Introduction
Cervical cancer was the fourth most common malignant
tumour worldwide in 2018, with a high mortality rate among women
(1). In 2018, 530,000 women
worldwide were diagnosed with cervical cancer, and 60% of patients
with cervical cancer are likely to die (1,2). Human
papillomavirus (HPV) is the main risk factor of cervical cancer,
and the widespread use of HPV vaccines and virus screening has
reduced cervical cancer incidence (3,4).
Although treatment methods, such as radiotherapy, chemotherapy and
surgery, have been used in the last two decades to improve the
survival rate of patients with cervical cancer (5), the underlying mechanism of this tumour
remains largely unknown. Therefore, oncogenes involved in cervical
cancer carcinogenesis require further exploration.
MicroRNAs (miRNAs/miRs) are non-coding RNAs, 19–25
nucleotides in length, which are involved in the
post-transcriptional modification of RNAs by binding to the 3′
untranslated region (UTR) of target genes (6,7).
Researchers have studied the effect of miR-186 on several types of
cancer, including gastric cancer, ovarian cancer, multiple myeloma
and melanoma (8–11). Several studies have demonstrated that
miR-186 promotes cell apoptosis and inhibits cell proliferation,
aerobic glycolysis and metastasis (12–15).
Additionally, this miRNA functions as a tumour suppressor in breast
cancer by inhibiting the expression of epiregulin (EREG), which
promotes glycolysis and enhances cell proliferation (16). Although no studies have explored the
function of miR-186-3p in cervical cancer, miR-186-5p has been
reported to be downregulated in HPV-infected cervical cancer cells
(17). Previous evidence has
demonstrated that miR-186 suppresses the epithelial-mesenchymal
transition of cervical cancer cells, which promotes apoptosis of
cervical cancer cells (18).
Furthermore, the long non-coding RNA (lncRNA) antisense RNA in the
INK4 locus promotes the progression of cervical cancer by sponging
miR-186 (19). However, to the best
of our knowledge, the function and underlying mechanism of
miR-186-3p remain elusive.
Minichromosome maintenance complex component 2
(MCM2) is located on chromosome 3q21.3 and comprises 17 exons
(20). It encodes minichromosome
maintenance complex (MCM) involved in the initiation of eukaryotic
genome replication (21). MCM2 is
frequently upregulated in breast cancer (22), melanoma (23), ovarian cancer (24) and oral squamous cell carcinoma
(25). Several bioinformatics
studies have suggested that MCM2 is a key gene for diagnosis and
prediction of the occurrence of cervical cancer (26–28).
Although a study regarding differentially expressed miRNAs and
genes has also revealed aberrant MCM2 expression (29), to the best of our knowledge,
researchers are yet to clarify the oncogenic function of MCM2 in
cervical cancer and its regulatory network, especially the
interaction between miR-186-3p and MCM2.
The present study aimed to investigate the oncogenic
effects of MCM2 and miR-186-3p in cervical cancer. It was
hypothesised that by directly targeting MCM2, miR-186-3p suppresses
MCM2 expression and cervical cancer progression. These results may
provide novel insights into the oncogenic functions of miR-186-3p
and MCM2 in cervical cancer and enhance the understanding of
oncogenes and their regulatory network profiles.
Materials and methods
Bioinformatics analysis
The mRNA expression profiles [GSE7803 (30) and GSE63514 (31)] were downloaded from Gene Expression
Omnibus DataSets (https://www.ncbi.nlm.nih.gov/gds/?term=) and were used
to screen the upregulated genes with adjusted P-values set at
<0.05 and log fold change at >1.5. Subsequently, the Search
Tool for the Retrieval of Interacting Genes/Proteins database
(STRING; version 11.0; http://string-db.org/) was used for biological process
enrichment analysis of upregulated genes. Next, the expression
pattern of upregulated genes was further analysed using data from
The Cancer Genome Atlas (TCGA; Project ID, CESC; http://portal.gdc.cancer.gov/). Finally, the
miRNA was identified using TargetScan 7.1 (http://www.targetscan.org/vert_71/) and TarBase v8
(http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=tarbasev8/index),
which could predict the upstream miRNAs of a key gene. Venny 2.1.0
(http://bioinfogp.cnb.csic.es/tools/venny/) was used to
overlap the miRNAs from the two databases (TargetScan 7.1 and
TarBase v8).
Clinical specimens
Cervical cancer tissues and corresponding normal
tissues (3 cm from tumour tissues) were collected from 48 female
patients (mean age, 48 years; age range, 36–67 years) diagnosed
with cervical cancer at The First Affiliated Hospital of Hebei
North University (Zhangjiakou, China) between September 2017 and
October 2018. Biopsy samples were obtained and stored in liquid
nitrogen (−196°C). The inclusion criteria were: i) Patients were
diagnosed with cervical cancer; ii) patient's information had
obtained; and iii) patients signed the consent forms. The exclusion
criteria were: i) Patients had other types of disease; and ii)
patients underwent chemotherapy, radiotherapy or other therapies.
Diagnostic test results were independently confirmed by two
pathologists. The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Hebei North
University (Zhangjiakou, China). The clinical characteristics of
the patients are summarised in Table
I. All samples were graded as I, II, III and IV based on The
International Federation of Gynecology and Obstetrics staging
system (32).
 | Table I.Clinical characteristics of 48
patients with cervical cancer. |
Table I.
Clinical characteristics of 48
patients with cervical cancer.
|
Characteristics | No. (%) (n=48) |
|---|
| Age, years |
|
|
≤48 | 34 (70.8) |
|
>48 | 14 (29.2) |
| Tumor
differentiation |
|
|
Well | 6
(12.5) |
|
Moderate | 18 (37.5) |
|
Poor | 24 (50.0) |
| Tumor size, cm |
|
| ≤4 | 37 (77.1) |
|
>4 | 11 (22.9) |
| Histological
type |
|
|
Squamous carcinoma | 32 (66.7) |
|
Adenocarcinoma | 10 (20.8) |
|
Other | 6
(12.5) |
| FIGO stage |
|
| I | 11 (22.9) |
| II | 33 (68.7) |
|
III | 3
(6.3) |
| IV | 1
(2.1) |
| Lymph nodes
status |
|
|
Positive | 17 (35.4) |
|
Negative | 31 (64.6) |
| Vascular
invasion |
|
|
Positive | 20 (41.7) |
|
Negative | 28 (58.3) |
Cell culture and transfection
Human cervical cancer cell lines (HeLa, CaSki, SiHa
and C33A) and a normal cervical epithelial cell line (HcerEpic)
were purchased from the National Infrastructure of Cell Line
Resource. The small interfering RNA (siRNA/si) of MCM2 (si-MCM2;
5′-CAGGTGACAGACTTTATCAAA-3′) and the scrambled negative control
(si-NC; 5′-UUCUCCGAACGUGUCACGUTT-3′) were obtained from Shanghai
GenePharma Co., Ltd., and the miR-186-3p mimic
(5′-GCCCAAAGGUGAAUUUUUUGGG-3′), miR-186-3p inhibitor
(5′-CCCAAAAAAUUCACCUUUGGGC-3′), mimic-NC
(5′-UCACAACCUCCUAGAAAGAGUAGA-3′) and inhibitor-NC
(5′-UUUGUACUACACAAAAGUACUG-3′) were purchased from Guangzhou
RiboBio Co., Ltd. All cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with
penicillin (100 U/ml), streptomycin (100 µg/ml) and FBS [10% (v/v);
Gibco; Thermo Fisher Scientific, Inc.]. The cells were cultured and
incubated in air containing 5% CO2 at 37°C. All cell
transfections were performed using Lipofectamine® 2000
Transfection Reagent (Thermo Fisher Scientific, Inc.). The
transfection concentration of si-MCM2, si-NC, miR-186-3p mimic,
mimic-NC, miR-186-3p inhibitor and inhibitor-NC was 50 nM. After 48
h of transfection at 37°C, transfection efficiency was determined
using reverse transcription-quantitative PCR (RT-qPCR). The cells
in the control (CON) group were not transfected. The cell function
experiments were performed at 48 h after transfection.
RT-qPCR
All cells were harvested and allowed to reach ~90%
confluence as described previously (33). The cells were first washed with PBS
(pH 7.4), and total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. For tissues, total RNA was also extracted
using TRIzol® reagent (Thermo Fisher Scientific, Inc.).
Subsequently, cDNA was synthesised using the PrimeScript RT-PCR Kit
(Takara Bio, Inc.) at 37°C for 15 min and 85°C for 5 sec. RT-qPCR
was performed using a One Step SYBR PrimeScript RT-PCR Kit (Takara
Bio, Inc.) with the following thermocycling conditions: 42°C for 5
min, 95°C for 10 sec, 40 cycles of 95°C for 5 sec and 60°C for 30
sec. Gene expression was examined using the 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
2−ΔΔCq method (34) was
used to calculate relative expression. U6 and GAPDH were used as
reference genes for miRNA and mRNA, respectively. The primer
sequences used for RT-qPCR are listed in Table II.
 | Table II.Primer sequences for reverse
transcription-quantitative PCR. |
Table II.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequences
(5′-3′) |
|---|
| miR-186-3p | Forward:
GCCCAAAGGTGAATTTTTTGGG |
|
| Reverse:
CAGTGCGTGTCGTGGAGT |
| U6 | Forward:
CTCGCTTCGGCAGCACA |
|
| Reverse:
AACGCTTCACGAATTTGCGT |
| MCM2 | Forward:
AGCACTTGATGAACTCGGGG |
|
| Reverse:
AAGCCAACAGATACCAGCGT |
| GAPDH | Forward:
GGAGCGAGATCCCTCCAAAAT |
|
| Reverse:
GGCTGTTGTCAT-ACTTCTCATGG |
Western blotting
First, 5×105 HeLa and SiHa cells were
suspended in lysis buffer [50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5
mM MgCl2, 10% glycerol, 1% Triton X-100 and 0.1% sodium
dodecyl sulfate (SDS)] containing the protease inhibitor cocktail
(Roche Diagnostics) on ice for 30 min. Subsequently, the samples
were centrifuged for 15 min at 12,000 × g at 4°C. After the
supernatants were collected, protein concentrations were measured
using the Dc Bio-Rad Protein Assay Kit (Bio-Rad Laboratories,
Inc.). Proteins (20 µg/lane) extracted from the cells were
separated using a 10% SDS polyacrylamide gel and subsequently
transferred to PVDF membranes. The membranes were blocked with 5%
non-fat milk for 2 h at 25°C and then incubated overnight at 4°C
with the anti-MCM2 antibody (dilution, 1:1,000; cat. no. ab4461;
Abcam) and the anti-GAPDH antibody (dilution, 1:1,000; cat. no.
ab128915; Abcam) followed by the HRP Anti-Rabbit IgG antibody
(dilution, 1:5,000; cat. no. ab270144; Abcam) for 2 h at 25°C. The
blots were developed using the Immobilon Western Chemiluminescent
HRP Substrate (cat. no. WBKLS0500; Merck KGaA). Densitometry was
performed using ImageJ 1.48 (National Institutes of Health).
MTT assay
HeLa and SiHa cells at 60% confluence were
transfected using Lipofectamine® 2000 Transfection
Reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Subsequently, the cells were treated with
trypsin at 37°C for 60 sec and seeded into 96-well plates at a
density of 3×103 cells/well. After a transfection period
of 24 h, 20 µl MTT was added to the 96-well plates. Three
replicates were performed for each group. After 4 h, the
supernatant was discarded, 150 µl DMSO was added to each well, and
the mixture was incubated at 37°C for 10 min. Finally, the
absorbance value of the cells at 570 nm was measured at 0, 24, 48
and 72 h.
5-bromo-2-deoxyuridine (BrdU)
assay
This assay was performed using the BrdU Cell
Proliferation ELISA kit (cat. no. ab126556; Abcam). BrdU was first
dissolved in DMSO (Thermo Fisher Scientific, Inc.). The HeLa and
SiHa cells were seeded in 96-well plates at a density of
3×103 cells/well and incubated for 48 h at 37°C.
Subsequently, the BrdU solution was added to label the cells
according to the manufacturer's protocol. Next, the labelling
solution was removed, and the cells were washed twice with PBS,
fixed with paraformaldehyde at 4°C for 20 min and treated with
Triton X-100 permeabilization buffer. The cells were subsequently
treated with the anti-BrdU antibody (100 µl/well) at room
temperature for 1 h. After adding the anti-mouse IgG antibody (100
µl/well) and incubating the mixture at room temperature for 30 min,
the absorbance value at 450 nm was determined using a microplate
reader.
Cell adhesion assay
To test the adhesive ability of cervical cancer
cells, the HeLa and SiHa cells (3×104 cells/well) were
seeded in 6-well plates coated with type I collagen (BD
Biosciences). After a 60-min incubation, the non-adherent cells
were removed, and MTT (Sigma-Aldrich; Merck KGaA) was added for 4
h. The medium was then replaced with 150 µl DMSO for 20 min.
Subsequently, the absorbance value was determined at 570 nm.
Cell migration assay
A chamber (cat. no. 3422; Corning, Inc.) in a
24-well plate was prepared for the cell migration assay using HeLa
and SiHa cells. Cell culture medium with 10% FBS was added to the
lower chamber, and 200 µl cell suspensions (2×105 cells)
in serum-free cell culture medium were added to the upper chamber
for a 24-h incubation at 37°C. Subsequently, the cells that
migrated to the lower chamber were fixed with 100% methanol for 30
min at 25°C and stained with 0.5% crystal violet (cat. no. C0775;
Sigma-Aldrich; Merck KGaA) for 20 min at 25°C. Finally, images of
five different fields from each chamber were randomly captured
using a light microscope (Olympus Corporation).
Determination of caspase-3/7
activity
Cell apoptosis was detected using a caspase-3/7
activity assay kit (cat. no. E607103; Sangon Biotech Co., Ltd.)
according to the manufacturer's protocol. The HeLa and SiHa cells
(2×104 cells/well) were seeded into a 96-well plate and
cultured for 48 h. The cells were lysed and centrifuged at 1,000 ×
g for 20 min at 4°C. Subsequently, the protein concentration was
determined using a BCA assay (Thermo Fisher Scientific, Inc.). The
supernatant containing 50 µg protein was added to the caspase-3/7
assay loading solution (100 µl) and the mixture was incubated at
37°C for 1 h in the absence of light. Finally, caspase-3/7 activity
was determined at excitation/emission = 490/525 nm using a
microplate reader.
Cell cycle assay
The cell cycle kit was obtained from Beckman
Coulter, Inc. (cat. no. C03551) for the cell cycle assay. HeLa and
SiHa cells (3×106 cells/well) were seeded in a 6-well
plate and transfected with si-MCM2 and NC siRNA for 24 h. The cells
were harvested using trypsin, fixed with 70% ethanol at 4°C
overnight, incubated with RNase A at 37°C for 1 h, and stained with
propidium iodide (included in the cell cycle kit) for 20 min at
4°C. The cells in different phases of the cell cycle were measured
using a flow cytometer (FACSCalibur; BD Biosciences) and the
results were analysed using ModFit LT v3.3 software (Verity
Software House, Inc.).
RNA pull-down
The HeLa and SiHa cells were transfected with 50 nM
biotinylated miR-186-3p (Bio-miR-186-3p;
5′-GCCCAAAGGUGAAUUUUUUGGG-biotin-3′; Guangzhou RiboBio Co., Ltd.)
and biotinylated NC (Bio-NC; 5′-UCACAACCUCCUAGAAAGAGUAGA-biotin-3′;
Guangzhou RiboBio Co., Ltd.) using Lipofectamine® 2000
Transfection Reagent (Thermo Fisher Scientific, Inc.). After a
transfection period of 48 h at 37°C, the cells were harvested,
lysed using 500 µl lysis buffer (25 mM Tris-HCl pH 7.0, 70 mM KCl,
2.5 mM EDTA, 80 U/ml of a RNAse inhibitor) and centrifuged at
12,000 × g at 4°C for 15 min. A total of 10 µl M-280 streptavidin
magnetic beads (cat. no. 11205D; Invitrogen; Thermo Fisher
Scientific, Inc.) washed with washing buffer containing 250 µg
RNase-free BSA (Thermo Fisher Scientific, Inc.) and 100 µg yeast
tRNA in 500 µl 25 mM Tris-HCl (pH 7.5), 70 mM KCl, 2.5 mM EDTA and
0.05% NP-40 were subsequently utilized to incubate the 500 µl cell
lysates. The beads were coated with RNase-free BSA and yeast tRNA
(both from Sigma-Aldrich; Merck KGaA) to prevent non-specific
binding of RNA or protein complexes. Subsequently, the beads were
incubated for 3 h at 4°C and washed with cold lysis buffer three
times and with high salt buffer (0.1% SDS, 1% Triton X-100, 2 mM
EDTA, 20 mM Tris-HCl, pH 8.0, and 500 mM NaCl). The
biotin-miRNA/mRNA complex was collected after centrifugation at
5,000 × g for 30 sec at 4°C. Finally, the combined RNA was
extracted using TRIzol reagent, and the relative expression levels
of MCM2 were measured using RT-qPCR.
Dual-luciferase reporter assay
Fragments of the 3′ UTR region of MCM2, which
contained the putative binding site for miR-186-3p, were cloned
into the luciferase reporter vector (psiCHECK-2), which was named
the wild-type (wt) vector. The mutant (mut) vector harboured the
mutated binding site of MCM2 3′ UTR. The HeLa and SiHa cells at a
density of 2×105 cells/well were seeded in 24-well
plates and cultured for 24 h. When the density of the cells
increased by 50%, Lipofectamine® 2000 Transfection
Reagent was used to transfect 100 ng pMIR-REPORT plasmid (Addgene,
Inc.), which contained firefly luciferase, 60 pmol mimic-NC or
miR-186-3p mimic, and 10 ng psiCHECK-2-wt or psiCHECK-2-mut
plasmid. Subsequently, the cells were harvested, and the
Renilla and firefly activities were examined using the
Dual-Luciferase Reporter Assay Kit (Promega Corporation) after 48 h
of transfection. Finally, the luciferase activity was calculated
and normalized to Renilla activity.
Statistical analysis
SPSS 13.0 (SPSS, Inc.) and GraphPad 7.0 (GraphPad
Software, Inc.) were used for statistical analyses. The statistical
comparison between two groups was performed using a paired
Student's t-test, while that among more than two groups was
performed using one-way ANOVA with Dunnett's or Tukey's post hoc
test. Pearson's correlation analysis was conducted to investigate
the correlation between MCM2 expression and miR-186-3p expression.
All data are presented as the mean ± standard deviation of three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
MCM2 is a key gene in cervical
cancer
The mRNA expression profile was downloaded from the
Gene Expression Omnibus DataSets. With adjusted P values set at
<0.05 and log fold change at >1.5, 115 upregulated genes were
identified in both the GSE7803 dataset and the GSE63514 dataset
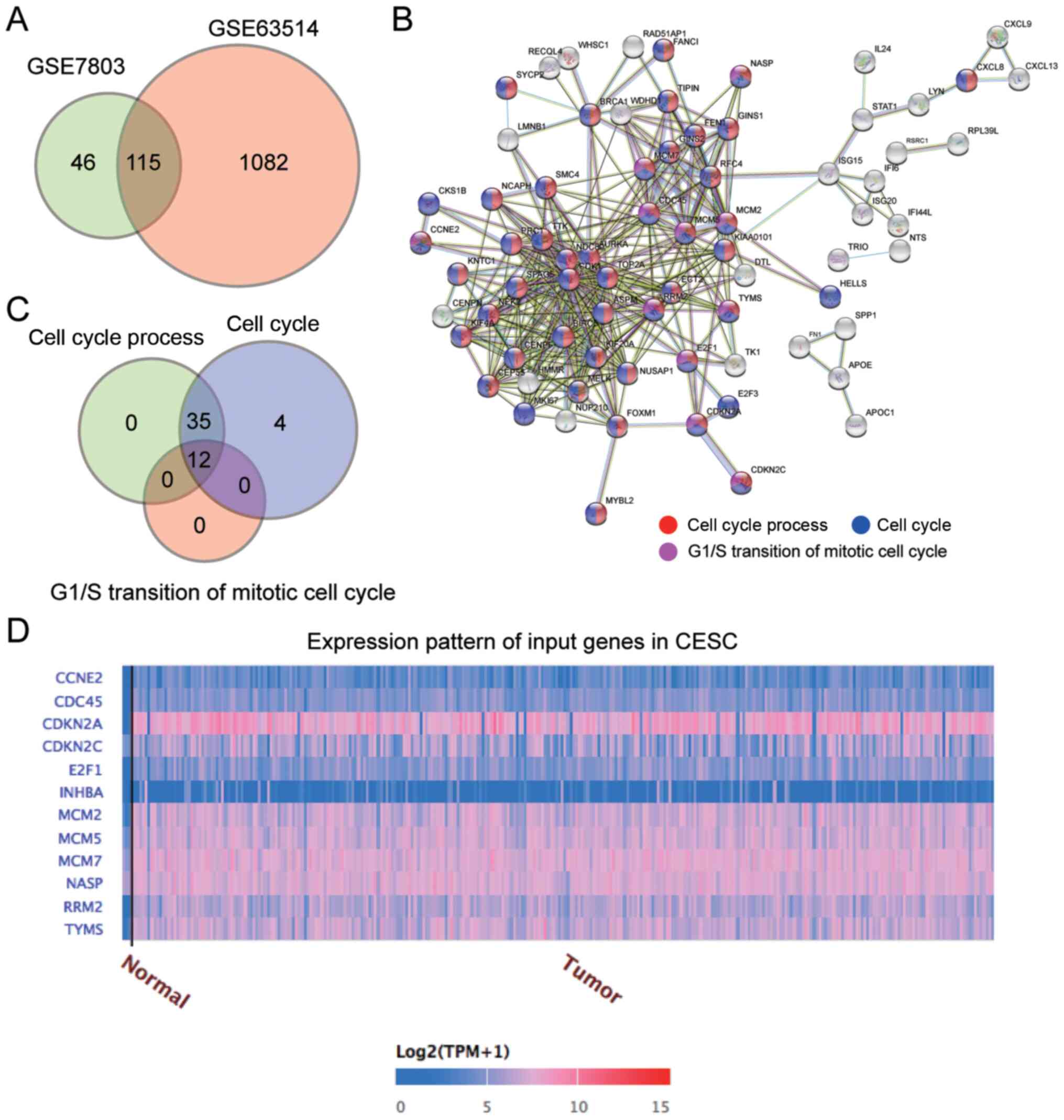
(Fig. 1A). These 115 genes were
uploaded to the STRING database for biological process enrichment
analysis, which revealed that the ‘Cell cycle process’, ‘Cell
cycle’ and ‘G1/S transition of the mitotic cell cycle’
were the key biological processes (Fig.
1B). A total of 12 genes involved in these three biological
processes were screened (Fig. 1C).
Data from TCGA revealed that among the 12 genes, the expression
levels of CDKN2A, MCM2, RRM2 and TYMS were upregulated in cervical
squamous cell carcinoma (Fig. 1D).
After reviewing the literature, it was identified that MCM2
expression is upregulated in clinical samples of cervical cancer
(26,28,35);
however, to the best of our knowledge, its function and regulatory
mechanisms in cervical cancer cells have not yet been explored.
Therefore, MCM2 was identified as the gene of interest.
MCM2 expression is upregulated in
cervical cancer tissues and cell lines
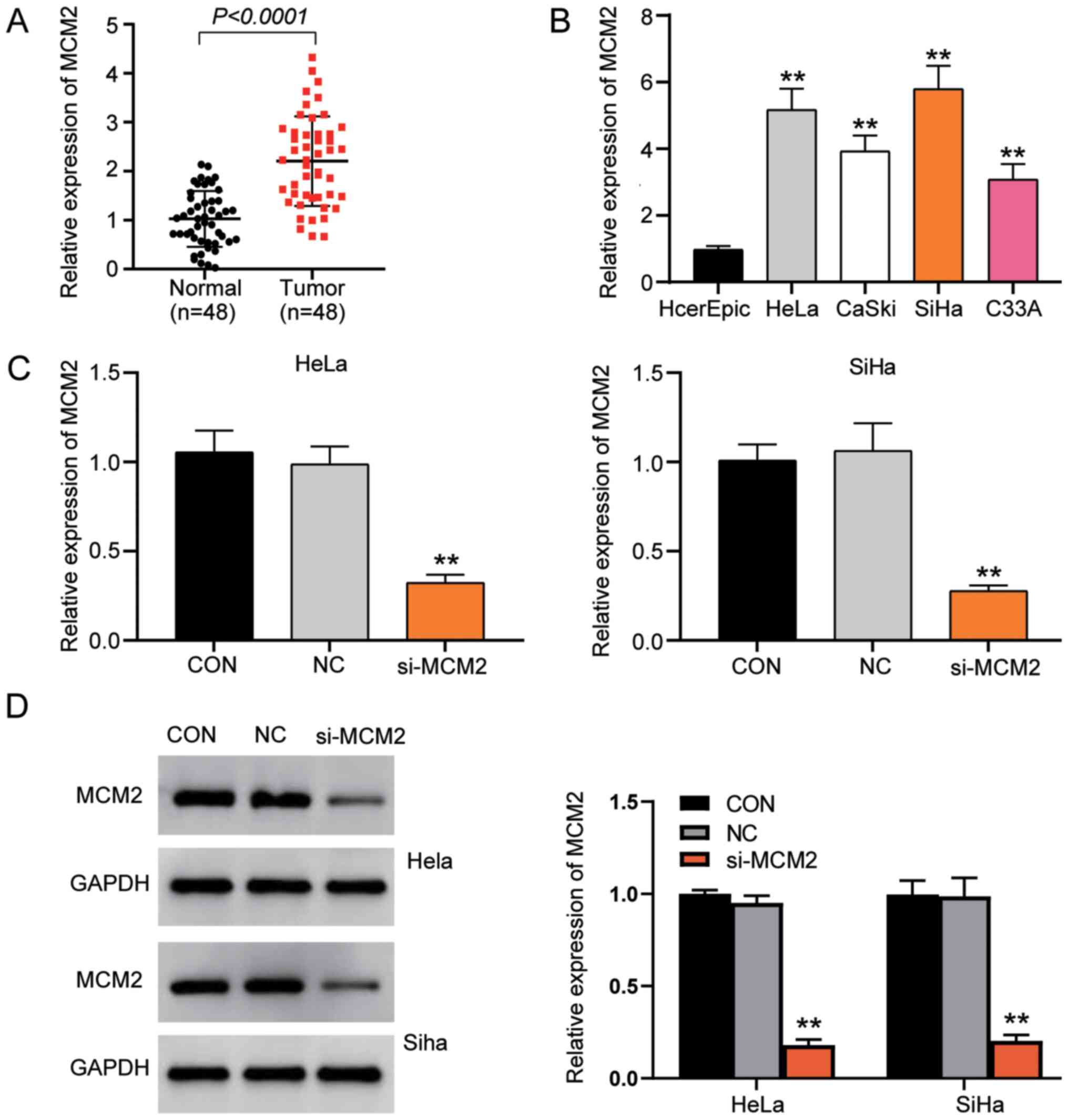
MCM2 expression in cervical cancer and normal
tissues was first examined using RT-qPCR. The results revealed that
the expression levels of MCM2 were significantly upregulated in
tumour tissues compared with normal tissues (Fig. 2A). In addition, RT-qPCR demonstrated
that the expression levels of MCM2 mRNA were higher in cervical
cancer cell lines (HeLa, CaSki, SiHa and C33A) than in the normal
cervical epithelial cell line (HCerEpiC; Fig. 2B). Since the expression levels of
MCM2 in HeLa and SiHa cells were higher than those in the other
cell lines, these two cell lines were selected to conduct MCM2
knockdown experiments using siRNA to verify the function of MCM2 in
cervical cancer. The results revealed that MCM2 expression was
successfully downregulated with a gene silencing efficiency >70%
in the si-MCM2 group compared with the control (CON) group
(Fig. 2C). Additionally, it was
identified that protein expression in the si-MCM2 group was
decreased by 80% compared with that in the CON group (Fig. 2D).
Effects of MCM2 knockdown on cervical
cancer cells
To evaluate the oncogenic function of MCM2, several
experiments were performed using cervical cancer cells. The results
of the MTT assay demonstrated that the proliferation of cancer
cells was significantly reduced after transfection with si-MCM2 at
48 and 72 h compared with that of cells in the CON group (Fig. 3A). Similar results were observed
using the BrdU assay, demonstrating that si-MCM2 inhibited cell
proliferation compared with the CON group (Fig. 3B). Next, the present study examined
whether MCM2 enhanced cell adhesion, and it was revealed that the
reduced MCM2 expression by artificial knockdown impaired the
adhesion capability of the cells by >50% (Fig. 3C). Additionally, the present study
investigated the effect of MCM2 on the apoptosis of cancer cells
using a caspase-3/7 activity assay. MCM2 knockdown increased the
caspase-3/7 activity by 6-fold compared with that in the CON group,
suggesting that MCM2 acted as a strong anti-apoptotic agent
(Fig. 3D). Additionally, cell
migration was significantly downregulated following MCM2 knockdown
(Fig. 3E). Flow cytometric analyses
revealed that the MCM2 downregulation increased the proportion of
cells in the G0/G1 phase, whereas the
proportion of cells in the G2/M phase was reduced
compared with that in the CON group, indicating that silencing of
MCM2 induced G0/G1 phase arrest (Fig. 3F and G). Overall, these results
suggested that MCM2 served a crucial role in promoting cell
proliferation and inhibiting cell apoptosis in cervical cancer
cells.
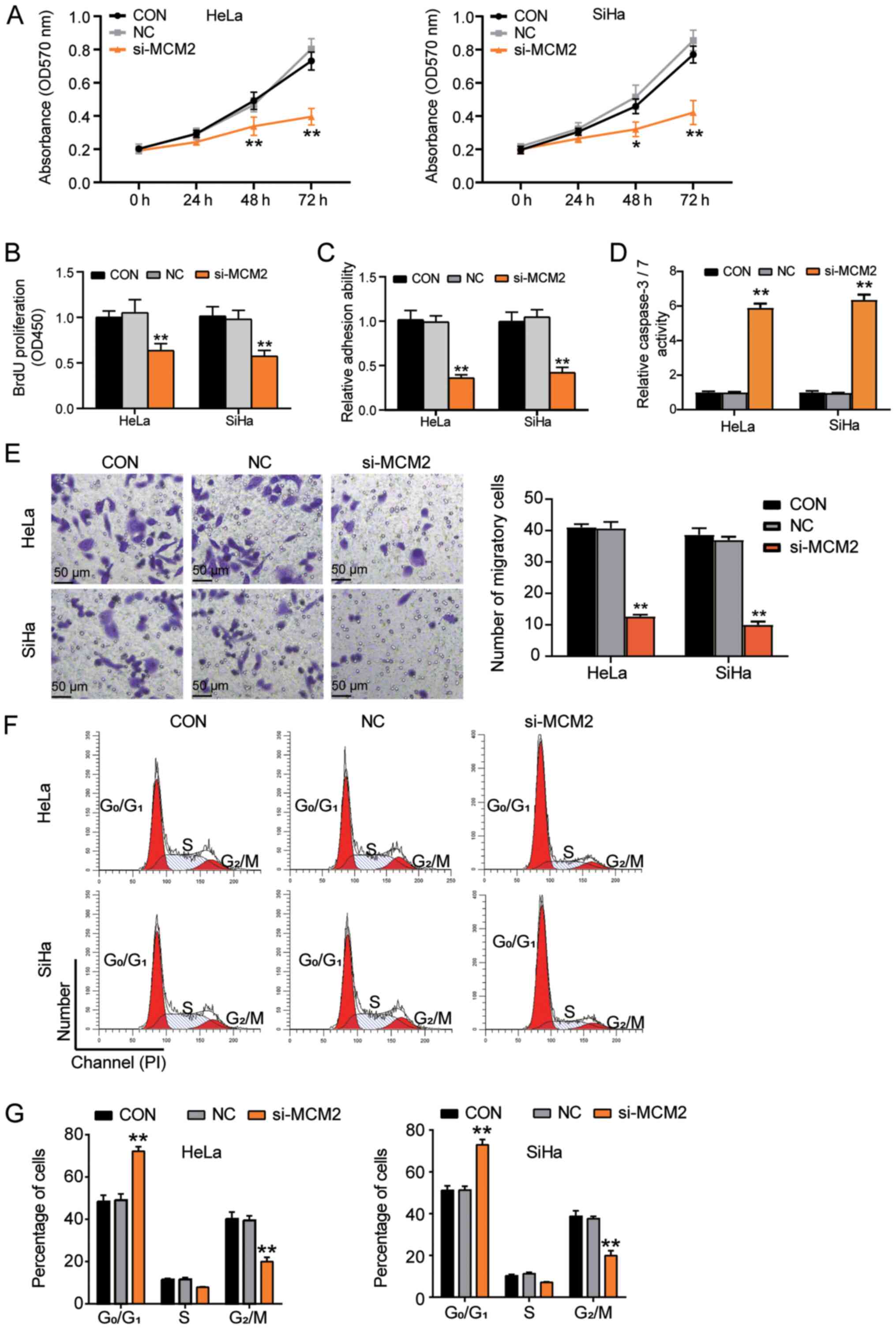 | Figure 3.Knockdown of MCM2 attenuates
proliferation, cell adhesion and G0/G1 to S
transition, but enhances apoptosis of cervical cancer cells. (A)
Cell proliferation was decreased after silencing of MCM2 expression
at 48 and 72 h. (B) Knockdown of MCM2 inhibited cell proliferation
examined using a BrdU assay. (C) Cell adhesion ability assay for
the si-MCM2 group, NC group and the CON group. (D) Effect of MCM2
on cell apoptosis was examined using a caspase-3/7 activity assay.
(E) Cell migration assay for si-MCM2 group, NC group and the
control group. Scale bar, 50 µm. (F) Representative images of cell
cycle in different transfection groups. (G) Cell cycle distribution
in different transfection groups was calculated. *P<0.05,
**P<0.001 vs. CON group (one-way ANOVA with Tukey's post hoc
test). BrdU, 5-bromo-2-deoxyuridine; CON, blank control; MCM2,
minichromosome maintenance complex component 2; NC, negative
control; OD, optical density; si, small interfering RNA. |
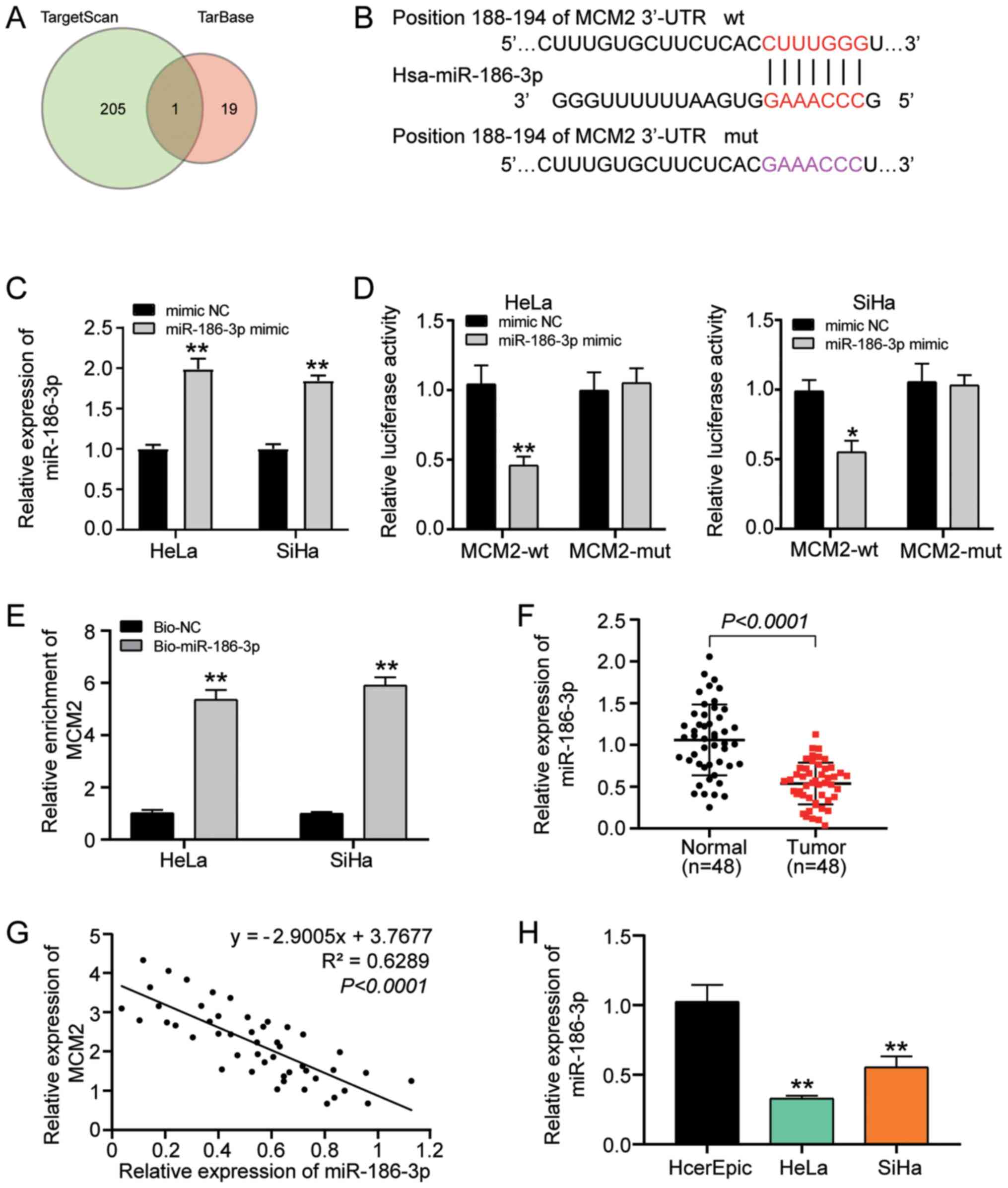
Effect of miR-186-3p on MCM2
The online softwares TargetScan and TarBase were
used to explore the underlying mechanism by which MCM2 regulates
cervical cancer cell behaviours and to identify miRNAs that could
regulate MCM2 expression. After Venny 2.1.0 analysis, it was
identified that miR-186-3p was the only miRNA identified by both
databases (Fig. 4A). Furthermore,
miR-186-3p targeted the 3′ UTR region of MCM2 (Fig. 4B). Subsequently, the miR-186-3p mimic
and its negative control (mimic NC) were successfully transfected
into HeLa and SiHa cells (Fig. 4C).
Additionally, a wt and a mut plasmid encoding the 3′ UTR region of
MCM2 were constructed and a luciferase reporter assay was performed
to verify the effect of miR-186-3p on MCM2. The results
demonstrated that miR-186-3p overexpression significantly decreased
the luciferase activity of MCM2-wt-3′ UTR to half of that of the
mimic NC group; however, the mut counterpart did not exhibit the
same tendency, demonstrating that miR-186-3p bound to the 3′ UTR of
MCM2 (Fig. 4D). The interaction
between miR-186-3p and MCM2 was assessed using an RNA pull-down
assay, and it was revealed that the relative expression levels of
MCM2 increased after transfection with Bio-miR-186-3p compared with
Bio-NC (Fig. 4E). Furthermore, the
expression pattern of miR-186-3p in cervical cancer tissues was
analysed. The findings revealed that the expression levels of
miR-186-3p were downregulated in tumour tissues (Fig. 4F) and that these were negatively
correlated with MCM2 expression (Fig.
4G). Furthermore, the expression levels of miR-186-3p in normal
cervical epithelial cells and cancer cells were measured and it was
revealed that miR-186-3p expression was downregulated in HeLa and
SiHa cells (Fig. 4H). Overall, these
findings indicated that miR-186-3p could target MCM2 and was
negatively associated with MCM2 expression.
Effects of MCM2 on cervical cancer
cells could be reversed by miR-186-3p
The aforementioned data demonstrated that miR-186-3p
could bind to MCM2. The antitumor function of si-MCM2 in cervical
cancer has also been demonstrated (Fig.
3). The present study further explored the roles of miR-186-3p
in cervical cancer and revealed that the expression levels of MCM2
were increased in the inhibitor group compared with the CON group;
however, MCM2 knockdown did not affect the expression levels of
miR-186-3p (Figs. 5A and S1). It was also observed that miR-186-3p
inhibitor enhanced cell proliferation at 48 and 72 h; however, the
co-transfection of si-MCM2 and miR-186-3p inhibitor reversed the
effect of si-MCM2 (Fig. 5B). In
addition, the BrdU assay results demonstrated that cell
proliferation was induced by silencing of miR-186-3p; however, the
co-transfection of si-MCM2 and miR-186-3p inhibitor reversed the
effect of si-MCM2 (Fig. 5C).
Furthermore, miR-186-3p inhibitor enhanced the adhesive ability of
the cells but MCM2 downregulation reversed the positive effect of
miR-186-3p inhibitor (Fig. 5D).
Additionally, the miR-186-3p inhibitor inhibited caspase-3/7
activity by 80% but the inhibitory effect of miR-186-3p inhibitor
could be reversed by inhibiting MCM2 expression (Fig. 5E). The migration assay results
demonstrated that miR-186-3p inhibitor increased cell migration by
2-fold, and co-transfection of si-MCM2 and miR-186-3p inhibitor
reversed the effect of si-MCM2 (Fig.
5F). When investigating the effect of miR-186-3p on the cell
cycle, it was identified that the miR-186-3p inhibitor reversed the
G0/G1 phase arrest caused by si-MCM2
(Fig. 5G and H). Collectively, these
results suggested that miR-186-3p suppressed cervical cancer
tumours. By downregulating MCM2 expression, miR-186-3p impeded the
proliferation and migration of cervical cancer cells and enhanced
cervical cancer cell apoptosis.
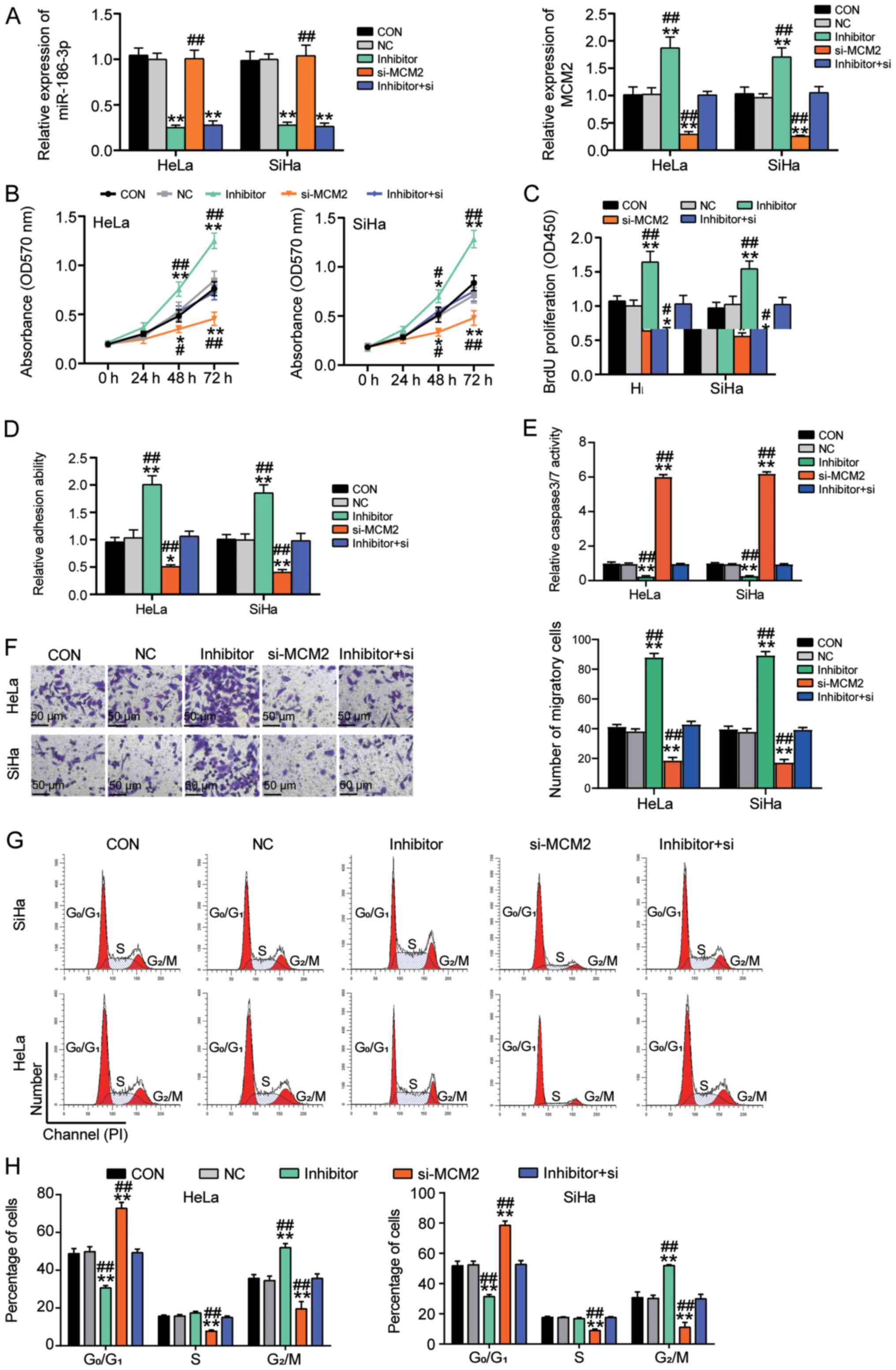 | Figure 5.Effect of MCM2 on cervical cancer
cells could be reversed by negative regulation from miR-186-3p. (A)
Expression levels of miR-186-3p and MCM2 in the CON, NC, miR-186-3p
inhibitor, si-MCM2 and inhibitor+si-MCM2 groups measured by reverse
transcription-quantitative PCR. (B) MTT assays were performed to
evaluate the proliferation of cells in the CON, NC, miR-186-3p
inhibitor, si-MCM2 and inhibitor+si-MCM2 groups. (C) BrdU assays
were performed to validate the effect of miR-186-3p on cell
proliferation. (D) Cell adhesion assays were performed for the CON,
NC, miR-186-3p inhibitor, si-MCM2 and inhibitor+si-MCM2 groups. (E)
Caspase-3/7 activity in the CON, NC, miR-186-3p inhibitor, si-MCM2
and inhibitor+si-MCM2 groups to assess the effect on apoptosis
mediated by miR-186-3p. (F) Cell migration assays were performed
for cells in the CON, NC, miR-186-3p inhibitor, si-MCM2 and
inhibitor+si-MCM2 groups. Scale bar, 50 µm. (G) Representative
images of cell cycle in the CON, NC, miR-186-3p inhibitor, si-MCM2
and inhibitor+si-MCM2 groups. (H) Cell cycle distribution in the
CON, NC, miR-186-3p inhibitor, si-MCM2 and inhibitor+si-MCM2 groups
was calculated *P<0.05, **P<0.001 vs. CON group (one-way
ANOVA with Tukey's post hoc test). #P<0.05,
##P<0.001 vs. inhibitor+si group (one-way ANOVA with
Tukey's post hoc test). BrdU, 5-bromo-2-deoxyuridine; CON, blank
control; MCM2, minichromosome maintenance complex component 2;
miR-186-3p, microRNA-186-3p; NC, negative control; OD, optical
density; si, small interfering RNA. |
Discussion
Cervical cancer is one of the most malignant cancer
types and has a high mortality rate among women (1). Annually, >500,000 women are
diagnosed with cervical cancer, and this tumour is responsible for
>300,000 deaths worldwide (36).
Although HPV infection has been demonstrated to be the main cause
of cervical cancer, several unknown oncogenes promote cervical
cancer progression (37). The
present study revealed that MCM2 influenced cervical cancer cells
and its expression was higher in tumour tissues than in non-tumour
tissues. Knocking down MCM2 attenuated the proliferation and
G0/G1 to S phase transition of cervical
cancer cells. Furthermore, miR-186-3p inhibitor increased the
expression of MCM2 by binding to the 3′ UTR region of the mRNA. The
results revealed that by targeting MCM2, miR-186-3p could reverse
the inhibitory effect on cell proliferation,
G0/G1 to S phase transition and cell
adhesion.
The cell cycle involves a series of events that
occur within a cell, and these events include cell proliferation
and division (38). Drugs target
vital molecules that regulate the cell cycle. For instance,
CDK4/CDK6 influences several malignant tumours, including breast
cancer, oesophageal cancer and acute myeloid leukaemia (39–42).
Although drugs targeting cell cycle molecules have not been
approved for cervical cancer therapy, the genes regulating the cell
cycle are under investigation (43,44). For
example, the inhibition of ASF1B induces cell cycle arrest in
cervical cancer cells (43). Shen
et al (45) demonstrated that
claudin 1 induces cell cycle arrest in cervical squamous cell
carcinoma. In the present study, it was revealed that MCM2
expression was upregulated in cervical cancer tissues and cell
lines and that silencing MCM2 induced cell cycle arrest at the
G0/G1 phase. These findings suggest that MCM2
regulates the cell cycle and promotes the development of cervical
cancer.
Furthermore, the MCM family is encoded by components
involved in the initiation of genome replication (46). Upregulation of the MCM family is
associated with the invasion of bladder cancer (47) and poor prognosis of breast cancer
(48). Previous studies (25,28) have
reported that HPV-infected cervical cancer cells exhibit high MCM2
expression, which is associated with a high risk of cervical
cancer. Based on a clinical study on MCM2 in cervical cancer, the
present study further explored the functions of MCM2 in cervical
cancer cells and revealed that MCM2 acted as a tumour promoter in
cervical cancer by inducing cell proliferation, cell adhesion and
cell cycle progression.
In the last three decades, miRNAs have been revealed
to regulate gene expression. In humans, >1,000 types of miRNAs
influence the expression of more than one-third of the target genes
and contribute to multiple alterations in cellular functions
(49,50). As a member of the miRNA family,
miR-186-3p decreases the expression levels of CDK1 and causes
G2/M cell cycle arrest in hepatocytes (51), indicating that this miRNA can
regulate the cell cycle. These results are in agreement with the
present findings. In breast cancer, the downregulation of
miR-186-3p increases EREG expression, promotes aerobic glycolysis
and accelerates cell proliferation (16), and this was verified in the present
study. Indeed, low expression levels of miR-186 not only contribute
to the anti-apoptosis ability and enhancement of metastasis in
cervical cancer cells (18) but also
act as a sponge of lncRNA to facilitate cervical cancer progression
(19,52). Consistent with these findings, the
present study revealed that miR-186-3p was downregulated in
cervical cancer and inhibited cell proliferation, cell cycle
progression and cell adhesion. Furthermore, miR-186-3p regulated
the progression of cervical cancer cells by targeting MCM2.
Therefore, the present study demonstrated that miR-186-3p regulated
MCM2 expression in cervical cancer, thereby facilitating cell
proliferation and G0/G1 to S phase transition
and reducing cell apoptosis by inhibiting MCM2 expression.
Although the present study demonstrated the
oncogenic effects of MCM2 in terms of facilitating cell
proliferation and influencing cell apoptosis, the present study had
some limitations. No in vivo experiments were performed, and
all results were based on a cellular function investigation and
analysis of patient tissues. In addition, the present study did not
investigate the downstream mechanism of MCM2 in accelerating cell
proliferation. Therefore, future studies should explore downstream
signalling pathways and key proteins involved in the process.
In summary, the present study suggested that by
targeting MCM2, miR-186-3p inhibitor contributed to the
proliferation, adhesion, migration and cell cycle progression of
cervical cancer cells and inhibited apoptosis of cervical cancer
cells. This research finding may enrich the understanding of the
miRNA/oncogene regulatory axis in cervical cancer and provide novel
insights for cervical cancer treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhangjiakou
City's 2020 Municipal Science and Technology Plan Self-Financing
Project (grant no. 2021053D).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XRL, XS, XHH, XYL, XYZ, NY, HM and ZLZ performed the
experiments and data analysis. XRL conceived and designed the
study. XS wrote the paper. XRL, XHH, XYL, XYZ, NY, HM and ZLZ
reviewed and edited the manuscript. HM and ZLZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Hebei North
University (Zhangjiakou, China). The processing of clinical tissue
samples was in strict compliance with the ethical standards of the
Declaration of Helsinki. All patients signed the written informed
consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brisson M and Drolet M: Global elimination
of cervical cancer as a public health problem. Lancet Oncol.
20:319–321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simms KT, Steinberg J, Caruana M, Smith
MA, Lew JB, Soerjomataram I, Castle PE, Bray F and Canfell K:
Impact of scaled up human papillomavirus vaccination and cervical
screening and the potential for global elimination of cervical
cancer in 181 countries, 2020-99: A modelling study. Lancet Oncol.
20:394–407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin C, Slama J, Gonzalez P, Goodman MT,
Xia N, Kreimer AR, Wu T, Hessol NA, Shvetsov Y, Ortiz AP, et al:
Cervical determinants of anal HPV infection and high-grade anal
lesions in women: A collaborative pooled analysis. Lancet Infect
Dis. 19:880–891. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang J, Cai H, Xiao ZX, Wang H and Yang P:
Effect of radiotherapy on the survival of cervical cancer patients:
An analysis based on SEER database. Medicine (Baltimore).
98:e164212019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inui M, Martello G and Piccolo S: MicroRNA
control of signal transduction. Nat Rev Mol Cell Biol. 11:252–263.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Y, Zhou Y, Qu W, Deng M and Zhang C: A
Lasso regression model for the construction of microRNA-target
regulatory networks. Bioinformatics. 27:2406–2413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao C, Sun D, Zhang L and Song L: miR-186
affects the proliferation, invasion and migration of human gastric
cancer by inhibition of Twist1. Oncotarget. 7:79956–79963. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong S, Wang R, Wang H, Ding Q, Zhou X,
Wang J, Zhang K, Long Y, Lu S, Hong T, et al: HOXD-AS1 promotes the
epithelial to mesenchymal transition of ovarian cancer cells by
regulating miR-186-5p and PIK3R3. J Exp Clin Cancer Res.
38:1102019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Zhang G, Yu W, Gao N and Peng J:
miR-186 inhibits cell proliferation in multiple myeloma by
repressing Jagged1. Biochem Biophys Res Commun. 469:692–697. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su BB, Zhou SW, Gan CB and Zhang XN:
miR-186 inhibits cell proliferation and invasion in human cutaneous
malignant melanoma. J Cancer Res Ther. 14 (Suppl):S60–S64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu H, Yuan S and Lu X: miR-186 suppressed
CYLD expression and promoted cell proliferation in human melanoma.
Oncol Lett. 12:2301–2306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Wang Y, Bai R, Yang K and Tian Z:
miR-186 inhibited aerobic glycolysis in gastric cancer via HIF-1α
regulation. Oncogenesis. 5:e2242016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao K, He L, Gan Y, Zeng Q, Dai Y and Tan
J: miR-186 suppresses the growth and metastasis of bladder cancer
by targeting NSBP1. Diagn Pathol. 10:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang R, Bao H, Zhang S, Li R, Chen L and
Zhu Y: miR-186-5p Promotes Apoptosis by Targeting IGF-1 in SH-SY5Y
OGD/R Model. Int J Biol Sci. 14:1791–1799. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He M, Jin Q, Chen C, Liu Y, Ye X, Jiang Y,
Ji F, Qian H, Gan D, Yue S, et al: The miR-186-3p/EREG axis
orchestrates tamoxifen resistance and aerobic glycolysis in breast
cancer cells. Oncogene. 38:5551–5565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Honegger A, Schilling D, Bastian S,
Sponagel J, Kuryshev V, Sültmann H, Scheffner M, Hoppe-Seyler K and
Hoppe-Seyler F: Dependence of intracellular and exosomal microRNAs
on viral E6/E7 oncogene expression in HPV-positive tumor cells.
PLoS Pathog. 11:e10047122015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Wang J, Hu Y, Xie H, Liu M and Tang
H: Upregulation of kazrin F by miR-186 suppresses apoptosis but
promotes epithelial-mesenchymal transition to contribute to
malignancy in human cervical cancer cells. Chin J Cancer Res.
29:45–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang JJ, Wang DD, Du CX and Wang Y: Long
Noncoding RNA ANRIL Promotes Cervical Cancer Development by Acting
as a Sponge of miR-186. Oncol Res. 26:345–352. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kucherlapati M: Examining transcriptional
changes to DNA replication and repair factors over uveal melanoma
subtypes. BMC Cancer. 18:8182018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lei M, Kawasaki Y, Young MR, Kihara M,
Sugino A and Tye BK: Mcm2 is a target of regulation by Cdc7-Dbf4
during the initiation of DNA synthesis. Genes Dev. 11:3365–3374.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Issac MSM, Yousef E, Tahir MR and Gaboury
LA: MCM2, MCM4, and MCM6 in Breast Cancer: Clinical Utility in
Diagnosis and Prognosis. Neoplasia. 21:1015–1035. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gad SA, Ali HEA, Gaballa R, Abdelsalam RM,
Zerfaoui M, Ali HI, Salama SH, Kenawy SA, Kandil E and Abd Elmageed
ZY: Targeting CDC7 sensitizes resistance melanoma cells to
BRAFV600E-specific inhibitor by blocking the CDC7/MCM2-7 pathway.
Sci Rep. 9:141972019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aihemaiti G, Kurata M, Nogawa D, Yamamoto
A, Mineo T, Onishi I, Kinowaki Y, Jin XH, Tatsuzawa A, Miyasaka N,
et al: Subcellular localization of MCM2 correlates with the
prognosis of ovarian clear cell carcinoma. Oncotarget.
9:28213–28225. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al-Hazmi N, Alhazzazi T, Williams G,
Stoeber K and Al-Dabbagh R: DNA replication licensing factor MCM2,
geminin, and Ki67 define proliferative state and are linked with
survival in oral squamous cell carcinoma. Eur J Oral Sci.
126:186–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaur G, Balasubramaniam SD, Lee YJ,
Balakrishnan V and Oon CE: Minichromosome Maintenance Complex (MCM)
Genes Profiling and MCM2 Protein Expression in Cervical Cancer
Development. Asian Pac J Cancer Prev. 20:3043–3049. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang X, Xu Y, Lu L, Jiao Y, Liu J, Wang L
and Zhao H: Identification of key candidate genes and small
molecule drugs in cervical cancer by bioinformatics strategy.
Cancer Manag Res. 10:3533–3549. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amaro Filho SM, Nuovo GJ, Cunha CB, Ramos
Pereira LO, Oliveira-Silva M, Russomano F, Pires A and Nicol AF:
Correlation of MCM2 detection with stage and virology of cervical
cancer. Int J Biol Markers. 29:e363–e371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Z, Zhou Y, Shi F, Cao Y, Dinh TLA, Wan
J and Zhao M: Investigation of differentially-expressed microRNAs
and genes in cervical cancer using an integrated bioinformatics
analysis. Oncol Lett. 13:2784–2790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhai Y, Kuick R, Nan B, Ota I, Weiss SJ,
Trimble CL, Fearon ER and Cho KR: Gene expression analysis of
preinvasive and invasive cervical squamous cell carcinomas
identifies HOXC10 as a key mediator of invasion. Cancer Res.
67:10163–10172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
den Boon JA, Pyeon D, Wang SS, Horswill M,
Schiffman M, Sherman M, Zuna RE, Wang Z, Hewitt SM, Pearson R, et
al: Molecular transitions from papillomavirus infection to cervical
precancer and cancer: Role of stromal estrogen receptor signaling.
Proc Natl Acad Sci USA. 112:E3255–E3264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsuo K, Machida H, Mandelbaum RS,
Konishi I and Mikami M: Validation of the 2018 FIGO cervical cancer
staging system. Gynecol Oncol. 152:87–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Powazniak Y, Kempfer AC, Pereyra JC,
Palomino JP and Lazzari MA: VWF and ADAMTS13 behavior in
estradiol-treated HUVEC. Eur J Haematol. 86:140–147. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng J: Diagnostic value of MCM2
immunocytochemical staining in cervical lesions and its
relationship with HPV infection. Int J Clin Exp Pathol. 8:875–880.
2015.PubMed/NCBI
|
|
36
|
Smith RA, Manassaram-Baptiste D, Brooks D,
Doroshenk M, Fedewa S, Saslow D, Brawley OW and Wender R: Cancer
screening in the United States, 2015: A review of current American
cancer society guidelines and current issues in cancer screening.
CA Cancer J Clin. 65:30–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brisson M, Kim JJ, Canfell K, Drolet M,
Gingras G, Burger EA, Martin D, Simms KT, Bénard É, Boily MC, et
al: Impact of HPV vaccination and cervical screening on cervical
cancer elimination: A comparative modelling analysis in 78
low-income and lower-middle-income countries. Lancet. 395:575–590.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petroni G, Formenti SC, Chen-Kiang S and
Galluzzi L: Immunomodulation by anticancer cell cycle inhibitors.
Nat Rev Immunol. 20:669–679. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Romero-Pozuelo J, Figlia G, Kaya O,
Martin-Villalba A and Teleman AA: Cdk4 and Cdk6 Couple the
Cell-Cycle Machinery to Cell Growth via mTORC1. Cell Rep.
31:1075042020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qie S, Yoshida A, Parnham S, Oleinik N,
Beeson GC, Beeson CC, Ogretmen B, Bass AJ, Wong KK, Rustgi AK, et
al: Targeting glutamine-addiction and overcoming CDK4/6 inhibitor
resistance in human esophageal squamous cell carcinoma. Nat Commun.
10:12962019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Whittle JR, Vaillant F, Surgenor E,
Policheni AN, Giner G, Capaldo BD, Chen HR, Liu HK, Dekkers JF,
Sachs N, et al: Dual Targeting of CDK4/6 and BCL2 Pathways Augments
Tumor Response in Estrogen Receptor-Positive Breast Cancer. Clin
Cancer Res. 26:4120–4134. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schmoellerl J, Barbosa IAM, Eder T,
Brandstoetter T, Schmidt L, Maurer B, Troester S, Pham HTT,
Sagarajit M, Ebner J, et al: CDK6 is an essential direct target of
NUP98 fusion proteins in acute myeloid leukemia. Blood.
136:387–400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu X, Song J, Zhang Y, Wang H, Sun H,
Feng X, Hou M, Chen G, Tang Q and Ji M: ASF1B promotes cervical
cancer progression through stabilization of CDK9. Cell Death Dis.
11:7052020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee S, Ho JY, Liu JJ, Lee H, Park JY, Baik
M, Ko M, Lee SU, Choi YJ and Hur SY: CKD-602, a topoisomerase I
inhibitor, induces apoptosis and cell-cycle arrest and inhibits
invasion in cervical cancer. Mol Med. 25:232019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shen Z, Song W, Qian L, Zhu J, Li Y, Li M,
Zhang T, Zhao W, Zhou Y and Yang X: Effect of claudin 1 on cell
proliferation, migration and apoptosis in human cervical squamous
cell carcinoma. Oncol Rep. 45:606–618. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Forsburg SL: Eukaryotic MCM proteins:
Beyond replication initiation. Microbiol Mol Biol Rev. 68:109–131.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roupret M, Gontero P, McCracken SRC,
Dudderidge T, Stockley J, Kennedy A, Rodriguez O, Sieverink C,
Vanié F, Allasia M, et al: Diagnostic Accuracy of MCM5 for the
Detection of Recurrence in Nonmuscle Invasive Bladder Cancer
Followup: A Blinded, Prospective Cohort, Multicenter European
Study. J Urol. 204:685–690. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao Y, Wang Y, Zhu F, Zhang J, Ma X and
Zhang D: Gene expression profiling revealed MCM3 to be a better
marker than Ki67 in prognosis of invasive ductal breast carcinoma
patients. Clin Exp Med. 20:249–259. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rachagani S, Macha MA, Heimann N,
Seshacharyulu P, Haridas D, Chugh S and Batra SK: Clinical
implications of miRNAs in the pathogenesis, diagnosis and therapy
of pancreatic cancer. Adv Drug Deliv Rev. 81:16–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Garofalo M and Croce CM: Role of microRNAs
in maintaining cancer stem cells. Adv Drug Deliv Rev. 81:53–61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang R, Wei M, Yang F, Sheng Y and Ji L:
Diosbulbin B induced G2/M cell cycle arrest in hepatocytes by
miRNA-186-3p and miRNA-378a-5p-mediated the decreased expression of
CDK1. Toxicol Appl Pharmacol. 357:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Y, Guo R, Qiao Y, Han L and Liu M:
LncRNA NNT-AS1 contributes to the cisplatin resistance of cervical
cancer through NNT-AS1/miR-186/HMGB1 axis. Cancer Cell Int.
20:1902020. View Article : Google Scholar : PubMed/NCBI
|