Introduction
Breast cancer is one of the most dangerous invasive
cancer in women that has a global prevalence (1). Although the overall mortality rate of
patients with breast cancer has decreased, it is continuing to
emerge as a major health issue in women worldwide. At present,
surgical treatment is the best option. Chemotherapy and
radiotherapy are used to inhibit the growth and spread of tumors,
and after many years of medical advancement and improvement, both
chemotherapy and radiotherapy have been shown to prolong the lives
of patients (2). The proliferation
and recurrence rates of breast cancer cells are very high, and some
patients develop drug resistance, which may cause side effects.
Therefore, identifying non-toxic and efficacious natural compounds
for the treatment of breast cancer is of utmost importance.
Traditional Chinese medicine has been widely used in
China. Due to its non-toxic effects and efficacy, it is often used
in combination with other medicines. With the continuous progress
in modern medicine, preventing and treating the recurrence and
metastasis of breast cancer using genetic technology and molecular
biology methods will become a trend in future breast cancer
research. Eupafolin is a flavonoid, which has anti-inflammatory,
anti-viral, anti-angiogenesis and anti-tumor activities (3). Angiogenesis is closely associated with
tumor development and metastasis, and Eupafolin can inhibit the
activation of VEGFR2 and its associated signaling pathways. The
molecular mechanism of the anti-cancer effect of Eupafolin may be
associated with the activation of caspase-3 (4), the downregulation of vascular
endothelial growth factor (VEGF) (5), and inhibition of the Akt signaling
pathway (6). However, the underlying
mechanism of its anticancer effect in breast cancer remains
unclear. Therefore, understanding the effect of Eupafolin on breast
cancer and identifying the mechanism of action will help in the
management of breast cancer.
Proteins involved in the PI3K/Akt/mTOR pathway are
abnormally expressed in several tumors, thereby leading to the
progression of breast cancer, gastric cancer, nasal cancer and
pancreatic cancer, among others. This pathway is closely associated
with tumor proliferation, autophagy and migration (7,8). Several
studies show that targeting this pathway using drugs or drug
combinations is effective in the treatment of tumors (9). Therefore, research on drugs targeting
the PI3K/Akt/mTOR pathway may have great significance in the
management of breast cancer.
Therefore, the aim of the present study was to
investigate whether Eupafolin could inhibit the proliferation and
apoptosis of breast cancer cells (EO771 cell line), and to identify
its possible underlying mechanism of action. Experimental results
showed that Eupafolin significantly inhibited the proliferation of
EO771 cells by modulating the PI3K/Akt/mTOR pathway, causing
G0/G1 phase arrest, and promoting
apoptosis.
Materials and methods
Cell culture and processing
The mouse breast cancer cell line, EO771, selected
for the present study was obtained from Binsui Biotechnology Co.,
Ltd. EO771 cells were cultured in Dulbecco's Modified Eagle's
Medium (DMEM; Gibco; Thermo Fisher Scientific Inc.), with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), and
maintained in an atmosphere of 5% CO2 in an incubator at
37°C. Eupafolin (purity ≥99%) was purchased from Yuanye
Biotechnology. In the present study, Eupafolin was dissolved in
dimethyl sulfoxide (DMSO; Beijing Solarbio Science & Technology
Co., Ltd.) at different concentrations (0, 25, 50 and 100 µM).
Subsequently, cells were treated with Eupafolin for different
periods.
Cell viability test
Cell viability was determined using Cell Counting
kit-8 (CCK-8; MedChemExpress). In brief, 5,000 EO771 cells were
seeded into 96-well plates, treated with 0, 25, 50 and 100 µM
Eupafolin, and incubated at 37°C for 24, 36 and 48 h. Then, 10 µl
CCK-8 solution was added to each well and the cells were incubated
for 1.5 h. A microplate reader was used to measure the absorbance
at 450 nm (TECAN).
Determination of apoptosis and cell
cycle
Apoptosis was measured according to the instructions
provided with the Annexin-V/Fluorescein isothiocyanate (FITC)
apoptosis detection kit (Ebisson). After drug treatment for 24 h,
cells were digested with trypsin without EDTA, collected,
centrifuged at 500 × g for 5 min at 37°C, resuspended in pre-cooled
PBS, centrifuged at 500 × g for 5 min to discard the supernatant,
resuspended in 300 µl 1X binding buffer; 5 µl sample was mixed with
FITC-Annexin V. Then, 5 µl propidium iodide (PI) staining solution
was added to 200 µl 1X binding buffer 5 min prior to detection.
Flow cytometry was performed using FACS (Thermo Fisher Scientific,
Inc.). For cell cycle analysis, the cells were harvested and fixed
in 70% ethanol at 4°C overnight. Next, cells were resuspended in
500 µl 1X PI solution (Baihao) for 30 min at 37°C. Flow cytometry
analysis was performed using FACS (Thermo Fisher Scientific, Inc.).
The collected data were analyzed using ModFit LT (version 2.0;
Verity Software House, Inc.) to determine cell cycle
distribution.
Cell scratch test
A total of 10,000 EO771 cells was seeded into 6-well
culture plates in DMEM containing 10% FBS, and placed in an
incubator until the cell density was 90% or higher. Using a sterile
100-µl plastic pipette, a wound was created; the cell debris was
removed by washing with PBS and images were captured using an
inverted light microscope (Olympus Corporation) with a digital
camera (magnification, ×80) at 0 h. A total of 3 ml of FBS-free
medium was added per well; then, 25, 50 and 100 µM Eupafolin was
added for 24 h. Cells were washed with PBS and images were obtained
using microscopy. Healing areas were analyzed using ImageJ software
(version 1.51; National Institutes of Health). The experiment was
performed at least in triplicate.
Cell migration and invasion
experiments
A Transwell (Corning, Inc.) assay was performed to
determine cell migration and invasion abilities. After Eupafolin
treatment, cells to be tested in the logarithmic growth phase were
digested, resuspended in serum-free medium, and adjusted to a
density of (1–10)×105 cells/ml. A volume of
500 µl DMEM containing 10% FBS was added to the lower chamber of
each transwell; tweezers were used to place the cells in a new
24-well plate. A volume of 100 µl of the cell suspension was added
to the upper chamber, and the transwells were placed in an
incubator for 24 h. Next, the cells were removed and the medium was
aspirated. In a new 24-well plate, 600 µl 4% paraformaldehyde was
added. The transwell was placed at room temperature and cells were
fixed for 20–30 min. The fixative solution was removed and cells
were stained at 37°C using 0.2% crystal violet for 10 min. Then,
the cells were washed three times with PBS to remove the unbound
crystal violet. The upper surface of the chamber was gently wiped
with a cotton swab. Excess crystal violet was removed prior to
microscopy. After drying, five fields were randomly selected and
the cells were observed and counted under an inverted light
microscope with a digital camera (magnification, ×80). The cell
invasion test procedure that was used was similar to that of the
cell migration assay, except that the upper chamber was covered
with BD Matrigel™ Matrix (Corning, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
According to the instructions provided with the kit,
TRIzol reagent (Ruan) was used to extract total RNA, and cDNA was
generated using the FastKing RT kit (Tiangen Biotech Co., Ltd) with
2 µg RNA, according to the manufacturer's instructions. The primers
used were obtained from NCBI. A real-time fluorescent quantitative
PCR detection system (Eppendorf) was used to perform the RT-qPCR
reactions using SYBR-Green (Tiangen Biotech Co., Ltd.) and a total
of 20 µl reaction mixture. The 2−ΔΔCq method (10) was used to analyze gene expression
levels. Primer sequences were as follows: Matrix metalloprotease
(MMP)2 forward, 5′-CTGCAGGTGGTCATAG-3′ and reverse,
5′-TGGTGTGCAGCGATGAAGAT-3′; MMP9 forward,
5′-CTTCACCGGCTAAACCACCT-3′ and reverse, 5′-CTTCACCGGCTAAACCACCT-3′;
VEGFA forward, 5′-ATAGGAGAGATGAGCTTCC-3′ and reverse,
5′-TCTGCATTCACATCTGCTGTGC-3′; β-actin forward,
5′-GTCGAGTCGCGTCCACC-3′ and reverse, 5′-GTCATCCATGGCGAACTGGT-3′;
cyclin D1 forward, 5′-CTGTGCTGCGAAGTGGAAACCAT-3′ and reverse,
5′-TTCATGGCCAGCGGGAAGACCTC-3′; CDK4 forward,
5′-CGAGCGTAAGGCTGATGGAT-3′ and reverse, 5′-CCAGGCCGCTTAGAAACTGA-3′;
and CDK6 forward, 5′-AGCCCTGCTGTGGAAGAAAA-3′ and reverse,
5′-TAGACGGACCGACCTTCTCG-3′. The qPCR reaction conditions were as
follows: Initial denaturation for 15 min at 95°C, followed by 40
cycles of denaturation for 10 sec at 95°C, annealing for 30 sec at
60°C and extension for 20 sec at 72°C.
Western blotting
After treating the cells with Eupafolin for 24 h,
EO771 cells were harvested, washed twice with PBS, and lysed on ice
with RIPA lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd.) for 30 min. Then, the BCA method was used to determine
the protein concentration: 5X loading buffer was added and proteins
were denatured at 95°C by boiling in a water bath for 10 min. A
total of 20 µg protein sample was added to each well, separated
using 12% SDS-PAGE at 120 V, and then transferred to PVDF membranes
(Thermo Fisher Scientific, Inc.). Skimmed milk powder (5%) was used
to block the PVDF membranes for 1 h at room temperature. Next, the
membranes were incubated with primary antibodies directed against
Bcl-2 (cat. no. 4223), Bax (cat. no. 2772), cleaved caspase 3 (cat.
no. 9661), PI3K (cat. no. 4257), p-PI3K (cat. no. 17366), Akt (cat.
no. 4691), p-Akt (cat. no. 4060), Mtor (cat. no. 2983), p-mTOR
(cat. no. 5536) and GAPDH (cat. no. 5174) (all 1:1,000; Cell
Signaling Technology, Inc.) at 4°C overnight. Subsequently,
membranes were washed three times with PBS, then incubated with a
corresponding horseradish peroxidase-conjugated secondary antibody
(1:2,000; cat. no. A0208; Beyotime Institute of Biotechnology) for
1.5 h at room temperature and washed five times with PBS. Then,
protein bands were visualized using an enhanced chemiluminescence
assay kit (Beyotime Institute of Biotechnology) and photographed
using an imaging system (Tanon). Finally, data were analyzed using
ImageJ software (version 1.8.0; National Institutes of Health).
Statistical analysis
All experiments were repeated ≥3 times. The data are
presented as the mean ± SD and were analyzed using SPSS (v.20.0;
IBM Corp.). Differences between multiple groups were analyzed using
one-way ANOVA followed by Dunnett's post hoc test. Results with
P<0.05; P<0.01; and P<0.001 were considered as
statistically significant; ‘ns’ indicates P>0.05.
Results
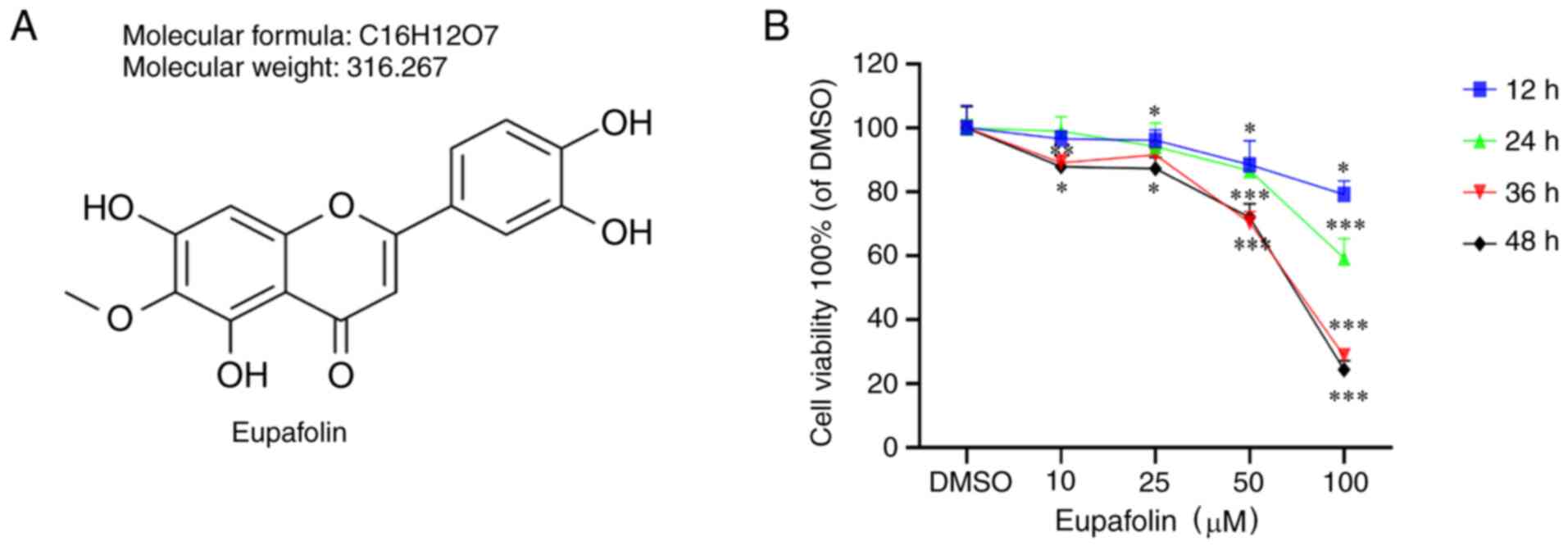
Eupafolin decreases the viability and
proliferation of breast cancer cells
EO771 cells were treated with Eupafolin for 24, 36
and 48 h to study the effects of the compound (Fig. 1A) on cell proliferation. The results
of the CCK-8 assay indicated that Eupafolin inhibited the viability
of EO771 cells, and the inhibitory effect was proportional to the
treatment time and dose (Fig.
1B).
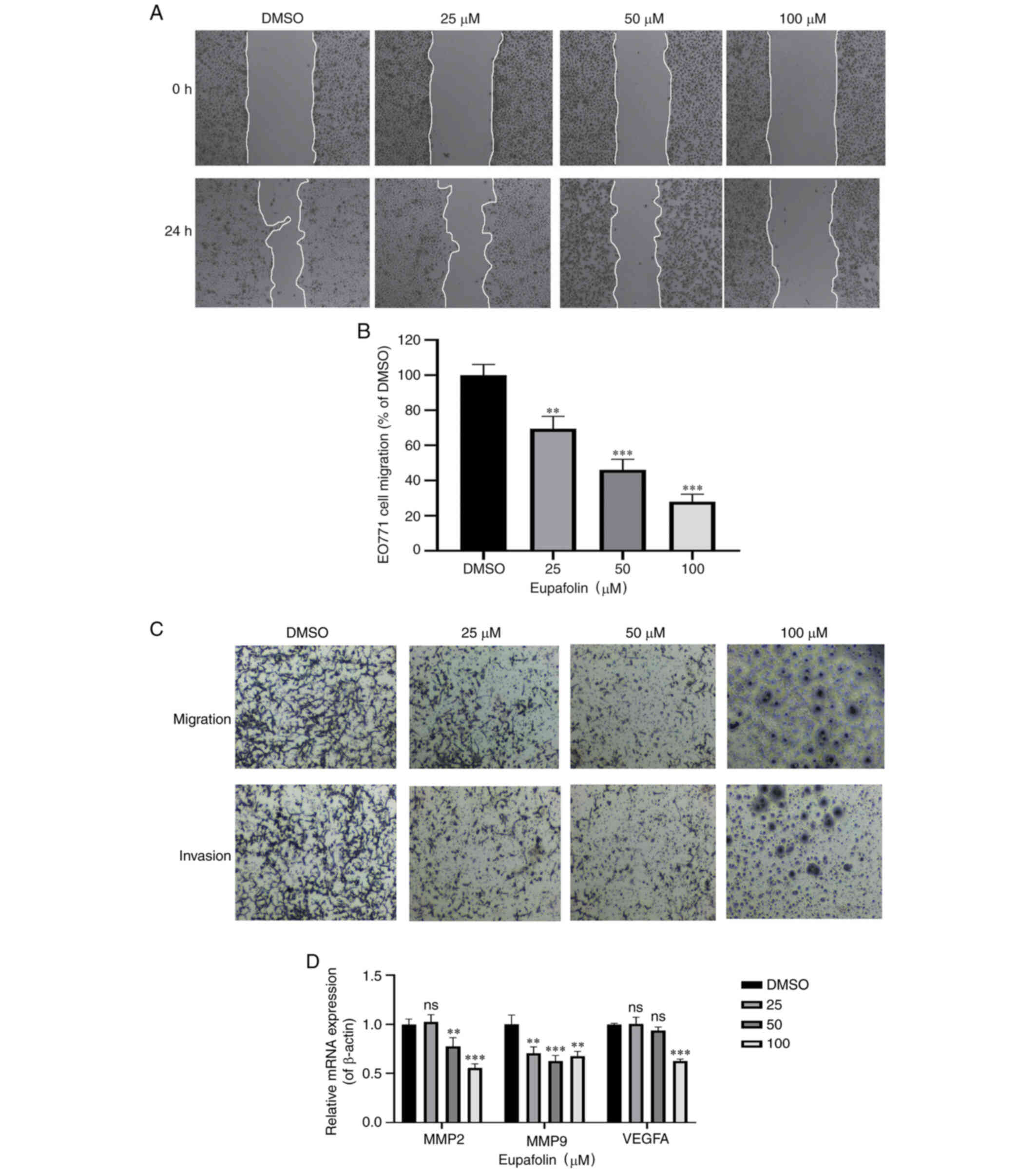
Eupafolin inhibits the invasion and
migration of breast cancer cells
The scratch test was used to determine the wound
healing time of EO771 cells. Furthermore, a Transwell assay was
performed to determine the ratio of invasion and migration of EO771
cells (Fig. 2A and B). Results
showed that compared with the control cells, Eupafolin
significantly decreased the migration and invasion of EO771 cells
(Fig. 2C). MMP2, MMP9 and VEGF-A are
positively associated with the migration ability of tumor cells and
can be used as marker genes. Therefore, their expression following
Eupafolin treatment was tested, and the results showed that
Eupafolin inhibited MMP2, MMP9 and VEGF-A (Fig. 2D). Taken together, these results
indicate that Eupafolin prevented further deterioration of breast
cancer cells.
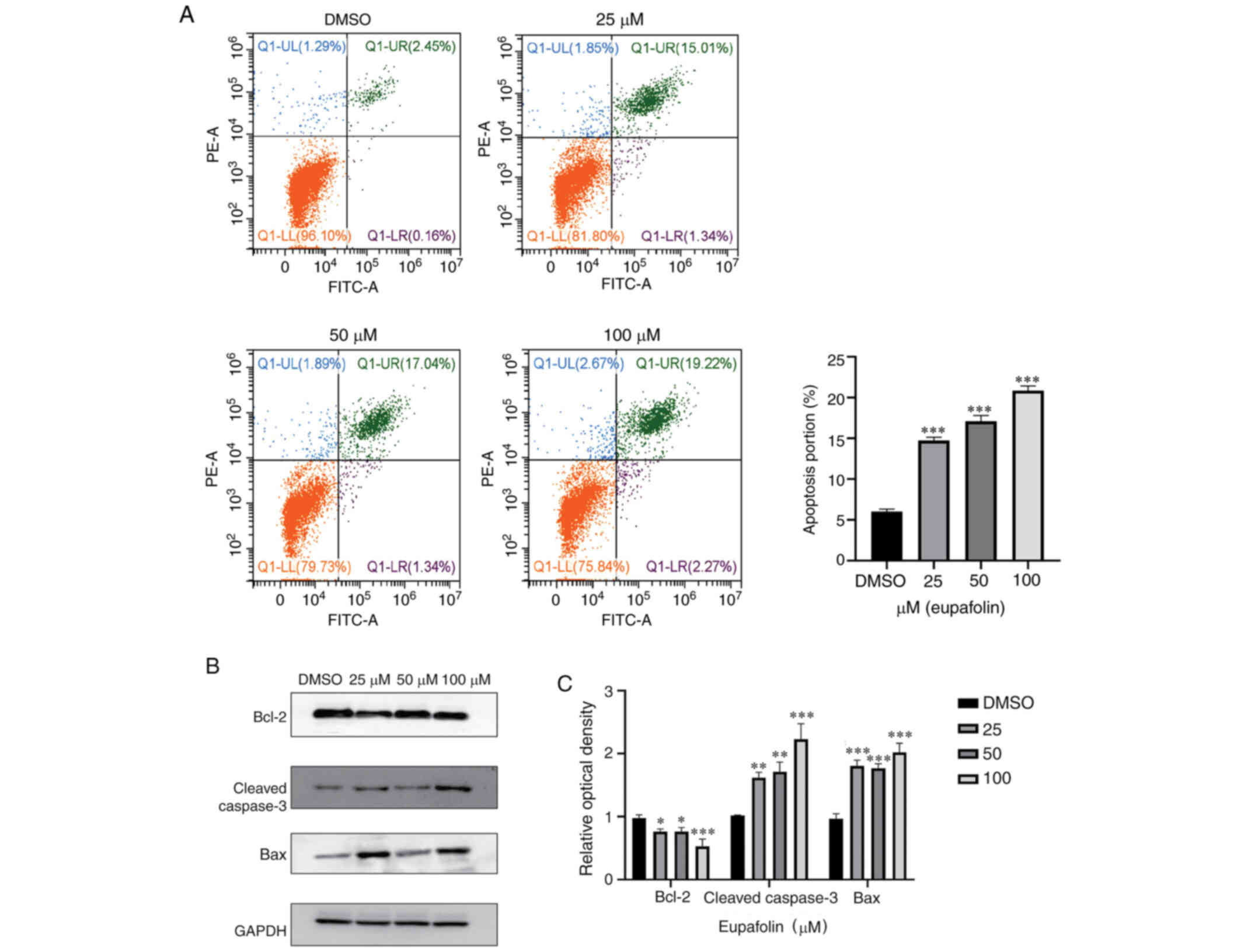
Eupafolin induces apoptosis of breast
cancer cells
Next, whether Eupafolin could induce apoptosis in
breast cancer cells was tested. Flow cytometry was used to analyze
the apoptosis ratio of EO771 cells following treatment with
Eupafolin. The results showed that compared with the control, 100
µM Eupafolin increased the apoptosis rate of EO771 cells by 18%
(Fig. 3A). To further understand the
specific mechanism of Eupafolin in causing apoptosis in EO771
cells, the expression of apoptosis-associated proteins was
evaluated using western blotting. The protein levels of cleaved
caspase 3 and Bax were increased, whereas Bcl-2 protein levels were
decreased (Fig. 3B and C).
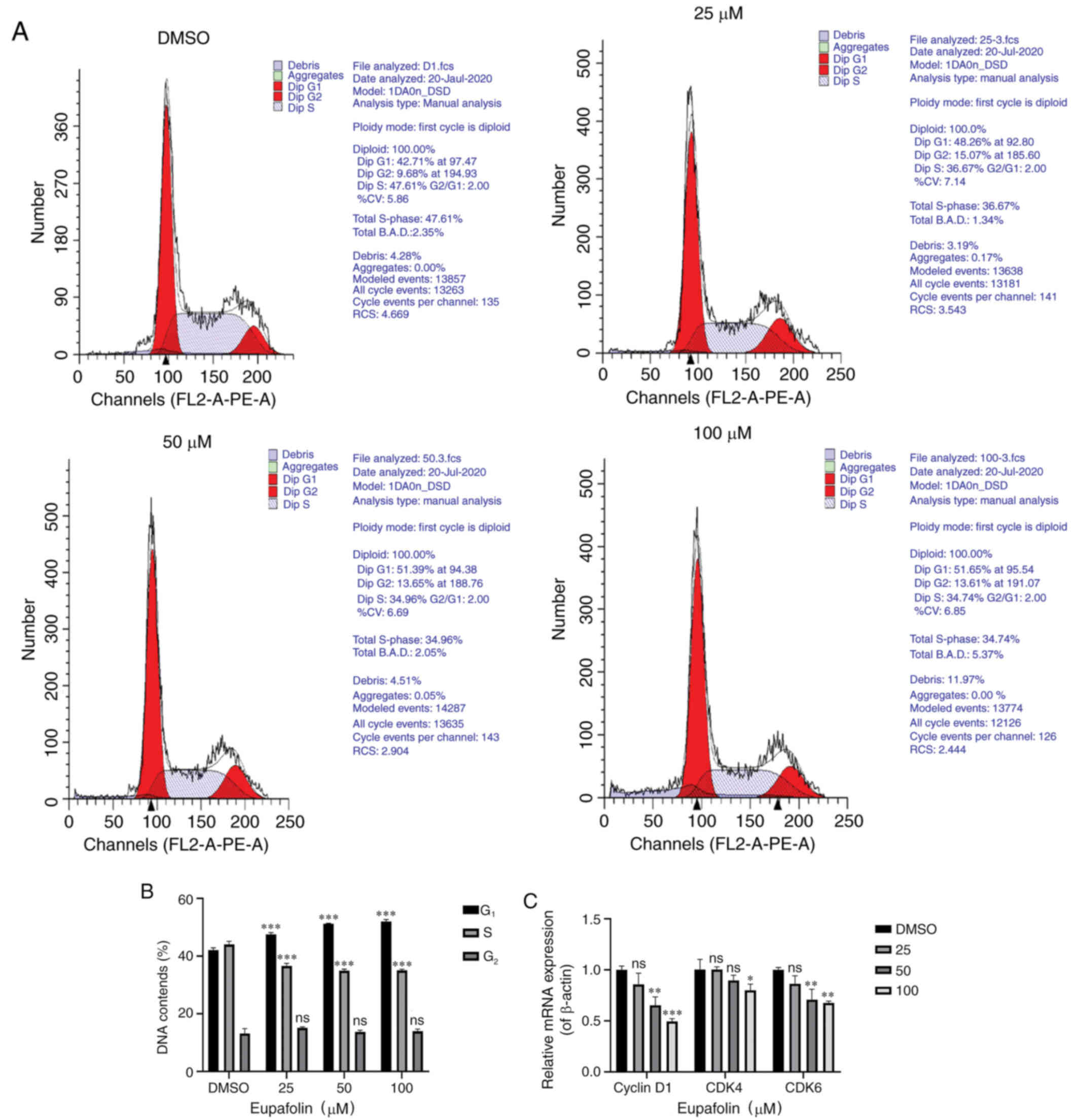
Eupafolin induces
G0/G1 phase arrest in breast cancer
cells
After staining with PI, cell cycle analysis of EO771
cells treated with various concentrations of Eupafolin was
performed using flow cytometry. Eupafolin induced
G0/G1 phase arrest in EO771 cells (Fig. 4A and B). In addition, RT-qPCR was
used to determine the mRNA levels of cycle-associated genes. The
results showed that Eupafolin inhibited the expression of cyclin
D1, CDK4 and CDK6 mRNA (Fig.
4C).
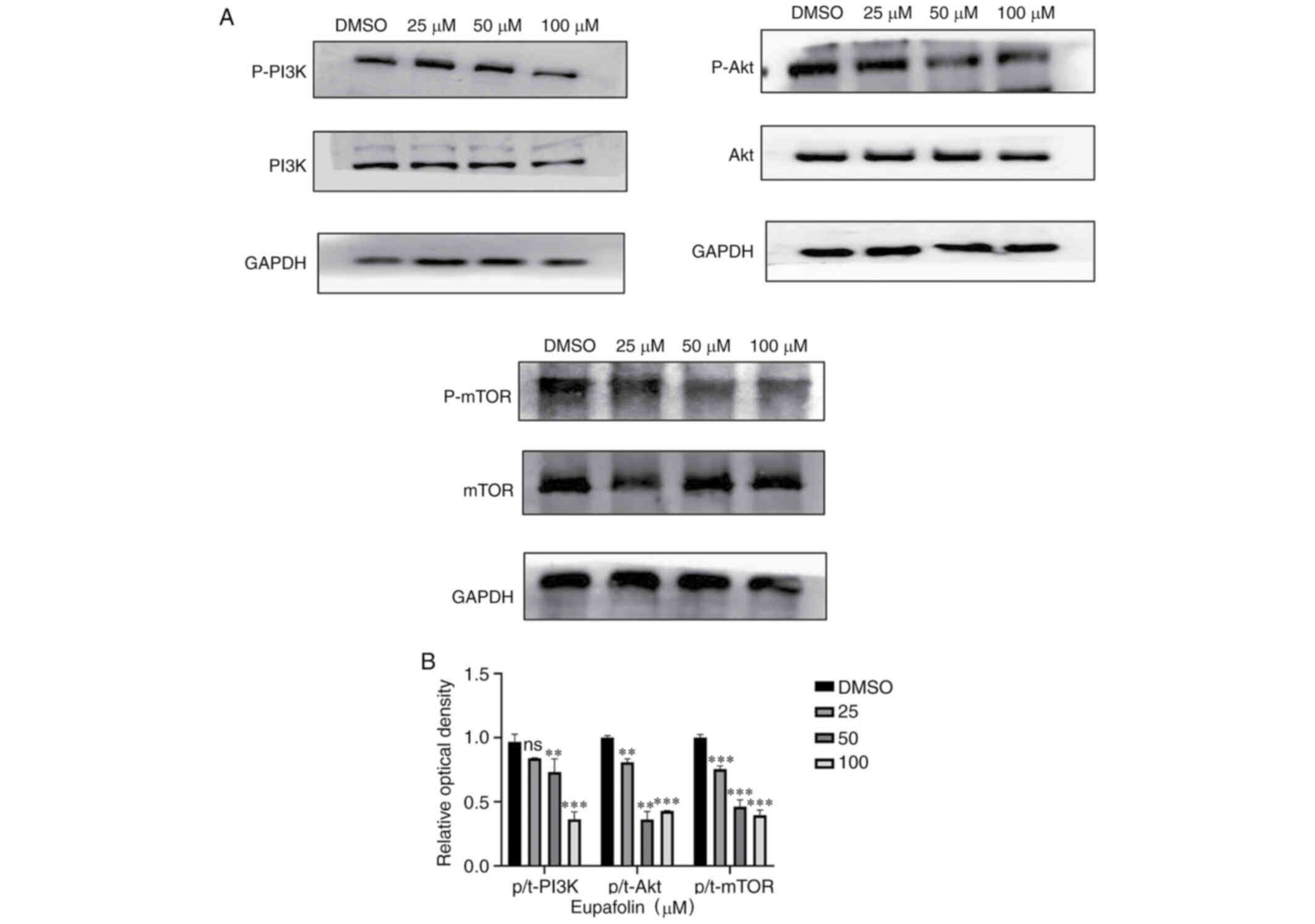
Eupafolin downregulates the
PI3K/Akt/mTOR pathway
The aforementioned data indicated that Eupafolin had
an impact on the proliferation and apoptosis of EO771 cells.
PI3K/Akt/mTOR was found to play an important role in the occurrence
of many tumors. Therefore, whether Eupafolin affected EO771 cells
through this pathway was tested. Results from western blotting
showed that Eupafolin significantly decreased protein levels of
p-PI3K, p-Akt and p-mTOR. Taken together, these results indicate
that Eupafolin affected the proliferation and apoptosis of EO771
cells through the PI3K/Akt/mTOR pathway (Fig. 5A and B).
Discussion
The GLOBOCAN 2018 Global Cancer Analysis Annual
Report released by the World Health Organization in 2018 indicates
that annually, 2.088 million new cases of breast cancer and 627,000
deaths occur worldwide (1). China's
incidence rate ranks 120 in the world (11). Although the total incidence of breast
cancer in China is lower than that in other developed countries,
the trend shows a gradual increase (1). Current breast cancer treatment
strategies mainly focus on chemotherapy and radiotherapy; however,
these treatment strategies almost inevitably have side effects,
which have become the bottleneck of clinical tumor treatment
(12). Since natural compounds have
advantages of higher efficacy and lower toxicity, many natural
compounds have been used clinically (13). Eupafolin is a natural compound
extracted from plants. Previous studies have reported that
Eupafolin has anti-inflammatory and anti-tumor effects (3). For example, Eupafolin can inhibit the
viability of esophageal cancer cells in vivo and in
vitro by targeting T-LAK, and by downregulating Mcl-1 and
upregulating Mcl-1 (4). Bim enhances
TRAIL-mediated apoptosis of renal cancer cells (14). In addition, Eupafolin can induce
apoptosis in HeLa human cervical cancer cells by inhibiting the
expression of associated apoptosis proteins (3). However, the role of Eupafolin in breast
cancer and its possible underlying mechanism of action have not yet
been elucidated. Therefore, the present study aimed to determine
the effect of Eupafolin on breast cancer cells. Our results showed
that Eupafolin treatment had a significant inhibitory effect on
breast cancer cell proliferation, promoted cell apoptosis, and
induced G0/G1 phase arrest. Therefore, the
data indicated that Eupafolin could inhibit the proliferation of
breast cancer cells and induce apoptosis thereof, via
downregulating the PI3K/Akt/mTOR pathway.
The anti-breast cancer proliferation activity of
Eupafolin is shown in Fig. 1. Within
48 h of treatment, the inhibitory effect of Eupafolin increased in
a time- and dose-dependent manner. MMP degrades various protein
components in the extracellular matrix, destroys the histological
barrier of tumor cell invasion, and plays a key role in tumor
invasion and metastasis (15). VEGF
is abnormally expressed in liver cancer and plays an important role
in liver cancer neovascularization and tumor growth (5). The treatment of cancer by targeting
VEGF and its receptor, VEGFR, is a much-explored topic in drug
research (16). After treatment with
different concentrations of Eupafolin, the migration and invasion
ability of breast cancer cells decreased significantly.
Furthermore, the mRNA levels of associated genes were detected
using RT-qPCR, and the present data showed that mRNA levels of
MMP2, MMP9 and VEGFA were significantly decreased (Fig. 2). Caspase 3 is the most important
terminal splicing enzyme in apoptosis and plays an important part
in the cell-killing mechanism (17).
In addition, Bcl-2 was one of the first members of the Bcl-2
protein family reported to regulate cell death. In cancer, Bcl-2
can prevent apoptosis of some cells and is highly expressed in
cancer cells that occur in lymph nodes and other organs of the
immune system (18). The balance
between Bcl-2 and Bax protein determines cell survival or
apoptosis. Flow cytometry results in the present study revealed
that the apoptosis rate of cells increased significantly. Moreover,
protein levels of the pro-apoptotic proteins, Bax and cleaved
caspase 3, increased significantly, whereas protein levels of Bcl-2
decreased significantly after Eupafolin treatment (Fig. 3). The decrease or resistance of cell
apoptosis often leads to further malignancy (19). Therefore, in some studies, it was
shown that the apoptosis rate of tumor cells can be increased by
activating or inhibiting various signaling pathways (20,21).
Naringin has been shown to inhibit the PI3K/Akt/mTOR pathway and
promote the apoptosis of thyroid cancer cells (22), whereas hyperoside-induced breast
cancer cell apoptosis is achieved via the reactive oxygen species
(ROS)-mediated NF-κB signaling pathway (23).
Several studies have shown that there are critically
important phases in the cell cycle: G1 to S and
G2 to M, which occur in a period of complex and active
molecular level changes, which are easily affected by external
conditions. If tumor growth needs to be suitably controlled, it can
be achieved via two mechanisms. Cyclin-dependent protein kinases
are a group of serine/threonine protein kinases, which can drive
the cell cycle. Each cyclin-dependent kinase (CDK) binds to a
different type of cyclin to form a complex, which regulates the
transition of cells from the G1 to the S phase, or from
the G2 to the M phase and exit from the M phase.
Increased expression of CDK and cyclin has been observed in most
cancer cells; the increased activity of CDK may be associated with
uncontrolled cell proliferation (24). When cells are damaged, the CDK
inhibitor genes are upregulated, and the G1
phase-associated CDK complex is downregulated. Among them, both
CDK4 and CDK6 can bind to cyclin D1 and play an important role in
the G1/S phase (25). If
the expression of G1 cell cycle-associated proteins
increases significantly, it causes the abnormal proliferation of
tumor cells (26). Because the
expression of CDK inhibitors in these cells may be insufficient,
the G1 phase of the cell cycle arrest provides cells
with a repair mechanism or follows the pathological changes
(27). Therefore, targeting the
cell-cycle disorders have been considered promising targets for
cancer treatment (28). Results from
flow cytometry showed that cells in the G0/G1
phase increased and those in the S phase decreased in
Eupafolin-treated cells. To the best of our knowledge, cancer
occurs or develops through the activation of CDKs that regulate the
cell cycle. Inhibiting the expression or function of CDK is
becoming one of the promising strategies in cancer treatment
(24). Among these proteins, cyclin
D1, CDK4 and CDK6 are the key regulators of the
G0-G1 phase (29). It was found that the mRNA levels of
CDK4, CKD6 and cyclin D1 were decreased when cells were treated
with 100 µM Eupafolin, thus preventing the progression of the cell
cycle from the G1 to S phase (Fig. 4).
To further understand the underlying anti-cancer
mechanisms of Eupafolin in breast cancer cells, western blotting
was performed to determine the proteins of the PI3K/Akt/mTOR
pathway that were involved in tumor cell growth, differentiation,
and apoptosis (22,30). Generally, the expression of Akt in
tumors is higher when compared with that in normal tissue, and the
activation of Akt can activate many downstream target genes and
regulate cell proliferation and cycle through several signaling
pathways (31). Many studies have
shown that the PI3k/Akt/mTOR pathway, as a therapeutic target, can
effectively inhibit the further progression of tumors, including
breast cancer, non-small cell lung cancer, esophageal cancer,
gastric cancer and liver cancer (32). Astragaloside IV upregulates Nrf2
through the PI3K/Akt/mTOR signaling pathway to regulate
inflammation and oxidative stress, thereby effectively inhibiting
breast cancer cell metastasis (12).
The combined use of compound Sophora flavescens and gefitinib can
upregulate autophagy in lung cancer by inhibiting the PI3K/Akt/mTOR
pathway (33). In addition, in
animal models, the addition of Akt allosteric inhibitors and dual
PI3K and mTOR inhibitors can inhibit the PI3K/Akt/mTOR pathway and
inhibit the growth of esophageal cancer (34). These findings are consistent with the
present results. In the present study, results from western
blotting indicated that Eupafolin decreased the phosphorylation of
PI3K, Akt, and mTOR proteins, but did not change their total
protein levels (Fig. 5).
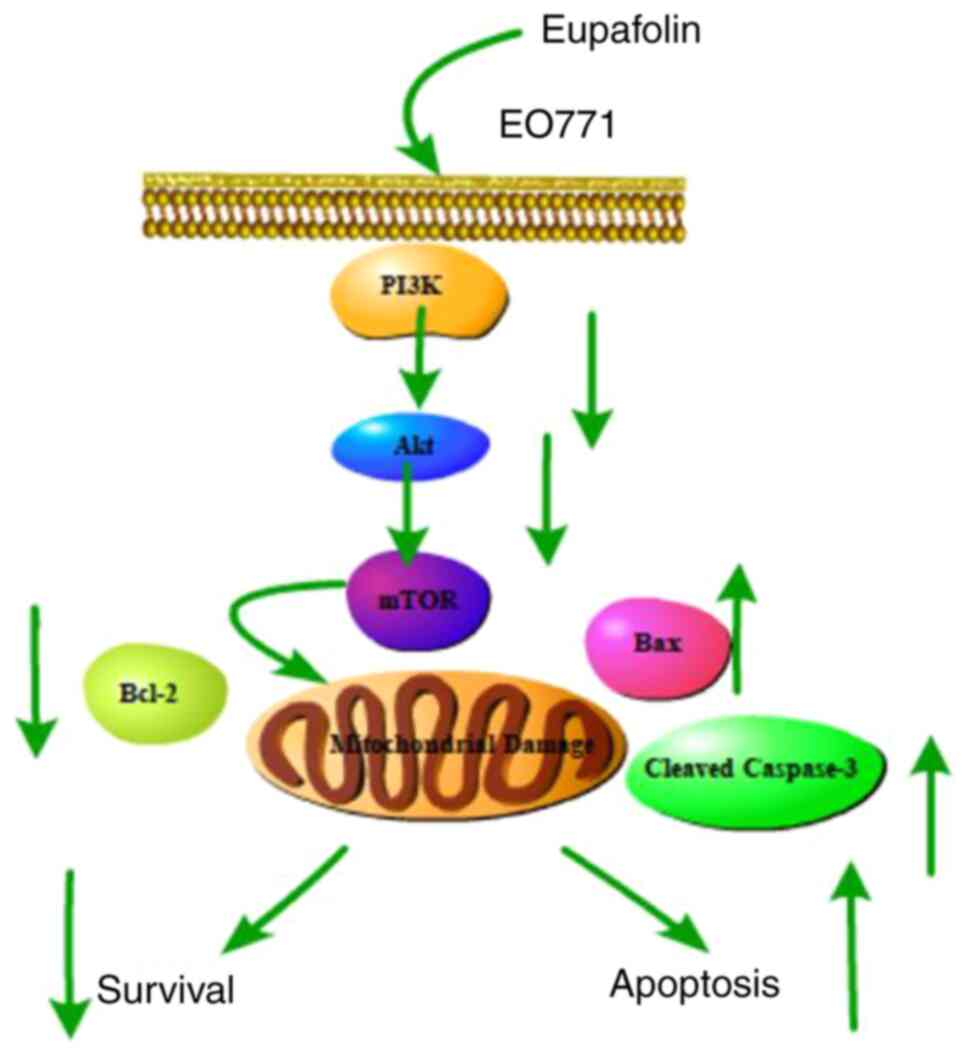
Taken together, the present findings showed that
Eupafolin significantly inhibited the proliferation and migration
of breast cancer cells, promoted apoptosis, and caused
G0/G1 phase arrest, which was achieved by
inhibiting the PI3K/Akt/mTOR pathway (Fig. 6). Thus, these results provide a
theoretical basis for the use of Eupafolin in subsequent clinical
trials. However, further research is needed to identify whether
Eupafolin inhibits the growth of breast cancer cells through other
pathways and target genes.
Acknowledgements
Not applicable.
Funding
This study was supported by Jilin Province Science
and Technology Development Project (grant no. 20200703014ZP).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
JW, XZ, JZ and YD conceptualized the study and
performed experimental research. XZ, SH and HP generated and
analyzed the data. JW, XZ, BY and QL performed data analysis and
edited the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Watkins EJ: Overview of breast cancer.
JAAPA. 32:13–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao W, Ge S and Sun J: Ailanthone exerts
anticancer effect by up-regulating miR-148a expression in
MDA-MB-231 breast cancer cells and inhibiting proliferation,
migration and invasion. Biomed Pharmacother. 109:1062–1069. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung KS, Choi JH, Back NI, Choi MS, Kang
EK, Chung HG, Jeong TS and Lee KT: Eupafolin, a flavonoid isolated
from Artemisia princeps, induced apoptosis in human cervical
adenocarcinoma HeLa cells. Mol Nutr Food Res. 54:1318–1328. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang H, Wu D, Xu D, Yu H, Zhao Z, Ma D
and Jin J: Eupafolin exhibits potent anti-angiogenic and antitumor
activity in hepatocellular carcinoma. Int J Biol Sci. 13:701–711.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan X, Tao J, Cai Z, Fredimoses M, Wu J,
Jiang Z, Zhang K and Li S: Eupafolin suppresses esophagus cancer
growth by targeting T-LAK cell-originated protein kinase. Front
Pharmacol. 10:12482019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu K, Park C, Chen H, Hwang J,
Thimmegowda NR, Bae EY, Lee KW, Kim HG, Liu H, Soung NK, et al:
Eupafolin suppresses prostate cancer by targeting
phosphatidylinositol 3-kinase-mediated Akt signaling. Mol Carcinog.
54:751–760. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Am JU, Gong WJ, Su Y and Mou ZB:
Imperatorin shows selective antitumor effects in SGC-7901 human
gastric adenocarcinoma cells by inducing apoptosis, cell cycle
arrest and targeting PI3K/Akt/m-TOR signalling pathway. J BUON.
22:1471–1476. 2017.PubMed/NCBI
|
|
8
|
Zhang Y, Zhang R and Ni H: Eriodictyol
exerts potent anticancer activity against A549 human lung cancer
cell line by inducing mitochondrial-mediated apoptosis, G2/M cell
cycle arrest and inhibition of m-TOR/PI3K/Akt signalling pathway.
Arch Med Sci. 16:446–452. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao JG, Zhang L, Xiang XJ, Yu F, Ye WL,
Wu DP, Wang JF and Xiong JP: Amarogentin secoiridoid inhibits in
vivo cancer cell growth in xenograft mice model and induces
apoptosis in human gastric cancer cells (SNU-16) through G2/M cell
cycle arrest and PI3K/Akt signalling pathway. J BUON. 21:609–617.
2016.PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thorat MA and Balasubramanian R: Breast
cancer prevention in high-risk women. Best Pract Res Clin Obstet
Gynaecol. 65:18–31. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang XQ, Yao C, Bian WH, Chen X, Xue JX,
Zhu ZY, Ying Y, Xu YL and Wang C: Effects of Astragaloside IV on
treatment of breast cancer cells execute possibly through
regulation of Nrf2 via PI3K/AKT/mTOR signaling pathway. Food Sci
Nutr. 7:3403–3413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lai ZR, Ho YL, Huang SC, Huang TH, Lai SC,
Tsai JC, Wang CY, Huang GJ and Chang YS: Antioxidant,
anti-inflammatory and antiproliferative activities of Kalanchoe
gracilis (L.) DC Stem. Am J Chin Med. 39:1275–1290. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han MA, Min KJ, Woo SM, Seo BR and Kwon
TK: Eupafolin enhances TRAIL-mediated apoptosis through cathepsin
S-induced down-regulation of Mcl-1 expression and AMPK-mediated Bim
up-regulation in renal carcinoma Caki cells. Oncotarget.
7:65707–65720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Hu Z, Wang Z, Cui Y and Cui X:
Angiopoietin-like protein 2 is an important facilitator of tumor
proliferation, metastasis, angiogenesis and glycolysis in
osteosarcoma. Am J Transl Res. 11:6341–6355. 2019.PubMed/NCBI
|
|
16
|
Aksenenko MB, Palkina NV, Sergeeva ON, Yu
Sergeeva E, Kiriehenko AK and Ruksha TG: miR-155 overexpression is
followed by downregulation of its target gene, NFE2L2, and altered
pattern of VEGFA expression in the liver of melanoma B16-bearing
mice at the premetastatic stage. Int J Exp Pathol. 100:311–319.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng C, Ke Z, Song Y, Yao Y, Hu X, Zhang
M, Li H and Yin J: Annexin A3 is associated with a poor prognosis
in breast cancer and participates in the modulation of apoptosis in
vitro by affecting the Bcl-2/Bax balance. Exp Mol Pathol. 95:23–31.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao Z, Zhang G, Xie C and Zhou Y: miR-34b
regulates cervical cancer cell proliferation and apoptosis. Artif
Cells Nanomed Biotechnol. 47:2042–2047. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu R, Chen Y, Shou T, Hu J, Chen J and
Qing C: TRIM67 promotes NFκB pathway and cell apoptosis in
GA-13315-treated lung cancer cells. Mol Med Rep. 20:2936–2944.
2019.PubMed/NCBI
|
|
21
|
Wu G, Zheng H, Xu J, Guo Y, Zheng G, Ma C,
Hao S, Liu X, Chen H, Wei S, et al: miR-429 suppresses cell growth
and induces apoptosis of human thyroid cancer cell by targeting
ZEB1. Artif Cells Nanomed Biotechnol. 47:548–554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou J, Xia L and Zhang Y: Naringin
inhibits thyroid cancer cell proliferation and induces cell
apoptosis through repressing PI3K/AKT pathway. Pathol Res Pract.
215:1527072019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu J, Zhang T, Zhu X, Yang C, Wang Y,
Zhou N, Ju B, Zhou T, Deng G and Qiu C: Hyperoside induces breast
cancer cells apoptosis via ROS-Mediated NF-κB signaling pathway.
Int J Mol Sci. 21:1312019. View Article : Google Scholar
|
|
24
|
Noori S and Hassan ZM: Tehranolide
inhibits proliferation of MCF-7 human breast cancer cells by
inducing G0/G1 arrest and apoptosis. Free Radic Biol Med.
52:1987–1999. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suryavanshi S, Choudhari A, Raina P and
Kaul-Ghanekar R: A polyherbal formulation, HC9 regulated cell
growth and expression of cell cycle and chromatin modulatory
proteins in breast cancer cell lines. J Ethnopharmacol.
242:1120222019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahn H, Im E, Lee DY, Lee HJ, Jung JH and
Kim SH: Antitumor Effect of pyrogallol via miR-134 mediated S phase
arrest and inhibition of PI3K/AKT/Skp2/cMyc signaling in
hepatocellular carcinoma. Int J Mol Sci. 20:39852019. View Article : Google Scholar
|
|
27
|
Hunter T and Pines J: Cyclins and cancer.
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park W, Park S, Song G and Lim W:
Inhibitory effects of osthole on human breast cancer cell
progression via induction of cell cycle arrest, mitochondrial
dysfunction, and ER stress. Nutrients. 11:27772019. View Article : Google Scholar
|
|
29
|
Gao X, Wang Y, Li Y, Wang Y, Yan M, Sun H,
Chen S and Pan X: Huganpian, a traditional Chinese medicine,
inhibits liver cancer growth in vitro and in vivo by inducing
autophagy and cell cycle arrest. Biomed Pharmacother.
120:1094692019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Sun L, Zhang S, Zhang S and Li W:
GINS2 facilitates epithelial-to-mesenchymal transition in
non-small-cell lung cancer through modulating PI3K/Akt and MEK/ERK
signaling. J Cell Physiol. 235:7747–7756. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo L and Yang T: Oxymatrine inhibits the
proliferation and invasion of breast cancer cells via the PI3K
pathway. Cancer Manag Res. 11:10499–10508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murugan AK: Special issue: PI3K/Akt
signaling in human cancer. Semin Cancer Biol. 59:1–2. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Qu Z, Yao H, Sun L, Harata-Lee Y,
Cui J, Aung TN, Liu X, You R, Wang W, et al: An effective drug
sensitizing agent increases gefitinib treatment by down regulating
PI3K/Akt/mTOR pathway and up regulating autophagy in non-small cell
lung cancer. Biomed Pharmacother. 118:1091692019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi N, Yu H and Chen T: Inhibition of
esophageal cancer growth through the suppression of PI3K/AKT/mTOR
signaling pathway. Onco Targets Ther. 12:7637–7647. 2019.
View Article : Google Scholar : PubMed/NCBI
|