Introduction
Endometrial carcinoma (EC) originates from the
endometrium and is ranked as the most prevalent reproductive
malignancy (1). EC patients are
mostly diagnosed in early clinical stages, but late-stage EC is
frequently diagnosed with invasive metastasis and a high relapse
rate (2). Therefore, the prognosis
is usually poor with the occurrence of metastases and recurrence
(3). To date, common treatments for
EC mainly include radiation, chemotherapy, hormonal therapy, and
surgeries with bilateral salpingo-oophorectomy and hysterectomy.
These methods remain mainstream therapies in EC treatment and are
generally only effective in patients at early stages of this
disease (4). Therefore, it is urgent
to elucidate the mechanism which occurs in EC pathogenesis for the
identification of promising therapeutic strategies.
MicroRNA (miRNA/miR) is a type of small non-coding
RNA (18–24 nucleotides in length) that could silence the target
genes via inhibition of translation or degradation of mRNAs
(5). A growing body of studies has
revealed that miRs participate in regulating a variety of cellular
processes, including metastasis, metabolism, differentiation and
growth (6–8). Increasing evidence has revealed that
aberrant miR expression is correlated with the genesis and
development of multiple tumors, serving as either tumor suppressors
or oncogenes. Evidence from Zhang et al revealed that
miR-1299 was a tumor suppressor in prostate carcinoma, inhibiting
cell growth and metastasis via regulation of never in mitosis gene
A-related kinase 2 (NEK2) (9). Peng
et al revealed that miR-31-5p enhanced colorectal carcinoma
cell proliferation and metastasis by targeting NUMB (10). Zhen et al reported that
miR-524 induced thyroid cancer cell apoptosis and suppressed cell
proliferation through sperm-associated antigen 9 (SPAG9) (11). Moreover, emerging evidence has
revealed that multiple miRs are implicated in EC. miR-486-5p could
promote EC cell proliferation, migration, and invasion by targeting
MARK1 (12); miR-101-3p induced
autophagy in EC cells by targeting EZH2 (13); miR-522 facilitated the proliferation,
invasion and migration of EC cells by directly binding to monoamine
oxidase B (14). However, the roles
of miR-15a-5p in EC have rarely been clarified. The present study
aimed to elucidate the functions of miR-15a-5p in EC progression.
Vascular endothelial growth factor (VEGF) plays crucial roles in
tumor angiogenesis and is correlated with increased tumor
metastasis and relapse (15). VEGFA
is a potent angiogenic inducer of the VEGF family and increased
VEGFA expression has been detected in various human tumors
(16,17). Recent studies have revealed that
VEGFA may enhance cancer growth and progression in preclinical
hepatocellular carcinoma models and genomic VEGFA amplification was
identified as a predictive marker for hepatocellular carcinoma
(18,19). Overexpressed VEGFA was detected in
colorectal cancer tissues and upregulation of VEGFA reversed the
suppressive roles mediated by miR-150-5p overexpression in
colorectal cancer (20). In EC, VEGF
was indicated to function as a prognostic marker (21). miR-34a-5p downregulated VEGFA in
endometrial stem cells, contributing to the pathogenesis of
endometriosis (22). In the present
study, the correlation between miR-15a-5p and VEGFA in EC
progression was investigated.
Materials and methods
Cell culture and transfection
Human EC cell lines (AN3CA, HEC-1B, HEC-1A, and
RL95-2) and endometrial stromal cell line T-HESCs were maintained
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS; both Gibco; Thermo Fisher Scientific,
Inc.) in a humidified incubator of 5% CO2 at 37°C.
miR-15a-5p mimic, inhibitor and negative controls
were constructed by Shanghai GenePharma Co., Ltd. miR-15a-5p mimics
(50 nM) were transfected into HEC-1A cells and miR-15a-5p inhibitor
(50 nM) was transfected into AN3CA cells in accordance with the
instructions of Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 48 h. The primer
sequences were as follows: miR-15a-5p mimic (sense,
5′-UAGCAGCACAUAAUGGUUUGUG-3′ and antisense,
5′-CAAACCAUUAUGUGCUGCUAUU-3′); mimic control (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′); miR-15a-5p inhibitor
(5′-CACAAACCAUUAUGUGCUGCUA-3′); inhibitor control
(5′-CAGUACUUUUGUGUAGUACAA-3′). The cells were inoculated in a
6-well plate with an inoculation density of ~2.5×106
cells/well, and incubated in a 37°C incubator. Logarithmically
growing cells were selected and inoculated in a culture plate, and
the cell confluence was ~80% before transfection. Subsequent
experimentations were performed at 48-h after transfection.
Clinical specimens
A total of 49 pairs of EC tissue samples and
adjacent para-tumor tissues were acquired from the Weifang People's
Hospital (Weifang, China) from August 2015 to June 2018. The age
range of patients was 43–68 years (mean age, 51 years old). The
inclusion criteria used were as follows: i) The EC tissue specimens
were confirmed by pathology, and the adjacent para-carcinoma tissue
specimens were confirmed by pathology that there was no tumor cell
invasion; ii) the patients had not received immunotherapy,
radiotherapy and chemotherapy or other antitumor treatments before
surgery. The exclusion criteria were as follows: Patients who also
had other i) malignant tumors; ii) severe cardiovascular and
cerebrovascular diseases; and iii) hematological diseases. The
tissue specimens were snap-frozen in liquid nitrogen after surgical
resection and reserved at −80°C for further usage. In the present
study, no patient had received prior irradiation or chemotherapy.
Informed consent was obtained from the subjects for the use of
their samples for experimentation. The present study conformed to
the Code of Ethics from the Ethics Committee of Weifang People's
Hospital.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from EC/normal tissues and
cells by TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). To detect miR-15a-5p expression, TaqMan MicroRNA Reverse
Transcription Kit (Thermo Fisher Scientific, Inc.) was used to
reverse transcribe total RNA into cDNA. For VEGFA detection, cDNA
was synthesized by Prime Script RT Reagent Kit (Takara
Biotechnology Co., Ltd.). Thereafter, qPCR was conducted on an ABI
7500 Detection System with SYBR Premix Ex Taq™ kit (Takara
Biotechnology Co., Ltd.) or a TaqMan MicroRNA Assay Kit (Thermo
Fisher Scientific, Inc.) for VEGFA or miR-15a-5p, respectively. The
thermocycling conditions were as follows: Pre-denaturation at 94°C
for 5 min, followed by 40 cycles of denaturation at 94°C for 30
sec, annealing at 55°C for 30 sec and extension at 72°C for 90 sec.
The relative expression levels were analyzed using the
2−ΔΔCq method (23), with
normalization to GAPDH or U6. The primers are presented in Table I.
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Primer | Sequence |
|---|
| miR-15a-5p | F:
5′-TAAGGCACGCGGTGAATGCC-3′ |
|
| R:
5′-GCCTGGGTCTCACCATGTAG-3′ |
| U6 | F:
5′-CTCGCTTCGGCAGCACA-3′ |
|
| R:
5′-AACGCTTCACGAATTTGCGT-3′ |
| VEGFA | F:
5′-TGGCTCACTGGCTTGCTCTA-3′ |
|
| R:
5′-ATCCAACTGCACCGTCACAG-3′ |
| GAPDH | F:
5′-CCAGGTGGTCTCCTCTGACTT-3′ |
|
| R:
5′-GTTGCTGTAGCCAAATTCGTTGT-3′ |
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay
Transfected EC cells (5×103) were seeded
into 96-well plates. Then, cell viability was assessed at indicated
time-points (0, 24, 48 and 72 h) after transfection by MTT assay.
In brief, 10 µl MTT solution (0.5 mg/ml; Sigma-Aldrich; Merck KGaA)
was added into the cells, followed by incubation in a humidified
chamber at 37°C for 4 h. Subsequently, 150 µl DMSO (Sigma-Aldrich;
Merck KGaA) was added to each well for full dissolution of
crystallization. The absorbance at 490 nm was detected by a
microplate reader (BioTek Instruments, Inc.).
Transwell assay
The influence of miR-15a-5p on EC cell invasion and
migration was assessed by Transwell chamber with an 8-µm pore size
membrane (Corning, Inc.). For invasion assays, cells in FBS-free
medium were added to the upper Matrigel-coated Transwell chamber.
For the migration assay, the chamber was not coated with Matrigel.
In addition, the medium containing 10% FBS was added into the
bottom chamber. The cells were cultured at 37°C for 48 h. The
migrated/invasive cells were fixed with 95% ethyl alcohol for 15
min at room temperature and stained with 0.1% crystal violet for 10
min at room temperature. Finally, the stained cells were quantified
under a light microscope (BX-42; Olympus Corporation;
magnification, ×200).
Western blot analysis
Total protein lysate was obtained by ice-cold
radioimmunoprecipitation lysis buffer (RIPA; Thermo Fisher
Scientific, Inc.), and quantified with a bicinchoninic acid (BCA)
assay kit (Pierce; Thermo Fisher Scientific, Inc.). Then, 30 µg
lysate was separated by 10% sodium dodecyl sulphate-polyacrylamide
gel electrophoresis (SDS-PAGE), and transferred onto a
polyvinylidene fluoride (PVDF) membrane. The membrane was blocked
with 5% skim milk at room temperature for 1 h in Tris-Buffered
Saline and 0.1% Tween-20 (TBST). Thereafter, the membranes were
incubated overnight at 4°C with a primary antibody, followed by a
2-h incubation with secondary antibody goat anti-rabbit IgG H&L
(HRP) (1:4,000; product code ab7090) at room temperature. The
primary antibodies were as follows: Antibodies against VEGFA
(1:10,000; product code ab52917), cyclin D1 (1:1,000; product code
ab40754), c-Myc (1:1,000; product code ab32072), β-catenin
(1:1,000; product code ab68183), p-GSK3β (1:1,000; product code
ab131097), total GSK3β (1:1,000; product code ab227208), E-cadherin
(1:2,000; product code ab133597), N-cadherin (1:2,000; product code
ab207608), vimentin (1:1,000; product code ab137321) and GAPDH
(1:1,000; product code ab128915). All antibodies were obtained from
Abcam. GAPDH served as an internal control. Finally, the membranes
were visualized with the ECL reagent (EMD Millipore). ImageJ
software (version 1.48; National Institutes of Health) was used for
densitometry.
Bioinformatic analysis
The putative human target genes of miR-15a-5p were
analyzed using the TargetScan (version 6.0; targetscan.org/) (24,25).
Luciferase reporter assay
Two types of VEGFA 3′UTR fragments [wild-type (WT)
or mutant (MUT)] were inserted into the pGL3 vector (Promega
Corporation) to obtain the VEGFA 3′UTR-WT or -MUT reporter. EC
cells were co-transfected with miR-15a-5p mimics and VEGFA 3′UTR-WT
or -MUT by Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h of transfection, the Dual-Luciferase
Reporter Assay System (Promega Corporation) was applied to evaluate
the luciferase activity. Relative firefly luciferase activity was
normalized to Renilla luciferase activity.
In vivo tumor xenograft mouse
model
The lentiviral plasmid containing miR-15a-5p
(lenti-miR-15a-5p) or a control (lenti-control) were constructed
and synthesized by Shanghai GenePharma Co., Ltd. A three-plasmid
system and 9 µg lentiviral vectors were co-transfected into the 293
cells (Invitrogen; Thermo Fisher Scientific, Inc.) through
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The rate of lentiviral plasmid: Packaging
vector: Envelope was 1:1:1. Lentivirus-containing medium was
collected 48 h after transfection and used to infect HEC-1A cells
(5×106 cells/well, in six-well plates) at a multiplicity
of infection (MOI) of 20. Subsequently, 48 h after lentiviral
infection, 1 µg/ml puromycin was used to select the infected cells.
After 72 h, transfected cells were suspended in DMEM and injected
subcutaneously into the dorsal flank of the nude mice. For in
vivo xenograft studies, a total of 12 BALB/c nude mice (aged
4–6 weeks; female; 20–25 g) were obtained from Beijing Vital River
Laboratory Animal Technology Co., Ltd. and the animal experiments
were approved by the Animal Care and Use Committee of Weifang
People's Hospital. The nude mice were housed in pathogen-free
environment with a 12-h light/dark cycle, temperature (26°C),
humidity (50%) and free access to food and water. HEC-1A cells
(5×106) packaged with lentiviral vector containing
miR-15a-5p (lenti-miR-15a-5p) or a control (lenti-control) were
suspended in DMEM and injected subcutaneously into the dorsal flank
of the nude mice (n=6 per group). Then, 10 days after inoculation,
tumor volumes were measured every 3 days following the formula:
Volume (mm3)=(length × width2)/2.
Subsequently, the mice were sacrificed by cervical dislocation
after 28 days. All surgical procedures were performed under 1%
sodium pentobarbital anesthesia 70 mg/kg, and all efforts were made
to minimize suffering.
Statistical analysis
All experiments were conducted at least thrice.
Statistical analyses were performed by Statistical Product and
Service Solutions (SPSS) software version 17.0 (SPSS Inc.).
Comparisons of two or more groups were determined by unpaired
Student's t-test or one-way ANOVA analysis along with Dunnett's
post hoc test. The Kaplan-Meier analysis along with log-rank test
was applied to analyze the overall survival (OS) of EC patients.
Comparison of the tumor volume between the two groups was performed
by unpaired t-test. Statistical analysis for miR-15a-5p expression
and the clinicopathological characteristics was performed by the
χ2 test. P<0.05 indicated a statistically significant
difference.
Results
Downregulated miR-15a-5p is associated
with unfavorable prognosis in EC patients
To explore the functional roles of miR-15a-5p in EC,
miR-15a-5p levels in EC tissues and cell lines were detected.
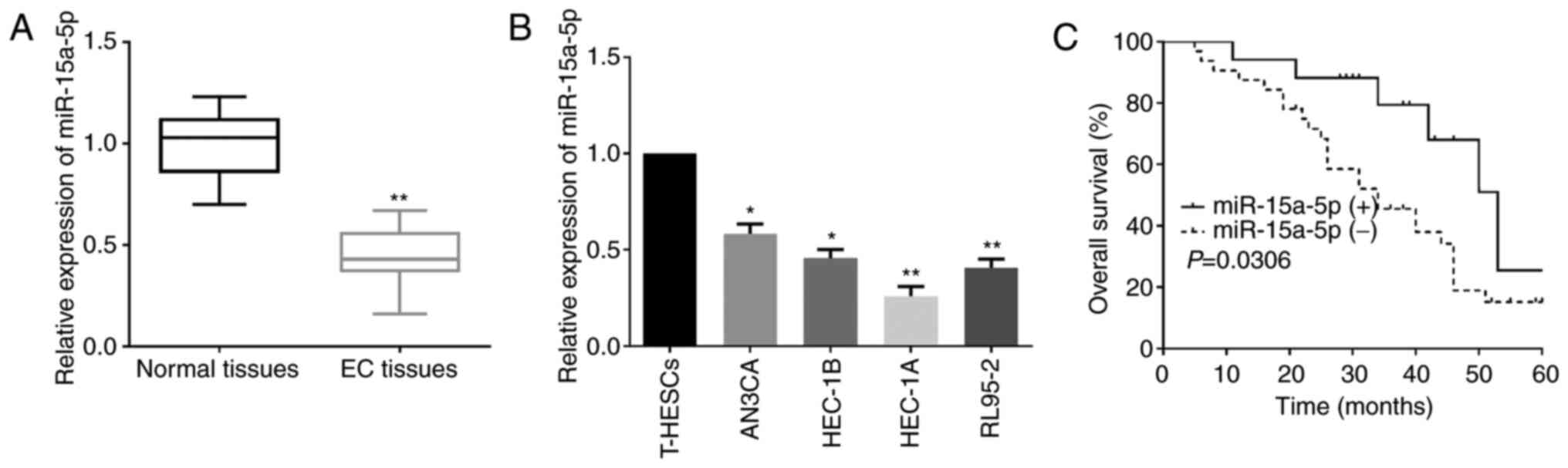
According to RT-qPCR, it was revealed that miR-15a-5p expression in
EC tissues was lower than that in the matched normal tissue samples
(Fig. 1A). Similarly, compared to
T-HESCs, EC cells had significantly lower miR-15a-5p expression
(Fig. 1B). Moreover, to investigate
the clinicopathological significance of miR-15a-5p in EC
progression, the EC patients were grouped into a high-miR-15a-5p
expression group and a low-miR-15a-5p expression group based on the
median miR-15a-5p expression level. As revealed in Table II, patients in the low-miR-15a-5p
expression group exhibited aggressive clinicopathological
phenotypes in comparison to patients in the high-miR-15a-5p
expression group. Additionally, low miR-15a-5p expression was also
associated with unfavorable prognosis of EC patients as
demonstrated by Kaplan-Meier analysis (Fig. 1C).
 | Table II.Association of miR-15a-5p expression
with the clinicopathological characteristics of EC patients. |
Table II.
Association of miR-15a-5p expression
with the clinicopathological characteristics of EC patients.
|
|
|
miR-15a-5pa expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
Features | Cases (n=49) | High (n=17) | Low (n=32) | P-value |
|---|
| Age (years) |
|
|
| 0.203 |
|
>56 | 26 | 7 | 19 |
|
|
≤56 | 23 | 10 | 13 |
|
| Pathology
classification |
|
|
| 0.061 |
| Well +
moderate | 24 | 9 | 15 |
|
|
Poor | 25 | 8 | 17 |
|
| FIGO stage |
|
|
| 0.031b |
|
I–II | 22 | 13 | 9 |
|
|
III–IV | 27 | 4 | 23 |
|
| Lymph-node
metastasis |
|
|
| 0.012b |
|
Yes | 29 | 5 | 24 |
|
| No | 20 | 12 | 8 |
|
| ER status |
|
|
| 0.078 |
|
Positive | 20 | 9 | 11 |
|
|
Negative | 39 | 8 | 21 |
|
| PR status |
|
|
| 0.092 |
|
Positive | 23 | 10 | 13 |
|
|
Negative | 26 | 7 | 19 |
|
miR-15a-5p suppresses EC cell
viability
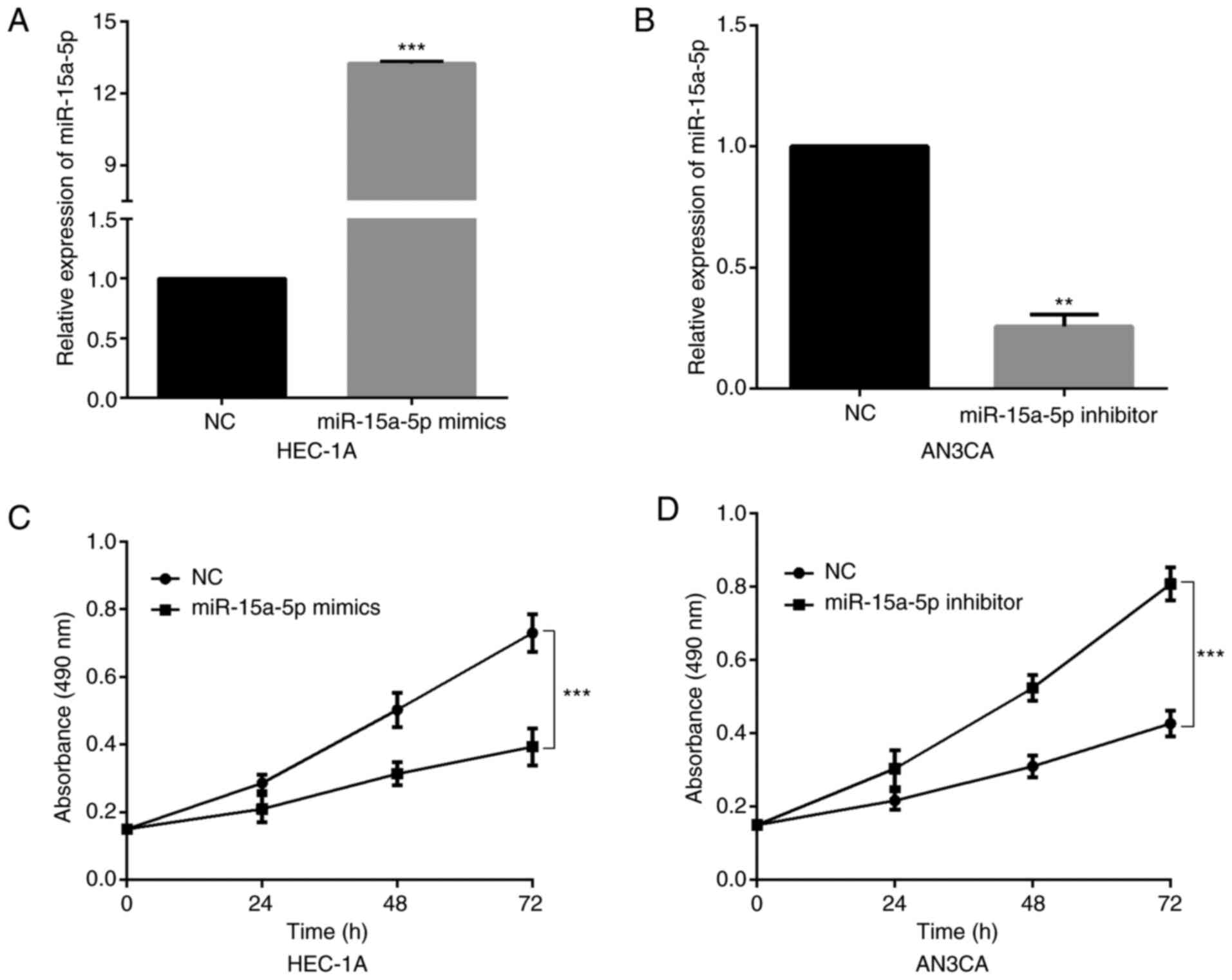
Since the decreased miR-15a-5p expression in EC was
confirmed, functional assays were further carried out to determine
its specific roles in EC. Firstly, miR-15a-5p mimics or inhibitor
were transfected into HEC-1A and AN3CA cells according to their
relatively low and high endogenous miR-15a-5p expression. As
revealed in Fig. 2A and B, the
successful overexpression or inhibition of miR-15a-5p was confirmed
by RT-qPCR. Then, an MTT assay was performed to detect the
proliferation ability of transfected cells. The results revealed
that miR-15a-5p upregulation significantly suppressed HEC-1A cell
proliferation (Fig. 2C). Conversely,
the viability of AN3CA cells was significantly increased by
miR-15a-5p inhibitor (Fig. 2D).
miR-15a-5p inhibits EC cell invasion
and migration
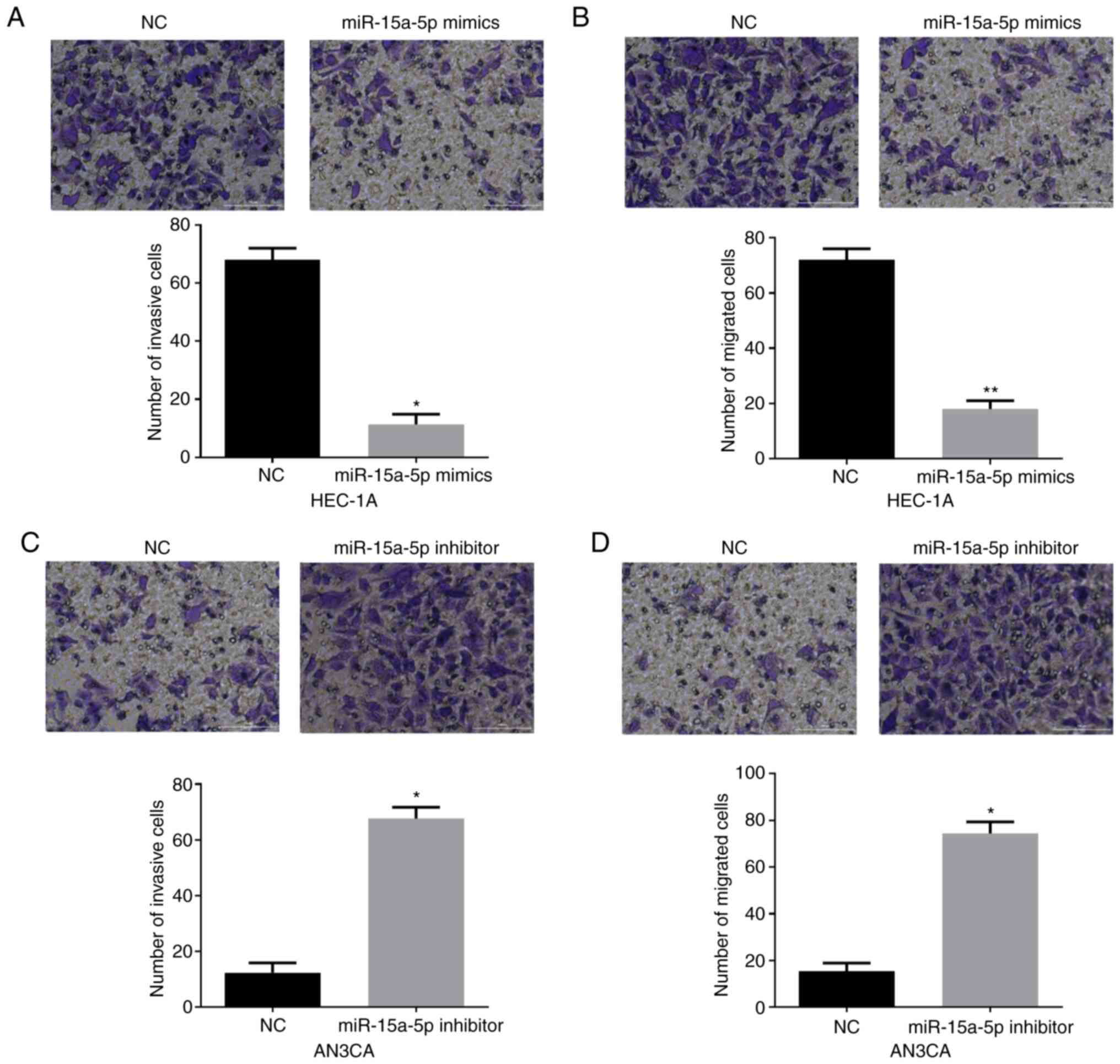
Subsequently, Transwell assays were performed to
explore the effects of miR-15a-5p on EC cell invasion and
migration. In Fig. 3A and B, it was
observed that both the invasion and migration capacities of HEC-1A
cells were significantly impaired by miR-15a-5p mimics. Conversely,
miR-15a-5p silencing in AN3CA cells significantly facilitated cell
invasion and migration (Fig. 3C and
D). In sum, all these data revealed that miR-15a-5p functioned
as a tumor suppressor in EC.
miR-15a-5p directly targets and
negatively regulates VEGFA
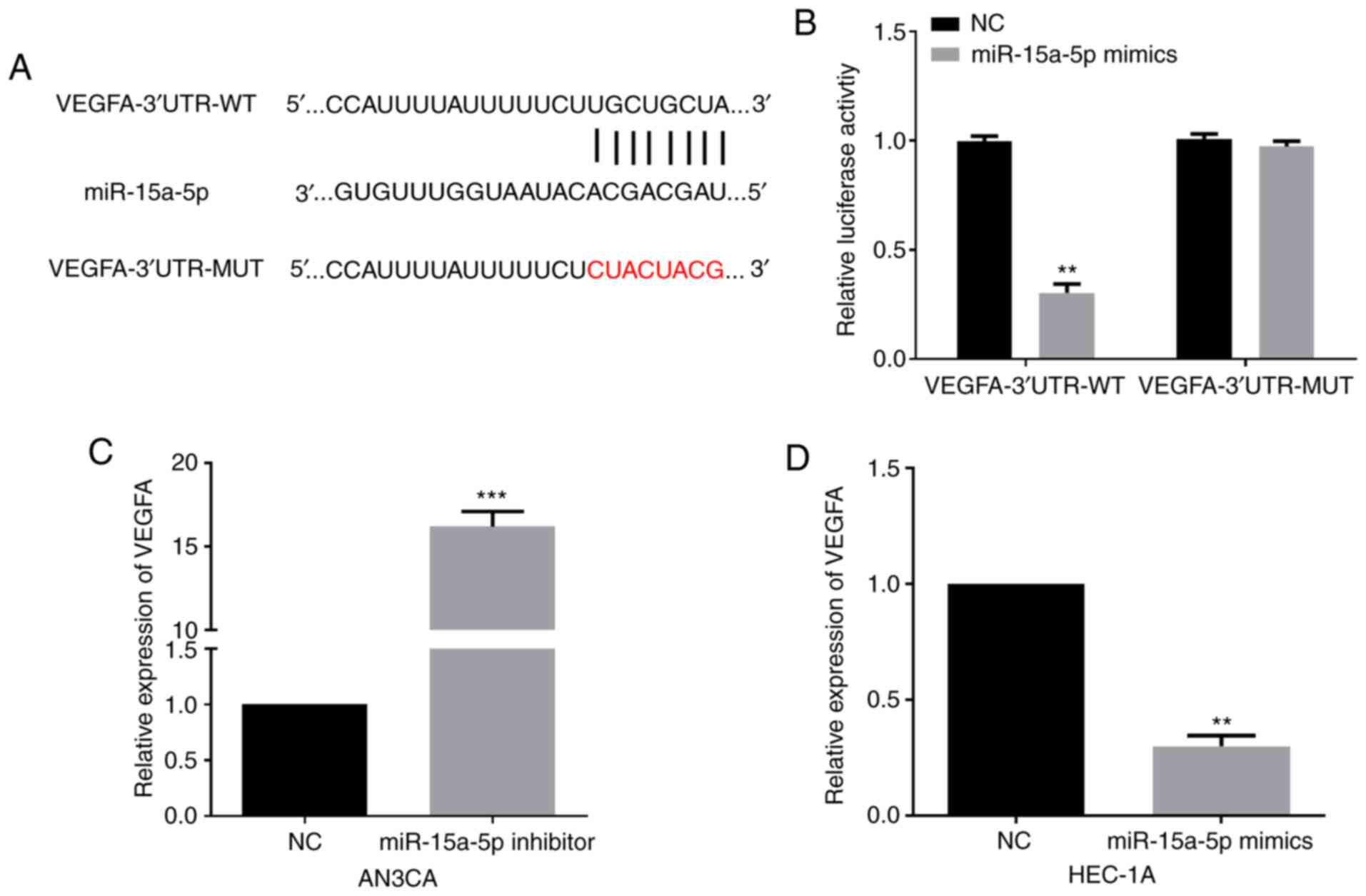
Mechanistically, the target genes of miR-15a-5p were
identified by Targetscan, and the results revealed that VEGFA was a
candidate target of miR-15a-5p. The targeting sites between VEGFA
and miR-15a-5p are presented in Fig.
4A. Then, a luciferase reporter assay was conducted to verify
the association. Findings revealed that the luciferase activity of
VEGFA-3′UTR-WT was significantly decreased by miR-15a-5p mimics in
EC cells, whereas no evident variation on luciferase activity of
VEGFA-3′UTR-MUT was detected (Fig.
4B). Moreover, the regulatory roles of miR-15a-5p in VEGFA
expression were examined by performing western blotting. miR-15a-5p
silencing significantly enhanced VEGFA levels in AN3CA cells
(Fig. 4C). In addition, VEGFA levels
in the miR-15a-5p-overexpressed HEC-1A cells were significantly
decreased (Fig. 4D). The
aforementioned collective data revealed that VEGFA was a direct
target of miR-15a-5p.
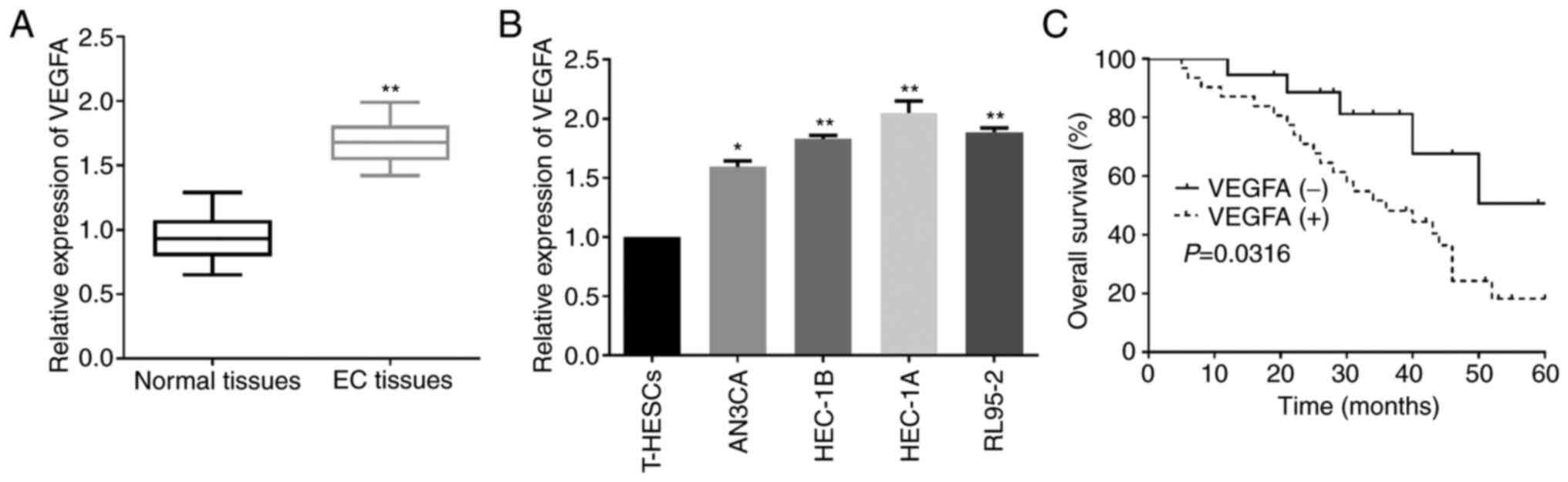
Upregulated VEGFA in EC indicates poor
prognosis of EC patients
Since VEGFA was confirmed as a target of miR-15a-5p,
the clinical value of VEGFA in EC development was further explored.
As indicated by RT-qPCR, VEGFA was significantly upregulated in EC
tissues when compared to that in the adjacent non-tumor tissue
samples (Fig. 5A). Similarly, the
increased VEGFA levels were also identified in EC cell lines
(Fig. 5B). Subsequently,
Kaplan-Meier analysis was performed to clarify the role of the
ectopic VEGFA expression in EC patients. As revealed in Fig. 5C, high VEGFA expression resulted in a
significantly poorer prognosis of EC patients than low VEGFA
expression. Data revealed that VEGFA was partially involved in the
functions of miR-15a-5p in EC.
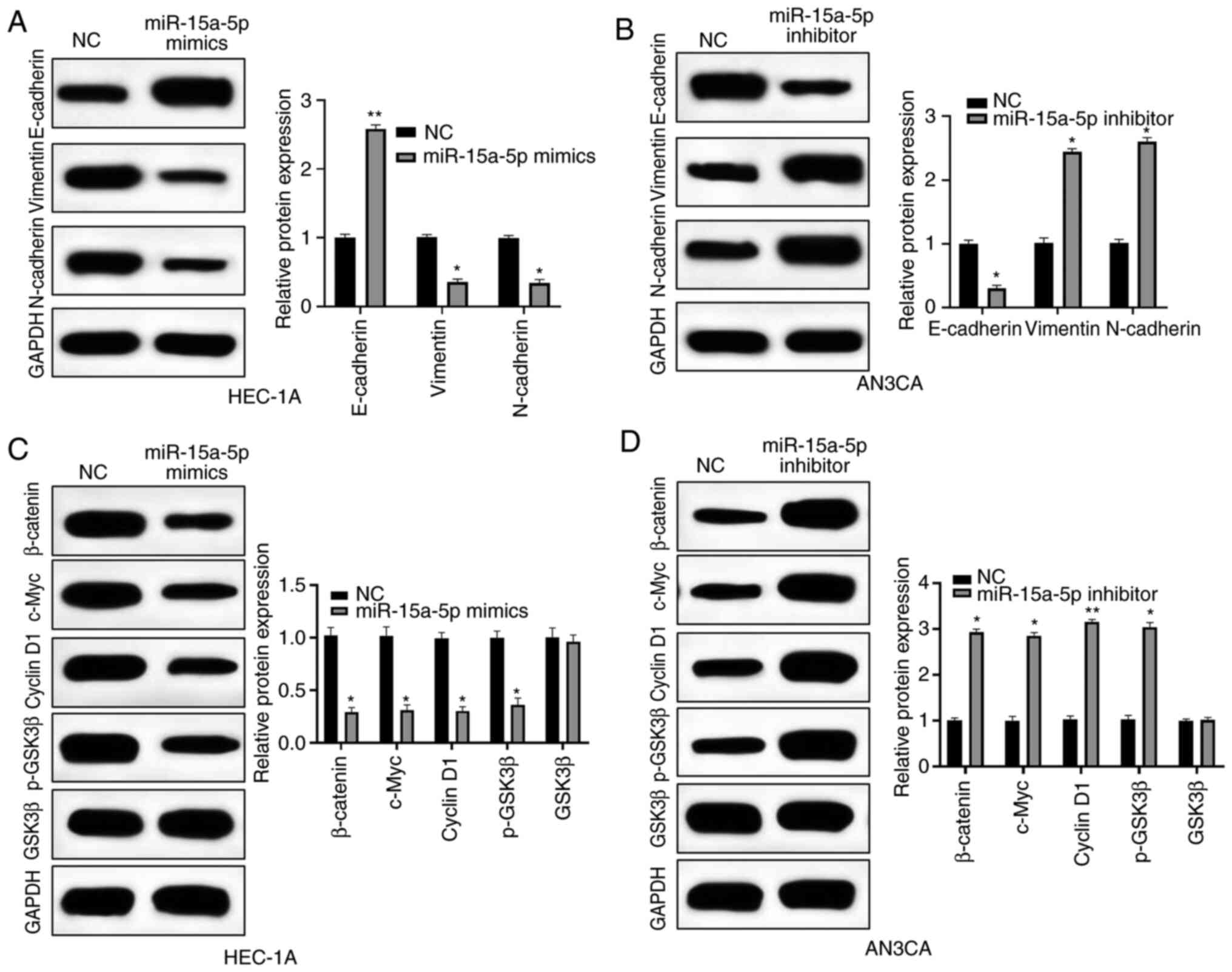
miR-15a-5p regulates the Wnt/β-catenin
signaling pathway and epithelial-mesenchymal transition (EMT) in EC
cells
The potential mechanism of miR-15a-5p was further
investigated by western blot analysis. Firstly, the expression
levels of EMT-related proteins were detected. In HEC-1A cells,
miR-15a-5p mimics significantly increased the expression level of
E-cadherin while significantly decreasing vimentin and N-cadherin
expression levels (Fig. 6A). In
contrast, E-cadherin was downregulated whereas vimentin and
N-cadherin were upregulated by miR-15a-5p inhibitor in AN3CA cells
(Fig. 6B). Expression levels of
Wnt/β-catenin-related proteins, including activated β-catenin,
c-Myc, cyclin D1, and p-GSK3β were significantly inhibited by
miR-15a-5p overexpression (Fig. 6C).
Moreover, in AN3CA cells, miR-15a-5p inhibitor had the opposite
effects on the Wnt/β-catenin pathway (Fig. 6D). Collectively, miR-15a-5p could
block EMT and Wnt/β-catenin pathway of EC cells.
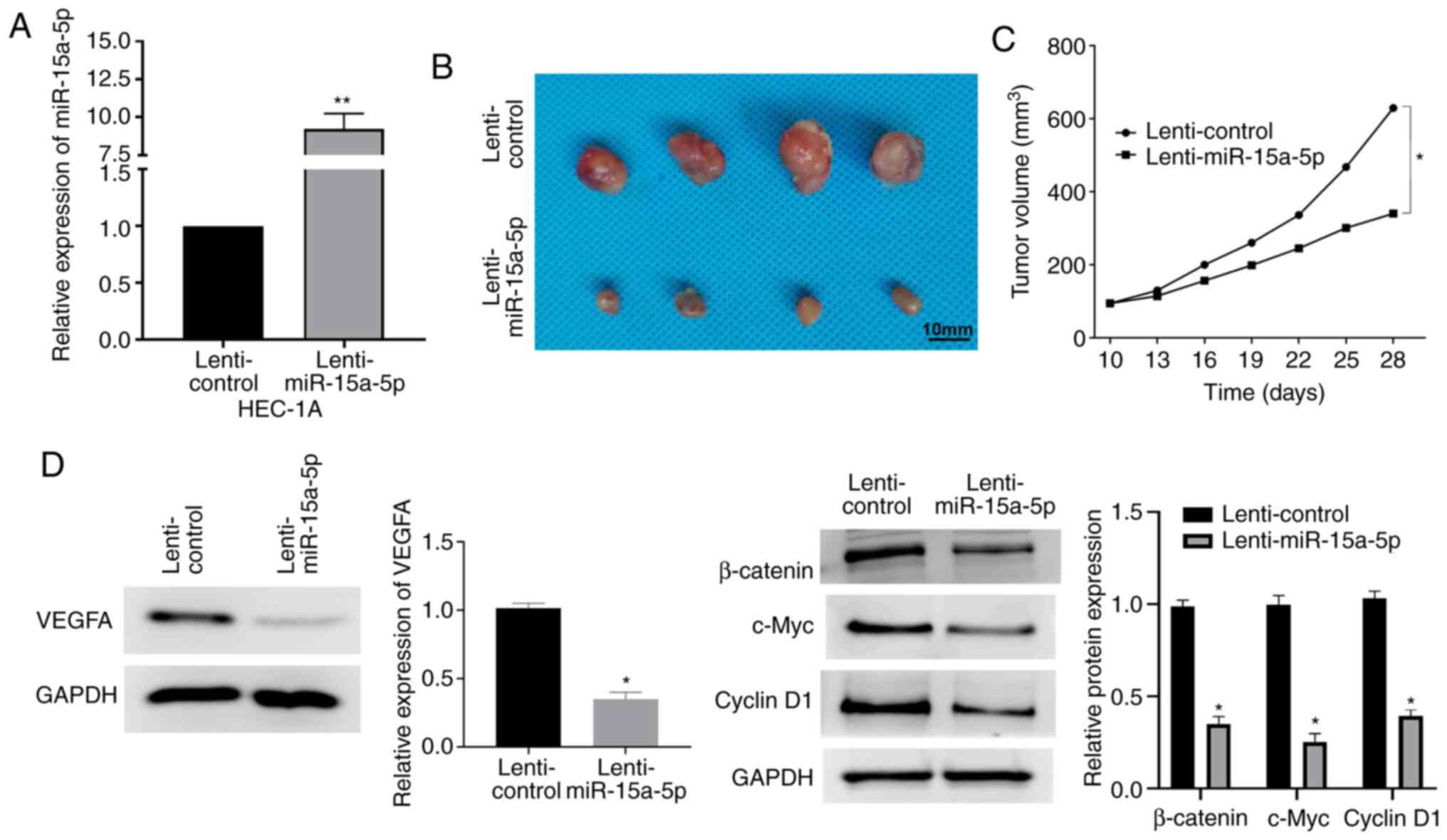
miR-15a-5p suppresses EC tumorigenesis
in vivo
The effect of miR-15a-5p on EC in vivo was
further investigated by tumorigenicity analysis in mice xenografts.
Firstly, the expression of miR-15a-5p in HEC-1A cells, which were
transfected with lenti-miR-15a-5p, was detected. As revealed in
Fig. 7A, miR-15a-5p was stably
upregulated in cells with transfection of lenti-miR-15a-5p. As
revealed by a significant decrease in tumor volumes, miR-15a-5p
overexpression markedly suppressed tumor growth in mice xenografts
compared with controls (Fig. 7B and
C). Moreover, the regulatory functions of miR-15a-5p in
regulating VEGFA expression and the Wnt/β-catenin pathway in an
in vivo mouse model were confirmed by western blotting. The
results revealed that the expression levels of VEGFA and
Wnt/β-catenin pathway-related genes were inhibited by miR-15a-5p
(Fig. 7D).
Discussion
Generally, advanced EC patients usually have a high
recurrence rate and poor prognosis, and existing therapeutic
methods for EC patients have various shortcomings (26). In brief, chemotherapy seriously
affects the quality of life, radiotherapy causes gastrointestinal
reactions, ovarian resection induces menopausal symptoms in
premenopausal women, and uterus removal leads to infertility
(27). Moreover, they also severely
affect the treatment and diagnosis progression of EC. Moreover, the
molecular biology of EC genesis and progression remain indistinct.
Hence, to explore the EC pathogenesis and identify novel diagnostic
and therapeutic biomarkers for EC prognosis is of great
significance. In recent years, differentially expressed miRNAs have
been identified in EC, indicating their importance in EC
development (28).
Tumorigenesis progresses by losing adhesion and
tight junctions as well as gaining invasive features, leading to
EMT, which is characterized by suppression of E-cadherin expression
and enhancement of N-cadherin expression (29). EMT is a pivotal step in tumor
invasion and metastasis, and the understanding of EMT has greatly
improved owing to the identification of miRs, which regulate
downstream events and signaling pathways (30). Furthermore, in EC progression,
molecular events still require further exploration and miR-15a-5p
may play important roles in EMT. As a critical developmental
pathway, Wnt/β-catenin signaling is considered important for cell
differentiation, self-regeneration, growth, developmental decisions
in tissue homeostasis, embryonic development, and tumorigenesis
(31,32). Abnormal activation of the
Wnt/β-catenin pathway may lead to the development of multiple human
tumors (33). Previous studies have
revealed that Wnt/β-catenin is involved in EC progression (34,35). For
instance, the Wnt/β-catenin pathway was involved in the functions
of the hsa_circ_0002577/miR-197/CTNND1 axis in EC development
(34). Moreover, a study by Chen
et al revealed that miR-202 inhibited cell migration and
invasion through targeting FGF2 and inactivating Wnt/β-catenin
signaling in EC (36). In the
present study, the functions of Wnt/β-catenin signaling in EC
progression regulated by miR-15a-5p were further explored.
Accumulating studies have revealed that miR-15a-5p is a pivotal
regulator in numerous human tumors. For instance, miR-15a-5p was
identified as a prognostic predictor in recurrent colorectal
adenocarcinoma (37). Moreover,
miR-15a-5p could suppress hepatocellular carcinoma division and
proliferation via targeting brain-derived neurotrophic factor
(BDNF) (38). As indicated in the
present study, miR-15a-5p was revealed to be underexpressed in EC,
which indicated aggressive phenotypes and poor prognosis of EC
patients. In addition, it was also verified that miR-15a-5p
overexpression could inhibit EC progression both in vivo and
in vitro. Data also revealed that miR-15a-5p could target
VEGFA and thereby regulated its expression, suggesting that VEGFA
was an essential regulator of miR-15a-5p-mediated functions in EC.
It is well known that EMT and Wnt/β-catenin signaling are crucial
factors in EC progression, and the present study revealed that
miR-15a-5p exerted its roles in EC via blocking EMT and
Wnt/β-catenin.
In conclusion, decreased miR-15a-5p expression was
associated with poor prognosis and malignant clinicopathologic
features of EC patients. Moreover, miR-15a-5p overexpression could
downregulate VEGFA in EC cells, resulting in suppression of cell
growth, invasion and migration. Additionally, it was also verified
that miR-15a-5p could regulate EMT and the Wnt/β-catenin pathway.
The aforementioned data may provide novel insights into the
identification of promising therapeutic and diagnostic strategies
for EC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HW and QY made substantial contributions to the
conception and design of the study. JL and WC performed all
experiments. XJ collected and interpreted the data and performed
the statistical analysis. YW performed the literature research,
collected samples/clinical data, and wrote and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from the subjects for
the use of their samples for experimentation. The present study
conformed to the Code of Ethics from the Ethics Committee of
Weifang People's Hospital (Weifang, China). Animal experiments were
approved by the Animal Care and Use Committee of Weifang People's
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mahecha AM and Wang H: The influence of
vascular endothelial growth factor-A and matrix metalloproteinase-2
and −9 in angiogenesis, metastasis, and prognosis of endometrial
cancer. Onco Targets Ther. 10:4617–4624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ray M and Fleming G: Management of
advanced-stage and recurrent endometrial cancer. Semin Oncol.
36:145–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davidson BA, Foote J, Clark LH, Broadwater
G, Ehrisman J, Gehrig P, Graybill W, Alvarez Secord A and
Havrilesky LJ: Tumor grade and chemotherapy response in
endometrioid endometrial cancer. Gynecol Oncol Rep. 17:3–6. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen HX, Xu XX, Tan BZ, Zhang Z and Zhou
XD: MicroRNA-29b inhibits angiogenesis by targeting VEGFA through
the MAPK/ERK and PI3K/Akt signaling pathways in endometrial
carcinoma. Cell Physiol Biochem. 41:933–946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krist B, Florczyk U,
Pietraszek-Gremplewicz K, Jozkowicz A and Dulak J: The Role of
miR-378a in metabolism, angiogenesis, and muscle biology. Int J
Endocrinol. 2015:2817562015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Srivastava AK, Banerjee A, Cui T, Han C,
Cai S, Liu L, Wu D, Cui R, Li Z, Zhang X, et al: Inhibition of
miR-328-3p impairs cancer stem cell function and prevents
metastasis in ovarian cancer. Cancer Res. 79:2314–2326. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kouri FM, Hurley LA, Daniel WL, Day ES,
Hua Y, Hao L, Peng CY, Merkel TJ, Queisser MA, Ritner C, et al:
miR-182 integrates apoptosis, growth, and differentiation programs
in glioblastoma. Genes Dev. 29:732–745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang FB, Du Y, Tian Y, Ji ZG and Yang PQ:
miR-1299 functions as a tumor suppressor to inhibit the
proliferation and metastasis of prostate cancer by targeting NEK2.
Eur Rev Med Pharmacol Sci. 23:530–538. 2019.PubMed/NCBI
|
|
10
|
Peng H, Wang L, Su Q, Yi K, Du J and Wang
Z: miR-31-5p promotes the cell growth, migration and invasion of
colorectal cancer cells by targeting NUMB. Biomed Pharmacother.
109:208–216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhen Z, Dong F, Shen H, Wang QG, Yang L
and Hu J: miR-524 inhibits cell proliferation and induces cell
apoptosis in thyroid cancer via targeting SPAG9. Eur Rev Med
Pharmacol Sci. 22:3812–3818. 2018.PubMed/NCBI
|
|
12
|
Zheng X, Xu K, Zhu L, Mao M, Zhang F and
Cui L: miR-486-5p Act as a biomarker in endometrial carcinoma:
Promotes cell proliferation, migration, invasion by targeting
MARK1. Onco Targets Ther. 13:4843–4853. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C and Liu B: miR-101-3p induces
autophagy in endometrial carcinoma cells by targeting EZH2. Arch
Gynecol Obstet. 297:1539–1548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang HC, Han YY, Zhang XM, Xiao N, Jiang
T, Zhu S, Wang EP and Chen CB: miR-522 facilitates the prosperities
of endometrial carcinoma cells by directly binding to monoamine
oxidase B. Kaohsiung J Med Sci. 35:598–606. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sales CB, Buim ME, de Souza RO, de Faro
Valverde L, Mathias Machado MC, Reis MG, Soares FA, Ramos EA and
Gurgel Rocha CA: Elevated VEGFA mRNA levels in oral squamous cell
carcinomas and tumor margins: A preliminary study. J Oral Pathol
Med. 45:481–485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Claesson-Welsh L and Welsh M: VEGFA and
tumour angiogenesis. J Intern Med. 273:114–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng T, Li Z, Li D and Wang S: MACC1
promotes angiogenesis in cholangiocarcinoma by upregulating VEGFA.
Onco Targets Ther. 12:1893–1903. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horwitz E, Stein I, Andreozzi M, Nemeth J,
Shoham A, Pappo O, Schweitzer N, Tornillo L, Kanarek N, Quagliata
L, et al: Human and mouse VEGFA-amplified hepatocellular carcinomas
are highly sensitive to sorafenib treatment. Cancer Discov.
4:730–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo X and Feng GS: VEGFA genomic
amplification tailors treatment of HCCs with sorafenib. Cancer
Discov. 4:640–641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Xu X, Pan B, Zeng K, Xu M, Liu X,
He B, Pan Y, Sun H and Wang S: miR-150-5p suppresses tumor
progression by targeting VEGFA in colorectal cancer. Aging (Albany
NY). 10:3421–3437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guset G, Costi S, Lazar E, Dema A,
Cornianu M, Vernic C and Păiuşan L: Expression of vascular
endothelial growth factor (VEGF) and assessment of microvascular
density with CD34 as prognostic markers for endometrial carcinoma.
Rom J Morphol Embryol. 51:677–682. 2010.PubMed/NCBI
|
|
22
|
Panda H, Pelakh L, Chuang TD, Luo X,
Bukulmez O and Chegini N: Endometrial miR-200c is altered during
transformation into cancerous states and targets the expression of
ZEBs, VEGFA, FLT1, IKKβ, KLF9, and FBLN5. Reprod Sci. 19:786–796.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garcia DM, Baek D, Shin C, Bell GW,
Grimson A and Bartel DP: Weak seed-pairing stability and high
target-site abundance decrease the proficiency of lsy-6 and other
microRNAs. Nat Struct Mol Biol. 18:1139–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
SGO Clinical Practice Endometrial Cancer
Working Group, ; Burke WM, Orr J, Leitao M, Salom E, Gehrig P,
Olawaiye AB, Brewer M, Boruta D, Villella J, et al: Endometrial
cancer: A review and current management strategies: Part I. Gynecol
Oncol. 134:385–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang CH, Zhang XY, Zhou LN, Wan Y, Song
LL, Gu WL, Liu R, Ma YN, Meng HR, Tian YL and Zhang Y: LncRNA SNHG8
participates in the development of endometrial carcinoma through
regulating c-MET expression by miR-152. Eur Rev Med Pharmacol Sci.
22:1629–1637. 2018.PubMed/NCBI
|
|
28
|
Teague EM, Print CG and Hull ML: The role
of microRNAs in endometriosis and associated reproductive
conditions. Hum Reprod Update. 16:142–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lamouille S, Connolly E, Smyth JW, Akhurst
RJ and Derynck R: TGF-β-induced activation of mTOR complex 2 drives
epithelial-mesenchymal transition and cell invasion. J Cell Sci.
125:1259–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bullock MD, Sayan AE, Packham GK and
Mirnezami AH: MicroRNAs: Critical regulators of epithelial to
mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in
cancer progression. Biol Cell. 104:3–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nusse R and Clevers H: Wnt/beta-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Majidinia M, Aghazadeh J,
Jahanban-Esfahlani R and Yousefi B: The roles of Wnt/beta-catenin
pathway in tissue development and regenerative medicine. J Cell
Physiol. 233:5598–5612. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen YN, He HG, Shi Y, Cao J, Yuan JY,
Wang ZC, Shi CF, Zhu N, Wei YP, Liu F, et al: Kruppel-like factor 8
promotes cancer stem cell-like traits in hepatocellular carcinoma
through Wnt/β-catenin signaling. Mol Carcinog. 56:751–760. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen Q, He T and Yuan H: Hsa_circ_0002577
promotes endometrial carcinoma progression via regulating
miR-197/CTNND1 axis and activating Wnt/β-catenin pathway. Cell
Cycle. 18:1229–1240. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park SA, Kim LK, Kim YT, Heo TH and Kim
HJ: Long non-coding RNA steroid receptor activator promotes the
progression of endometrial cancer via Wnt/β-catenin signaling
pathway. Int J Biol Sci. 16:99–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen P, Xing T, Wang Q, Liu A, Liu H, Hu
Y, Ji Y, Song Y and Wang D: MicroRNA-202 inhibits cell migration
and invasion through targeting FGF2 and inactivating Wnt/β-catenin
signaling in endometrial carcinoma. Biosci Rep. 39:BSR201906802019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kontos CK, Tsiakanikas P, Avgeris M,
Papadopoulos IN and Scorilas A: miR-15a-5p, A novel prognostic
biomarker, predicting recurrent colorectal adenocarcinoma. Mol
Diagn Ther. 21:453–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Long J, Jiang C, Liu B, Fang S and Kuang
M: MicroRNA-15a-5p suppresses cancer proliferation and division in
human hepatocellular carcinoma by targeting BDNF. Tumour Biol.
37:5821–5828. 2016. View Article : Google Scholar : PubMed/NCBI
|