Introduction
Aquaporins (AQPs), a family of small (30
kDa/monomer) water channel proteins that are integral membrane
proteins and expressed in all living organisms, serve a vital role
in water homeostasis by regulating cellular water transport. To
date, a total of 13 human isoforms (AQP0-AQP12) have been found to
be expressed in a cell- and tissue-dependent manner (1,2). In
addition to water and glycerol, several other chemical substances,
such as nitrate (3),
arsenite/antimony (4,5), ammonia (6), nitric oxide (7), carbon dioxide (8), hydrogen peroxide (9), urea (10), silicon (11) and anions (12), also enter and exit cells through
AQPs. In recent years, AQP5 has been observed in the epithelium of
various types of tissue, including that of the lacrimal (13), salivary (14) and airway submucosal glands (15), as well as type I lung cells (16) and pancreatic cells (17). As AQP5 has been observed in various
pathological conditions, there has been an increasing amount of
interest in its implication in carcinogenesis. An increasing number
of studies (18,19) have shown that AQP5 is abundantly
expressed in different types of tumors, such as respiratory system
tumors, digestive system tumors, reproductive system tumors, and
may serve as a prognostic biomarker to assess how aggressive a
cancer may be. Therefore, as AQP5 may play an important role in
tumor development, it has become a novel target for antitumor
therapy.
Structure of AQP5
Raina et al (20) were the first to clone AQP5 cDNA from
rat submandibular gland cells and confirmed that the gene was
located at the distal end of chromosome 15 in 1995. Thereafter, an
increasing number of studies on AQP5 have emerged. AQP5 is composed
of 265 amino acids and has a molecular weight of 28 kDa. However,
the AQP5 protein (27 kDa) can also be found in mouse lung tissues,
and the AQP5 protein (29 kDa) in mouse submandibular glands
(21). This may be due to different
tissue-specific modifications. Similar to other AQPs, AQP5 exists
as a tetramer in the cell membrane, and each monomer can be used as
an independent water channel. A sequence analysis revealed that the
AQP5 monomer is composed of a peptide chain, which has an
N-terminus and C-terminus located on the inner side of the cell
membrane (21). The peptide chain
contains six α-helix-rich tandem hydrophobic transmembrane regions
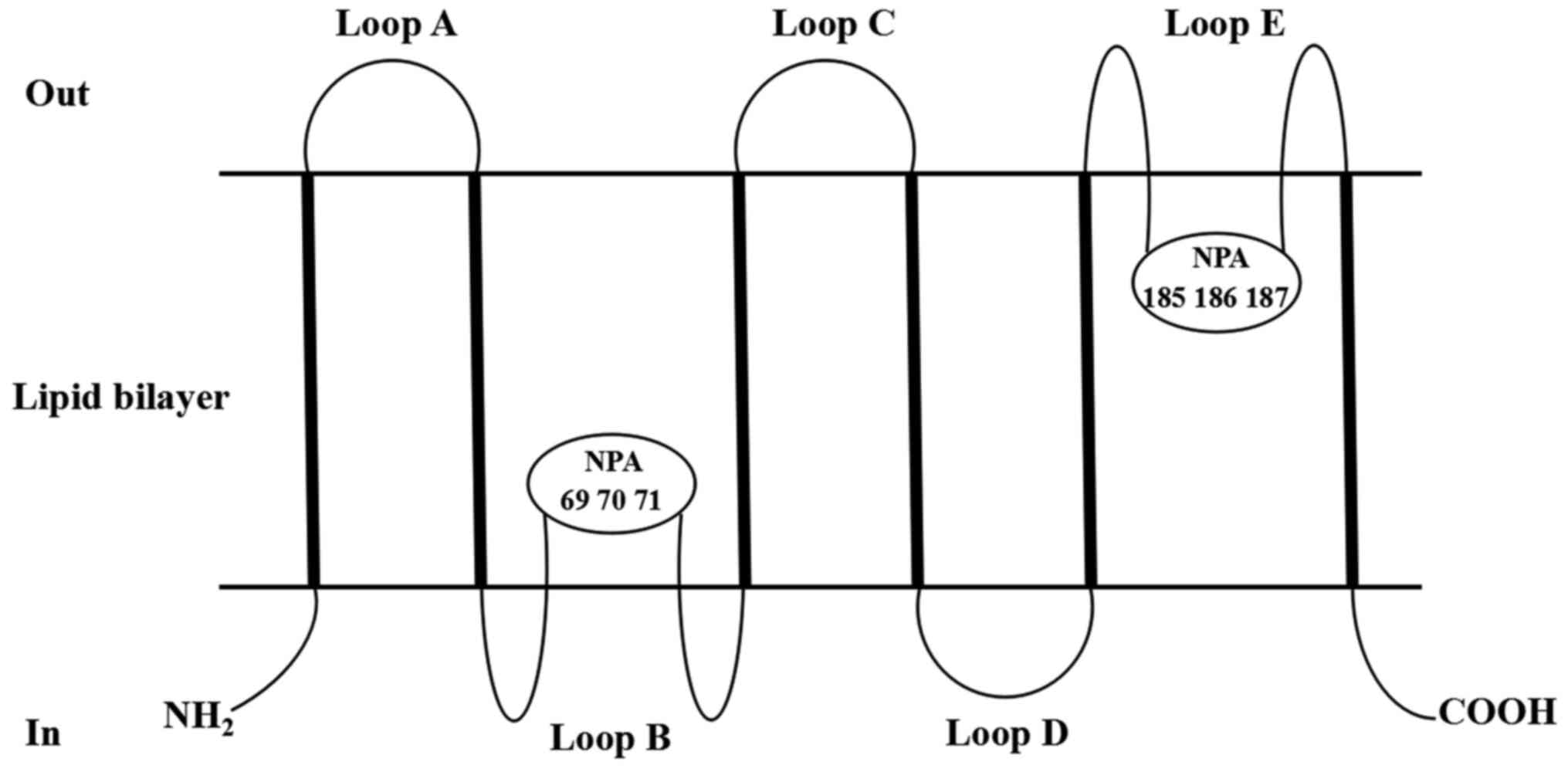
and is connected by five loops (A-E loops) (21). The AQP5 is embedded in the lipid
bilayer. As shown in Fig. 1, which
is adapted from Raine et al (20) and Woo et al (22) as appropriate, the A, C and E rings
are located outside the cell, and the B and D rings are located
inside the cell. The B and E loops contain highly conserved amino
acid sequences and aspartyl-proline-alanine (NPA) motifs (20,22). The
NPA motif is a common feature of AQP molecules and is known to be
located at positions 69, 70, 71, 185, 186 and 187 (20,22). The
B and E rings are embedded into the cell membrane at the NPA to
fold into a single-pore channel with the narrowest diameter being
0.3 nm, which the same size as a single water molecule (20,22). The
entire AQP5 protein is embedded into the cell membrane in an
hourglass-like structure (20,22). Due
to the particular structure of AQP5, it serves an important role in
the transmembrane transport of water.
Effect of AQP5 on cancer
The metabolic mechanisms of various tumor types are
closely associated with the flow of water molecules across
biofilms, and AQP-mediated fluid transport is the main way in which
water enters and exits cells (1).
Therefore, AQPs may serve an important role in tumor growth,
invasion and metastasis (1).
Furthermore, AQP5 is a vital member of the AQP family, and appears
to be a potential target for oncotherapy (18,19).
Cellular level
Cancer cell proliferation
A number of studies have shown that AQP5 can
participate in tumorigenesis and tumor development by affecting
tumor proliferation and apoptosis (23–28).
AQP5 knockdown in human breast cancer cells (MCF7 cells) was
associated with decreased cell proliferation (23). Zhang et al (24) reported enhanced proliferation
potential in lung cancer cells with high AQP5 expression, and
decreased proliferation potential in lung cancer cells with low
AQP5 expression. In the aforementioned study, in mice that were
injected with AQP5 cells within 2 weeks, tumor growth was found to
increase significantly compared with the control group. It was also
found that upregulated AQP5 was closely associated with the
proliferation of digestive tumor cells, such as esophageal
(25), gastric (26) and colon cancer cells (27). In a study on human chronic
myelogenous leukemia (CML), it was first reported that AQP5 small
interfering RNA (siRNA)-treated LAMA-84 CML cells exhibited a
marked decrease in cell proliferation when compared with control
siRNA-treated cells (28).
Furthermore, control siRNA-treated cells exhibited a higher number
of viable cells than AQP5 siRNA-treated cells (28). Therefore, these results confirmed
that AQP5 can not only promote cell proliferation, but also inhibit
cell apoptosis.
Cancer cell metastasis
Tumor cells can reach distant sites via the blood or
lymphatic vessels, which is a phenomenon known as tumor metastasis
(29). In order to determine whether
AQP was associated with cell migration and its exact underlying
mechanism, Chen et al (30)
used short hairpin RNA to inhibit the expression of the AQP5 gene,
and the results revealed that the migration potential of lung
adenocarcinoma SPC-A1 cells were significantly weakened. As
aforementioned, AQP5 was not only closely associated with the
proliferation of breast cancer cells, but also with their migration
(23). A semi-quantitative
assessment demonstrated that the AQP5 labeling intensity was
significantly higher in invasive carcinoma with lymph node
metastasis, compared with that without lymph node metastasis
(23). Furthermore, a high AQP5
expression level was associated with aggressive lymph node status
and the presence of distant metastasis in esophageal (31), gastric (26), colon (32) and prostate cancer (33). In ovarian cancer, the expression of
AQP5 was positively correlated with lymph node metastasis, but not
associated with International Federation of Gynecology and
Obstetrics stage, grade and histological type (34). A previous study demonstrated that the
overexpression of AQP5 and Ki-67 was significantly associated with
lymph node involvement in cervical cancer, and a positive
correlation was identified between AQP5 and Ki-67 expression levels
(35). Furthermore, the data also
revealed that the overexpression of AQP5 and Ki-67 was associated
with a less favorable prognosis. Since it was proven by the
aforementioned studies that AQP5 was associated with the ability of
tumor cells to invade and metastasize, it can be concluded that
AQP5 may serve as a potential novel target for to prevent tumor
metastasis.
Molecular level
AQP5 and its upstream and downstream signaling
pathways serve an important role in tumor proliferation, invasion
and migration (19). The reticular
activating system (Ras) pathway is a signaling pathway that plays
an important role in the development of cancer; its basic structure
is Ras/Raf/MEK/mitogen-activated protein kinase (MAPK) (36). Extracellular-signal-regulated kinase
(ERK) is one of the main subfamilies of MAPK, and is associated
with cell proliferation and differentiation (36). There was an experimental study which
demonstrated that AQP5 was able to activate the Ras pathway and its
downstream pathway via phosphorylation of the PKA consensus site in
its cytoplasmic loop D, thereby directly promoting cell
proliferation (37). In lung cancer
cells, the proliferative ability of cells was found to be
positively correlated with the expression level of AQP5 (24). At the same time, the expression
levels of proliferation-associated proteins, such as proliferating
cell nuclear antigen and c-Myc, were also enhanced in the cells
expressing AQP5. In AQP5 cells with a high expression level,
epidermal growth factor receptor (EGFR) phosphorylation was
enhanced, and the ERK and MAPK signaling pathways were activated,
while the activity of the EGFR/ERK/p38 MAPK pathway was decreased
following the deletion of AQP5. This result was consistent with the
results of the study by Yang et al (38), in which AQP5 gene silencing inhibited
the proliferation, reduced the migration and promoted the apoptosis
rates of human glioma cells by suppressing the EGFR/ERK/p38 MAPK
signaling pathway. In colorectal cancer, Kang et al
(39) found that the overexpression
of AQP5 increased the phosphorylation of retinoblastoma (Rb)
protein, which may be achieved by activating the Ras/ERK/Rb
signaling pathway.
Nuclear factor-κB (NF-κB), as the upstream regulator
of AQP5, inhibits the expression of AQP5 (40). The NF-κB pathway has been reported to
downregulate the expression of AQP5 by inhibiting cAMP-response
element binding protein (CREB) phosphorylation, or by competitive
binding to the CREB-binding protein (40). Therefore, the expression levels of
NF-κB and AQP5 are expected to be negatively correlated. However,
the regulatory relationship between NF-κB and AQP5 was reversed in
a CAOV3 ovarian cancer cell line (41). In the aforementioned study, a
positive correlation was identified between the AQP5 protein
expression and NF-κB, and the growth rate of CAOV3 cells was
decreased by the inhibition of the AQP5 protein. In an SKOV3
ovarian cancer cell line, the positive correlation between AQP5 and
NF-κB was verified again (42).
Pyrrolidine dithiocarbamate (PDTC), a specific blocker of NF-κB,
downregulated the expression of NF-κB and decreased its content in
the nucleus and cytoplasm, resulting in a decrease in the mRNA and
protein expression levels of AQP5 (42). At the same time, this effect also
markedly decreased tumor cell proliferation (42). Therefore, these results suggested
that the downregulation of AQP5 may inhibit tumor growth and be
associated with NF-κB (42). In
conclusion, the correlation between NF-κB and AQP5, which was
reversed in certain tumors, may reflect the difference between
normal and tumor tissue.
Chae et al (28) were the first to report that AKT
phosphorylation was enhanced in CML cell lines overexpressing AQP5,
suggesting that AQP5 may regulate tumor cell proliferation and
apoptosis via the AKT signaling pathway.
Distant metastasis is the leading cause of death in
patients with malignant tumors (43). Epithelial-to-mesenchymal transition
(EMT) is one of the key molecular steps in distant metastasis
(43). During EMT, epithelial cells
lose intercellular connections and normal polarity, and acquire
more mobile spindle-like interstitial phenotypes, promoting the
invasion and migration of cancer cells (43). In colon cancer cells, Cairicoside E
(CE), a resin glycoside isolated from Ipomoea cairica
(Convolvulaceae), inhibited the migration of colon cancer
cells by inhibiting EMT (44).
Notably, CE had no significant effect on EMT when AQP5 was silenced
(44). These results indicated that
the inhibitory effect of CE on EMT depends on the downregulation of
AQP5. In addition, it was found that transforming growth factor β1
(TGF-β1) promoted AQP5 expression, while AQP5-overexpression
upregulated p-Smad2/3, thereby activating EMT (44). Another study demonstrated that
AQP5-silencing inhibited the migration and invasion of colorectal
cancer cells, which was accompanied by altered expression of
EMT-associated molecules and weakened Wnt/β-catenin signaling
transduction (45).
AQP5-silencing-induced changes to the cell phenotype were partly
restored following the reactivation of the Wnt/β-catenin pathway
(45).
In this section, the regulatory effects of different
AQP5-related signaling pathways on different tumors were
summarized. As shown in Fig. 2, AQP5
can affect tumor cell proliferation, apoptosis and migration via
multiple signaling pathways. However, whether these pathways
interact with each other to participate in the regulation of tumor
cell pathophysiology by AQP5 requires further investigation.
Therefore, the development of therapeutic strategies using AQP5 as
a drug target, although promising, requires an increased
understanding of the molecular mechanisms involved in tumor
biology.
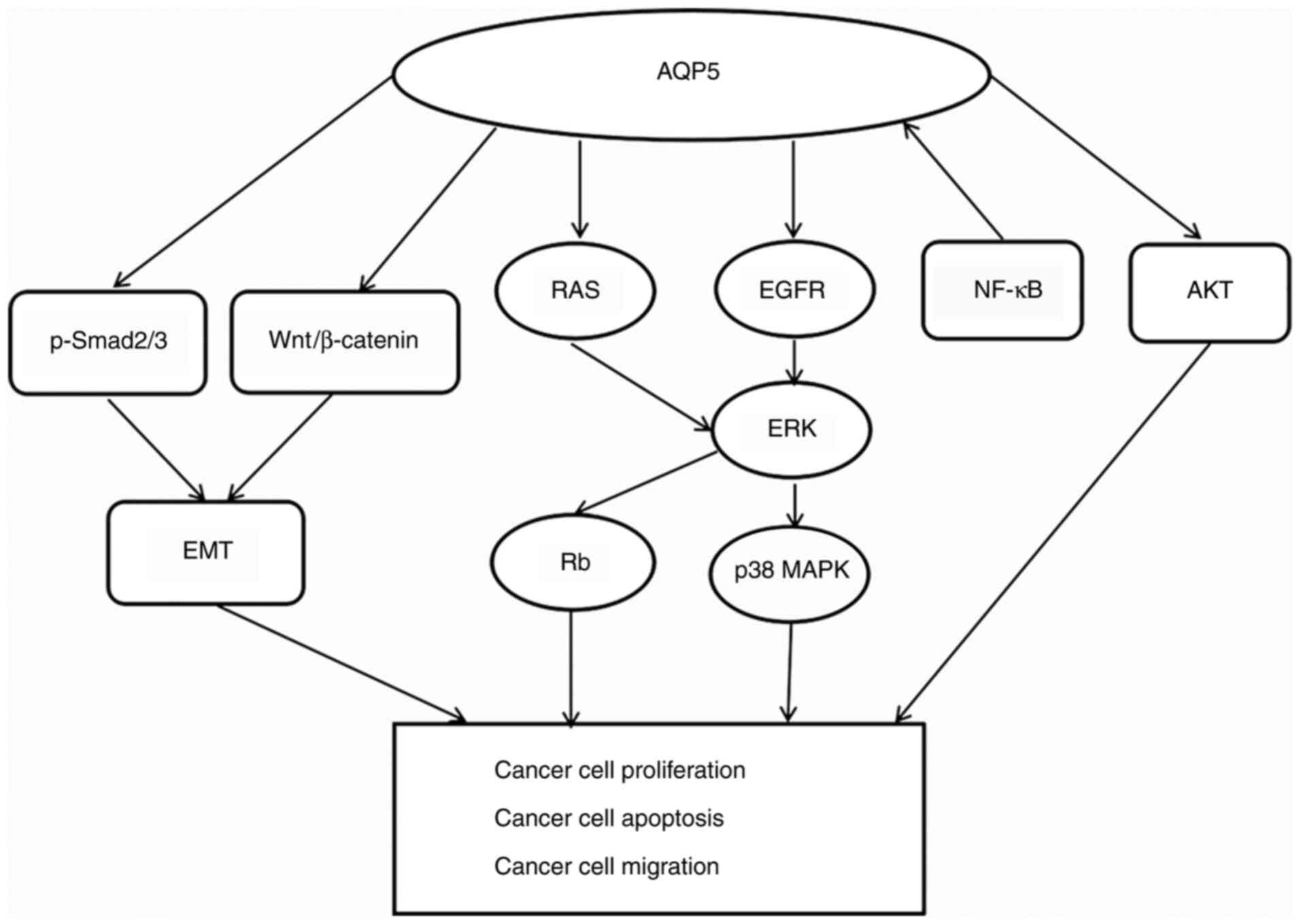 | Figure 2.Signaling pathways associated with
AQP5 in cancer. AQP5 is able to activate the EGFR/ERK/p38 MAPK
pathway and also increase the phosphorylation of Rb protein by
activating the Ras/ERK/Rb signaling pathway. In ovarian cancer, it
was found that there was a positive correlation between AQP5
protein expression and NF-κB. However, this is not the case in all
cancer cells, as detailed in the manuscript. AQP5 can also enhance
the phosphorylation of AKT to promote tumor cell proliferation. EMT
is one of the key molecular steps in cancer distant metastasis.
Overexpression of AQP5 can upregulate p-Smad2/3 and Wnt/β-catenin,
thereby activating EMT. In summary, AQP5 participates in tumor
proliferation and migration through a variety of complex signaling
pathways. AQP5, aquaporin-5; EGFR, epidermal growth factor
receptor; ERK, extracellular-signal-regulated kinase; MAPK,
mitogen-activated protein kinase; NF-κB, nuclear factor-κB; Rb,
retinoblastoma protein; Ras, reticular activating system; EMT,
epithelial-mesenchymal transition; TGF-β, tumor growth factor-β; p,
phosphorylated. |
Expression of AQP5 in cancer
Due to the distinct structural characteristics and
abnormal expression of AQP5 in different tumor types, an increasing
interest in its involvement in cancer has emerged. In tumors of the
digestive system, it was found that AQP5 was upregulated in tongue
(46), esophageal (25), gastric (26,47),
liver (48), bile duct (49) and colon cancer types (26,37,39), but
was downregulated in salivary gland adenoid cystic carcinoma
(46). In ovarian tumors, Yang et
al (34) reported that the
expression of AQP5 was associated with the pathological
characteristics of the tumor. It was found that the expression
level of AQP5 in malignant and borderline ovarian tumors was
significantly higher than that in benign tumors and normal tissues.
Secondly, the subcellular localization of AQP5 was also different
between benign and malignant tumors. AQP5 was primarily located in
the basal membrane of benign tumors, as well as the parietal
membrane and basal membrane of borderline tumors, and was scattered
at the plasma membrane of malignant tumor cells. In addition, AQP5
expression was found to be increased in cervical (35) and endometrial cancer (50). In lung cancer (51), patients with a positive AQP5
expression had a higher tumor recurrence rate and shorter
tumor-free survival time than those with a negative AQP5
expression. However, Machida et al (52) reported that the expression level of
AQP5 was positively correlated with the degree of cell
differentiation, but not prognosis. Therefore, the effect of AQP5
on the prognosis of lung cancer needs to be further studied. AQP5
overexpression was found to be associated with metastasis, poor
prognosis, higher tumor grade and tumor recurrence in breast cancer
(23,53). In addition to the aforementioned
studies, there are some other studies on the expression of AQP5 in
malignant tumor tissues. AQP5 was found to be upregulated in
primary glioblastomas and astrocytomas (54). However, due to the low number of
samples tested, further research is required to confirm these
preliminary findings. In prostate cancer, AQP5-overexpression was
associated with Tumor-Node-Metastasis stage (33,55),
lymph node metastasis (33) and the
cumulative survival rate (33,56). It
can therefore be concluded that AQP5 is an independent prognostic
indicator in prostate cancer.
Unlike the aforementioned studies that focused on
solid tumors, to the best of our knowledge, there is currently a
limited number of studies that focus on AQP5 in hematological
tumors. Chae et al (28)
reported that the AQP5 expression level was relatively higher in
patients with CML diagnosed at the advanced or blast phases when
compared with those diagnosed at the chronic phase, and those with
a high AQP5 expression level were more likely to develop drug
resistance.
Influence of drugs and compounds on
AQP5
Over the past few decades, significant progress has
been made in understanding the fundamental role of AQP5 in various
types of cancer. Further research on AQP5 revealed several drugs or
compounds that can regulate the expression of AQP5. Therefore, AQP5
has potential as a powerful target for the development of new
anticancer drugs.
Direct AQP5 inhibitors
Radiotherapy serves a vital role in the treatment of
head and neck tumors, particularly nasopharyngeal carcinoma.
However, even after low-dose radiotherapy, most patients still
develop sinusitis, mucositis, bronchitis, pneumonia, fibrosis or
soft tissue necrosis. AQP5 is the main AQP in the airway mucosa,
and the rate-limiting barrier in mucin production (57,58). The
expression of AQP5 is closely associated with cilia function
(59) and bronchoconstriction
(60,61). To the best of our knowledge, Liu
et al (62) were the first to
report that ovatodiolide can enhance AQP5 membrane transport and
reverse the inhibitory effect of histamine on CREB phosphorylation,
thereby reducing the side effects of radiotherapy on nasopharyngeal
carcinoma. Therefore, they inferred that treatment with
ovatodiolide is expected to restore normal nasopharyngeal
physiology in patients with nasopharyngeal carcinoma following
radiotherapy. Acetazolamide is an AQP inhibitor that is able to
decrease AQP expression and AQP-related functions (63,64).
Huang et al (26) reported
that the expression of AQP5 was significantly increased in gastric
cancer tissues, and the inhibition of AQP5 expression by
acetazolamide decreased the proliferative and invasive abilities of
gastric cancer cells. In addition, AQP5 was involved in the
differentiation of gastric cancer cells. The expression of AQP5 was
significantly upregulated in intestinal gastric cancer cells, while
that of AQP5 was not identified in intestinal metaplasia and the
diffuse type of the gastric cancer tissues. Treatment of MKN45
gastric cancer cells that stably expressed AQP5 with HgCl2, an
inhibitor of AQPs, significantly decreased the proportion of
differentiated cells and the activity of alkaline phosphatase
(65). Heavy metal compounds are
certainly effective AQP inhibitors, but their toxicity and lack of
specificity substantially limit their therapeutic potential.
Direct modulation of AQP5, even by therapeutically
unpalatable heavy metal ions, are the best demonstration that AQP5
inhibiting drugs can have a therapeutic effect in specific tumor
types. By studying these direct regulators of AQP5, it may be
possible to provide new development directions for tumor targeted
therapy.
Genetic (siRNA) silencing of AQP5
expression
Chemotherapy is one of the main treatments for
advanced cancer; however, its effectiveness is limited (66). One of the main reasons for that is
the drug resistance of tumor cells. Multidrug resistance can be
divided into two categories (66).
The first is the classic drug resistance mechanism, involving
energy-dependent drug pumps, which is mediated by drug-resistant
membrane glycoproteins, including P-glycoprotein 1 (P-gp),
multidrug resistance-associated protein 2 and resistance-related
protein (66). The other is a
resistance mechanism involving resistance enzymes, such as DNA
topoisomerase II (TOPO II), thymidylate synthase (TS) and protein
kinase C (66). AQP5 expression was
found to be increased in human colon cancer tissues and was
positively correlated with P-gp, glutathione S-transferase Pi and
TOPO II (27). Following the use of
siRNA-mediated genes to silence AQP5 expression in HT-29 colon
cancer cell lines, the sensitivity of these cells to chemotherapy
drugs cisplatin (DDP) and 5-fluorouracil (5-FU) was increased
(27). In addition, resistance to
imatinib mesylate, a tyrosine-kinase inhibitor used in CML
treatment at the chronic phase, was also associated with higher
levels of AQP5 expression (28).
siRNA targeting AQP5 reduced the cell proliferation rate in CML
cells.
The effectiveness of certain Traditional Chinese
Medicine (TCM) agents in the treatment of diseases has been widely
accepted. Youyou Tu, chief scientist, Institute of Chinese Materia
Medica China Academy of Chinese Medical Sciences Beijing (Beijing,
China), received the Nobel Prize in Physiology or Medicine in 2015
for her discovery of artemisinin, a drug used to treat malaria,
which saved millions of lives around the world (67). In recent years, certain natural
plant-derived compounds have attracted increasing attention in the
field of antitumor drug development. The Realgar-Indigo naturalis
formula has been proven to be effective in treating human acute
promyelocytic leukemia (68).
Furthermore, CE, a resin glycoside isolated from Ipomoea
cairica, was found to be cytotoxic to tumor cells in a study by
Yu et al (69). A follow-up
study revealed that CE had no significant effect on EMT markers and
p-Smad2/3 following AQP5 silencing, indicating that CE may also
inhibit the EMT process by downregulating AQP5 in CRC cells,
thereby inhibiting the metastasis of cancer cells (44). In summary, gene silencing of AQP5 by
siRNA is also a reasonable method to directly inhibit AQP5. If
certain drugs can suppress tumor growth by silencing the AQP5 gene,
this may represent a major advancement for antitumor therapies.
Indirect effectors of AQP5
expression
Ovarian cancer appears to be associated with AQP5.
Epigallocatechin gallate (a potential cancer drug), PDTC (an NF-κB
specific inhibitor) and DDP (first-line drug in the defense against
cancer), all inhibit proliferation and induce apoptosis of ovarian
cancer cells by downregulating AQP5 expression (41,42);
These drugs can downregulate AQP5, which might all be associated
with the nuclear transcription factor NF-κB. It has been reported
that hyperosmotic stress induced by sorbitol treatment reduced AQP5
expression in breast cancer MCF7 cells, which was also associated
with a significant reduction in cell proliferation and migration
(23).
In addition, there are certain physical environments
that can exert antitumor effects by activating the expression of
AQP5. A previous study has shown that the hypotonic solution at low
temperature increases the initial water influx by activating AQP5,
thereby enhancing the cytocidal effect on gastric cancer cells
(70). Therefore, low-temperature
lavage under hypotonic conditions may have the potential to become
a new method of reducing the peritoneal recurrence of gastric
cancer following radical surgery. As mentioned above drugs and
certain physical environments can suppress tumors by regulating the
expression of AQP5, which may not be a strong evidence in the
development of antitumor drugs (23,41,42,70),.
However, it also provides new evidence for AQP5 to become a target
and prognostic indicator of antitumor therapy.
In conclusion, multiple substances can affect the
regulatory effect of AQP5 on tumors. The substances include TCM and
western medicine agents, as well as compounds. A previous study
(71) showed that puerarin and 5-FU
have a significant synergistic effect on gastric cancer cells.
Puerarin can enhance the antiproliferative effect of 5-FU and
decrease the dose required for 5-FU treatment without increasing
toxicity. It was therefore hypothesized that combining TCM and
western medicine may achieve more favorable anticancer effects than
each of them alone, by synergistically inhibiting AQP5. That is to
say, this combination could not only enhance the therapeutic
effect, but also decrease the adverse side effects of various
drugs. Of course, the exact method of effectively combining TCM and
western medicine to maximize the therapeutic effect is a very
complex subject that requires further study.
Conclusions and perspectives
The incidence of cancer has been increasing annually
due to the pressure of fast-paced life, environmental pollution,
bad living habits, such as smoking, alcoholism, etc. In order to
improve cancer treatment, a large number of targeted studies are
constantly being performed. After establishing the anticancer
effects of AQP1, there has been considerable interest in the
development of other AQPs for use in antitumor therapy (29). A number of studies have proven that
AQP5 can be an effective therapeutic target against cancer. In the
present study, the structural characteristics of AQP5 were first
briefly described. Secondly, the effects of AQP5 and its expression
in different tumor types were summarized. In addition, a
comprehensive summary of drugs and compounds that can affect the
antitumor effect of AQP5 was provided. AQP5 participates in all
stages of malignant tumors, including tumor occurrence,
development, recurrence, metastasis, drug resistance and prognosis.
Although several potential AQP modulators have been identified,
discovering better modulators, including those that have the
ability to target specificity, is not without its challenges
(72). In addition, the exact
molecular mechanism underlying the role of AQP5 in tumors has not
yet been fully elucidated. To the best of our knowledge, a genetic
link between AQP5 and any known tumor types has not yet been
identified. This may be due to the current research on AQP5 being
relatively new. Therefore, more comprehensive and in-depth research
is required to build on these findings. In conclusion, with the
continuous increase in research on AQP5, the investigation into
various treatment methods based on the expression and regulation of
AQP5, with the aim of intervening in the biological behavior of
tumors, will provide new ideas and methods for the clinical
treatment of cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LW collected and analysed the data, and was a major
contributor in drafting the original manuscript. DH, HZ, QX, CG and
WC collected and analysed the data. YZ reviewed and edited the
manuscript. LW and YZ confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
AQP5
|
aquaporin-5
|
|
AQPs
|
aquaporins
|
|
CE
|
Cairicoside E
|
|
CML
|
chronic myelogenous leukemia
|
|
CREB
|
cAMP-response element binding
protein
|
|
DDP
|
cisplatin
|
|
EGFR
|
epidermal growth factor receptor
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
ERK
|
extracellular-signal-regulated
kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
NPA
|
aspartyl-proline-alanine
|
|
P-gp
|
P-glycoprotein 1
|
|
PDTC
|
pyrrolidine dithiocarbamate
|
|
Ras
|
reticular activating system
|
|
siRNA
|
small interfering RNA
|
|
TCM
|
Traditional Chinese Medicine
|
|
TGF-β1
|
transforming growth factor β1
|
|
TOPO II
|
DNA topoisomerase II
|
References
|
1
|
Takata K, Matsuzaki T and Tajika Y:
Aquaporins: Water channel proteins of the cell membrane. Prog
Histochem Cytochem. 39:1–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishibashi K, Tanaka Y and Morishita Y: The
role of mammalian superaquaporins inside the cell. Biochim Biophys
Acta. 1840:1507–1512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ikeda M, Beitz E, Kozono D, Guggino WB,
Agre P and Yasui M: Characterization of aquaporin-6 as a nitrate
channel in mammalian cells. Requirement of pore-lining residue
threonine 63. J Biol Chem. 277:39873–39879. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanders OI, Rensing C, Kuroda M, Mitra B
and Rosen BP: Antimonite is accumulated by the glycerol facilitator
GlpF in Escherichia coli. J Bacteriol. 179:3365–3367. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Shen J, Carbrey JM, Mukhopadhyay R,
Agre P and Rosen BP: Arsenite transport by mammalian
aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA.
99:6053–6058. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holm LM, Jahn TP, Møller AL, Schjoerring
JK, Ferri D, Klaerke DA and Zeuthen T: NH3 and NH4+
permeability in aquaporin-expressing Xenopus oocytes.
Pflugers Arch. 450:415–428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herrera M, Hong NJ and Garvin JL:
Aquaporin-1 transports NO across cell membranes. Hypertension.
48:157–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakhoul NL, Davis BA, Romero MF and Boron
WF: Effect of expressing the water channel aquaporin-1 on the
CO2 permeability of Xenopus oocytes. Am J
Physiol. 274:C543–C548. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bienert GP, Møller AL, Kristiansen KA,
Schulz A, Møller IM, Schjoerring JK and Jahn TP: Specific
aquaporins facilitate the diffusion of hydrogen peroxide across
membranes. J Biol Chem. 282:1183–1192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma T, Yang B and Verkman AS: Cloning of a
novel water and urea-permeable aquaporin from mouse expressed
strongly in colon, placenta, liver, and heart. Biochem Biophys Res
Commun. 240:324–328. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma JF, Tamai K, Yamaji N, Mitani N,
Konishi S, Katsuhara M, Ishiguro M, Murata Y and Yano M: A silicon
transporter in rice. Nature. 440:688–691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yasui M, Hazama A, Kwon TH, Nielsen S,
Guggino WB and Agre P: Rapid gating and anion permeability of an
intracellular aquaporin. Nature. 402:184–187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gresz V, Kwon TH, Gong H, Agre P, Steward
MC, King LS and Nielsen S: Immunolocalization of AQP-5 in rat
parotid and submandibular salivary glands after stimulation or
inhibition of secretion in vivo. Am J Physiol Gastrointest Liver
Physiol. 287:G151–G161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krane CM, Melvin JE, Nguyen HV, Richardson
L, Towne JE, Doetschman T and Menon AG: Salivary acinar cells from
aquaporin 5-deficient mice have decreased membrane water
permeability and altered cell volume regulation. J Biol Chem.
276:23413–23420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kreda SM, Gynn MC, Fenstermacher DA,
Boucher RC and Gabriel SE: Expression and localization of
epithelial aquaporins in the adult human lung. Am J Respir Cell Mol
Biol. 24:224–234. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dobbs LG, Gonzalez R, Matthay MA, Carter
EP, Allen L and Verkman AS: Highly water-permeable type I alveolar
epithelial cells confer high water permeability between the
airspace and vasculature in rat lung. Proc Natl Acad Sci USA.
95:2991–2996. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burghardt B, Elkaer ML, Kwon TH, Rácz GZ,
Varga G, Steward MC and Nielsen S: Distribution of aquaporin water
channels AQP1 and AQP5 in the ductal system of the human pancreas.
Gut. 52:1008–1016. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moosavi MS and Elham Y: Aquaporins 1, 3
and 5 in Different Tumors, their Expression, Prognosis Value and
Role as New Therapeutic Targets. Pathol Oncol Res. 26:615–625.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Direito I, Madeira A, Brito MA and Soveral
G: Aquaporin-5: From structure to function and dysfunction in
cancer. Cell Mol Life Sci. 73:1623–1640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raina S, Preston GM, Guggino WB and Agre
P: Molecular cloning and characterization of an aquaporin cDNA from
salivary, lacrimal, and respiratory tissues. J Biol Chem.
270:1908–1912. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horsefield R, Nordén K, Fellert M,
Backmark A, Törnroth-Horsefield S, Terwisscha van Scheltinga AC,
Kvassman J, Kjellbom P, Johanson U and Neutze R: High-resolution
X-ray structure of human aquaporin 5. Proc Natl Acad Sci USA.
105:13327–13332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woo J, Lee J, Chae YK, Kim MS, Baek JH,
Park JC, Park MJ, Smith IM, Trink B, Ratovitski E, et al:
Overexpression of AQP5, a putative oncogene, promotes cell growth
and transformation. Cancer Lett. 264:54–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung HJ, Park JY, Jeon HS and Kwon TH:
Aquaporin-5: A marker protein for proliferation and migration of
human breast cancer cells. PLoS One. 6:e284922011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Chen Z, Song Y, Zhang P, Hu J and
Bai C: Expression of aquaporin 5 increases proliferation and
metastasis potential of lung cancer. J Pathol. 221:210–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimizu H, Shiozaki A, Ichikawa D,
Fujiwara H, Konishi H, Ishii H, Komatsu S, Kubota T, Okamoto K,
Kishimoto M, et al: The expression and role of Aquaporin 5 in
esophageal squamous cell carcinoma. J Gastroenterol. 49:655–666.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang YH, Zhou XY, Wang HM, Xu H, Chen J
and Lv NH: Aquaporin 5 promotes the proliferation and migration of
human gastric carcinoma cells. Tumour Biol. 34:1743–1751. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi X, Wu S, Yang Y, Tang L, Wang Y, Dong
J, Lü B, Jiang G and Zhao W: AQP5 silencing suppresses p38 MAPK
signaling and improves drug resistance in colon cancer cells.
Tumour Biol. 35:7035–7045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chae YK, Kang SK, Kim MS, Woo J, Lee J,
Chang S, Kim DW, Kim M, Park S, Kim I, et al: Human AQP5 plays a
role in the progression of chronic myelogenous leukemia (CML). PLoS
One. 3:e25942008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Zhang Y, Wu X and Yu G:
Aquaporins: New Targets for Cancer Therapy. Technol Cancer Res
Treat. 15:821–828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Z, Zhang Z, Gu Y and Bai C: Impaired
migration and cell volume regulation in aquaporin 5-deficient
SPC-A1 cells. Respir Physiol Neurobiol. 176:110–117. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu S, Zhang S, Jiang H, Yang Y and Jiang
Y: Co-expression of AQP3 and AQP5 in esophageal squamous cell
carcinoma correlates with aggressive tumor progression and poor
prognosis. Med Oncol. 30:6362013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang BW, Kim JG, Lee SJ, Chae YS and Jeong
JY, Yoon GS, Park SY, Kim HJ, Park JS, Choi GS and Jeong JY:
Expression of aquaporin-1, aquaporin-3, and aquaporin-5 correlates
with nodal metastasis in colon cancer. Oncology. 88:369–376. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Wang Z, Chong T, Chen H, Li H, Li G,
Zhai X and Li Y: Over-expression of a poor prognostic marker in
prostate cancer: AQP5 promotes cells growth and local invasion.
World J Surg Oncol. 12:2842014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang JH, Shi YF, Cheng Q and Deng L:
Expression and localization of aquaporin-5 in the epithelial
ovarian tumors. Gynecol Oncol. 100:294–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang T, Zhao C, Chen D and Zhou Z:
Overexpression of AQP5 in cervical cancer: Correlation with
clinicopathological features and prognosis. Med Oncol.
29:1998–2004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Woo J, Lee J, Kim MS, Jang SJ, Sidransky D
and Moon C: The effect of aquaporin 5 overexpression on the Ras
signaling pathway. Biochem Biophys Res Commun. 367:291–298. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Zhang JN, Chen WL, Wang GS, Mao Q,
Li SQ, Xiong WH, Lin YY, Ge JW, Li XX, et al: Effects of AQP5 gene
silencing on proliferation, migration and apoptosis of human glioma
cells through regulating EGFR/ERK/p38 MAPK signaling pathway.
Oncotarget. 8:38444–38455. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang SK, Chae YK, Woo J, Kim MS, Park JC,
Lee J, Soria JC, Jang SJ, Sidransky D and Moon C: Role of human
aquaporin 5 in colorectal carcinogenesis. Am J Pathol. 173:518–525.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang W and Zheng M: Nuclear factor kappa B
pathway down-regulates aquaporin 5 in the nasal mucosa of rats with
allergic rhinitis. Eur Arch Otorhinolaryngol. 268:73–81. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang J, Yan C, Zheng W and Chen X:
Proliferation inhibition of cisplatin and aquaporin 5 expression in
human ovarian cancer cell CAOV3. Arch Gynecol Obstet. 285:239–245.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan C, Yang J, Shen L and Chen X:
Inhibitory effect of Epigallocatechin gallate on ovarian cancer
cell proliferation associated with aquaporin 5 expression. Arch
Gynecol Obstet. 285:459–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen C, Ma T, Zhang C, Zhang H, Bai L,
Kong L and Luo J: Down-regulation of aquaporin 5-mediated
epithelial-mesenchymal transition and anti-metastatic effect by
natural product Cairicoside E in colorectal cancer. Mol Carcinog.
56:2692–2705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang W, Li Q, Yang T, Li D, Ding F, Sun H
and Bai G: Anti-cancer effect of Aquaporin 5 silencing in
colorectal cancer cells in association with inhibition of
Wnt/β-catenin pathway. Cytotechnology. 70:615–624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ishimoto S, Wada K, Usami Y, Tanaka N,
Aikawa T, Okura M, Nakajima A, Kogo M and Kamisaki Y: Differential
expression of aquaporin 5 and aquaporin 3 in squamous cell
carcinoma and adenoid cystic carcinoma. Int J Oncol. 41:67–75.
2012.PubMed/NCBI
|
|
47
|
Tan SH, Swathi Y, Tan S, Goh J, Seishima
R, Murakami K, Oshima M, Tsuji T, Phuah P, Tan LT, et al: AQP5
enriches for stem cells and cancer origins in the distal stomach.
Nature. 578:437–443. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo X, Sun T, Yang M, Li Z, Li Z and Gao
Y: Prognostic value of combined aquaporin 3 and aquaporin 5
overexpression in hepatocellular carcinoma. BioMed Res Int.
2013:2065252013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sekine S, Shimada Y, Nagata T, Moriyama M,
Omura T, Watanabe T, Hori R, Yoshioka I, Okumura T, Sawada S, et
al: Prognostic significance of aquaporins in human biliary tract
carcinoma. Oncol Rep. 27:1741–1747. 2012.PubMed/NCBI
|
|
50
|
Jiang XX, Xu KH, Ma JY, Tian YH, Guo XY,
Lin J and Wu RJ: Reduced migration of Ishikawa cells associated
with downregulation of aquaporin-5. Oncol Lett. 4:257–261. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chae YK, Woo J, Kim MJ, Kang SK, Kim MS,
Lee J, Lee SK, Gong G, Kim YH, Soria JC, et al: Expression of
aquaporin 5 (AQP5) promotes tumor invasion in human non small cell
lung cancer. PLoS One. 3:e21622008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Machida Y, Ueda Y, Shimasaki M, Sato K,
Sagawa M, Katsuda S and Sakuma T: Relationship of aquaporin 1, 3,
and 5 expression in lung cancer cells to cellular differentiation,
invasive growth, and metastasis potential. Hum Pathol. 42:669–678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shi Z, Zhang T, Luo L, Zhao H, Cheng J,
Xiang J and Zhao C: Aquaporins in human breast cancer:
Identification and involvement in carcinogenesis of breast cancer.
J Surg Oncol. 106:267–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
McCoy E and Sontheimer H: Expression and
function of water channels (aquaporins) in migrating malignant
astrocytes. Glia. 55:1034–1043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee H, Lee M, Byun SS, Lee SE and Hong SK:
Evaluation of Prostate Cancer Stage Groups Updated in the 8th
Edition of the American Joint Committee on Cancer
Tumor-Node-Metastasis Staging Manual. Clin Genitourin Cancer.
17:e221–e226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pust A, Kylies D, Hube-Magg C, Kluth M,
Minner S, Koop C, Grob T, Graefen M, Salomon G, Tsourlakis MC, et
al: Aquaporin 5 expression is frequent in prostate cancer and shows
a dichotomous correlation with tumor phenotype and PSA recurrence.
Hum Pathol. 48:102–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hansel NN, Sidhaye V, Rafaels NM, Gao L,
Gao P, Williams R, Connett JE, Beaty TH, Mathias RA, Wise RA, et
al: Aquaporin 5 polymorphisms and rate of lung function decline in
chronic obstructive pulmonary disease. PLoS One. 5:e142262010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shen Y, Wang Y, Chen Z, Wang D, Wang X,
Jin M and Bai C: Role of aquaporin 5 in antigen-induced airway
inflammation and mucous hyperproduction in mice. J Cell Mol Med.
15:1355–1363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shikani AH, Sidhaye VK, Basaraba RJ,
Shikani HJ, Alqudah MA, Kirk N, Cope E and Leid JG: Mucosal
expression of aquaporin 5 and epithelial barrier proteins in
chronic rhinosinusitis with and without nasal polyps. Am J
Otolaryngol. 35:377–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Krane CM, Fortner CN, Hand AR, McGraw DW,
Lorenz JN, Wert SE, Towne JE, Paul RJ, Whitsett JA and Menon AG:
Aquaporin 5-deficient mouse lungs are hyperresponsive to
cholinergic stimulation. Proc Natl Acad Sci USA. 98:14114–14119.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Aggarwal NR, Chau E, Garibaldi BT, Mock
JR, Sussan T, Rao K, Rao K, Menon AG, D'Alessio FR, Damarla M, et
al: Aquaporin 5 regulates cigarette smoke induced emphysema by
modulating barrier and immune properties of the epithelium. Tissue
Barriers. 1:e252482013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu SC, Huang CM, Chang YL, Bamodu OA, Yeh
CT, Wang HW, Lee FP and Lin CS: Ovatodiolide suppresses
inflammatory response in BEAS-2B cells by regulating the CREB/AQP5
pathway, and sensitizes nasopharyngeal carcinoma cells to radiation
therapy. Eur J Pharmacol. 859:1725482019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ameli PA, Madan M, Chigurupati S, Yu A,
Chan SL and Pattisapu JV: Effect of acetazolamide on aquaporin-1
and fluid flow in cultured choroid plexus. Acta Neurochir Suppl
(Wien). 113:59–64. 2012. View Article : Google Scholar
|
|
64
|
Tanimura Y, Hiroaki Y and Fujiyoshi Y:
Acetazolamide reversibly inhibits water conduction by aquaporin-4.
J Struct Biol. 166:16–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Watanabe T, Fujii T, Oya T, Horikawa N,
Tabuchi Y, Takahashi Y, Morii M, Takeguchi N, Tsukada K and Sakai
H: Involvement of aquaporin-5 in differentiation of human gastric
cancer cells. J Physiol Sci. 59:113–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Beck WT: The cell biology of multiple drug
resistance. Biochem Pharmacol. 36:2879–2887. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mikić D: The 2015 Nobel Prize Laureates in
Physiology or Medicine. Vojnosanit Pregl. 72:951–952. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang L, Zhou GB, Liu P, Song JH, Liang Y,
Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al: Dissection of
mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as
an effective treatment for promyelocytic leukemia. Proc Natl Acad
Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yu B, Luo J, Wang J, Zhang D, Yu S and
Kong L: Pentasaccharide resin glycosides from Ipomoea
cairica and their cytotoxic activities. Phytochemistry.
95:421–427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shiozaki A, Yamazato Y, Kosuga T, Kudou M,
Shoda K, Arita T, Konishi H, Komatsu S, Kubota T, Fujiwara H, et
al: Effect of low temperature on the regulation of cell volume
after hypotonic shock in gastric cancer cells. Int J Oncol.
55:905–914. 2019.PubMed/NCBI
|
|
71
|
Guo XF, Yang ZR, Wang J, Lei XF, Lv XG and
Dong WG: Synergistic antitumor effect of puerarin combined with
5-fluorouracil on gastric carcinoma. Mol Med Rep. 11:2562–2568.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Verkman AS, Anderson MO and Papadopoulos
MC: Aquaporins: Important but elusive drug targets. Nat Rev Drug
Discov. 13:259–277. 2014. View Article : Google Scholar : PubMed/NCBI
|