Introduction
Cervical cancer is the fourth most frequently
diagnosed cancer and the fourth leading cause of cancer-associated
death in women worldwide, with an estimated 570,000 new cases and
311,000 deaths reported in 2018 (1).
The number of patients with cervical cancer is increasing,
particularly in developing countries; moreover, it ranks second as
the cause of mortality among women, after breast cancer (1). Patients with cervical cancer are
currently treated with radical hysterectomy in addition to pelvic
lymph node dissection, or concurrent platinum-based
chemoradiotherapy (CRT), and course of treatment is generally
determined by tumor stage and size (2–4).
Notably, treatment at early stages is directly associated with
improved clinical outcomes (5). A
meta-analysis of patients treated by CRT reported that the 5-year
overall survival (OS) rate for stage I and II was >80%, but it
was 40–60 and 10–40% for stage III and IVA, respectively (6). Furthermore, current CRT using
image-guided brachytherapy (IGBT) achieved significant local
control if treatment was started early (7). A multicentre retrospective study
(RetroEMBRACE) reported that the 5-year local control rate for
definitive external beam radiotherapy (EBRT) with or without
chemotherapy followed by IGBT for stage IB, IIB and IIIB was 98, 91
and 75%, respectively (7). However,
since clinical outcomes after CRT for advanced diseases remain
unsatisfactory, the establishment of biomarkers that affect
survival, local control and distant metastasis is a critical
issue.
The development of immune checkpoint therapy using
anti-programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1)
antibodies caused a paradigm shift in cancer therapy. Notably, it
highlighted the importance of antitumor immunity in cancer
treatment. PD-L1 which is expressed on the surface of various
cells, including tumor cells, activated T cells and
antigen-presenting cells, such as dendritic cells,
macrophages/monocytes and B cells is the major ligand for PD-1
(8,9). In tumor microenvironments, the
interaction between PD-L1 on tumor cells and PD-1 on T cells
induces T-cell exhaustion, resulting in tumor escape from host
immune surveillance (10,11). Indeed, cancers with high PD-L1
expression are associated with a poor prognosis due to the
suppression of immune function (12). By contrast, CD8+
tumor-infiltrating lymphocytes (TILs), which serve an important
role in immune response for eliminating tumor cells, have been
reported as biomarkers for clinical outcomes of cervical cancer
(13–15). However, for cervical cancer,
conflicting results have been reported for the prognostic value of
PD-L1 and CD8+ TILs and, thus, their utility for
prognosis remains unclear (16–18).
Additionally, while immune responses induced by radiotherapy (RT)
have been characterized in several preclinical models (19), immune responses in patients with
cervical cancer have not been sufficiently analyzed. The present
study aimed to analyze the alterations and associations between
patient outcomes and PD-L1 expression or density of CD8+
TILs using biopsy specimens before and during RT in patients with
cervical cancer. The findings of the present study suggested that
CD8+ TILs density have potential to be a predictive
biomarker for patients with cervical cancer treated with
CRT/RT.
Materials and methods
Patients and tumor
characteristics
In the present retrospective analysis, 75
consecutive patients with uterine cervical squamous cell carcinoma
(median age, 62 years; range, 32–87 years) who underwent concurrent
platinum-based CRT or RT alone between August 2009 and November
2013 at the Gunma University Hospital (Maebashi, Japan) were
enrolled. Herein, the abbreviation ‘RT’ represents both CRT/RT
unless otherwise stated. Paired tumor specimens obtained from all
patients before RT (pre-RT) and after 10 Gy RT (post-10 Gy) were
used for pathological analysis and immunohistochemistry (IHC). Most
specimens in both groups (~89% in the RT alone group and ~96% in
the CRT group) were collected on days 8–9 (range, 5–11 days;
Table SI). The number of days
between application of 10 Gy RT and biopsy of the specimens was in
the range of 0–4 days. Of these, 60/75 (80%) were performed on the
same day or one day later (Table
SII). Specimens were used to examine PD-L1 expression and
stromal CD8+ TILs. Patient characteristics were recorded
for tumor stage [stages IB, IIA, IIB, IIIA, IIIB and IVA according
to the International Federation of Gynecology and Obstetrics [FIGO
classification 2008 (20)], age and
lymph node metastasis (Table I). The
Institutional Review Board for clinical trials of Gunma University
approved the study protocol.
 | Table I.Characteristics of patients with
uterine cervical squamous cell carcinoma enrolled the present study
(n=75). |
Table I.
Characteristics of patients with
uterine cervical squamous cell carcinoma enrolled the present study
(n=75).
|
Characteristics | Value |
|---|
| Observation period
(range), months | 63 (8–120) |
| Median age (range),
years | 62 (32–87) |
| Treatment, n
(%) |
|
| RT
alone | 27 (36) |
|
Concurrent CRT | 48 (64) |
| FIGO stage, n
(%) |
|
| IB | 11 (15) |
| II | 31 (41) |
|
III | 31 (41) |
|
IVA | 2 (3) |
| Lymph node
metastasis in pelvis, n (%) |
|
| + | 36 (48) |
| - | 39 (52) |
| Para-aortic lymph
node metastasis, n (%) |
|
| + | 6 (8) |
| - | 69 (92) |
Treatment
All patients underwent definitive RT, involving a
combination of EBRT and intracavitary brachytherapy (ICBT). EBRT
was typically administered using a four-field technique and 10 MV
X-ray. The most common EBRT dose and fractionation regimen was 50
Gy in 25 fractions. EBRT was performed with a combination of
whole-pelvic irradiation (20-40 Gy) followed by 3-cm-wide central
shielding irradiation. In patients with para-aortic lymph node
metastases, pelvic irradiation fields were extended to include the
gross metastatic region (2 Gy/fraction, 1 fraction/day, 5
fractions/week). For patients with lymph node metastases,
additional boost irradiation of 6–8 Gy in 3–4 fractions was
administered. ICBT was performed once per week, concurrently with
the central shielding EBRT. EBRT was skipped on the day of ICBT
administration. Three-dimensional IGBT was performed with a
high-dose rate source using an 192Ir remote afterloading
system (microSelectron; Elekta Instrument AB) for all patients. The
prescribed dose of each ICBT was determined to cover 90% of
high-risk clinical target volume with a 6 Gy total dose.
Interstitial brachytherapy was added along with ICBT for bulky
and/or asymmetric tumors. ICBT was most commonly performed four
times.
Concurrent chemotherapy was administered to 63.0% of
the patients (48/75). Cisplatin was typically administered weekly
at a dose of 40 mg/m2 for patients treated with CRT.
Follow-up and assessment of clinical
outcomes
After the completion of CRT/RT, patients were
followed up every 1–3 months for the first two years and every 3–6
months for three subsequent years by radiation oncologists. During
each follow-up examination, disease status was assessed in terms of
locoregional control (LC) and progression-free survival (PFS). OS
was defined as the term from initial RT until death as a result of
any cause or the date of last follow up. LC was defined as no
evidence of tumor regrowth or recurrence in the pelvic region. PFS
was defined as no evidence of tumor regrowth or recurrence in the
pelvic region or distant metastasis.
IHC analysis for PD-L1 expression and
CD8+ TILs
PD-L1 expression on tumor cells and density of
stromal CD8+ TILs were evaluated with IHC using biopsy
samples excised from the cervical cancer samples pre-RT and post-10
Gy. Biopsied samples were fixed in 10% buffered formalin for 24 h
at room temperature, then dehydrated, degreased and
paraffin-embedded. Paraffin sections (4-µm-thick) were dewaxed in
xylene at room temperature and rehydrated using a graded ethanol
series. Endogenous peroxidase activity was blocked with a 10 min
incubation at room temperature in 0.3% hydrogen peroxide.
Subsequently, PD-L1 sections were heated in 1 mmol/l
ethylenediaminetetraacetic acid (pH 8.0) and CD8 sections were
heated in 0.01 mol/l citric acid (pH 6.0) at 121°C for 10 min for
antigen retrieval. After blocking by 10% goat normal serum for
PD-L1 sections and 10% rabbit normal serum for CD8 sections with a
20 min incubation at room temperature, sections were incubated
overnight with primary antibodies at 4°C. Next day, the sections
were incubated with biotin-labeled secondary antibodies followed by
peroxidase-labeled streptavidin (Histofine; SAB-PO (rabbit) cat.
no. 424032 and SAB-PO (mouse) cat. no. 424022; Nichirei Biosciences
Inc.; Nichirei Corporation), both for 20 min each at room
temperature. Then, sections were incubated for 5 min with
diaminobenzidine at room temperature for detecting the molecules.
The following primary antibodies were used: Monoclonal anti-PD-L1
antibody (1:100; clone E1L3N; rabbit IgG; cat. no. 13684; Cell
Signaling Technology, Inc.) and monoclonal anti-CD8 antibody
(1:800; clone C8/144B; mouse IgG; cat. no. M7103; Dako; Agilent
Technologies, Inc.).
All immunostaining images were obtained using a
KEYENCE light microscope (BZ-9000; Keyence Corporation;
magnification, ×400). PD-L1+ tumor cells and
CD8+ cells were automatically counted using the visual
inspection application software BZ-X analyzer JP ver.1.4.1 (Keyence
Corporation). Percentages of tumor cells with cell surface staining
for PD-L1 were recorded and presented as tumor proportion score
(TPS). When the TPS was >1%, the sample was classified as
PD-L1+. A minimum of 100 tumor cells was evaluated to
calculate the TPS. The percentage of CD8+ TILs among
total nucleated cells in the stromal compartments was defined as
stromal CD8+ TIL density (21). To calculate stromal CD8+
TIL density, the CD8+ cells in hotspot areas of the
specimens were counted; hotspot areas were defined as those
containing the highest density of nucleated cells (22). The tumor area was manually excluded.
The quality of tumor samples was carefully evaluated and validated
independently by 2 co-authors (Department of Human Pathology, Gunma
University Graduate School of Medicine) of the present study who
were pathologists.
Statistical analysis
The OS, LC and PFS rates were calculated using the
Kaplan-Meier method, and the log-rank test was used to confirm
significant differences. Continuous data were compared with a
non-parametric test (Wilcoxon signed-rank test for paired data).
Receiver operating characteristic (ROC) curve analyses were
performed to determine the optimal cut-off value of CD8+
TIL density. Univariate analyses were performed using Cox
proportional hazards model. P<0.05 was considered to indicate a
statistically significant difference. Bonferroni correction was
used to adjust the familywise error rate from multiple comparisons
for P-values. All statistical analyses were performed using SPSS
26.0 for Mac (IBM Corp.).
Results
Clinical characteristics and
outcomes
Median follow-up duration was 63 months (range,
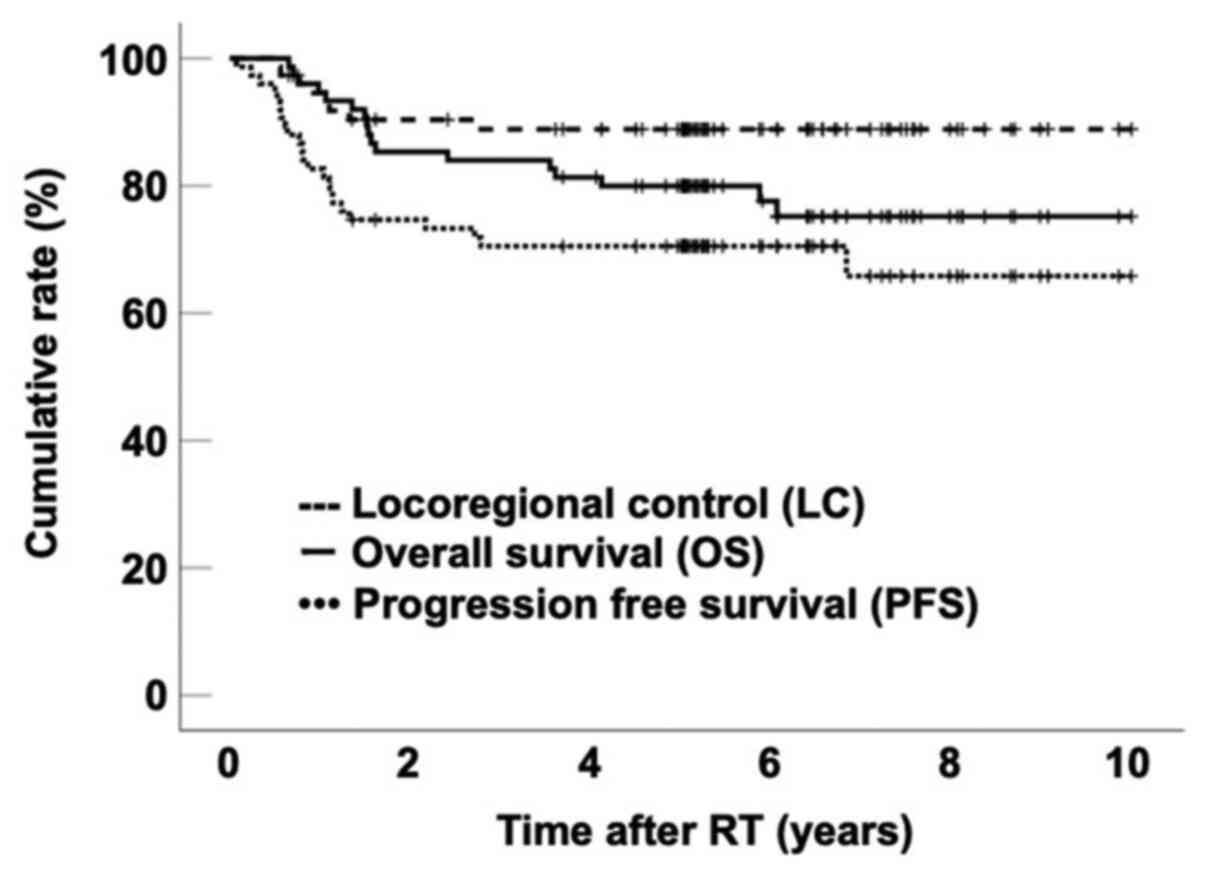
8–120 months). The 5-year OS, LC and PFS rates for all patients
were 80.0% (95% CI, 70.9–89.0%), 89.0% (95% CI, 81.6–96.2%) and
70.5% (95% CI, 60.1–80.9%), respectively (Fig. 1). Patient clinical characteristics
are shown in Table I.
PD-L1 expression and clinical
outcomes
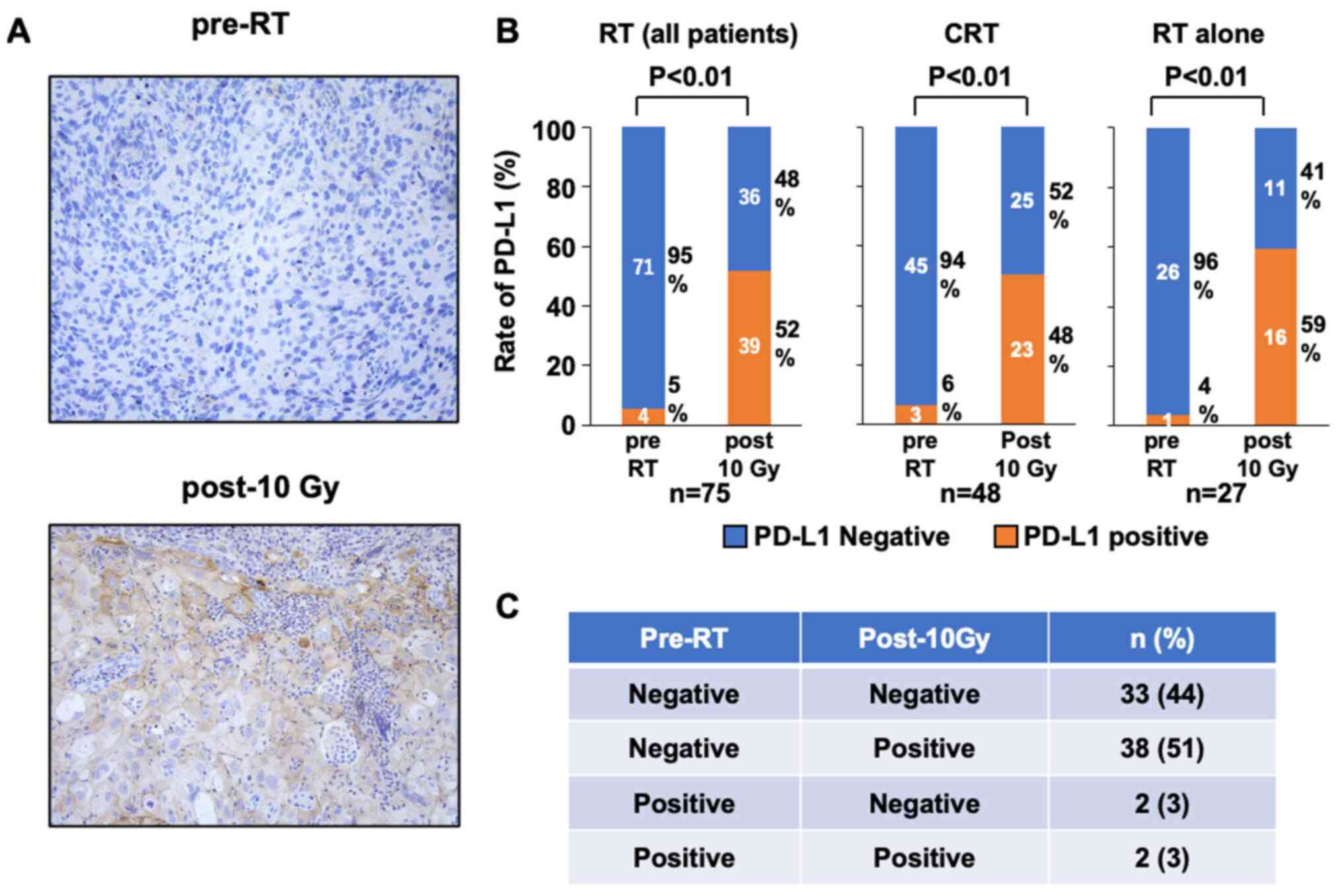
To investigate the alteration of PD-L1 expression
post-10 Gy, the rate of PD-L1+ cells in the IHC samples
pre-RT and post-10 Gy was analyzed (Fig.
2A). In pre-RT samples, 4 patients (5%) were positive and 71
patients (95%) were negative; in post-10 Gy samples, 39 patients
(52%) were positive and 36 patients (48%) were negative (Fig. 2B). In all the patient groups, the
percentage of PD-L1+ patients significantly increased
from 5 to 52% between pre-RT and post-10 Gy (P<0.01; Fig. 2B). In patients treated with CRT, the
percentage of PD-L1+ patients significantly increased
from 6 to 48% between pre-RT and post-10 Gy (P<0.01; Fig. 2B). In patients treated with RT alone,
the percentage of PD-L1+ patients significantly
increased from 4 to 59% between pre-RT and post-10 Gy (P<0.01;
Fig. 2B). Therefore, RT
significantly upregulated PD-L1 expression in patients with uterine
cervical squamous cell carcinoma. When compared with PD-L1
expression before RT, 38 patients (51%) exhibited an increase, two
patients (3%) exhibited a decrease and 35 patients (47%) exhibited
no change post-10 Gy RT (Fig.
2C).
Subsequently, the association between pre-RT and
post-10 Gy PD-L1 expression and clinical outcome was examined. OS,
LC and PFS did not display significant differences with positive
and negative PD-L1 expression for both pre-RT and post-10 Gy
samples (Fig. S1A-F). Furthermore,
patients were categorized into three groups according to changes in
PD-L1 expression. It was revealed that PD-L1 alteration exhibited
no significant association with clinical outcome (Fig. S1G-I).
CD8+ TILs and clinical
outcomes
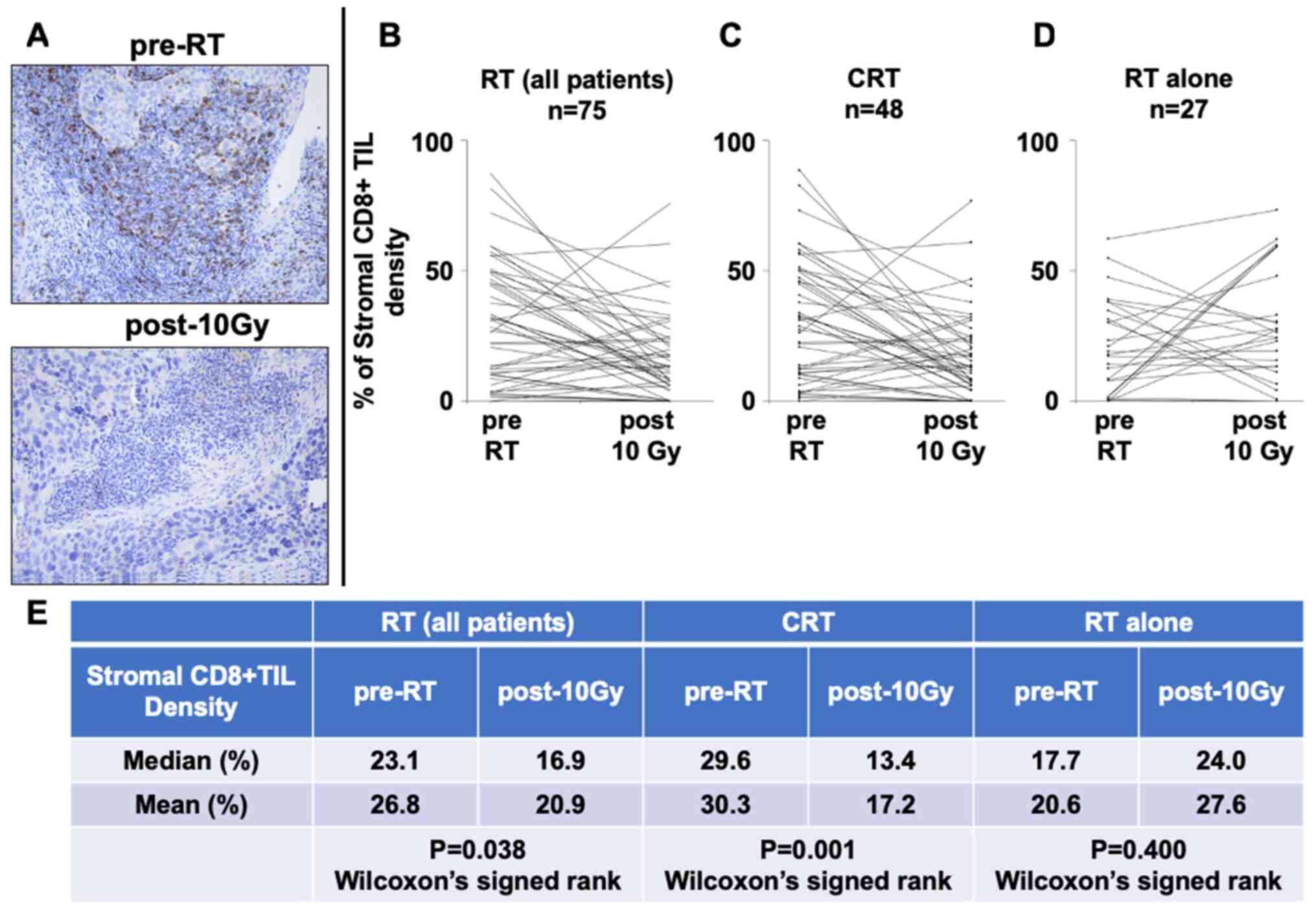
To investigate the alteration of CD8+
TILs in tumor tissues post-10 Gy, the density of stromal
CD8+ TILs in the IHC samples both pre-RT and post-10 Gy
was analyzed (Fig. 3A). IHC staining
revealed that 31 patients (41.3%) exhibited an increase, while 44
patients (58.7%) exhibited a decrease in stromal CD8+
TIL density after 10 Gy (Fig. 3B).
Stromal CD8+ TIL density was significantly decreased in
post-10 Gy samples (median density, 23.1% pre-RT vs. 16.9% post-10
Gy; P=0.038; Fig. 3B and E).
According to the treatment type, stromal CD8+ TIL
density was significantly decreased after CRT (median density,
29.6% pre-RT vs. 13.4% post-10 Gy; P=0.001; Fig. 3C and E). By contrast, stromal
CD8+ TIL density tended to increase after RT alone
(median, 17.7% pre-RT vs. 24.0% post-10 Gy; P=0.400; Fig. 3D and E). No significant difference
was observed in the density of pre-RT CD8+ TILs between
the CRT and RT alone groups (data not shown). Furthermore, to
evaluate the optimal cut-off value for CD8+ TIL density
in the present study, a ROC curve analysis was performed. The
association between clinical outcome and stromal CD8+
TIL density was assessed in all 75 patients. ROC curve analysis
revealed that both pre-RT and post-10 Gy CD8+ TIL
density exhibited significant prognostic value for predicting death
and tumor recurrence, with optimal cut-off values of 32.2 and
16.9%, respectively (Fig. S2).
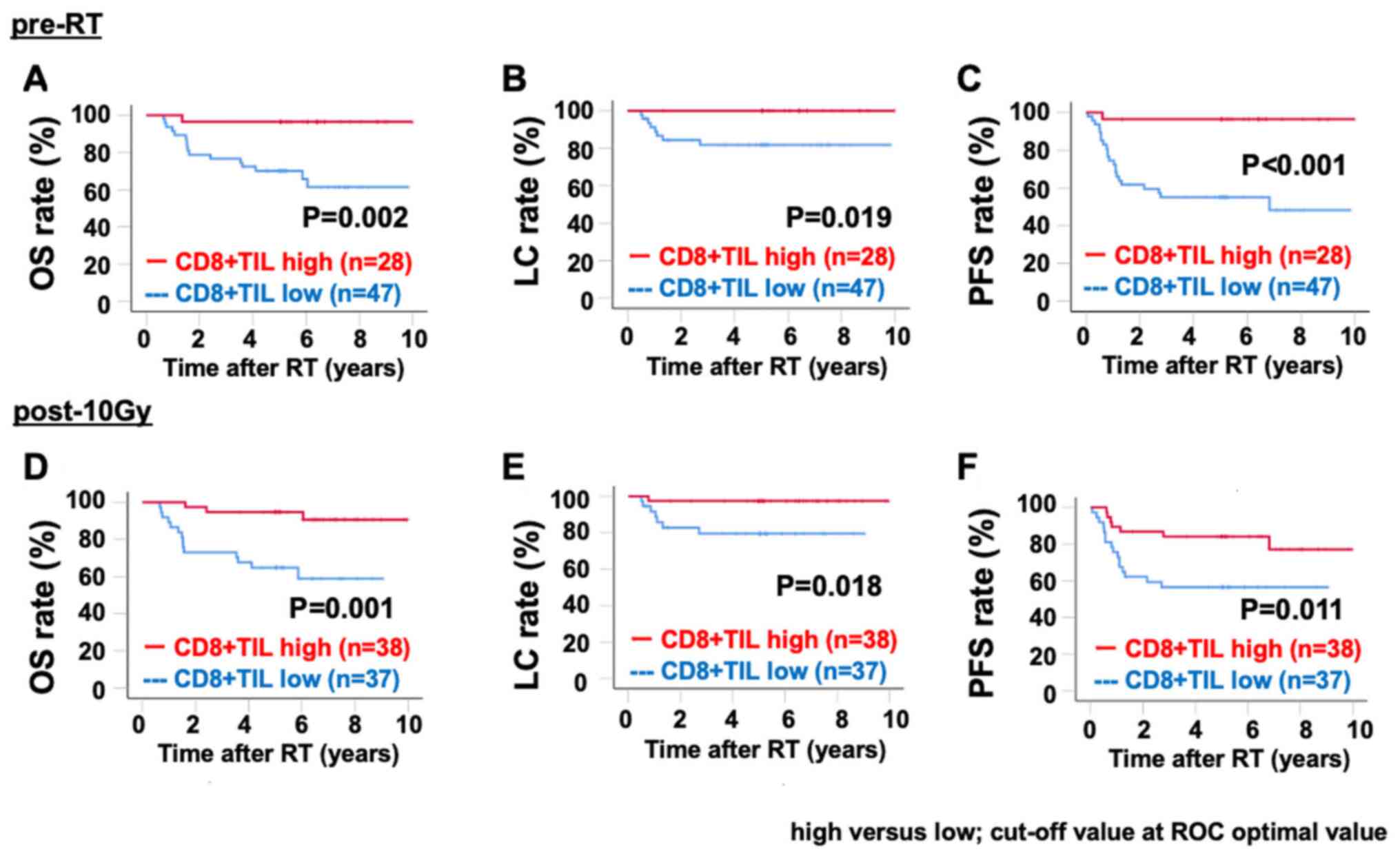
Notably, patients with high stromal CD8+ TIL density
(based on the ROC optimal cut-off values) both pre-RT and post-10
Gy exhibited significantly improved OS, LC and PFS compared with
patients with low stromal CD8+ TIL density (Fig. 4A-F).
Furthermore, to analyze the effects of each factor
on prognosis, univariate analyses using Cox proportional hazards
model were performed (Tables
II–IV). Univariate analyses
revealed that FIGO stages III–IVA and lymph node metastasis in the
pelvis were significantly associated with unfavorable OS and PFS
(Tables II and IV). Univariate analyses revealed that
para-aortic lymph node metastasis in the pelvis were significantly
associated with unfavorable LC (Table
III). Notably, high stromal CD8+ TILs in both pre-RT
and post-10 Gy groups exhibited a significant association with
improved OS and PFS (Tables II and
IV).
 | Table II.Univariate analysis of overall
survival. |
Table II.
Univariate analysis of overall
survival.
|
| Univariate
analysis |
|---|
|
|
|
|---|
| Factor | Hazard ratio (95%
CI) | P-value |
|---|
| Age (≥62 years vs.
<62 years) | 0.868
(0.334–2.252) | 0.770 |
| Concurrent
chemotherapy (no vs. yes) | 0.223
(0.211–1.423) | 0.548 |
| FIGO stage (I+II
vs. III+IV) | 7.041
(2.020–24.541) | 0.002 |
| PeLN (positive vs.
negative) | 0.277
(0.090–0.851) | 0.025 |
| PALN (positive vs.
negative) | 0.365
(0.105–1.273) | 0.114 |
| CD8+
TILs (pre-RT) (low vs. high) | 0.086
(0.110–6.46) | 0.017 |
| CD8+
TILs (post-10 Gy) (low vs. high) | 0.160
(0.046–0.560) | 0.004 |
 | Table IV.Univariate analysis of
progression-free survival. |
Table IV.
Univariate analysis of
progression-free survival.
|
| Univariate
analysis |
|---|
|
|
|
|---|
| Factor | Hazard ratio (95%
CI) | P-value |
|---|
| Age (≥62 years vs.
<62 years) | 0.822
(0.355–1.906) | 0.648 |
| Concurrent
chemotherapy (no vs. yes) | 0.463
(0.200–1.070) | 0.072 |
| FIGO stage (I+II
vs. III+IV) | 4.264
(1.664–10.927) | 0.003 |
| PeLN (positive vs.
negative) | 0.318
(0.124–0.816) | 0.017 |
| PALN (positive vs.
negative) | 0.293
(0.098–0.874) | 0.280 |
| CD8+
TILs (pre-RT) (low vs. high) | 0.302
(0.102–0.894) | 0.031 |
| CD8+
TILs (post-10 Gy) (low vs. high) | 0.368
(0.150–0.905) | 0.030 |
 | Table III.Univariate analysis of locoregional
control duration. |
Table III.
Univariate analysis of locoregional
control duration.
|
| Univariate
analysis |
|---|
|
|
|
|---|
| Factor | Hazard ratio (95%
CI) | P-value |
|---|
| Age (≥62 years vs.
<62 years) | 0.630
(0.105–3.792) | 0.614 |
| Concurrent
chemotherapy (no vs. yes) | 0.321
(0.053–1.935) | 0.215 |
| FIGO stage (I+II
vs. III+IV) | 2.104
(0.350–12.638) | 0.416 |
| PeLN (positive vs.
negative) | 1.403
(0.233–8.457) | 0.711 |
| PALN (positive vs.
negative) | 0.126
(0.021–0.757) | 0.024 |
| CD8+
TILs (pre-RT) (low vs. high) | 0.337
(0.037–3.053) | 0.334 |
| CD8+
TILs (post-10 Gy) (low vs. high) | 0.187
(0.021–1.703) | 0.137 |
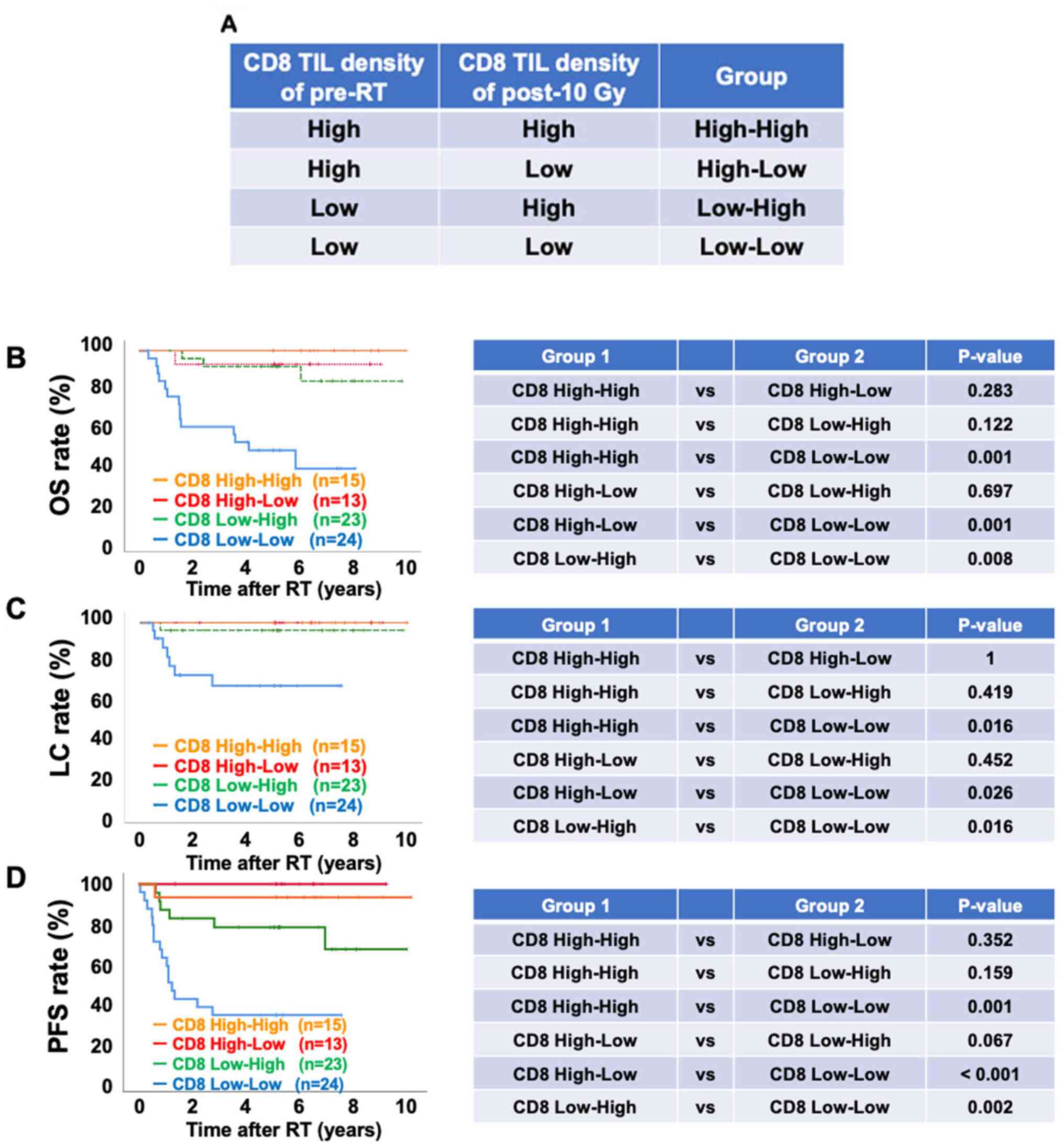
Finally, patients were classified into four groups
according to stromal CD8+ TIL density pre-RT and post-10
Gy [i) High (pre-RT CD8 density)-high (post-10 Gy CD8 density)
defined as CD8 High-High; ii) high (pre-RT CD8 density)-low
(post-10 Gy CD8 density) defined as CD8 high-low; ii) low (pre-RT
CD8 density)-high (post-10 Gy CD8 density) defined as CD8 low-high
and iv) low (pre-RT CD8 density)-low (post-10 Gy CD8 density)
defined as CD8 low-low) (Fig. 5A).
Notably, the group displaying low stromal CD8+ TIL
density both pre-RT and post-10 Gy (CD8 Low-Low group) exhibited a
significantly lower OS, LC and PFS compared with the other groups
(Fig. 5B-D). Overall, the current
data suggested that low stromal CD8+ TIL density either
pre-RT or post-10 Gy may be a critical predictive biomarker for
patients with cervical cancer treated with definitive RT.
Association between PD-L1 expression
and CD8+ TILs
To clarify the association between PD-L1 expression
and CD8+ TIL density, the changes in PD-L1 expression
and CD8+ TIL density in the 10-Gy irradiated samples
were analyzed. It was revealed that 68% (21/31) of the increased
CD8+ TIL density group also exhibited an increase in
PD-L1 expression, whereas 61% (27/44) of the decreased
CD8+ TIL density group exhibited no increase in PD-L1
expression (Table V). Thus, there
was a significant association between an elevation in
CD8+ TIL density and the induction of PD-L1 expression
(P=0.013; Table V). Furthermore, the
present study investigated whether changes in CD8+ TILs
and PD-L1 expression in response to 10 Gy affected clinical
outcomes in patients. However, a log-rank test did not indicate a
significant association between changes in CD8+ TIL
density and PD-L1 expression with prognosis (Fig. S3).
 | Table V.Alterations in PD-L1 expression and
CD8+ TIL density. |
Table V.
Alterations in PD-L1 expression and
CD8+ TIL density.
|
| PD-L1
expression |
|
|---|
|
|
|
|
|---|
| CD8+ TIL
density |
Unchanged/decreased, n (%) | Increased, n
(%) | P-value |
|---|
| Decreased | 27 (61) | 17 (39) | 0.013 |
| Increased | 10 (32) | 21 (68) |
|
Discussion
In the present study, it was demonstrated that the
PD-L1+ rate was significantly increased from 5% pre-RT
to 52% post-10 Gy, and the median density of stromal
CD8+ TILs was significantly decreased from 23.1% pre-RT
to 16.9% post-10 Gy in patients with cervical squamous cancer.
However, stromal CD8+ TIL density tended to increase
from a median of 17.7% pre-RT to 24.0% post-10 Gy in patients who
received RT alone, suggesting that chemotherapy may be involved in
the alternation. With regard to the association of stromal
CD8+ TIL density with prognosis, univariate analyses
revealed that stromal CD8+ TIL density pre-RT and
post-10 Gy was significantly associated with improved OS and PFS.
By contrast, PD-L1 expression did not show any significant
association with the clinical outcomes, despite its upregulation
post-10 Gy. To the best of our knowledge, the present study was the
first to assess CD8+ TILs and PD-L1 expression of biopsy
specimens by IHC during RT (post-10 Gy).
Notably, the present study revealed that stromal
CD8+ TIL density tended to increase only in patients
receiving RT alone. Consistent with the current data, neoadjuvant
RT alone increased stromal CD8+ TIL density in patients
with rectal cancer (23). In
addition, the induction of CD8+ TILs into the irradiated
field after RT alone has been demonstrated in a mouse model
(24). In contrast to the results of
RT alone, there was a significant decrease in stromal
CD8+ TIL density in the overall RT and CRT groups. This
decrease may have been caused by a decrease in the number of
systemic immune cells due to chemotherapy. However, increased
CD8+ TIL ratio after neoadjuvant CRT has been observed
in patients with colorectal cancer (23), non-small-cell carcinoma (25) and esophageal cancer (26). Thus, at present, changes in
CD8+ TIL levels in tumor tissues caused by CRT/RT are
controversial. Indeed, preclinical models have revealed that
chemotherapy induces immunogenic cell death, which may recruit
CD8+ TILs into the tumor tissues (27–29). The
elucidation of the molecular mechanism underlying CD8+
TILs migration into tumors is required to understand the current
controversial results dependent on the modality, tissue
specificity, type of cancer and other factors.
The present study demonstrated that high density of
CD8+ TILs may contribute to improved outcomes, whereas
CRT/RT was less effective in tumors with low density of
CD8+ TILs before treatment. Consistent with the present
results, clinical studies have indicated that high levels of
CD8+ TILs in tumor tissues before treatment are
associated with improved outcomes in patients with cervical cancer
(13,14). Similarly, post-CRT stromal
CD8+ TILs have been associated with improved OS in
patients with rectal cancer (30).
The idea that tumors harboring high levels of CD8+ TIL
exhibit improved outcomes with RT is also supported by our previous
data indicating that the depletion of CD8+ T cells using
an anti-CD8 antibody significantly suppresses the effect of RT on
tumor growth delay (31). Thus, the
present study confirmed the aforementioned previous findings.
PD-L1 upregulation by RT has been demonstrated in
several preclinical models (32,33). A
recent similar clinical study revealed PD-L1 upregulation after RT
in patients with squamous cervical cancer (14). The novel aspect of the present study
is that PD-L1 and CD8+ TILs were assessed in the
biopsied specimens during RT. Our previous in vitro study
indicated that PD-L1 upregulation after DNA damage by X-ray
irradiation was not maintained for >14 days (28). Thus, the current post-10 Gy samples
may reflect the DNA damage-induced PD-L1 upregulation more
directly. In particular circumstances involving CD8+
TILs and PD-L1 upregulation after RT, anti-PD-1/PD-L1 antibodies in
combination with RT may be more effective, because PD-L1 expression
is considered as a predictive biomarker of response rate for
anti-PD-1/PD-L1 antibody (34).
Based on this idea, several studies have started clinically testing
the combined use of an anti-PD-1/PD-L1 antibody during or after RT,
suggesting promising outcomes (35–37).
A limitation of the present study is its
retrospective evaluation of 75 cases from a single institute. The
number of cases is small, and various clinical stages (stages I–IV)
were included. Therefore, a larger analysis is required to clarify
the association between clinical outcomes and PD-L1 expression or
CD8+ TILs. In addition, to identify novel prognostic
biomarkers, evaluation of other factors affecting the immune
response in tumor microenvironments, such as HLA class I
expression, myeloid-derived suppressor cells, M2 tumor-associated
macrophages and regulatory T cells, may be important. Furthermore,
knowledge of patients' characteristics leading to PD-L1
upregulation and CD8+ TILs alteration after CRT/RT will
be valuable. The present data suggest the potential of PD-1/PD-L1
blockade immunotherapy for patients with increased PD-L1 expression
after 10 Gy. However, it is currently not appropriate to use
anti-PD-1/PD-L1 antibodies instead of cisplatin, which is the
current standard of care for patients with cervical cancer
(38,39). Future studies should also explore
this treatment method for patients with other types of cancer.
In summary, the present study revealed that CRT/RT
induced PD-L1 upregulation in patients with cervical cancer and
affected the density of stromal CD8+ TILs. Stromal
CD8+ TIL density may be a predictive biomarker both
pre-RT and post-10 Gy. Therefore, careful follow-up may be crucial
for patients with cervical cancer with low CD8+ TILs
before RT or no increase in CD8+ TILs during RT. To
investigate the clinical significance of radiation-induced PD-L1
upregulation and CD8+ TILs alteration, additional
studies with large cohorts are required.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mr. Koji Isoda
(Gunma University, Maebashi, Japan) for his technical assistance in
performing the immunohistochemical analysis.
Funding
The present study was supported by Japan Society for
the Promotion of Science KAKENHI (grant nos. JP17H04713 and
JP19K08195), the Takeda Science Foundation, the Uehara Memorial
Foundation, the Astellas Foundation for Research on Metabolic
Disorders, The Kanae Foundation for the Promotion of Medical
Science, the Yasuda Memorial Medicine Foundation and the Nakajima
Foundation. Additionally, the present study was supported by the
Program of the network-type Joint Usage/Research Center for
Radiation Disaster Medical Science of Hiroshima University,
Nagasaki University and Fukushima Medical University, and the
Grants-in-Aid from the Ministry of Education, Culture, Sports,
Science and Technology of Japan for programs for Leading Graduate
Schools, Cultivating Global Leaders in Heavy Ion Therapeutics and
Engineering.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM summarized the patient information. YM, HS, TKu,
TOi, KS, HI, HY and AS performed the experiments and analyzed the
data. YM, HS, TBMP, SK and AS were involved in drafting the
manuscript and made substantial contributions to analysis and
interpretation of data. YM, KM, SEN, TKu, KA, YY, NO, TKa, KO, TN
and TOh coordinated the clinics, performed the treatment,
participated in the follow-up of the patients, obtained specimens
and acquired data. TN and TOh contributed
reagents/materials/analysis tools and gave final approval of the
manuscript version to be published. The authenticity of all the raw
data was assessed by YM and HS. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board for clinical trials
of Gunma University (Maebashi, Japan) approved the study protocol
(approval no. HS2020-015). This study is a retrospective and
observational study. All patients provided their informed consent
to participate in the study using the opt-out approach by public
notice at internet site of the Institutional Review Board for
clinical trials of Gunma University (Maebashi, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: An update of
radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stehman FB, Ali S, Keys HM, Muderspach LI,
Chafe WE, Gallup DG, Walker JL and Gersell D: Radiation therapy
with or without weekly cisplatin for bulky stage 1B cervical
carcinoma: Follow-up of a gynecologic oncology group trial. Am J
Obstet Gynecol. 197:503.e1–e6. 2007. View Article : Google Scholar
|
|
4
|
Zhao H, Li L, Su H, Lin B, Zhang X, Xue S,
Fei Z, Zhao L, Pan Q, Jin X and Xie C: Concurrent
paclitaxel/cisplatin chemoradiotherapy with or without
consolidation chemotherapy in high-risk early-stage cervical cancer
patients following radical hysterectomy: Preliminary results of a
phase III randomized study. Oncotarget. 7:70969–70978. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lea JS and Lin KY: Cervical cancer. Obstet
Gynecol Clin North Am. 39:233–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chemoradiotherapy for Cervical Cancer
Meta-Analysis Collaboration, . Reducing uncertainties about the
effects of chemoradiotherapy for cervical cancer: A systematic
review and meta-analysis of individual patient data from 18
randomized trials. J Clin Oncol. 26:5802–5812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sturdza A, Pötter R, Fokdal LU, Haie-Meder
C, Tan LT, Mazeron R, Petric P, Šegedin B, Jurgenliemk-Schulz IM,
Nomden C, et al: Image guided brachytherapy in locally advanced
cervical cancer: Improved pelvic control and survival in
RetroEMBRACE, a multicenter cohort study. Radiother Oncol.
120:428–433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishida M, Iwai Y, Tanaka Y, Okazaki T,
Freeman GJ, Minato N and Honjo T: Differential expression of PD-L1
and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of
lymphohematopoietic tissues. Immunol Lett. 84:57–62. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamazaki T, Akiba H, Iwai H, Matsuda H,
Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al:
Expression of programmed death 1 ligands by murine T cells and APC.
J Immunol. 169:5538–5545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Liu F and Liu L: Prognostic
significance of PD-L1 in solid tumor: An updated meta-analysis.
Medicine. 96:e63692017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Xia B, Zheng T and Lou G:
Immunoscore system combining CD8 and PD-1/PD-L1: A novel approach
that predicts the clinical outcomes for cervical cancer. Int J Biol
Markers. 35:65–73. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuchiya T, Someya M, Takada Y, Hasegawa
T, Kitagawa M, Fukushima Y, Gocho T, Hori M, Nakata K, Hirohashi Y,
et al: Association between radiotherapy-induced alteration of
programmed death ligand 1 and survival in patients with uterine
cervical cancer undergoing preoperative radiotherapy. Strahlenther
Onkol. 196:725–735. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyasaka Y, Yoshimoto Y, Murata K, Noda
SE, Ando K, Ebara T, Okonogi N, Kaminuma T, Yamada S, Ikota H, et
al: Treatment outcomes of patients with adenocarcinoma of the
uterine cervix after definitive radiotherapy and the prognostic
impact of tumor-infiltrating CD8+ lymphocytes in pre-treatment
biopsy specimens: A multi-institutional retrospective study. J
Radiat Res. 61:275–284. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karim R, Jordanova ES, Piersma SJ, Kenter
GG, Chen L, Boer JM, Melief CJ and van der Burg SH: Tumor-expressed
B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and
survival of patients with cervical carcinoma. Clin Cancer Res.
15:6341–6347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Enwere EK, Kornaga EN, Dean M, Koulis TA,
Phan T, Kalantarian M, Köbel M, Ghatage P, Magliocco AM,
Lees-Miller SP and Doll CM: Expression of PD-L1 and presence of
CD8-positive T cells in pre-treatment specimens of locally advanced
cervical cancer. Mod Pathol. 30:577–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu X, Dong M, Liu Z, Mi Y, Yang J, Zhang
Z, Liu K, Jiang L, Zhang Y, Dong S and Shi Y: Elevated PD-L1
expression predicts poor survival outcomes in patients with
cervical cancer. Cancer Cell Int. 19:1462019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carvalho HA and Villar RC: Radiotherapy
and immune response: The systemic effects of a local treatment.
Clinics (Sao Paulo). 73 (Suppl 1):e557s2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donnem T, Hald SM, Paulsen EE, Richardsen
E, Al-Saad S, Kilvaer TK, Brustugun OT, Helland A, Lund-Iversen M,
Poehl M, et al: Stromal CD8+ T-cell Density-A promising supplement
to TNM staging in non-small cell lung cancer. Clin Cancer Res.
21:2635–2643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feldmeyer L, Hudgens CW, Ray-Lyons G,
Nagarajan P, Aung PP, Curry JL, Torres-Cabala CA, Mino B,
Rodriguez-Canales J, Reuben A, et al: Density, distribution, and
composition of immune infiltrates correlate with survival in merkel
cell carcinoma. Clin Cancer Res. 22:5553–5563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teng F, Mu D, Meng X, Kong L, Zhu H, Liu
S, Zhang J and Yu J: Tumor infiltrating lymphocytes (TILs) before
and after neoadjuvant chemoradiotherapy and its clinical utility
for rectal cancer. Am J Cancer Res. 5:2064–2074. 2015.PubMed/NCBI
|
|
24
|
Arina A, Beckett M, Fernandez C, Zheng W,
Pitroda S, Chmura SJ, Luke JJ, Forde M, Hou Y, Burnette B, et al:
Tumor-reprogrammed resident T cells resist radiation to control
tumors. Nat Commun. 10:39592019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoneda K, Kuwata T, Kanayama M, Mori M,
Kawanami T, Yatera K, Ohguri T, Hisaoka M, Nakayama T and Tanaka F:
Alteration in tumoural PD-L1 expression and stromal CD8-positive
tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy
for non-small cell lung cancer. Br J Cancer. 121:490–496. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kelly RJ, Zaidi AH, Smith MA, Omstead AN,
Kosovec JE, Matsui D, Martin SA, DiCarlo C, Werts ED, Silverman JF,
et al: The dynamic and transient immune microenvironment in locally
advanced esophageal adenocarcinoma post chemoradiation. Ann Surg.
268:992–999. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen G and Emens LA: Chemoimmunotherapy:
Reengineering tumor immunity. Cancer Immunol Immunother.
62:203–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van der Most RG, Currie AJ, Cleaver AL,
Salmons J, Nowak AK, Mahendran S, Larma I, Prosser A, Robinson BW,
Smyth MJ, et al: Cyclophosphamide chemotherapy sensitizes tumor
cells to TRAIL-dependent CD8 T cell-mediated immune attack
resulting in suppression of tumor growth. PLoS One. 4:e69822009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kodumudi KN, Woan K, Gilvary DL, Sahakian
E, Wei S and Djeu JY: A novel chemoimmunomodulating property of
docetaxel: Suppression of myeloid-derived suppressor cells in tumor
bearers. Clin Cancer Res. 16:4583–4594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shinto E, Hase K, Hashiguchi Y, Sekizawa
A, Ueno H, Shikina A, Kajiwara Y, Kobayashi H, Ishiguro M and
Yamamoto J: CD8+ and FOXP3+ tumor-infiltrating T cells before and
after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 21
(Suppl 3):S414–S421. 2014. View Article : Google Scholar
|
|
31
|
Yoshimoto Y, Suzuki Y, Mimura K, Ando K,
Oike T, Sato H, Okonogi N, Maruyama T, Izawa S, Noda SE, et al:
Radiotherapy-induced anti-tumor immunity contributes to the
therapeutic efficacy of irradiation and can be augmented by CTLA-4
blockade in a mouse model. PLoS One. 9:e925722014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walle T, Martinez Monge R, Cerwenka A,
Ajona D, Melero I and Lecanda F: Radiation effects on antitumor
immune responses: Current perspectives and challenges. Ther Adv Med
Oncol. 10:17588340177425752018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sato H, Niimi A, Yasuhara T, Permata TBM,
Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S, et al:
DNA double-strand break repair pathway regulates PD-L1 expression
in cancer cells. Nat Commun. 8:17512017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sindoni A, Minutoli F, Ascenti G and
Pergolizzi S: Combination of immune checkpoint inhibitors and
radiotherapy: Review of the literature. Crit Rev Oncol Hematol.
113:63–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karam SD and Raben D: Radioimmunotherapy
for the treatment of head and neck cancer. Lancet Oncol.
20:e404–e416. 2019. View Article : Google Scholar
|
|
37
|
Sato H, Okonogi N and Nakano T: Rationale
of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy
for cancer treatment. Int J Clin Oncol. 25:801–809. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rose PG, Bundy BN, Watkins EB, Thigpen JT,
Deppe G, Maiman MA, Clarke-Pearson DL and Insalaco S: Concurrent
cisplatin-based radiotherapy and chemotherapy for locally advanced
cervical cancer. N Engl J Med. 340:1144–1153. 1999. View Article : Google Scholar : PubMed/NCBI
|