Introduction
Circular RNAs (circRNAs) are non-coding RNAs with
covalently closed loop structures formed by reverse splicing
(1). Unlike linear RNAs, circRNAs
have no exposed end and are not easily degraded by conventional
intracellular degradation mechanisms. They are thus relatively
stable in cells. Due to the limitations of technology and the low
abundance of circRNAs in cells, circRNAs have not been well
described (2). In the recent years,
thanks to improvements in technology and algorithms, circRNAs have
been implicated in numerous human diseases. For example, circSLC8A1
is downregulated in bladder cancer tissues and cell lines and its
expression is associated with the pathological and histological
stages of bladder cancer. In breast cancer, circTADA2As is a
promising prognostic biomarker for patients with large
triple-negative breast cancer (TNBC). Furthermore, the high
expression of circRanGAP1 is closely related to the advanced TNM
staging, lymph node metastasis and poor survival rate of patients
with gastric cancer (3–5). Several studies have demonstrated that
ncRNAs are differentially expressed in tumour and normal tissues
and cells, including pediatric acute lymphoblastic leukemia and
thyroid carcinoma (6,7). Some circRNAs have been reported to
promote or inhibit cancer cell proliferation, migration and
invasion. For example, Li et al (8) reported that circ-ITCH is expressed at
lower levels in oesophageal carcinoma tissues compared with
adjacent tissues, and that circ-ITCH can inhibit cell proliferation
and tumorigenic ability by repressing the Wnt pathway. CircRNAs can
bind to intracellular regulatory proteins, thereby affecting
cellular metabolic activity. A study by Du et al (9) reported that circ-FOXO3 can interact
with important regulators of cell cycle progression, including
CDK6, p16, and p27, in order to regulate cell cycle progression of
cancer cells.
Due to the expanded use of diagnostic imaging and
surveillance, the prevalence of TC has risen worldwide (10). In 2013, 33,939 patients with TC
(7,146 men and 26,793 women) were identified and the death toll was
4,974 (2,292 men and 2,682 women) in China (11). A steady increase has been observed in
TC incidence worldwide, whereas the death rate has only slightly
increased (12). According to
previous studies, a number of genes and signaling pathways that
play an important role in TC have been identified. Most patients
with TC exhibit overactivated MAPK signalling, which plays an
important role in the regulation of cell proliferation (13). The regulation of cellular metabolic
pathways by circRNA is increasingly documented (14). For example, the circRNA circZFR was
reported to promote the expression of C8orf4 in papillary TC (PTC)
cells as a competitive endogenous RNA (ceRNA) of the microRNA (miR)
miR-1261, thus regulating the carcinogenic effect of the
miR-1261/C8orf4 axis (15).
A previous study by Hsiao et al (16) reported a novel carcinogenic function
of circCCDC66 in colon cancer. The results demonstrated that
circCCDC66 is highly upregulated in colorectal cancer and serves as
a new cancer-related circRNA. Furthermore, the study showed that
88% of patients with colon cancer have high circCCDC66 expression,
and that patients with higher circCCDC66 expression have a lower
overall survival rate compared with patients with lower circCCDC66
expression. The dysregulation of circCCDC66 might therefore have a
prognostic potential in human cancers. This study also reported
that the suppression of circCCDC66 in cancer cells can inhibit
tumour invasiveness in xenograft mouse models. In addition,
circCCDC66 knockout results in decreased miRNA-targeted oncogene
expression in circCCDC66-mediated sponge processes. Furthermore,
the overexpression of circCCDC66 can promote cancer cell
proliferation and colony formation, suggesting therefore that
circCCDC66 might be considered as a critical driver of cancer
aetiology. The overexpression of circCCDC66 can stimulate oncogenes
by inhibiting miRNAs that are involved in the suppression of these
oncogenes. circCCDC66 acts as a miRNA sponge, affecting the
expression of miR-211, leading to the upregulation of SRCIN1 and
promoting cell proliferation in non-small cell lung cancer
(17). In osteosarcoma, circCCDC66
promotes the malignant phenotype of osteosarcoma by stimulating
miR-338-3p to upregulate the expression of PTP1B (18). The present study aimed to investigate
the effects of circCCDC66 on the proliferation, migratory and
invasive abilities and glycolysis of TC cells.
Materials and methods
Human tissue samples
Samples of TC tissues and matched adjacent normal
tissues were collected from nine patients with TC (age range, 32–69
years; mean age, 43.7±6.9 years; sex ratio, four men and 18 women)
who underwent surgery at Qingdao Municipal Hospital. All subjects
were recruited between April 2019 and December 2019. This study was
approved by the Ethics Committee of Qingdao Municipal Hospital
(approval no. 2019QMH126) and all patients provided informed
consent.
Cell culture and transfection
The cell lines Nthy-ori 3-1, CAL62 and TPC1 were
purchased from the American Type Culture Collection (ATCC). All
cells were cultured in DMEM (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.) and were places at 37°C in a humidified incubator
containing 5% CO2.
Cell transfection was carried out using
Lipofectamine® 2000 Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturers' instruction when
cells in culture reached 60–80% confluence. Small interfering
(si)RNAs targeting circCCDC66 (si-circCCDC66#1,
5′-CAAUUAGAGCAUCAGGAAA-3′; si-circCCDC66#2,
5′-GAGCAUCAGGAAACAGUAC-3′) and negative control (si-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′) were purchased from Shanghai
GenePharma Co., Ltd. miR-211-5p mimic and negative control were
purchased from Guangzhou RiboBio Co., Ltd (cat. no.
miR10000268-1-5). The transfection concentrations of
oligonucleotides were as follows: si-NC, 40 nM; si-circCCDC66, 40
nM; miR-211-5p mimic, 50 nM; and miRNA control, 50 nM.
Lipofectamine® 2000 Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and si-RNAs or miR-mimic were diluted into
serum-free DMEM medium, mixed together and incubated for 20 min at
room temperature. This solution was subsequently was added to CAL62
and TPC1 cells and transfected for 4–6 h at 37°C in a humidified
incubator containing 5% CO2.
Cell fractionation
The PARIS™ kit (cat. no. AM1921; Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract cytoplasmic and
nuclear RNA. Briefly, CAL62 and TPC1 cells were incubated with the
lysis solution from the kit on ice for 10 min following two washes
with PBS. Samples were collected, centrifuged at 500 × g for 30 min
at 4°C, and cytoplasmic RNA was extracted from the supernatant,
whereas nuclear RNA was extracted from the nuclear pellet. Samples
were stored at −80°C until further use. GAPDH and U6 were used as
the cytoplasmic RNA and nuclear RNA controls, respectively.
Online analysis of downstream target
of circCCDC66
CircRNAs are known to exert their functions through
sponging miRNAs and subsequently regulating downstream genes
expression in cells (19,20). RegRNA2.0 (http://regrna2.mbc.nctu.edu.tw/index.html) was
therefore used to predict the potential miRNA associated with
circCCDC66. RegRNA2.0 is an integrated web server used to identify
functional RNA motifs and sites. miRcode (http://www.mircode.org/mircode/) was subsequently used
to predict the miRNA target. MiRcode provides the ‘whole
transcriptome’ human miRNA target predictions based on the
comprehensive GENCODE gene annotation.
Dual-luciferase reporter assay
To generate luciferase reporter expression
constructs, full-length circCCDC66 or the 3′-UTR of PDK4 containing
the expected binding sites was inserted into luciferase reporter
DNA pGL3 vector (Promega Corporation). circCCDC66 mutant DNA or
PDK4 3′-UTR mutant DNA was cloned into luciferase reporter DNA pGL3
vector. Plasmids were transfected with miRNAs in 293T cells (ATCC)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h. The measurement of luciferase activity
was carried out with a Dual-Luciferase Reporter Assay System. The
relative activity was normalized to Renilla luciferase
activity.
Reverse transcription quantitative
(RT-q) PCR
Total RNA was extracted from tissues or cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), which
was then reverse transcribed into cDNA. The cDNA was generated
using the Moloney Murine Leukemia Virus (M-MLV) first strand kit
(Thermo Fisher Scientific, Inc.) or miRNA reverse transcription kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol. RT-qPCR reactions were conducted using a SYBR Green PCR
Kit (Takara Biotechnology Co., Ltd). The thermocycling conditions
are as follows: 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec and 60°C for 20 sec. All primers were purchased from
Guangzhou RiboBio Co., Ltd. The relative expression levels were
normalized to endogenous control and were expressed as
2−ΔΔCq (21). GAPDH and
U6 were used as endogenous control. The sequences of the primers
used were as follows: circCCDC66, forward
5′-TCTCTTGGACCCAGCTCAG-3′, reverse 5′-TGAATCAAAGTGCATTGCCC-3′;
miR-211-5p, forward 5′-GATGCTGTAATGGATGATATGA-3′; reverse,
5′-ATTGGAACGATACAGAGAAGATT-3′; PDK4, forward
5′-GGAAGCATTGATCCTAACTGTGA-3′, reverse,
5′-GGTGAGAAGGAACATACACGATG-3′; GAPDH, forward
5′-GGAGCGAGATCCCTCCAAAAT-3′, reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′; and U6, forward
5′-GCTCGCTTCGGCAGCACA-3′ and reverse,
5′-AGGTATTCGCACCAGAGGA-3′.
Cell proliferation assay
CAL62 and TPC1 cells (2×103 cells/well)
were seeded in a 96-well plate and five wells were used for
technical replicates. Cell proliferation was assessed with a CCK-8
kit (Dojindo Molecular Technologies, Inc.) according to the
manufacturers' protocol. The absorbance was measured at 450 nm on a
microplate reader.
Cell migration and invasion
assays
CAL62 and TPC1 cells (5×104 cells/well)
were seeded in the upper chamber of a Transwell plate (Corning,
Inc.) containing DMEM supplemented with 1% FBS, which was coated
with (invasion assay) or without (migration assay) Matrigel mix (BD
Biosciences). Medium containing 15% FBS was added to the lower
chamber. After incubation for 48 h, methanol was used to fix the
cells for 10 min at room temperature and cells were subsequently
stained with DAPI for 10 min at room temperature. A fluorescent
microscope (magnification, ×200) was used to count the number of
cells in each treatment group (CAL62 cells transfected with
si-circCCDC66, CAL62 cells transfected with si-NC, TPC1 cells
transfected with si-circCCDC66 and TPC1 cells transfected with
si-NC) and six areas were randomly selected to calculate the
average value.
Detection of glucose consumption
The metabolism of malignant tumors is explained by
the Warburg effect, which is the metabolic shift from oxidative
phosphorylation (OXPHOS) to glycolysis in tumor cells (22). The effect of glycolysis on tumor
growth is crucial. As previously described, glucose consumption was
analysed. CAL62 and TPC1 cells (5×104 cells/well) were
seeded into 24-well plates for 48 h. The cell supernatants were
collected after 48 h of transfection. A glucose assay kit (cat. no.
K606-100; BioVision, Inc.) was used to determine glucose levels in
the supernatant according to the manufacturers' instructions.
Statistical analysis
Data from at least three independent experiments
were analysed using SPSS 10.0 (SPSS, Inc.) and were presented as
the means ± standard deviation. Normality of data was evaluated
using Shapiro-Wilk normality test. Homoscedasticity of data was
evaluated using F test. The differences between two groups were
compared using Student's t-test. The differences among more than
two groups were compared using one-way ANOVA followed by the Least
Significance Difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
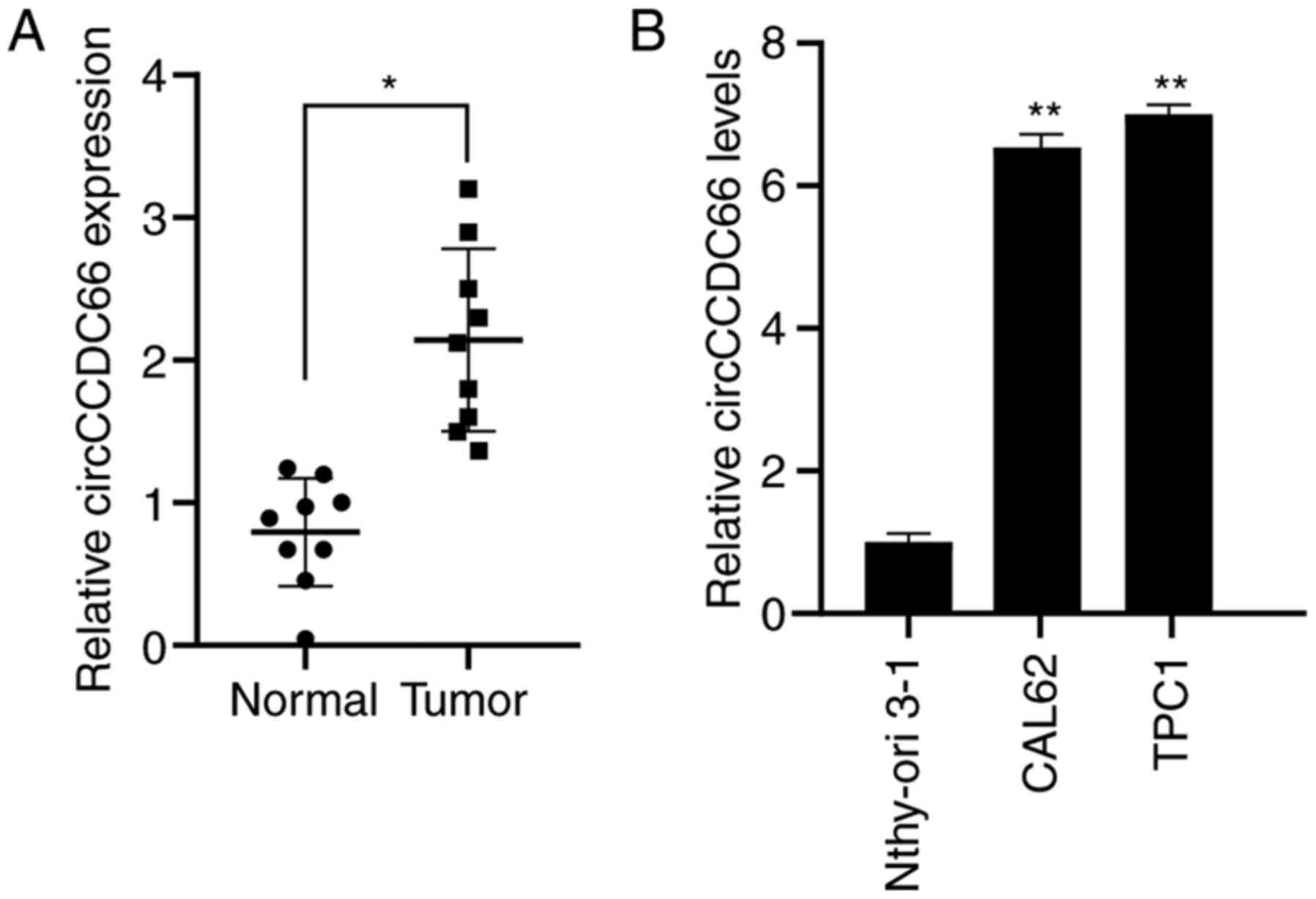
circCCDC66 is upregulated in TC
tissues
The results from RT-qPCR demonstrated that
circCCDC66 was significantly overexpressed in tumour tissues
compared with normal tissues (Fig.
1A). In addition, circCCDC66 expression levels were assessed in
the TC cell lines TPC1 and CAL62 and the normal thyroid cell line
Nthy-ori 3-1. The results showed that circCCDC66 was significantly
overexpressed in TC cells compared with normal thyroid cells
(Fig. 1B).
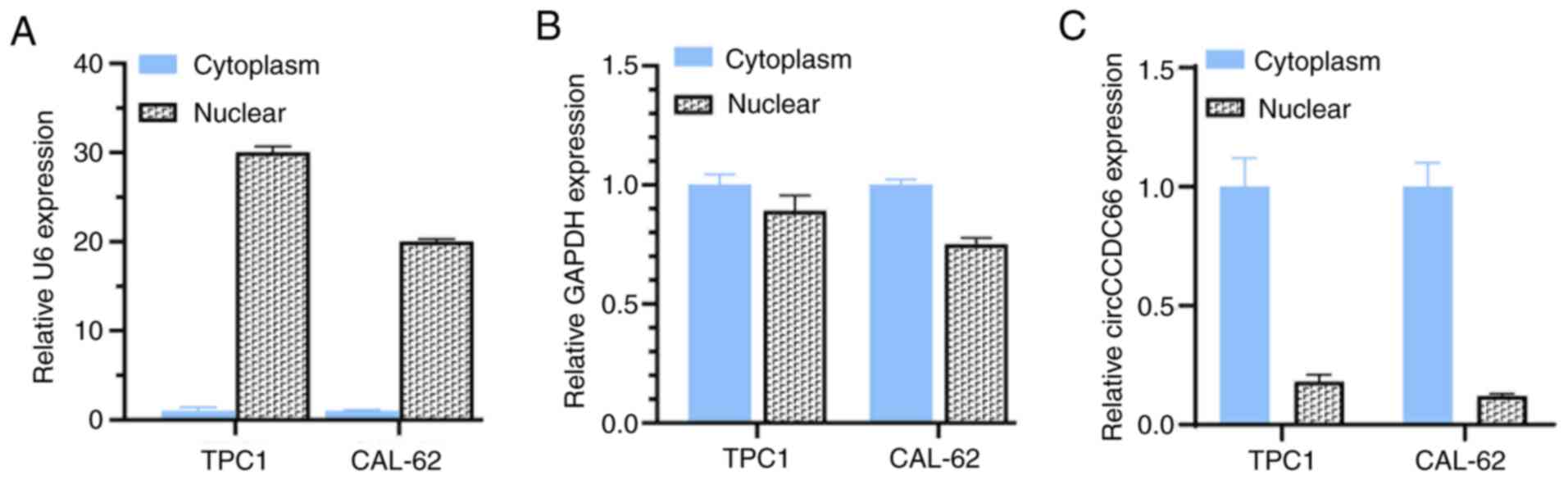
circCCDC66 knockdown inhibits TC cell
proliferation
To investigate the biological function of circCCDC66
in TC cells, its subcellular localization was assessed in TPC1 and
CAL62 cells. U6 in the nucleus and GAPDH in the cytoplasm were
detected as nuclear and cytoplasmic markers (Fig. 2A and B). The results from RT-qPCR
demonstrated that circCCDC66 was mainly located in the cytoplasm,
similarly to GAPDH (Fig. 2C). TC
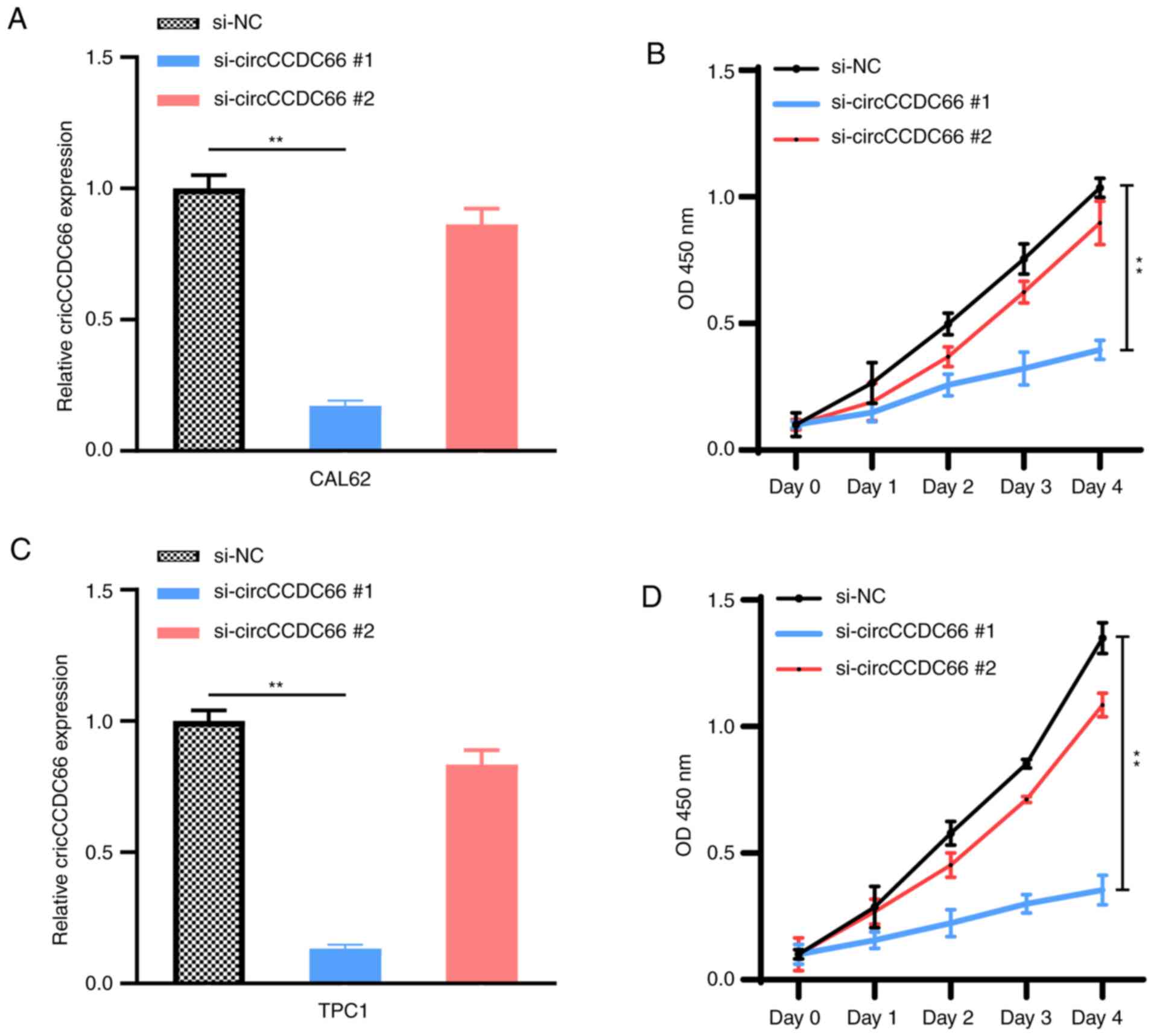
cells (TPC1 and CAL62) were then transfected with si-circCCDC66#1
and si-circCCDC66#2 in order to knockdown circCCDC66 (Fig. 3A and C). The effect of circCCDC66
knockdown on TC cell proliferation was evaluated with a CCK-8
assay. Compared with control siRNA-transfected cells, the
proliferation of TPC1 and CAL62 cells transfected with
si-circCCDC66#1 was significantly decreased (Fig. 3B and D), suggesting that circCCDC66
knockdown could inhibit TC cell proliferation. Since the efficiency
of knockdown and proliferation ability of si-circCCDC66#2 was not
satisfactory, only si-circCCDC66#1 was used for subsequent
experiments (represented as si-circCCDC66).
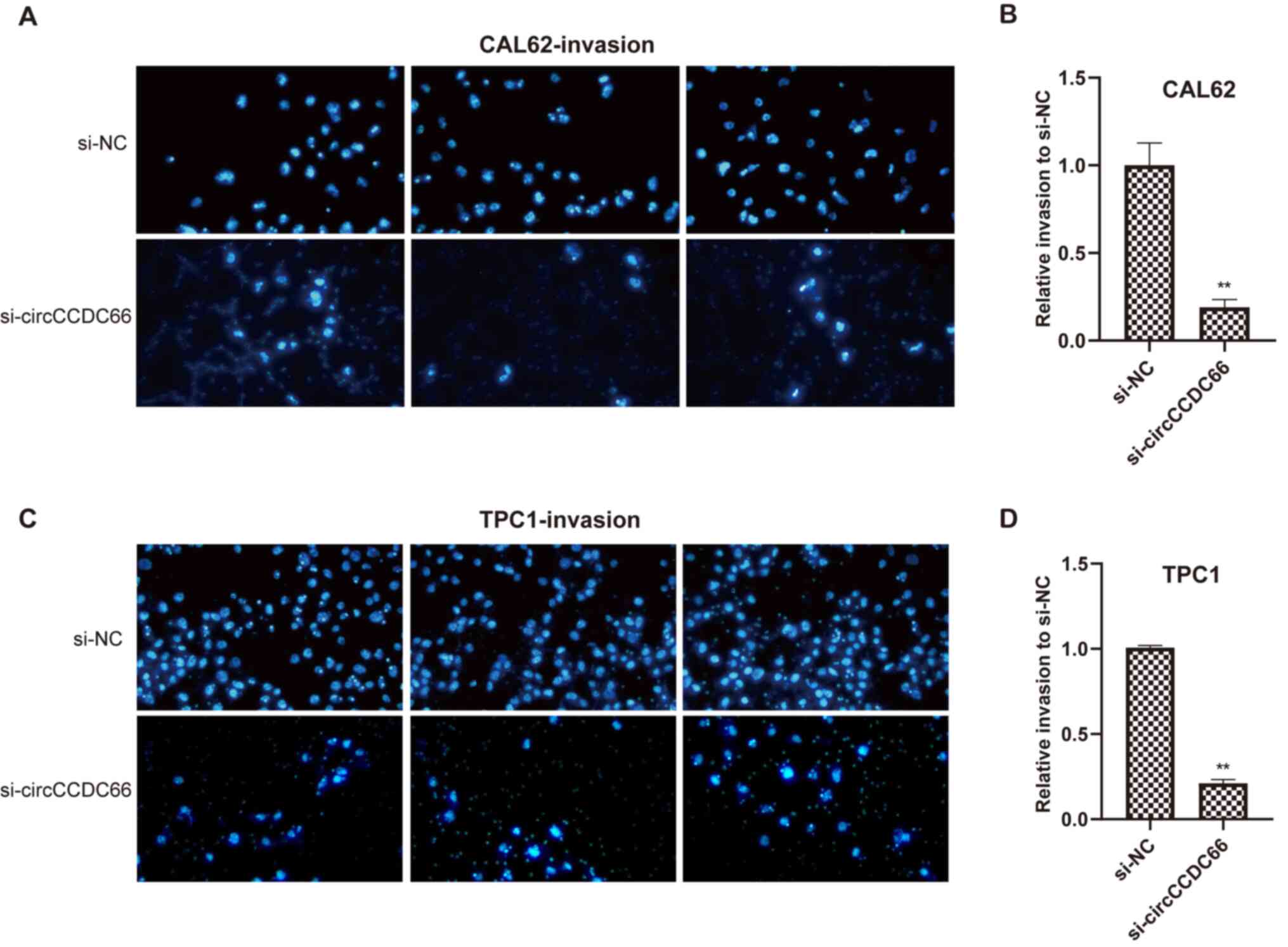
circCCDC66 silencing inhibits TC cell
migratory and invasive abilities in vitro
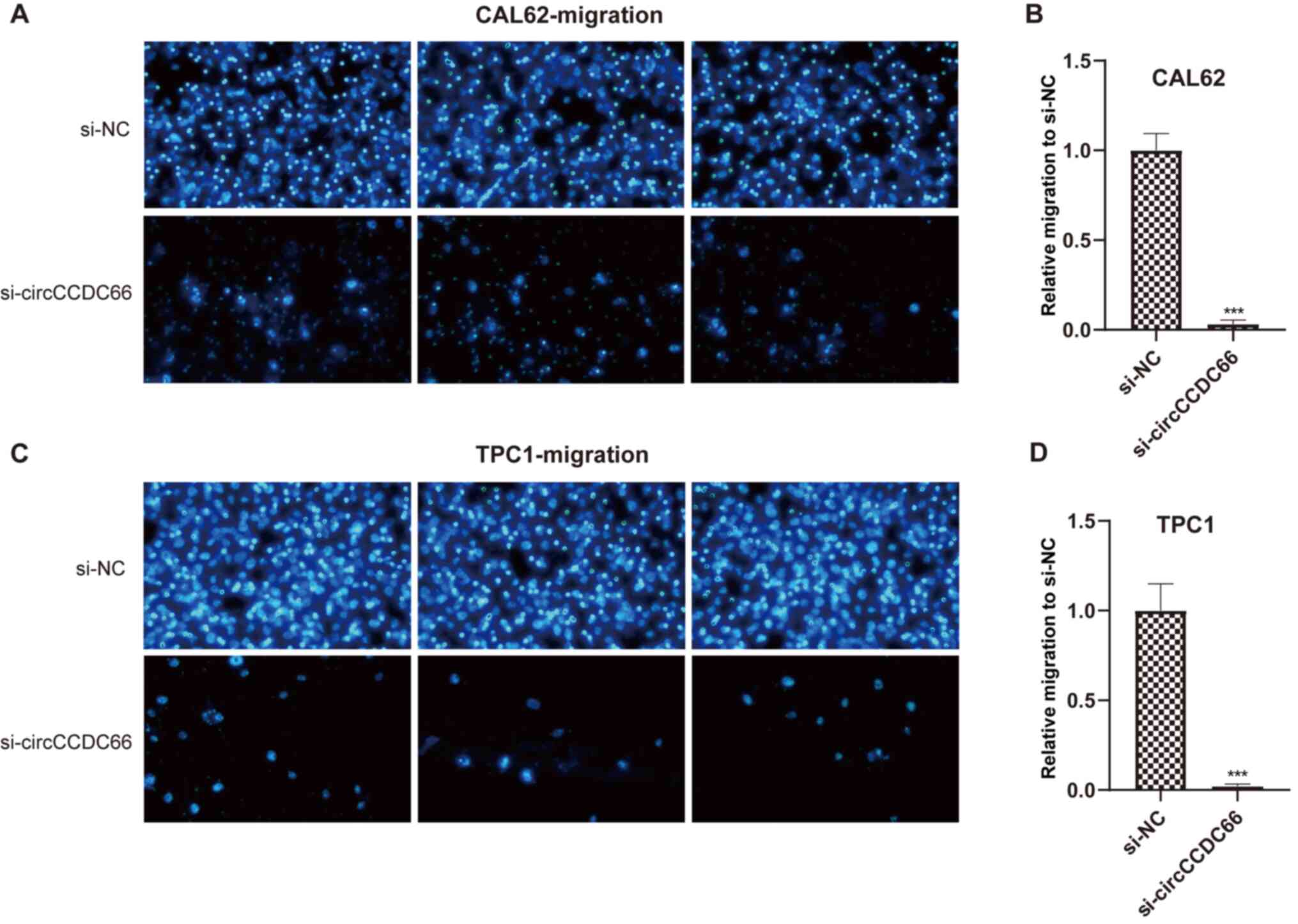
Whether circCCDC66 knockdown could affect the
metastatic ability of the TC cells TPC1 and CAL62 was evaluated
using Transwell assays. As presented in Figs. 4 and 5, circCCDC66 silencing resulted in a
smaller number of migrating cells, suggesting that the knockdown of
circCCDC66 inhibited TC cell migratory and invasive abilities.
circCCDC66 knockdown inhibits TC
glycolysis in vitro
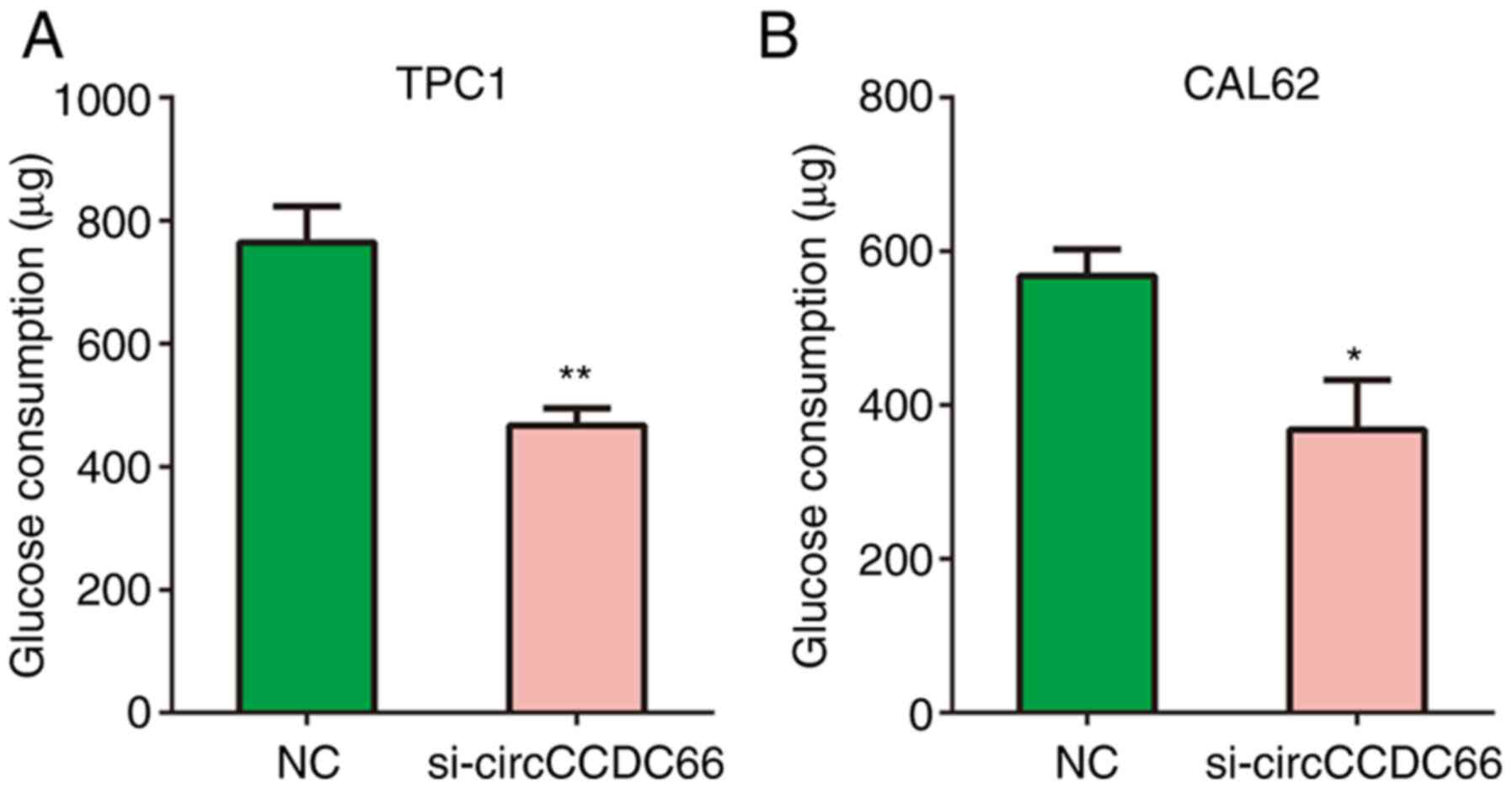
Glycolysis serves a key role in cancer progression
(23). Therefore, the effects of
circCCDC66 knockdown on glycolytic metabolism in TPC1 and CAL62
cells were analysed in the present study. The results from Fig. 6A and B demonstrated that circCCDC66
knockdown significantly reduced the glucose consumption of TPC1 and
CAL62 cells compared with control cells.
circCCDC66 serves as a sponge of
miR-211-5p to promote PDK4 expression
To explore the mechanism by which circCCDC66 may
serve important roles in TC cells, the mechanism by which
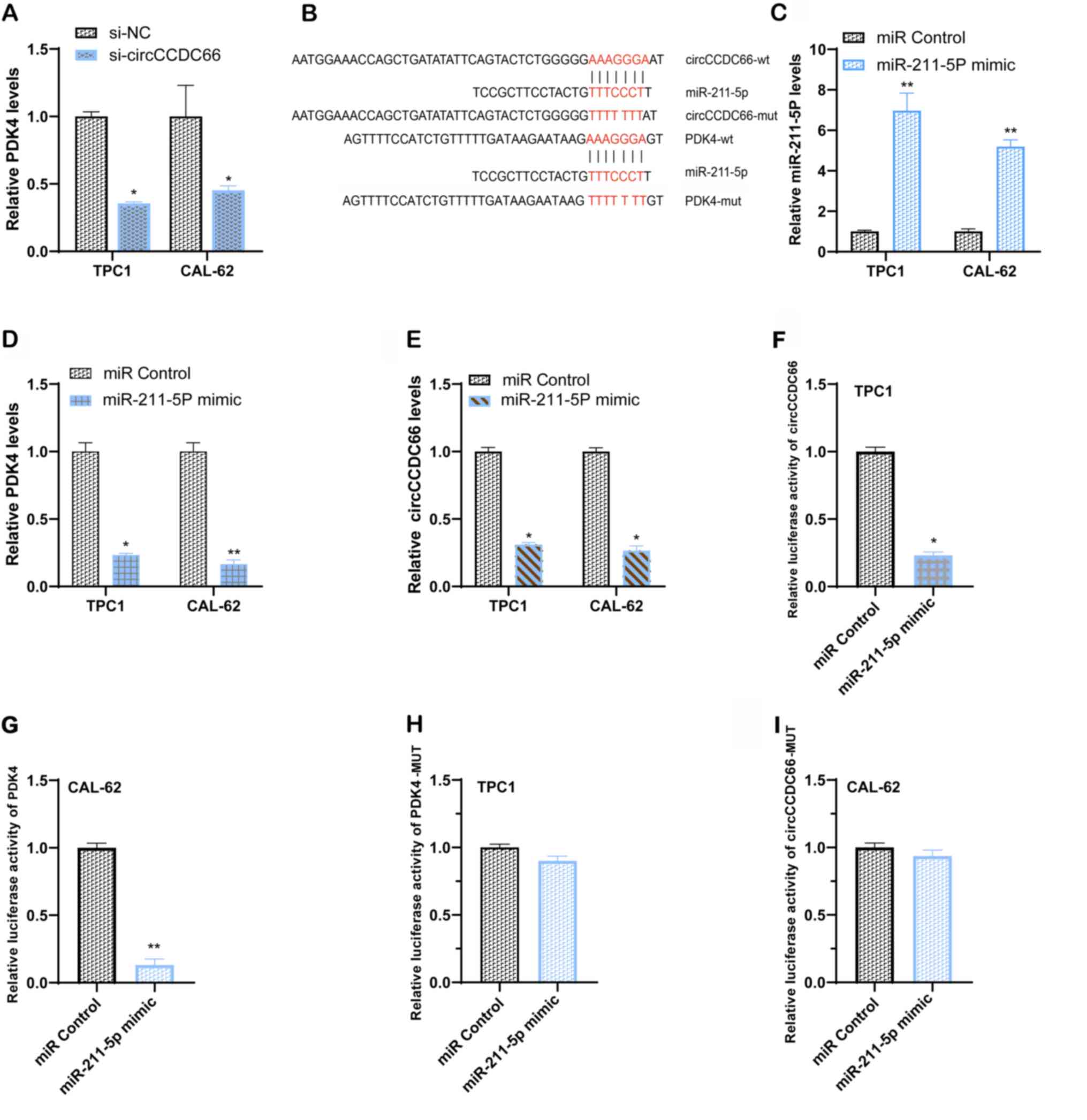
circCCDC66 may regulate PDK4 expression was examined. The
expression level of PDK4 was decreased following circCCDC66
silencing in TPC1 and CAL62 cells (Fig.
7A). circCCDC66 might act as a competing endogenous RNA and a
sponge of miRNA and subsequently affect the expression of target
genes. The results demonstrated that miR-211-5p and PDK4 were
predicted as potential downstream targets associated with
circCCDC66 function with RegRNA2.0 (http://regrna2.mbc.nctu.edu.tw/index.html) and miRcode
(http://www.mircode.org/mircode/;
Fig. 7B). Subsequently, the
expression of PDK4 following miR-211-5p overexpression was
determined. The results demonstrated that TC cells successfully
transfected with miR-211-5p mimic (Fig.
7C) presented a lower PDK4 expression compared with control
cells (Fig. 7D). Furthermore, the
expression of circCCDC66 in both TC cell lines was inhibited
following transfection with miR-211-5p-mimic (Fig. 7E).
In addition, to investigate the binding between
miR-211-5p and circCCDC66 or the 3′-UTR of PDK4, a luciferase
reporter assay was performed. The results demonstrated that a
miR-211-5p-mimic could decrease the luciferase activity when
wild-type circCCDC66 and wild-type 3′-UTR of PDK4 were expressed,
which was not the case with mutant circCCDC66 and mutant 3′-UTR of
PDK4 (Fig. 7F-I). These findings
suggested that circCCDC66 may act as a sponge for miR-211-5p and
promote PDK4 expression.
Discussion
High levels of metastasis and invasion are key
characteristics of cancer cells (24). The present study revealed that
circCCDC66 served a crucial stimulating role in TC cell
proliferation and migratory and invasive abilities. The results
demonstrated that circCCDC66 was expressed at higher levels in TC
tissues compared with normal tissues and was also highly expressed
in TC cells compared with normal cells. In addition, circCCDC66
knockdown inhibited the proliferation, glycolysis and migratory and
invasive abilities of TC cells. Furthermore, circCCDC66 silencing
decreased the expression of PDK4 by acting as a ceRNA and sponging
miR-211-5p.
CircRNAs have been used as biomarkers for tumour
detection due to their differential expression in tumour tissues
and normal tissues (25). At the
same time, the importance of numerous circRNAs in TC cells has also
been reported. For example, Cui et al (26) performed a microarray analysis of
circRNAs in TC and reported that 98 different circRNAs were
abnormally regulated. The circRNA circZFR promotes the expression
of C8orf4 by sponging miR-1261 and further promotes the
proliferation and invasion of TC cells. The circRNA circ-ITCH
inhibits cancer progression via the miR-22-3p/CBL/beta-catenin
axis. However, the underlying mechanism of circCCDC66 in TC remains
unclear.
The present study evaluated the role and underlying
mechanism of circCCDC66 on the biological behaviour of TC cells.
The results demonstrated that circCCDC66 was highly expressed in TC
tissues and cells compared with normal thyroid tissues and cells,
suggesting that circCCDC66 may promote the progression of TC. The
results also demonstrated that the proliferation and migratory and
invasive abilities of TC cells was significantly inhibited
following circCCDC66 silencing. In addition, circCCDC66 was
reported to bind to miR-211-5p and inhibit its expression.
Subsequently, the overexpression of miR-211-5p in TC cells
significant decreased the expression of PDK4 and circCCDC66. A
luciferase assay demonstrated that miR-211-5p decreased the
activity of pmiR-RB-reporter vectors containing PDK4 or circCCDC66
regions. These finding indicated that circCCDC66 may competitively
inhibit the binding of miR-211-5p to PDK4, thereby affecting TC
cell proliferation and invasiveness.
miR-211-5p has been found to be associated with
metabolic activity in a variety of cancer cells. For example, the
progression of TNBC is inhibited by miR-211-5p via targeting SET
binding protein 1, suggesting that miR-211-5p might act as a
potential prognostic biomarker and therapeutic target for TNBC
(27). Furthermore, a previous study
reported that the expression of snail family transcriptional
repressor 1 (SNAI1) is decreased following miR-211-5p
overexpression. Luciferase reporter assays showed that miR-211-5p
can target the 3′-UTR of SNAI1. In addition, miR-211-5p reduces the
weight of xenograft tumours and inhibits tumour metastasis in mice.
These results indicate that the decrease in SNAIL1 expression
mediated by miR-211-5p could help to inhibit the progression of
renal cell carcinoma (28). Zinc
finger E-box binding homeobox 2 (ZEB2) has also been reported to be
a target of miR-211-5p and miR-211-5p serves an inhibitory role in
hepatocellular carcinoma by inhibiting the expression of ZEB2
(29).
Pyruvate dehydrogenase kinase and phosphatase
strictly regulate the pyruvate dehydrogenase complex through
phosphorylation, thus regulating the irreversible reaction of
pyruvate oxidative decarboxylation and affecting the glucose
metabolism process and sugar homeostasis (30). Impaired glucose homeostasis is one of
the risk factors for metabolic diseases, including obesity, type 2
diabetes and cancer (31). Previous
studies reported that PDK4 is highly expressed in breast tumour
tissues, and that higher PDK4 expression is associated with a worse
prognosis in patients. A study reported that miR-211 can suppress
PDK4 expression in breast cancer cells, while lower miR-211
expression levels are correlated with a worse overall survival,
indicating that miR-211 and PDK4 expression levels are negatively
correlated (32). In addition, the
suppression of PDK4 by miR-211 can induce a dominant phenotype of
oxidative phosphorylation, including decreased glucose and
increased expression of key enzymes from pyruvate dehydrogenase and
tricarboxylic acid cycle (33).
These studies suggested that the effect of circCCDC66 on glycolysis
might be mediated by PDK4 in TC cells.
The present study had several limitations. Although
glucose consumption is a marker of glycolysis in cancer cells, it
is not sufficient for evaluating glycolysis. Other experiment,
including lactate production and oxygen consumption, should be
performed. In future research, the signal pathways and metabolic
pathways of circCCDC66 regulating glycolysis will be investigated.
In addition, future investigation will further verify that PDK4 and
miR-211-5p may affect the proliferation and migratory and invasive
abilities of TC cells.
In conclusion, the present study demonstrated that
circCCDC66 may promote TC cell proliferation, migratory and
invasive abilities, and glycolysis in vitro by sponging
miR-211-5p and increasing PDK4 expression. These findings suggested
that targeting circCCDC66 may be considered as a promising
therapeutic strategy for TC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HR, ZS, CC and WM participated in the concept and
design of the study. ZS and CC participated in data acquisition,
analysis and interpretation. HR and WM participated in methodology
design. ZS participated in sample collection. HR, ZS and WM
participated in writing and revising the manuscript. All authors
read and approved the final version.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Qingdao Municipal Hospital (approval no. 2019QMH126) and all
patients provided informed consent to participate to this
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
circRNA
|
circular RNA
|
|
ceRNA
|
competitive endogenous RNA
|
|
TC
|
thyroid cancer
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Zhang JW, Long KR, Wang X, LI MZ and Ma
JD: The research advance of circular RNA. Chin J Anim Veterinary
Sci,. 11:2151–2158. 2016.(In Chinese).
|
|
2
|
Cai H, Li Y, Niringiyumukiza JD, Su P and
Xiang W: Circular RNA involvement in aging: An emerging player with
great potential. Mech Ageing Dev. 178:16–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu Q, Liu T, Feng H, Yang R, Zhao X, Chen
W, Jiang B, Qin H, Guo X, Liu M, et al: Circular RNA circSLC8A1
acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer
progression via regulating PTEN. Mol Cancer. 18:1112019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu JZ, Shao CC, Wang XJ, Zhao X, Chen JQ,
Ouyang YX, Feng J, Zhang F, Huang WH, Ying Q, et al: circTADA2As
suppress breast cancer progression and metastasis via targeting
miR-203a-3p/SOCS3 axis. Cell Death Dis. 10:1752019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu J, Wang YH, Yoon C, Huang XY, Xu Y, Xie
JW, Wang JB, Lin JX, Chen QY, Cao LL, et al: Circular RNA
circ-RanGAP1 regulates VEGFA expression by targeting miR-877-3p to
facilitate gastric cancer invasion and metastasis. Cancer Lett.
471:38–48. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gaffo E, Boldrin E, Dal Molin A, Bresolin
S, Bonizzato A, Trentin L, Frasson C, Debatin KM, Meyer LH, Te
Kronnie G and Bortoluzzi S: Circular RNA differential expression in
blood cell populations and exploration of circRNA deregulation in
pediatric acute lymphoblastic leukemia. Sci Rep. 9:146702019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiong X, Zhu H and Chen X: Low expression
of long noncoding RNA CASC2 indicates a poor prognosis and promotes
tumorigenesis in thyroid carcinoma. Biomed Pharmacother.
93:391–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Li Z, Guo F, Qin X, Liu B, Lei Z,
Song Z, Sun L, Zhang HT, You J and Zhou Q: MiR-223 regulates
migration and invasion by targeting Artemin in human esophageal
carcinoma. J Biomed Sci. 18:242011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Durante C, Grani G, Lamartina L, Filetti
S, Mandel SJ and Cooper DS: The diagnosis and management of Thyroid
Nodules: A review. JAMA. 319:914–924. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cong S, Fang LW, Bao HL, Feng YJ, Wang N,
Yin P, Li YC, Duan XN and Zhou MG: (Disease burden of thyroid
cancer in the Chinese population, in 1990 and 2013). Zhonghua Liu
Xing Bing Xue Za Zhi. 37:773–777. 2016.PubMed/NCBI
|
|
12
|
Schmidt B and Davies L: The rising
incidence of thyroid cancer: Contributions from healthcare practice
and biologic risk factors. Management of Differentiated Thyroid
Cancer. Springer; Cham: pp. 1–13. 2017, View Article : Google Scholar
|
|
13
|
Murugan AK, Dong J, Xie J and Xing M: MEK1
mutations, but not ERK2 mutations, occur in melanomas and colon
carcinomas, but none in thyroid carcinomas. Cell Cycle.
8:2122–2124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Wang J, Zheng Y, Zhang J, Chen S
and Zhao F: Comprehensive identification of internal structure and
alternative splicing events in circular RNAs. Nat Commun.
7:120602016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei H, Pan L, Tao D and Li R: Circular RNA
circZFR contributes to papillary thyroid cancer cell proliferation
and invasion by sponging miR-1261 and facilitating C8orf4
expression. Biochem Biophys Res Commun. 503:56–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsiao KY, Lin YC, Gupta SK, Chang N, Yen
L, Sun HS and Tsai SJ: Noncoding effects of circular RNA CCDC66
promote colon cancer growth and metastasis. Cancer Res.
77:2339–2350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong W, Yu S, Zhuang Y, Zhang Q, Wang J
and Gao X: SRCIN1 regulated by circCCDC66/miR-211 is upregulated
and promotes cell proliferation in non-small-cell lung cancer.
Biomed Res Int. 2020:53076412020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiang D, Li Y and Lin Y: Circular RNA
circCCDC66 contributes to malignant phenotype of osteosarcoma by
Sponging miR-338-3p to upregulate the expression of PTP1B. Biomed
Res Int. 2020:46371092020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang M, Chen B, Ru Z and Cong L: CircRNA
circ-ITCH suppresses papillary thyroid cancer progression through
miR-22-3p/CBL/β-catenin pathway. Biochem Biophys Res Commun.
504:283–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu H, Liu Y, Cheng P, Wang C, Liu Y, Zhou
W, Xu Y and Ji G: CircRNA_0000392 promotes colorectal cancer
progression through the miR-193a-5p/PIK3R3/AKT axis. J Exp Clin
Cancer Res. 39:2832020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nahm JH, Kim HM and Koo JS:
Glycolysis-related protein expression in thyroid cancer. Tumour
Biol. 39:10104283176959222017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Zhou X, Shan B, Han J, Wang F, Fan
X, Lv Y, Chang L and Liu W: Downregulation of microRNA-33a promotes
cyclin-dependent kinase 6, cyclin D1 and PIM1 expression and
gastric cancer cell proliferation. Mol Med Rep. 12:6491–6500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim Y, Williams KC, Gavin CT, Jardine E,
Chambers AF and Leong HS: Quantification of cancer cell
extravasation in vivo. Nat Protoc. 11:937–948. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang KW and Dong M: Role of circular RNAs
in gastric cancer: Recent advances and prospects. World J
Gastrointest Oncol. 11:459–469. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui S, Qian Z, Chen Y, Li L, Li P and Ding
H: Screening of up- and downregulation of circRNAs in HBV-related
hepatocellular carcinoma by microarray. Oncol Lett. 15:423–432.
2018.PubMed/NCBI
|
|
27
|
Chen LL, Zhang ZJ, Yi ZB and Li JJ:
MicroRNA-211-5p suppresses tumour cell proliferation, invasion,
migration and metastasis in triple-negative breast cancer by
directly targeting SETBP1. Br J Cancer. 117:78–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang K, Jin W, Jin P, Fei X, Wang X and
Chen X: MiR-211-5p suppresses metastatic behavior by targeting
SNAI1 in Renal cancer. Mol Cancer Res. 15:448–456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang G, Wen L, Deng W, Jian Z and Zheng
H: Regulatory role of miR-211-5p in hepatocellular carcinoma
metastasis by targeting ZEB2. Biomed Pharmacother. 90:806–812.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shan C, Kang HB, Elf S, Xie J, Gu TL,
Aguiar M, Lonning S, Hitosugi T, Chung TW, Arellano M, et al:
Tyr-94 phosphorylation inhibits pyruvate dehydrogenase phosphatase
1 and promotes tumor growth. J Biol Chem. 289:21413–21422. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sui W, Shi Z, Xue W, Ou M, Zhu Y, Chen J,
Lin H, Liu F and Dai Y: Circular RNA and gene expression profiles
in gastric cancer based on microarray chip technology. Oncol Rep.
37:1804–1814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun S, Zhao M, Han Y, Juanzi W, Peng W and
Liu J: PDK4 mRNA Expression in Breast Cancer and Its Relationship
with Prognosis. Cancer Res Prev Treat. 45:73–76. 2018.
|
|
33
|
Mazar J, Richardson A, Qi F, Lee B, Duran
A, Govindarajan S, Shelley J, Brill LM, Li JL, Han X, Moscat J and
Perera RJ: MicroRNA-211 modulates energy metabolism in human
melanoma cells by destabilizing HIF1-α and downregulating PDK4.
AACR. 74:S9782014.
|