Introduction
Cancer is a high mortality malignant disease with
biological characteristics, such as abnormal cell differentiation
and proliferation, uncontrolled growth, invasiveness and
metastasis. Several patients with cancer have different degrees of
metastasis following diagnosis or treatment (1,2). Thus,
cancer is an important cause of mortality and high-cost medical
expenses (3). Tumour occurrence is
associated with the expression levels of tumour suppressor genes
and oncogenes (4–6). Accordingly, it is essential to identify
biomarkers for the early diagnosis and prognosis of cancer, and to
develop effective therapies to prolong the overall survival time of
patients with cancer. The human genome is pervasively transcribed,
while <2% transcripts encode proteins. According to their size,
non-coding RNAs (ncRNAs) are subdivided into two major categories:
Short ncRNAs [sncRNAs, <200 nucleotides (nt)] and long ncRNAs
(lncRNAs, >200 nt). MicroRNAs (miRNAs/miRs) and endogenous short
interfering RNAs are sncRNAs (7).
MiRNAs are short single-stranded RNA sequences (usually 19–23 nt)
from a precursor of ~70 nt, which can control gene expression
during several physiological and developmental processes, thus,
they play vital roles in post-transcriptional regulation by
directly binding the miRNA response element in target mRNAs
(8–10).
lncRNAs include multiple well-studied ncRNAs, such
as the most representative, HOX antisense intergenic RNA (HOTAIR)
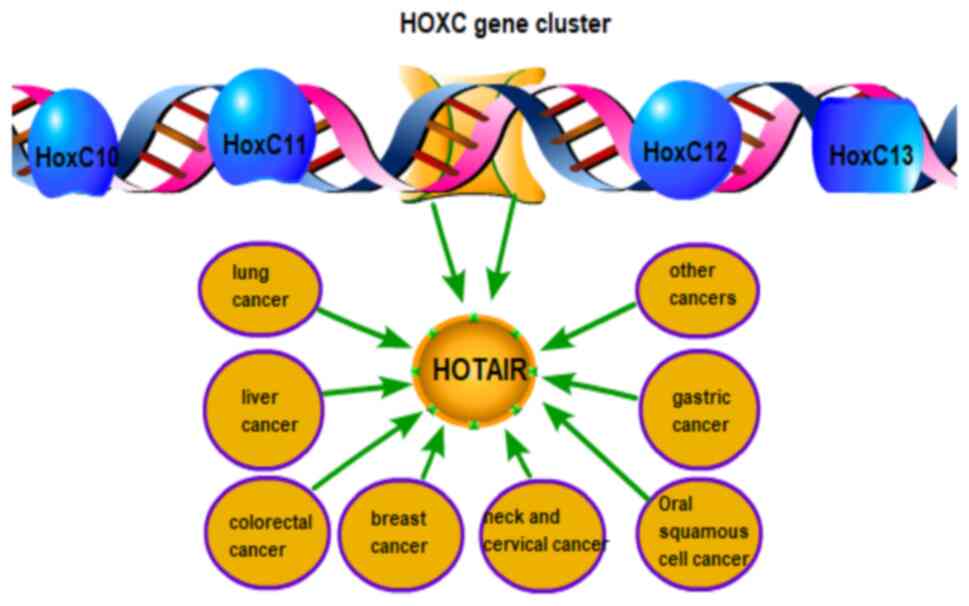
(11). HOTAIR is expressed by the
homeobox C gene (HOXC) locus and is located in the HOXC cluster
between the HOXC11 and HOXC12 genes on human chromosome 12q13.13
(12). Increasing evidence suggests
that HOTAIR is strongly associated with different types of cancer
(13) (Fig. 1). For example, HOTAIR plays a role in
metastasis progression through canonical and noncanonical pathways
in breast carcinoma, hepatocellular carcinoma (HCC), lung
carcinoma, gastric carcinoma and oral squamous cell carcinoma
(14–18) (Table
I). In addition, HOTAIR participates in regulating cell cycle
progression, tumour proliferation, epithelial-to-mesenchymal
transition (EMT) and tumour migration and invasion by altering gene
expression (19).
 | Table I.Expression and signalling pathways
associated with tumour metastasis of HOTAIR. |
Table I.
Expression and signalling pathways
associated with tumour metastasis of HOTAIR.
| Cancer type | Expression | Pathways | Molecules | Refs. |
|---|
| Breast cancer | Up | TGF-β,
PI3K/AKT | miR-7, TGF-β1,
p53 | (27,31,35,58,60) |
| Lung cancer | Up | TGF-β, VEGF | EMT, MMP-2,
MMP-9 | (37,72,79) |
| Liver cancer | Up | TGF-β, Wnt/β,
VEGF | miR-217-5p, EMT,
MMP-9, RBM38 | (32,44,71) |
| Colorectal
cancer | Up | TGF-β, Wnt/β,
PI3K/AKT | EMT, miR-326,
miR-203a-3p, IGF2BP2 | (28,47,61) |
| ESCC | Up | Wnt/β | WIF-1 | (50) |
| Renal cancer | Up | TGF-β,
PI3K/AKT | miR-203, claudin,
E-cadherin, PTEN, miR-217 | (29,30) |
| Gastric cancer | Up | Wnt/β,
PI3K/AKT | E-cadherin,
miR-34a | (36,38,46) |
| Esophageal
cancer | Up |
| miR-204 | (33) |
| OSCC | Up | TGF-β | E-cadherin | (34) |
| HNSCC | Up | PI3K/AKT, VEGF | miR-206, MMP-9 | (64,74) |
| Cervical
cancer | Up |
| E-cadherin | (39) |
| Bladder cancer | Up |
| EMT | (40) |
| Ovarian cancer | Up | Wnt/β |
| (49) |
| Leukemia | Up | Wnt/β,
PI3K/AKT | Cyclin D1, GSK-3β,
c-Myc, miR-143 | (48,62) |
| Osteosarcoma | Up | PI3K/AKT, VEGF | E-cadherin,
N-cadherin, p-mTOR, p-PI3K, pAKT, p27, MMP-9, MMP-2 | (59) |
| Melanoma | Up | PI3K/AKT | miR-152-3p | (47) |
| Glioma | Up | PI3K/AKT, VEGF | miR-206, FGF-1,
MMP-9, MMP-7 | (66,73) |
According to a recent study, HOTAIR promotes tumour
metastasis in several ways, including: i) Inhibition by competing
with miRNAs, downregulating the expression levels of EMT-related
proteins, ii) controlling angiogenesis to provide tumour tissue
with the oxygen and nutrients necessary for metabolism, iii)
promoting tumour progression by regulating the expression of genes
associated with cell metabolism; iv) controlling gene silencing to
regulate tumour metastasis and v) playing a role in cargo loading,
such as in exosomes, to promote tumour cell metastasis (Fig. 2).
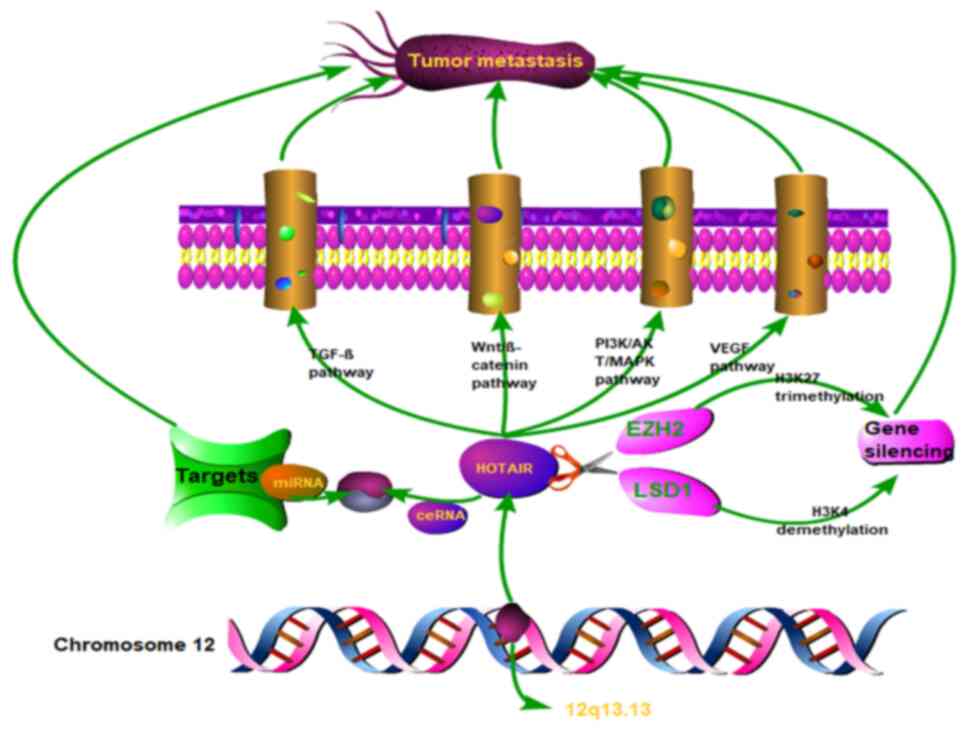 | Figure 2.Overview of HOTAIR and tumour
metastasis. This figure illustrates the chromosome where HOTAIR is
located, summarizes the functions and molecular mechanisms of
HOTAIR-mediated gene silencing, association between HOTAIR and
miRNA, and four associated signalling pathways. These include: i)
Act as a ceRNA to compete with miRNA, ii) regulate gene silencing
by H3K27 trimethylation and H3K4 demethylation and iii) promote
metastasis through influence on the TGF-β, Wnt/β-catenin,
PI3K/AKT/MAPK and VEGF pathways. HOTAIR, HOX antisense intergenic
RNA; miRNA, microRNA; ceRNA, competitive endogenous RNA; TGFβ,
transforming growth factor β; VEGF, vascular endothelial growth
factor; EZH2, enhancer of zeste homolog 2. |
The present review discusses the pathways and
molecular mechanisms of HOTAIR in tumour metastasis, emphasizes the
function of HOTAIR in human malignant tumour metastasis, and
provides a foundation for its application in tumour diagnosis,
prognosis and medical treatment of various tumours.
TGF-β pathway
The EMT is the process of epithelial cell
transformation or transdifferentiation from the epithelial to the
mesenchymal cell type (20). In this
process, cells lose their epithelial properties and can migrate,
breaking away from the epithelial cell colony and moving to
surrounding and distant tissues (20). The EMT and mesenchymal-to-epithelial
transformation (MET) processes are crucial during embryonic
development (organogenesis and regeneration), and are triggered
during tumour invasion and metastasis (21).
The transforming growth factor β (TGF-β) signalling
pathway comprises a series of signal transmission processes
mediated by transforming growth factor. In humans, it includes the
TGFβ subtype, bone morphogenetic protein, activin, and growth and
differentiation factor, which has an indispensable effect on cell
proliferation and metastasis (22).
TGF-β regulates the EMT primarily through a group of transcription
factors, including the basic helix-loop-helix proteins Twist and
E47, zinc finger proteins Snail and Slug (also called Snail2), and
zinc finger and homeodomain proteins ZEB1, ZEB2 (23), and FOXC2 (24), which inhibit E-cadherin expression (a
key process in the EMT), and the expression levels of other
epithelial markers. E-cadherin is a transmembrane glycoprotein that
connects epithelial cells together at adhesion junctions and is
closely associated with the EMT (25). E-cadherin exerts its
tumour-suppressing role primarily by sequestering β-catenin,
preventing its binding to lymphoid enhancer factor/T cell factor
(26). Increasing evidence suggests
that HOTAIR is associated with the EMT process through regulation
of the TGF-β pathway. The molecular mechanism occurs mainly through
two processes. On one hand, HOTAIR regulates the expression of key
EMT-related genes, such as E-cadherin, N-cadherin and Snail, to
promote tumour metastasis by acting as a competitive endogenous RNA
(ceRNA) that competes with a miRNA for binding sites on mRNA
(13). On the other hand, HOTAIR
promotes gene transcription, and thus regulates gene expression,
controlling the EMT through epigenetic mechanisms (19,27).
Wu et al (28)
demonstrated that silencing HOTAIR improves the capability of LoVo
colon cancer cells to invade, proliferate and migrate, and promotes
their apoptosis by inhibiting the EMT. In addition, Dasgupta et
al (29) reported that the
miR-203-HOTAIR interaction suppresses metastatic genes, such as
those for E-cadherin and claudin, regulating the EMT and metastasis
in renal cell carcinoma (RCC). Furthermore, downregulation of
HOTAIR significantly decreases the migration and expression levels
of the epithelial markers, Vimentin and Snail, that are induced by
TGF-β1, while E-cadherin expression markedly increases, which
promotes RCC cell migration, proliferation and the EMT through
negative modulation of miR-217 (30). For miR-217, HOTAIR plays a ceRNA role
and modulates HIF-1a expression by sponging miR-217 to promote the
EMT through the miR-217/HIF-1a/AXL axis (30). Similarly, HOTAIR represses miR-7
expression, resulting in upregulated SETDB1 expression in stem
cells of breast cancer, which promotes the EMT (31). There is also evidence to suggest that
HOTAIR has an important effect on the proliferation, invasion and
migration of liver tumour cells and the EMT by sponging miR-217-5p
(32).
In addition to acting as a ceRNA, HOTAIR can also
drive EMT through epigenetic mechanisms, according to recent
studies. For example, HOTAIR specifically regulates HOXC8
expression and changes protein levels associated with cell
proliferative, invasive and migratory abilities, promoting the
invasion and pervasion of oesophageal cancer cells (33). Wu et al (34) demonstrated that HOTAIR is highly
expressed in oral squamous cell carcinoma (OSCC) with regional
lymph node metastasis (N1) compared with OSCC without N1. It was
also reported that HOTAIR inhibits E-cadherin expression by
associating with enhancer of zeste homolog 2 (EZH2) and H3K27me3,
improving the metastatic and invasive capacities of OSCC cells, and
promoting EMT (34). Notably, the
progression and migration of breast cancer is also regulated by the
TGF-β1/HOTAIR axis (35). Secretion
of TGF-β1 by CAF activates the TGF-β1/Smad pathway in breast cancer
cells, increasing HOTAIR transcription and modifying histones in
the CDK5 signalling pathway, which leads to changes in the tumour
microenvironment (35). In gastric
cancer, HOTAIR negatively regulates E-cadherin mRNA and protein
expression levels (36). In
addition, HOTAIR inhibits E-cadherin expression by acetylating
histone H3K27 to methylate the E-cadherin promoter, thereby
promoting EMT (36). Liu et
al (37) demonstrated that
HOTAIR and its two fragments, HOTAIR 3′ and HOTAIR 5′, promote cell
proliferation of non-small cell lung cancer (NSCLC) in the
G1/S phase by restricting the cell cycle through the
regulation of the Rb-E2F pathway in vitro and through the
EMT pathway, which affects cancer metastasis and invasion. Liu
et al (38) reported that
HOTAIR recruitment and combination with polycomb repressive complex
2 (PRC2) epigenetically inhibits the control target C-Met
(HGF/C-Met/Snail pathway) and miR-34a associated with Snail, thus
promoting the procession of the GC cell EMT and acceleration of
tumour migration. HOTAIR plays a key role in cervical cancer cell
metastasis and invasive as transfection with si-HOTAIR increases
E-cadherin expression and decreases the EMT transcription factors,
Snail and Twist (39). Berrondo
et al (40) demonstrated that
lack of HOTAIR expression in urothelial bladder cancer (UBC) cell
lines regulates the expression levels of genes associated with EMT,
including SNAI1, TWIST1, ZEB1, ZO1, LAMB3, matrix metalloproteinase
(MMP)1 and LAMC2, to decrease migration and invasion.
TGF-β promotes EMT through transcription and
post-transcriptional adjustment of a set of transcription elements
that inhibit epithelial characteristics, including cell junctions
and the expression of polar complex components, and enhancing
mesenchymal characteristics (41).
As a key factor triggering tumour promotion, HOTAIR enhances
invasiveness and metastatic ability by inducing EMT through both
ceRNAs and epigenetic mechanisms in the tumour microenvironment
(19).
Wnt/β-catenin pathway
The Wnt/β-catenin pathway plays a pivotal role in
regulating cellular processes and angiogenesis in tumours,
affecting oncogenic processes (42).
Owing to genic and epigenetic alterations, abnormal activation of
Wnt/β-catenin signalling is associated with different types of
tumours, such as ovarian cancer and liver cancer (43). HOTAIR activates the Wnt/β-catenin
signalling pathway to promote tumour progression (15).
As early as 2016, it was demonstrated that HOTAIR is
highly expressed in liver cancer tissues compared with adjacent
normal tissues, and its expression is significantly associated with
poor tumour metastasis (44).
Overexpression of HOTAIR partially promotes HCC processes by
activating the Wnt/β-catenin signalling pathway, and regulating the
proliferation, invasion and tumorigenic ability of liver cancer
cells in vivo (44). HOTAIR
has also been reported to specifically act in HeLa cells, playing
an indispensable role in their proliferation, migration and
invasion, as HOTAIR participates in downregulating TET1 expression
to promote the activity of Wnt/β-catenin signalling (45). The primary effect is to increase the
methylation pattern and decrease the expression levels of SOX17,
PCDH10, AJAP1 and MAGI2 genes (38).
Cheng et al (46)
demonstrated that miR-34a in Wnt/β-catenin signalling regulates
HOTAIR in GC tissues and cell lines, which promotes tumour growth
in vivo. It has also been reported that HOTAIR gene
knockdown is negatively associated with the overexpression of
miR-203a-3p, which affects cell proliferation and metastasis
(47). miR-203a-3p targets β-catenin
and GRG5, while overexpression of miR-203a-3p and HOTAIR knockdown
suppress Wnt/β-catenin signalling (47). In addition, overexpression of HOTAIR
activates Wnt/β-catenin, which increases cyclin D1, GSK-3β and
c-Myc expression, as well as enhancing survival and the
proliferation of leukaemia cells (48). Furthermore, cell cycle progression is
promoted by overexpression of HOTAIR through activation of
Wnt/β-catenin signalling, which in turn promotes ovarian cancer
cell proliferation and invasion (49). HOTAIR directly controls WIF-1
expression by promoting the methylation of its histone H3K27 in the
promoter region (50). Wnt/β-catenin
signalling is triggered by suppressing WIF-1, thereby promoting
tumour invasion and migration (50).
Aberrant activation of Wnt/β-catenin signalling, generally caused
by genetic and epigenetic alterations, is associated with a few
types of tumour migration (51). The
combination of HOTAIR expression and genetic alterations may
decreases Wnt/β-catenin signalling activity and increase the mRNA
levels of its negative regulators (45).
PI3K/AKT/MAPK pathway
PI3K/Akt signalling is associated with cell
proliferation, apoptosis and metastasis (52,53).
HOTAIR plays an important role in tumour metastasis in this pathway
by affecting the expression of related upstream and downstream
genes (54).
According to Dasgupta et al (29), overexpression of miR-203 supresses
HOTAIR, resulting in upregulated PTEN expression, a main regulator
of the PI3K/Akt pathway (55).
miR-203 mimic mediated induction of PTEN resulted in the induction
of p21 in ACHN and Caki-1 cells (56,57) and
upregulated mRNA and protein expression levels of the downstream
molecule, p21, which induces the PTEN gene and suppresses the
effectors of downstream pathways that control proliferation and
metastasis. Cheng et al (46)
also indicated that the PI3K/Akt signalling pathway is regulated by
HOTAIR through miR-34a, which promotes tumour growth. In addition,
Yu et al (58) demonstrated
that HOTAIR knockdown in MCF-7 cells increases p53 expression and
decreases AKT and JNK expression, resulting in decreased cell
migration and invasion. HOTAIR silencing decreases the expression
levels of p-mTOR, p-PI3K and p-AKT, thus, HOTAIR promotes
osteosarcoma cell proliferation by activating AKT/mTOR signalling
(59). In addition, HOTAIR knockdown
restrains the proliferation and metastasis of breast cancer cells
by decreasing the phosphorylation of PI3K/AKT/mTOR (60). Pan et al (61) reported that the HOTAIR/miR-326/FUT6
axis regulates the fucosylation of CD44 to facilitate colorectal
cancer progression through the PI3K/AKT/mTOR pathway. Both HOTAIR
inhibition and overexpression of miR-143 inhibit the proliferation
and promote the apoptosis of chronic leukaemia KCL22 and K562 cells
through changes to PI3K/AKT pathway proteins (62). HOTAIR functions as a ceRNA, competing
with miR-152-3p to release c-MET mRNA. This leads to downstream
PI3K/Akt/mTOR signalling pathway activation, and melanoma cell
proliferation and metastasis (63).
It is speculated that HOTAIR competitively binds to miR-206 and
restrains its expression. miR-206 targets STC2 to inhibit head and
neck squamous cell cancer (HNSCC) cell proliferation, migration and
invasion through the PI3K/Akt signalling pathway (64). Thus, HOTAIR silencing inhibits the
proliferation, migration and invasion of HNSCC cells, while
increasing their rate of apoptosis. Fibroblast growth factor-1
(FGF1) belongs to the fibroblast family and activates the PI3K/AKT
and MEK1/2 signalling pathways (65,66).
HOTAIR inhibits FGF1 expression by upregulating miR-326 expression,
which inhibits the proliferation, invasion and migration of cells
in the G0/G1 phase, promotes apoptosis and
induces cell cycle arrest (66).
Taken together, these results suggest that the
PI3K/Akt/MAPK signalling pathway is significant for the
transduction of membrane receptor signals into cells, which
functions in tumour metastasis by influencing tumour cell migration
and adhesion, as well as tumour cell angiogenesis.
Vascular endothelial growth factor (VEGF)
pathway
It is well-known that tumour angiogenesis plays an
important role in tumour invasion and migration. Among angiogenesis
factors, VEGF, a highly specific vascular endothelial growth
factor, plays a vital role in the extracellular matrix and vascular
endothelial cells (67). HOTAIR is
directly involved in promoting VEGFA transcription. The results of
a dual-luciferase report assay revealed that the transcription of
the 2.3 kb VEGFA promoter is activated by HOTAIR (68). The degradation of extracellular
matrix proteins is closely associated with MMPs, increasing the
potential of cancer cells to invade, proliferate and migrate
(69). In addition, glucose
regulatory protein 78, a member of the heat shock protein 70
family, has been recognized as an anti-angiogenic target of HOTAIR
in NPC cells (68,70). A previous study hypothesized that
HOTAIR knockdown decreases proliferation of cells and is associated
with decreased MMP-9 and VEGF expression, which play vital roles in
the motility and migration of cells in HCC (71). Liu et al (72) demonstrated that HOTAIR is upregulated
in NSCLC tissues, which promotes cell invasion and migration,
partially through the downregulation of HOXA5; in addition, HOTAIR
knockdown decreases MMP-2 and MMP-9 protein expression levels in
NSCLC cells. Collectively, these results suggest that HOTAIR may
affect the potential of NSCLC cells to invade and migrate by
altering the expression levels of MMPs and HOXA5 (72). In addition, Zhao et al
(73) assessed 123 patients
undergoing surgery for glioma and demonstrated that HOTAIR may
facilitate invasion by increasing the expression levels of MMP-9,
MMP-7 and VEGF. Kim et al reported that HOTAIR accelerates
the invasiveness of cervical cancer cells by upregulating VEGF and
MMP-9 expression (74). HOTAIR
expression is significantly upregulated in patients with
osteosarcoma (OS), and is associated with lymph node metastasis
(59). In addition, HOTAIR mediates
the proliferation of OS cells by regulating the expression levels
of cyclin E, CDK2, CDK4 and p27, MMP-2, MMP-9 and CD44, which
promotes OS cell invasion and metastasis (59). Taken together, these results suggest
that HOTAIR promotes tumour cell metastasis by regulating
VEGF-related specific proteins, such as extracellular matrix
proteins; however, the specific molecular mechanisms are yet to be
investigated.
Others
Epigenetic modification is defined as heritable
changes that appear in gene expression of unchanged DNA sequences.
In addition, chromatin remodelling is an important part of
epigenetics (75). A single lncRNA
can serve as the interface of DNA and specific chromatin
remodelling activities, leading to diseases, such as cancer and
activities, such as tumour metastasis (76–78).
HOTAIR acts as a molecular scaffold to connect and
target PRC2 and LSD1, histone modification complexes, and
subsequently reprograms chromatin states by coupling histone H3K27
methylation and H3K4 demethylation to promote cancer metastasis
through epigenetic gene silencing (27). Gupta et al (27) proposed that enforced expression of
HOTAIR in epithelial cancer cells induced genome-wide re-targeting
of PRC2 to an occupancy pattern more resembling embryonic
fibroblasts, leading to altered histone H3 lysine 27 methylation,
gene expression, and increased cancer invasiveness and metastasis
in a manner dependent on PRC2. Recently, Wu et al (28) reported that proliferation, invasion
and metastasis can be inhibited by silencing HOTAIR, and the
apoptosis of LoVo colon cancer cells can be expedited by supressing
IGF2BP2. In addition, protein modification plays an indispensable
role in epigenetic modifications. HOTAIR functions in the promotion
of the migration and invasion of HCC cells by inhibiting RBM38, an
RNA-binding protein, suggesting that HOTAIR and RBM38 play
significant roles in the progression of HCC (79). Notably, RBM38 may have an important
function in the TGF-β signalling pathway, according to an
integrated KEGG pathway analysis; however, further studies are
required to confirm these findings.
Increasing evidence suggests that cancer cells
support tumour progression by secreting extracellular vesicles
(EVs), and several lncRNAs appear in EVs in different tumour cells
(80). lncRNAs from exosomes act as
signalling molecules for intercellular communication, and play
indispensable roles in tumour invasion and metastasis, including
HOTAIR (81). The expression levels
of SNAI1, TWIST1, MMP1, LAMB3, ZEB1, ZO-1, JAM2, LAMC2 and ABL2 in
UBC cells are changed by exosomal lncRNA-HOTAIR, which induces the
migration and invasion of carcinoma cells (40). It has been demonstrated that HOTAIR
originating from carcinoma cells exhibits the possibility of being
liberated in the tumor microenvironment by EVs and delivered to
endothelium cells, thereby accelerating tumour metastasis (82,83).
Although the specific association between exosomal HOTAIR and
cancer metastasis remains unclear, the role of HOTAIR is worthy of
further investigation.
Prospects and challenges
Tumour metastasis refers to tumour cells leaving
their primary growth sites (local infiltration and intravascular
infiltration), undergoing systemic displacement (surviving in the
circulation, retention in distant organs and extravasation), and
adapting for survival and reproduction in the foreign
microenvironment of distant tissues (84). With the development of medical
technology, surgical resection and adjuvant therapy can cure
limited primary tumours; however, metastatic diseases remain
largely incurable. This explains why >90% of cancer mortality is
attributed to metastasis instead of the primary tumours caused by
these malignant lesions (85).
HOTAIR is abundant in saliva, clinical blood samples
and exosomes. Owing to its structural stability and cell/tissue
type specificity, HOTAIR is stable, sensitive, specific and easy to
detect (40,86). Early detection of HOTAIR in body
fluid samples may facilitate its role as a biomarker for the
diagnosis of different types of carcinomas (87). A previous study demonstrated that
serum exosomes containing HOTAIR act as potential diagnostic and
prognostic biomarkers for breast cancer, and its high expression is
associated with response to treatment (88). In addition, Wang et al
(89) demonstrated that HOTAIR is
significantly associated with clinical parameters of laryngeal
squamous cell carcinoma (LSCC), suggesting its potential as a
valuable biomarker for screening LSCC and a promising predictive
tool for patients with LSCC patients. Similar results have been
reported in lung cancer, stomach cancer and liver cancer (90–93). In
summary, HOTAIR functions as follows: i) Promotes epigenetic
activation/repression of tumour suppressor gene function, ii)
affects the inhibition of target gene expression by competitively
binding miRNA and iii) modifies genes to ensure their compatibility
with transcription factors, while ribosomes can
post-transcriptionally interact with splicing factors (30).
The complexity of the molecular mechanism of HOTAIR
was unexpected; thus, more detailed studies are required. It is
speculated that a better understanding of the biological functions
of HOTAIR in cancer pathogenesis will provide in-depth knowledge
about malignant tumours, and help identify basic disease processes
and provide useful treatment for patients who suffer from tumours.
In addition, investigations to elucidate the molecular mechanisms
of how HOTAIR is expressed and regulated, such as the
characteristics of HOTAIR protein biochemistry and structure and
HOTAIR-DNA interactions, may help identify potential therapeutic
treatment tactics for different types of cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81904129), the
National Natural Science Foundation of China (grant no. 81904129),
the Shanghai Pudong New Area Health Commission's 2019 Joint Public
Relations Project (grant no. PW2019D-7), the three-year action plan
(grant no. 2018-2020) for further accelerating the development of
Chinese medicine in Shanghai (grant no. ZY(2018–2020)-ZYBZ-01) and
the 2019 Shanghai Chinese and Western Medicine Clinical
Collaboration Pilot Project Funding (grant no. ZXYXZ-201909).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LC conceived this review, searched, analyzed and
drafted the initial manuscript. XQ and ZW participated in
discussing, collecting literature and revising the manuscript for
important intellectual details. XZ conceived and revised the
manuscript for important intellectual details. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dušek L, Mužík J, Malúšková D, Májek O,
Pavlík T, Koptíková J, Gregor J, Brabec P and Abrahámová J:
Epidemiology of screening-targeted cancers according to new data of
the Czech national cancer registry. Klin Onkol. 27 (Suppl
2):S19–S39. 2014.(In Czech). View Article : Google Scholar
|
|
3
|
Rosenberg AR, Kroon L, Chen L, Li CI and
Jones B: Insurance status and risk of cancer mortality among
adolescents and young adults. Cancer. 121:1279–1286. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van der Weyden L, Arends MJ, Rust AG,
Poulogiannis G, McIntyre RE and Adams DJ: Increased tumorigenesis
associated with loss of the tumor suppressor gene Cadm1. Mol
Cancer. 11:292012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan XH, Nama S, Gopal F, Rizk P, Ramasamy
S, Sundaram G, Ow GS, Ivshina AV, Tanavde V, Haybaeck J, et al:
Targeting glioma stem cells by functional inhibition of a
prosurvival oncomiR-138 in malignant gliomas. Cell Rep. 2:591–602.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shroff EH, Eberlin LS, Dang VM, Gouw AM,
Gabay M, Adam SJ, Bellovin DI, Tran PT, Philbrick WM, Garcia-Ocana
A, et al: MYC oncogene overexpression drives renal cell carcinoma
in a mouse model through glutamine metabolism. Proc Natl Acad Sci
USA. 112:6539–6544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Zhang P, Wang L, Piao HL and Ma
L: Long non-coding RNA HOTAIR in carcinogenesis and metastasis.
Acta Biochim Biophys Sin (Shanghai). 46:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Selbach M, Schwanhäusser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wan Y and Chang HY: HOTAIR: Flight of
noncoding RNAs in cancer metastasis. Cell Cycle. 9:3391–3392. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao Y, Baker D and Ten Dijke P:
TGF-β-mediated epithelial-mesenchymal transition and cancer
metastasis. Int J Mol Sci. 20:27672019. View Article : Google Scholar
|
|
14
|
Qin W, Kang P, Xu Y, Leng K, Li Z, Huang
L, Gao J, Cui Y and Zhong X: Long non-coding RNA HOTAIR promotes
tumorigenesis and forecasts a poor prognosis in cholangiocarcinoma.
Sci Rep. 8:121762018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Tian H, Yang J and Gong Z: Long
noncoding RNAs regulate cell growth, proliferation, and apoptosis.
DNA Cell Biol. 35:459–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rossi MN and Antonangeli F: LncRNAs: New
players in apoptosis control. Int J Cell Biol. 2014:4738572014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou S, He Y, Yang S, Hu J, Zhang Q, Chen
W, Xu H, Zhang H, Zhong S, Zhao J and Tang J: The regulatory roles
of lncRNAs in the process of breast cancer invasion and metastasis.
Biosci Rep. 38:BSR201807722018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou T, Ding JW, Wang XA and Zheng XX:
Long noncoding RNAs and atherosclerosis. Atherosclerosis.
248:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhan A and Mandal SS: LncRNA HOTAIR: A
master regulator of chromatin dynamics and cancer. Biochim Biophys
Acta. 1856:151–164. 2015.PubMed/NCBI
|
|
20
|
Nieto MA: The ins and outs of the
epithelial to mesenchymal transition in health and disease. Annu
Rev Cell Dev Biol. 27:347–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skrypek N, Goossens S, De Smedt E,
Vandamme N and Berx G: Epithelial-to-mesenchymal transition:
Epigenetic reprogramming driving cellular plasticity. Trends Genet.
33:943–959. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Syed V: TGF-β signaling in cancer. J Cell
Biochem. 117:1279–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mani SA, Yang J, Brooks M, Schwaninger G,
Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL and Weinberg
RA: Mesenchyme forkhead 1 (FOXC2) plays a key role in metastasis
and is associated with aggressive basal-like breast cancers. Proc
Natl Acad Sci USA. 104:10069–10074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
David CJ, Huang YH, Chen M, Su J, Zou Y,
Bardeesy N, Iacobuzio-Donahue CA and Massagué J: TGF-β tumor
suppression through a lethal EMT. Cell. 164:1015–1030. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong SHM, Fang CM, Chuah LH, Leong CO and
Ngai SC: E-cadherin: Its dysregulation in carcinogenesis and
clinical implications. Crit Rev Oncol Hematol. 121:11–22. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu XL, Lu RY, Wang LK, Wang YY, Dai YJ,
Wang CY, Yang YJ, Guo F, Xue J and Yang DD: Long noncoding RNA
HOTAIR silencing inhibits invasion and proliferation of human colon
cancer LoVo cells via regulating IGF2BP2. J Cell Biochem. Oct
18–2018.(Epub ahead of print). View Article : Google Scholar
|
|
29
|
Dasgupta P, Kulkarni P, Majid S, Shahryari
V, Hashimoto Y, Bhat NS, Shiina M, Deng G, Saini S, Tabatabai ZL,
et al: MicroRNA-203 inhibits long noncoding RNA HOTAIR and
regulates tumorigenesis through epithelial-to-mesenchymal
transition pathway in renal cell carcinoma. Mol Cancer Ther.
17:1061–1069. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hong Q, Li O, Zheng W, Xiao WZ, Zhang L,
Wu D, Cai GY, He JC and Chen XM: LncRNA HOTAIR regulates HIF-1α/AXL
signaling through inhibition of miR-217 in renal cell carcinoma.
Cell Death Dis. 8:e27722017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang H, Cai K, Wang J, Wang X, Cheng K,
Shi F, Jiang L, Zhang Y and Dou J: MiR-7, inhibited indirectly by
lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of
breast cancer stem cells by downregulating the STAT3 pathway. Stem
Cells. 32:2858–2868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong X and Zhu Z: Long noncoding RNA
HOTAIR contributes to progression in hepatocellular carcinoma by
sponging miR-217-5p. Cancer Biother Radiopharm. 35:387–396. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang AH, Tan P, Zhuang Y, Zhang XT, Yu ZB
and Li LN: Down-regulation of long non-coding RNA HOTAIR inhibits
invasion and migration of oesophageal cancer cells via
up-regulation of microRNA-204. J Cell Mol Med. 23:6595–6610. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Y, Zhang L, Zhang L, Wang Y, Li H, Ren
X, Wei F, Yu W, Liu T, Wang X, et al: Long non-coding RNA HOTAIR
promotes tumor cell invasion and metastasis by recruiting EZH2 and
repressing E-cadherin in oral squamous cell carcinoma. Int J Oncol.
46:2586–2594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren Y, Jia HH, Xu YQ, Zhou X, Zhao XH,
Wang YF, Song X, Zhu ZY, Sun T, Dou Y, et al: Paracrine and
epigenetic control of CAF-induced metastasis: The role of HOTAIR
stimulated by TGF-ß1 secretion. Mol Cancer. 17:52018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song Y, Wang R, Li LW, Liu X, Wang YF,
Wang QX and Zhang Q: Long non-coding RNA HOTAIR mediates the
switching of histone H3 lysine 27 acetylation to methylation to
promote epithelial-to-mesenchymal transition in gastric cancer. Int
J Oncol. 54:77–86. 2019.PubMed/NCBI
|
|
37
|
Liu M, Zhang H, Li Y, Wang R, Li Y, Zhang
H, Ren D, Liu H, Kang C and Chen J: HOTAIR, a long noncoding RNA,
is a marker of abnormal cell cycle regulation in lung cancer.
Cancer Sci. 109:2717–2733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu YW, Sun M, Xia R, Zhang EB, Liu XH,
Zhang ZH, Xu TP, De W, Liu BR and Wang ZX: LincHOTAIR
epigenetically silences miR34a by binding to PRC2 to promote the
epithelial-to-mesenchymal transition in human gastric cancer. Cell
Death Dis. 6:e18022015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim HJ, Yim GW, Baek SM, Kim JW and Kim
YT: Long noncoding RNA HOTAIR is associated with human cervical
cancer progression. Gynecol Oncol. 133 (Suppl 1):S52014. View Article : Google Scholar
|
|
40
|
Berrondo C, Flax J, Kucherov V, Siebert A,
Osinski T, Rosenberg A, Fucile C, Richheimer S and Beckham CJ:
Expression of the long non-coding RNA HOTAIR correlates with
disease progression in bladder cancer and is contained in bladder
cancer patient urinary exosomes. PLoS One. 11:e01472362016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heldin CH, Vanlandewijck M and Moustakas
A: Regulation of EMT by TGFβ in cancer. FEBS Lett. 586:1959–1970.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ochoa-Hernández AB, Juárez-Vázquez CI,
Rosales-Reynoso MA and Barros-Núñez P: WNT-β-catenin signaling
pathway and its relationship with cancer. Cir Cir. 80:389–398.
2012.(In Spanish). PubMed/NCBI
|
|
44
|
Gao JZ, Li J, Du JL and Li XL: Long
non-coding RNA HOTAIR is a marker for hepatocellular carcinoma
progression and tumor recurrence. Oncol Lett. 11:1791–1798. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Salmerón-Bárcenas EG, Illades-Aguiar B,
Del Moral-Hernández O, Ortega-Soto A and Hernández-Sotelo D: HOTAIR
knockdown decreased the activity Wnt/β-catenin signaling pathway
and increased the mRNA levels of its negative regulators in Hela
cells. Cell Physiol Biochem. 53:948–960. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheng C, Qin Y, Zhi Q, Wang J and Qin C:
Knockdown of long non-coding RNA HOTAIR inhibits cisplatin
resistance of gastric cancer cells through inhibiting the PI3K/Akt
and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int
J Biol Macromol. 107:2620–2629. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xiao Z, Qu Z, Chen Z, Fang Z, Zhou K,
Huang Z, Guo X and Zhang Y: LncRNA HOTAIR is a prognostic biomarker
for the proliferation and chemoresistance of colorectal cancer via
MiR-203a-3p-mediated Wnt/β-catenin signaling pathway. Cell Physiol
Biochem. 46:1275–1285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li GJ, Ding H and Miao D: Long-noncoding
RNA HOTAIR inhibits immunologic rejection of mouse leukemia cells
through activating the Wnt/β-catenin signaling pathway in a mouse
model of leukemia. J Cell Physiol. 234:10386–10396. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li J, Yang S, Su N, Wang Y, Yu J, Qiu H
and He X: Overexpression of long non-coding RNA HOTAIR leads to
chemoresistance by activating the Wnt/β-catenin pathway in human
ovarian cancer. Tumour Biol. 37:2057–2065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ge XS, Ma HJ, Zheng XH, Ruan HL, Liao XY,
Xue WQ, Chen YB, Zhang Y and Jia WH: HOTAIR, a prognostic factor in
esophageal squamous cell carcinoma, inhibits WIF-1 expression and
activates Wnt pathway. Cancer Sci. 104:1675–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ying Y and Tao Q: Epigenetic disruption of
the WNT/beta-catenin signaling pathway in human cancers.
Epigenetics. 4:307–312. 2009. View Article : Google Scholar
|
|
52
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Petrulea MS, Plantinga TS, Smit JW,
Georgescu CE and Netea-Maier RT: PI3K/Akt/mTOR: A promising
therapeutic target for non-medullary thyroid carcinoma. Cancer
Treat Rev. 41:707–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen J, Lin C, Yong W, Ye Y and Huang Z:
Calycosin and genistein induce apoptosis by inactivation of
HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells.
Cell Physiol Biochem. 35:722–728. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hales EC, Taub JW and Matherly LH: New
insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling
axis: Targeted therapy of γ-secretase inhibitor resistant T-cell
acute lymphoblastic leukemia. Cell Signal. 26:149–161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Salmena L, Carracedo A and Pandolfi PP:
Tenets of PTEN tumor suppression. Cell. 133:403–414. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun H, Lesche R, Li DM, Liliental J, Zhang
H, Gao J, Gavrilova N, Mueller B, Liu X and Wu H: PTEN modulates
cell cycle progression and cell survival by regulating
phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B
signaling pathway. Proc Natl Acad Sci USA. 96:6199–6204. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yu Y, Lv F, Liang D, Yang Q, Zhang B, Lin
H, Wang X, Qian G, Xu J and You W: HOTAIR may regulate
proliferation, apoptosis, migration and invasion of MCF-7 cells
through regulating the P53/Akt/JNK signaling pathway. Biomed
Pharmacother. 90:555–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li E, Zhao Z, Ma B and Zhang J: Long
noncoding RNA HOTAIR promotes the proliferation and metastasis of
osteosarcoma cells through the AKT/mTOR signaling pathway. Exp Ther
Med. 14:5321–5328. 2017.PubMed/NCBI
|
|
60
|
Li Z, Qian J, Li J and Zhu C: Knockdown of
lncRNA-HOTAIR downregulates the drug-resistance of breast cancer
cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp
Ther Med. 18:435–442. 2019.PubMed/NCBI
|
|
61
|
Pan S, Liu Y, Liu Q, Xiao Y, Liu B, Ren X,
Qi X, Zhou H, Zeng C and Jia L: HOTAIR/miR-326/FUT6 axis
facilitates colorectal cancer progression through regulating
fucosylation of CD44 via PI3K/AKT/mTOR pathway. Biochim Biophys
Acta Mol Cell Res. 1866:750–760. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li Z and Luo J: Epigenetic regulation of
HOTAIR in advanced chronic myeloid leukemia. Cancer Manag Res.
10:5349–5362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Luan W, Li R, Liu L, Ni X, Shi Y, Xia Y,
Wang J, Lu F and Xu B: Long non-coding RNA HOTAIR acts as a
competing endogenous RNA to promote malignant melanoma progression
by sponging miR-152-3p. Oncotarget. 8:85401–85414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li T, Qin Y, Zhen Z, Shen H, Cong T,
Schiferle E and Xiao S: Long non-coding RNA HOTAIR/microRNA-206
sponge regulates STC2 and further influences cell biological
functions in head and neck squamous cell carcinoma. Cell Prolif.
52:e126512019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Itoh N and Ornitz DM: Fibroblast growth
factors: From molecular evolution to roles in development,
metabolism and disease. J Biochem. 149:121–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ke J, Yao YL, Zheng J, Wang P, Liu YH, Ma
J, Li Z, Liu XB, Li ZQ, Wang ZH and Xue YX: Knockdown of long
non-coding RNA HOTAIR inhibits malignant biological behaviors of
human glioma cells via modulation of miR-326. Oncotarget.
6:21934–21949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling-in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fu WM, Lu YF, Hu BG, Liang WC, Zhu X, Yang
HD, Li G and Zhang JF: Long noncoding RNA Hotair mediated
angiogenesis in nasopharyngeal carcinoma by direct and indirect
signaling pathways. Oncotarget. 7:4712–4723. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Burger RA: Role of vascular endothelial
growth factor inhibitors in the treatment of gynecologic
malignancies. J Gynecol Oncol. 21:3–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Z and Li Z: Glucose regulated protein
78: A critical link between tumor microenvironment and cancer
hallmarks. Biochim Biophys Acta. 1826:13–22. 2012.PubMed/NCBI
|
|
71
|
Geng YJ, Xie SL, Li Q, Ma J and Wang GY:
Large intervening non-coding RNA HOTAIR is associated with
hepatocellular carcinoma progression. J Int Med Res. 39:2119–2128.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX and
De W: The long non-coding RNA HOTAIR indicates a poor prognosis and
promotes metastasis in non-small cell lung cancer. BMC Cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhao WH, Yuan HY, Ren XY, Huang K and Guo
ZY: Association between expression of HOTAIR and invasiveness of
gliomas, and its predictive value. Adv Clin Exp Med. 28:1179–1183.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kim HJ, Lee DW, Yim GW, Nam EJ, Kim S, Kim
SW and Kim YT: Long non-coding RNA HOTAIR is associated with human
cervical cancer progression. Int J Oncol. 46:521–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Flavahan WA, Gaskell E and Bernstein BE:
Epigenetic plasticity and the hallmarks of cancer. Science.
357:eaal23802017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Khalil AM, Guttman M, Huarte M, Garber M,
Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van
Oudenaarden A, et al: Many human large intergenic noncoding RNAs
associate with chromatin-modifying complexes and affect gene
expression. Proc Natl Acad Sci USA. 106:11667–11672. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ding C, Cheng S, Yang Z, Lv Z, Xiao H, Du
C, Peng C, Xie H, Zhou L, Wu J and Zheng S: Long non-coding RNA
HOTAIR promotes cell migration and invasion via down-regulation of
RNA binding motif protein 38 in hepatocellular carcinoma cells. Int
J Mol Sci. 15:4060–4076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Godlewski J, Krichevsky AM, Johnson MD,
Chiocca EA and Bronisz A: Belonging to a network-microRNAs,
extracellular vesicles, and the glioblastoma microenvironment.
Neuro Oncol. 17:652–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fan Q, Yang L, Zhang X, Peng X, Wei S, Su
D, Zhai Z, Hua X and Li H: The emerging role of exosome-derived
non-coding RNAs in cancer biology. Cancer Lett. 414:107–115. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gezer U, Özgür E, Cetinkaya M, Isin M and
Dalay N: Long non-coding RNAs with low expression levels in cells
are enriched in secreted exosomes. Cell Biol Int. 38:1076–1079.
2014.PubMed/NCBI
|
|
83
|
Ma X, Li Z, Li T, Zhu L, Li Z and Tian NJ:
Long non-coding RNA HOTAIR enhances angiogenesis by induction of
VEGFA expression in glioma cells and transmission to endothelial
cells via glioma cell derived-extracellular vesicles. Am J Transl
Res. 9:5012–5021. 2017.PubMed/NCBI
|
|
84
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang C, Ji Q, Yang Y, Li Q and Wang Z:
Exosome: Function and Role in cancer metastasis and drug
resistance. Technol Cancer Res Treat. 17:15330338187634502018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang H, Fu H, Xu W and Zhang X: Exosomal
non-coding RNAs: A promising cancer biomarker. Clin Chem Lab Med.
54:1871–1879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tang S, Zheng K, Tang Y, Li Z, Zou T and
Liu D: Overexpression of serum exosomal HOTAIR is correlated with
poor survival and poor response to chemotherapy in breast cancer
patients. J Biosci. 44:372019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang J, Zhou Y, Lu J, Sun Y, Xiao H, Liu M
and Tian L: Combined detection of serum exosomal miR-21 and HOTAIR
as diagnostic and prognostic biomarkers for laryngeal squamous cell
carcinoma. Med Oncol. 31:1482014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang C, Xu L, Deng G, Ding Y, Bi K, Jin
H, Shu J, Yang J, Deng H, Wang Z and Wang Y: Exosomal HOTAIR
promotes proliferation, migration and invasion of lung cancer by
sponging miR-203. Sci China Life Sci. 63:1265–1268. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Poulet C, Njock MS, Moermans C, Louis E,
Louis R, Malaise M and Guiot J: Exosomal long non-coding RNAs in
lung diseases. Int J Mol Sci. 21:35802020. View Article : Google Scholar
|
|
92
|
Yao Z, Jia C, Tai Y, Liang H, Zhong Z,
Xiong Z, Deng M and Zhang Q: Serum exosomal long noncoding RNAs
lnc-FAM72D-3 and lnc-EPC1-4 as diagnostic biomarkers for
hepatocellular carcinoma. Aging (Albany NY). 12:11843–11863. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang J, Qiu WQ, Zhu H, Liu H, Sun JH,
Chen Y, Shen H, Qian CL and Shen ZY: HOTAIR contributes to the
carcinogenesis of gastric cancer via modulating cellular and
exosomal miRNAs level. Cell Death Dis. 11:7802020. View Article : Google Scholar : PubMed/NCBI
|