Introduction
Cervical cancer is one of the most common
malignancies, threatening women's physical and mental health
worldwide, especially in developing countries (1). A total of ~570,000 cases of cervical
cancer and 311,000 deaths from the disease occurred globally in
2018 (2). Cervical cancer is the
fourth most common cancer among women, after breast cancer (2.1
million cases), colorectal cancer (0.8 million) and lung cancer
(0.7 million) (2). China and India
together contribute more than a third of the global cervical cancer
burden, with 106,000 cases in China and 97,000 cases in India, and
48,000 deaths in China and 60,000 deaths in India (2). Cervical cancer is divided into squamous
cell carcinoma, adenocarcinoma, adenosquamous carcinoma and
neuroendorine carcinoma according to pathological characteristics
(3). Almost all cervical cancer is
induced by human papillomavirus infection (HPV) 16 and 18, which
generally spreads through sexual activity and an impaired immune
system (4,5). In recent years, regular Pap smear
screening has detected cervical cancer at an earlier stage to
reduce the number of associated deaths (6,7).
However, advanced cervical cancer still has a high mortality rate,
which results in 5-year survival rates ranging from 16% (stage IVA)
to 58% (stage IIB) (8). Hence, it is
of great importance to explore the molecular mechanisms, potential
prediction and treatment targets of cervical cancer.
The dynamins family belongs to the guanylate
triphosphatases superfamily, which is involved in the regulation of
the pathogenesis of a variety of carcinomas (9). For example, dynamin-2 upregulation is
associated with the progression of bladder cancer (10). Dynamin 1 and 2 can promote the
proliferation and metastasis of cancer cells, whereas dynamin 3
(DNM3) is generally considered as a candidate tumor suppressor
(11). The sequence analysis of the
DNM3 promoter demonstrated that the DNM3 promoter is
hypermethylated in hepatocellular carcinoma (HCC) tissues and DNM3
is expressed at significantly lower levels in these tissues
(12). Patients with HCC with low
expression of DNM3 generally have a poorer prognosis compared with
those with high DNM3 expression (12). Furthermore, overexpression of DNM3
induces cell cycle arrest and reduces cell proliferation of HCC
cell lines by activating p53 (13).
In addition, DNM3 overexpression can activate nitric oxide (NO)
synthases to generate NO, and the increased NO production can
induce reactive oxygen species accumulation and activate cell
apoptosis of HCC cells (14).
However, the precise tumor suppressive mechanisms of DNM3 in
cervical cancer remain unclear.
The present study aimed to investigate the
expression pattern and biological functions of DNM3 in cervical
cancer. The findings of this study may be beneficial for the
prognosis or treatment of cervical cancer.
Materials and methods
Bioinformatics analysis
The mRNA expression level of DNM3 was analyzed in
306 cases of cervical squamous cell carcinoma and endocervical
adenocarcinoma (CESC) tissues and 13 cases of normal cervical
tissues using the online Gene Expression Profiling Interactive
Analysis (GEPIA) database (http://gepia.cancer-pku.cn/) (15). RNA sequencing data analyzed by GEPIA
is generated by large consortium projects, such as The Cancer
Genome Atlas (TCGA) and Genotype-Tissue Expression project (GTex),
using the output of a standard processing pipeline for RNA
sequencing data (15). For survival
analysis, GEPIA uses the log-rank test (Mantel-Cox test) for
hypothesis evaluation (15).
Tissue collection
A total of 41 pairs of fresh cervical cancer tissues
and normal adjacent tissues (<3 cm) were collected from patients
diagnosed with cervical cancer who underwent surgical resection at
Linyi Cancer Hospital (Linyi, China) between July 2018 and May
2019. The age range of the participants was 19–67 years, with a
median age of 56 years. The exclusion criteria of the patients
were: i) Had any other malignant tumors or serious lesions; and ii)
received radiotherapy or any other preoperative treatment. The
Ethics Committee of Linyi Cancer Hospital approved the study and
all the participants provided written informed consent.
Immunohistochemistry (IHC)
Clinical samples that had been collected were fixed
in formalin at 4°C for 12 h and then embedded in paraffin.
Subsequently, the tissue was cut into 4-µm paraffin sections using
a cryostat. The paraffin sections were dewaxed, rehydrated and
blocked with 0.3% H2O2. Antigen retrieval was
performed by microwaving in 0.01 M citrate buffer for 10 min. After
blocking with 5% goat serum for 1 h at room temperature, the slides
were exposed to rabbit anti-DNM3 (1:100; cat. no. 14737-1-AP;
Proteintech Group, Inc.) for 2 h at room temperature and
subsequently incubated with horseradish peroxidase (HRP)-labeled
goat anti-mouse/rabbit IgG polymer (1:5,000; cat. no. 160101405L;
Fuzhou Maixin Biotech Co., Ltd.) at room temperature for 20 min.
Immune response was detected by the enhanced DAB chromogenic kit
(cat. no. 1705252031; Fuzhou Maixin Biotech Co., Ltd.) at room
temperature for 3 min, and hematoxylin was used for counterstaining
at room temperature for 5 min. Finally, a light upright microscope
system (Nikon Corporation) was used to obtain images.
The immunostaining score of DNM3 was the product of
the score of the positive staining cells ratio (R) and the staining
intensity score (S). R was divided into 4 levels: i) 0 (<5%,
negative); ii) 1 (5-25%, sporadic); iii) 2 (25-50%, focus); and iv)
3 (>51%, diffuse). S was also divided into 4 levels: i) 0
(negative); ii) 1 (weak); iii) 2 (medium); and iv) 3 (strong).
Finally, a total of 0–3 was considered to represent low expression
and 4–9 was considered to represent high expression.
Cell culture and transfection
Human cervical cancer cell lines (Caski, SiHa, Hela
and C33A) and normal cervical cell line H8 used in the present
study were purchased from the ATCC. The cells were cultured in
RPMI-1640 (Hyclone; GE Healthcare Life Sciences) supplemented with
10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific
Inc.), 100 U/ml penicillin and 0.1 mg/ml streptomycin
(Sigma-Aldrich; Merck KGaA). The cells were grown in a humidified
incubator with 5% CO2 at 37°C. When the cells were in
their exponential growth phase, they were washed with PBS and
treated with trypsin-EDTA (Beijing Solarbio Science and Technology
Co., Ltd.), and then the cells were resuspended and seeded into
6-well plates with a density of 5×104 for further
experiments.
DNM3 cDNA was cloned into pcDNA3.1 plasmid (DNM3, 5
nM) (V87020; Thermo Fisher Scientific, Inc.). The empty pcDNA3.1
plasmid (Thermo Fisher Scientific, Inc.) was used as a negative
control (NC, 5 nM). When the cell density reached 80% confluence,
plasmid transfection was performed according to the manufacturer's
instructions using Lipofectamine 2000® (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37 C for 6 h. After 6 h, medium
was changed to complete medium, and the cells were cultured for a
further 24 h prior to subsequent experimentation.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA from cells was extracted using a TRIzol
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The isolated RNAs were used as
templates and reverse transcribed to obtain the first-strand cDNA
using a HiFiScript cDNA Synthesis kit (CoWin Biosciences) according
to the manufacturer's instructions. Quantitative real-time PCR
analysis was performed using the Magic SYBR mixture (CoWin
Biosciences). The cycling conditions were as follows: Denaturation
at 95°C for 5 min, followed by 40 cycles of 95°C for 30 sec, 60°C
for 45 sec and 72°C for 30 sec. The relative quantification was
performed by the 2−∆∆Cq method (16). β-actin was used as the internal
reference. The primer sequences were as follows: DNM3 forward,
5′-GCTCACCATCAGCAACATTGGC-3′ and reverse,
5′-CCGAACTTTCAGGTTGTCCAAGG-3′; and β-actin, forward,
5′-CCCGAGCCGTGTTTCCT-3′ and reverse,
5′-GTCCCAGTTGGTGACGATGC-3′.
Immunofluorescence assay
SiHa and C33A cells (1,000 cells/well) were plated
in 6-well chamber slides. After 24 h, cells were fixed in 4%
paraformaldehyde for 30 min at room temperature and permeabilized
with 0.1% Triton X-100. Cells were probed for DNM3 primary
antibodies (1:1,000; cat. no. 14737-1-AP; Proteintech Group, Inc.)
overnight at 4°C and FITC-labeled secondary antibody (1:200; cat.
no. CL488-66484; Proteintech Group, Inc.) for 1 h at room
temperature. Cell nuclei were stained with DAPI (Sigma-Aldrich;
Merck KGaA) for 5 min in the dark at room temperature. Images were
captured using a fluorescence microscope (IX2-SL; Olympus
Corporation) (magnification, ×400).
Cell Counting Kit-8 assay
Following transfection, SiHa and C33A cells were
cultured for 24 h. Next, the cells were digested by trypsin-EDTA
solution, resuspended and counted. The cells (1,000/well) were
seeded into 96-well plates, each well containing 100 µl RPMI-1640
medium (10% FBS). The plates were cultured in a humidified
incubator with 5% CO2 at 37°C. Cell viability was
measured every 24 h. For each measurement, 10 µl CCK-8 (Beijing
Solarbio Science and Technology Co., Ltd.) reagent was added into
the wells and incubated for 1.5 h. Finally, absorbance was measured
by a microplate reader at 450 nm and the growth curve drawn.
Plate clone formation assay
Following transfection for 24 h, C33A and SiHa cells
were resuspended and counted. The cells were seeded into 60-mm
plates (500 cells/plate) containing 5 ml RPMI-1640 medium (10% FBS)
and cultured for 2 weeks at 37°C. Finally, the cells were fixed
with 4% paraformaldehyde for 30 min at room temperature and then
stained with 0.1% crystal violet at room temperature for 30 min.
Images of visible colonies were captured with a HP Scanjet G4010
scanner (HP Spectre) and counted manually.
Transwell migration and invasion
assays
Cell migration and invasion detection was performed
using Transwell chambers (EMD Millipore). The transfected C33A and
SiHa cells were resuspended in serum-free medium. For the invasion
assay, 1×105 cells in 100 µl serum-free medium were
seeded into the top chamber coated with Matrigel (BD Biosciences).
Complete medium (500 µl) containing 10% FBS was added to the bottom
wells. After incubating for 12 h at 37°C, the non-invaded cells in
the upper chamber were removed with a cotton swab. Subsequently,
the invaded cells were stained with 0.1% crystal violet at room
temperature for 10 min. Five fields per filter were counted using a
light microscope (Nikon TE2000; Nikon Corporation) (magnification,
×40). For the migration assay, 5×103 cells were added to
the top chambers. Except for the Matrigel coating, the migration
assay was the same as the invasion assay. Matrigel (10 µl) (1:8)
was added to the upper chamber for 4 h at 37°C.
Analysis of apoptosis by flow
cytometry
Following transfection, C33A and SiHa cells were
cultured in complete medium for 24 h at 37°C, and then cultured in
serum-free medium for another 24 h at 37°C to induce apoptosis. The
cells were digested with EDTA-free trypsin, and collected with the
1X binding buffer. The cell density was adjusted to
1×106/ml and then 5 µl Annexin V/FITC (Beijing 4A
Biotech Co., Ltd.) was added to a 100 µl cell suspension and
incubated at room temperature for 5 min. After that, 10 µl
propidium iodide (PI) and 400 µl PBS buffer were added to the cell
suspension. The analysis of apoptosis was used a FACSCalibur Flow
Cytometer (BD Biosciences). Data were analyzed using FlowJo (v.4.5;
TreeStar, Inc.).
Western blotting
After 48 h of transfection, C33A and SiHa cells were
lysed with RIPA lysis buffer (CoWin Biosciences) with protease
inhibitor cocktail (CoWin Biosciences) to collect total protein.
The protein concentration was measured by using bicinchoninic acid
(BCA) protein assay kit (CoWin Biosciences). The samples were
boiled at 95°C for 5 min in LDS sample buffer (Invitrogen; Thermo
Fisher Scientific, Inc.). Protein (20 µg) samples was loaded onto
the 10% SDS-PAGE gel. After electrophoresis, the protein was
transferred to a PVDF membrane (EMD Millipore). After transferring,
the PVDF membrane was blocked with 5% skimmed milk for 1 h at room
temperature. Then, the membranes were incubated with primary
antibodies against DNM3 (1:1,000; cat. no. 14737-1-AP; Proteintech
Group, Inc.), Bcl-2 (1:2,000; cat. no. 60178-1-Ig; ProteinTech
Group, Inc.), Bax (1:1,000; cat. no. 50599-2-Ig; ProteinTech Group,
Inc.), cleaved caspase3 (1:1,000; cat. no. 19677-1-AP; ProteinTech
Group, Inc.); E-cadherin (1:1,000; cat. no. ab194982; Abcam),
N-cadherin (1:1,000; cat. no. ab18203; Abcam), vimentin (1:1,000;
cat. no. ab8978; Abcam) and GAPDH (1:500; cat. no. ab8245; Abcam)
at 4°C overnight, followed by incubation with anti-rabbit IgG
(1:2,000; cat. no. GTX300119; GeneTex, Inc.) or anti-mouse IgG
(1:2,000; cat. no. GTX300120; GeneTex, Inc.) secondary antibodies
for 1 h at room temperature. Finally, the protein bands were
visualized by ECL reagents (Proteintech Group, Inc.). GAPDH was
used as a protein-loading control. Data were analyzed by Quantity
One software (v.4.6; Bio-Rad Laboratories, Inc.).
Statistical analysis
SPSS 18.0 (SPSS Inc.) software was used for
statistical analysis. All assays were performed in triplicate. The
data from individual experiments was presented as the mean ± SD.
The difference between the two groups was determined by unpaired
Student's t-test. The difference between multiple groups was
determined by one-way analysis of variance (ANOVA) and a post hoc
Scheffe's test. Table I was analyzed
by the χ2 test, and Table
II was analyzed by the Fisher's test. Kaplan-Meier survival
analysis was used to evaluate the prognosis. The log-rank test was
used to compare the survival curves. P<0.05 was considered to
indicate a statistically significant difference.
 | Table I.DNM3 expression in cervical cancer
tissues (n=41) compared with normal adjacent tissue. |
Table I.
DNM3 expression in cervical cancer
tissues (n=41) compared with normal adjacent tissue.
|
| DNM3 expression |
|
|---|
|
|
|
|
|---|
| Group | Low, n (%) | High, n (%) | P-value |
|---|
| Cervical cancer | 23 (56.1) | 18 (43.9) | 0.007a |
| Normal adjacent | 10 (24.4) | 31 (75.6) |
|
 | Table II.DNM3 expression associated with
clinicopathological parameters of patients with cervical
cancer. |
Table II.
DNM3 expression associated with
clinicopathological parameters of patients with cervical
cancer.
| Clinicopathological
parameters | N | DNM3 Low, n (%) | DNM3 High, n (%) | P-value |
|---|
| Age, years |
|
<45 | 17 | 10 (0.588) | 7 (0.412) | 0.930 |
| ≥45 | 24 | 15 (0.625) | 9 (0.375) |
|
| Tumor diameter,
cm |
|
<3 | 15 | 9 (0.600) | 6 (0.400) | 0.956 |
| ≥3 | 26 | 14 (0.538) | 12 (0.462) |
|
| Pathological
grading |
| I–II | 28 | 12 (0.429) | 16 (0.571) | 0.030a |
|
II–III | 13 | 11 (0.846) | 2 (0.154) |
|
Results
DNM3 is significantly expressed at low
levels in human cervical cancer tissues and cells compared with
normal cervical tissues and cells
First, the GEPIA online database was used to analyze
the mRNA level of DNM3 in CESC tissues (n=306) and normal cervical
tissues (n=13). Expression analysis demonstrated that the mRNA
expression of DNM3 in CESC tissue was significantly lower compared
with that in normal cervical tissue (P<0.05; Fig. 1A). In addition, 41 clinical pairs of
cervical cancer and corresponding adjacent normal tissues were used
for IHC analysis of DNM3. The findings demonstrated that DNM3 was
expressed at high levels in 75.6% (31/41) of adjacent tissues and
43.9% (18/41) of cervical cancer tissues (P=0.007; Fig. 1B; Table
I). These data suggested that DNM3 was expressed at a
significantly lower level in human cervical cancer tissues compared
with that in normal tissues. In addition, the low expression of
DNM3 was significantly associated with high pathological grading of
cervical cancer (P=0.030; Table
II). However, the expression level of DNM3 had no significant
association with patient age (P=0.792) or tumor size (P=0.956;
Table II). In further analysis of
the association between DNM3 expression and the progression of
cervical cancer, it was found that the survival of patients with
low DNM3 expression was significantly improved compared with
patients with high DNM3 expression (log-rank P=0.0068; Fig. 1C). The information regarding patients
survival times were obtained from the GEPIA online database. The
results of RT-qPCR demonstrated that the mRNA level of DNM3 in the
cervical cancer Caski, SiHa, Hela and C33A cell lines was
significantly lower compared with that of the normal cervical cell
line H8 (P<0.01; Fig. 1D). Two
cervical cancer cell lines, C33A and SiHa, with low DNM3 expression
were selected for follow-up experiments. In addition, the results
of the immunofluorescence assay demonstrated that DNM3 was mainly
located in the cytoplasm (Fig.
1E).
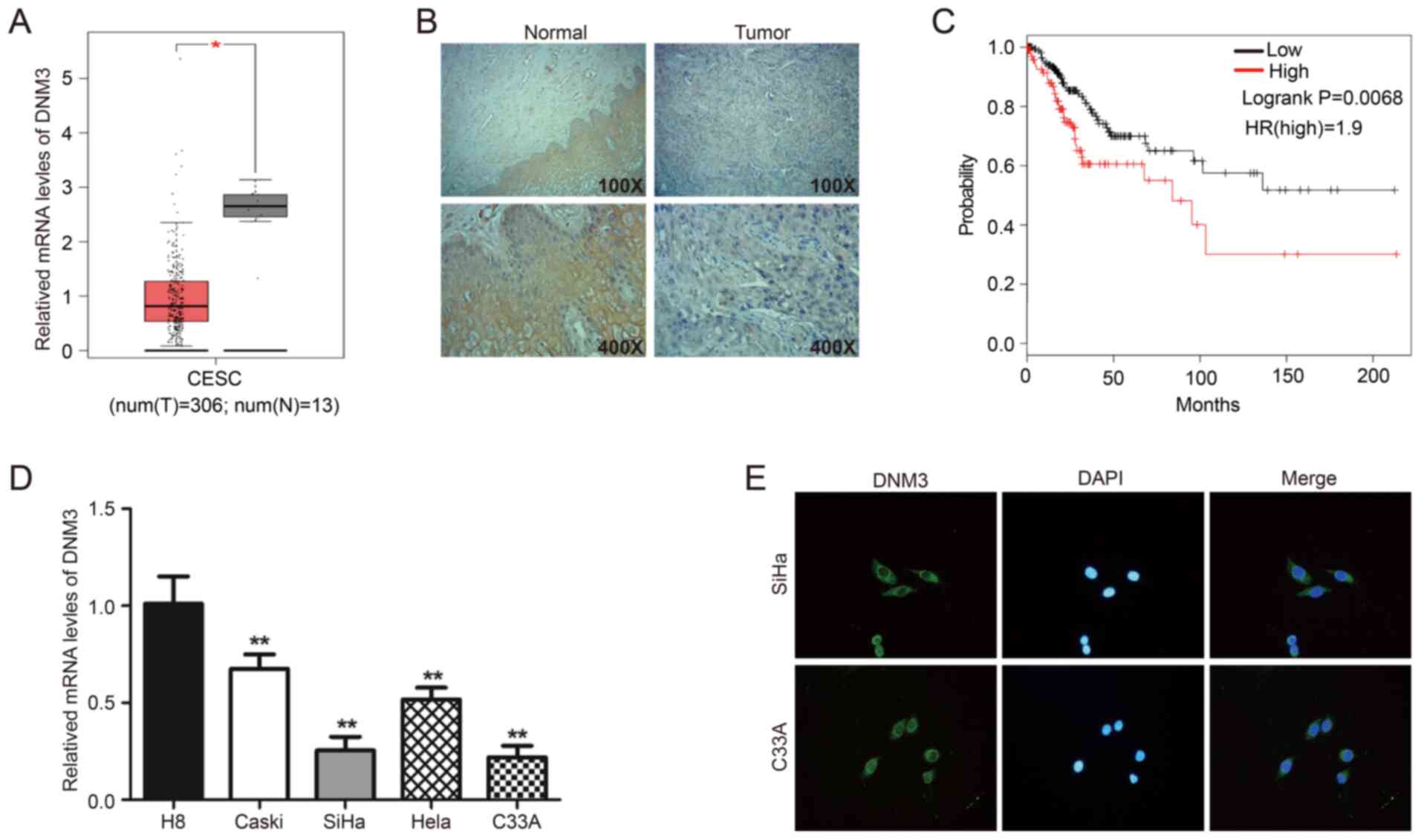 | Figure 1.DNM3 is expressed at significantly low
levels in human cervical cancer tissues and cells compared with
normal cervical tissues and cells. (A) Bioinformatics analysis of
the expression profile of DNM3 in cervical squamous cell carcinoma
and endocervical adenocarcinoma and normal tissues. All data are
from the GEPIA online database. (B) Immunohistochemistry staining
of DNM3 protein in human cervical cancer tissue specimens. (C)
Overall survival curve of the human cervical cancer patients with
different DNM3 expression levels. All data are from the GEPIA
online database. (D) The endogenous expression of DNM3 in cervical
carcinoma cell lines (Caski, SiHa, Hela and C33A) was higher
compared with that in the normal cervical cell line H8, as
determined by RT-qPCR. (E) Immunofluorescence staining for DNM3
protein in C33A and SiHa cells (magnification, ×400). *P<0.05,
**P<0.01. DNM3, dynamin 3; GEPIA, Gene Expression Profiling
Interactive Analysis; RT-q, reverse transcription quantitative; t,
tumor; n, normal; HR, hazard ratio. |
DNM3 overexpression suppresses the
proliferation of cervical carcinoma cells
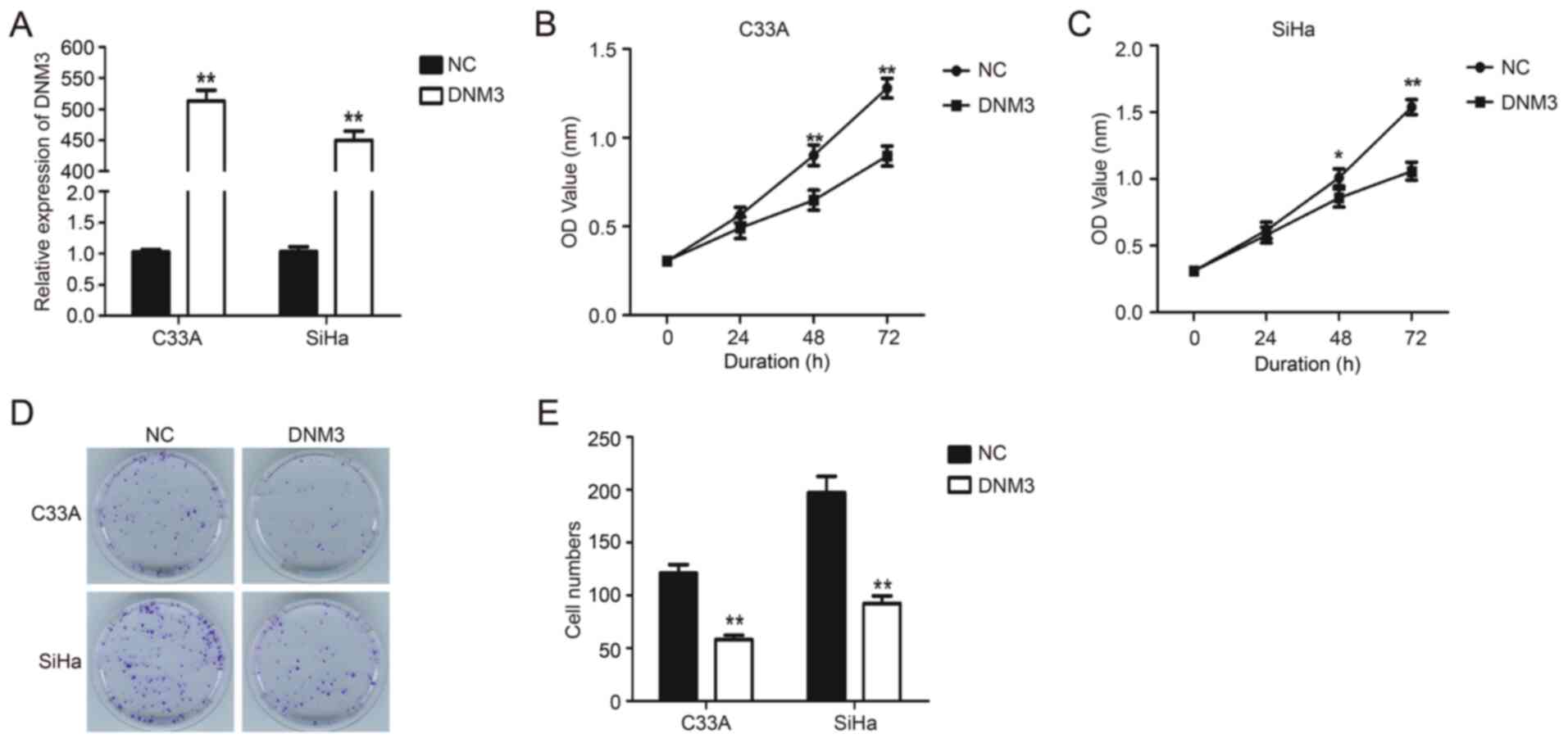
DNM3 expression plasmid was transfected into C33A
and SiHa cells to upregulate the expression of DNM3 (P<0.01;
Fig. 2A). Results of the CCK-8
experiment demonstrated that the proliferation of C33A and SiHa
cells with DNM3 overexpression was significantly inhibited compared
with that of the control group (P<0.01; Fig. 2B and C). Similarly, the results of
plate clone formation demonstrated that DNM3 overexpression greatly
reduced the number of cell clones compared with the control group
in both cell lines (P<0.01; Fig. 2D
and E). These data indicated that the overexpression of DNM3
suppressed the proliferation of human cervical carcinoma cells.
DNM3 overexpression suppresses the
migration and invasion of cervical carcinoma cells
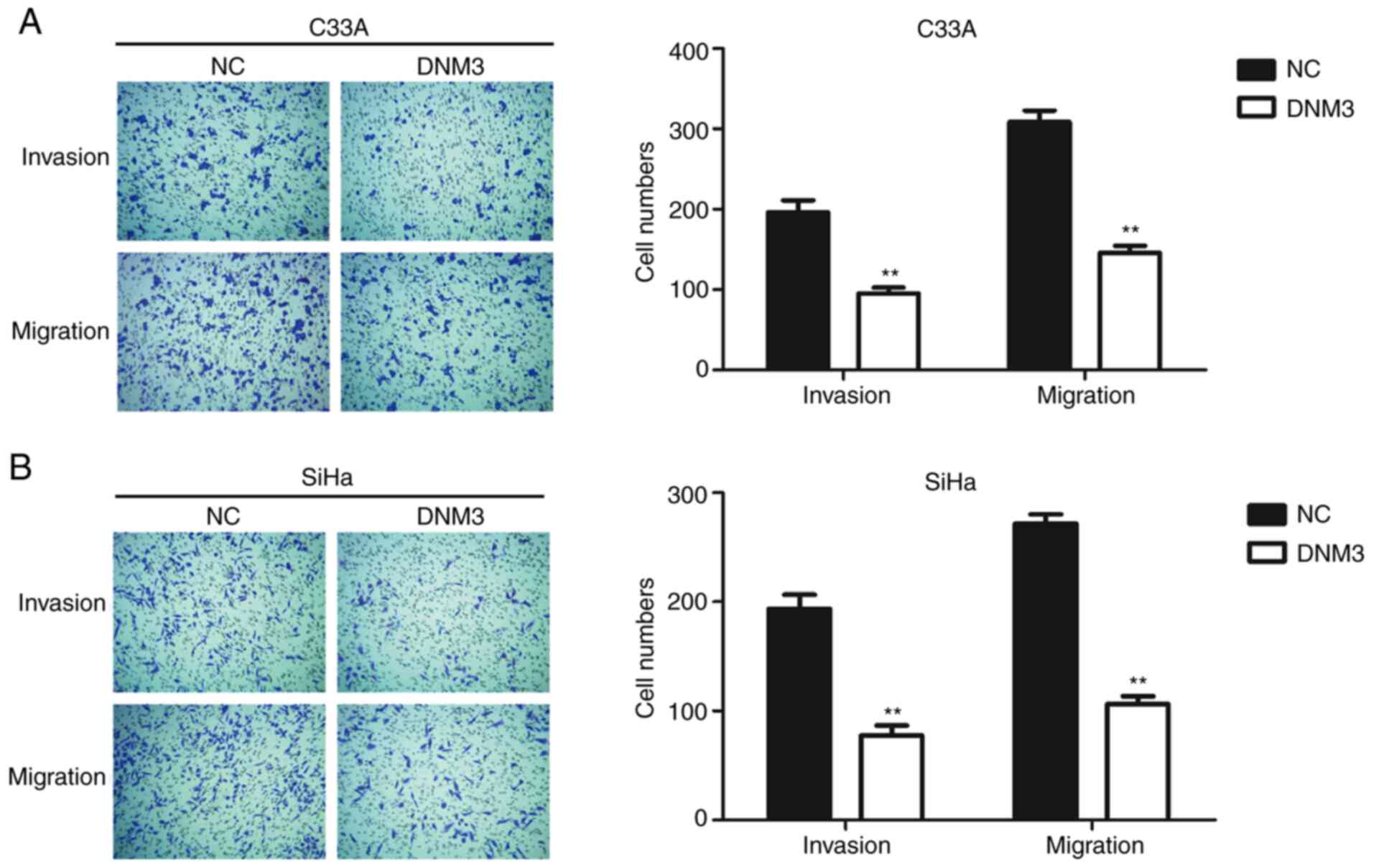
Cancer cells are generally characterized by aberrant
motility (17). In order to further
investigate the role of DNM3 in regulating cancer cell motility,
Transwell assays were performed. The invasion and migration
abilities of C33A and SiHa cells with high expression of DNM3 were
inhibited significantly compared with those of the control group
(P<0.01; Fig. 3).
DNM3 overexpression induces apoptosis
of cervical carcinoma cells
Flow cytometry and western blotting were performed
to evaluate the effect of DNM3 overexpression on the apoptosis of
C33A and SiHa cells. The results of flow cytometry demonstrated
that DNM3 overexpression significantly promoted the apoptosis of
C33A (P<0.01; Fig. 4A) and SiHa
(P<0.01; Fig. 4B) cells compared
with the negative control (NC) group, respectively. These findings
were further validated by western blotting. Bcl2 is an
antiapoptotic protein, Bax is a proapoptotic protein and caspase-3
is an apoptotic executioner (18).
The aforementioned proteins serve an important role in the process
of apoptosis (19,20). The results demonstrated that
following DNM3 overexpression, the level of Bcl2 was downregulated,
while the expression of Bax and cleaved caspase-3 was upregulated
in both cell lines compared with the NC group (P<0.01; Fig. 4C). These data indicated that DNM3
promoted the apoptosis of cervical cancer cells.
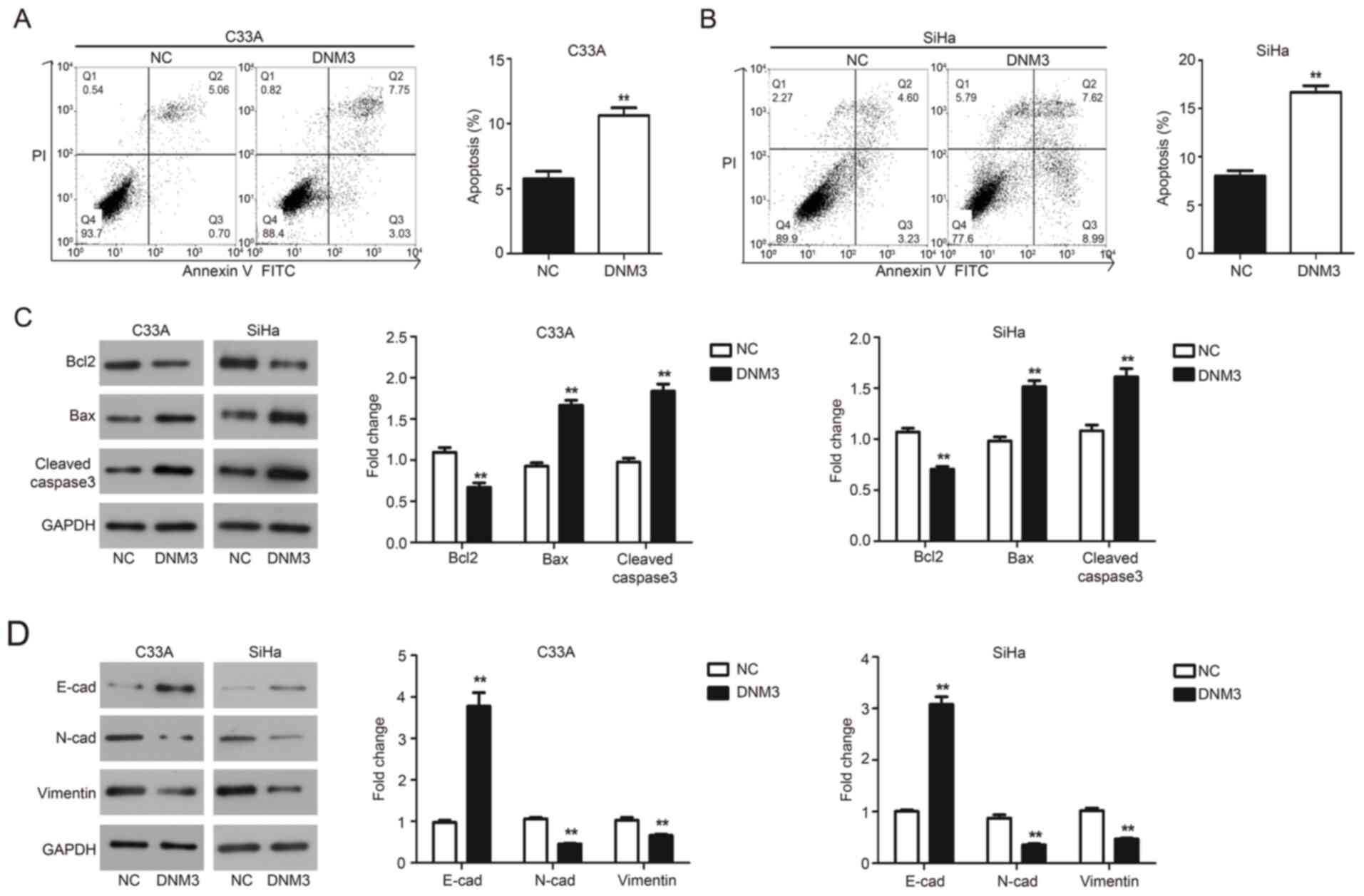 | Figure 4.DNM3 overexpression promotes cell
apoptosis and inhibits epithelial mesenchymal transition of C33A
and SiHa cells. (A and B) C33A and SiHa cells were transfected with
NC or DNM3 expression plasmid, and then cell apoptosis was
determined using double staining with Annexin V/PI by flow
cytometry. Statistical analysis of cell numbers after flow
cytometry was presented. (C) C33A and SiHa cells were transfected
with NC or DNM3 expression plasmid for 24 h. Next, the protein
expression of Bcl2, Bax, cleaved caspase-3 and GAPDH was assessed
by western blotting. Relative amounts of proteins were normalized
to GAPDH in C33A and SiHa cells. (D) Protein expression of
E-cadherin, N-cadherin, vimentin and GAPDH by western blotting.
Relative amounts of proteins were normalized to GAPDH in C33A and
SiHa cells. **P<0.01. DNM3, dynamin 3; NC, negative control; PI,
propidium iodide; E-cad, E-cadherin; N-cad, N-cadherin. |
DNM3 overexpression suppresses the EMT
of cervical carcinoma cells
EMT is a developmental program and related to the
progression and metastasis of cancer (21). The present study investigated the
involvement of EMT in cervical cancer tumor progression. Epithelial
and mesenchymal markers were assessed by western blotting and the
results demonstrated that C33A and SiHa cells overexpressing DNM3
exhibited a significant upregulation of E-cadherin expression;
meanwhile the expression of mesenchymal markers N-cadherin and
vimentin was significantly downregulated compared with the NC group
(P<0.01; Fig. 4D).
Discussion
DNM3 serves a vital role in the regulation of tumor
progression (22). Numerous emerging
studies have demonstrated that DNM3 exhibits decreased expression
in some types of tumor tissues, such as colon cancer (22) and hepatocellular carcinoma (10). By contrast, one study demonstrated
that the expression of DNM3 is upregulated in glioblastoma
multiforme (GBM). In GBM orthotopic xenograft models, DNM3
expression was increased in original tumors and exosomes. The mRNA
levels of DNM3 also increased in recurrence tumors and exosomes
from recurrent tumor xenografts (23). To the best of our knowledge, there
are no previous studies on the association between DNM3 expression
and cervical cancer. The present study evaluated the expression of
DNM3 in cervical tumors. The expression of DNM3 decreased in
cervical cancerous tissues compared with that in normal adjacent
tissues. The present study also examined the mRNA expression
profile of DNM3 in four different cervical carcinoma cell lines
(Caski, SiHa, Hela and C33A) and one normal cell line (H8) by
RT-qPCR. The results demonstrated that the expression of DNM3 was
downregulated in cervical carcinoma cells compared with that in
normal cervical cells. In addition, using immunofluorescence
analysis in the present study, it was demonstrated that DNM3 was
located in the cytoplasm. In the present study, the methylation
status of the DNM3 promoter region was not assessed in
cervical cancer tissues and cells. Promoter hypermethylation may be
related to decreased expression. This should be investigated by
future studies.
In the present study, the function of DNM3 in
cervical cancer cells was assessed by function analyses, such as
proliferation, apoptosis, migration and invasion of SiHa and C33A
cells. The results of CCK-8 and clone formation assays demonstrated
that DNM3 overexpression significantly inhibited the proliferation
of SiHa and C33A cells compared with NC groups. Cervical cancer
generally develops distant metastases through the hematogenous
route. In the present study, the findings of the Transwell assay
demonstrated that the overexpression of DNM3 reduced cell migration
and invasion of SiHa and C33A cells compared with NC groups. Flow
cytometry analysis revealed that the overexpression of DNM3
promoted the apoptosis of SiHa and C33A cells.
The present study demonstrated that the
overexpression of DNM3 inhibits the EMT process. EMT is a crucial
process in the regulation of cell migration and invasion (21). Combined with the results of the
Transwell assay, the present study revealed that the overexpression
of DNM3 may inhibit the migration and invasion of cancer cells by
suppressing the EMT process. However, the association between DNM3
and EMT needs further investigation.
In conclusion, the present study demonstrated the
functions of DNM3 in cervical carcinoma cells. The expression of
DNM3 was downregulated in cervical cancer tissues and cells
compared with normal cervical tissues and cells. Meanwhile, the
overexpression of DNM3 inhibited the proliferation, migration and
invasion of cervical cancer cells and promoted apoptosis. Based on
the findings of the present study, DNM3 may be a new potential
prognostic biomarker and therapeutic target for cervical
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Author's contributions
JF designed the study, performed experiments,
analyzed data and wrote the manuscript. The author read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Linyi Cancer Hospital (Linyi, China). All patients
provided written informed consent for participation in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vinh-Hung V, Bourgain C, Vlastos G, Cserni
G, De Ridder M, Storme G and Vlastos AT: Prognostic value of
histopathology and trends in cervical cancer: A SEER population
study. BMC Cancer. 7:1642007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denny L, Adewole I, Anorlu R, Dreyer G,
Moodley M, Smith T, Snyman L, Wiredu E, Molijn A, Quint W, et al:
Human papillomavirus prevalence and type distribution in invasive
cervical cancer in sub-Saharan Africa. Int J Cancer. 134:1389–1398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu XX, Zhou JS, Yuan SH, Yu H and Lou HM:
Distribution of HPV genotype in invasive cervical carcinoma and
cervical intraepithelial neoplasia in Zhejiang province, southeast
China: Establishing the baseline for surveillance. Int J Environ
Res Public Health. 12:10794–10805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McDowell M, Pardee DJ, Peitzmeier S,
Reisner SL, Agénor M, Alizaga N, Bernstein I and Potter J: Cervical
cancer screening preferences among trans-masculine individuals:
Patient-collected human papillomavirus vaginal swabs versus
provider-administered pap tests. LGBT Health. 4:252–259. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bosgraaf RP, Ketelaars PJ, Verhoef VM,
Massuger LF, Meijer CJ, Melchers WJ and Bekkers RL: Reasons for
non-attendance to cervical screening and preferences for HPV
self-sampling in Dutch women. Prev Med. 64:108–113. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heymann JA and Hinshaw JE: Dynamins at a
glance. J Cell Sci. 122((Pt 19)): 3427–3431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raja SA, Shah STA, Tariq A, Bibi N, Sughra
K, Yousuf A, Khawaja A, Nawaz M, Mehmood A, Khan MJ and Hussain A:
Caveolin1 and dynamin2 overexpression is associated with the
progression of bladder cancer. Oncol Lett. 18:219–226.
2019.PubMed/NCBI
|
|
11
|
Meng J: Distinct functions of dynamin
isoforms in tumorigenesis and their potential as therapeutic
targets in cancer. Oncotarget. 8:41701–41716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inokawa Y, Nomoto S, Hishida M, Hayashi M,
Kanda M, Nishikawa Y, Takeda S, Fujiwara M, Koike M, Sugimoto H, et
al: Dynamin 3: A new candidate tumor suppressor gene in
hepatocellular carcinoma detected by triple combination array
analysis. Onco Targets Ther. 6:1417–1424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z, Chen C, Guo W, Zheng S, Sun Z and
Geng X: DNM3 attenuates hepatocellular carcinoma growth by
activating P53. Med Sci Monit. 22:197–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu C, Yao J and Sun P: Dynamin 3
suppresses growth and induces apoptosis of hepatocellular carcinoma
cells by activating inducible nitric oxide synthase production.
Oncol Lett. 13:4776–4784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res.
45(W1):W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using quantitative PCR and the
2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trepat X, Chen Z and Jacobson K: Cell
migration. Compr Physiol. 2:2369–2392. 2012.PubMed/NCBI
|
|
18
|
Dietrich JB: Apoptosis and anti-apoptosis
genes in the Bcl-2 family. Arch Physiol Biochem. 105:125–135.
1997.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brambilla E, Negoescu A, Gazzeri S,
Lantuejoul S, Moro D, Brambilla C and Coll JL: Apoptosis-related
factors p53, Bcl2, and Bax in neuroendocrine lung tumors. Am J
Pathol. 149:1941–1952. 1996.PubMed/NCBI
|
|
20
|
Levesley J, Steele L, Bruning-Richardson
A, Davison A, Zhou J, Ding C, Lawler S and Short SC: Selective
BCL-XL inhibition promotes apoptosis in combination with MLN8237 in
medulloblastoma and pediatric glioblastoma cells. Neuro Oncol.
20:203–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suarez-Carmona M, Lesage J, Cataldo D and
Gilles C: EMT and inflammation: Inseparable actors of cancer
progression. Mol Oncol. 11:805–823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Y, Guan L, Han Y, Zhou Y, Li X, Liu Y,
Zhang X, Zhang W, Li X, Wang S and Lu W: siPRDX2-elevated DNM3
inhibits the proliferation and metastasis of colon cancer cells via
AKT signaling pathway. Cancer Manag Res. 11:5799–5811. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang JK, Yang JP, Tong J, Jing SY, Fan B,
Wang F, Sun GZ and Jiao BH: Exosomal miR-221 targets DNM3 to induce
tumor progression and temozolomide resistance in glioma. J
Neurooncol. 131:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|