Introduction
The human digestive system is composed of a
digestive tube (comprising the oral cavity, pharynx, esophagus,
stomach, small intestine and colorectum) and digestive gland
organs, including salivary glands, liver, gallbladder and pancreas
(1). The most common malignant
tumors of the digestive system include esophageal cancer, gastric
cancer, colorectal cancer, liver cancer and pancreatic cancer
(1). Due to the high incidence rate
of digestive system cancers, digestive tract tumors accounted for
43.3% of cancer incidence in China from 2000–2015, a comprehensive
study of their underlying molecular biology would cast light on
effective methods of treatment (2).
However, an accurate in vitro research model that would
improve our understanding of the complete picture of tumor
development is currently lacking.
Current in vitro cancer research models
mainly focus on two-dimensional (2D) models, namely, tissue-lice
cultures and 2D cell-line cultures (1). Tissue section models can capture
transient interactions between physiologically relevant cellular
tissues, although they often lose their phenotype quickly and are
difficult to maintain for longer time periods (3). Currently, the most widely used tumor
research model is based on patient-derived cancer cell lines
(PDCs); however, although PDC culture models are economical, simple
and the cell lines proliferate infinitely, they still have serious
defects, such as the loss of genetic phenotype and heterogeneity,
the lack of immune microenvironment and vascular network system,
and cannot reproduce the morphology and function of the original
tumor tissue (4).
Organoids are derived from multipotent tissue
progenitors (adult stem cells) and cancer cells, consisting of
multiple cell types with remarkable self-renewal and
self-organizing abilities, which maintain the key structural and
functional properties of organs (5,6). The
organoid model is more representative of typical physiological
conditions compared with other models (4). Currently, the organoids for normal
tissues, including the stomach (7),
small intestine (8), liver (9), pancreas (10) and prostate (11), have been established successfully.
Compared with PDCs, the patient-derived organoids (PDOs) not only
reflect histopathological characteristics and the genomic and
transcriptomic profiles of the original tumor, but also
recapitulate the components of the tumor microenvironment (12). With the development of gene-editing
technologies, including gene knockdown, gene overexpression and
mutations, the successful establishment of organoids is of great
significance for the study of solid tumors. In addition, organoids
only require a small piece of tissue containing stem cells obtained
from biopsy in the patient, and the injury inflicted on the patient
is minimal (13).
The present review summarizes the currently
developed digestive system solid tumor organoids and discusses
their potential applications in future research.
Development and merit of organoids
In the 1960s, three-dimensional (3D) culture models
were developed, although these were mainly used to simulate the
processes and investigate the molecular mechanisms of tissue and
organ formation in vitro (14). In 2009, Sato et al (15) were the first to produce an organoid
with small intestinal villi and crypt structure from adult stem
cells. Since then, tissue culture has regained its status as a
leading contemporary technique, as it was ranked as one of the top
10 breakthroughs in 2013 and 2017 by Science and Nature
Methods magazines, respectively. As the technology has
advanced, organoids have been extensively used as a powerful tool
of tumor research, particularly regarding the digestive system
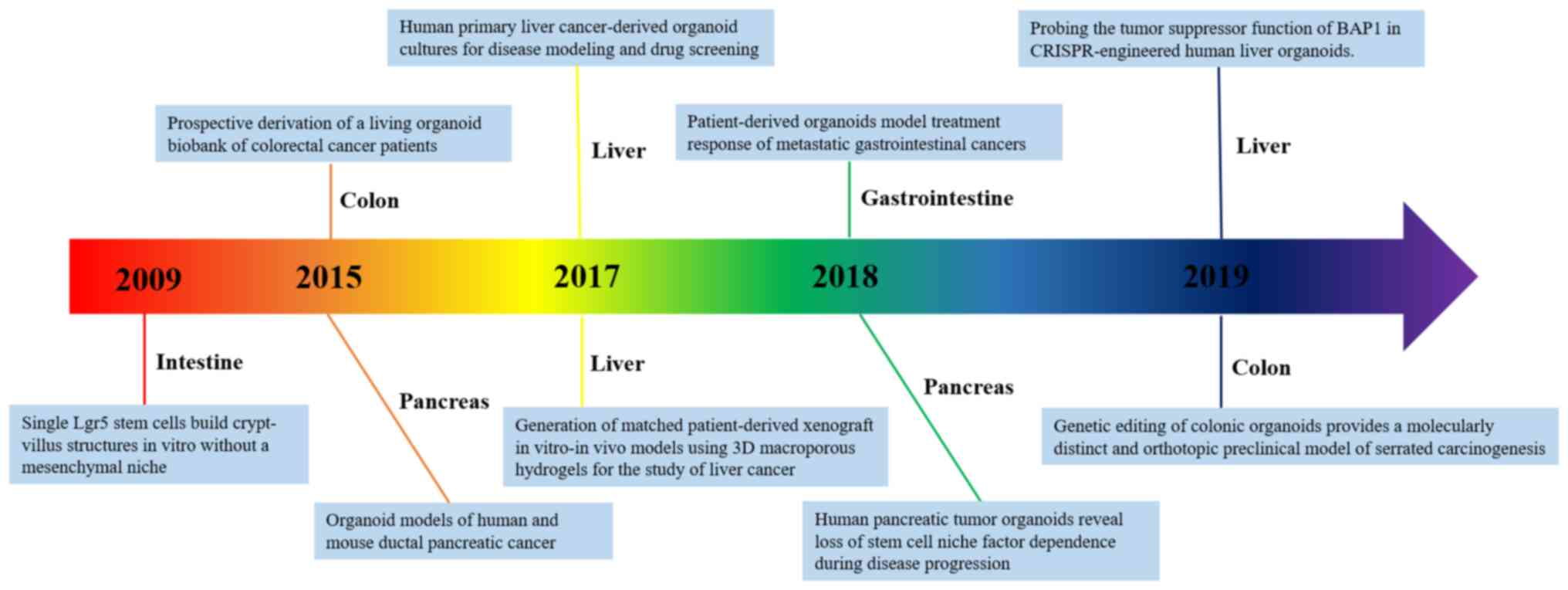
(Fig. 1) (7–11).
Tumor organoid construction is associated with
significantly higher success rates and lower costs compared with
traditional 2D culture and tumor tissue xenotransplantation, which
is convenient for gene modification and large-scale drug screening
(1). In addition, the organoid model
retains the tissue characteristics of the tumor during the
experimental process, which is useful in terms of tumor
microenvironment research, and provides a more realistic
environment for tumor drug development (Table I) (2–6). Neal
et al (16) developed PDOs
using air-liquid interface technology. The PDOs model successfully
retained the same intrinsic fibrous matrix and diversity of immune
cell components that were observed in the original tumor tissues,
which was confirmed at the genetic level. Furthermore,
tumor-infiltrating lymphocytes in PDOs accurately retained the
T-cell receptor profile in the original tumor.
 | Table I.Characteristics of tissue sections,
2D culture cell lines and 3D organoids. |
Table I.
Characteristics of tissue sections,
2D culture cell lines and 3D organoids.
| Variable | Initiation
efficiency | Heterogeneity | Genetic
manipulation | Drug screens |
|---|
| Tissue | Low | High |
Not
amenable | Low-throughput |
| 2D culture
cell | Low | Low | Amenable |
High-throughput |
| 3D organoids | High | High | Amenable |
High-throughput |
Application of organoids in digestive system
solid tumor cancer research
Digestive system solid tumors accounted for ~35.4%
of cancer mortality rates worldwide in 2019 (17). The growth condition flexibility of
organoids enable them to precisely simulate heterogeneity and
pathophysiology of digestive system solid tumors, which is useful
for gaining a better understanding of the biological behaviors of
the disease (4). In addition, the
introduction of gene-editing technology for organoids has exhibited
great advantages in terms of studying the pathogenesis of digestive
system solid tumor treatment (12).
Phenotypical and genotypical analyses of PDOs have confirmed that
these are highly similar to the original tumor (12). The molecular profile of tumor
organoids was demonstrated to match the results of drug screening,
indicating that PDOs can complement existing methods to identify
cancer weaknesses and improve the treatment response. Thus, it is
reasonable to speculate that, in the future, organoids will serve
important roles in drug development, personalized therapy, gene
therapy and regenerative medicine (Table II).
 | Table II.Characteristics of tissue sections,
2D culture cell lines and 3D organoids. |
Table II.
Characteristics of tissue sections,
2D culture cell lines and 3D organoids.
| Application | Cancer | Refs. |
|---|
| Tumor development
mechanism | Gastric cancer,
pancreatic cancer, colorectal cancer | (9,19,20,33,36,39,46) |
| Drug screening and
personalized treatment | Liver cancer,
colorectal cancer | (23,24,29,30) |
| Malignant Tumor
Organoid Biological Bank | Colorectal cancer,
gastric cancer | (27) |
Mechanism of tumor development
From the onset of the mutant single cell to the
progression of the lesion, the way the tumor develops and
deteriorates is an interrelated and multistep process (5). It is important to study the exact
mechanism underpinning each step in the overall process of tumor
development and progression. Organoids consist of multiple cells
that specifically mimic the microenvironment of cancer development
(18–20). How to construct models in
vitro at different stages of tumorigenesis and development is
one of the key research areas of organoids (18). Bartfeld et al (19) developed p53 gene-mutation gastric
cancer organoids with Nulin-3-selective medium, which revealed that
Nutli3 can markedly inhibit the proliferation of normal gastric
organoids, and has no effect on gastric organoids, which indirectly
suggests that the occurrence and development of gastric cancer may
be studied in vitro. Other researchers have also used
gene-editing technology to mutate certain genes in normal gastric
organoids to simulate the evolutionary process of gastric cancer,
suggesting that deletion of p53 or mutation of KRAS contribute to
the occurrence of gastric cancer (20).
Drug screening and personalized
treatment
Organoids can proliferate infinitely in
vitro, which maximizes the characteristics of cancer cells
without inducing novel mutations during long-term culture (21). Thus, they provide an ideal model for
drug testing and screening, which has good prospects in terms of
the applicability of the technique in combination with traditional
treatment methods, and this should prove useful in treating tumors
(21). Based on the organoids and
high-content screening technologies, Wenzel et al (22) screened out nine substances from two
commercially available drug libraries that specifically target
dormant cancer cells that are predominantly located in the internal
core area of the tumor tissues, and are resistant to treatment with
conventional chemotherapy or radiotherapy. Arena et al
(23) suggested that one colorectal
cancer subpopulation with poor prognosis and limited therapeutic
options may be sensitive to poly(ADP-ribose) polymerase inhibitors,
and drug-screening assays based on PDOs may be used to screen
populations of cells that are sensitive to olaparib. In addition to
drug screening, Yao et al (24) constructed a PDO biobank from patients
with locally advanced rectal cancer (LARC). The PDOs were able to
identify patients with LARC that were sensitive to neoadjuvant
chemoradiation, thereby improving the treatment of this disease,
highlighting that PDOs may be used to predict chemotherapy or
radiotherapy responses. From the perspective of personal precision
treatment, organoids can be used for expanding limited cancer
samples from patients, for the screening of drugs, and as a
therapeutic avenue for specific patients, assessing the
side-effects of drugs and therapies in patients (25). In summary, organoids provide helpful
assistance in terms of the accurate medical treatment of individual
patients with digestive system solid tumors.
The malignant tumor organoid
biological bank
The Malignant Tumor Organoid Biological bank is a
biological sample bank for malignant tumors that combines targeted
genes with drug action, thereby providing a basis for risk
stratification and individualized treatment options (26). In 2015, the Sanger and the Hubrecht
Institutes established the first living organoid biobank (27). The researchers involved examined 83
different types of experimental drugs and cancer drugs, and
concluded that organoids with different genetic backgrounds are
associated with different drug sensitivities.
Application of organoids in digestive system
solid tumors
Liver cancer-derived organoid
According to the results of a 2019 survey, liver
cancer is the second most lethal cancer worldwide among men
(17). 2D culture systems fail to
preserve the histological architecture and gene expression patterns
of the original tumor, and are unable to mimic the real
communication between cancer and stomal cells (28). To better understand the processes of
tumorigenesis and pathogenesis, and to develop curative treatments,
organoids provide the most precise model for accurately simulating
tumor biology behavior in patients (28).
Researchers at Cambridge University were the first
to develop a mini tumor called a ‘tumoroid’ (<0.5 cm) to imitate
three of the most common subtypes of primary liver cancer, namely
hepatocellular carcinoma (HCC), cholangiocarcinoma (CC) and
combined HCC/CC (CHC) tumors (29).
The tumoroid panel was used to screen 29 specific liver cancer
target drugs (29). One of the
screened drugs was able to specifically inhibit the activity of
extracellular signal-regulated kinase (29). The ERK inhibitor SCH772984 markedly
inhibited the growth of liver tumors in mice and beneficial
therapeutic effects on three types of liver cancers were observed,
suggesting its potential therapeutic value for primary liver cancer
(29). This discovery is now
considered to be the milestone for the usage of organoids in liver
cancer research (29). Scientists
from the National University of Singapore later designed a novel
method to culture cancerous hepatocytes obtained from
HCC-patient-derived xenografts (HCC-PDXs) for drug screening
(30). To maintain the exact shape
and function of hepatocytes so that they grew like tumor organoids,
cells were cultured on a 3D macroporous cellulosic sponge system
with optimized biochemical and mechanical properties (30). With the development of this
technology, engineered organoids have continued to attract great
attention, and the most attractive advantage is high-throughput
drug screening (30). The genetic
characteristics and heterogeneity of these models may make it
possible to radically alter the screening and development of HCC
drugs (9). Using CRISPR/Cas9 to
study the role of mutated genes in the formation and progression of
liver tumors, researchers have demonstrated that BAP1-mutated
organoids have characteristics that distinguish them from healthy
organoids (9). BAP1-mutated
organoids have been reported to grow faster, move more easily and
fuse more easily with other organoids, and these features are
similar to aggressive malignancies (9). The morphological and behavioral changes
of the organoid were reversed following introduction of unmutated
BAP1 (9). Due to the ease with which
this organoid permitted the function of mutated genes to be
analyzed, its suitability for use in a wider context in the study
of liver cancer was demonstrated (9).
Due to a lack of effective treatments for liver
cancer, the development of liver cancer-derived organoids may
accurately reflect the characteristics of this disease and the
surrounding environment, which may facilitate the selection of more
clinically efficacious drugs, thereby saving on costs and improving
clinical transformation efficiency.
Pancreatic cancer-derived
organoids
Pancreatic cancer is known as ‘the stubborn bastion
of 21st century medicine’, with a 5-year survival rate <8%
(31). To better understand
pancreatic cancer development and progression, it is necessary to
isolate cancer cells for analysis from the primary tumor tissue.
However, nearly 85% of patients with pancreatic cancer will have
already progressed to an advanced stage at the time of diagnosis,
and thus are not suitable for surgery (32). Both normal cells and cancerous cells
cultured in the laboratory were derived from a suboptimal
environment that failed to reflect the important characteristics of
tumor cells (12). The limited
treatment options available for patients with pancreatic cancer has
adversely affected their prognosis. Thus, 3D culture strategies of
organoids can shed light on this disease.
In 2015, the Cold Spring Harbor Laboratory (CSHL)
successfully established pancreatic organoids from murine and human
tissues (33). The organoid was able
to cultivate murine and human pancreatic cancer tissues (33). In addition, following transplantation
into mice, the organoid was demonstrated to accurately imitate the
pathogenesis of pancreatic ductal adenocarcinoma (PDA) and
successfully recapitulate the full spectrum of tumor development
(33). The identified molecular
pathways based on the organoid specifically reflected the
progression of the disease and provided the therapeutic target
(34). Huang et al (35) established exocrine progenitor
organoids derived from human pluripotent stem cells. These
researchers developed five tumor organoids and tested their
response to gemcitabine and epigenetic inhibitors. The results
demonstrated that the response of different organoids to the drugs
varied and was positively correlated with drug-resistant
biomarkers, findings that were consistent with the clinical
response of patients. Using a small sample, pancreatic cancer
organoids can retain the sensitivity of the patient's tissue to
novel agents in vitro, which indicates the feasibility of
using organoid-based techniques to study tumor heterogeneity
(35). The greatest advantage of
this technology is being able to establish a living sample library
using multiple patient tumor tissues, and to study the specific
pathogenesis of each patient, both of which are beneficial for
targeted research and for rapid individualized drug testing
(35).
Using tumor and normal tissue organoids from
patients with pancreatic cancer, Chio et al (36) reported that the expression level of
nuclear factor erythroid 2-related factor 2 (NRF2) was higher in
the tumor organoids. NRF2 knockdown exhibited a different response
since tumor organoids were not able to grow; however, the normal
tissue organoids were not affected. Furthermore, this group studied
changes in the oxidation level in the cells and confirmed that NRF2
exerts a notable effect on the proliferation of pancreatic tumor
cells, suggesting that decreasing the levels of antioxidants kills
tumors.
Researchers from the CSHL established the organoid
based on cancer-associated fibroblasts (CAFs) and cancer cells from
the patient tumor samples (37). The
results demonstrated that myofibroblastic CAFs (myCAFs) higher
levels of α-smooth muscle actin (αSMA), which approximated to the
behavior of tumor cells in human and mouse tumor tissues. The
researchers also discovered that co-culture resulted in the
formation of dense matrix tissue. Inflammatory CAFs lacked elevated
αSMA expression, which synthesizes and secretes interleukin 6
(IL-6), and is relatively distant from cancer cells in human and
mouse PDA tumors (37). A previous
study demonstrated that IL-6 is associated with the processes of
cancer cell proliferation and cachexia in numerous patients with
pancreatic cancer (38). This study
reported that the behavior of stromal tissue in PDA is not uniform,
which provides an opportunity to develop therapeutic drugs that can
specifically target the stromal cells. Using the pancreatic
organoid technique, this research group confirmed that FOXA1
activation induces the reprogramming of enhancers, which causes
pancreatic cancer cells to metastasize (39).
CSHL also demonstrated that it is feasible to
cultivate organoids using endoscopic ultrasonography (EUS)-derived
pancreatic tumor samples (40). PDA
organoids derived from the EUS samples can be developed within 2
weeks, with a success rate of 87%. Establishment of PDA organoid
lines for ≥5 passages or cycles of growth (P5) was also
demonstrated, with a success rate of 66% (40). The advent of EUS-guided fine-needle
biopsy (EUS-FNB) technology may be used in the chest, abdomen and
pelvis, and in the future, this technique is likely to be used to
prepare organoids from a variety of different tumors for research
purposes (40).
In addition to demonstrating the success of organoid
development and the consistency between the organoids and the
original tumors, in terms of pathology and genetics, Seino et
al (41) also used organoids for
tumor microenvironment research. They identified an association
between the degree of malignancy of pancreatic adenocarcinoma
(PDAC) and growth factors in the microenvironment, and demonstrated
the association between GATA-6 and Wnt in the niche. As CAFs
provide a Wnt niche for PDAC, the supportive role of CAFs in
pancreatic cancer was further confirmed (41). Their experimental results were
verified using CRISPR-Cas9 technology. The concept of ‘engineering
organoids’ in the article was innovative (41). Their research enabled pancreatic
cancer to be classified as Wnt- and R-Spondin-dependent,
Wnt-dependent, and Wnt and R-Spondin-independent (41). This classification is of great value
in clinical practice and may be used for individual treatment
(41).
Organoid technology not only has the potential to
fill in the void of cancer genetics, but it also provides
theoretical guidance for pancreatic cancer (42). The characteristics of pancreatic
cancer organoids include maintaining the differentiation state
tissue structure and phenotypic heterogeneity of the primary
pancreatic cancer (42). Thus,
organoid technology allows a new research direction for studying
the biological characteristics of pancreatic cancer, enabling more
effective anti-pancreatic cancer drugs to be investigated and the
formulation of personalized treatment plans.
Gastrointestinal cancer-derived
organoid
The first human colonic organoid model was achieved
at Cincinnati Children's Hospital Medical Center using pluripotent
stem cells (43). This technique has
enabled the use of a human-like model to perform unprecedented
research on colonic diseases, which will prove to be invaluable in
the future. Transforming growth factor-β (TGF-β) can promote the
proliferation and distant metastasis of colorectal cancer cells,
and induce fibroblasts to differentiate into myofibroblasts, which
promotes the metastasis of colorectal cancer (44). Using the colorectal cancer organoids,
Usui et al (45) concluded
that fibroblasts secrete TGF-β. Utilizing combined organoids and
CRISPR/Cas9 genetic engineering technology, Australian scientists
demonstrated that elevated expression levels of v-raf murine
sarcoma viral oncogene homolog B1 were involved in the progression
of serrated colorectal cancer (46).
Organoid models can also be applied in the case of
genetic manipulation in gastrointestinal cancer (47–49).
Nadauld et al (47)
demonstrated that TGF-β receptor 2 was able to inhibit the
metastasis of gastric cancer based on the organoids in
vitro, which was consistent with the results demonstrated in
vivo with cadherin 1−/−/Tp53−/− gastric
cancer mice (47). In examining
mutations of the genes, Apc, Tp53, Kras and Smad4 in
gastrointestinal organoids, Li et al (48) reported that they exhibited aggressive
histological features on subsequent living transplants. Another
research group constructed the largest known number of gastric
cancer organoid biobanks, and considered in depth the genomic
variation, molecular typing, chemosensitivity and sensitivity of
targeted therapies for gastric cancer (49). This study is of great value in terms
of comprehending the pathogenesis of gastric cancer, and in
promoting the development of novel drugs for gastrointestinal
cancer-targeted therapy.
The metastatic organoid model has rarely been
constructed. The application of PDO-tested cancer drugs may assist
specialists in treating patients with more personalized therapies
specifically targeted for tumor metastasis (50). Researchers successfully cultivated
into organoids 71 colorectal cancer, gastric cancer and other
digestive system cancers with metastatic tumor samples from
patients, and subsequently screened 55 anticancer metastasis drugs
(50). It was demonstrated that the
histology, molecular structure and function of the organoids
remained highly consistent with those of the primary tumor tissues,
thereby confirming the usefulness of their characteristics
(50). When predicting patients'
response to drugs with PDOs, the overall sensitivity was 100%, the
specificity was 93%, the positive predictive value was 88% and the
negative predictive value was 100% (50). Organoids cultivated from the
patients' samples were thereby demonstrated to have predictive
ability. In addition to the potential for personalized treatment,
organoids can also fulfill a crucial role in drug development
(50). Optimizing the simulation of
how tumors are expected to react to treatment can be helpful in
terms of accelerating the process of drug discovery, and even in
terms of decreasing the dependence on animal experiments (50).
Gastrointestinal organoids provide a basic
experimental model, superior to experimental animal models and
conventional cell culture models in several aspects. As newly
generated preclinical tumor models, the gastrointestinal cancer
organoids have had a major role in the study of the biological
characteristics and underlying molecular mechanism of
gastrointestinal cancer and have been demonstrated to simulate the
occurrence and development of tumors. There are promising prospects
with their application in the development and screening of
antitumor drugs, targeted tumor therapies and personalized
medicine, and their combined application with PDXs (51).
Summary and outlook
Limitations
Recently, research on organoids has achieved
considerable progress. However, limitations exist between
theoretical in vitro studies and clinical applications due
to the technical bottlenecks of the culture system. First,
organoids mainly rely on animal-derived Matrigel or collagen I.
Matrigel, or animal-derived Matrigel components, are complex and
their quality is difficult to control (5). Exotic pathogens are also potential risk
factors (52). In addition, the
organoid system ignores the effects of stromal cells (52). The current existing organoid cultures
are devoid of intestinal microbiota, vascular endothelial cells,
neurons and immune cells, so their role in the physiology and the
pathology of tumor processes does not come into consideration
(52). Organoid is only supposed to
reflect the physiological or pathological characteristics of local
tissues, rather than the effects of systemic inflammatory responses
and autonomic nerves (53). The
recent emergence of a new organoid culture model has allowed the
co-culturing of epithelial organoids and mesenchymal cells, which
will enable the further study of the interactions between tumor and
mesenchymal cells (54), and nerves
and vascular tissues. Fong et al (55) co-cultured tumor cells derived from
the PDX model of prostate cancer with osteoblasts, and the 3D model
formed in this culture system was able to successfully maintain the
proliferative activity of cells, and maintain the state of
osteogenesis. Thus, this type of organoid model can be applied to
observe the interaction between tumor cells and the tumor
microenvironment, including stromal cells. However, additional
research is required for further investigation of the organoid
culture system. For example, the addition of stromal cells,
adipocytes or lymphocytes would make the organoid culture system
even more similar to the living tumor microenvironment, thereby
providing a more ideal model for studying the association between
the microenvironment and the tumor. Furthermore, organoids lack
homogeneity (56). As the organoids
are cultivated in 3D Matrigel, a certain differential effect is
exerted by the Matrigel on their growth (5). Organoids on the periphery of Matrigel
generally tend to be larger, whereas their size in the middle of
Matrigel is smaller (56). This size
heterogeneity creates a problem in terms of the quantification and
analysis of high-flux organoid cells (56). Organoid transplants still face many
challenges (57). Although these
organoids composed of many cells can simulate the internal
structure of real organs in certain aspects, other structural
characteristics closely associated with the function and
development of real organs cannot be simulated as of now, including
the lack of a vascular system (57).
Thus far, organoids would be better described as miniature and
simple organ models, rather than being a ‘reduced version’ of the
real organ (57). For example, a
neurologist who used brain organoids to study the Zika virus
considers that researchers have so far been unable to give
organoids for immune systems the integrity they require to
replicate exactly what happens in the body when screening drugs
(58). Thus, organoid
transplantations still face many challenges. In general, organoids
cannot be successfully transplanted into mice in place of real
organs due to their lack of vascular system and functionality
(56,57).
Perspective
Organoids have unique advantages in tumor research:
i) They maintain high heterogeneity of tumor cells; ii) they
maintain contact polarity between tumor cells and the
microenvironment matrix to better simulate the tumor
microenvironment in vivo; iii) organoid cultures derived
from clinical tissue are highly efficient and time-saving and iv)
tumor organoids also have the advantages of genetic manipulation of
tumor cell lines and 3D complex-system characteristics of mouse PDX
models (59).
However, further studies are required to optimize
the culture techniques, as organoids derived from different human
tissues may have the potential to promote relevant medical
technologies; for example, establishing models of rare diseases
in vitro, biological 3D printing, screening of patient's
individual drugs and therapies, primary screening of toxicity and
side effects, high-throughput drug screening, transplantation of
organoids in vivo and the study of tissue regeneration. The
future application of organoids will be broad and diverse, and it
is hoped that the development of organoids will be an indispensable
foundation for precision medicine in the future.
Acknowledgements
Not applicable.
Funding
The present review was supported by grants from the
Natural Science Foundation of Jiangsu Province (grant no.
BK20191223), the Key Program of Jiangsu Commission of Health (grant
no. K2019024), the Young Medical Talents of Jiangsu (grant no.
QNRC2016833), the Six Talent Peals Project of Jiangsu Province
(grant no. WSW-039), the Six for One Project of Jiangsu Province
(grant no. LGY2018093) and the Postgraduate Research & Practice
Innovation Program of Jiangsu Province (grant no. SJCX19_1175).
Availability of data and materials
Not applicable.
Authors' contributions
XY and XX wrote the draft of the manuscript. MW and
HZ contributed to the conception of the work and organized the
structure of the manuscript. DW revised the manuscript for
important intellectual content. HZ and DW confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lau H, Kranenburg O, Xiao H and Yu J:
Organoid models of gastrointestinal cancers in basic and
translational research. Nat Rev Gastroenterol Hepatol. 17:203–222.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei W, Zeng H, Zheng R, Zhang S, An L,
Chen R, Wang S, Sun K, Matsuda T, Bray F and He J: Cancer
registration in China and its role in cancer prevention and
control. Lancet Oncol. 21:e342–e349. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gähwiler BH, Capogna M, Debanne D,
McKinney RA and Thompson SM: Organotypic slice cultures: A
technique has come of age. Trends Neurosci. 20:471–477. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fatehullah A, Tan SH and Barker N:
Organoids as an in vitro model of human development and disease.
Nat Cell Biol. 18:246–254. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clevers H: Modeling development and
disease with organoids. Cell. 165:1586–1597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dutta D, Heo I and Clevers H: Disease
modeling in stem cell-derived 3D organoid systems. Trends Mol Med.
23:393–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Lau BT, Andor N, Grimes SM, Handy
C, Wood-Bouwens C and Ji HP: Single-cell transcriptome analysis
identifies distinct cell types and niche signaling in a primary
gastric organoid model. Sci Rep. 9:45362019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hartl L, Huelsz-Prince G, van Zon J and
Tans SJ: Apical constriction is necessary for crypt formation in
small intestinal organoids. Dev Biol. 450:76–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Artegiani B, van Voorthuijsen L, Lindeboom
RGH, Seinstra D, Heo I, Tapia P, López-Iglesias C, Postrach D,
Dayton T, Oka R, et al: Probing the tumor suppressor function of
BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell.
24:927–943.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuura T, Maru Y, Izumiya M, Hoshi D,
Kato S, Ochiai M, Hori M, Yamamoto S, Tatsuno K, Imai T, et al:
Organoid-based ex vivo reconstitution of Kras-driven pancreatic
ductal carcinogenesis. Carcinogenesis. 41:490–501. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shu Y and Chua CW: An organoid assay for
long-term maintenance and propagation of mouse prostate luminal
epithelial progenitors and cancer cells. Methods Mol Biol.
1940:231–254. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao D and Chen Y: Organoid development in
cancer genome discovery. Curr Opin Genet Dev. 30:42–48. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schumacher D, Andrieux G, Boehnke K, Keil
M, Silvestri A, Silvestrov M, Keilholz U, Haybaeck J, Erdmann G,
Sachse C, et al: Heterogeneous pathway activation and drug response
modelled in colorectal-tumor-derived 3D cultures. PLoS Genet.
15:e10080762019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Corrò C, Novellasdemunt L and Li VSW: A
brief history of organoids. Am J Physiol Cell Physiol.
319:C151–C165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato T, Vries RG, Snippert HJ, van de
Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters
PJ and Clevers H: Single Lgr5 stem cells build crypt-villus
structures in vitro without a mesenchymal niche. Nature.
459:262–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neal J, Li X, Zhu J, Giangarra V,
Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, et
al: Organoid modeling of the tumor immune microenvironment. Cell.
175:1972–1988.e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wild CP, Weiderpass E and Stewart BW:
World Cancer Report: Cancer Research for Cancer Prevention. World
Health Organisation; Geneva, Switzerland: pp. 23–33. 2020,
PubMed/NCBI
|
|
18
|
Liu HD, Xia BR, Jin MZ and Lou G: Organoid
of ovarian cancer: genomic analysis and drug screening. Clinical
and Translational Oncology. 22:1240–1251. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartfeld S, Bayram T, van de Wetering M,
Huch M, Begthel H, Kujala P, Vries R, Peters PJ and Clevers H: In
vitro expansion of human gastric epithelial stem cells and their
responses to bacterial infection. Gastroenterology. 148:126–136.e6.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bertaux-Skeirik N, Centeno J, Gao J, Gabre
J and Zavros Y: Oncogenic transformation of human-derived gastric
organoids. Methods Mol Biol. 1576:205–213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagle PW, Plukker JTM, Muijs CT, van Luijk
P and Coppes RP: Patient-derived tumor organoids for prediction of
cancer treatment response. Semin Cancer Biol. 53:258–264. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wenzel C, Riefke B, Gründemann S, Krebs A,
Christian S, Prinz F, Osterland M, Golfier S, Räse S, Ansari N, et
al: 3D high-content screening for the identification of compounds
that target cells in dormant tumor spheroid regions. Exp Cell Res.
323:131–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arena S, Corti G, Durinikova E, Montone M,
Reilly NM, Russo M, Lorenzato A, Arcella P, Lazzari L, Rospo G, et
al: A subset of colorectal cancers with cross-sensitivity to
olaparib and oxaliplatin. Clin Cancer Res. 26:1372–1384. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L,
Xia F, Fu G, Deng Y, Pan M, et al: Patient-derived organoids
predict chemoradiation responses of locally advanced rectal cancer.
Cell Stem Cell. 26:17–26.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skardal A, Shupe T and Atala A:
Organoid-on-a-chip and body-on-a-chip systems for drug screening
and disease modeling. Drug Discov Today. 21:1399–1411. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saito Y: Establishment of an organoid bank
of biliary tract and pancreatic cancers and its application for
personalized therapy and future treatment. J Gastroenterol Hepatol.
34:1906–1910. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van de Wetering M, Francies HE, Francis
JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J,
Taylor-Weiner A, Kester L, et al: Prospective derivation of a
living organoid biobank of colorectal cancer patients. Cell.
161:933–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kondo J and Inoue MJC: Application of
cancer organoid model for drug screening and personalized therapy.
Cells. 8:4702019. View Article : Google Scholar
|
|
29
|
Broutier L, Mastrogiovanni G, Verstegen
MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R,
Sidorova O, Gaspersz MP, et al: Human primary liver cancer-derived
organoid cultures for disease modeling and drug screening. Nat Med.
23:1424–1435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fong ELS, Toh TB, Lin QXX, Liu Z, Hooi L,
Mohd Abdul Rashid MB, Benoukraf T, Chow EK, Huynh TH and Yu H:
Generation of matched patient-derived xenograft in vitro-in vivo
models using 3D macroporous hydrogels for the study of liver
cancer. Biomaterials. 159:229–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang HC and Kuo CJ: Personalizing
pancreatic cancer organoids with hPSCs. Nat Med. 21:1249–1251.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boj SF, Hwang CI, Baker LA, Chio II, Engle
DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, et al:
Organoid models of human and mouse ductal pancreatic cancer. Cell.
160:324–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patman G: Pancreatic cancer: From normal
to metastases-a whole gamut of pancreatic organoids. Nat Rev
Gastroenterol Hepatol. 12:612015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang L, Holtzinger A, Jagan I, BeGora M,
Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, et
al: Ductal pancreatic cancer modeling and drug screening using
human pluripotent stem cell- and patient-derived tumor organoids.
Nat Med. 21:1364–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chio IIC, Jafarnejad SM, Ponz-Sarvise M,
Park Y, Rivera K, Palm W, Wilson J, Sangar V, Hao Y, Öhlund D, et
al: NRF2 promotes tumor maintenance by modulating mRNA translation
in pancreatic cancer. Cell. 166:963–976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Öhlund D, Handly-Santana A, Biffi G,
Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA,
Lee EJ, et al: Distinct populations of inflammatory fibroblasts and
myofibroblasts in pancreatic cancer. J Exp Med. 214:579–596. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lesina M, Kurkowski MU, Ludes K, Rose-John
S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S,
et al: Stat3/Socs3 activation by IL-6 transsignaling promotes
progression of pancreatic intraepithelial neoplasia and development
of pancreatic cancer. Cancer Cell. 19:456–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roe JS, Hwang CI, Somerville TDD, Milazzo
JP, Lee EJ, Da Silva B, Maiorino L, Tiriac H, Young CM, Miyabayashi
K, et al: Enhancer reprogramming promotes pancreatic cancer
metastasis. Cell. 170:875–888.e20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tiriac H, Bucobo J, Tzimas D, Grewel S,
Lacomb JF, Rowehl LM, Nagula S, Wu M, Kim J, Sasson A, et al:
Successful creation of pancreatic cancer organoids by means of
EUS-guided fine-needle biopsy sampling for personalized cancer
treatment. Gastrointest Endosc. 87:1474–1480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seino T, Kawasaki S, Shimokawa M, Tamagawa
H, Toshimitsu K, Fujii M, Ohta Y, Matano M, Nanki K, Kawasaki K, et
al: Human pancreatic tumor organoids reveal loss of stem cell niche
factor dependence during disease progression. Cell Stem Cell.
22:454–467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frappart PO, Walter K, Gout J, Beutel AK,
Morawe M, Arnold F, Breunig M, Barth TF, Marienfeld R, Schulte L,
et al: Pancreatic cancer-derived organoids-a disease modeling tool
to predict drug response. United European Gastroenterol J.
8:594–606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Múnera JO, Sundaram N, Rankin SA, Hill D,
Watson C, Mahe M, Vallance JE, Shroyer NF, Sinagoga KL,
Zarzoso-Lacoste A, et al: Differentiation of human pluripotent stem
cells into colonic organoids via transient activation of BMP
signaling. Cell Stem Cell. 21:51–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Webber JP, Spary LK, Sanders AJ, Chowdhury
R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD,
et al: Differentiation of tumour-promoting stromal myofibroblasts
by cancer exosomes. Oncogene. 34:290–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Usui T, Sakurai M, Enjoji S, Kawasaki H,
Umata K, Ohama T, Fujiwara N, Yabe R, Tsuji S, Yamawaki H, et al:
Establishment of a novel model for anticancer drug resistance in
three-dimensional primary culture of tumor microenvironment. Stem
Cells Int. 2016:70538722016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lannagan TRM, Lee YK, Wang T, Roper J,
Bettington ML, Fennell L, Vrbanac L, Jonavicius L, Somashekar R,
Gieniec K, et al: Genetic editing of colonic organoids provides a
molecularly distinct and orthotopic preclinical model of serrated
carcinogenesis. Gut. 68:684–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nadauld LD, Garcia S, Natsoulis G, Bell
JM, Miotke L, Hopmans ES, Xu H, Pai RK, Palm C, Regan JF, et al:
Metastatic tumor evolution and organoid modeling implicate TGFBR2
as a cancer driver in diffuse gastric cancer. Genome Biol.
15:4282014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li X, Nadauld L, Ootani A, Corney DC, Pai
RK, Gevaert O, Cantrell MA, Rack PG, Neal JT, Chan CW, et al:
Oncogenic transformation of diverse gastrointestinal tissues in
primary organoid culture. Nat Med. 20:769–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Seidlitz T, Merker SR, Rothe A, Zakrzewski
F, von Neubeck C, Grützmann K, Sommer U, Schweitzer C, Schölch S,
Uhlemann H, et al: Human gastric cancer modelling using organoids.
Gut. 68:207–217. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vlachogiannis G, Hedayat S, Vatsiou A,
Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford
I, Burke R, et al: Patient-derived organoids model treatment
response of metastatic gastrointestinal cancers. Science.
359:920–926. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Singh A, Poling HM, Spence JR, Wells JM
and Helmrath MA: Gastrointestinal organoids: A next-generation tool
for modeling human development. Am J Physiol Gastrointest Liver
Physiol. 319:G375–G381. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu H, Lyu X, Yi M, Zhao W, Song Y and Wu
K: Organoid technology and applications in cancer research. J
Hematol Oncol. 11:1162018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Foulke-Abel J, In J, Kovbasnjuk O, Zachos
NC, Ettayebi K, Blutt SE, Hyser JM, Zeng XL, Crawford SE, Broughman
JR, et al: Human enteroids as an ex-vivo model of host-pathogen
interactions in the gastrointestinal tract. Exp Biol Med (Maywood).
239:1124–1134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sachs N, Tsukamoto Y, Kujala P, Peters PJ
and Clevers H: Intestinal epithelial organoids fuse to form
self-organizing tubes in floating collagen gels. Development.
144:1107–1112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fong EL, Wan X, Yang J, Morgado M, Mikos
AG, Harrington DA, Navone NM and Farach-Carson MC: A 3D in vitro
model of patient-derived prostate cancer xenograft for controlled
interrogation of in vivo tumor-stromal interactions. Biomaterials.
77:164–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang S, Wan Z and Kamm RD: Vascularized
organoids on a chip: Strategies for engineering organoids with
functional vasculature. Lab Chip. 21:473–488. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang C, Jin M, Zhao J, Chen J and Jin W:
Organoid models of glioblastoma: Advances, applications and
challenges. Am J Cancer Res. 10:2242–2257. 2020.PubMed/NCBI
|
|
58
|
Date S and Sato T: Mini-gut organoids:
Reconstitution of the stem cell niche. Annu Rev Cell Dev Biol.
31:269–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kim J, Koo BK and Knoblich JA: Human
organoids: Model systems for human biology and medicine. Nat Rev
Mol Cell Biol. 21:571–584. 2020. View Article : Google Scholar : PubMed/NCBI
|