Introduction
Cervical cancer ranks fourth in terms of both
incidence and mortality, according to data reported by GLOBOCAN
2018, with an estimated 311,000 deaths annually in 2018 (1).
Human papillomavirus (HPV), which is spread
primarily through sexual contact, has been established as the
essential causative agent for cervical intraepithelial lesions and
cervical cancer (2,3). There are >200 genotypes of HPV that
have been identified, of which 15 are oncogenic and classified as
high-risk HPVs (hr-HPVs) (4,5), and result in the development of ≥99.7%
of cervical cancer cases (6).
The prevalence of HPV is 11–12% worldwide (7), and the two most widespread types of
hr-HPV are HPV-16 and −18. A survey of healthy women conducted by
the International Agency for Research on Cancer confirmed that
there is geographic variation in HPV infection rates and types; for
example, HPV-45 and −33 are most prevalent in Africa, HPV-33 and
−31 are most prevalent across Europe, HPV-31, −33 and −45 are most
prevalent in the USA, and HPV-58 and −52 are the most prevalent in
Asia (5). Furthermore, the
Retrospective International Survey and HPV Time Trends Study Group
analyzed the prevalence of HPV subtypes in cervical cancer and
observed that the geographical variations in HPV distribution in
healthy women was associated with the variation in cancer biology
(4). Therefore, it is important to
survey the distribution of regional HPV genotypes, in order to
guide the effectiveness of vaccination programs, calculate health
economics and develop improved vaccines (8).
China, with its vast territory, has a population
that comprises 19% of the total global population and is currently
unvaccinated (8). Although several
reports have demonstrated that HPV-16, −18, −52 and −58 are the
most common genotypes (9–11) in Zhejiang Province, no specific type
of HPV has been demonstrated to be of the greatest concern. The
present study utilized a model based on a Geographic Information
System (GIS) to represent the spatial distribution of HPV and
perform accurate public health analyses (12–14).
This should aid in determining future healthcare, and enable new
therapeutic techniques and approaches to be more accurately
focused.
The aim of the present study was to establish the
urban baseline distribution of hr-HPV genotypes in Zhejiang
Province, China, in order to recommend appropriate specific
vaccines for this population.
Patients and methods
Patients
Participants were enrolled during their health
examination between January 2016 and December 2017 in Zhejiang
Cancer Hospital (Hangzhou, China). The inclusion criteria were as
follows: i) Mentally and physically competent; ii) never received a
HPV vaccine; iii) stably located (census registration) in Zhejiang;
iv) a permanent resident of Zhejiang (>60% of the year resides
in a permanent residence), and v) had previously had sexual
intercourse. Of these 8,897 participants, 2,669 did not meet the
inclusion criteria and 1,668 missed the follow-up. Thus, a total of
4,560 women were enrolled in the present study. Each participant
provided written informed consent prior to the start of the study.
The protocol was approved by the Ethics Committee of Zhejiang
Cancer Hospital (approval no. IRB-2019-75).
HPV detection and typing
A senior gynecologist performed the pelvic
examination, and collected samples of exfoliated cervical cells for
HPV DNA detection. The HPV GenoArray Test kit (HybriBio Ltd.) was
used to differentiate between 13 hr-HPV genotypes (HPV-16, −18,
−31, −33, −35, −39, −45, −51, −52, −56, −58, −59 and −68).
All the exfoliated cervical cell samples were
obtained by cytobrush and collected in Surepath™ solution (TriPath
Imaging, Inc.). Next, 0.05 g of the samples was centrifuged at
20,784 × g for 1 min at 4°C to remove supernatant and the pellet
was resuspended in 200 ml PBS buffer. DNA extracts were prepared
with the QIAamp DNA Blood Mini kit 3 (Qiagen, Inc.) following the
Blood and Body Fluid Spin protocol. HPV genotyping by HybriMax used
an HPV GenoArray Test kit (HybriBio Ltd.) according to the
manufacturer's instructions (15). A
negative control (pure water) and a positive control (HPV DNA from
low density gene chip hybrid membrane in HPV GenoArray test kit)
were used to avoid false-positive reactions. HPV genotyping was
performed following a HPV GenoArray test kit (HybriBio, Ltd.).
Heat maps
All the detailed geographical information of the
participants in the present study was obtained using EsgynDB (Esgyn
Corporation). EsgynDB is a large database with platforms such as
Hadoop, Mobile Measurement Partner, stream processing and in-memory
databases, and fusion of internal data (operation, business and
management support systems), that can use the internet to extract
multidimensional data. The GIS is one of these functions, which
possesses omnibearing position data, and has improved data
acquisition and analysis capability when compared with GRPS
positioning. In order to determine the spatial distribution of the
4,560 women, their locations were pin-pointed according to
longitude and latitude coordinates using the GIS. The accuracy of
detection is 0.01 degrees of longitude and latitude (the longitude
distance is ~1,000 m, and the latitude distance is ~1,113 m; thus,
each unit is 1,000×1,113 m). To decrease the risk of bias, data for
the unit were discarded if the total population in each unit was
<3 study participants. The heat maps were generated directly in
the database, based on the HPV positive infection rate (0–100%).
EsgynDB is a platform for managing big data and performing
analyses, but is not available populated with the information on
subject location that was used in the present study.
Statistical analysis
Data analyses were performed using SPSS 20 software
(IBM Corp.). The χ2 test was performed to compare hr-HPV
genotype distributions across regions. P<0.05 was considered to
indicate a statistically significant difference.
Results
HPV positive rate and infection
status
The mean age of the enrolled women was 49.05 years
(range, 12–90 years). Of the 4,560 cervical samples, 1,886 (41.4%)
had detectable HPV. The 13 hr-HPV genotypes had infection rates
that increased with age; the most prevalent type was HPV-16
(19.85%), followed by HPV-58, −52 and −18 with lower rates (7.74,
5.15 and 3.55%, respectively), and other HPV types with rates
ranging from 2.96 down to 0.35% (Table
I). The participants were mainly from Hangzhou, Ningbo,
Wenzhou, Huzhou, Jiaxing, Shaoxing, Jinhua and Taizhou. There were
few data available from Zhoushan, Quzhou and Lishui, as well as
other rural or remote areas (Fig.
1). The proportions of single-, co-, tri-, tetra- and more
genotype infections are presented in Table II. A total of 1,604 (35.18%)
participants were infected with a single HPV genotype, and 282
participants were infected with more than one HPV genotype, with
242 (5.31%), 34 (0.75%), and 5 (0.11%) having co-, tri- and
tetra-genotype infections, respectively.
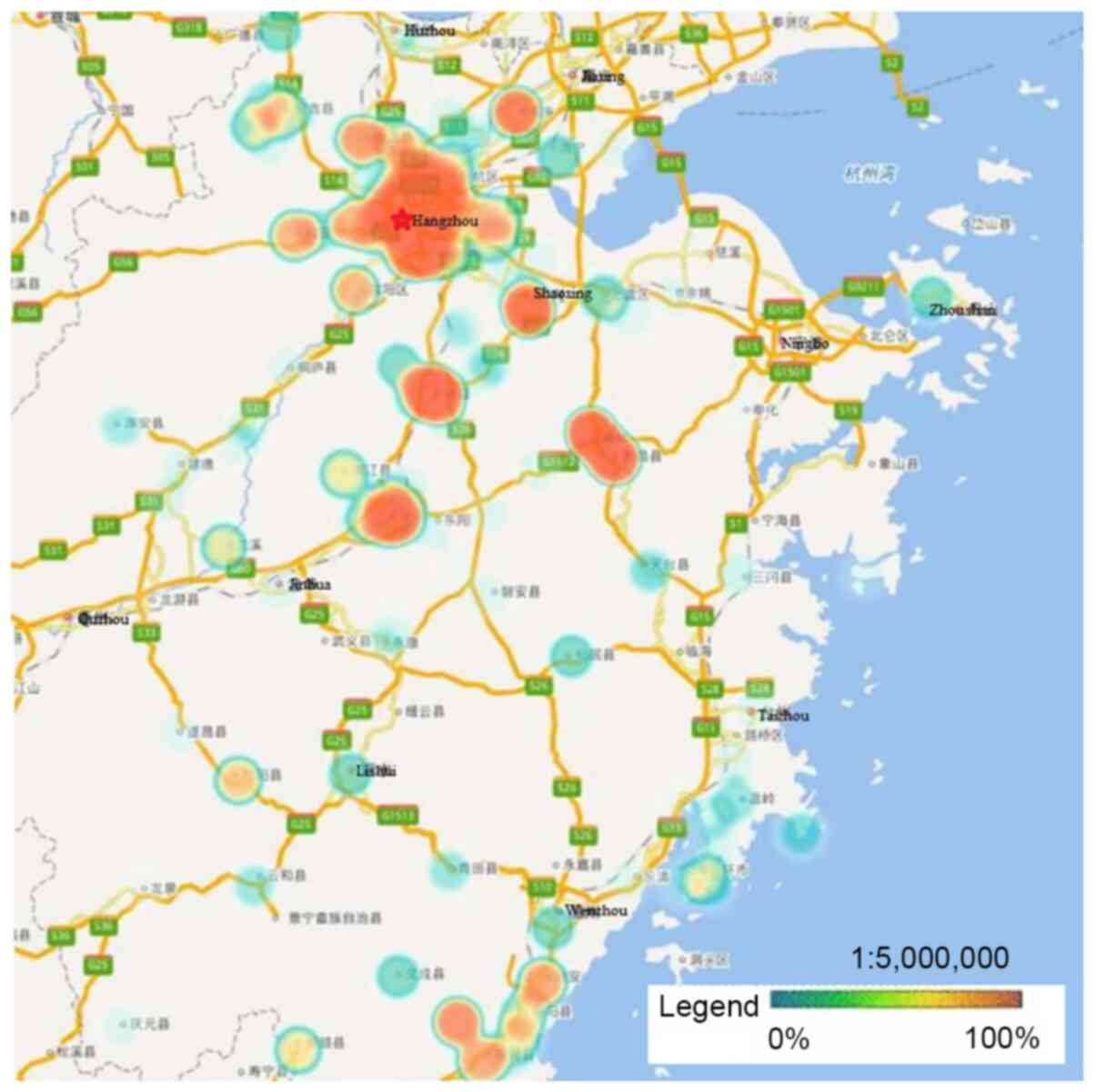
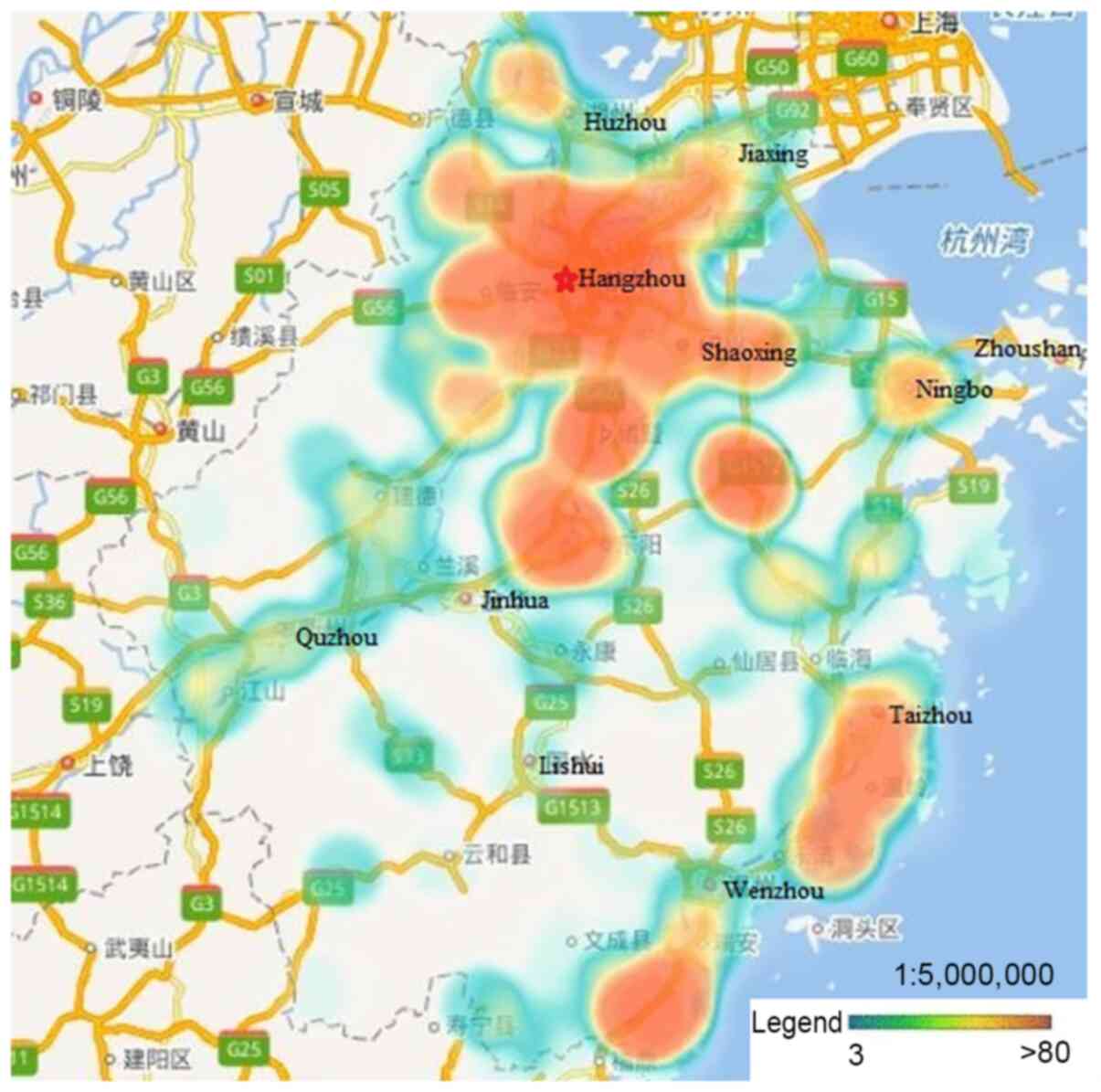 | Figure 1.Distribution of study participants.
Participants were mainly from Hangzhou, Ningbo, Wenzhou, Huzhou,
Jiaxing, Shaoxing, Jinhua and Taizhou, with few data from Zhoushan,
Quzhou, Lishui, and other rural or remote areas. |
 | Table I.Epidemiological distribution of HPV
genotype infection rates (%) according to age. |
Table I.
Epidemiological distribution of HPV
genotype infection rates (%) according to age.
|
| Age (years) |
|
|---|
|
|
|
|
|---|
| Genotype | <30 | 30–39 | 40–49 | 50–59 | 60–69 | ≥70 | Total |
|---|
| HPV16 | 15.53 | 14.23 | 17.72 | 21.59 | 26.71 | 26.78 | 19.85 |
| HPV18 | 2.48 | 2.53 | 2.72 | 4.60 | 5.04 | 2.73 | 3.55 |
| HPV31 | 0.00 | 1.33 | 1.46 | 2.88 | 2.52 | 3.28 | 2.01 |
| HPV33 | 3.73 | 3.06 | 2.79 | 2.42 | 4.45 | 1.64 | 2.96 |
| HPV35 | 0.00 | 0.13 | 0.33 | 0.39 | 0.74 | 0.00 | 0.35 |
| HPV39 | 4.35 | 1.86 | 2.06 | 2.34 | 2.67 | 0.01 | 2.24 |
| HPV45 | 0.62 | 0.53 | 0.13 | 0.70 | 0.30 | 0.00 | 0.39 |
| HPV51 | 2.48 | 1.46 | 1.53 | 2.49 | 2.82 | 3.28 | 2.08 |
| HPV52 | 3.73 | 3.06 | 5.04 | 4.52 | 7.57 | 11.48 | 5.15 |
| HPV56 | 0.00 | 0.53 | 0.46 | 0.86 | 0.89 | 2.73 | 0.72 |
| HPV58 | 5.59 | 5.19 | 6.64 | 8.03 | 11.42 | 13.66 | 7.74 |
| HPV59 | 0.62 | 0.13 | 0.40 | 1.17 | 0.45 | 0.00 | 0.57 |
| HPV68 | 1.86 | 1.20 | 0.86 | 0.94 | 0.74 | 0.55 | 0.94 |
| Total | 32.92 | 31.38 | 42.14 | 44.58 | 54.45 | 55.19 | 41.36 |
 | Table II.Numbers of positive HPV tests
according to genotype and infection status. |
Table II.
Numbers of positive HPV tests
according to genotype and infection status.
|
|
| Multiple-genotype
infection |
|---|
|
|
|
|
|---|
| Genotype | Single-genotype
infection | Co- | Tri- | Tetra- | Penta- |
|---|
| HPV16 | 735 | 149 | 17 | 3 | 1 |
| HPV18 | 120 | 37 | 3 | 2 | 0 |
| HPV31 | 62 | 21 | 7 | 1 | 1 |
| HPV33 | 81 | 38 | 14 | 2 | 0 |
| HPV35 | 6 | 7 | 2 | 1 | 0 |
| HPV39 | 61 | 33 | 7 | 1 | 0 |
| HPV45 | 8 | 9 | 0 | 1 | 0 |
| HPV51 | 51 | 31 | 12 | 0 | 1 |
| HPV52 | 161 | 58 | 12 | 3 | 1 |
| HPV56 | 18 | 10 | 3 | 1 | 1 |
| HPV58 | 267 | 67 | 17 | 2 | 0 |
| HPV59 | 15 | 7 | 3 | 1 | 0 |
| HPV68 | 19 | 17 | 5 | 2 | 0 |
| Total | 1,604 | 484 | 102 | 20 | 5 |
| No. of
patients | 1,604 | 242 | 34 | 5 | 1 |
HPV geographical distribution
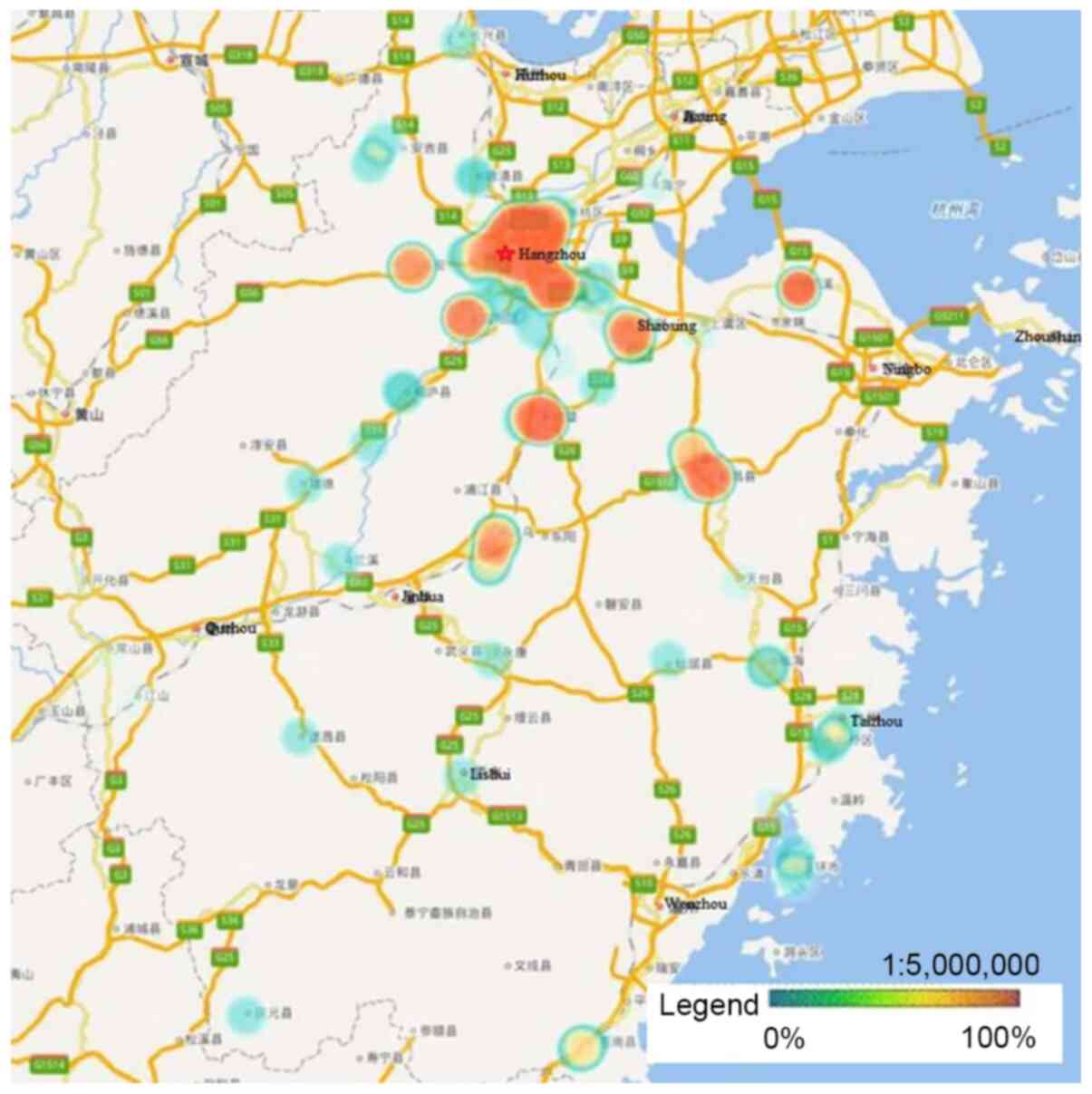
HPV-16, −58, −52 and −18 were the four most
prevalent genotypes observed in Zhejiang Province, and the ranking
of each varied by geographical region. Hangzhou, the capital city
of Zhejiang Province, had a significantly higher rate of HPV
infection than other regions. Heat maps revealed that areas with
high HPV infection rates tended to be have dense traffic networks,
particularly transport nodes. Specifically, HPV-16 and −58
infections were mainly distributed in the northern and central
regions of Zhejiang Province, including Hangzhou and Shaoxing,
where they were more prevalent than in the southern regions
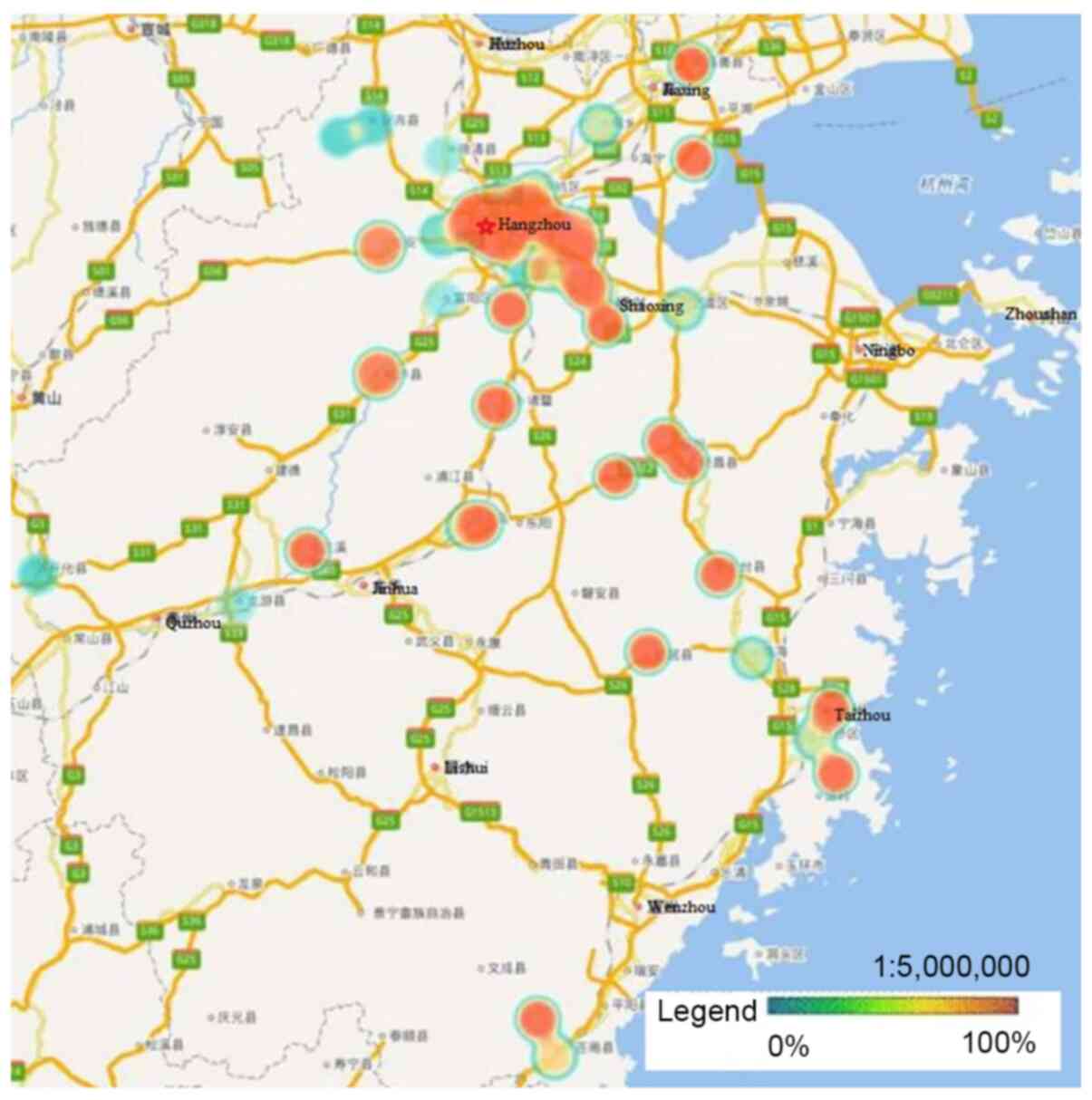
(P<0.05; Figs. 2 and 3). Unlike HPV-16 and −58, HPV-18 infection
was widespread throughout Zhejiang Province, but had much lower
infection rates in Ningbo and Huzhou compared with the rest of the
province (P<0.05; Fig. 4).
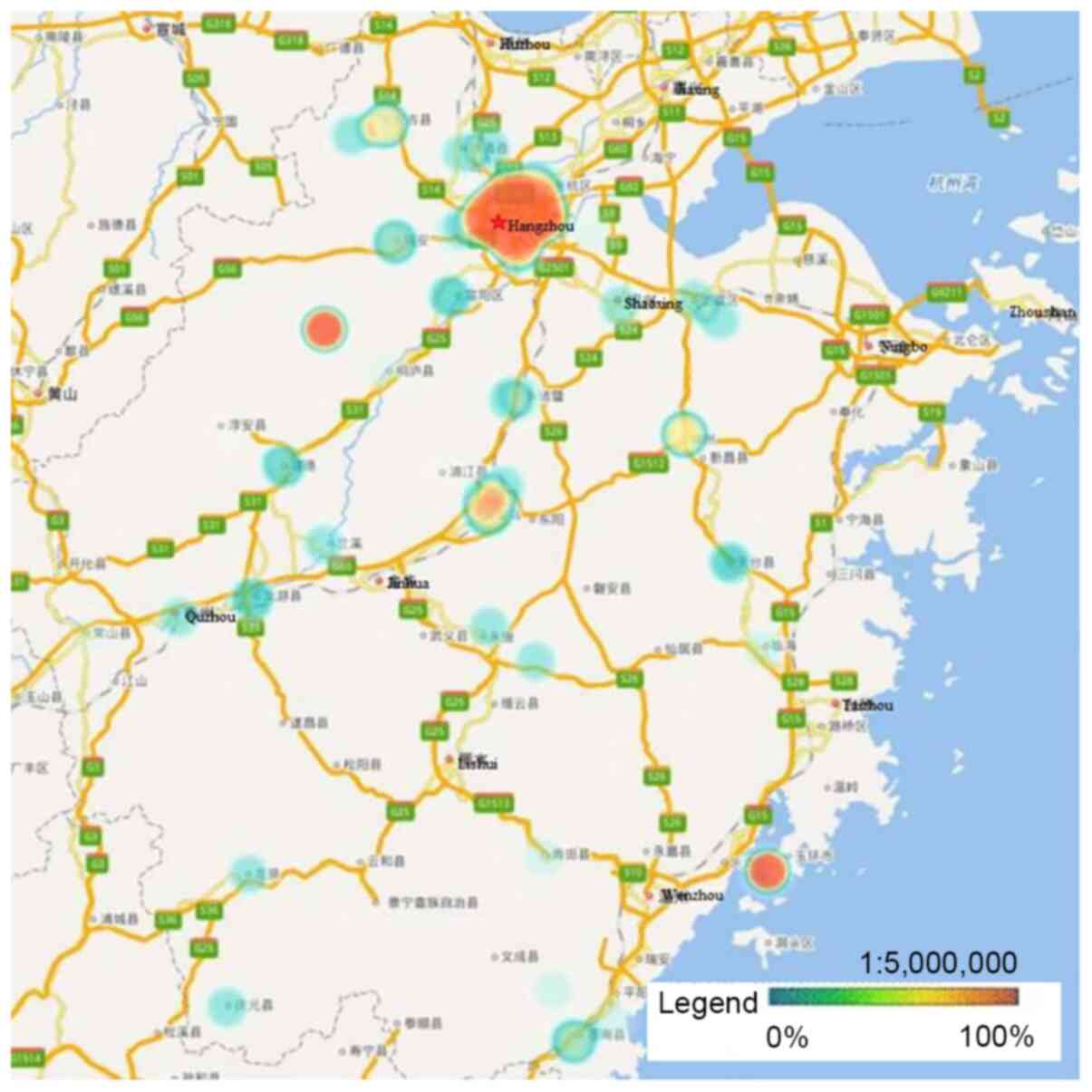
Notably, the high infection rate of HPV-52 was mainly distributed
in Hangzhou and the eastern coastal areas of Wenzhou, with a lower
infection rate in the center of Zhejiang Province (P<0.05;
Fig. 5).
Discussion
To the best of our knowledge, no urban-specific
baseline prevalence data of HPV genotypes according to location in
Zhejiang Province have been described. Such data would help in
healthcare planning, and enable researchers to identify new
therapeutic techniques and approaches more accurately. The present
study revealed this information using heat maps, which showed that
high HPV infection rates were associated with areas with dense
traffic networks, particularly transport nodes, and were much
higher in such areas than in remote areas. This finding indicates
that high population density and mobility may be risk factors for
HPV infection. Other possible reasons are that people from urban
areas usually are more sexually active, and have larger incomes,
higher education levels, improved hygiene standards and greater
medical check-up awareness compared with those from rural areas,
which results in a high infection and detection rates in the urban
population (16). However, this
finding contrasts with previous findings from a study in French
Guiana, in which the prevalence of HPV infection was high in remote
villages (17). The reasons for this
may be that the HPV vaccine is more accessible in urban
populations, which reduces HPV infection (18,19).
HPV-16, −58, −52 and −18 were the most prevalent
types of HPV in the population analyzed in the present study, which
was consistent with previous studies conducted in Asia (20,21).
Therefore, it is likely that bivalent or quadrivalent vaccines
alone will not meet regional requirements due to the prevalence of
other HR-HPV genotypes, such as HPV-52 and HPV-58, that are not
targeted by these vaccines. Further analysis revealed that the
distribution of HPV genotypes varied by geographical region;
Hangzhou had a high incidence of the four most prevalent HPV
genotypes, while Ningbo had a low incidence of these HPVs. Another
phenomenon was that HPV-16 and −58 infections were mainly
distributed in the northern and central regions, where their
infection rates were significantly higher than in the southern
regions. HPV-18 infection was found to be widespread throughout
Zhejiang Province, but had a much lower infection rate in Ningbo
and Huzhou. Wenzhou had relatively low rates of HPV-52 and −58
infections, but higher rates of HPV-16 and −18 infections, which is
consistent with the epidemiological characteristics of European
countries (3). A potential reason
for this is emigration from Europe and the USA. Furthermore,
infection with a single genotype of HPV was more frequently
observed (35.18%) than multiple infections (6.18%). Given the high
prevalence rate of hr-HPV infection in Zhejiang, an emphasis on
vaccination and expanded and innovative screening is important,
particularly in large cities. Specific vaccination programs have
been implemented in clinical practice since 2017 in Australia
(22), and Nygård et al
(23) demonstrated that an effective
HPV vaccination program could decrease the incidence of cervical
pre-invasive neoplasia by 51.5-66.6%. In the present study, one of
the positive aspects of using heat maps to display the spatial
distribution of HPV infection by geographical region in Zhejiang
Province is that it enables potentially appropriate vaccines to be
identified. The data suggest that for individuals in Ningbo or
Wenzhou, bivalent or quadrivalent vaccines may be suitable, but for
those in Hangzhou and Shaoxing, nonavalent vaccines are strongly
recommended.
The present study has several limitations. First,
only participants who received health examinations in Zhejiang
Cancer Hospital were enrolled, and this could produce a certain
level of bias. Another potential bias is that urban populations are
more likely than rural populations to have health examinations,
mainly due to greater income followed by check-up awareness. More
importantly, the genotypes of HPV that were detected and the
presence of multi-infections cause bias. If the patterns of
multi-infection elsewhere in China are consistent with the pattern
of geographical distribution of the virus detected in the present
study, the cities of China with the most migration, the communities
that mostly migrate to these cities, and whether the migration is
temporary or permanent should be determined; this would help to
distinguish whether the HPV geographical distribution is specific
or imported. In addition to analyzing the large-scale geographic
variations in HPV genotype distribution, a future larger scale,
population-based study should be conducted to obtain comprehensive
information on the prevalence and genotype distribution of HPV in
Chinese populations.
In conclusion, the data in the present study
indicate that HPV-16, −58, −52 and −18 are the four most prevalent
genotypes of HPV in Zhejiang Province. Heat maps displaying the
spatial distribution of HPV infection demonstrate that the
genotypes vary by geographical region.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Medical Health
Science and Technology Project of Zhejiang Provincial Health
Commission (grant nos. 2018KY276 and 2019PY022).
Availability of data and materials
The data that support the findings of this study are
available from EsgynDB but restrictions apply to the availability
of these data, which were used under license for the current study,
and so are not publicly available.
Authors' contributions
ZN and JX are responsible for the original concept
of the study and, with all co-authors, designed the study. TT and
YLG were responsible for data processing. JQZ, AWZ and AJY were
responsible for data cleaning and analyses. JX drafted the
manuscript, which was revised by ZN. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Each participant provided written informed consent
prior to the start of the study. The protocol was approved by the
Ethics Committee of Zhejiang Cancer Hospital (approval no.
IRB-2019-75).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burchell AN, Winer RL, de Sanjosé S and
Franco EL: Chapter 6: Epidemiology and transmission dynamics of
genital HPV infection. Vaccine. 24 (Suppl 3):S3/52–61. 2006.
View Article : Google Scholar
|
|
3
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ;
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group, : Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al: Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clifford GM, Gallus S, Herrero R, Muñoz N,
Snijders PJ, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E,
et al: Worldwide distribution of human papillomavirus types in
cytologically normal women in the International Agency for Research
on Cancer HPV prevalence surveys: A pooled analysis. Lancet.
366:991–998. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bruni L, Diaz M, Castellsagué X, Ferrer E,
Bosch FX and de Sanjosé S: Cervical human papillomavirus prevalence
in 5 continents: Meta-analysis of 1 million women with normal
cytological findings. J Infect Dis. 202:1789–1799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu XX, Zhou JS, Yuan SH, Yu H and Lou HM:
Distribution of HPV genotype in invasive cervical carcinoma and
cervical intraepithelial neoplasiain zhejiang province, southeast
China: Establishing the baseline for surveillance. Int J Environ
Res Public Health. 12:10794–10805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang S, Afonina I, Miller BA and Beckmann
AM: Human papillomavirus types 52 and 58 are prevalent in cervical
cancers from Chinese women. Int J Cancer. 70:408–411. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo KW, Wong YF, Chan MK, Li JC, Poon JS,
Wang VW, Zhu SN, Zhang TM, He ZG, Wu QL, et al: Prevalence of human
papillomavirus in cervical cancer: A multicenter study in China.
Int J Cancer. 100:327–331. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Rose B, Huang X, Liao G, Carter J,
Wu X and Thompson C: Comparative analysis of characteristics of
women with cervical cancer in high-versus low-incidence regions.
Gynecol Oncol. 94:803–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Requia WJ, Roig HL, Adams MD, Zanobetti A
and Koutrakis P: Mapping distance-decay of cardiorespiratory
disease risk related to neighborhood environments. Environ Res.
151:203–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Svendsen ER, Gonzales M, Mukerjee S, Smith
L, Ross M, Walsh D, Rhoney S, Andrews G, Ozkaynak H and Neas LM:
GIS-modeled indicators of traffic-related air pollutants and
adverse pulmonary health among children in El Paso, Texas. Am J
Epidemiol. 176 (Suppl 7):S131–S141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khoury MJ, Iademarco MF and Riley WT:
Precision Public Health for the Era of Precision Medicine. Am J
Prev Med. 50:398–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tao P, Zheng W, Wang Y and Bian ML:
Sensitive HPV genotyping based on the flow-through hybridization
and gene chip. J Biomed Biotechnol. 2012:9387802012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zou H, Meng X, Jia T, Zhu C, Chen X, Li X,
Xu J, Ma W and Zhang X: Awareness and acceptance of human
papillomavirus (HPV) vaccination among males attending a major
sexual health clinic in Wuxi, China: A cross-sectional study. Hum
Vaccin Immunother. 12:1551–1559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adenis A, Dufit V, Douine M, Corlin F,
Ayhan G, Najioullah F, Molinie V, Brousse P, Carles G, Lacoste V,
et al: High prevalence of HPV infection in the remote villages of
French Guiana: An epidemiological study. Epidemiol Infect.
145:1276–1284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lynge E, Clausen LB, Guignard R and Poll
P: What happens when organization of cervical cancer screening is
delayed or stopped. J Med Screen. 13:41–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sankaranarayanan R, Nene BM, Shastri SS,
Jayant K, Muwonge R, Budukh AM, Hingmire S, Malvi SG, Thorat R,
Kothari A, et al: HPV screening for cervical cancer in rural India.
N Engl J Med. 360:1385–1394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sukvirach S, Smith JS, Tunsakul S, Muñoz
N, Kesararat V, Opasatian O, Chichareon S, Kaenploy V, Ashley R,
Meijer CJ, et al: Population-based human papillomavirus prevalence
in Lampang and Songkla, Thailand. J Infect Dis. 187:1246–1256.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, Hang D, Yang L, Feng X, Lyu Z, Xie
S, Zhou J, Wu L, Li X, Li N, et al: Persistence of type-specific
human papillomavirus infection among Daqing City women in China
with normal cytology: A pilot prospective study. Oncotarget.
8:81455–81461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mammas IN, Spandidos DA and Sourvinos G:
Genomic diversity of human papillomaviruses (HPV) and clinical
implications: An overview in adulthood and childhood. Infect. Genet
Evol. 21:220–226. 2014. View Article : Google Scholar
|
|
23
|
Nygård M, Hansen BT, Dillner J, Munk C,
Oddsson K, Tryggvadottir L, Hortlund M, Liaw KL, Dasbach EJ and
Kjær SK: Targeting human papillomavirus to reduce the burden of
cervical, vulvar and vaginal cancer and pre-invasive neoplasia:
Establishing the baseline for surveillance. PLoS One. 9:e883232014.
View Article : Google Scholar : PubMed/NCBI
|