Introduction
Osteosarcoma is a malignant tumor derived from bone,
and it most commonly occurs in adolescents, accounting for 10% of
solid tumors in those who are 15–19 years old (1–3). In
2014, there were ~24,000 newly diagnosed cases of primary bone
cancer and 17,200 deaths due to primary bone cancer in China
(4). The crude incidence rate of
primary bone cancer is 1.76/100,000, and the age-standardized
incidence rate by the Chinese standard population is 1.35/100,000
(4).
MicroRNAs (miRNAs) are small, highly conserved,
non-coding RNA molecules involved in regulating gene expression.
They target mRNA molecules for cleavage or translational
repression, resulting in the degradation of mRNA and/or the
inhibition of mRNA translation (5).
Studies have shown that miRNAs may serve important roles in the
molecular mechanism of osteosarcoma (6,7).
In the present study, a novel miRNA that may serve a
role in the pathogenesis of osteosarcoma was identified. Raw miRNA
expression data was downloaded from The Cancer Genome Atlas (TCGA),
which is a landmark cancer genomics program that characterizes
>20,000 primary cancers of all types. The aim of the TCGA
database is to create a comprehensive ‘atlas’ of cancer genomic
profiles by cataloging and discovering major cancer-causing genomic
alterations (8). By searching for
novel miRNAs associated with the survival of patients with
osteosarcoma, miR-1226-3p was identified as a prime candidate. Some
studies have revealed that miR-1226-3p functions as a tumor
suppressor and may be associated with tamoxifen resistance in
breast cancer (9,10). Furthermore, in a recent study, the
role of miR-1226-3p in hepatocellular carcinoma was evaluated,
revealing that miR-1226-3p expression was downregulated in patients
with hepatocellular carcinoma with progressive disease that
developed following sorafenib treatment through the inhibition of
dual specificity protein phosphatase 4 protein expression (11). However, the role of miR-1226-3p in
osteosarcoma remains unknown and was therefore investigated in the
present study.
Materials and methods
Patients and tissue samples
A total of 35 pairs of osteosarcoma and matched
adjacent normal tissues (~1 cm from tumor) were collected from
patients (sex rate female/male, 17/18; age range, 6–60 years; mean
age, 14.2 years) at the Department of Pediatric Surgery, West China
Hospital, Sichuan University (Chengdu, China). The clinical data of
the 35 patients are listed in Table
SI. The stage of the 35 patients was based on the TNM staging
system (12). The patient
recruitment took place between February 2013 and December 2014. The
histopathological diagnoses of the 35 paired samples were reviewed
by a senior pathologist in the Department of Pathology of West
China Hospital. The Ethics Committee of Sichuan University approved
the usage of the patients' clinical data and tissue samples, and
all patients or their guardians provided written informed consent
for the use of their clinical information and tissue samples. The
inclusion criteria were as follows: i) Histological diagnosis of
high-grade osteosarcoma, ii) no prior history of lenvatinib
treatment, iii) life expectancy of ≥12 weeks, and iv) adequate
organ function per blood work results. The exclusion criteria were:
i) Any active infection, and ii) history of radiotherapy or
chemotherapy.
Bioinformatics analysis
For TCGA analysis, the pan-cancer miRNA expression
dataset (pancanMiRs_EBadjOnProtocolPlatform
WithoutRepsWithUnCorrect MiRs_08_04_16.xena) and clinical
information dataset (Survival_Supplemental
Table_S1_20171025_xena_sp) were downloaded using the Xena Browser
(https://xenabrowser.net/). The datasets were
processed with the dplyr and tribble packages (13). The miRNA expression dataset and
clinical information dataset were then merged, and the miRNA
information from patients with sarcoma was extracted for survival
analysis. The survminer package (https://cran.r-project.org/web/packages/survminer/index.html)
was installed in the R language for survival analysis. The clinical
data of the patients from TCGA are listed in Table SII. In summary, the total number of
patients with osteosarcoma from TCGA was 368 (sex ratio
male:female, 160:208; mean age ± SD, 66±14.77 years; age range,
20–90 years). The most common clinicopathological subtypes were
myxofibrosarcoma (11%), leiomyosarcoma (42%) and dedifferentiated
liposarcoma (20%).
Cell culture
Human osteosarcoma SaOS-2 cells (ATCC HTB-85™) and
fetal human osteoblastic hFOB 1.19 cells were purchased from the
American Type Culture Collection. SaOS-2 cells were cultured in
McCoy's 5A (Modified) medium (cat. no. 16600108; Thermo Fisher
Scientific, Inc.) with 10% FBS (cat. no. 16140071; Thermo Fisher
Scientific, Inc.). hFOB 1.19 cells were cultured in DMEM (cat. no.
30030; Thermo Fisher Scientific, Inc.) with 2.5 mM L-glutamine, 0.3
mg/ml G418 and 10% FBS. The cells were placed in a cell incubator
with 5% CO2 at 37°C.
Transfections
miR-1226-3p mimics and miR-1226-3p antisense
oligonucleotides (ASOs) were separately transfected into cells in
order to increase or decrease miR-1226-3p expression, respectively.
The miR-1226-3p mimics, miR-1226-3p ASOs and the respective
scrambled negative controls (NCs) were designed and constructed by
Sangon Biotech Co., Ltd. The sequences of the miR-1226-3p mimics
and miR-1226-3p ASOs were as follows: miR-1226-3p mimics,
5′-UCACCAGCCCUGUGUUCCCUAG-3′; miR-1226-3p ASOs,
5′-AGUGGCGGGACACAAGGGAAAAAA-3′; miR-1226-3p NC mimics,
5′-CGGUACGAUCGCGGCGGGAUAUC-3′; and miR-1226-3p NC ASOs,
5′-GCCAUGCUAGCGCCGCCCUAUAG-3′. Transfections were performed using
Lipofectamine® 2000 (cat. no. 11668019; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, cells were seeded into 24-well plates at a density of
5×104 cells/well. miR-1226-3p mimics or miR-1226-3p ASOs
(final concentration, 300 nM) were separately diluted in 50 µl
Opti-MEM™ Reduced Serum Medium (Gibco; Thermo Fisher Scientific,
Inc.) without serum. Lipofectamine 2000 was mixed gently before
use, and 1 µl was diluted in 50 µl Opti-MEM Reduced Serum Medium.
After incubation for 5 min, the miRNAs (miR-1226-3p mimics or
miR-1226-3p ASOs) were separately combined with the diluted
Lipofectamine 2000, then mixed gently and incubated for 20 min at
room temperature. Finally, miRNA mimic-Lipofectamine 2000 or miRNA
ASO-Lipofectamine 2000 mixtures were added to the wells containing
cells and medium. Cells were transfected for 20 min at 37°C. TRAF3
overexpression was achieved via transfection of the pcDNA3.1-TRAF3
plasmid. The pcDNA3.1-TRAF3 plasmid and NC plasmid (containing a
scrambled shNC sequence) was designed and constructed by Sangon
Biotech Co., Ltd. Similarly, the plasmids (500 ng) were transfected
into cells using Lipofectamine 2000 as aforementioned for 20 min at
37°C, and then cells were incubated at 37°C overnight. Subsequent
experiments were performed the next day.
Reverse transcription-quantitative
(RT-q)PCR
The expression levels of miR-1226-3p in tissues and
cells were analyzed by RT-qPCR using the 2−∆∆Cq method
for quantification (14). In detail,
total RNA was extracted using the TRIzol™ Plus RNA Purification kit
(cat. no. 12183555; Thermo Fisher Scientific, Inc.). Total RNA was
reverse transcribed into cDNA using the All-in-One™ miRNA
First-Strand cDNA Synthesis kit (cat. no. 18091050; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
miR-1226-3p expression was evaluated using the SuperScript™ III
Platinum™ SYBR™ One-Step Green qPCR kit (cat. no. 11736051; Thermo
Fisher Scientific, Inc.). TNF receptor-associated factor 3 (TRAF3)
mRNA expression was assayed using TaqMan™ Fast Advanced Master Mix
(cat. no. 4444557; Thermo Fisher Scientific, Inc.). The sequences
of the involved primers were as follows: miR-1226-3p-forward,
5′-GTCACCAGCCCTGTGT-3′ and reverse, 5′-GCAGGGTCCGAGGTAATTC-3′; U6
forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; TRAF3 forward,
5′-CTCACAAGTGCAGCGTCCAG-3′ and reverse,
5′-GCTCCACTCCTTCAGCAGGTT-3′; and GAPDH forward,
5′-GTCTCCTCTGACTTCAACAGCG-3′ and reverse,
5′-ACCACCCTGTTGCTGTAGCCAA-3′. The primers were designed and
synthesized by Sangon Biotech Co., Ltd. miR-1226-3p expression was
normalized to U6, which was used as the internal reference
(15). The qPCR conditions for miRNA
detection were as follows: 95°C for 10 min, followed by 38 cycles
at 95°C for 1 min and 60°C for 30 sec. The qPCR conditions for
TRAF3 detection were as follows: 95°C for 10 sec, followed by 40
cycles at 95°C for 5 sec and 60°C for 20 sec. GAPDH was used as the
internal reference for mRNA.
Mutation site and target gene
prediction
The potential targets of miR-1226-3p were predicted
using TargetScan software (http://www.targetscan.org/vert_72/; version 7.2)
(16–20). TargetScan predicts the biological
targets of miRNAs by searching for the presence of conserved 8mer,
7mer and 6mer sites that match the seed region of each miRNA. The
mutation in the 3′-untranslated region (3′-UTR) of TRAF3 was
generated using the GeneArt™ Site-Directed Mutagenesis System
according to the manufacturer's protocol (cat. no. A13282; Thermo
Fisher Scientific, Inc.).
Apoptosis analysis
The apoptosis rate was analyzed using the Dead Cell
Apoptosis kit with Annexin V-FITC and PI (cat. no. V13242; Thermo
Fisher Scientific, Inc.). In detail, the cells were harvested and
washed once in cold PBS (1×106 cells/tube). After
centrifuging the washed cells in PBS (300 × g at 4°C for 5 min),
the supernatant was discarded, the cells were resuspended in 1X
Annexin-binding buffer (100 µl), and then Annexin V-FTIC (5 µl) and
PI (1 µl) were added. After the mixture was incubated at room
temperature for 15 min, the apoptosis rate was analyzed using a BD
FACSVerse™ flow cytometer (BD Biosciences) with a 488-nm excitation
laser. The data were analyzed using BD FACSuite™ version 1.01 (BD
Biosciences). Cells in the right quadrants (early and late
apoptosis) were chosen for apoptosis assay for quantification.
MTT assay
The proliferation of SaOS-2 cells was analysed using
the MTT assay. SaOS-2 cells were seeded into 96-well plates at a
density of 5×105 cells/well. MTT reagent was added into
the culture medium at a final concentration of 0.1 mg/ml. The
purple formazan crystals were dissolved using 100 µl DMSO, and
optical density was measured using a microplate reader (Multiskan
Sky; Thermo Fisher Scientific, Inc.) at a wavelength of 570 nm
(21).
Western blot analysis
The SaOS-2 cells were detached and then lysed with
radioimmunoprecipitation assay (RIPA) buffer (cat. no. 89900;
Thermo Fisher Scientific, Inc.) containing protease inhibitor (cat.
no. 78420; Thermo Fisher Scientific, Inc.). Cold RIPA buffer (1 ml)
was used for 5×106 cells. The mixture was agitated for
30 min at 4°C and then centrifuged at 300 × g at 4°C for 5 min. The
protein concentration of the aspirated supernatant was analyzed
using the BCA Protein Assay kit (cat. no. ab102536; Abcam). Protein
samples (30 µg/lane) were loaded and run at 80 V for 2 h via 12%
SDS-PAGE. The proteins were transferred onto polyvinylidene
fluoride membranes that were then immersed in 5% skimmed milk for 1
h for blocking at room temperature. The membranes were cut and
incubated overnight at 4°C separately with primary antibodies
against TRAF3 (1:1,000; cat. no. ab36988; Abcam) and GAPDH
(1:1,000; cat. no. ab181602; Abcam). After washing 3 times for 10
min each time at room temperature in TBS with 0.1% Tween-20, the
membranes were incubated with HRP-conjugated goat anti-rabbit
secondary antibody (1:500; cat. no. ab205718; Abcam) at room
temperature for 2 h. The protein bands were visualized using
Pierce™ ECL Western blotting substrate (cat. no. 32209; Thermo
Fisher Scientific, Inc.). The images were acquired using the
FluorChem System (ProteinSimple), and the software used for
analysis was AlphaView Stand Alone (ProteinSimple; version
3.5.0).
Luciferase reporter assay
The luciferase reporter assay was performed using
the Dual-Luciferase Reporter Assay System (cat. no. E1910; Promega
Corporation). The 3′-UTR of the TRAF3 gene was amplified by PCR and
cloned downstream of the luciferase gene in the pGL/Promoter vector
(Sangon Biotech Co., Ltd.) to the wild-type plasmids. The
Renilla luciferase gene in the vector acted as a control
reporter for normalization. SaOS-2 cells were co-transfected with
miR-1226-3p mimics and the luciferase-containing wild-type or
mutant 3′-UTRs of TRAF3 using Lipofectamine® 2000 (cat.
no. 11668019; Thermo Fisher Scientific, Inc.). The cells were
collected after 24 h of transfection, and luciferase activity was
analyzed according to the manufacturer's protocol.
Statistical analysis
Experiments were repeated 3 times independently.
Statistical analysis was performed using GraphPad Prism version 5.0
(GraphPad Software, Inc.). All results were expressed as the mean ±
SD. Overall survival was defined as the length of time from the
date of cancer diagnosis to the date of death from any cause. The
Kaplan-Meier method was used to draw the survival curves, and the
log-rank test was used to calculate the P-value. Differences
between two groups were analyzed by Student's t-tests. The
differences between pairs of tumor and normal tissues were analyzed
by paired Student's t-test. The difference between two groups, such
as miR-1226-3p expression in two different cell lines or in
transfected or control cells, or the OD values in treated or
control cells were assessed by unpaired Student's t-test.
Differences among 3 groups were assessed by one-way ANOVA followed
by Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
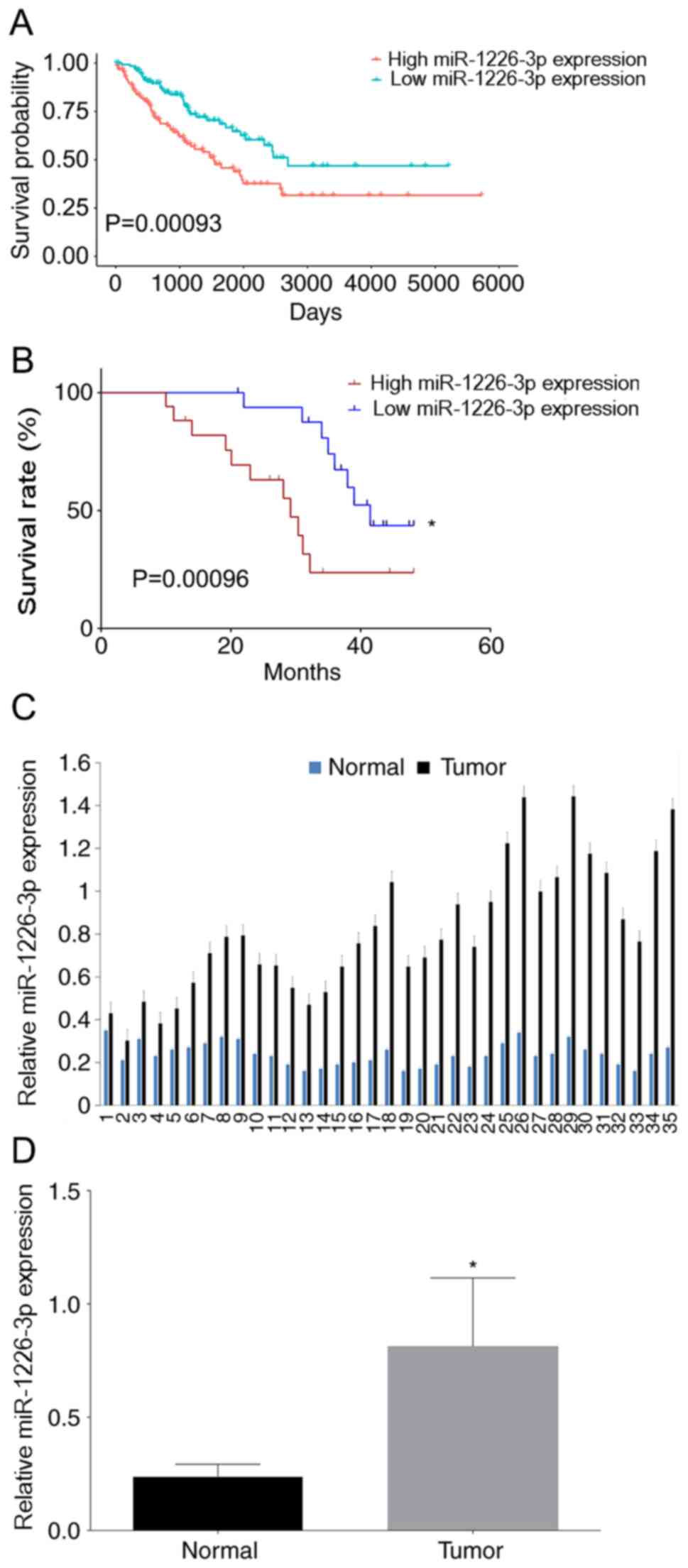
High miR-1226-3p expression is
associated with lower overall survival rates
The role of miR-1226-3p was initially assessed in
the survival of patients with sarcoma in TCGA database since
osteosarcoma accounts for a major portion of sarcoma. There were
256 patients with sarcoma in the data frame. The median miR-1226-3p
expression value was 0.97±1.03, and patients were divided into 2
groups based on the median miR-1226-3p expression value to perform
survival analysis. It was revealed that patients with higher
miR-1226-3p expression in tumor tissues had lower overall survival
rates than patients with lower miR-1226-3p expression (Fig. 1A). Subsequently, survival was
analyzed in the 35 matched pairs of osteosarcoma and adjacent
normal tissues collected for the present study. miR-1226-3p
expression in tissues was analyzed by RT-qPCR. The 35 patients with
osteosarcoma patients were divided into 2 groups according to the
median miR-1226-3p expression value (0.76), and then survival
analysis was performed. The Kaplan-Meier method was used to draw
the survival curves, and the log-rank test was used to calculate
the P-value. Consistently with TCGA data, patients with higher
miR-1226-3p expression in tumor tissues exhibited lower overall
survival rates than patients with lower miR-1226-3p expression
(Fig. 1B). Moreover, miR-1226-3p
expression in tumor tissues was higher than that in adjacent normal
tissues (Fig. 1C), with the mean
miR-1226-3p expression value in tumor tissues being significantly
higher than that in normal tissues (Fig.
1D). Thus, it was hypothesized that miR-1226-3p served a role
in the molecular mechanism of osteosarcoma.
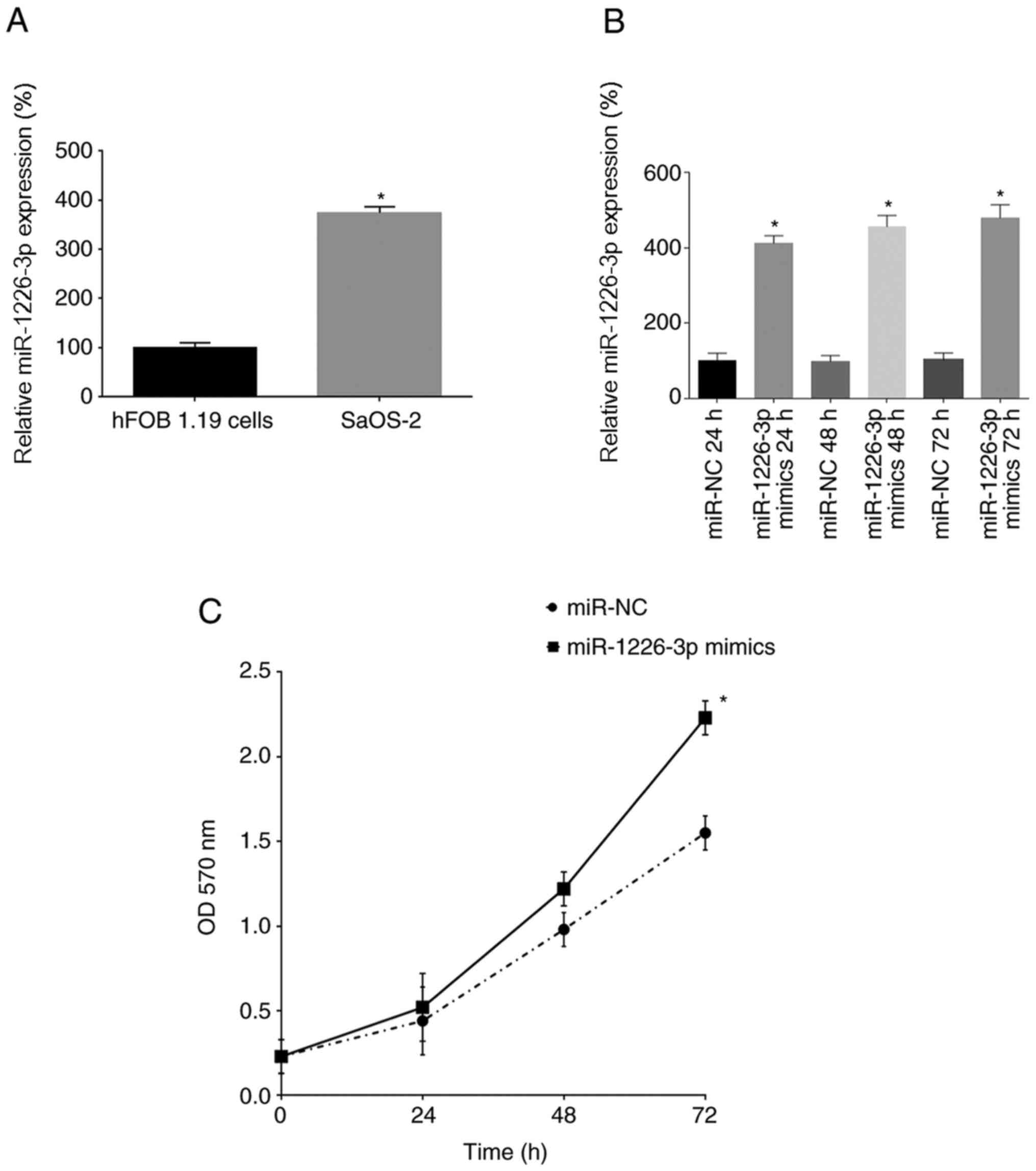
miR-1226-3p promotes SaOS-2 cell
proliferation
Cell experiments were used to analyze the function
of miR-1226-3p in osteosarcoma. First, miR-1226-3p expression was
analyzed in hFOB 1.19 and SaOS-2 cells by RT-qPCR. It was revealed
that SaOS-2 cells exhibited significantly higher miR-1226-3p
expression than hFOB 1.19 cells (Fig.
2A). Next, miR-1226-3p was overexpressed in SaOS-2 cells by
transfection with miR-1226-3p mimics, and miR-1226-3p expression
was analyzed at 24, 48 and 72 h after transfection. miR-1226-3p
mimics significantly increased miR-1226-3p expression in SaOS-2
cells transfected for 24, 48 and 72 h compared with their
respective NCs (Fig. 2B). Using MTT
analysis, the proliferation of SaOS-2 cells was assessed after
transfection with the miR-1226-3p mimics, revealing that
miR-1226-3p mimics significantly promoted the proliferation of
SaOS-2 cells after 72 h (Fig.
2C).
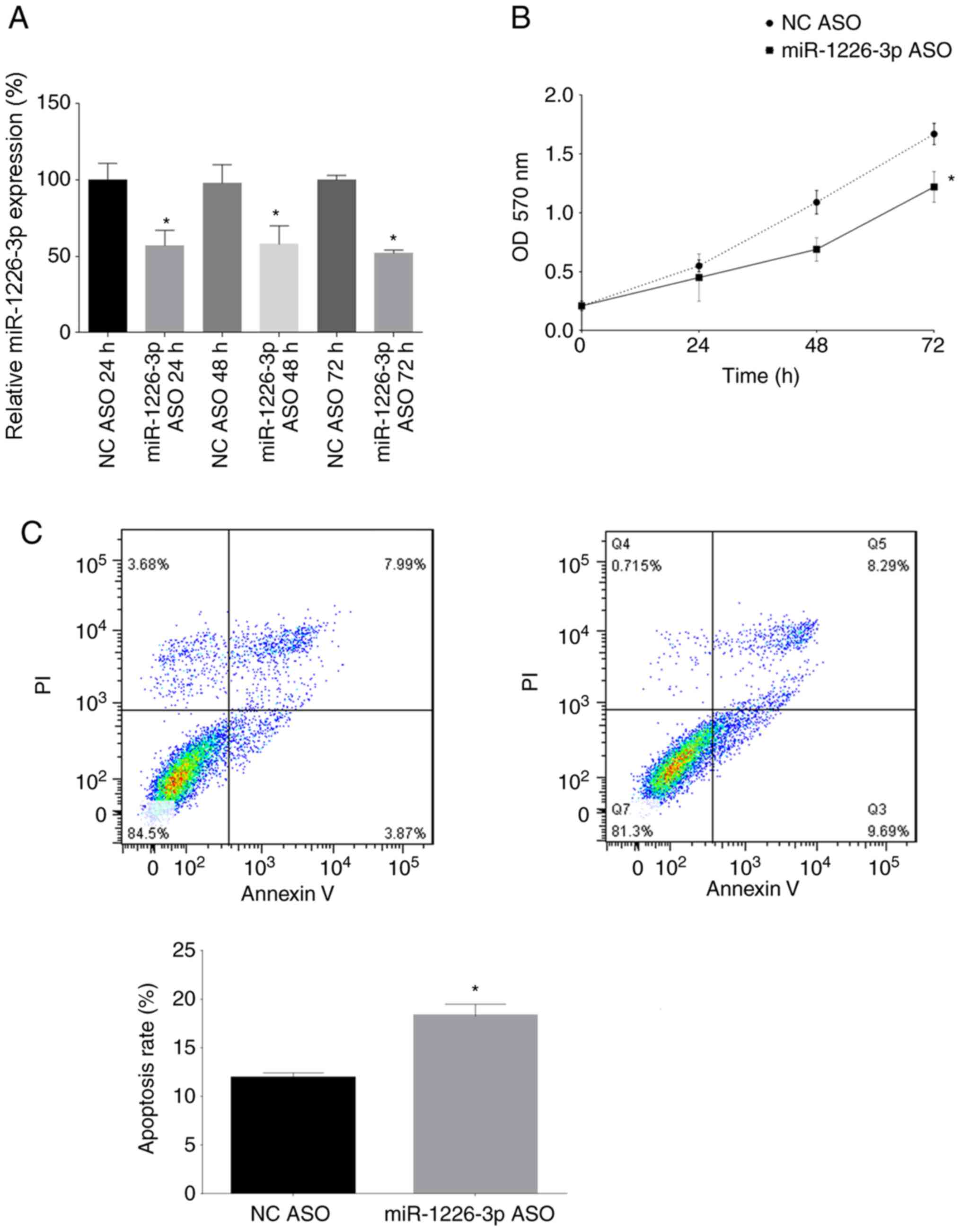
Inhibition of miR-1226-3p decreases
SaOS-2 cell proliferation and promotes apoptosis
miR-1226-3p expression was inhibited in SaOS-2 cells
by transfection with miR-1226-3p ASOs. At 24, 48 and 72 h after
transfection, miR-1226-3p expression in SaOS-2 cells was assessed
by RT-qPCR. miR-1226-3p ASOs significantly decreased miR-1226-3p
expression in SaOS-2 cells transfected for 24, 48 and 72 h compared
with their respective NCs (Fig. 3A).
In addition, the proliferation of SaOS-2 cells was analyzed 24, 48
and 72 h after transfection with miR-1226-3p ASOs, revealing that
the miR-1226-3p ASOs significantly inhibited the proliferation of
SaOS-2 cells after 72 h (Fig. 3B).
Finally, the apoptosis rate of SaOS-2 cells was assessed 24 h after
transfection with the miR-1226-3p ASOs, revealing that the
miR-1226-3p ASOs significantly promoted the apoptosis rate of
SaOS-2 cells (Fig. 3C).
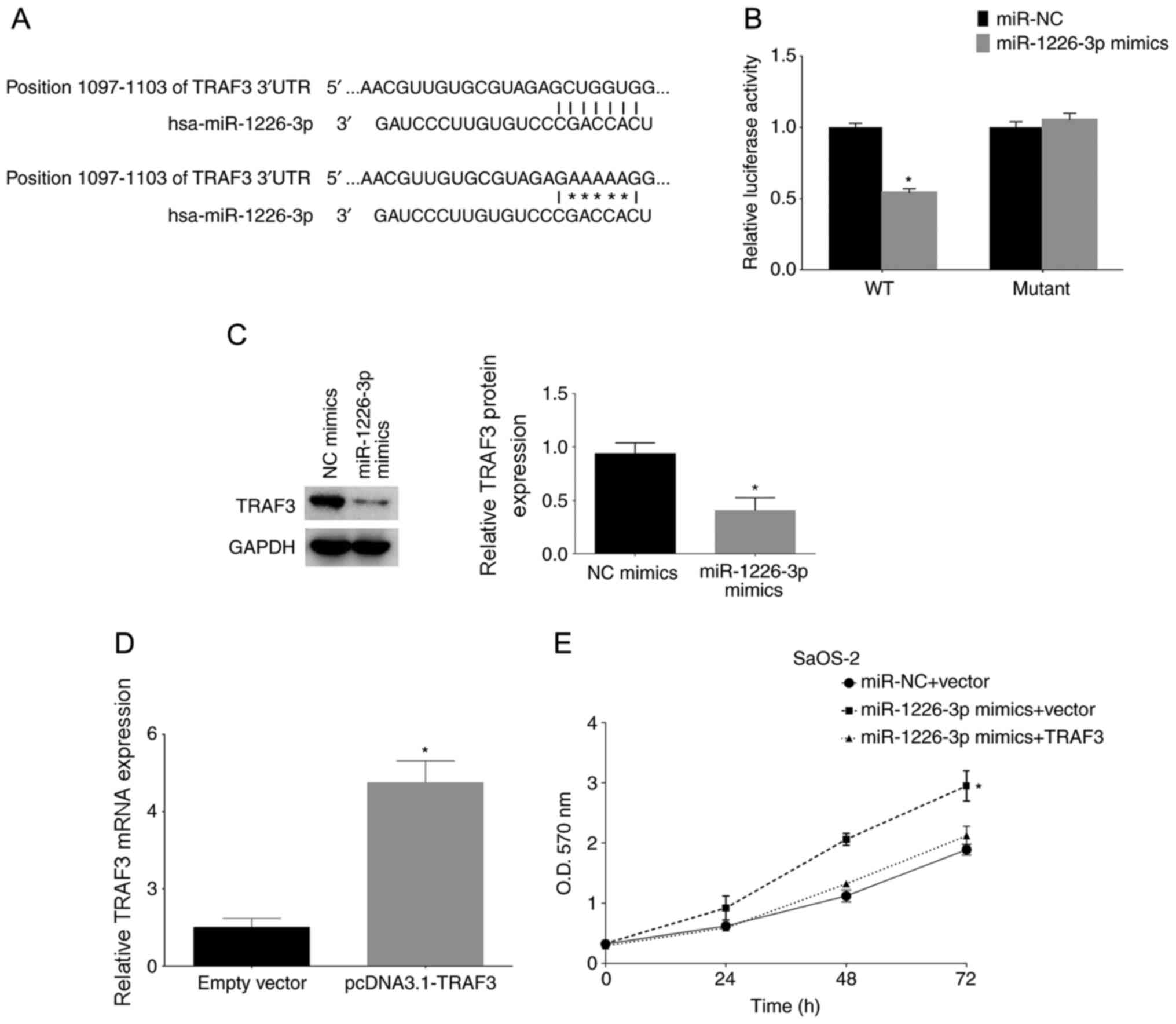
miR-1226-3p targets TRAF3
The possible genes targeted by miR-1226-3p were
analyzed using bioinformatics methods. It was revealed that the
TRAF3 gene was targeted by miR-1226-3p. The binding sites are shown
in Fig. 4A. TRAF3 wild-type and
mutant 3′-UTRs were cloned and inserted into luciferase reporter
plasmids. The luciferase assay results revealed that miR-1226-3p
mimics significantly decreased luciferase activity by directly
binding to the 3′-UTR of TRAF3; however, mutation of the putative
miR-1226-3p binding sites in the 3′-UTR of TRAF3 abrogated the
luciferase response to miR-1226-3p mimics (Fig. 4B). Subsequently, TRAF3 protein
expression was analyzed after miR-1226-3p mimic transfection,
revealing that miR-1226-3p mimic transfection significantly
decreased TRAF3 protein expression in SaOS-2 cells, as shown by
western blotting (Fig. 4C). Finally,
SaOS-2 cells were transfected with miR-1226-3p mimics and TRAF3
overexpression plasmid (pcDNA3.1-TRAF3). The effect of
pcDNA3.1-TRAF3 was confirmed by RT-qPCR (Fig. 4D). The results of the MTT assay of
the TRAF3-overexpressing cells after miR-1226-3p mimic transfection
revealed that TRAF3 overexpression reversed the proliferative
effect of miR-362-3p mimics on these cells (Fig. 4E).
Discussion
The present study analyzed the function of
miR-1226-3p in osteosarcoma. It was revealed that higher
miR-1226-3p expression decreased the overall survival rates of
patients with osteosarcoma, and data from experiments in SaOS-2
cells demonstrated that miR-1226-3p promoted cell proliferation.
Moreover, miR-1226-3p inhibition increased the apoptosis rate in
osteosarcoma cells. Furthermore, TRAF3 was identified as a target
gene of miR-1226-3p. To the best of our knowledge, the current
study is the first to reveal the role of miR-1226-3p in
osteosarcoma.
According to the apoptosis analysis, there appeared
to be some late apoptotic cells in both groups, for which there may
be two possible reasons: One is that the duration of PI staining,
since as the duration of PI staining increases, the number of
PI+ cells may increase, and the other is that there may
be some dead cells following transfection and PBS washing.
A similar study has revealed that hepatocellular
carcinoma tissues exhibit higher miR-1226-3p expression compared
with normal tissues (11).
Consistently, the current data indicated that osteosarcoma tissues
exhibited higher miR-1226-3p expression than normal tissues. In
addition to the aforementioned study, the present study revealed
that miR-1226-3 targeted the TRAF3 protein to serve its role in
osteosarcoma. However, another study involving human breast cancer
cell lines has demonstrated that miR-1226 targets mucin 1
oncoprotein and induces cell death (9). Therefore, it seems that miR-1226-3 may
serve different roles in different types of cancer.
The TRAF3 protein has an important role in the
immune response. TRAF proteins are basic components of signaling
pathways activated by TNF receptor or toll-like receptor family
members (22). TRAF3, which is a
specific TRAF protein, is involved in positive and negative
regulatory functions in multiple signaling pathways (10). TRAF3 is a highly versatile regulator
that positively controls type I interferon production but
negatively regulates mitogen-activated protein kinase activation
and alternative nuclear factor-κB signaling (23,24).
Notably, miR-214 functions as an oncogene in human osteosarcoma by
targeting TRAF3 (25). Thus, TRAF3
was further analyzed in the present study.
The role of TRAF3 in cancer has been the subject of
several studies. The TRAF3 protein acts as an anti-inflammatory
factor and is required for optimal innate immunity in myeloid cells
(26). Furthermore, the TRAF3 gene
is a novel tumor suppressor gene in macrophages (26). In addition, the TRAF3 protein
regulates the oncogenic proteins Pim2 and c-Myc to reduce survival
in normal and malignant B cells (27). Moreover, the TRAF3 protein can
interact with the glucocorticoid modulatory element-binding protein
1 protein and modulate its anti-apoptotic function (28). The current data indicated that TRAF3
overexpression partly decreased the proliferative effect of
miR-1226-3p in SaOS-2 cells. TRAF3 overexpression may therefore
also increase apoptosis, which will be investigated in a future
study.
The present study has some limitations. First,
SaOS-2 cells were used as models and hFOB 1.19 cells were used as
controls. hFOB 1.19 cells are derived from osteoblasts, while
Saos-2 cells are human osteosarcoma cells with several osteoblastic
features, and they lack both Rb and intact p53 (29,30).
However, the p53 protein is mutated in most patients with
osteosarcoma (80–90%) (31).
Therefore, p53 may affect the function of miR-1226-3p in
osteosarcoma, which will be investigated in a future study. Another
limitation is that there is a lack of comparison in miR-1226
expression across a panel of OS cell lines, as well as a lack if
in vivo data.
The present results revealed that TRAF3 may be
inhibited by miR-1226-3p, suggesting that miR-1226-3p may serve a
role in the immune response network. This study has clinical
significance since it revealed a possible molecular target and
indicator of osteosarcoma prognosis. Patients with osteosarcoma may
benefit from miR-1226-3p regulation, which requires further
investigation. Overall, the present study revealed that miR-1226-3p
may serve a tumor-promoting role in osteosarcoma.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Innovation
and Cultivation Foundation of Naval General Hospital (grant no.
CXPY-201818).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article. The datasets generated and/or
analyzed during the current study are available in the Xena Browser
(https://xenabrowser.net/).
Authors' contributions
YL, TA and JL collected patient data. YL, DS and TA
performed PCR, western blotting and other molecular experiments. QY
performed the bioinformatics analysis and luciferase reporter
assay. YL and SN contributed to the study design and manuscript
writing. JL and SN contributed to data analysis and revising the
manuscript for important intellectual content. All authors confirm
the authenticity of the raw data, and have read and approved the
final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Sichuan University (Chengdu,
China) approved the usage of the patients' clinical data and tissue
samples, and all patients or their guardians provided written
informed consent for the use of their clinical information and
tissue samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biermann JS, Adkins DR, Agulnik M,
Benjamin RS, Brigman B, Butrynski JE, Cheong D, Chow W, Curry WT,
Frassica DA, et al: Bone cancer. J Natl Compr Canc Netw.
11:688–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia L, Zheng R, Xu Y, Xu X, Zhang S, Zeng
H, Lin L and Chen W: Incidence and mortality of primary bone
cancers in China, 2014. Chin J Cancer Res. 31:135–143. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasaki R, Osaki M and Okada F:
MicroRNA-based diagnosis and treatment of metastatic human
osteosarcoma. Cancers (Basel). 11:5532019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sampson VB, Yoo S, Kumar A, Vetter NS and
Kolb EA: MicroRNAs and potential targets in osteosarcoma: Review.
Front Pediatr. 3:692015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomczak K, Czerwińska P and Wiznerowicz M:
The cancer genome atlas (TCGA): An immeasurable source of
knowledge. Contemp Oncol (Pozn). 19:A68–A77. 2015.PubMed/NCBI
|
|
9
|
Jin C, Rajabi H and Kufe D: miR-1226
targets expression of the mucin 1 oncoprotein and induces cell
death. Int J Oncol. 37:61–69. 2010.PubMed/NCBI
|
|
10
|
Zhou Q, Zeng H, Ye P, Shi Y, Guo J and
Long X: Differential microRNA profiles between
fulvestrant-resistant and tamoxifen-resistant human breast cancer
cells. Anticancer Drugs. 29:539–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Tan W, Li W, Li W, Zhu S, Zhong J,
Shang C and Chen Y: miR-1226-3p promotes sorafenib sensitivity of
hepatocellular carcinoma via downregulation of DUSP4 expression. J
Cancer. 10:2745–2753. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th edition.
Springer; Heidelberg: 2017, View Article : Google Scholar
|
|
13
|
Wickham H, François R, Henry L and Müller
K: dplyr: A grammar of data manipulation. R package version 0.7.6,
2018. https://CRAN.R-project.org/package=dplyrDecember
16–2019
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song B, Zhang C, Li G, Jin G and Liu C:
miR-940 inhibited pancreatic ductal adenocarcinoma growth by
targeting MyD88. Cell Physiol Biochem. 35:1167–1177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garcia DM, Baek D, Shin C, Bell GW,
Grimson A and Bartel DP: Weak seed-pairing stability and high
target-site abundance decrease the proficiency of lsy-6 and other
microRNAs. Nat Struct Mol Biol. 18:1139–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee S, Paulson KG, Murchison EP, Afanasiev
OK, Alkan C, Leonard JH, Byrd DR, Hannon GJ and Nghiem P:
Identification and validation of a novel mature microRNA encoded by
the merkel cell polyomavirus in human merkel cell carcinomas. J
Clin Virol. 52:272–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Zheng Y, You J, Xiong J, Ying S,
Xie L, Song X, Yao Y, Jin Z and Zhang C: Tumor suppressor role of
miR-876-5p in gastric cancer. Oncol Lett. 20:1281–1287. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hacker H, Tseng PH and Karin M: Expanding
TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev
Immunol. 11:457–468. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tseng PH, Matsuzawa A, Zhang W, Mino T,
Vignali DA and Karin M: Different modes of ubiquitination of the
adaptor TRAF3 selectively activate the expression of type I
interferons and proinflammatory cytokines. Nat Immunol. 11:70–75.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perkins DJ, Polumuri SK, Pennini ME, Lai
W, Xie P and Vogel SN: Reprogramming of murine macrophages through
TLR2 confers viral resistance via TRAF3-mediated, enhanced
interferon production. PLoS Pathog. 9:e10034792013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rehei AL, Zhang L, Fu YX, Mu WB, Yang DS,
Liu Y, Zhou SJ and Younusi A: MicroRNA-214 functions as an oncogene
in human osteosarcoma by targeting TRAF3. Eur Rev Med Pharmacol
Sci. 22:5156–5164. 2018.PubMed/NCBI
|
|
26
|
Lalani AI, Luo C, Han Y and Xie P: TRAF3:
A novel tumor suppressor gene in macrophages. Macrophage (Houst).
2:e10092015.PubMed/NCBI
|
|
27
|
Whillock AL, Mambetsariev N, Lin WW, Stunz
LL and Bishop GA: TRAF3 regulates the oncogenic proteins Pim2 and
c-Myc to restrain survival in normal and malignant B cells. Sci
Rep. 9:128842019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kotsaris G, Kerselidou D, Koutsoubaris D,
Constantinou E, Malamas G, Garyfallos DA and Ηatzivassiliou EG:
TRAF3 can interact with GMEB1 and modulate its anti-apoptotic
function. J Biol Res (Thessalon). 27:72020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marcellus RC, Teodoro JG, Charbonneau R,
Shore GC and Branton PE: Expression of p53 in Saos-2 osteosarcoma
cells induces apoptosis which can be inhibited by Bcl-2 or the
adenovirus E1B-55 kDa protein. Cell Growth Differ. 7:1643–1650.
1996.PubMed/NCBI
|
|
30
|
Li W, Fan J, Hochhauser D, Banerjee D,
Zielinski Z, Almasan A, Yin Y, Kelly R, Wahl GM and Bertino JR:
Lack of functional retinoblastoma protein mediates increased
resistance to antimetabolites in human sarcoma cell lines. Proc
Natl Acad Sci USA. 92:10436–10440. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|