Introduction
ErbB2 (or HER2) overexpression is found in 25–30% of
breast cancer and 4–50% of gastric cancer cases (1–3).
Trastuzumab is an anti-ErbB2 (human) antibody which is used for the
clinical treatment of ErbB2-amplified metastatic breast, metastatic
gastric and gastro-esophageal junction cancer (4,5).
However, ~70% of patients with cancer do not respond to
trastuzumab, and most of trastuzumab-responsive patients develop
resistance to trastuzumab within 1 year of treatment initiation
(6–8).
Despite cancer progression on ErbB2-directed
therapies (9–12), ErbB2 remains considered as a valid
therapeutic target. Pertuzumab is another anti-ErbB2 (human)
antibody, that binds to an epitope of ErbB2 different from
trastuzumab (9,13). Among the strategies to overcome
trastuzumab resistance, the combination of pertuzumab and
trastuzumab has provided clinical benefits (9,13).
However, the objective response rate is 24.2% and the complete
response rate is ~8% (9). Therefore,
there is an urgent need to develop novel strategies to overcome
trastuzumab resistance.
It is well established that pertuzumab recognizes
domain II of ErbB2, while trastuzumab binds to domain IV. When
cells are stimulated with the ErbB3 ligand, pertuzumab efficiently
inhibits the formation of the ErbB2-ErbB3 complex (14), whereas the same effect is not
observed with trastuzumab (14).
Notably, in the absence of the ErbB3 ligand, the extent of
ErbB2-ErbB3 complex formation is markedly decreased with
trastuzumab treatment, whereas pertuzumab induces a very minor
decrease in the formation of the ErbB2-ErbB3 complex (15). The combination of trastuzumab and
pertuzumab provides a complementary mechanism of action that
synergistically inhibits the proliferation of ErbB2-overexpressing
breast cancer cell lines, both in vitro and in vivo
(16,17). Trastuzumab predominantly interferes
with ligand-independent ErbB2-ErbB3 complex formation, whereas
pertuzumab inhibits ligand-induced ErbB2 heterodimerization
(14,15). The clinical success of the
combination of pertuzumab and trastuzumab may be partially
explained by the ability to inhibit ErbB2 heterodimerization more
thoroughly.
In previous studies, an ErbB2 domain I-specific
human antibody, H2-18, was developed, which exhibited a more potent
antitumor activity than trastuzumab and pertuzumab, either alone or
in combination, in trastuzumab-resistant breast and gastric cancer
cells (13,18). H2-18 functions by potently inducing
programmed cell death (PCD), a different mechanism of action from
either trastuzumab or pertuzumab (13,18).
Therefore, it was speculated that the two anti-ErbB2 antibodies,
H2-18 and trastuzumab, which have different mechanisms of action,
may also achieve a synergistic effect on the inhibition of
trastuzumab-resistant cancer.
In the present study, the in vivo and in
vitro antitumor capability of H2-18 plus trastuzumab in the
trastuzumab-sensitive gastric cancer cell line, NCI-N87, and
trastuzumab-resistant gastric cancer cell line, NCI-N87-TraRT, was
investigated. Additionally, the antitumor effect of H2-18 plus
trastuzumab was compared with that of pertuzumab plus
trastuzumab.
Materials and methods
Antibodies
The H2-18 antibody was expressed and purified using
a method as previously described (19). The recombinant antibody was purified
by affinity chromatography on Protein A Sepharose (GE Healthcare).
The purified antibodies were analyzed via 10% SDS-PAGE under
non-reducing and reducing conditions, followed by Coomassie
Brilliant Blue staining. Under reducing conditions, the H2-18
antibody yielded two protein bands with a molecular mass of ~50 kDa
(heavy chain) and ~25 kDa (light chain), respectively (Fig. S1). The SDS-PAGE analysis under
non-reducing conditions exhibited a single band at ~150 kDa for the
H2-18 antibody (Fig. S1).
The anti-ErbB2 antibodies trastuzumab and pertuzumab
and the anti-CD20 antibody rituximab were expressed and purified by
a similar method described in previous studies (20–22). The
drug concentrations used in the present experiments and the ratio
of anti-ErbB2 antibodies in antibody combinations were based on
previous experiments (13,23).
Cell lines and mice
NCI-N87 is an ErbB2-amplified human gastric cancer
cell line. BT-474 is an ErbB2-amplified human breast cancer cell
line. All the cell lines were obtained from the American Type
Culture Collection and were routinely cultured in DMEM (Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, and 100 µg/m streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified
incubator with 5% CO2. NCI-N87 cells were treated
consecutively with trastuzumab (10 µg/ml) for 2 years to obtain a
trastuzumab-resistant subline cell line, termed NCI-N87-TraRT. The
cells were authenticated by morphologic and isoenzyme analyses,
various times during the study period. They were routinely checked
for mycoplasma contamination using Hoechst staining, which was
consistently found to be negative. The BALB/c nude mice were
obtained from the Shanghai Experimental Animal Center of the
Chinese Academy of Sciences. The cages with food and water were
changed twice a week, and the mice were fed ad libitum. The
targeted conditions for the animal room environment and photo
period were as follows: Temperature, 20–26°C; humidity, 40–70%;
light/dark cycle, 12/12 h. All the animals were treated according
to the guidelines of the Committee on Animals of the Second
Military Medical University (Shanghai, China). The study was
approved by the Committee on Animals of the Second Military Medical
University.
Detection of ErbB2 expression
BT-474, NCI-N87 and NCI-N87-TraRT cells were treated
with anti-ErbB2 primary antibodies (trastuzumab, 10 µg/ml) or
control anti-CD20 antibodies (rituximab, 10 µg/ml) for 1 h on ice
at 4°C. After washing 3 times, secondary goat anti-human IgG
H&L (FITC) (1:100; cat. no., ab6854; Abcam) were added and
incubated with cells for 40 min at 0°C. After washing 3 times, the
cells were resuspended in PBS, measured using a FACSCalibur flow
cytometer (Becton, Dickinson and Company), then analyzed using
FlowJo v7.6.1 software (FlowJo LLC).
Cell proliferation experiments
The cells were seeded in a 96-well plate at a
density of 4×103 cells/well in a humidified chamber at
37°C with 5% CO2. After attachment, cells were treated
with control IgG (5 µg/ml), trastuzumab (5 µg/ml), pertuzumab (5
µg/ml), H2-18 (5 µg/ml), trastuzumab plus pertuzumab (5 µg/ml for
both), trastuzumab plus H2-18 (5 µg/ml for both) or trastuzumab
plus pertuzumab plus H2-18 (5 µg/ml for each) at 37°C in the
humidified incubator with 5% CO2 for 5 days. The medium
was changed every 2 days. Finally, the cells were incubated with
Cell Counting Kit-8 (CCK-8) reagent for 60–90 min and the cell
proliferation was determined according to the manufacturer's
instructions (Dojindo Molecular Technologies, Inc.).
Analysis of single and combined drug
effects
Cells were seeded in a 96-well plate at a density of
4×103 cells/well. After attachment, the cells were
treated with a range of concentrations of the aforementioned
antibodies for 5 days. The media was refreshed every 2 days.
Finally, the CCK-8 assay was used to assess the cell proliferation
inhibition rate. The cell proliferation inhibition rate was
calculated as follows: [1-(treated cells/untreated cells)] ×100%.
Combination index (CI) values were calculated using the
Chou-Talalay method (24). CI values
<1.0 represented drug synergy, CI values=1.0 represented drug
addition and CI values >1.0 indicated drug antagonism.
Reactive oxygen species (ROS)
detection
The 2′,7′-dichlorofluorescin diacetate (DCFH-DA;
Sigma-Aldrich; Merck KGaA) was used to detect ROS. Cells were
seeded in a flat-bottomed 24-well plate at a density of
1×105 cells/well. After a 4-h incubation at 37°C with
control IgG (5 µg/ml), trastuzumab (5 µg/ml), pertuzumab (5 µg/ml),
H2-18 (5 µg/ml), trastuzumab plus pertuzumab (5 µg/ml for both),
trastuzumab plus H2-18 (5 µg/ml for both) or trastuzumab plus
pertuzumab plus H2-18 (5 µg/ml for each), the cells were incubated
with 10 µM DCFH-DA for 20 min at 37°C. After washing twice with
PBS, the fluorescence level of the cells treated with DCFH-DA was
measured using a FACSCalibur flow cytometer (Becton, Dickinson and
Company) and analyzed with FlowJo v7.6.1 software (FlowJo LLC).
Immunoblotting
Cells were seeded in a 24-well plate at a density of
1×105 cells/well in a humidified atmosphere at 37°C with
5% CO2. After attachment, the cells were incubated with
the aforementioned drugs. Subsequently, cells were lysed in RIPA
buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium
deoxycholate and 0.1% SDS) containing a protease inhibitor cocktail
(cat. no. 11836153001; Roche Diagnostics) and a phosphatase
inhibitor cocktail (cat. no. P5726; Sigma-Aldrich; Merck KGaA).
Protein concentrations were quantified using a BCA Protein Assay
kit (cat. no. 23225; Pierce: Thermo Fisher Scientific, Inc.). Equal
amounts of protein (15 µg/lane) were separated via 10% SDS-PAGE and
transferred to PVDF membranes. After blocking with 5% BSA (cat. no.
A1933; Sigma-Aldrich; Merck KGaA) for 1 h at 37°C, the membranes
were immunoblotted with antibodies against HER2 (cat. no. 4290s),
phosphorylated (p)-HER2 (cat. no. 2243s), HER3 (cat. no. 12708s),
p-HER3 (cat. no. 2842s), AKT (cat. no. 9272s), p-AKT (cat. no.
2965s), ERK1/2 (cat. no. 9102s), p-ERK1/2 (cat. no. 9106s), JNK
(cat. no. 9252s), p-JNK (cat. no. 9251s), c-jun (cat. no. 9165s),
p-c-jun (cat. no. 2361s), and GAPDH (1:2,000; cat. no. cat.
no.5174S) (all from Cell Signaling Technology, Inc.) overnight at
4°C. After washing with PBS-Tween 20 (0.05%), the membranes were
immunoblotted with anti-rabbit IgG HRP-conjugated secondary
antibody (1:3,000; cat. no. 7074s; Cell Signaling Technology, Inc.)
at room temperature for 1 h. Finally, the ECL reagents (Plus-ECL;
PerkinElmer, Inc.) or chemiluminescence reagents (EMD Millipore)
were used to detect the proteins. Each band was quantified using
TanonImage software (v1.0; Tanon Science and Technology Co.,
Ltd.).
Cell death assay
Cells were seeded at a density of 1×105
cells/well in a flat-bottomed 24-well plate in the DMEM
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.), 100
U/ml penicillin, and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified incubator with 5%
CO2. After 24 h of attachment at 37°C, cells were
treated with control IgG (5 µg/ml), trastuzumab (5 µg/ml),
pertuzumab (5 µg/ml), H2-18 (5 µg/ml), trastuzumab (5 µg/ml) plus
pertuzumab (5 µg/ml), trastuzumab (5 µg/ml) plus H2-18 (5 µg/ml)
and trastuzumab (5 µg/ml) plus pertuzumab (5 µg/ml) plus H2-18 (5
µg/ml). After 24 h of treatment at 37°C, the cells were harvested
and incubated with 5 µl 488-conjugated annexin-V and 0.1 µl PI
(both from Tianjin Sungene Biotech Co., Ltd) per well for 15 min at
room temperature. Finally, the cells were measured using a
FACSCalibur flow cytometer (Becton, Dickinson and Company) and
analyzed using FlowJo 7.6.1 software (FlowJo LLC).
Colony formation assay
Cells were seeded at a density of 800 cells/well in
a 6-well plate. After 24 h of attachment at 37°C, the cells were
treated with control IgG (10 µg/ml), trastuzumab (10 µg/ml),
pertuzumab (10 µg/ml), H2-18 (10 µg/ml), trastuzumab (10 µg/ml)
plus pertuzumab (10 µg/ml), trastuzumab (10 µg/ml) plus H2-18 (10
µg/ml), and trastuzumab (10 µg/ml) plus pertuzumab (10 µg/ml) plus
H2-18 (10 µg/ml) for 5 days at 37°C. The medium was changed every
3–4 days. After 5 days of treatment, the medium was replaced with
fresh RPMI 1640 medium and cultured for another 12 days at 37°C.
Finally, the colonies were fixed with 4% paraformaldehyde at room
temperature for 15 min and then stained with 0.1% crystal violet at
room temperature for ~10 min. The number of stained colonies
containing >50 cells were recorded under a light microscope
(×40).
In vivo animal experiments
NCI-N87 or NCI-N87-TraRT cells were resuspended in
PBS and implanted into the right mammary fat pad of 78 6-week-old
female BALB/c nude mice (1×107 cells/mouse; 78 mice in
total; weight, 17–20 g; in the NCI-N87 model 7 mice per cohort, 6
cohorts in total; in the NCI-N87-TraRT model 6 mice per cohort, 6
cohorts in total). When the average volume of tumors reached 100
mm3, the mice were randomly grouped into cohorts. The
mice were injected intravenously with control IgG (10 mg/kg),
trastuzumab (10 mg/kg), H2-18 (10 mg/kg), trastuzumab (10 mg/kg)
plus pertuzumab (10 mg/kg), trastuzumab (10 mg/kg) plus H2-18 (10
mg/kg) and trastuzumab (10 mg/kg) plus pertuzumab (10 mg/kg) plus
H2-18 (10 mg/kg), twice a week for 3 weeks. Tumors were measured
with digital calipers twice a week and the volumes were calculated
using the following formula: Volume (mm3)=length ×
(width)2/2. The duration of the experiment was 39 days.
The following humane endpoints were used: Loss of significant body
mass (emaciated); obvious body weight loss >20% of initial body
weight; animals could not get to adequate food or water; cachexia
appearance was found; and tumor volume reached ≥2,000
mm3. All the 78 nude mice were euthanized at the end of
the experiment or when they had reached these humane endpoints. The
mice were sacrificed by CO2 inhalation (with a flow rate
of 30%/min).
Statistical analysis
SPSS v19.0 software (SPSS, Inc.) was used for the
analysis. Normality tests were conducted before data analysis.
One-way ANOVA with Tukey's multiple comparison post-hoc test was
used to identify significant differences among multiple groups,
unless otherwise indicated. The in vivo animal experiments
were analyzed using Kruskal-Wallis test with Dunn's post-hoc test.
The data are presented as the mean ± SD (n=3). P<0.05 was
considered to indicate a statistically significant difference.
Results
Addition of H2-18 to trastuzumab
enhances its inhibitory effect on cell proliferation of
ErbB2-overexpressing cancer cell lines
The trastuzumab-resistant cancer cell line,
NCI-N87-TraRT, was derived from the trastuzumab-sensitive cancer
cell line, NCI-N87 (13). It has
been reported that NCI-N87 and BT-474 are high-ErbB2-expressing
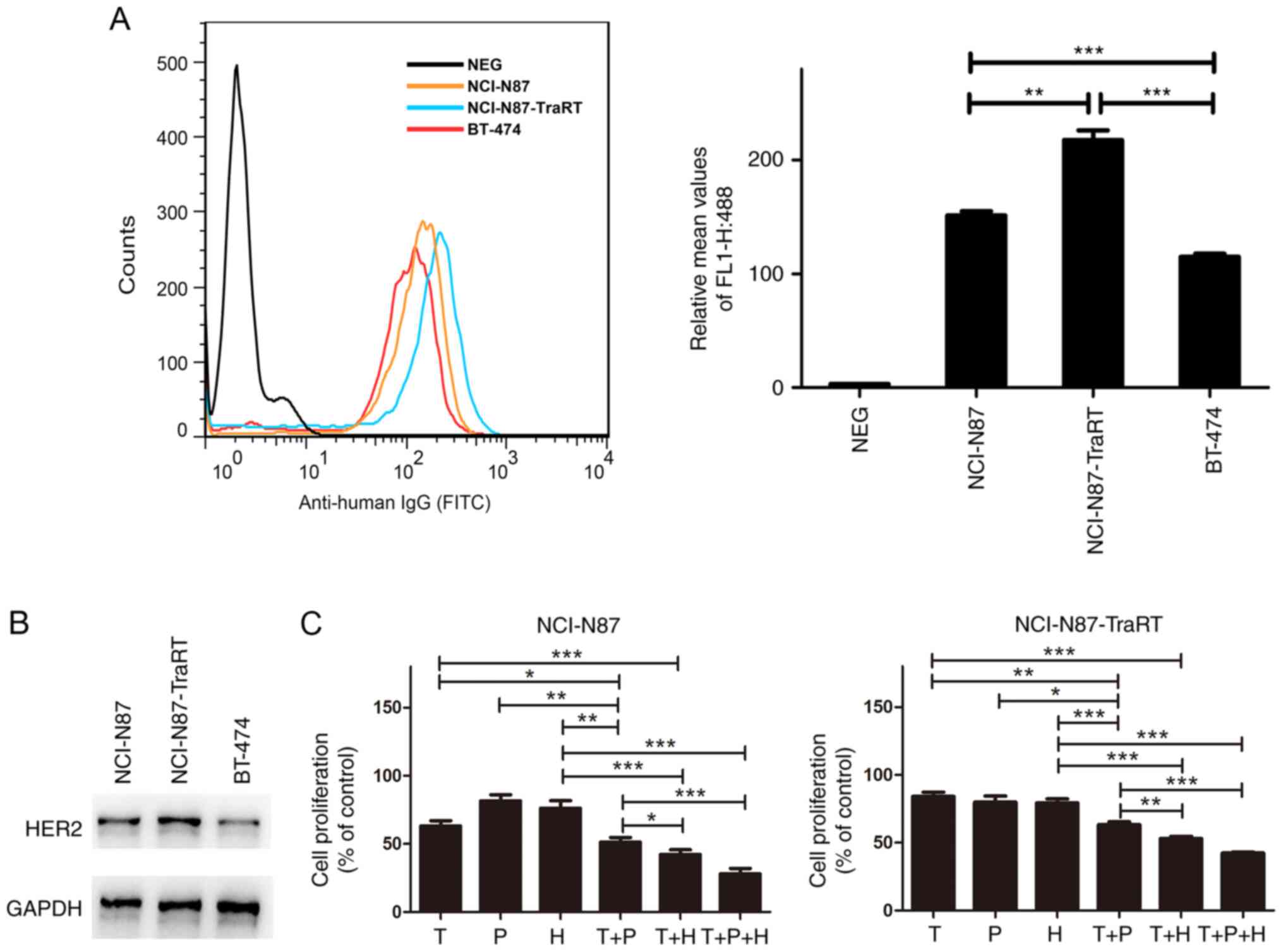
cell lines (25,26). Flow cytometry was used to examine
ErbB2 expression in the cancer cell lines, NCI-N87, NCI-N87-TraRT
and BT-474. The results revealed that ErbB2 expression in
NCI-N87-TraRT cells was significantly higher compared with that in
the NCI-N87 cells (Fig. 1A).
Compared with that in the ErbB2-amplified BT-474 cells, NCI-N87 and
NCI-N87-TraRT cells exhibited higher levels of ErbB2 receptor
(Fig. 1A). The protein expression
levels of ErbB2 in these cells were also confirmed using western
blotting (Fig. 1B).
Subsequently, CCK-8 assays were used to determine
the cell proliferation inhibitory effect of trastuzumab,
pertuzumab, H2-18, trastuzumab plus pertuzumab, trastuzumab plus
H2-18 and the combination of H2-18, trastuzumab and pertuzumab on
NCI-N87 and NCI-N87-TraRT cells. In both ErbB2-overexpressing
cancer cell lines, NCI-N87 and NCI-N87-TraRT, trastuzumab plus
pertuzumab exhibited a significantly more potent inhibitory effect
than trastuzumab, pertuzumab or H2-18 alone (Fig. 1C). Notably, trastuzumab plus H2-18
inhibited cell proliferation more effectively than trastuzumab plus
pertuzumab (Fig. 1C). Moreover, the
combination of H2-18, trastuzumab and pertuzumab exhibited the
maximal inhibitory effect among all of the monoclonal antibody
(mAb) combinations tested (Fig.
1C).
H2-18 and trastuzumab synergistically
inhibits the cell proliferation of both trastuzumab-sensitive and
trastuzumab-resistant cancer cells
The ErbB2-overexpressing trastuzumab-sensitive
cancer cell line, NCI-N87, and the trastuzumab-resistant cancer
cell line, NCI-N87-TraRT, were treated with various concentrations
of H2-18 alone, trastuzumab alone or trastuzumab plus H2-18. The
results revealed that H2-18 inhibited cell proliferation in a
dose-dependent manner in both cell lines (Fig. 2A and B). The method described by Chou
and Talalay was used to analyze the data. Synergy was defined as a
CI value (at ED90, ED75 or ED50) <1.0, antagonism as a CI value
>1.0 and additivity as a CI value of 1.0. The results
demonstrated that in both cell lines, H2-18 and trastuzumab
inhibited the in vitro cell proliferation synergistically
(Fig. 2A and B). In addition,
NCI-N87 and NCI-N87-TraRT cells were treated with various
concentrations of H2-18 alone, trastuzumab plus pertuzumab and the
combination of H2-18, trastuzumab and pertuzumab. Similar results
were observed (Fig. 2A and B), with
the combination of H2-18 with trastuzumab plus pertuzumab
synergistically inhibiting the in vitro proliferation of
NCI-N87 and NCI-N87-TraRT cells (Fig. 2A
and B).
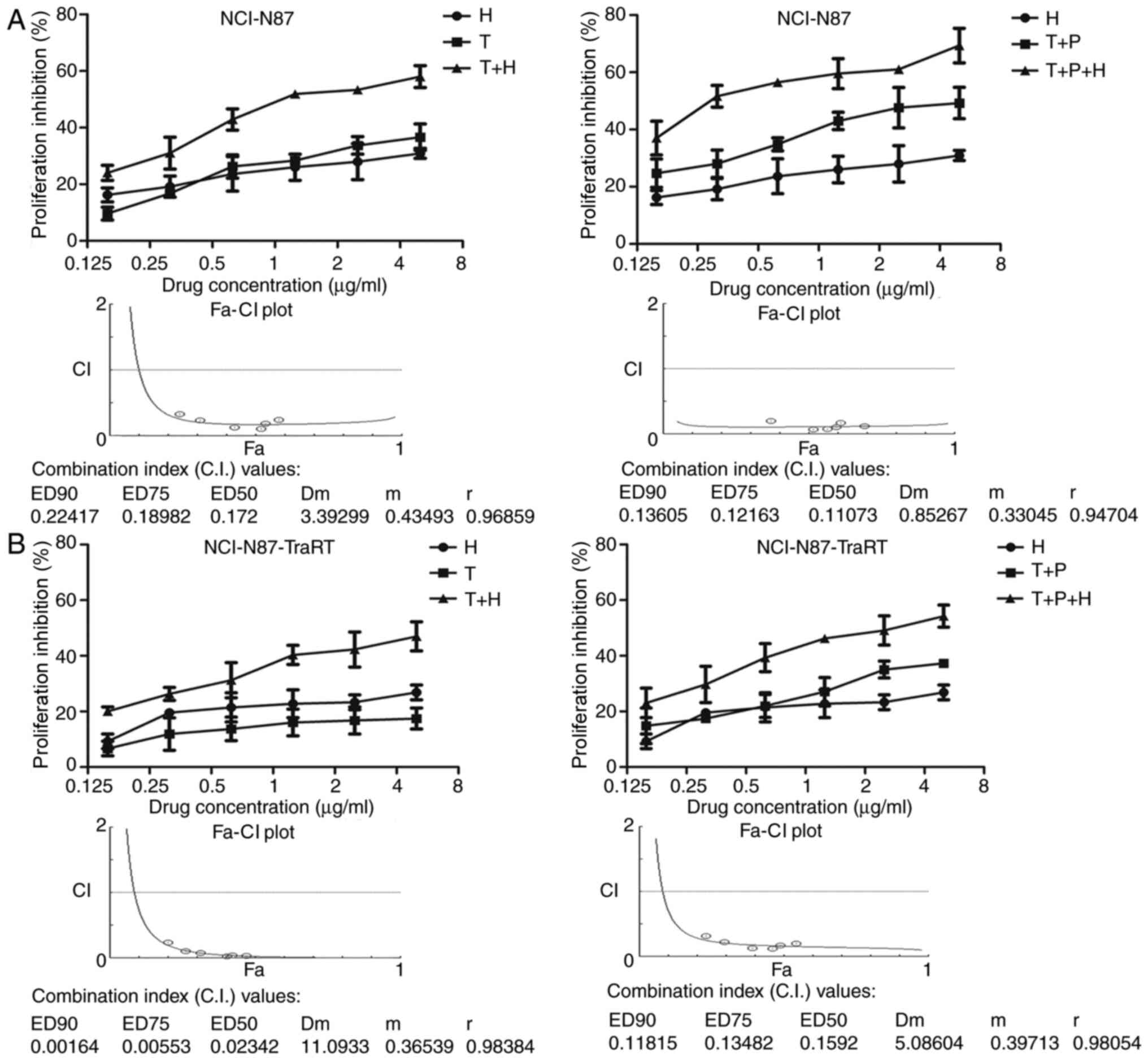 | Figure 2.H2-18 and trastuzumab synergistically
inhibit the proliferation of both trastuzumab-sensitive and
trastuzumab-resistant cancer cells. Cell Counting Kit-8 assays were
used to compare the proliferation of (A) NCI-N87 cells and (B)
NCI-N87-TraRT cells upon the indicated treatments. CI values were
calculated using the Chou-Talalay method. Drug synergy, addition
and antagonism were defined as CI values <1.0, =1.0 or >1.0,
respectively. The experiments were performed at least three times.
CI, combination index; T, trastuzumab; P, pertuzumab; H, H2-18; ED,
effective dose; Fa, given effect; Dm, dose of drugs that exhibit a
50% inhibition; m, slope; r, regression coefficient. |
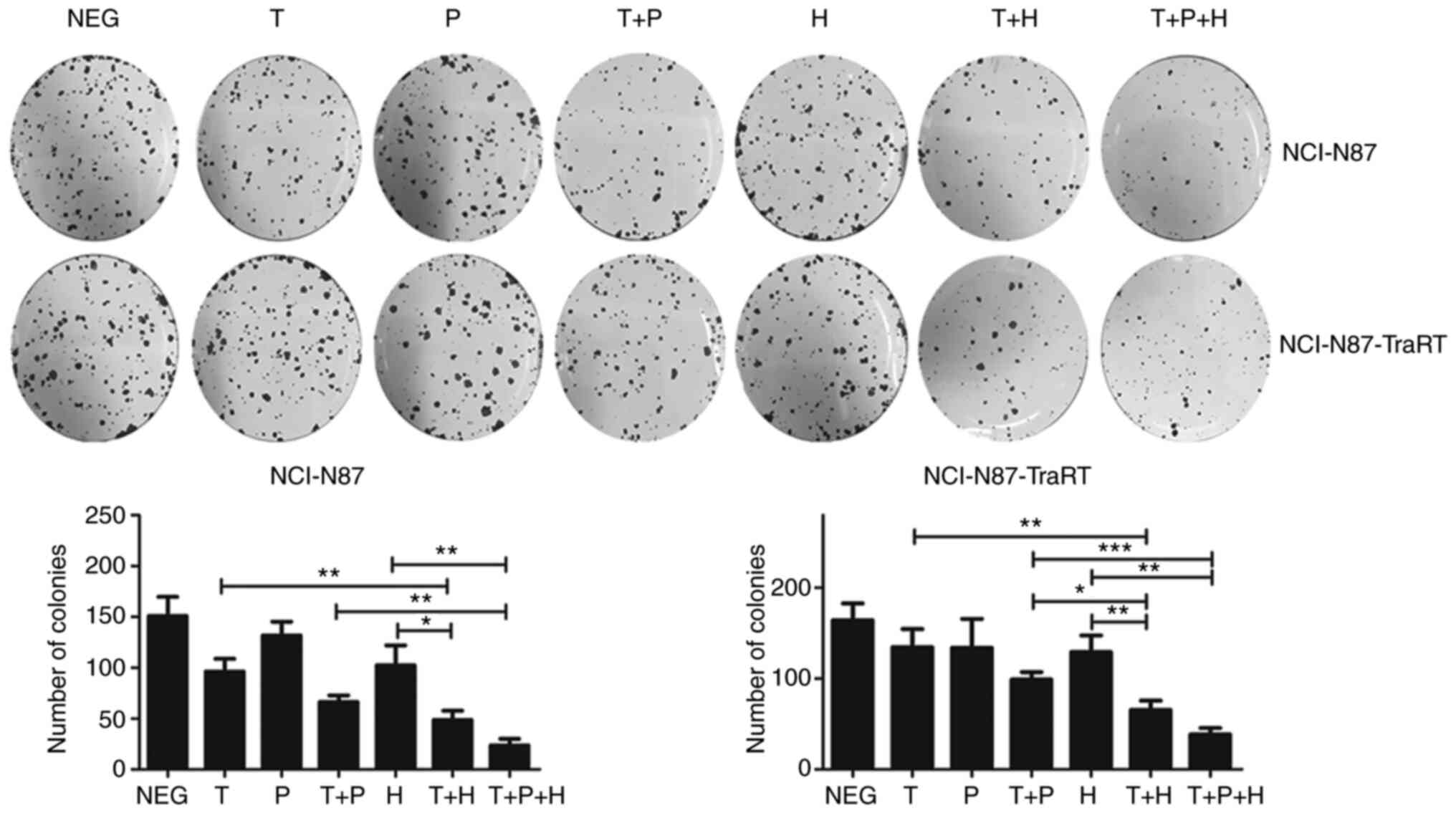
Trastuzumab plus H2-18 exhibits a more
potent inhibitory effect on colony formation compared with
trastuzumab plus pertuzumab in NCI-N87-TraRT cells
In the trastuzumab-sensitive NCI-N87 cell line,
trastuzumab plus H2-18 exhibited a similar inhibitory effect on the
colony forming ability as that of trastuzumab plus pertuzumab;
however, in trastuzumab-resistant NCI-N87-TraRT cells, trastuzumab
plus H2-18 significantly decreased the formation of colonies more
effectively than trastuzumab plus pertuzumab (Fig. 3). Notably, the combination of H2-18,
trastuzumab and pertuzumab inhibited colony formation more potently
than trastuzumab plus pertuzumab (Fig.
3).
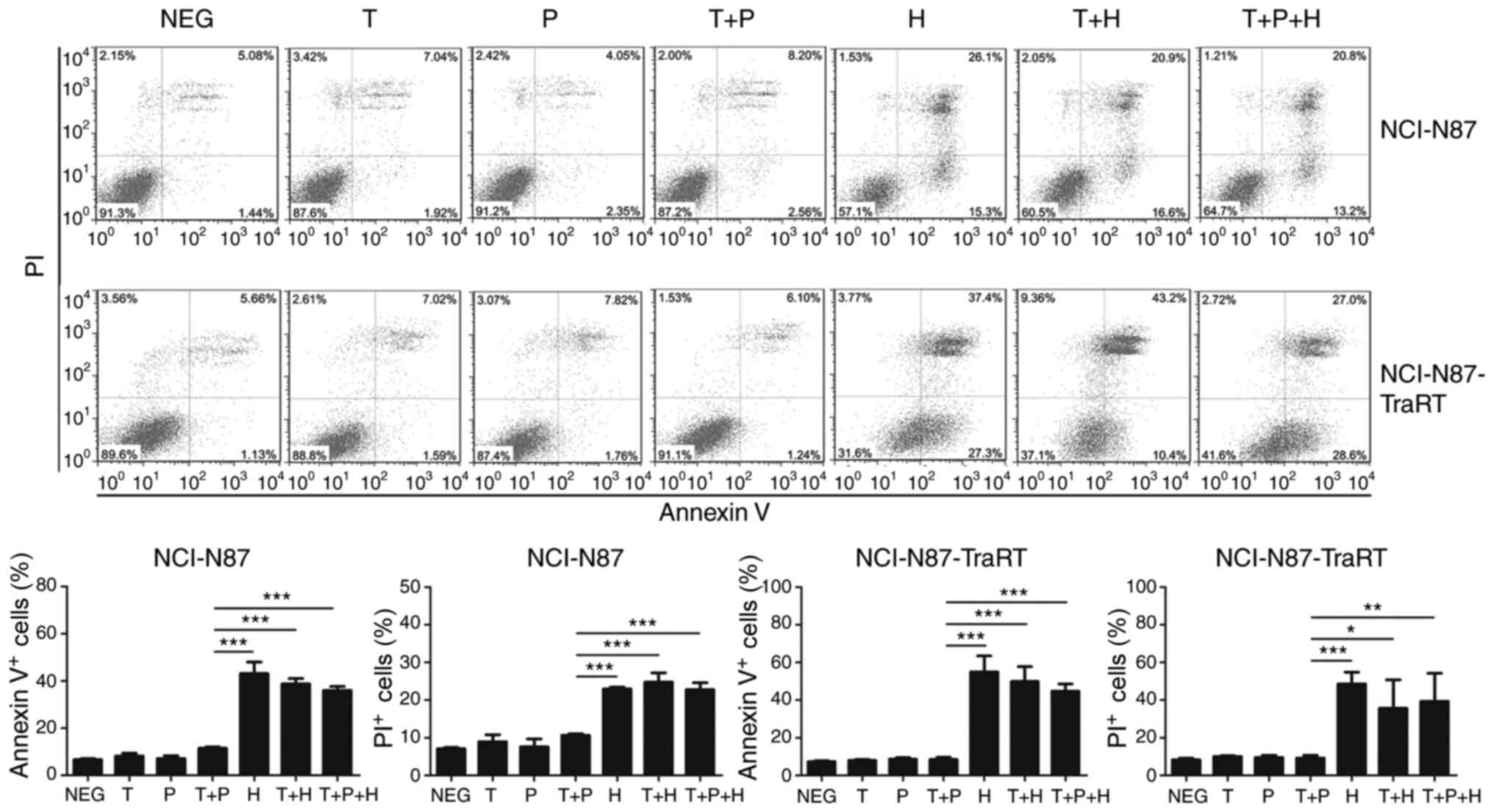
Trastuzumab plus H2-18 exhibits a
greater PCD inducing ability than trastuzumab plus pertuzumab
In both NCI-N87-TraRT and NCI-N87 cell lines,
trastuzumab, pertuzumab and trastuzumab plus pertuzumab did not
effectively induce cell death, whereas H2-18, trastuzumab plus
H2-18 and the combination of H2-18, trastuzumab and pertuzumab
significantly increased cell death (Fig.
4). However, trastuzumab plus H2-18 and the combination of
H2-18, trastuzumab and pertuzumab did not induce more annexin
V+ and PI+ cells than H2-18 alone (Fig. 4).
Trastuzumab plus H2-18 inhibits in
vivo tumor growth more potently than trastuzumab plus
pertuzumab
In the NCI-N87 tumor xenografts, trastuzumab plus
H2-18 exhibited a more potent inhibitory effect on tumor growth
compared with that in the mice treated with trastuzumab or H2-18
alone, and the combination of H2-18, trastuzumab and pertuzumab
further augmented this inhibitory effect (Fig. 5). In the NCI-N87-TraRT tumor
xenografts, trastuzumab alone did not effectively inhibit tumor
growth, whereas H2-18 alone was similar to trastuzumab plus
pertuzumab in terms of the inhibitory effect on tumor growth
(Fig. 5). Trastuzumab plus H2-18
exhibited a more potent ability to inhibit tumor growth than
trastuzumab plus pertuzumab (Fig.
5). Trastuzumab plus H2-18 and pertuzumab exhibited a more
potent inhibitory effect on tumor growth compared with that in the
mice treated with H2-18 and trastuzumab plus H2-18 (Fig. 5).
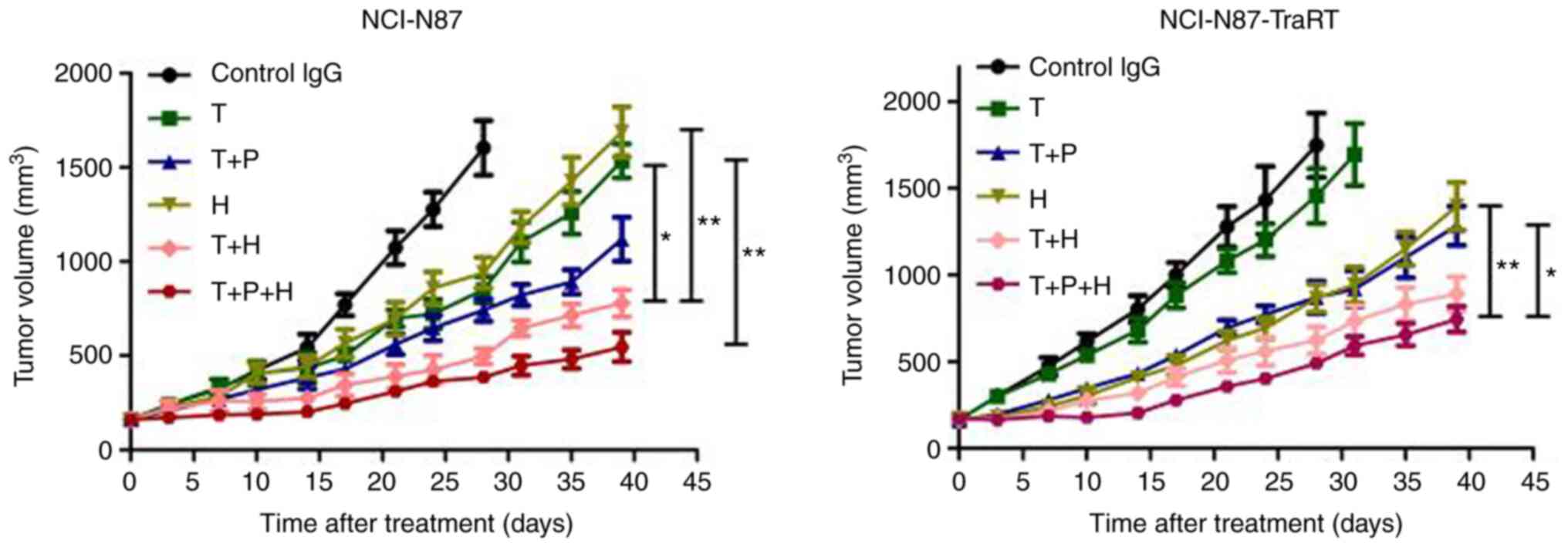 | Figure 5.Trastuzumab plus H2-18 inhibits in
vivo tumor growth more potently than trastuzumab plus
pertuzumab. Tumor volume of NCI-N87 and NCI-N87-TraRT gastric tumor
xenografts after treatment with control IgG (10 mg/kg, twice a
week), trastuzumab (10 mg/kg, twice a week), trastuzumab (10 mg/kg,
twice a week) plus pertuzumab (10 mg/kg, twice a week), H2-18 (10
mg/kg, twice a week), trastuzumab (10 mg/kg, twice a week) plus
H2-18 (10 mg/kg, twice a week) and trastuzumab (10 mg/kg, twice a
week) plus pertuzumab (10 mg/kg, twice a week) plus H2-18 (10
mg/kg, twice a week). The data are presented as the mean ± SEM.
*P<0.05 and **P<0.01 analyzed by Kruskal-Wallis test. T,
trastuzumab; P, pertuzumab; H, H2-18. |
Trastuzumab plus H2-18 significantly
induces ROS production compared with trastuzumab plus
pertuzumab
In both the NCI-N87-TraRT and NCI-N87 cell lines,
H2-18, trastuzumab plus H2-18 and the combination of H2-18,
trastuzumab and pertuzumab significantly induced ROS production
compared with that in the cells treated with trastuzumab and
pertuzumab alone and with trastuzumab plus pertuzumab (Fig. 6).
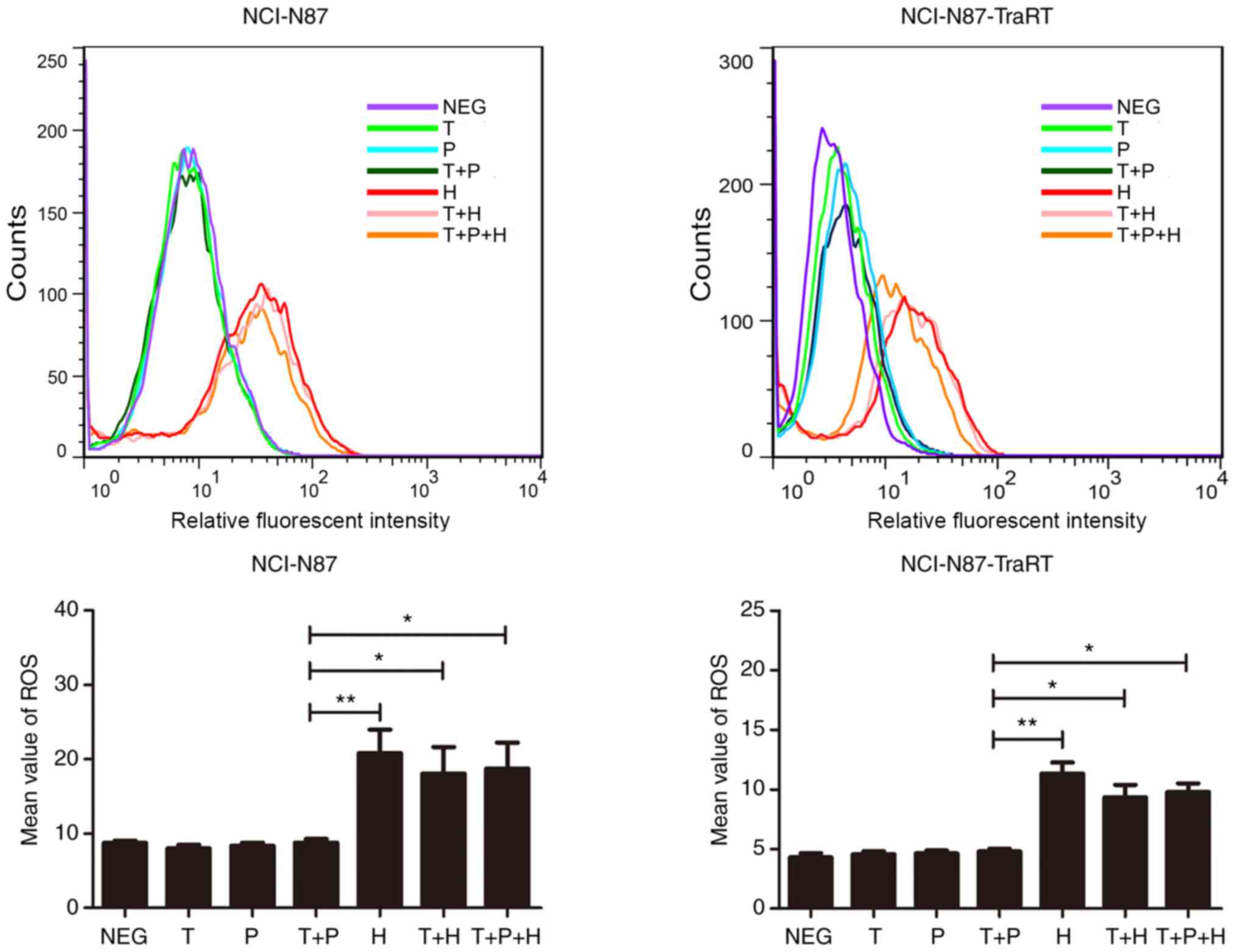 | Figure 6.Trastuzumab plus H2-18 activates ROS
production in both NCI-N87 and NCI-N87-TraRT cell lines.
2′,7′-dichlorofluorescin diacetate was detected by flow cytometry
to measure the levels of ROS production in NCI-N87 and
NCI-N87-TraRT cells treated with trastuzumab, pertuzumab,
trastuzumab plus pertuzumab, H2-18, trastuzumab plus H2-18 and
trastuzumab plus pertuzumab plus H2-18 for 4 h. The data are
presented as the mean ± SD (n=3). *P<0.05; **P<0.01. NEG,
negative control; T, trastuzumab; P, pertuzumab; H, H2-18; ROS,
reactive oxygen species. |
Trastuzumab plus H2-18 activates the
ROS-JNK-c-jun signaling pathway in both NCI-N87 and NCI-N87-TraRT
cell lines
As reported in a previous study, H2-18 induces
programmed cell death through activating the RIP1-ROS-JNK-c-jun
signaling pathway (18). Compared
with trastuzumab plus pertuzumab, trastuzumab plus H2-18
effectively activated the phosphorylation of both JNK and c-jun in
NCI-N87 cells and NCI-N87-TraRT cells (Fig. 7). The combination of H2-18,
trastuzumab and pertuzumab exhibited a similar effect on the
upregulation of p-JNK and p-c-jun to that of trastuzumab plus H2-18
(Fig. 7).
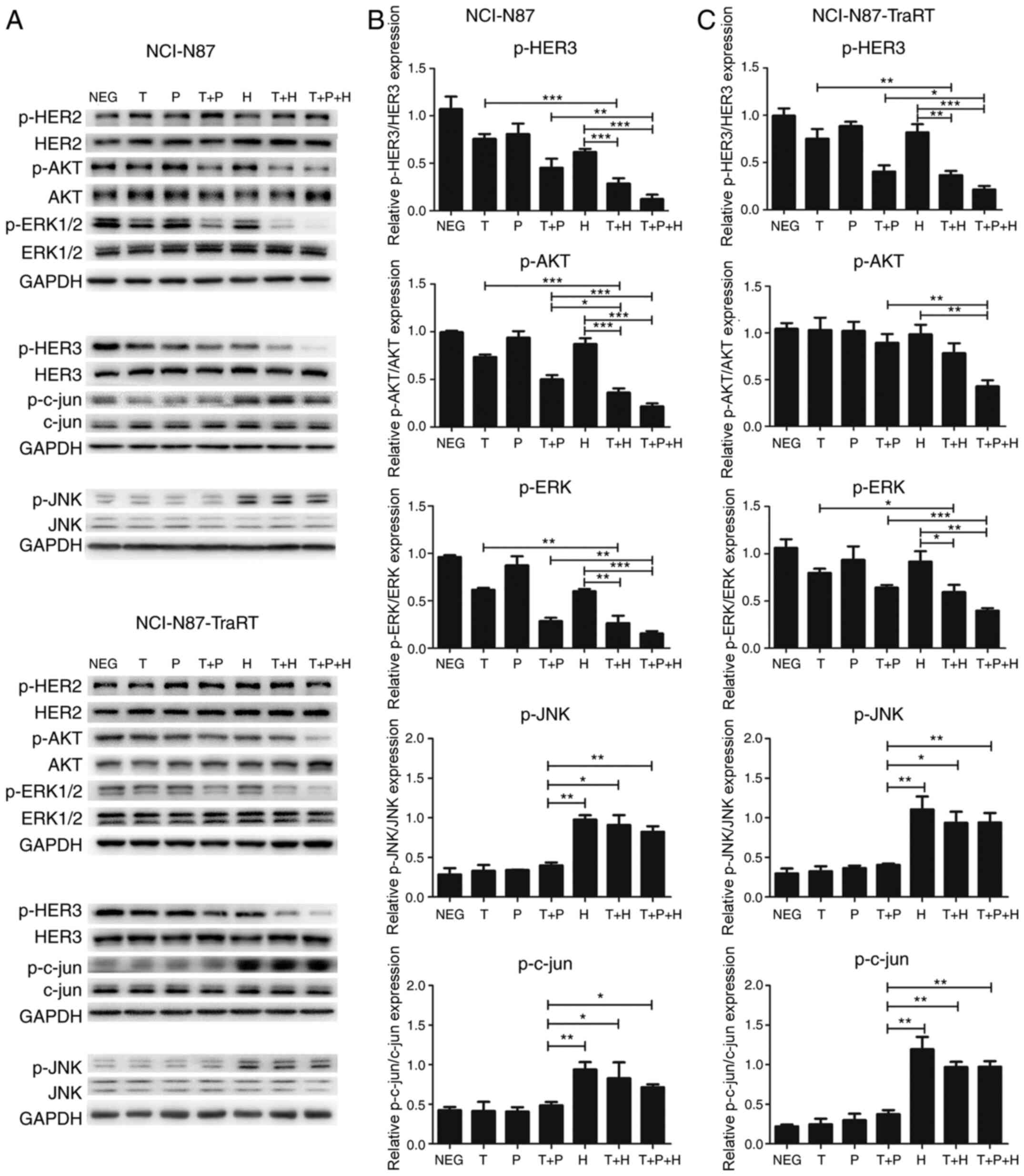 | Figure 7.Trastuzumab plus H2-18 activates the
ROS-JNK-c-jun signaling pathway and inhibits ErbB2 signaling
pathways in both NCI-N87 and NCI-N87-TraRT cell lines. (A)
Immunoblots examining the phosphorylation of HER2, HER3, AKT,
ERK1/2, JNK and c-jun in NCI-N87 and NCI-N87-TraRT cells treated
with trastuzumab, pertuzumab, trastuzumab plus pertuzumab, H2-18,
trastuzumab plus H2-18 and trastuzumab plus pertuzumab plus H2-18
for 4 h. Quantification of the phosphorylation of HER3, AKT, ERK,
JNK and c-jun in (B) NCI-N87 and (C) NCI-N87-TraRT cells following
the indicated drug treatments. The data are presented as the mean ±
SD. *P<0.05; **P<0.01; ***P<0.001. NEG, negative control;
T, trastuzumab; P, pertuzumab; H, H2-18; p-, phosphorylated. |
Trastuzumab plus H2-18 inhibits ErbB2
signaling pathways in both NCI-N87 and NCI-N87-TraRT cell
lines
Western blotting was used to determine the status of
ErbB2 signaling pathways in the NCI-N87 and NCI-N87-TraRT cells
treated with the various mAb combinations. The results revealed
that in NCI-N87 cells, all of the mAb combinations exhibited a more
potent ability to inhibit the expression levels of p-HER3, p-AKT
and p-ERK compared with any of the mAbs alone (Fig. 7A and B). Trastuzumab plus H2-18
inhibited p-AKT expression more effectively than trastuzumab plus
pertuzumab, whereas trastuzumab plus H2-18 and trastuzumab plus
pertuzumab exhibited a similar effect on the inhibition of p-HER3
and p-ERK expression (Fig. 7A and
B). In the NCI-N87-TraRT cells, the ability of trastuzumab plus
H2-18 to inhibit p-HER3, p-AKT and p-ERK expression was similar to
that of trastuzumab plus pertuzumab (Fig. 7A and C). The combination of H2-18,
trastuzumab and pertuzumab exhibited a significantly more potent
effect on the inhibition of p-HER3, p-AKT and p-ERK expression
compared with that in cells treated with trastuzumab plus
pertuzumab in both the NCI-N87 and NCI-N87-TraRT cell lines
(Fig. 7A-C). In addition, treatment
with all the combinations of the mAbs or alone had no significant
effect on the levels of HER2 and pHER2 (Fig. 7).
Discussion
The limited antitumor effect of trastuzumab, as well
as the common occurrence of trastuzumab resistance, has driven
numerous studies to develop new trastuzumab-based strategies
(27,28). Previous studies have demonstrated
that trastuzumab only partially inhibits ErbB2-dimer formation;
ErbB2 heterodimerization may still initiate signaling events that
confer resistance when ErbB2 is inhibited by trastuzumab (29,30). The
combination of the two anti-ErbB2 antibodies, pertuzumab and
trastuzumab, which have a complementary mechanism of action, has
been reported to overcome trastuzumab resistance (9). However, the response rate of
trastuzumab plus pertuzumab is currently unsatisfactory. The final
results of a phase 3 study, JACOB, revealed that the addition of
pertuzumab to trastuzumab for chemotherapy did not significantly
improve OS in patients with HER2+ metastatic gastric or
gastro-oesophageal junction cancer (31,32).
Therefore, further studies are required to investigate novel agents
for the treatment of gastric cancer.
A trastuzumab-resistant NCI-N87-TraRT cell line
derived from trastuzumab-sensitive NCI-N87 cells has been developed
after two years of trastuzumab treatment (13). In the present study, the growth
inhibition rate of NCI-N87-TraRT cells was very low, <20% when
treated with trastuzumab, while the growth inhibition rate of the
parental NCI-N87 cell line was ~40%.
An ErbB2 domain I-specific antibody, H2-18, has been
previously described (18). It is
well-known that trastuzumab and pertuzumab function by blocking
ErbB2 dimerization and inhibiting the activation of the main
downstream signaling pathways of ErbB2: PI3K/AKT and MAPK/ERK
signaling pathways (19). However,
neither trastuzumab nor pertuzumab significantly induced cell death
in the present study. In contrast to trastuzumab and pertuzumab,
H2-18 has a unique ability to overcome trastuzumab resistance both
in vitro and in vivo (18). The main mechanism of action
underlying the ability of H2-18 to overcome trastuzumab resistance
is to induce programmed cell death through the activation of the
RIP1-ROS-JNK-c-Jun signaling pathway (18). In the present study, it was found
that H2-18 plus trastuzumab was more effective than pertuzumab plus
trastuzumab in inhibiting cell proliferation in both the
trastuzumab-sensitive NCI-N87 cells and trastuzumab-resistant
NCI-N87-TraRT cells. Moreover, H2-18 and trastuzumab exhibited a
synergistic antitumor effect and a more potent ability to inhibit
the growth of NCI-N87-TraRT tumors than pertuzumab plus
trastuzumab. Further experiments demonstrated that trastuzumab plus
H2-18 decreased the formation of colonies more effectively than
trastuzumab plus pertuzumab in trastuzumab-resistant NCI-N87-TraRT
cells. Notably, H2-18 plus trastuzumab exhibited a more potent
ability to induce cell death than pertuzumab plus trastuzumab. The
expression levels of p-HER2, p-HER3, p-AKT and p-ERK in the
NCI-N87-TraRT cells treated with trastuzumab plus H2-18 were
similar to those in NCI-N87-TraT cells treated with trastuzumab
plus pertuzumab. However, in contrast to trastuzumab plus
pertuzumab, trastuzumab plus H2-18 effectively activated the
phosphorylation of both JNK and c-jun in NCI-N87 cells and
NCI-N87-TraRT cells. Thus, the superior antitumor efficacy of H2-18
plus trastuzumab over pertuzumab plus trastuzumab may be
attributable to its enhanced ability to inhibit colony formation
and to induce cell death.
In addition to H2-18 plus trastuzumab, the present
study investigated the antitumor effects of the combination of
H2-18, pertuzumab and trastuzumab. It is known that trastuzumab,
pertuzumab and H2-18 recognize different epitopes of the ErbB2
molecule. Trastuzumab recognizes domain IV of ErbB2, while
pertuzumab binds with domain II and H2-18 targets domain I. These
anti-ErbB2 antibodies have different mechanisms of action on the
molecule (18). The present results
revealed that H2-18 plus pertuzumab plus trastuzumab provided the
most potent antitumor effect compared with all of the other mAbs
alone, as well as all the other combinations of mAbs, in both N87
and NCI-N87-TraRT cell lines. Therefore, the present study
suggested that combination therapy of these various anti-ErbB2
antibodies may provide a novel strategy for the treatment of
ErbB2-amplified cancer. However, there were several limitations in
the present study, including the lack of safety and toxicity
studies, the lack of further mechanistic studies and the lack of
use of inhibitors of the JNK and c-jun signaling pathways. Thus,
further studies are required in the future.
In summary, the present study investigated the
antitumor effect of the combination of H2-18 and trastuzumab, as
well as their associated mechanisms of action. The present data
indicated that H2-18 plus trastuzumab exhibited a superior
antitumor effect over pertuzumab plus trastuzumab in
trastuzumab-resistant cancer cells. The strong antitumor efficacy
of H2-18 plus trastuzumab may be attributable to a number of
factors, including a more effective inhibition of colony formation
and more potent induction of cell death. The present data suggested
that H2-18 plus trastuzumab may have the potential to be an
effective strategy to circumvent trastuzumab resistance in
ErbB2-overexpressing cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81572996, 81830052,
81573004 and 81773275), the Construction Project of Shanghai Key
Laboratory of Molecular Imaging (grant no. 18DZ2260400), the
Shanghai Municipal Education Commission Class II Plateau
Disciplinary Construction Program of Medical Technology of Shanghai
University of Medicine and Health Sciences, (2018–2020), the
Shanghai Health and Family Planning Commission Foundation Youth
Project (grant no. 20164Y0272), the Medical Science and Technology
Project of Zhejiang Province (grant no. 2020391513), the Top-level
Clinical Discipline Project of Shanghai Pudong (grant no.
PWYgf2018-04) and the Pudong New District Health and Family
Planning Commission Youth Science and Technology Project (grant no.
PW2016B-4).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW, LW and YM performed the experiments. CW, LW, BL,
BZ, YS, YM, JD, LC and BL analyzed the data. LW and BL wrote the
manuscript. The authenticity of data was confirmed by CW, LW and
BL. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All the animals were treated according to the
guidelines of the Committee on Animals of the Second Military
Medical University (Shanghai, China). The study was approved by the
Committee on Animals of the Second Military Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A and
Press MF: Studies of the HER-2/neu proto-oncogene in human breast
and ovarian cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiel A and Ristimaki A: Targeted therapy
in gastric cancer. APMIS. 123:365–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D: Gastric cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
27:v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vogel CL, Cobleigh MA, Tripathy D, Gutheil
JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF,
Burchmore M, et al: Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing metastatic
breast cancer. J Clin Oncol. 20:719–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nahta R and Esteva FJ: HER2 therapy:
Molecular mechanisms of trastuzumab resistance. Breast Cancer Res.
8:2152006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nahta R, Yu D, Hung MC, Hortobagyi GN and
Esteva FJ: Mechanisms of disease: Understanding resistance to
HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol.
3:269–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baselga J, Gelmon KA, Verma S, Wardley A,
Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA, et al:
Phase II trial of pertuzumab and trastuzumab in patients with human
epidermal growth factor receptor 2-positive metastatic breast
cancer that progressed during prior trastuzumab therapy. J Clin
Oncol. 28:1138–1144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cortes J, Fumoleau P, Bianchi GV, Petrella
TM, Gelmon K, Pivot X, Verma S, Albanell J, Conte P, Lluch A, et
al: Pertuzumab monotherapy after trastuzumab-based treatment and
subsequent reintroduction of trastuzumab: Activity and tolerability
in patients with advanced human epidermal growth factor receptor
2-positive breast cancer. J Clin Oncol. 30:1594–1600. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blackwell KL, Burstein HJ, Storniolo AM,
Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff
J, et al: Randomized study of Lapatinib alone or in combination
with trastuzumab in women with ErbB2-positive,
trastuzumab-refractory metastatic breast cancer. J Clin Oncol.
28:1124–1130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
von Minckwitz G, du Bois A, Schmidt M,
Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann
M, Bauer W, et al: Trastuzumab beyond progression in human
epidermal growth factor receptor 2-positive advanced breast cancer:
A german breast group 26/breast international group 03–05 study. J
Clin Oncol. 27:1999–2006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Wang L, Yu X, Zhang Y, Meng Y,
Wang H, Yang Y, Gao J, Wei H, Zhao J, et al: Combating acquired
resistance to trastuzumab by an anti-ErbB2 fully human antibody.
Oncotarget. 8:42742–42751. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agus DB, Akita RW, Fox WD, Lewis GD,
Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K,
et al: Targeting ligand-activated ErbB2 signaling inhibits breast
and prostate tumor growth. Cancer Cell. 2:127–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Junttila TT, Akita RW, Parsons K, Fields
C, Lewis Phillips GD, Friedman LS, Sampath D and Sliwkowski MX:
Ligand-independent HER2/HER3/PI3K complex is disrupted by
trastuzumab and is effectively inhibited by the PI3K inhibitor
GDC-0941. Cancer Cell. 15:429–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nahta R, Hung MC and Esteva FJ: The
HER-2-targeting antibodies trastuzumab and pertuzumab
synergistically inhibit the survival of breast cancer cells. Cancer
Res. 64:2343–2346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scheuer W, Friess T, Burtscher H,
Bossenmaier B, Endl J and Hasmann M: Strongly enhanced antitumor
activity of trastuzumab and pertuzumab combination treatment on
HER2-positive human xenograft tumor models. Cancer Res.
69:9330–9336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu Q, Wang L, Zhang Y, Yu X, Wang C, Wang
H, Yang Y, Chong X, Xia T, Meng Y, et al: An anti-ErbB2 fully human
antibody circumvents trastuzumab resistance. Oncotarget.
7:67129–76141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B, Meng Y, Zheng L, Zhang X, Tong Q,
Tan W, Hu S, Li H, Chen Y, Song J, et al: Bispecific antibody to
ErbB2 overcomes trastuzumab resistance through comprehensive
blockade of ErbB2 heterodimerization. Cancer Res. 73:6471–6483.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adams CW, Allison DE, Flagella K, Presta
L, Clarke J, Dybdal N, McKeever K and Sliwkowski MX: Humanization
of a recombinant monoclonal antibody to produce a therapeutic HER
dimerization inhibitor, pertuzumab. Cancer Immunol Immunother.
55:717–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li B, Shi S, Qian W, Zhao L, Zhang D, Hou
S, Zheng L, Dai J, Zhao J, Wang H and Guo Y: Development of novel
tetravalent anti-CD20 antibodies with potent antitumor activity.
Cancer Res. 68:2400–2408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li B, Zhao L, Guo H, Wang C, Zhang X, Wu
L, Chen L, Tong Q, Qian W, Wang H and Guo Y: Characterization of a
rituximab variant with potent antitumor activity against
rituximab-resistant B-cell lymphoma. Blood. 114:5007–5015. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Chen J, Weng Z, Li Q, Zhao L, Yu
N, Deng L, Xu W, Yang Y, Zhu Z, et al: A new anti-HER2 antibody
that enhances the anti-tumor efficacy of trastuzumab and pertuzumab
with a distinct mechanism of action. Mol Immunol. 119:48–58. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoriko YK, Sei S, Naoki H and Kaori F:
Enhanced antitumor activity of trastuzumab emtansine (T-DM1) in
combination with pertuzumab in a HER2-positive gastric cancer
model. Oncol Rep. 30:1087–1093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stanley A, Ashrafi GH, Seddon AM and
Modjtahedi H: Synergistic effects of various Her inhibitors in
combination with IGF-1R, C-MET and Src targeting agents in breast
cancer cell lines. Sci Rep. 7:39642017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sampera A, Sánchez-Martín FJ, Arpí O, Visa
L, Iglesias M, Menéndez S, Gaye É, Dalmases A, Clavé S,
Gelabert-Baldrich M, et al: HER-family ligands promote acquired
resistance to trastuzumab in gastric cancer. Mol Cancer Ther.
18:2135–2145. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Derakhshani A, Rezaei Z, Safarpour H,
Sabri M, Mir A, Sanati MA, Vahidian F, Gholamiyan Moghadam A,
Aghadoukht A, Hajiasgharzadeh K and Baradaran B: Overcoming
trastuzumab resistance in HER2-positive breast cancer using
combination therapy. J Cell Physiol. 235:3142–3156. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ritter CA, Perez-Torres M, Rinehart C,
Guix M, Dugger T, Engelman JA and Arteaga CL: Human breast cancer
cells selected for resistance to trastuzumab in vivo overexpress
epidermal growth factor receptor and ErbB ligands and remain
dependent on the ErbB receptor network. Clin Cancer Res.
13:4909–4919. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumar R: ErbB-dependent signaling as a
determinant of trastuzumab resistance. Clin Cancer Res.
13:4657–4659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shitara K, Hara H, Yoshikawa T, Fujitani
K, Nishina T, Hosokawa A, Asakawa T, Kawakami S and Muro K:
Pertuzumab plus trastuzumab and chemotherapy for Japanese patients
with HER2-positive metastatic gastric or gastroesophageal junction
cancer: A subgroup analysis of the JACOB trial. Int J Clin Oncol.
25:301–311. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tabernero J, Hoff PM, Shen L, Ohtsu A,
Shah MA, Cheng K, Song C, Wu H, Eng-Wong J, Kim K and Kang YK:
Pertuzumab plus trastuzumab and chemotherapy for HER2-positive
metastatic gastric or gastro-oesophageal junction cancer (JACOB):
Final analysis of a double-blind, randomised, placebo-controlled
phase 3 study. Lancet Oncol. 19:1372–1384. 2018. View Article : Google Scholar : PubMed/NCBI
|