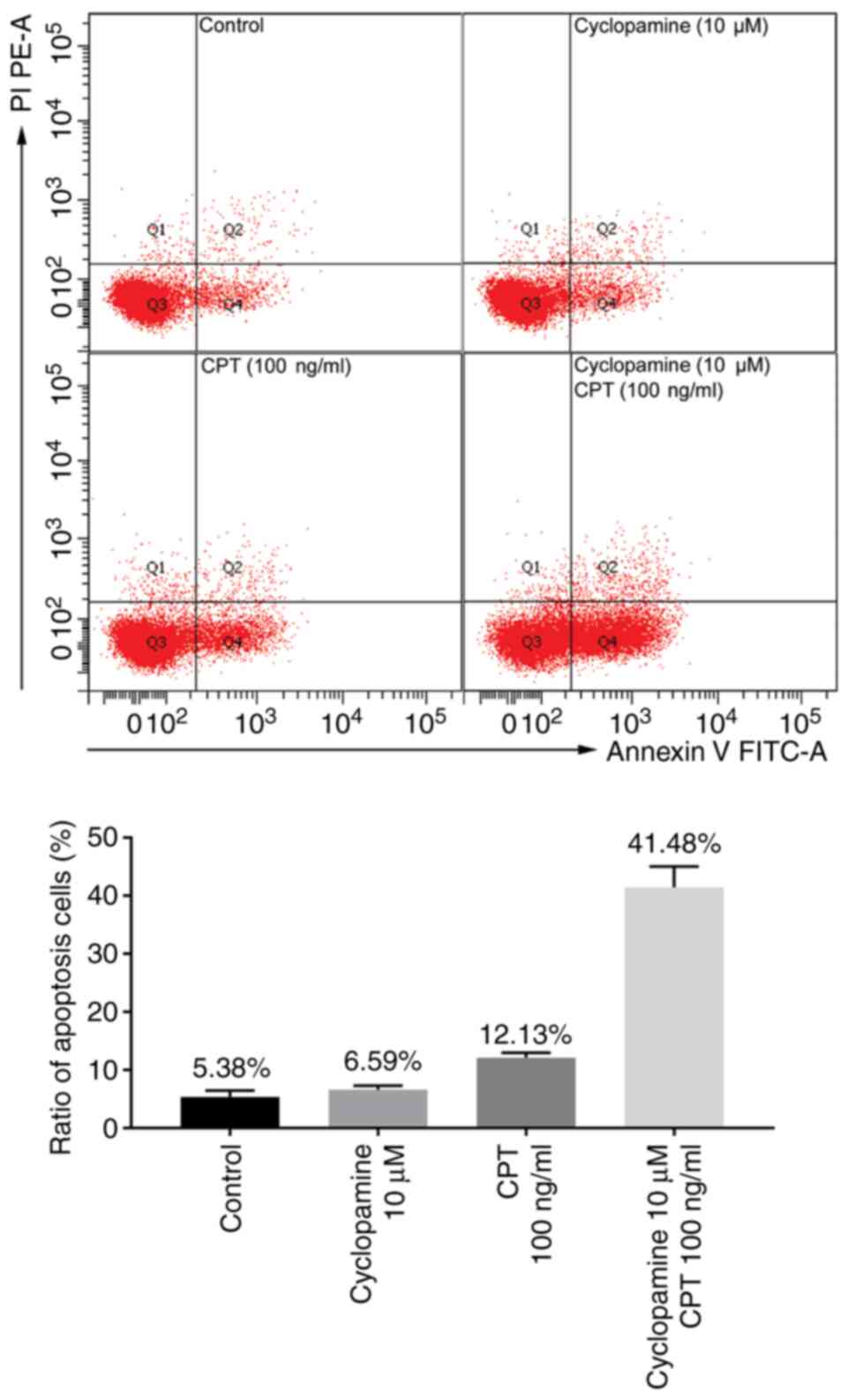
apoptosis in resistant SKO-007 cells
Annexin V and PI double staining and flow cytometry
were used to measure the induction of apoptosis by cyclopamine (10
µmol/l) and CPT (100 ng/ml) in SKO-007 cells. The percentage of
cells that were considered as early apoptotic when treated with
cyclopamine and CPT was 6.59±0.71% and 12.13±0.85%, respectively.
Combination of cyclopamine and CPT increased the proportion of
apoptotic cells (41.48±3.58%). Compared with the control cells
(5.38±1.08%), cyclopamine and CPT were able to increase the
proportion of apoptotic cells (P<0.05) and the combination of
the two drugs had a synergistic effect on induction of apoptosis (Q
value, 2.31; Fig. 1 and Table SII).
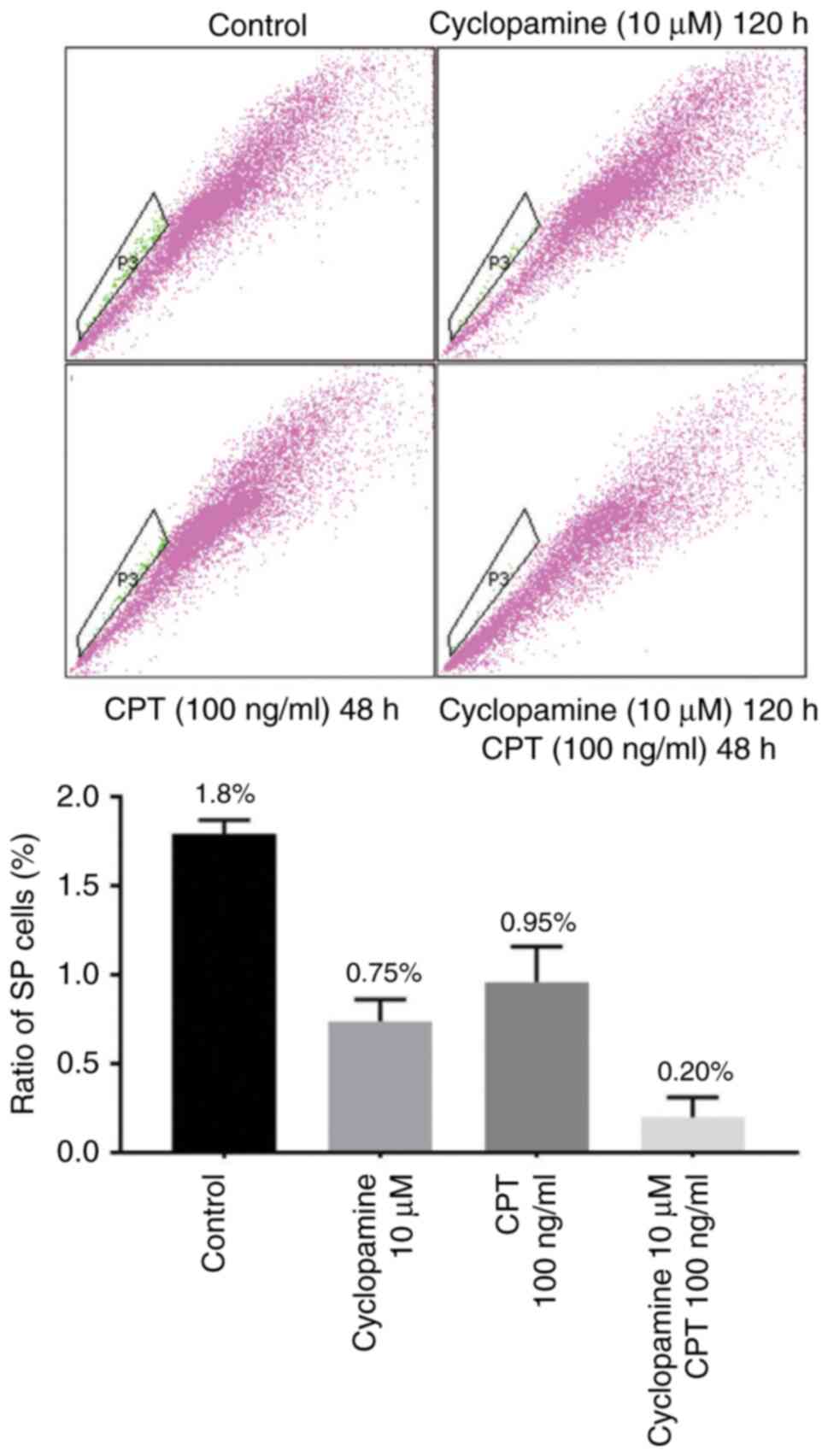
Cyclopamine combined with CPT
effectively decreases the proportion of SP cells
The proportion of SP cells was very low in SKO-007
cells; the percentage of SP cells was 1.79±0.08% in untreated
cells. The percentage of SP cells when treated with cyclopamine or
CPT were 0.74±0.12% and 0.96±0.20%, respectively. Treatment with a
combination of cyclopamine and CPT decreased the proportion of SP
cells (0.20±0.11%). Compared with the control cells, cyclopamine
and CPT were able to further decrease the proportion of SP cells
(P<0.05; Fig. 2 and Table SIII).
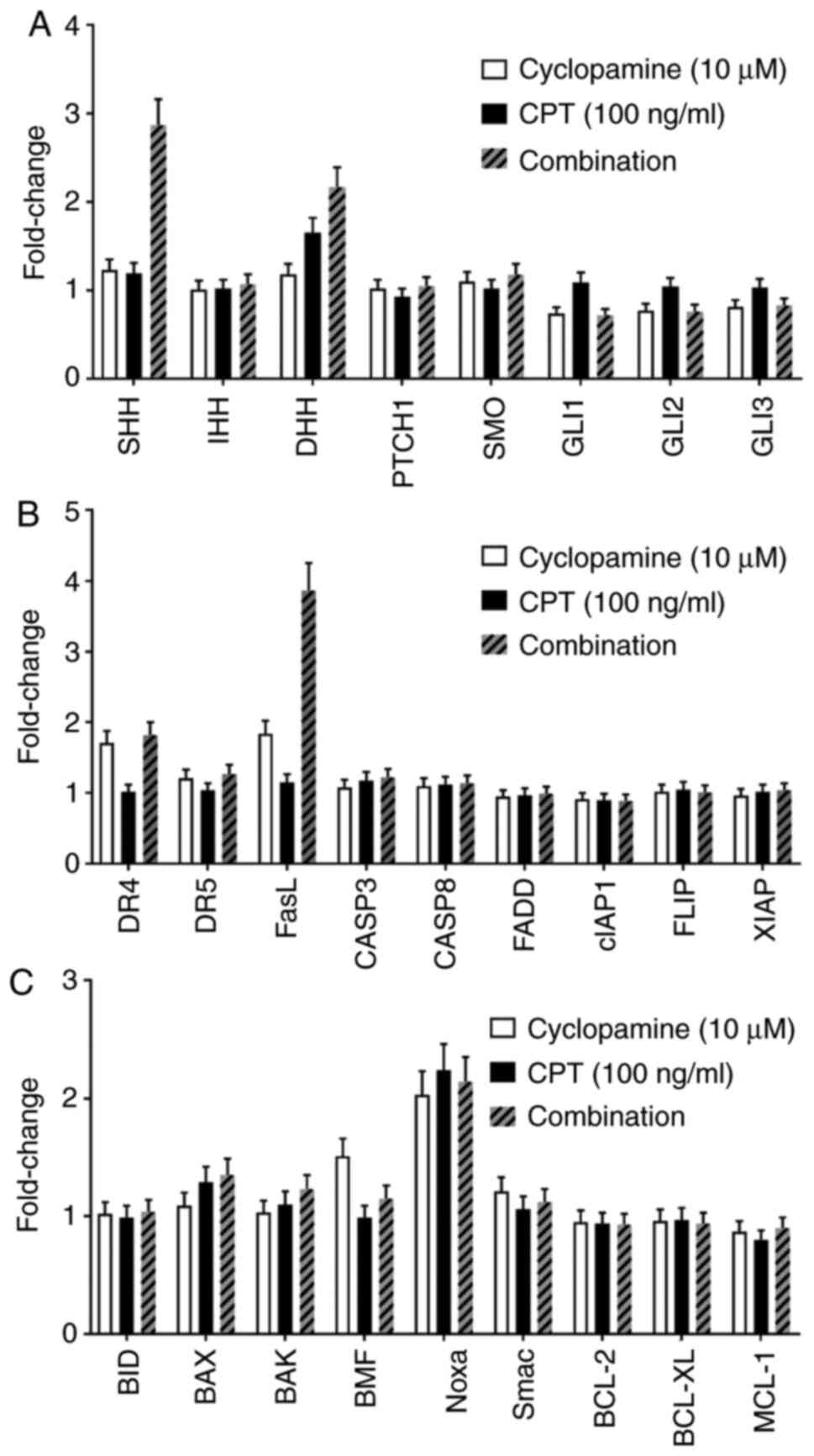
Cyclopamine sensitizes SKO-007 cells
to CPT through upregulation of DR4 expression
Results of qPCR analysis showed that cyclopamine
decreased the expression levels of GLI1/GLI2/GLI3; however, the
expression levels of other genes involved in the Hedgehog signaling
pathway were not significantly altered. The mRNA expression levels
of genes involved in the Hedgehog signaling pathway were not
notably altered following treatment with CPT. The expression levels
of GLI1/GLI2/GLI3 decreased and those of SHH and DHH increased when
SKO-007 cells were treated with cyclopamine combined with CPT.
Following treatment with cyclopamine, the expression levels of DR4
and FasL increased and those of other genes involved in
TRAIL-induced signaling pathways were not changed. The expression
levels of FasL further increased when SKO-007 cells were treated
with cyclopamine combined with CPT. However, there was no
significant effect of CPT treatment alone on the expression levels
of TRAIL signaling pathway genes. In the mitochondrial pathway of
apoptosis, the expression levels of Noxa were increased in SKO-007
cells treated with drugs, whereas expression of other genes
involved in this pathway were not notably altered (Fig. 3 and Table
SIV).
Discussion
TRAIL is a member of the tumor necrosis factor super
family, and exhibits antitumor, anti-virus and immunoregulatory
effects. There are five interacting partners: Death receptor 4
(DR4), DR5, decoy receptor 1 (DcR1), DcR2 and osteoprotegerin, and
TRAIL binds to the extracellular domain of these receptors. TRAIL
can induce cell apoptosis by activating apoptosis signaling
pathways when it binds with DR4 and DR5. However, extracellular
domains of the other three receptors are short and cannot activate
apoptotic signaling (14,15). TRAIL receptor expression levels are
different in normal tissues and tumor tissues. Major tumor cells
express death receptors but not DcR1 and DcR2; however, normal
cells express death receptors and decoy receptors. DcR1 and DcR2
cannot induce apoptosis; moreover, these two receptors may inhibit
apoptosis induced by DR4 and DR5. Thus, TRAIL can induce apoptosis
of tumor cells and has no harmful effects on normal cells (16). Therefore, TRAIL may serve as a
promising novel candidate drug for the treatment of MM (17,18).
However, none of the clinical trials utilizing TRAIL and the
antibodies against death receptors have exhibited objective
clinical benefits. Thus, recombinant gene technology has been used
to create analogues that exhibit enhanced function compared with
physiological TRAIL on tumor cells. To improve its activity, the
TRAIL protein was mutated to form CPT (6). Compared with wild-type TRAIL, CPT
exhibits excellent water solubility, higher bioactivity and
enhanced stability. In the phase 2 study of 71 patients with
relapsed or refractory MM, 47 patients were assigned to the CPT +
thalidomide and dexamethasone (TD) group, and 24 patients were
recruited to the TD group. The overall response rate in the CPT +
TD group was 38.3 vs. 25.0% in the TD group. The median progression
free survival time was 6.7 months for the CPT + TD group and 3.1
months for the TD group. The median duration of response for the
CPT + TD and TD groups were 7.1 and 3.2 months, respectively
(19). Other studies also showed
that CPT was effective against MM, but there were some patients who
were resistant to CPT (7,8). It is suggested that tumor cells may
survive CPT treatment though inactivation of signaling pathways
induced by TRAIL.
In myeloma cells, there is a rare subpopulation of
cells that possess the capacity for self-renewal and drug
resistance and these cells are termed myeloma stem cells (MSCs)
which are considered to underlie drug resistance (20). Treatments targeting MSCs are
effective in inhibiting the proliferation of myeloma cells
(21,22). The Hedgehog signaling pathway is one
of the major signaling pathways involved in the regulation of
self-renewal and differentiation of MSCs. Targeting this pathway
may improve the efficacy of chemotherapy in patients with MM
(23). Cyclopamine is the first
small molecule Hedgehog signaling pathway inhibitor that directly
targets the transmembrane receptor Smoothened. Cyclopamine can
induce apoptosis in certain types of cancer cells and exhibits
synergistic therapeutic effects when combined with other
anti-cancer drugs (9–12). In order to determine whether
cyclopamine can enhance the sensitivity of CPT to myeloma cells,
the effects of cyclopamine and CPT on myeloma cells were
assessed.
In the present study, the inhibitory effect of
cyclopamine on the proliferation of RPMI-8226 and SKO-007 cells was
weak. The inhibitory rate was <40% when a very high
concentration was used, which was close to the maximum
concentration. therefore, the 50% inhibiting concentration
(IC50) values were not calculated. Thus, RPMI-8226 and
SKO-007 cells were considered resistant to cyclopamine. As
described in a previous study, RPMI-8226 cells were sensitive to
CPT (24). The inhibitory rate of
low-dose treatment was >50% and that of the high dose was ~80%.
However, the proliferation of SKO-007 cells was not effectively
inhibited by CPT. The inhibitory rate was <30% when a very high
concentration was used, thus, SKO-007 cells were considered
resistant to CPT. To determine whether cyclopamine increased the
sensitivity of myeloma cells to CPT, SKO-007 cells, which were
resistant to cyclopamine and CPT, were used for subsequent
experiments. The results showed that a combination of cyclopamine
and CPT could significantly decrease cell proliferation. Moreover,
the Q value showed that cyclopamine combined with CPT could
synergistically inhibit the proliferation of SKO-007 cells.
It was shown that cyclopamine induced apoptosis in
SKO-007 cells; however, these cells were not sensitive to
cyclopamine. Similarly, SKO-007 cells were also resistant to
apoptosis induced by CPT. Cyclopamine could enhance induction of
CPT on SKO-007 cells and induction of apoptosis when combined.
Moreover, cyclopamine and CPT exhibited notable effects on the SP
cells, which were representative of MSCs. SP cells are rare in
SKO-007 cells and the proportion of these cells was decreased
following treatment with drugs. Combinations of these two drugs
significantly killed SP cells. This suggests that the combination
of cyclopamine and CPT may be able to eliminate MSCs, the root of
MM relapse.
The results of the present study showed that
cyclopamine was able to decrease the mRNA expression levels of
GLI1/GLI2/GLI3, all of which are involved in the Hedgehog signaling
pathway. The expression of SHH and DHH was increased when SKO-007
cells were treated with cyclopamine combined with CPT. It is
suggested that cyclopamine is an effective inhibitor of the
Hedgehog signaling pathway and the increased expression of SHH and
DHH may serve as feedback adjustment to low expression levels of
the downstream genes. The expression levels of DR4 are increased in
SKO-007 cells treated with cyclopamine. It was hypothesized that
the inhibition of the hedgehog signaling pathway may influence the
expression of TRAIL signaling pathway genes and increase DR4
expression. High levels of DR4 improve TRAIL signaling pathway
activity and increases CPT-induced apoptosis in SKO-007 cells.
Thus, cyclopamine enhanced the sensitivity of SKO-007 cells to CPT.
In addition, expression level changes of upstream genes in
apoptosis signaling pathways may be induced via a cascade reaction
of downstream genes and altered expression levels of associated
genes. Further experiments may better reveal the mechanisms
underlying the synergistic effects.
In summary, the present study demonstrated that
SKO-007 cells were resistant to cyclopamine and CPT. These two
drugs exhibit a synergistic effect on the inhibition of
proliferation and the induction of apoptosis in SKO-007 cells.
Moreover, the combinations of cyclopamine and CPT may inhibit MSCs,
which are hypothesized to be the root of MM relapse. Cyclopamine
may increase expression of DR4 and this may underlie the increased
sensitivity of SKO-007 cells to CPT.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (Youth Program; grant no.
81500164).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HW, HZ, and ZZ performed the experiments. HW and CG
confirm the authenticity of all the raw data. CG and WC conceived
and designed the study. CG wrote the manuscript. All authors read
and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martin T and Huff CA: Multiple myeloma:
Current advances and future directions. Clin Lymphoma Myeloma Leuk.
19:255–263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ziogas DC, Dimopoulos MA and Kastritis E:
Prognostic factors for multiple myeloma in the era of novel
therapies. Expert Rev Hematol. 11:863–879. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishida T: Therapeutic antibodies for
multiple myeloma. Jpn J Clin Oncol. 48:957–963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aljama MA, Sidiqi MH and Dingli D: Therapy
for relapsed multiple myeloma. Panminerva Med. 60:174–184. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gazitt Y: TRAIL is a potent inducer of
apoptosis in myeloma cells derived from multiple myeloma patients
and is not cytotoxic to hematopoietic stem cells. Leukemia.
13:1817–1824. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang F, Wang AP and Yang SF: Antitumor
activity of a novel recombinant mutant human tumor necrosis
factor-related apoptosis-inducing ligand. Acta Pharmacol Sin.
26:1373–1381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geng C, Hou J, Zhao Y, Ke X, Wang Z, Qiu
L, Xi H, Wang F, Wei N, Liu Y, et al: A multicenter, open-label
phase II study of recombinant CPT (Circularly Permuted TRAIL) plus
thalidomide in patients with relapsed and refractory multiple
myeloma. Am J Hematol. 89:1037–1042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leng Y, Qiu L, Hou J, Zhao Y, Zhang X,
Yang S, Xi H, Huang Z, Pan L and Chen W: Phase II open-label study
of recombinant circularly permuted TRAIL as a single-agent
treatment for relapsed or refractory multiple myeloma. Chin J
Cancer. 35:862016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan C, Wang Y, Liu Z, Sun Y, Wang X, Wei G
and Wei J: Metformin exerts anticancer effects through the
inhibition of the Sonic hedgehog signaling pathway in breast
cancer. Int J Mol Med. 36:204–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonnissen A, Isebaert S and Haustermans K:
Hedgehog signaling in prostate cancer and its therapeutic
implication. Int J Mol Sci. 14:13979–14007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang R, Mondal G, Wen D and Mahato RI:
Combination therapy of paclitaxel and cyclopamine polymer-drug
conjugates to treat advanced prostate cancer. Nanomedicine.
13:391–401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iovine V, Mori M, Calcaterra A, Berardozzi
S and Botta B: One hundred faces of cyclopamine. Curr Pharm Des.
22:1658–1681. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassanzadeh A, Farshdousti Hagh M, Alivand
MR, Akbari AAM, Shams Asenjan K, Saraei R and Solali S:
Down-regulation of intracellular anti-apoptotic proteins,
particularly c-FLIP by therapeutic agents; the novel view to
overcome resistance to TRAIL. J Cell Physiol. 233:6470–6485. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan X, Gajan A, Chu Q, Xiong H, Wu K and
Wu GS: Developing TRAIL/TRAIL death receptor-based cancer
therapies. Cancer Metastasis Rev. 37:733–748. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Testa U: TRAIL/TRAIL-R in hematologic
malignancies. J Cell Biochem. 110:21–34. 2010.PubMed/NCBI
|
|
17
|
Buckle CH, Neville-Webbe HL, Croucher PI
and Lawson MA: Targeting RANK/RANKL in the treatment of solid
tumours and myeloma. Curr Pharm Des. 16:1272–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arhoma A, Chantry AD, Haywood-Small SL and
Cross NA: SAHA-induced TRAIL-sensitisation of multiple myeloma
cells is enhanced in 3D cell culture. Exp Cell Res. 360:226–235.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leng Y, Hou J, Jin J, Zhang M, Ke X, Jiang
B, Pan L, Yang L, Zhou F, Wang J, et al: Circularly permuted TRAIL
plus thalidomide and dexamethasone versus thalidomide and
dexamethasone for relapsed/refractory multiple myeloma: A phase 2
study. Cancer Chemother Pharmacol. 79:1141–1149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao M, Kong Y, Yang G, Gao L and Shi J:
Multiple myeloma cancer stem cells. Oncotarget. 7:35466–35477.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi F, Li M, Wang J, Wu D, Pan M, Guo M
and Dou J: Induction of multiple myeloma cancer stem cell apoptosis
using conjugated anti-ABCG2 antibody with epirubicin-loaded
microbubbles. Stem Cell Res Ther. 9:1442018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi F, Li M, Wu S, Yang F, Di W, Pan M,
Zhao F, Luo S, Gu N and Dou J: Enhancing the anti-multiple myeloma
efficiency in a cancer stem cell xenograft model by conjugating the
ABCG2 antibody with microbubbles for a targeted delivery of
ultrasound mediated epirubicin. Biochem Pharmacol. 132:18–28. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Z, Xu J, He J, Zheng Y, Li H, Lu Y,
Qian J, Lin P, Weber DM, Yang J and Yi Q: A critical role of
autocrine sonic hedgehog signaling in human CD138+ myeloma cell
survival and drug resistance. Blood. 124:2061–2071. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Li Y, Sun W, Liu J and Chen W:
Synergistic effects of rmhTRAIL and 17-AAG on the proliferation and
apoptosis of multiple myeloma cells. Hematology. 23:620–625. 2018.
View Article : Google Scholar : PubMed/NCBI
|