Introduction
Breast cancer is an invasive cancer, ranking first
in the incidence of cancer in women; it accounts for 11.6% of the
total cancer incidences, and ~630,000 women worldwide died of
breast cancer in 2018, accounting for 15% of cancer-associated
deaths in women (1). Compared with
the global average, Chinese women have a lower incidence of breast
cancer and a higher mortality rate, seriously threatening women's
health (2). Breast cancer treatment
uses a comprehensive treatment model that includes surgery and
chemotherapy (3). The high
postoperative recurrence rate and post-surgical complications
within a short period following breast cancer surgery are
significant (4,5). Therefore, increased attention has been
focused on further elucidating the pathogenesis of breast cancer to
discover and develop novel treatment methods.
Modern medical research has revealed that the
formation and growth of tumors are associated with cellular
differentiation and apoptosis (6).
Apoptosis-induced inhibition of tumor cell proliferation is
currently an effective method for treating tumors (7). After numerous years of development,
traditional Chinese medicine has identified a set of unique
antitumor methods (8). Modern
pharmacological studies have demonstrated that traditional Chinese
medicine exerts antitumor effects by inhibiting cellular
proliferation, inducing apoptosis, regulating cellular signaling
pathways and inhibiting multidrug resistance (9–11).
Furthermore, traditional Chinese medicine is widely used in
chemotherapy and radiation therapy, which can effectively extend
the life expectancy of patients and improve the overall efficacy of
treatments (12–14).
As a Chinese herbal medicine, safflower promotes
blood circulation and removes blood stasis (15). The red flavonoid hydroxyl safflower
yellow A (HSYA) is an effective active ingredient in mitigating
numerous different types of tumor, such as liver cancer and glioma
(16–19). A novel flavonoid, hydroxyl safflower
yellow B (HSYB), which is an isomer of HSYA, has been demonstrated
to have a well-defined anti-breast cancer effect (20,21).
Doxorubicin (DOX), as an anthracycline anticancer drug, is a
classic breast cancer chemotherapeutic drug with a high sensitivity
to cancer cells that inhibits cell proliferation by inducing
apoptosis (22,23). Moreover, combination therapies can
exert synergistic therapeutic effects. However, to the best of our
knowledge, there are currently no studies on the combination of
HSYB and DOX for treating breast cancer. Hence, the present study
aimed to investigate the potential synergistic effects of combined
DOX and HSYB treatment.
Materials and methods
Chemicals and reagents
HSYB (purity, >95%) was donated by Dr Tao Weiwei
of Nanjing University of Traditional Chinese Medicine (Nanjing,
China). DOX hydrochloride (purity, >98%; cat. no. 113424) was
purchased from Beijing Bailingwei Technology Co., Ltd. Dulbecco's
modified Eagle's medium (DMEM) was purchased from Thermo Fisher
Scientific, Inc. Fetal bovine serum (FBS) was purchased from
Zhejiang Tianhang Biotechnology Co., Ltd. Dimethyl sulfoxide
(DMSO), Penicillin-Streptomycin, MTT, 0.25% trypsin digestion
solution (without phenol red), Hoechst 33258 staining solution,
Annexin V-FITC Apoptosis Detection kit, Reactive Oxygen Species
Assay kit and Mitochondrial Membrane Potential Assay kit with JC-1
were ordered from Beijing Solarbio Science & Technology Co.,
Ltd. Anti-cleaved caspase-3 (1:1,000; cat. no. 9662), anti-cleaved
caspase-9 (1:1,000; cat. no. 9508), anti-cytochrome c (1:1,000;
cat. no. 4280) and anti-BAX (1:1,000; cat. no. 3498) were purchased
from Cell Signaling Technology, Inc. Anti-Bcl-2 (1:1,000; cat. no.
ab196495), anti-caspase-9 (1:1,000; cat. no. ab25758) and
anti-caspase-3 (1:500; cat. no. ab13847) were purchased from Abcam.
Primary antibodies against β-actin (1:2,000; cat. no. TA-09) and
peroxidase-conjugated goat anti-mouse IgG secondary antibodies
(1:25,000; cat. no. ZB2305) were purchased from OriGene
Technologies, Inc.
Cell lines and cell culture
MCF-7 cells were obtained from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences. HaCaT cells
were purchased from Procell Life Science & Technology Co.,
Ltd.. The cells were cultured at 37°C with 5% CO2 in
DMEM with 10% FBS and 1% penicillin-streptomycin.
Determination of cellular
viability
HaCaT (1.0×104 cells/well) or MCF-7 cells
(0.7×104 cells/well) in 96-well plates were treated with
DOX (0.1–2 µg/ml), HSYB (5–30 µg/ml) or a combination of the two
for 24 h at 37°C. Subsequently, MTT (20 µl) was added to each well
and incubated for 4 h at 37°C. DMSO (150 µl) was then directly
added to the plate while shaking at low speed for 10 min at room
temperature. Finally, the optical density (OD) of the cells at 490
nm was determined using an enzyme-labeling instrument, and the
inhibition rate was calculated as follows: (1-OD value of
experimental group/OD value of control group) × 100%. In addition,
according to the Chou-Talalay combination index (CI) method
(24), the CI was calculated to
evaluate the effect of drug combination and to test the interaction
between two drugs with different concentrations. Data were analyzed
using Compusyn 2.0 software (Biosoft) to calculate CI and Fa
values. CI<1, CI=1 and CI>1 indicated synergistic, additive
and antagonistic effects, respectively.
Colony formation experiments
MCF-7 cells were inoculated into a 6-well plate (200
cells/well) and treated with DOX (0.4 µg/ml), HSYB (6 µg/ml) or a
combination of the two for 24 h at 37°C after attachment. The
complete fresh medium was replaced every three days. After ~12 days
of culture, the cells were fixed at room temperature with 4%
paraformaldehyde solution for 20 min. Subsequently, the cells were
washed with PBS and treated with crystal-violet staining solution
for 10 min at room temperature. Finally, the dye was cleaned with
PBS, and the stained cells were imaged using a camera. A colony was
defined as >50 cells.
Hoechst 33258 staining detection
An aseptic cover slide was placed into a 6-well
plate and inoculated with MCF-7 cells (1.4×105
cells/well). Cells were fixed with 4% paraformaldehyde for 20 min
at room temperature after treatment with DOX (0.4 µg/ml), HSYB (6
µg/ml) or a combination of the two for 24 h at 37°C. Subsequently,
the cells were washed with PBS and then stained with Hoechst 33258
for 10 min at room temperature. The staining solution was cleaned
with PBS, and the slides were observed under an inverted
fluorescence microscope (magnification, ×400).
Annexin V-FITC apoptosis
detection
MCF-7 cells (1.0×106 cells/group) treated
with DOX (0.4 µg/ml), HSYB (6 µg/ml) or a combination of the two
drugs were collected and resuspended in a staining buffer (500 µl)
containing Annexin V-FITC (5 µl), after which they were tested
using a FACSCanto II flow cytometer (Becton, Dickinson and
Company). The apoptosis rate was analyzed using the FACSDiva 6.1.3
software (Becton, Dickinson and Company).
Western blot analysis
MCF-7 cells (5.7×106 cells/well) were
seeded and treated as aforementioned in Petri dishes (100-mm).
Protein was extracted using RIPA lysis buffer (cat. no. R0010;
Beijing Solarbio Science & Technology Co., Ltd.) after the
cells were collected. The protein concentration was detected using
a BCA protein assay kit (cat. no. PC0020; Beijing Solarbio Science
& Technology Co., Ltd.). Proteins (80 µg/lane) were separated
via 12% SDS-PAGE and transferred to a PVDF membrane. Protein bands
were blocked with 5% BSA (cat. no. 232100; Becton, Dickinson and
Company) at room temperature for 3 h and were then incubated
overnight with primary antibodies at 4°C. The samples were washed
four times with TBS-Tween (TBST; 0.1% of Tween-20) buffer (6 min
per wash), incubated with secondary antibodies for 35 min at room
temperature and washed four times with TBST buffer (6 min per
wash). Finally, the protein bands were incubated with an ECL
chemiluminescence working buffer (cat. no. 34580; Thermo Fisher
Scientific, Inc.), and western blotting was imaged via a UVP
imaging system. ImageJ v1.43 software (National Institutes of
Health) was used for densitometry.
Detection of reactive oxygen species
(ROS) in cells
MCF-7 cells (1.8×105 cells/well) were
plated in Petri dishes (30-mm). Cells were treated with DOX (0.4
µg/ml), HSYB (6 µg/ml) or a combination of the two for 24 h at
37°C. The cells were detached from the plate with EDTA-containing
trypsin and resuspended in serum-free medium containing a DCFH-DA
probe (10 µmol/l). Subsequently, the cells were transferred to a
thermostatic chamber at 37°C for 20 min. Finally, the cells were
washed three times with TBST buffer, and the DCF fluorescence
intensity of the treated cells was determined using a FACSCanto II
flow cytometer. The DCF fluorescence intensity was analyzed using
the FACSDiva 6.1.3 software (Becton, Dickinson and Company). In
addition, aseptic cover slides were placed into a 6-well plate and
inoculated cells were imaged under an inverted fluorescence
microscope (magnification, ×200). The dye solutions used for ROS
detection were from a Reactive Oxygen Species Assay kit (cat. no.
CA1410; Beijing Solarbio Science & Technology Co., Ltd.).
Measurement of mitochondrial membrane
potential (MMP)
As aforementioned, DOX- and HSYB-treated cells were
treated with EDTA-containing trypsin. The cells were resuspended in
complete medium (500 µl) containing JC-1 staining working solution
(500 µl) and then incubated at 37°C for 20 min. Next, cells were
washed twice with the JC-1 staining buffer (1X). Finally, the JC-1
staining buffer (500 µl) was used to resuspend the cells, which
were subsequently analyzed using a FACSCanto II flow cytometer. The
ratio of red to green fluorescence was analyzed using the FACSDiva
6.1.3 software.
Statistical analysis
The SPSS 21.0 software (IBM Corp.) was used to
analyze the data presented as the mean ± SD. Significant
differences were determined using an unpaired Student's t-tests or
one-way ANOVA followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Combination of HSYB and DOX has a
synergistic effect on MCF-7 cells
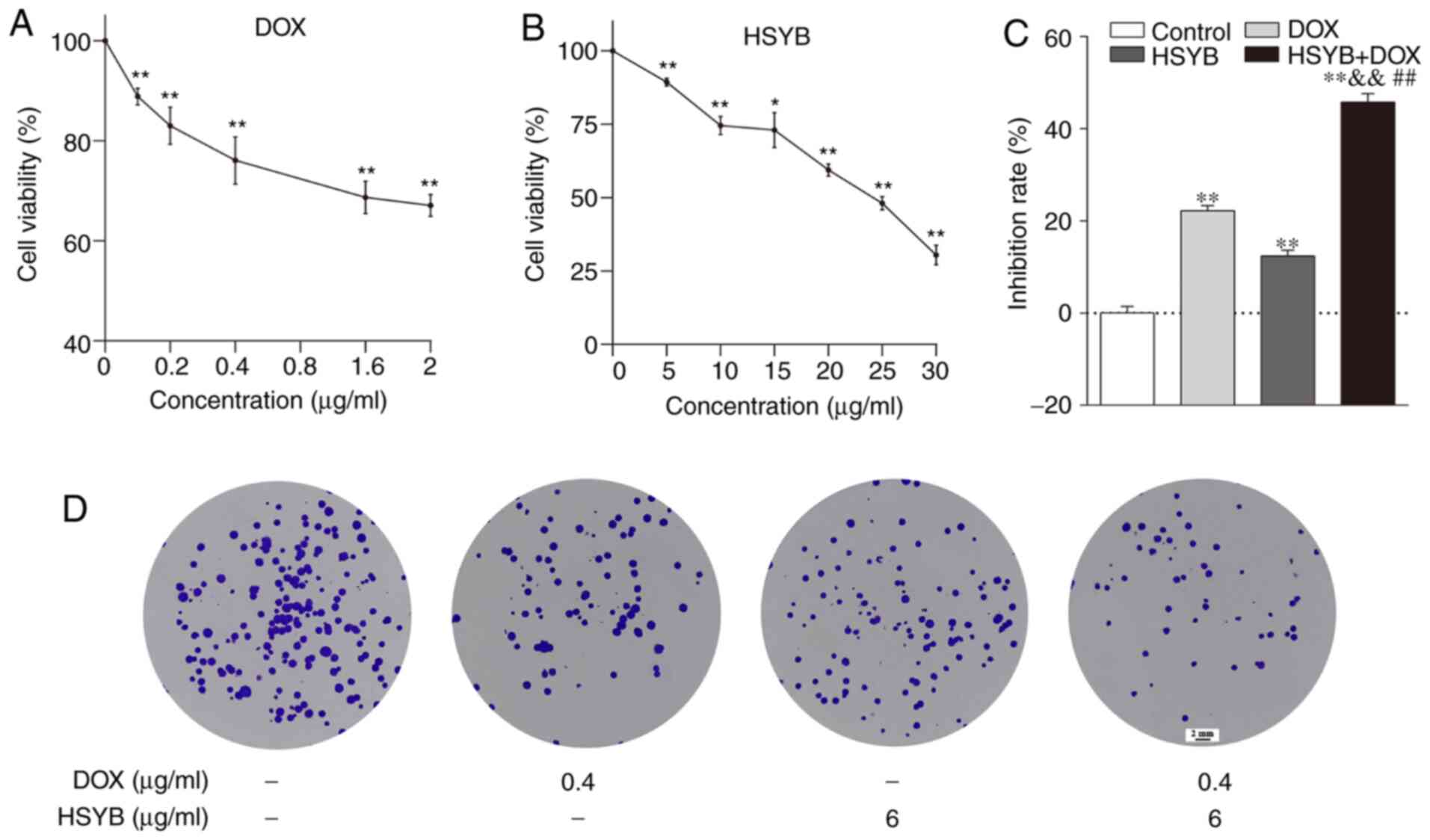
The viability of HSYB and/or DOX-treated MCF-7 cells
was assessed using the MTT method to investigate whether there was
a synergistic effect of HSYB and DOX. First, MCF-7 cells were
incubated with different concentrations of HSYB (0–30 µg/ml) or DOX
(0–2 µg/ml) for 24 h. The inhibitory effect of HSYB and DOX on
breast cancer MCF-7 cells was significantly decreased in a
concentration-dependent manner (Fig. 1A
and B). The IC50 values of HSYB and DOX were 22.12
µg/ml and 6.85 µg/ml, respectively, after 24 h of drug
administration. Subsequently, the effect of the combination of HSYB
and DOX for 24 h on the viability of MCF-7 cells was detected via
MTT assays. The Chou-Talalay method and Compusyn 2.0 software were
used to calculate the combination index (CI). The present results
demonstrated that HSYB synergistically enhanced the efficacy of DOX
against breast cancer MCF-7 cells. When HSYB (6 µg/ml) was combined
with DOX (0.4 µg/ml), the CI value was the smallest, and the effect
of coadministration was the most obvious (CI<1; Table SI). Therefore, these concentrations
were used for subsequent experiments. Compared with HSYB (6 µg/ml)
or DOX (0.4 µg/ml) alone, inhibition of cellular proliferation was
significantly enhanced after combined treatment with HSYB and DOX
(Fig. 1C).
Furthermore, the effects of HSYB, DOX or combination
therapy on cell colony formation were studied. There were fewer
cell colonies in the DOX or HSYB groups than in the untreated
(control) group, and after combined HSYB and DOX treatment, the
number of cell colonies was markedly decreased (Fig. 1D). Therefore, the combination of HSYB
and DOX enhanced the inhibition of MCF-7 cell proliferation. In
addition, the normal human keratinocyte HaCaT cell line was chosen
as a control group. The results revealed that DOX (0.1–2 µg/ml)
significantly inhibited the proliferation of HaCaT cells and had a
higher cytotoxicity in HaCaT cells than in MCF-7 cells (Fig. S1A). Additionally, the results
revealed that a high concentration (25 µg/ml) of HSYB significantly
inhibited the proliferation of HaCaT cells and had a higher
cytotoxicity in these cells than in MCF-7 cells; however, a low
concentration (5 µg/ml) of HSYB significantly promoted the
proliferation of HaCaT cells (Fig.
S1B). Based on these data, a lower concentration (6 µg/ml) of
HSYB was selected for the combined treatment with HSYB and DOX.
Combination of HSYB and DOX induces
apoptosis in MCF-7 cells
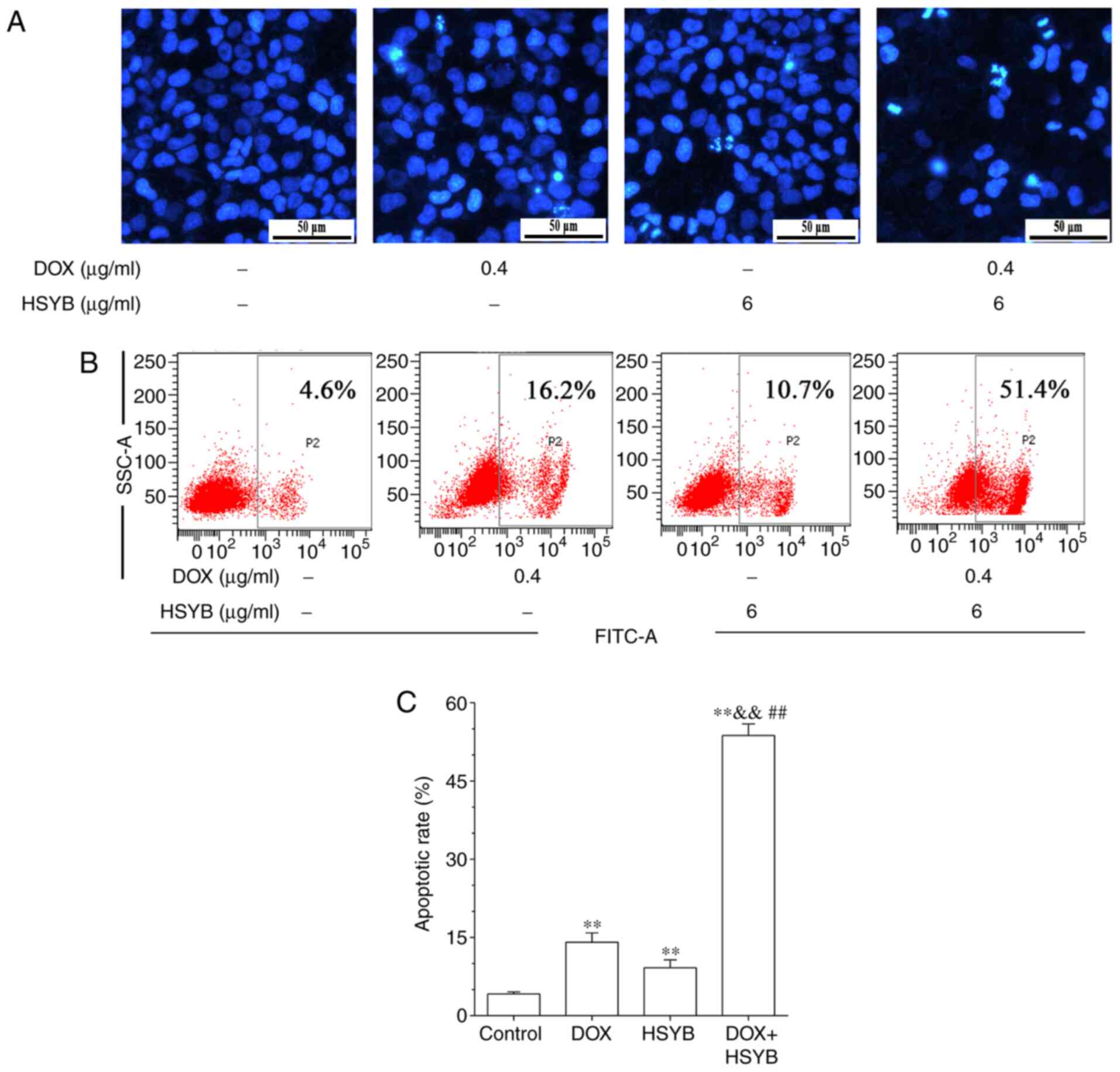
The synergistic effects of HSYB and DOX on MCF-7
apoptosis and cellular morphology were further investigated. Using
Hoechst 33258 staining, it was revealed that the HSYB and DOX
combined-treatment group exhibited typical apoptotic morphological
characteristics compared with the control and the single drug
groups, with the combined therapy group exhibiting a higher
concentration of nuclear chromatin and the formation of apoptotic
bodies (Fig. 2A).
The apoptotic rate of the HSYB and DOX combined-
treatment group in MCF-7 cells was further evaluated by Annexin
V-FITC staining. Treatment with DOX or HSYB alone significantly
increased apoptosis compared with the control group, but the
combined use of DOX and HSYB further significantly increased
apoptosis (Fig. 2B and C). The
apoptotic rate of MCF-7 cells increased from 4.2 to 14.1, 9.2 and
53.7% after treatment with DOX, HSYB and a combination of HSYB and
DOX, respectively (Fig. 2C).
Therefore, the present results confirmed that DOX combined with
HSYB significantly induced apoptosis of MCF-7 cells.
Combination of HSYB and DOX affects
the level of apoptosis-associated molecular proteins in MCF-7
cells
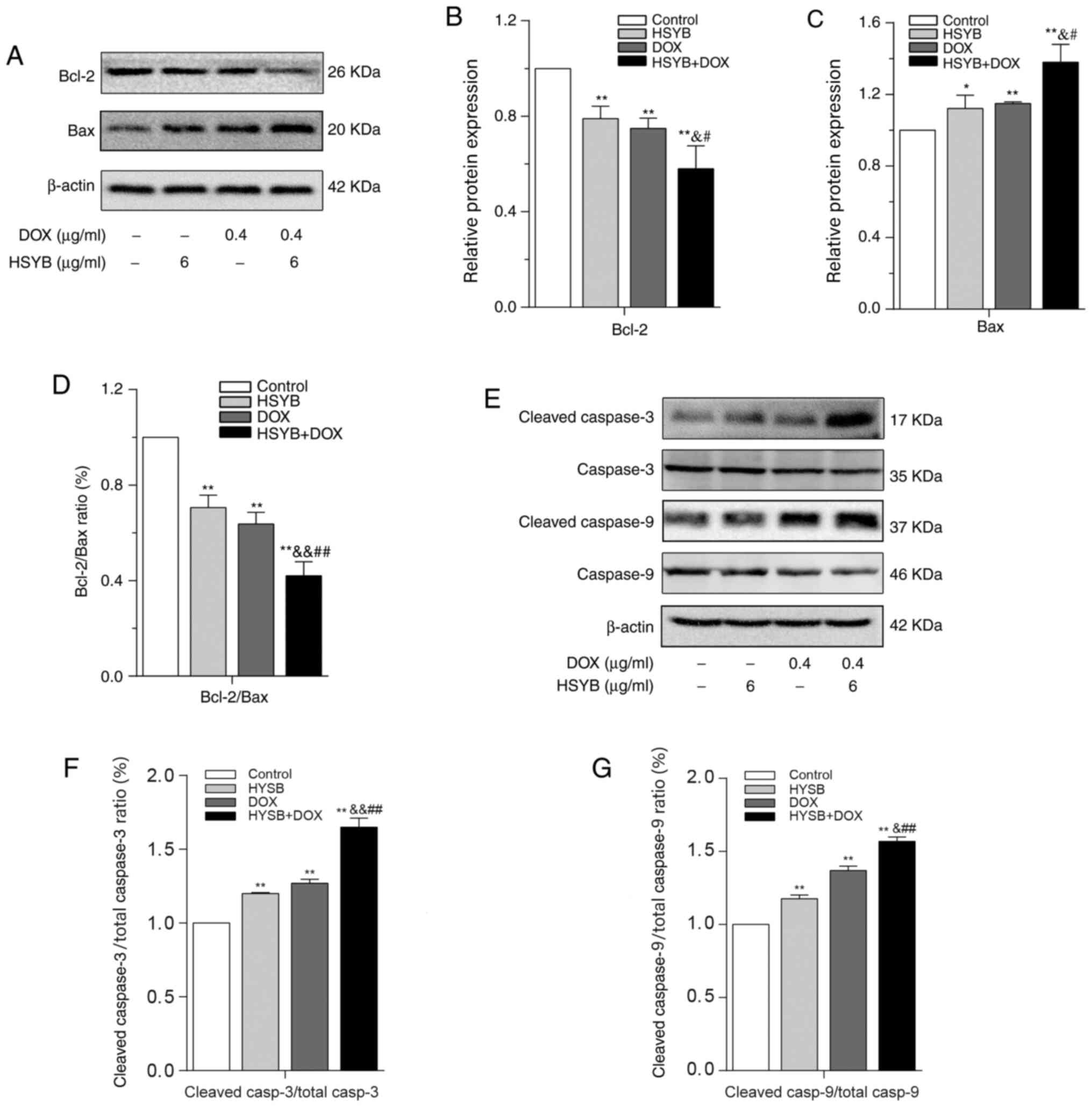
Western blotting was employed to identify the
synergistic effects of HSYB and DOX on the expression levels of
signaling molecules associated with apoptosis in MCF-7 cells. BAX
expression in MCF-7 cells in the single drug groups was
significantly upregulated compared with in the control group, while
the levels of BCL-2 and BCL-2/BAX were significantly decreased, and
this effect was even more marked in the HSYB and DOX
combined-treatment group (Fig.
3A-D). Further research revealed that the ratios of cleaved
caspase-3/total caspase-3 and cleaved caspase-9/total caspase-9 in
the HSYB and DOX combined-treatment group were significantly higher
compared with in the untreated and single drug groups (Fig. 3E-G). These findings demonstrated that
a combination of HSYB and DOX had the strongest regulatory effect
on apoptotic proteins, indicating that a combination of HSYB and
DOX synergistically induced apoptosis of MCF-7 cells.
Combination of HSYB and DOX increases
ROS levels in MCF-7 cells and activates the mitochondrial apoptotic
pathway
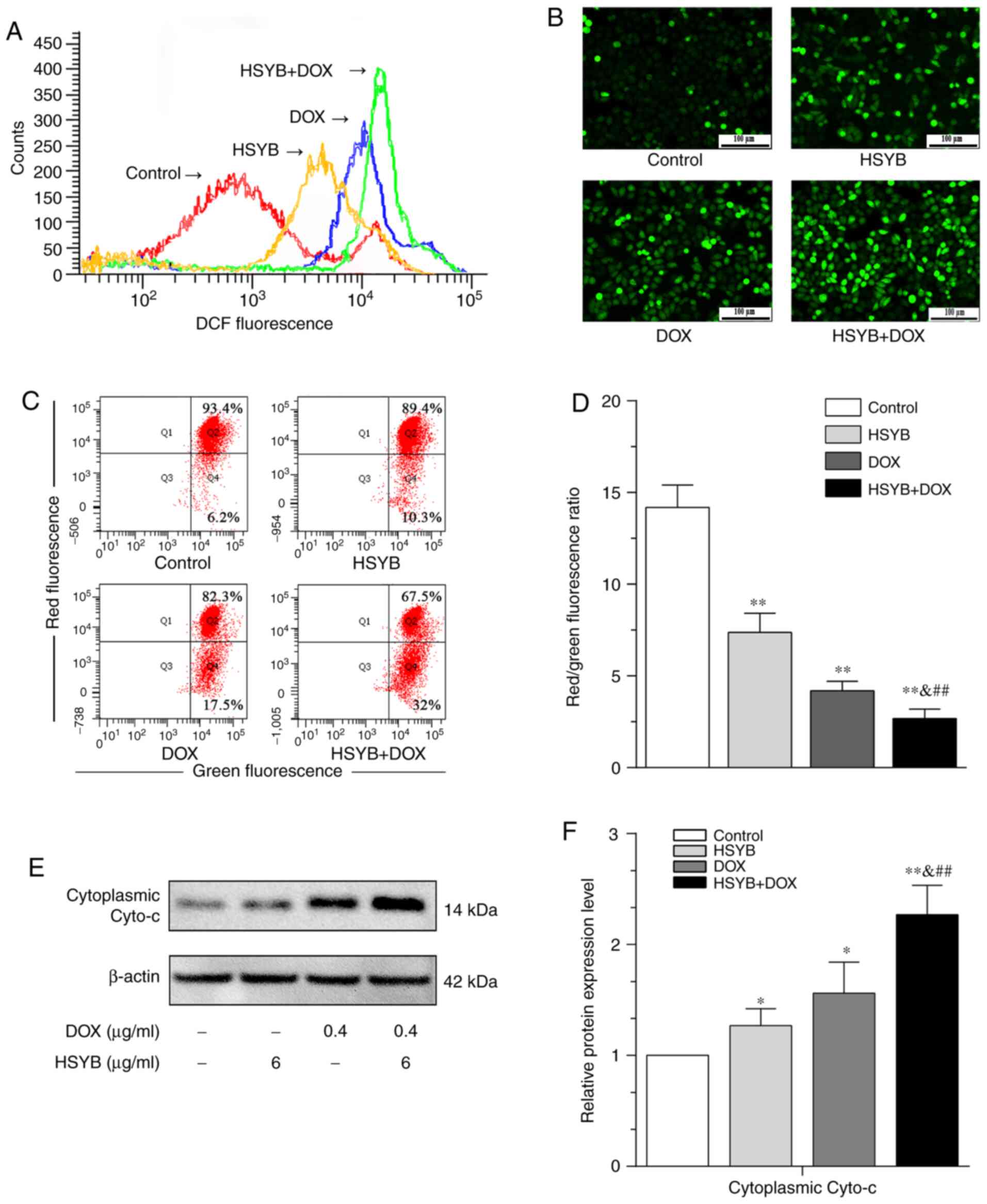
To further determine whether the synergistic effects
of HSYB and DOX in inducing apoptosis of MCF-7 cells were
associated with ROS and the mitochondrial apoptotic pathway, ROS
levels were detected by DCFH-DA staining and the MMP by JC-1
staining. The ROS levels of MCF-7 cells treated with HSYB and/or
DOX were higher than in control cells, and the combined-treatment
group exhibited the highest ROS levels (Fig. 4A and B). Furthermore, MMP was
significantly lower in cells treated with HSYB and/or DOX than in
the control group, and the combined-treatment group had the most
pronounced effect (Fig. 4C and D).
Additionally, the synergistic effects of HSYB and DOX on
cytoplasmic cytochrome c (Cyto-c) levels in MCF-7 cells were
detected by western blotting. It was revealed that the Cyto-c
levels of MCF-7 cells in the single drug groups were higher than in
the control group, with a further significant increase in the
combined-treatment group (Fig. 4E and
F). The current results confirmed that a combination of HSYB
and DOX promoted ROS levels in MCF-7 cells and activated the
mitochondrial apoptotic pathway.
Discussion
In most countries globally, breast cancer has the
highest incidence and mortality rates among female-specific types
of cancer (1). DOX represents the
first-line drug for breast cancer treatment; however, it also
induces side effects, such as cardiac toxicity (25,26).
Safflower can be used for treating angina pectoris or
cardiovascular/cerebrovascular diseases, and the effective
component, HSYA, exerts antitumor effects (18,27,28).
Recently, combinations of different drugs have been widely used to
treat tumors and have yielded effective results (29). A number of studies have obtained
encouraging results on the combination of Chinese herbal medicine
and chemotherapeutic drugs to treat tumors, indicating that
traditional Chinese medicine may be a safe and effective auxiliary
approach for tumor treatment (13,30–32). The
present study revealed that HSYB, a flavonoid component in
safflower, combined with DOX significantly inhibited breast cancer
MCF-7 cell proliferation.
Apoptosis is a form of programmed cell death that
requires the participation of various complex factors and is an
indispensable component in multicellular life processes (33,34).
Numerous studies have demonstrated that apoptosis is affected by
various apoptosis-associated factors, including BCL-2 (35,36).
Furthermore, BCL-2 and BAX serve opposite roles in the process of
apoptosis (37). BCL-2 inhibits
apoptosis and promotes cell proliferation (38,39)
while BAX is mostly distributed in the cytoplasm, it can destroy
the integrity of the mitochondrial membrane and promote apoptosis
after being activated (40–42).
Normally, BCL-2 expression is relatively stable
(43). When BAX is highly expressed,
numerous BAX/BAX homodimers are formed to induce apoptosis
(44). By contrast, when BCL-2
expression is upregulated, BCL-2/BAX heterodimers are formed
(45). Many BAX/BAX homodimers are
dissociated, decreasing the responsiveness of cells to apoptotic
signals, which inhibits apoptosis (46). Therefore, the ratio between these two
antagonistic proteins is key to cell survival (44,46).
With the decrease in BCL-2/BAX levels, the permeability of the
mitochondrial membrane is altered, Cyto-c and apoptosis-promoting
factors are released into the cytoplasm, and the apoptotic promoter
caspase-9 is activated, directly promoting apoptosis by activating
the downstream effector caspase-3 (47,48). In
the present study, the BCL-2/BAX ratio was significantly decreased,
the level of Cyto-c was increased and the levels of the
apoptotic promoter (cleaved caspase-9) and the apoptotic effector
(cleaved caspase-3) were also upregulated after combined treatment
with HSYB and DOX.
ROS are closely associated with the induction and
regulation of apoptosis. Increased intracellular ROS levels can
cause cellular damage, alter mitochondrial membrane permeability
and decrease MMP, thereby promoting Cyto-c release into the
cytoplasm and inducing apoptosis (49,50). In
the present study, the combination of HSYB and DOX in MCF-7 cells
increased ROS levels and decreased the MMP, as determined by
fluorescence microscopy and flow cytometry. Overall, the current
results revealed that a combination of HSYB and DOX synergistically
induced apoptosis of MCF-7 cells by increasing ROS levels,
downregulating BCL-2/BAX ratio, promoting Cyto-c release into the
cytoplasm and subsequently activating caspase-3 and caspase-9.
However, the present study presents some
limitations. First, the antitumor experiments testing HSYB and DOX
were only performed in a single cell line, the human breast cancer
MCF-7 cell line. Therefore, future studies should investigate the
effects of HSYB and DOX in other breast cancer cells in
vitro and in mouse models in vivo. Second, the effects
of the combination of HSYB and DOX on MCF-7 cell invasion and
migration were not assessed, which will require further
investigation in future studies.
In conclusion, the current study revealed that the
combination of HSYB and DOX synergistically induced apoptosis and
enhanced antitumor effects in breast cancer MCF-7 cells, indicating
that the combination of HSYB and DOX may be a new alternative for
clinical treatment of breast cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 31870338), the Key Research
and Development Program of Shandong Province of China (grant no.
2019GSF108214), Taishan Scholars Construction Engineering of
Shandong Province (grant no. tsqn201812099), the Dominant
Disciplines' Talent Team Development Scheme of Higher Education of
Shandong Province (grant no. 2016052410), the Introduction and
Cultivation Project for Young Creative Talents of Higher Education
of Shandong Province (grant no. 20191008189) and the Guidance Plan
of Science and Technology Research and Development of Shijiazhuang
(grant no. 171561533).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KL, ZQ, QZ and DL designed the experiments. KL and
DL obtained the experimental data and wrote the manuscript. KL and
CQ collected cell samples for Hoechst 33258 staining, apoptosis and
western blot analysis. CQ, XC, QJ and ML interpreted and analyzed
the data. KL and QJ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song QK, Wang XL, Zhou XN, Yang HB, Li YC,
Wu JP, Ren J and Lyerly HK: Breast cancer challenges and screening
in China: Lessons from current registry data and population
screening studies. Oncologist. 20:773–779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gradishar WJ, Anderson BO, Abraham J, Aft
R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD,
et al: Breast cancer, version 3.2020, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 18:452–478. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lissoni P, Sormani AL, Tancini G, Cattaneo
G, Archili C, Mandelli D, Crispino S, Paolorossi F and Barni S:
Postoperative hyperprolactinaemia and early recurrence rate in
breast cancer. Eur J Cancer. 26:953–956. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murthy BL, Thomson CS, Dodwell D, Shenoy
H, Mikeljevic JS, Forman D and Horgan K: Postoperative wound
complications and systemic recurrence in breast cancer. Br J
Cancer. 97:1211–1217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Macherey S, Mallmann P, Malter W, Doerr F,
Heldwein M, Wahlers T and Hekmat K: Lung metastasectomy for
pulmonary metastatic breast carcinoma. Geburtshilfe Frauenheilkd.
77:645–650. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ling CQ, Yue XQ and Ling C: Three
advantages of using traditional Chinese medicine to prevent and
treat tumor. J Integr Med. 12:331–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Li M, Wang X, Dang Z, Yu L, Wang X,
Jiang Y and Yang Z: Effects of adjuvant traditional Chinese
medicine therapy on long-term survival in patients with
hepatocellular carcinoma. Phytomedicine. 62:1529302019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bing Z, Cheng Z, Shi D, Liu X, Tian J, Yao
X, Zhang J, Wang Y and Yang K: Investigate the mechanisms of
Chinese medicine Fuzhengkangai towards EGFR mutation-positive lung
adenocarcinomas by network pharmacology. BMC Complement Altern Med.
18:2932018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gezici S and Şekeroğlu N: Current
perspectives in the application of medicinal plants against cancer:
Novel therapeutic agents. Anticancer Agents Med Chem. 19:101–111.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng MB, Wen QL, Cui YL, She B and Zhang
RM: Meta-analysis: Traditional Chinese medicine for improving
immune response in patients with unresectable hepatocellular
carcinoma after transcatheter arterial chemoembolization. Explore
(NY). 7:37–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo H, Liu JX, Xu L, Madebo T and Baak JP:
Traditional Chinese medicine herbal treatment may have a relevant
impact on the prognosis of patients with stage IV adenocarcinoma of
the lung treated with platinum-based chemotherapy or combined
targeted therapy and chemotherapy. Integr Cancer Ther. 10:127–137.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng LJ, Hu LP, Peng QL, Yang XL, Bai LL,
Yiu A, Li Y, Tian HY, Ye WC and Zhang DM: Hellebrigenin induces
cell cycle arrest and apoptosis in human hepatocellular carcinoma
HepG2 cells through inhibition of Akt. Chem Biol Interact.
219:184–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim I, Bae J and Kim BJ: Carthami flos
regulates gastrointestinal motility functions. Integr Med Res.
6:404–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
George VC, Dellaire G and Rupasinghe HPV:
Plant flavonoids in cancer chemoprevention: Role in genome
stability. J Nutr Biochem. 45:1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang F, Li J, Zhu J, Wang D, Chen S and
Bai X: Hydroxysafflor yellow A inhibits angiogenesis of
hepatocellular carcinoma via blocking ERK/MAPK and NF-κB signaling
pathway in H22 tumor-bearing mice. Eur J Pharmacol. 754:105–114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Wang Y and Guo ML: Identification
and mapping of a novel hydroxysafflor yellow A (HSYA) biosynthetic
gene in Carthamus tinctorius. Biochem Genet. 49:410–415.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ao H, Feng W and Peng C: Hydroxysafflor
yellow A: A promising therapeutic agent for a broad spectrum of
diseases. Evid Based Complement Alternat Med. 2018:82592802018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu C, Zhu W, Dong K, Pan Z, Chen Y, Chen
X, Liu X, Xu W, Lin H, Zheng Q and Li D: Inhibitory effect of
hydroxysafflor yellow B on the proliferation of human breast cancer
MCF-7 cells. Recent Pat Anticancer Drug Discov. 14:187–197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yue S, Tang Y, Xu C, Li S, Zhu Y and Duan
JA: Two new quinochalcone C-glycosides from the florets of
Carthamus tinctorius. Int J Mol Sci. 15:16760–16771. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bouchalova K, Cizkova M, Cwiertka K,
Trojanec R and Hajduch M: Triple negative breast cancer-current
status and prospective targeted treatment based on HER1 (EGFR),
TOP2A and C-MYC gene assessment. Biomed Pap Med Fac Univ Palacky
Olomouc Czech Repub. 153:13–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ku JM, Kim SR, Hong SH, Choi HS, Seo HS,
Shin YC and Ko SG: Cucurbitacin D induces cell cycle arrest and
apoptosis by inhibiting STAT3 and NF-κB signaling in doxorubicin-
resistant human breast carcinoma (MCF7/ADR) cells. Mol Cell
Biochem. 409:33–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren B, Ye L, Gong J, Ren H, Ding Y, Chen
X, Liu X, Lu P, Wei F, Xu W, et al: Alteronol enhances the
anti-tumor activity and reduces the toxicity of high-dose
adriamycin in breast cancer. Front Pharmacol. 10:2852019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Menna P, Paz OG, Chello M, Covino E,
Salvatorelli E and Minotti G: Anthracycline cardiotoxicity. Expert
Opin Drug Saf. 11 (Suppl 1):S21–S36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi SH, Lee AY, Park CH, Shin YS and Cho
EJ: Protective effect of Carthamus tinctorius L. seed on
oxidative stress and cognitive impairment induced by chronic
alcohol consumption in mice. Food Sci Biotechnol. 27:1475–1484.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xi S, Zhang Q, Xie H, Liu L, Liu C, Gao X,
Zhang J, Wu L, Qian L and Deng X: Effects of hydroxy safflor yellow
A on blood vessel and mRNA expression with VEGF and bFGF of
transplantation tumor with gastric adenocarcinoma cell line BGC-823
in nude mice. Zhongguo Zhong Yao Za Zhi. 34:605–610. 2009.(In
Chinese). PubMed/NCBI
|
|
29
|
Zheng Z, Zhu W, Yang B, Chai R, Liu T, Li
F, Ren G, Ji S, Liu S and Li G: The co-treatment of metformin with
flavone synergistically induces apoptosis through inhibition of
PI3K/AKT pathway in breast cancer cells. Oncol Lett. 15:5952–5958.
2018.PubMed/NCBI
|
|
30
|
Hong M, Wang N, Tan HY, Tsao SW and Feng
Y: MicroRNAs and Chinese medicinal herbs: New possibilities in
cancer therapy. Cancers (Basel). 7:1643–1657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu
L, Zhao M, Liu Q, Cheng Z, Zou J, et al: Naturally occurring
anti-cancer compounds: Shining from Chinese herbal medicine. Chin
Med. 14:482019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim HP, Son KH, Chang HW and Kang SS:
Anti-inflammatory plant flavonoids and cellular action mechanisms.
J Pharmacol Sci. 96:229–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin L and Zakeri ZF: Apoptosis in
development. Methods Mol Biol. 136:107–113. 2000.PubMed/NCBI
|
|
34
|
Weng D, Marty-Roix R, Ganesan S, Proulx
MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK,
Kelliher MA, et al: Caspase-8 and RIP kinases regulate
bacteria-induced innate immune responses and cell death. Proc Natl
Acad Sci USA. 111:7391–7396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brown R: The bcl-2 family of proteins. Br
Med Bull. 53:466–477. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roset R, Ortet L and Gil-Gomez G: Role of
Bcl-2 family members on apoptosis: What we have learned from
knock-out mice. Front Biosci. 12:4722–4730. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zou J, Ardecky R, Pinkerton AB, Sergienko
E, Su Y, Stonich D, Curpan RF, Simons PC, Zhai D, Diaz P, et al:
Selective Bcl-2 inhibitor probes. Probe reports from the NIH
molecular libraries program Bethesda (MD): National Center for
Biotechnology Information (US); 2010, https://www.ncbi.nlm.nih.gov/books/NBK133437/October
31–2011
|
|
40
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schindler CK, Shinoda S, Simon RP and
Henshall DC: Subcellular distribution of Bcl-2 family proteins and
14-3-3 within the hippocampus during seizure-induced neuronal death
in the rat. Neurosci Lett. 356:163–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tan KO, Fu NY, Sukumaran SK, Chan SL, Kang
JH, Poon KL, Chen BS and Yu VC: MAP-1 is a mitochondrial effector
of Bax. Proc Natl Acad Sci USA. 102:14623–14628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bruckheimer EM, Cho SH, Sarkiss M,
Herrmann J and McDonnell TJ: The Bcl-2 gene family and apoptosis.
Adv Biochem Eng Biotechnol. 62:75–105. 1998.PubMed/NCBI
|
|
44
|
Tsukahara S, Yamamoto S, Tin-Tin-Win-Shwe,
Ahmed S, Kunugita N, Arashidani K and Fujimaki H: Inhalation of
low-level formaldehyde increases the Bcl-2/Bax expression ratio in
the hippocampus of immunologically sensitized mice.
Neuroimmunomodulation. 13:63–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan Y, Ye C, Tian Q, Yan S, Zeng X, Xiao
C, Wang L and Wang H: miR-145 suppresses the proliferation,
invasion and migration of NSCLC cells by regulating the BAX/BCL-2
ratio and the caspase-3 cascade. Oncol Lett. 15:4337–4343.
2018.PubMed/NCBI
|
|
46
|
Walensky LD: BCL-2 in the crosshairs:
Tipping the balance of life and death. Cell Death Differ.
13:1339–1350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hastak K, Gupta S, Ahmad N, Agarwal MK,
Agarwal ML and Mukhtar H: Role of p53 and NF-kappaB in
epigallocatechin-3-gallate-induced apoptosis of LNCaP cells.
Oncogene. 22:4851–4859. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin HH, Chen JH, Huang CC and Wang CJ:
Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric
carcinoma cells involving JNK/p38 MAPK signaling activation. Int J
Cancer. 120:2306–2316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kinnally KW and Antonsson B: A tale of two
mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis.
12:857–868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Skulachev VP: Role of uncoupled and
non-coupled oxidations in maintenance of safely low levels of
oxygen and its one-electron reductants. Q Rev Biophys. 29:169–202.
1996. View Article : Google Scholar : PubMed/NCBI
|