Introduction
Acute myeloid leukemia (AML) is an aggressive highly
heterogeneous hematological malignancy associated with a poor
prognosis and high mortality rate (1). According to the SEER cancer statistics
of 2018, the age-adjusted incidence of AML was 4.3 per 100,000
annually in the United States while the mortality rate was 2.8 per
100,000 with an estimated median overall survival (OS) time of AML
of 8.5 months and 2-year and 5-year OS rates were 32.0 and 24.0%,
respectively (2). There is a high
incidence of extramedullary disease (EMD) in AML at the initial
diagnosis, during treatment and at relapse. Patients with AML and
EMD have a worse prognosis than those without EMD (3). Conventional chemotherapy and
hematopoietic stem cell transplantation remain the standard choice
of care for patients with AML (4).
However, resistance and poor tolerance to chemotherapy,
treatment-related mortality and the high relapse rate create an
urgent need to develop more effective and tolerable novel therapies
for patients with AML, such as immune-based therapies (4,5).
Inhibitory checkpoints are part of the normal immune
system that function to turn off an immune response. These
molecules interrupt T-cell activation and proliferation, and
decrease cytokine production, which is mandatory for the
establishment of peripheral tolerance during normal immune
responses (6). Research on the
immune microenvironment of AML has revealed that leukemia cells
manipulate the immune system by a dynamic process called
immunoediting, through which it takes advantage of the normal
inhibitory checkpoints by expressing the ligands of these
checkpoint receptors, thus resulting in T-cell exhaustion, a
process of gradual loss of T-cell function and downregulation of
the immune system. This concept may potentially explain immune
escape by both solid and hematological malignancies (6,7).
Exhausted T cells are characterized by increased
expression of several inhibitory receptors, such as programmed cell
death protein-1, T-cell immunoglobulin mucin domain 3 (TIM-3) and
lymphocyte activation gene-3 (8).
TIM-3 is a negative regulatory receptor expressed on
CD4+ and CD8+ T cells, T-regulatory cells and
dendritic cells; it plays a key role in inhibiting Th1 responses
and the expression of cytokines, such as tumor necrosis factor and
γ-interferon (8,9). There are four binding ligands that have
been identified for TIM-3, including carcinoembryonic antigen cell
adhesion molecule 1, high-mobility group protein B1,
phosphatidylserine and galectin-9 (Gal-9) (9).
Several studies have demonstrated a close
association between TIM-3 expression and tumor-associated immune
suppression (10,11). TIM-3 and its ligand, Gal-9, are
expressed on AML blast and leukemic stem cells (12). In addition, there is a reported
increase in the percentage of TIM-3-expressing CD8+ T
cells circulating in the blood of patients with AML compared with
that in healthy individuals (13).
However, further studies are required to confirm these results. The
present study aimed to investigate TIM-3 expression in patients
newly diagnosed with AML, as well as to determine its association
with different prognostic variables and further investigate the
impact of TIM-3 expression status on the clinical outcome.
Patients and methods
Patients
A total of 60 patients newly diagnosed with AML were
recruited from the Department of Internal Medicine, Clinical
Hematology and Bone Marrow Transplantation Unit, Ain-shams
University Hospitals (Cairo, Egypt) between January 2018 and
December 2018. All patients were treated and followed up for 12
months. A total of 15 healthy, age and sex matched individuals were
enrolled to serve as controls. The patients included 24 men and 36
women, age range, 20–68 years, mean age of 53.4±12.9 years, while
the healthy controls included 7 men and 8 women, with age range,
35–56 years, mean age of 46.9±6.3 years. The diagnosis was made
according to the criteria of the French-American-British (FAB)
classification and World Health Organization (14). The diagnosis was based on the
morphological findings from Wright-Giemsa-stained smears of bone
marrow aspirates, combined with immunophenotyping analyses of
leukemic cells using diagnostic kits [CD34, CD13, CD33,
myeloperoxidase, human leukocyte antigen-DR isotype (HLA-DR)
HLA-DR, CD117, CD2 and CD19; Beckman Coulter, Inc.] (15) and cytogenetic studies by fluorescence
in situ hybridization (FISH) using a locus-specific
identifier DNA probe (fluorophore-labeled; Abbott Molecular).
Dual-color FISH and visualization of hybridization signals by
fluorescence microscopy were performed to detect the presence of
recurrent cytogenetic abnormalities, as previously described by
Fröhling et al (16).
A total of three cytogenetic risk groups were
distinguished in AML, and patients with t(15;17), t(8;21) and
inv(16) were assigned to the ‘favorable risk’ group, while patients
with (3q), del(5q), −5/−7 or complex karyotype were assigned to the
‘poor risk’ group. The remaining patients, including normal
karyotype, were assigned to the ‘intermediate risk’ group, and
patients with intermediate or poor risk cytogenetics had an
unfavorable prognosis (17).
The present study was approved by the Ethical
Committee of Research, Faculty of Medicine, Ain Shams University,
and performed in accordance with the Declaration of Helsinki.
Written informed consent was provided by all participants prior to
the study start. The inclusion criteria for the patients were: i)
Age >18 years; and ii) newly diagnosed AML and fit to receive
chemotherapy. The exclusion criteria for the patients were: i)
Secondary AML; ii) relapsed AML; and iii) unfit to receive
chemotherapy. The included patients provided a detailed history,
subjected to a clinical examination, and had other data extracted
and recorded from the patient files. All patients received
induction standard chemotherapy in accordance with NCCN 2019
guidelines for AML (PETHEMA protocol for APL M3 and 3+7 protocol
for other types of AML) (17). By
the end of the first induction, all surviving patients were
assessed regarding their responsiveness to chemotherapy. Assessment
included a full blood work up, as well as a bone marrow (BM)
examination. Patients were classified into responders, who attained
complete remission (CR), and non-responders, who were refractory to
chemotherapy. CR was defined as an absolute neutrophilic count
>1,000/µl, a platelet count ≥100,000/µl and <5% BM blasts in
a normocellular marrow, with no evidence of EMD. Patients who had
achieved CR following induction chemotherapy were subjected to
consolidation with either allogeneic stem cell transplantation, if
they had poor risk cytogenetics and a matched sibling donor, or in
cases where donors were unavailable and/or patients had a good risk
from the start, high-dose chemotherapy (consolidation phase of
PETHEMA protocol for APL M3 and high-dose cytosine arabinoside for
other types of AML) (18). Overall
survival time was defined as the time from the date of diagnosis
until mortality. Patients still alive were censored at the end of
the 12 months as only 1-year survival was assessed in the current
study.
Reverse transcription-quantitative
(RT-q)PCR
Peripheral blood samples were withdrawn from
controls and from patients with AML prior to treatment, and
contained in vacutainer tubes containing Na2 EDTA to
determine the complete blood count and for total RNA purification
from human whole blood. Total RNA was extracted using extraction
buffer (present in the kit) and purified from human whole blood
using the QIAamp RNA blood mini kit (Qiagen GmbH), according to the
manufacturer's protocol. Total RNA was reverse transcribed into
cDNA using the High Capacity cDNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol, and stored at −20°C until subsequent
experimentation. qPCR was subsequently performed using the
Rotor-Gene Q® Real-Time PCR cycler (Qiagen GmbH), with
standard thermocycling conditions according to the Thermo Fisher
Scientific Inc. protocol (95°C for 10 min, followed by 40 cycles of
95°C for 15 sec and 60°C for 1 min) and the Taqman assay specific
for TIM-3 (catalog no. Hs00958618_m1; Thermo Fisher Scientific,
Inc.). Relative expression levels were normalized to the internal
reference gene GAPDH (catalog no. Hs02786624_g1). The primer
sequences used were as follows: TIM-3 forward,
5′-CCAAATCCCAGGCATAAT-3′ and reverse, 5′-AAGCGACAACCCAAAGGT-3′, and
GAPDH forward, 5′-GTCTCCTCTGACTTCAACAGCG-3′ and reverse,
5′-ACCACCCTGTTGCTGTAGCCAA-3′. The expression levels in unknown
samples were normalized and analyzed by the 2−∆∆Cq
method where ∆∆Cq=(Cqtarget gene-CqGAPDH)
sample-(Cq target gene-CqGAPDH) calibrator
(19).
Statistical analysis
Statistical analysis was performed using SPSS 23.0
software (IBM Corp.) and MedCalc 18.2.1 software (MedCalc Software
bvba). Data are presented as the mean ± standard deviation.
Unpaired Student's t-test was used to compare differences between
two groups. Skewed numerical data are presented as the median and
interquartile range, and the Mann-Whitney U test was used to
compare differences between two groups, while the Kruskal Wallis
test followed by the Conover post hoc test, Bonferrroni-adjusted
(critical P-value <0.005), was used to compare differences
between multiple groups. Categorical variables are presented as
number and percentage, and differences were compared using the
Fisher's exact test. Correlations were assessed using the
Spearman's rank correlation coefficient. Receiver operating
characteristic (ROC) curve analysis was performed to determine the
diagnostic value of TIM-3. Area under the curve (AUC) values
between 0.50–0.69 represent diagnostic tests with low accuracy,
while values between 0.7–0.9 represent moderate accuracy and values
>0.9 represent high accuracy (20). The Kaplan-Meier method and log-rank
test were performed to determine the prognostic value of TIM-3.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of patients
with AML
Demographic data and baseline characteristics of the
60 patients with AML are presented in Table I. Of the 60 patients, 8 (13.3%)
presented with EMD, including 6 men (75.0%) and 2 women (25.0%),
and the extramedullary sites of the disease were the skin (leukemia
cutis) in 3 cases and the central nervous system (CNS) in 5 cases
(3 cases with only spinal fluid involvement and 2 cases with
isolated CNS chloroma). The majority of patients with EMD (5 cases)
were classified as the M4 subtype, while 2 cases were the M1
subtype and 1 case was the M0 subtype. All cases exhibited
unfavourable cytogenetics.
 | Table I.Demographic data and clinical
characteristics of the studied groups. |
Table I.
Demographic data and clinical
characteristics of the studied groups.
| Characteristic | Control group | AML group |
|---|
| Sex, n (%) |
|
|
|
Male | 7 (46.7) | 24 (40.0) |
|
Female | 8 (53.3) | 36 (60.0) |
| Age,
yearsb | 46.9±6.3 | 53.4±12.9 |
| Hemoglobin,
g/dl | 14.5
(13.9–15.6) | 7.9 (5.9–9.6) |
| WBCs,
1×103 cells/µla | 5.1 (4.1–7.8) | 17.6
(3.2–43.2) |
| Absolute neutrophil
count, 1×103 cells/µla | 4.2 (3.6–5.9) | 1.9 (1–7.6) |
| Absolute
lymphocytic count, 1×103 cells/µla | 2.3 (1.9–2.6) | 1.6 (1–12.7) |
| PB blast count
(%)a | – | 34 (10–62) |
| BM blast count
(%)a | – | 36 (25–71) |
| TIM-3 fold
expressiona | 1.07
(0.84–1.12) | 2.99
(1.45–5.65) |
| Extramedullary
disease, n (%) |
|
|
| + | – | 8 (13.3) |
| − | – | 52 (86.7) |
| FAB classification,
n (%) | – |
|
| M0 | – | 8 (13.3) |
| M1 | – | 4 (6.7) |
| M2 | – | 12 (20.0) |
| M3 | – | 12 (20.0) |
| M4 | – | 24 (40.0) |
| Prognosis according
to cytogenetic studies, n (%) | – |
|
|
Favorable | – | 20 (33.3) |
|
Unfavorable | – | 40 (66.7) |
TIM-3 expression in patients with AML
and healthy individuals
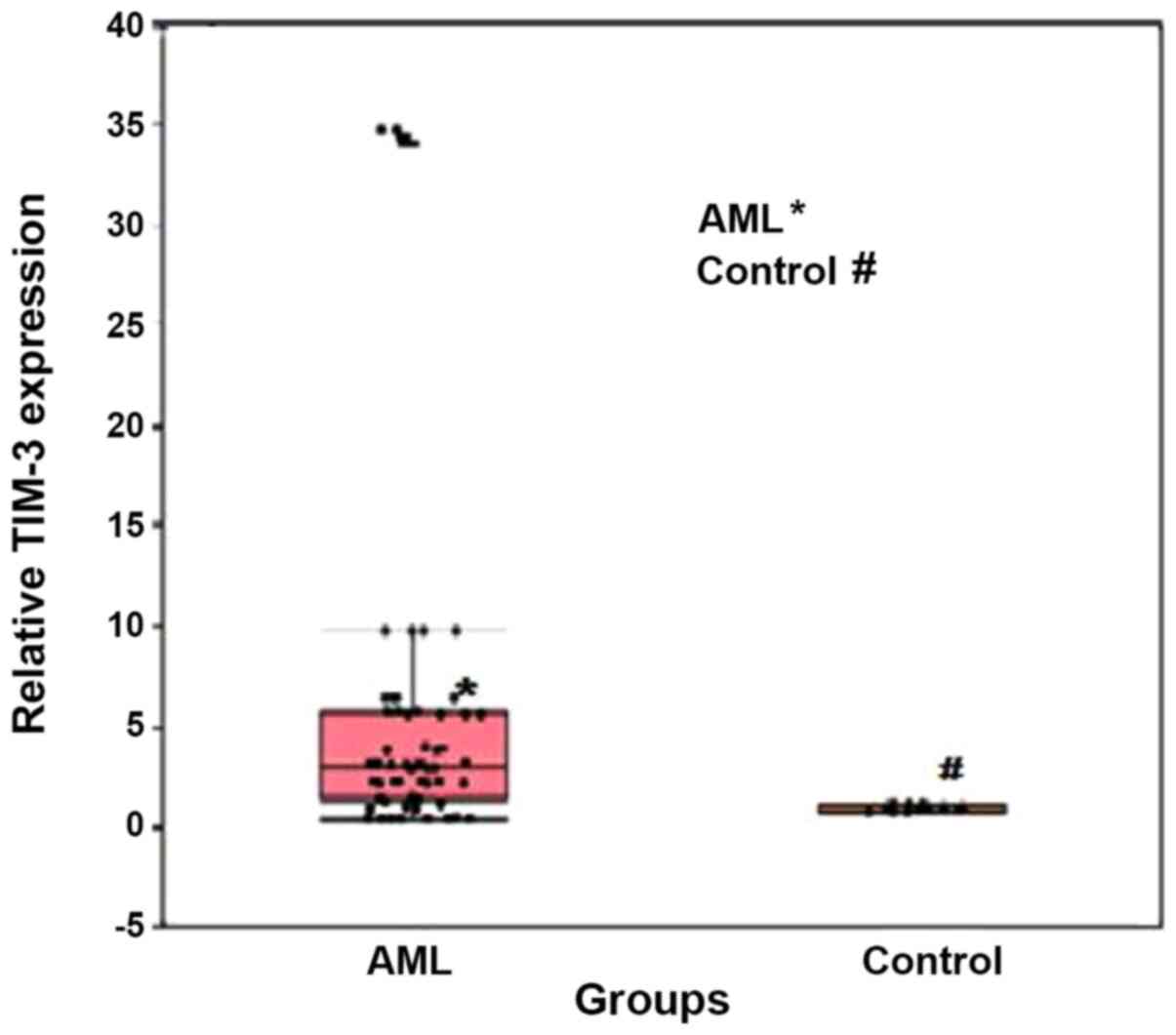
TIM-3 expression was significantly upregulated in
patients with AML compared with that in the healthy individuals,
with median relative expression levels of 2.99 (range, 1.45–5.65)
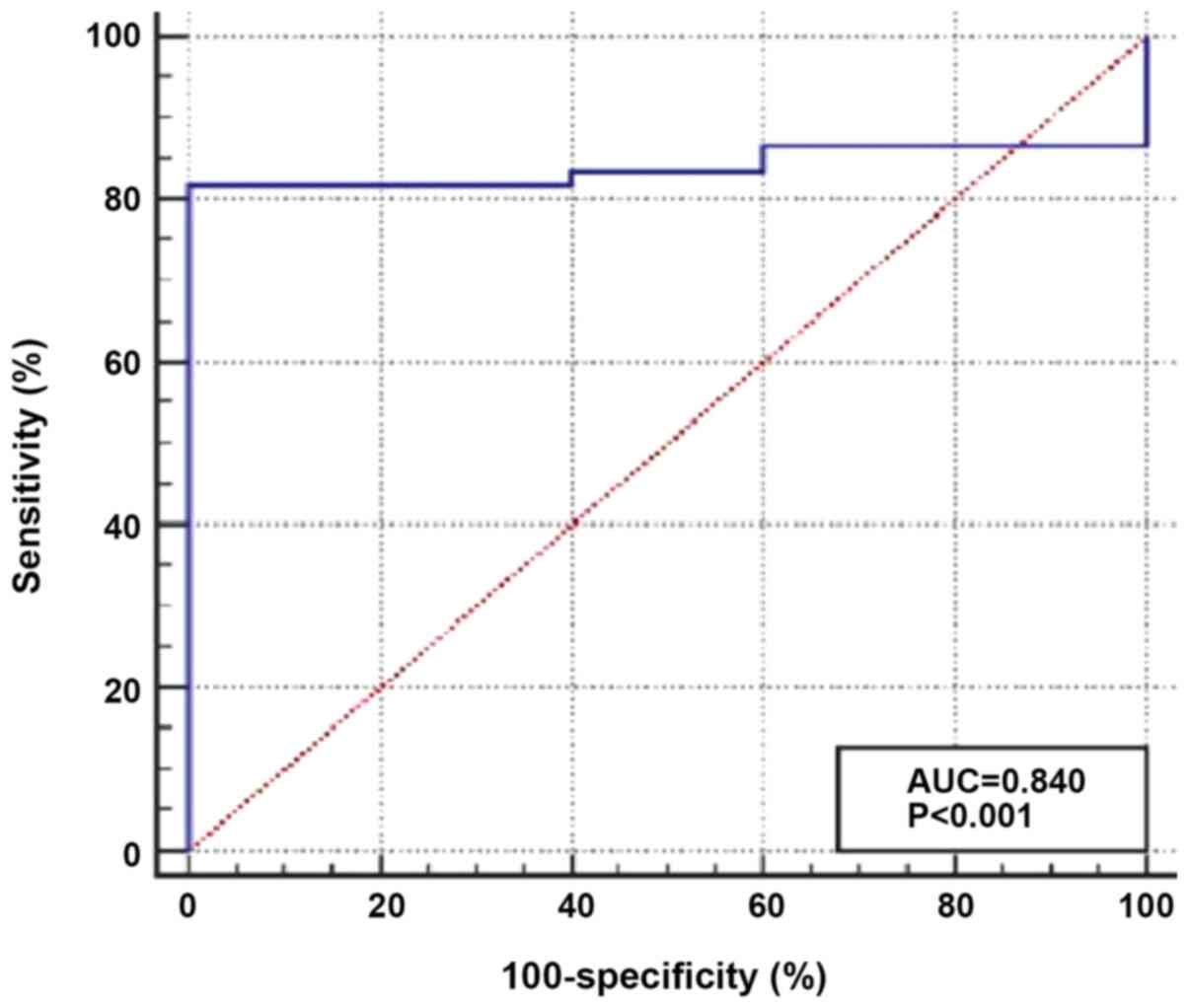
and 1.07 (0.84–1.12), respectively (P<0.001; Fig. 1). In addition, ROC curve analysis was
performed to determine the optimum cut-off value of TIM-3
expression for discrimination between patients with AML and healthy
individuals. The results demonstrated a moderate diagnostic value,
with an AUC value of 0.840 and optimum cut-off level >1.197. The
diagnostic sensitivity and specificity were 81.7 and 100.0%,
respectively (Fig. 2).
TIM-3 expression and EMD in patients
with AML
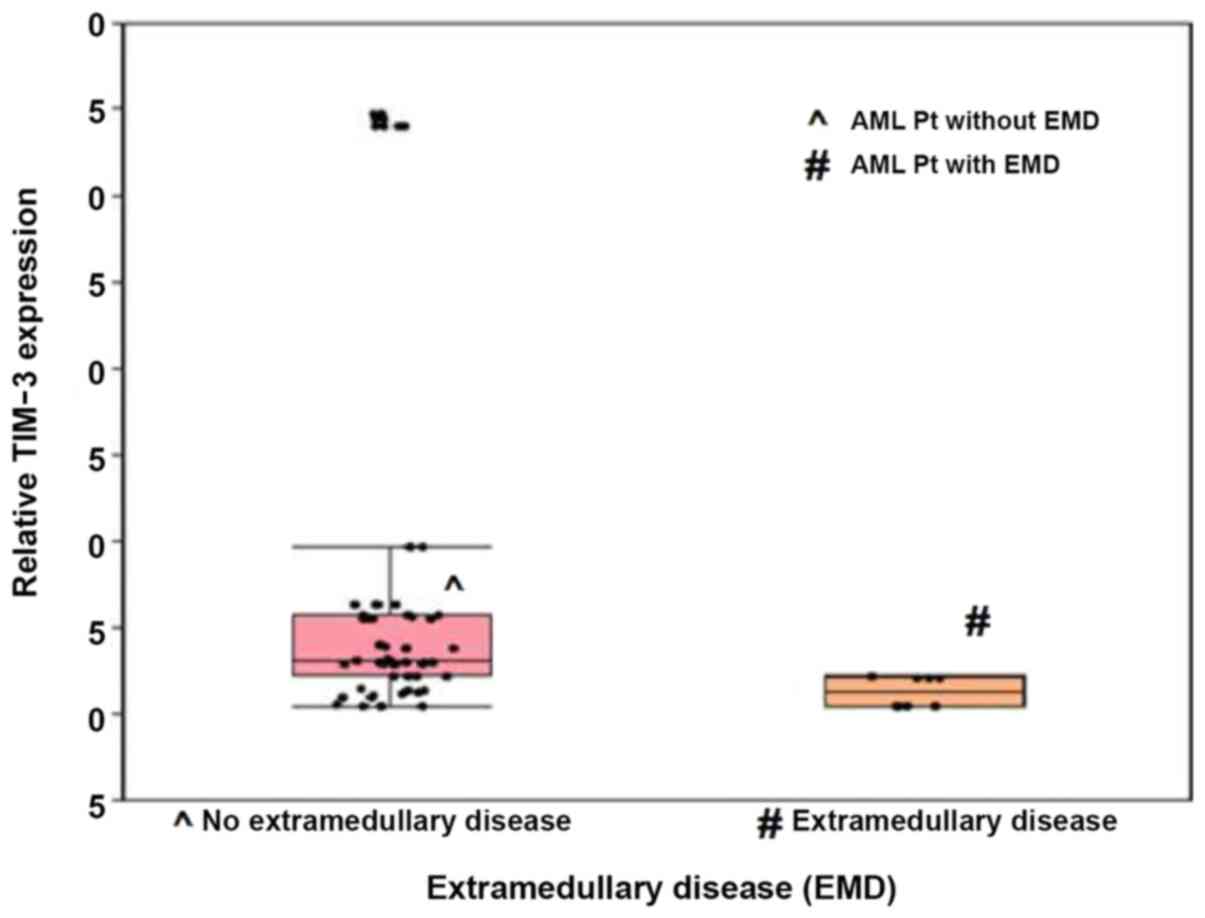
Patients with EMD exhibited significantly lower
median TIM-3 expression levels than those without EMD, and their
median expression levels were 1.3 and 3.1, respectively
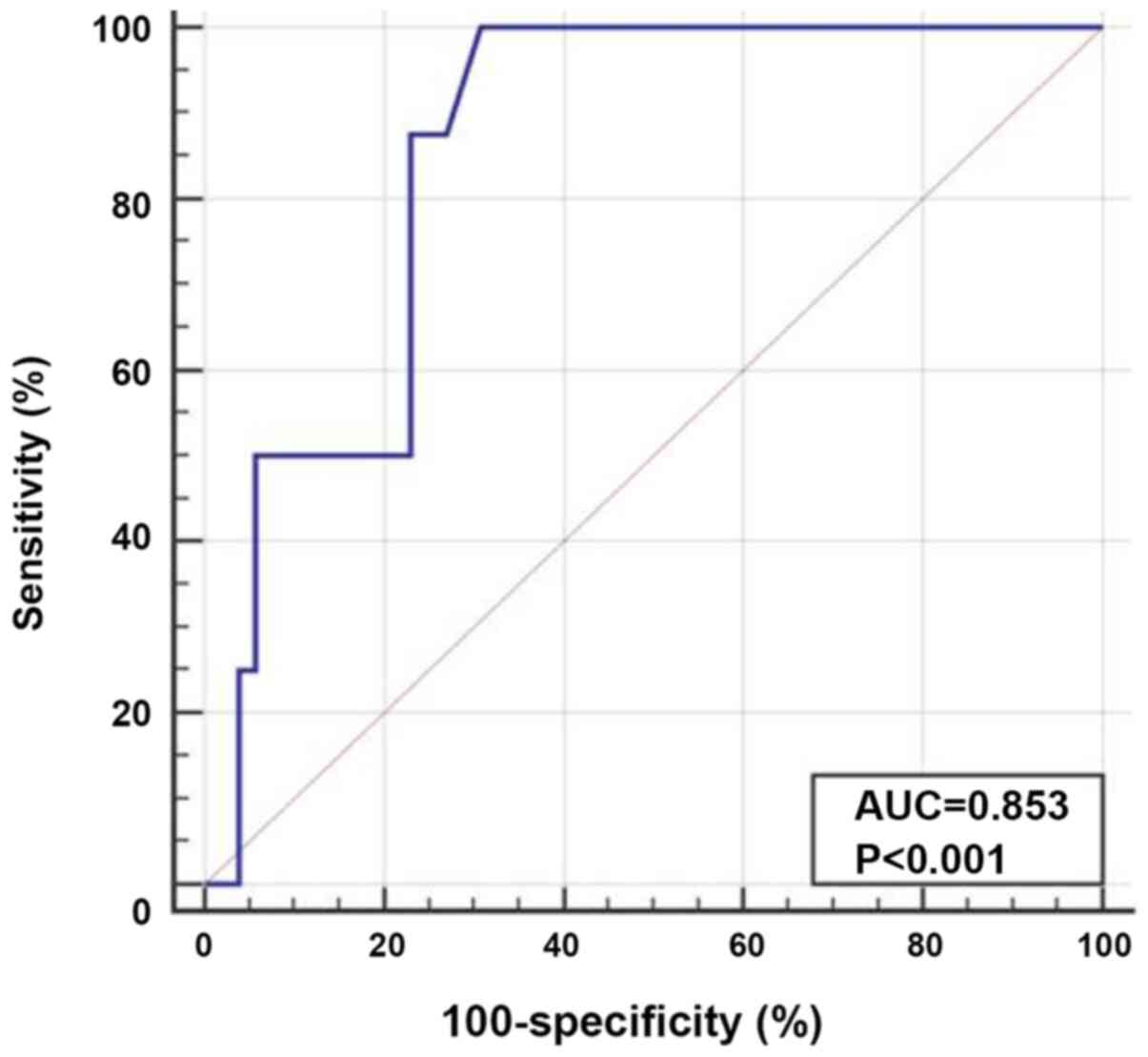
(P<0.001; Fig. 3). ROC curve
analysis was performed to determine the optimum cut-off value of
TIM-3 expression for the prediction of AML cases with EMD (AUC,
0.853; optimum cut-off level, ≤2.2). The diagnostic sensitivity and
specificity were 100.0 and 69.2%, respectively (Fig. 4).
Association between TIM-3 expression
and the clinicopathological characteristics of patients with
AML
TIM-3 expression was significantly associated with
hemoglobin (r=−0.450; P<0.001), peripheral blood blast (r=0.324;
P=0.01) and BM blasts (r=0.300; P=0.02) counts in patients with
AML. However, no statistically significant associations were
observed between TIM-3 expression and age, white blood cell and
platelet counts (Table II).
 | Table II.Correlation between TIM-3 expression
and variable clinicopathological parameters. |
Table II.
Correlation between TIM-3 expression
and variable clinicopathological parameters.
|
| TIM-3
expression |
|---|
|
|
|
|---|
| Variable | Spearman ρ | P-value |
|---|
| Age | 0.223 | 0.087 |
| TLC | 0.121 | 0.357 |
| Hemoglobin | −0.450 |
<0.001a |
| Platelets | −0.015 | 0.912 |
| PB blasts | 0.324 | 0.012b |
| BM blasts | 0.300 | 0.020b |
| Absolute neutrophil
count | 0.071 | 0.589 |
| Absolute
lymphocytic count | 0.063 | 0.635 |
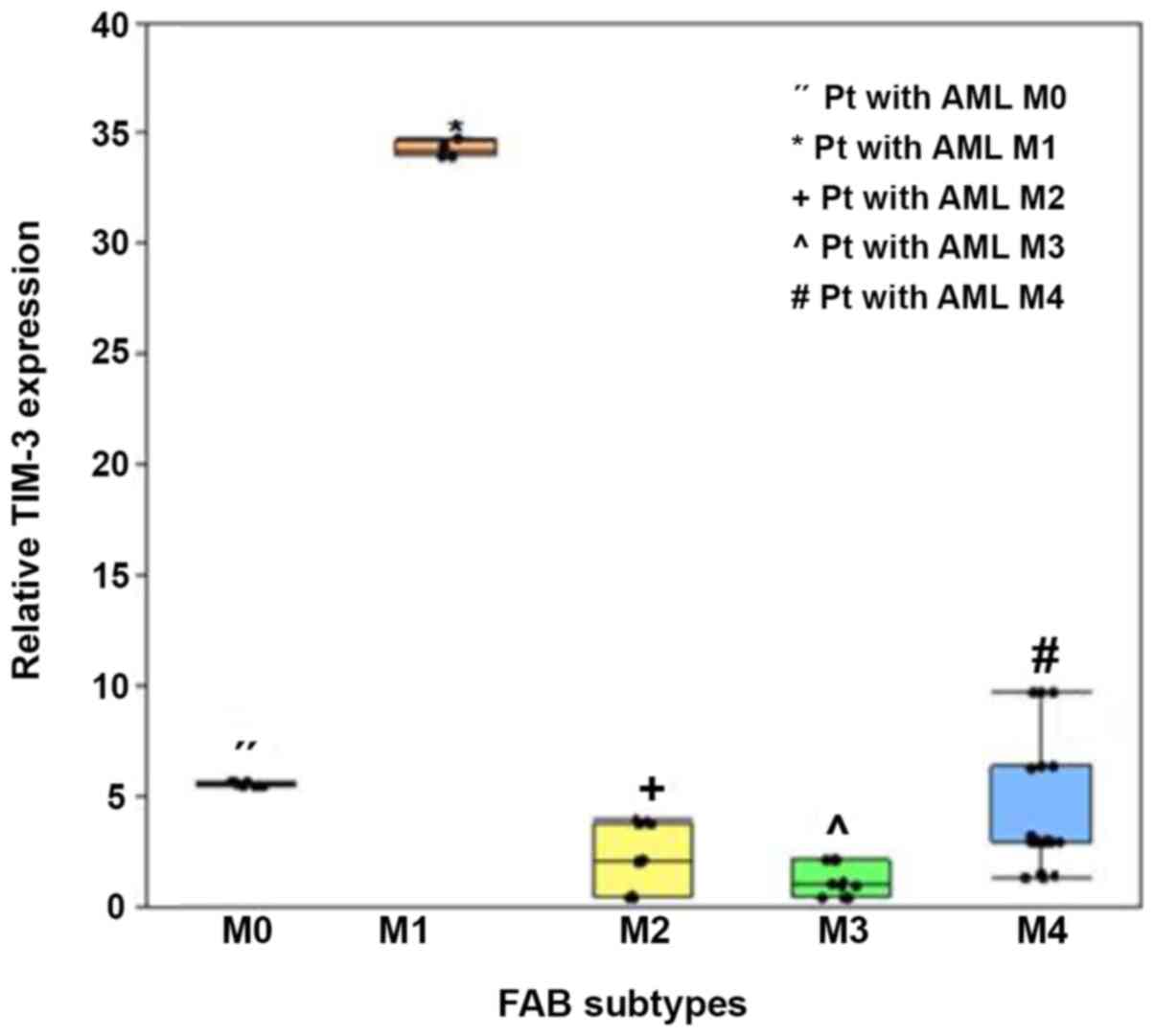
In addition, TIM-3 expression significantly varied
amongst the FAB subtypes (P<0.001), whereby expression was
significantly higher in patients with the M1 subtype (median, 34.2;
IQR, 34.00–34.59) and M4 subtype (median, 3; IQR, 2.94–6.36),
compared with the other subtypes (P<0.005; Fig. 5).
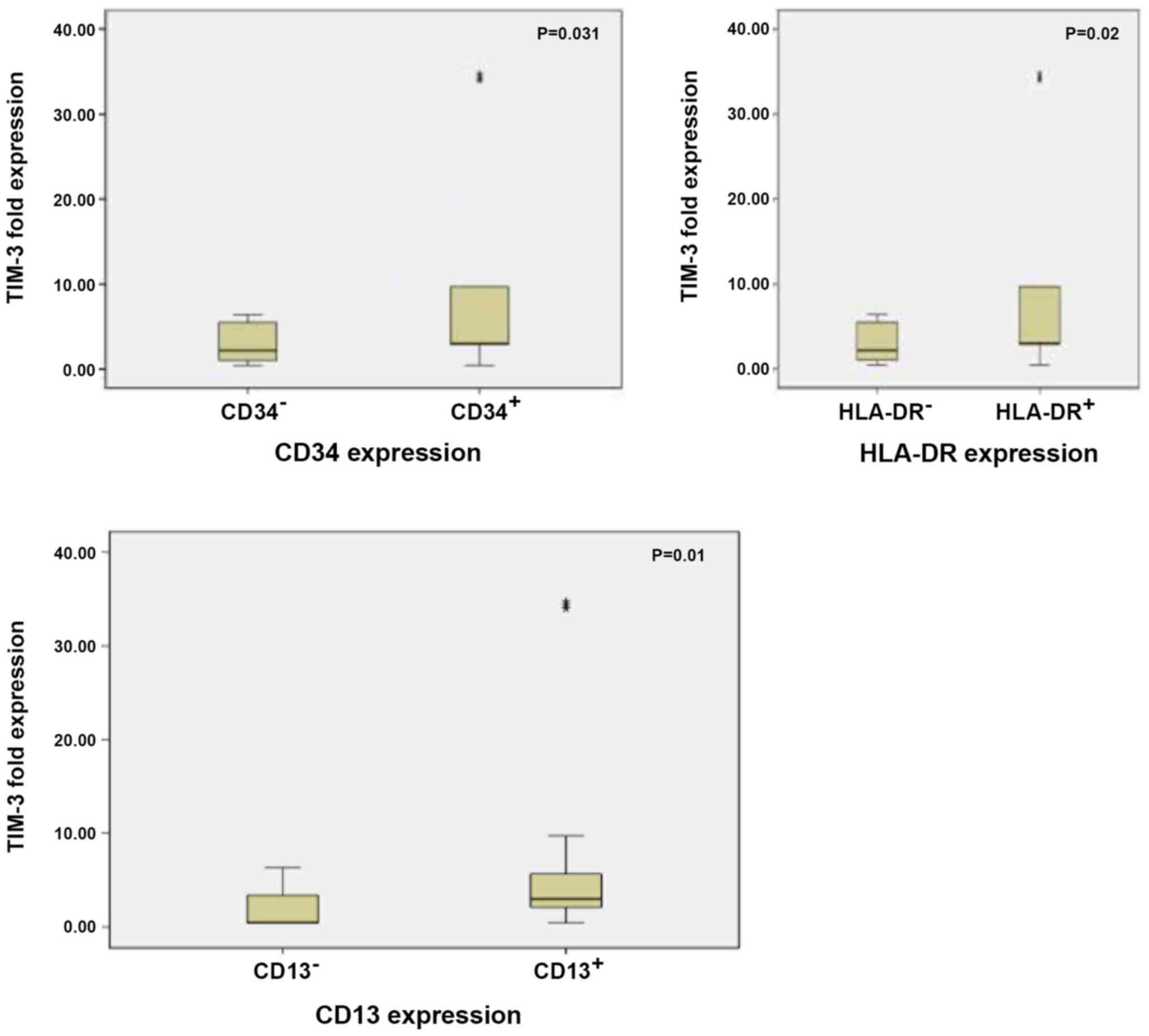
TIM-3 expression was significantly upregulated in
patients with CD34+, CD13+ and
HLA-DR+ BM blasts compared with those with
CD34−, CD13− and HLA-DR− BM blasts
(P=0.031, P=0.010 and P=0.020, respectively; Fig. 6). Patients with unfavourable
prognosis (intermediate and poor risk cytogenetics) exhibited
almost comparable expression levels of TIM-3 to patients with
favourable prognosis (favourable risk cytogenetics), with no
statistically significant differences between the two groups
(P=0.447; Table III).
 | Table III.Association between TIM-3 expression
and different cytogenetic risk groups. |
Table III.
Association between TIM-3 expression
and different cytogenetic risk groups.
|
| Cytogenetics |
|
|---|
|
|
|
|
|---|
|
| Favorable
(n=20) | Intermediate or
poor (n=40) |
|
|---|
|
|
|
|
|
|---|
| Variable | Median | Interquartile
range | Median | Interquartile
range | P-value |
|---|
| TIM-1 | 3.0 | 1.0–4.0 | 2.9 | 2.1–5.7 | 0.447 |
TIM-3 expression and clinical outcomes
in patients with AML
To assess the impact of TIM-3 expression on the
clinical outcomes of patients with AML, regarding their
responsiveness to induction chemotherapy and overall 1-year
survival during the first year, patients were assigned to two
groups, the low TIM-3 expression group (24 patients) and the high
TIM-3 expression group (36 patients), based on their median TIM-3
expression level. By the end of the induction cycle of treatment,
on Day 28 (D28), the study cohorts were compared regarding their
TIM-3 expression levels and treatment outcomes. A total of 12
patients (20%) in the high TIM-3 expression group died due to
pulmonary fungal infection, septicemia and bleeding complications.
However, high TIM-3 expression was not significantly associated
with a higher mortality risk by the end of the induction
chemotherapy (P<0.09). The 48 survivors (80%) underwent
re-evaluation of their disease response to chemotherapy, and were
further categorized into 36 responders (75%), including 12 patients
(33%) with high TIM-3 expression and 24 patients (66%) with low
TIM-3 expression. Conversely, the 12 non-responders (20%) with
refractory disease were all categorized into the high TIM-3
expression group. Thus, all cases of AML with low TIM-3 expression
were responders to induction chemotherapy, and of the patients with
high TIM-3 expression, only 12 of them were responders to induction
chemotherapy (Table IV). Comparison
of responders vs. non-responders in terms of TIM-3 expression
demonstrated that the responders had significantly lower TIM-3
expression levels compared with the non-responders (P=0.004;
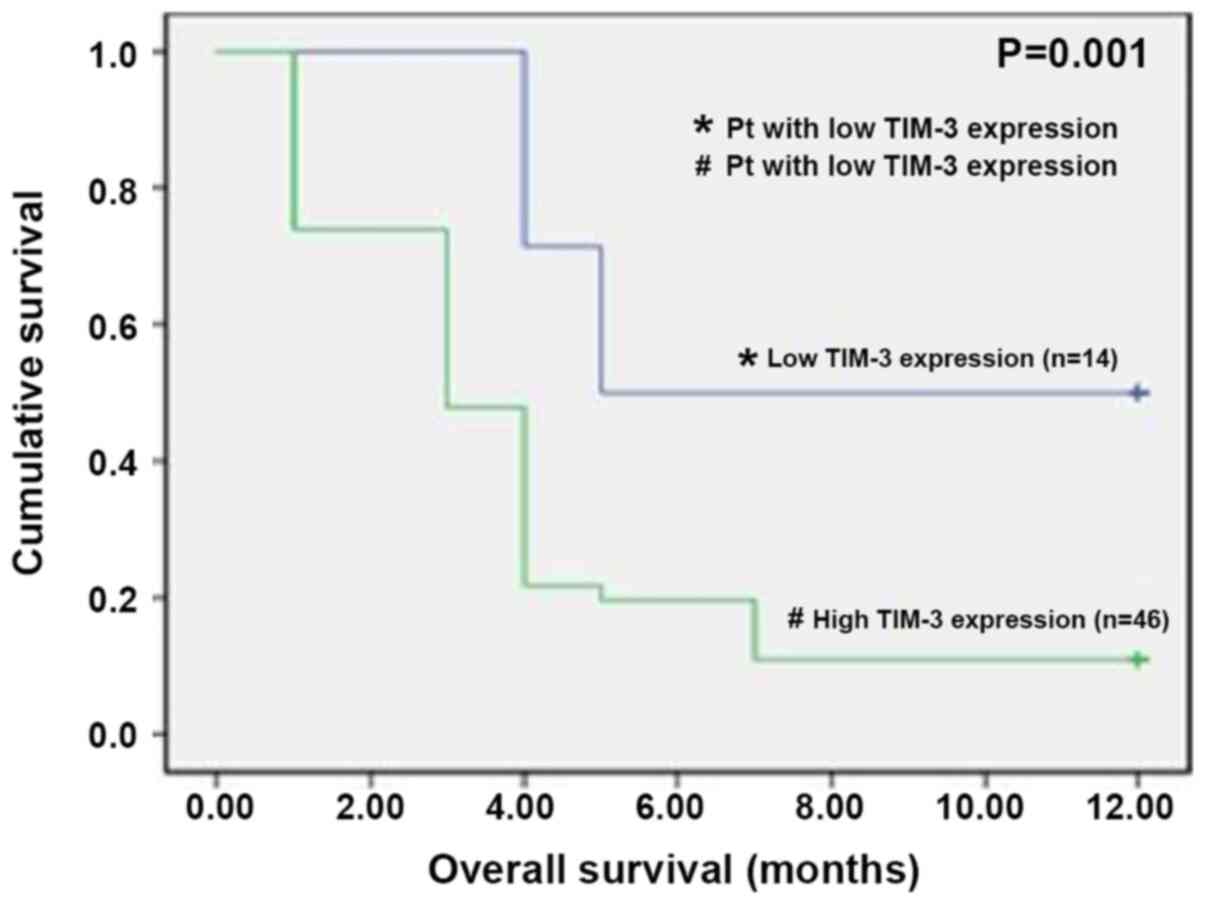
Table IV). Patients were followed
up for 12 months, and of the 60 patients recruited, only 12
patients were alive at the end of the follow-up period, 8 of who
had low TIM-3 expression and 4 with high TIM-3 expression.
Kaplan-Meier survival analysis demonstrated that patients with high
TIM-3 expression exhibited a significantly lower overall 1-year
survival rate compared with those with low TIM-3 expression
(P=0.001; Fig. 7).
 | Table IV.Correlation between TIM-3 expression
and post-induction chemotherapy outcome. |
Table IV.
Correlation between TIM-3 expression
and post-induction chemotherapy outcome.
| Different D28
treatment outcome | Patients, n
(%) | Median TIM-3
expression | P-value |
|---|
| Survival on
D28 |
|
|
|
|
Alive | 48 (80.0) | 2.59 | 0.09 |
|
Dead | 12 (20.0) | 3.05 |
|
| Response to
induction chemotherapy |
|
|
|
|
Responder | 36 (75.0) | 2.08 | 0.004 |
|
Resistant | 12 (25.0) | 5.5 |
|
Discussion
AML is the most common type of acute leukemia in
adults (21). Despite recent
advancements in the therapeutic landscape of AML, patient prognosis
remains poor (22). Thus, an
improved understanding of the immune checkpoints and immune
response to AML is imperative for appropriate development and
application of novel immunotherapies (23). TIM-3 is a key immune checkpoint in
tumor immune suppression; however, its role in AML remains unclear
(24). Thus, the present study
investigated TIM-3 expression in patients with AML and assessed its
clinical significance as a potential prognostic tool for adult AML.
The results demonstrated that TIM-3 expression was significantly
upregulated in patients with AML compared with that in the control
group. This result is consistent with previous findings, which have
demonstrated that TIM-3 expression is upregulated in bone marrow
samples of patients with AML via RT-qPCR analysis (25) and flow cytometric analysis of
peripheral blood T cells or bone marrow blasts (13,26). The
results of the present study also demonstrated that TIM-3
expression exhibited moderate diagnostic value in the AML cohort.
Research on the tumor microenvironment suggest that aberrant
overexpression of TIM-3 in patients with AML may be attributed to
the upregulated TIM-3 expression on the T-cell surface, mediating
T-cell exhaustion in response to the continuous stimulation of
malignant cell antigens, thus resulting in a weakened tumor immune
response (27). In addition, high
TIM-3 expression on AML blasts may also explain its high level in
studied AML cohorts (28). Focusing
on the association between TIM-3 expression and patient
characteristics, the present study demonstrated a positive
correlation between TIM-3 expression and bone marrow and peripheral
blast count. This result was explained in the study by Silva et
al (29), which analyzed the
total expression and surface presence of the TIM-3 receptor in
primary human AML blasts and healthy primary human leukocytes
isolated from human blood. It was reported that primary AML cells
generate more TIM-3 protein compared with healthy leukocytes,
including cell surface protein expression. In addition, only 30% of
TIM-3 molecules are externalized in primary healthy leukocytes,
while almost all TIM-3 protein is present on the cell surface of
primary AML cells, facilitating its ability to mediate
ligand-induced activation of the mammalian target of rapamycin
pathway (29).
The results of the present study demonstrated that
TIM-3 expression was significantly upregulated in patients with the
M1 subtype compared with that in patients with the other FAB
subtypes. Consistently, Darwish et al (25) reported that TIM-3 expression is
upregulated in M1 and M4 patient groups compared with the other FAB
subtypes. However, another study demonstrated that TIM-3 expression
is upregulated in the M4 subtype compared with the other FAB
subtypes (13).
The present study aimed to investigate the
association between TIM-3 expression and different prognostic
parameters in patients with AML. Cytogenetics analysis is one of
the most powerful independent prognostic factors in AML; however,
comparisons between TIM-3 expression in different cytogenetic risk
categories in the present study failed to prove any association
between cytogenetic risk groups and high TIM-3 expression.
Conversely, Xu et al (26)
confirmed a significant association between TIM-3 expression and
inv(16), which is a favorable cytogenetic abnormality, while Li
et al (13) reported that
high TIM-3 expression on CD8+ T cells in patients with
AML is significantly associated with unfavorable cytogenetic
abnormalities. The present study detected significantly higher
TIM-3 expression in patients with positive expression of surface
antigens CD34, CD13 and HLA-DR, which are considered predictors of
adverse outcome in AML (30,31). These results are consistent with
those in the study by Xu et al (26), which detected significantly positive
correlations between TIM-3 expression and CD34 and CD13 in patients
with AML. Several studies have reported that patients with AML and
EMD have a worse prognosis due to a poor response to chemotherapy
and a high relapse rate (32). To
the best of our knowledge, the present study was the first to
investigate the association between TIM-3 expression and EMD in
AML. Notably, the results demonstrated that TIM-3 expression was
significantly downregulated in patients with EMD compared with that
in those without EMD. In addition, TIM-3 expression exhibited
moderate value in the prediction of AML cases with EMD. The AML
cohort included 36 patients who exhibited high TIM-3 expression,
while the remaining 24 patients had low TIM-3 expression. The
impact of TIM-3 expression on the patient response to the induction
chemotherapy protocol demonstrated that patients with low TIM-3
expression achieved a higher rate of CR compared with that of
patients with high TIM-3 expression. Refractory cases exhibited
significantly higher TIM-3 expression compared with the responders.
These findings oppose those recorded in the study by Xu et
al (26), which reported that
patients with high TIM-3 expression achieved a significantly high
CR rate due to the acceleration of leukemic cell apoptosis by
ligand-dependent TIM-3 activation, which triggers tumor necrosis
factors and induces growth factors. However, there is contradicting
data to this explanation, reported in a study by Kikushige and
Miyamoto (33), which demonstrated
that Gal-9 produced by leukemic stem cells binds and stimulates
TIM-3 expression on AML cells, including leukemia stem cells, thus
supporting their survival and leukemia progression. Regarding the
impact of TIM-3 expression on the overall 1-year survival of the
patients assessed in the present study, the results demonstrated
that patients with high TIM-3 expression had a significantly lower
overall 1-year survival rate than compared with those with low
TIM-3 expression. These results are consistent with those reported
by Darwish et al (25), where
it was demonstrated that patients with AML and low TIM-3 expression
exhibit a longer overall survival time compared with those with
high TIM-3 expression.
The present study is not without limitations. First,
only 60 patients were included in the study cohort, and the
cytogenetic and molecular profiles were not complete for all
patients. Thus, further studies are required to confirm the
expression of the immune checkpoint gene TIM-3 in a larger sample
size, including full chromosomal and molecular studies. Secondly,
the present study failed to investigate TIM-3 expression following
chemotherapy, which would explore the correlation between the
follow-up TIM-3 expression level and remission status.
In conclusion, the results of the present study
demonstrated that TIM-3 expression was significantly upregulated in
patients with AML. In addition, high TIM-3 expression was
associated with a poor response to chemotherapy and a lower overall
survival rate, suggesting that TIM-3 may act as a biomarker for a
poor prognosis in AML. However, further studies are required to
confirm the use of TIM-3 as a potential therapeutic target for
AML.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data mentioned in this paper are available from
the corresponding author and will be provided upon request.
Authors' contributions
NAN wrote the manuscript. AMK, SMR and NSE
critically edited the manuscript. NAN, AMK, SMR and NSE helped to
design the study, perform the research and analyze the data. NAN,
SMR, NSE and AMK confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
Research, Faculty of Medicine, Ain Shams University (Cairo, Egypt)
and was conducted in accordance with the Declaration of Helsinki.
Consent was obtained from all individuals included in this study
for their participation.
Patient consent for publication
Consent was obtained from all individuals included
in this study to publish their scientific data in an aggregated
anonymized manner.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of AML in adults: 2017 ELN
recommendations from an international expert panel. Blood.
129:424–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shallis RM, Wang R, Davidoff A, Ma X and
Zeidan AM: Epidemiology of acute myeloid leukemia: Recent progress
and enduring challenges. Blood Rev. 36:70–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohammadiasl J, Khosravi A, Shahjahani M,
Azizidoost S and Saki N: Molecular and cellular aspects of
extramedullary manifestations of acute myeloid leukemia. J Cancer
Metastasis Treat. 2:44–50. 2016.
|
|
4
|
Wang ES: Treating acute myeloid leukemia
in older adults. Hematol Am Soc Hematol Educ Program. 2014:14–20.
2014. View Article : Google Scholar
|
|
5
|
Stahl M, Lu BY, Kim TK and Zeidan AM:
Novel therapies for acute myeloid leukemia: Are we finally breaking
the deadlock? Target Oncol. 12:413–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lamble AJ and Lind EF: Targeting the
immune microenvironment in acute myeloid leukemia: A focus on t
cell immunity. Front Oncol. 8:2132018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto T, Price DA, Casazza JP, Ferrari
G, Nason M, Chattopadhyay PK, Roederer M, Gostick E, Katsikis PD,
Douek DC, et al: Surface expression patterns of negative regulatory
molecules identify determinants of virus-specific CD8+
T-cell exhaustion in HIV infection. Blood. 117:4805–4815. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, TIM-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulation. Immunity. 44:989–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The TIM-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anderson AC: Tim-3, a negative regulator
of anti-tumor immunity. Curr Opin Immunol. 24:213–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kikushige Y, Shima T, Takayanagi S, Urata
S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki
Y and Akashi K: TIM-3 is a promising target to selectively kill
acute myeloid leukemia stem cells. Cell Stem Cell. 7:708–7017.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li C, Chen X, Yu X, Zhu Y, Ma C, Xia R, Ma
J, Gu C, Ye L and Wu D: TIM-3 is highly expressed in T cells in
acute myeloid leukemia and associated with clinicopathological
prognostic stratification. Int J Clin Exp Pathol. 7:6880–6888.
2014.PubMed/NCBI
|
|
14
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nunez R: Flow cytometry: Principles and
instrumentation. Curr Issues Mol Biol. 3:39–45. 2001.PubMed/NCBI
|
|
16
|
Fröhling S, Skelin S, Liebisch C, Scholl
C, Schlenk RF, Döhner H and Döhner K; Acute Myeloid Leukemia Study
Group, Ulm, : Comparison of cytogenetic and molecular cytogenetic
detection of chromosome abnormalities in 240 consecutive adult
patients with acute myeloid leukemia. J Clin Oncol. 20:2480–2485.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Donnell MR, Abboud CN, Altman J,
Appelbaum FR, Arber DA, Attar E, Borate U, Coutre SE, Damon LE,
Goorha S, et al: NCCN clinical practice guidelines acute myeloid
leukemia. J Natl Compr Canc Netw. 10:984–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Comprehensive Cancer Network:
Acute myeloid leukemia (Version 2. 2018). http://www.nccn.org/professionals/physician_gls/pdf/aml
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wians FH: Clinical laboratory tests:
Which, why, and what do the results mean? Laboratory Med.
40:105–113. 2009. View Article : Google Scholar
|
|
21
|
Song X, Peng Y, Wang X, Chen Y, Jin L,
Yang T, Qian M, Ni W, Tong X and Lan J: Incidence, survival, and
risk factors for adults with acute myeloid leukemia not otherwise
specified and acute myeloid leukemia with recurrent genetic
abnormalities: Analysis of the surveillance, epidemiology, and end
results (SEER) database, 2001–2013. Acta Haematol. 139:115–127.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prokocimer M, Molchadsky A and Rotter V:
Dysfunctional diversity of p53 proteins in adult acute myeloid
leukemia: Projections on diagnostic work up and therapy. Blood.
130:699–712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Radwan SM, Elleboudy NS, Nabih NA and
Kamal AM: The immune checkpoints cytotoxic T Lymphocyte Antigen-4
and Lymphocyte activation gene-3 expression is up-regulated in
acute myeloid leukemia. HLA. 96:3–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting TIM-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Darwish NHE, Sudha T, Godugu K, Elbaz O,
Abdelghaffar HA, Hassan EE and Mousa SA: Acute myeloid leukemia
stem cell markers in prognosis and targeted therapy: Potential
impact of BMI-1, TIM-3 and CLL-1. Oncotarget. 7:57811–57820. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu L, Xu J, Ma S, Li X, Zhu M, Chen S, Han
Y, Tang X, Fu Z, Qiu H, et al: High TIM-3 expression chemotherapy
sensitivity on AML blasts could enhance chemotherapy sensitivity.
Oncotarget. 8:102088–102096. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jan M, Chao MP, Cha AC, Alizadeh AA,
Gentles AJ, Weissman IL and Majeti R: Prospective separation of
normal and leukemic stem cells based on differential expression of
TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl
Acad Sci USA. 108:5009–5014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan J, Huang S, Huang J, Yu Z, Chen Y, Lu
Y, Li Y and Chen S: Increasing Tim-3+CD244+,
Tim-3+CD57+, and
Tim-3+PD-1+ T cells in patients with acute
myeloid leukemia. Asia Pac J Clin Oncol. 16:137–141. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silva IG, Gibbs BF, Bardelli M, Varani L
and Sumbayev VV: Differential expression and biochemical activity
of the immune receptor TIM-3 in healthy and malignant human myeloid
cells. Oncotarget. 6:33823–33833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Radwan SM, Hamdy NM, Hegab HM and
El-Mesallamy HO: Beclin-1 and hypoxia-inducible factor-1α genes
expression: Potential biomarkers in acute leukemia patients. Cancer
Biomark. 16:619–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sinha K: Prognostic significance of
flowcytometric immunophenotype in acute leukemias: Insights gained
at a tertiary care hospital. J Dental Med Sci. 18:55–58. 2019.
|
|
32
|
Jiang L, Yu G, Meng W, Wang Z, Meng F and
Ma W: Overexpression of amyloid precursor protein in acute myeloid
leukemia enhances extramedullary infiltration by MMP-2. Tumor Biol.
34:629–636. 2013. View Article : Google Scholar
|
|
33
|
Kikushige Y and Miyamoto T: TIM-3 as a
novel therapeutic target for eradicating acute myelogenous leukemia
stem cells. Int J Hematol. 98:627–633. 2013. View Article : Google Scholar : PubMed/NCBI
|