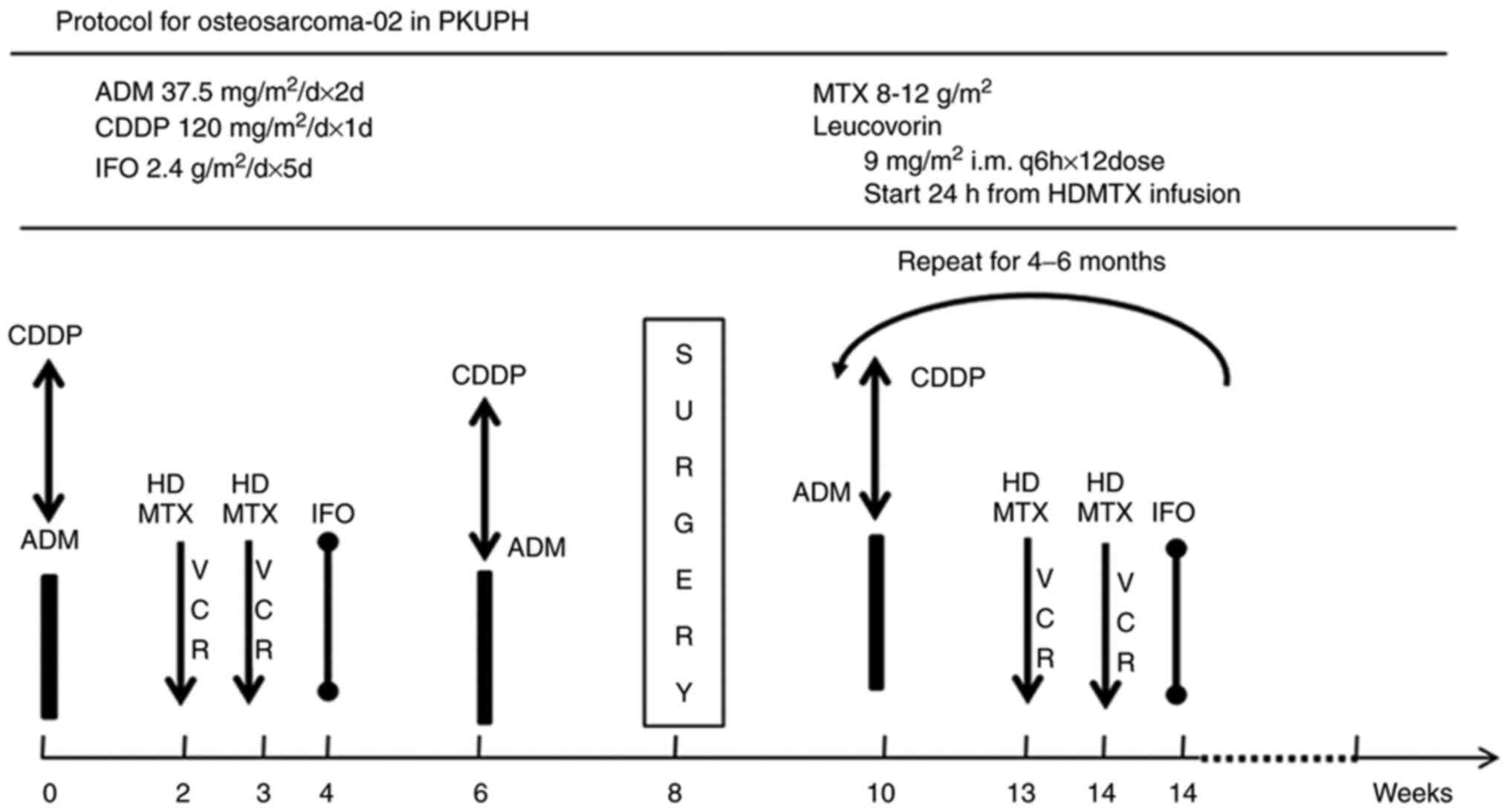
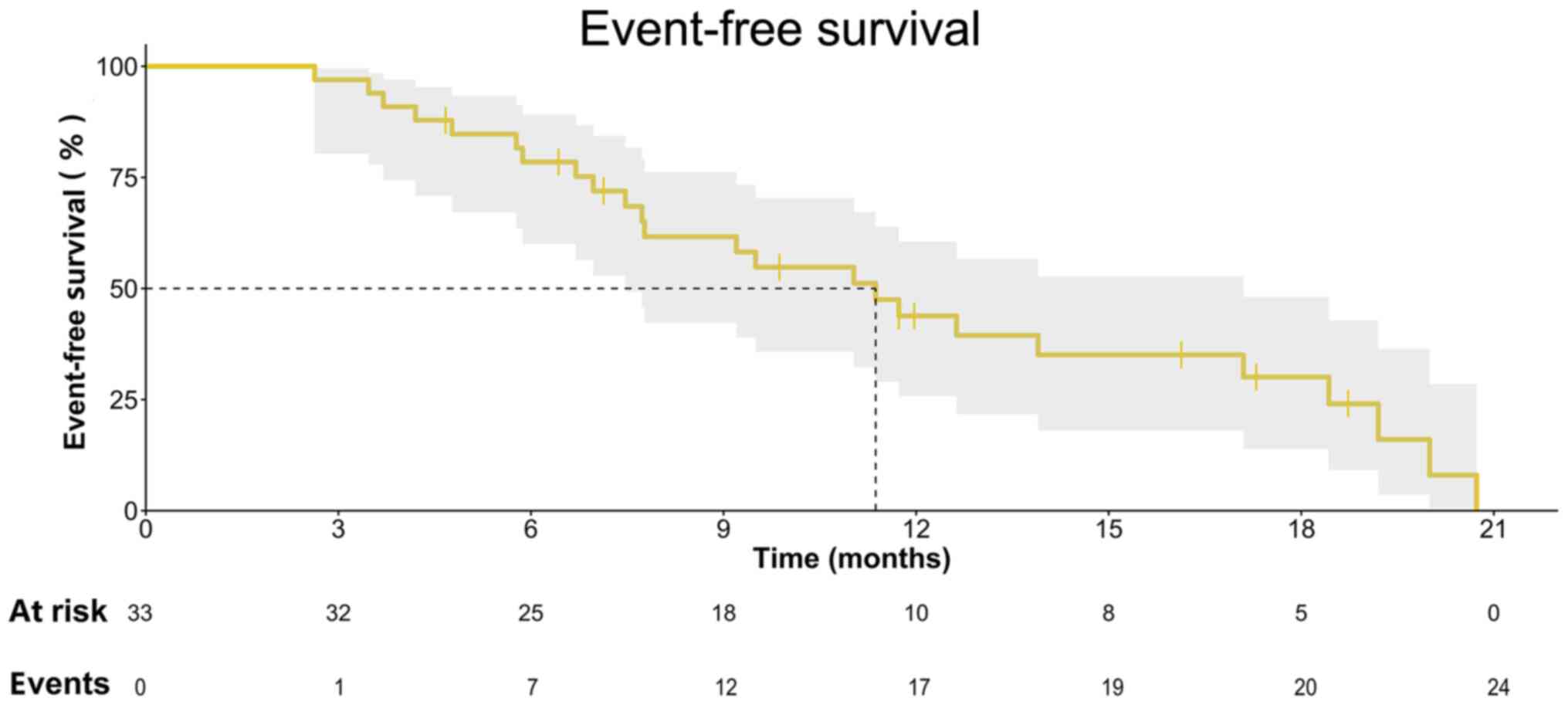
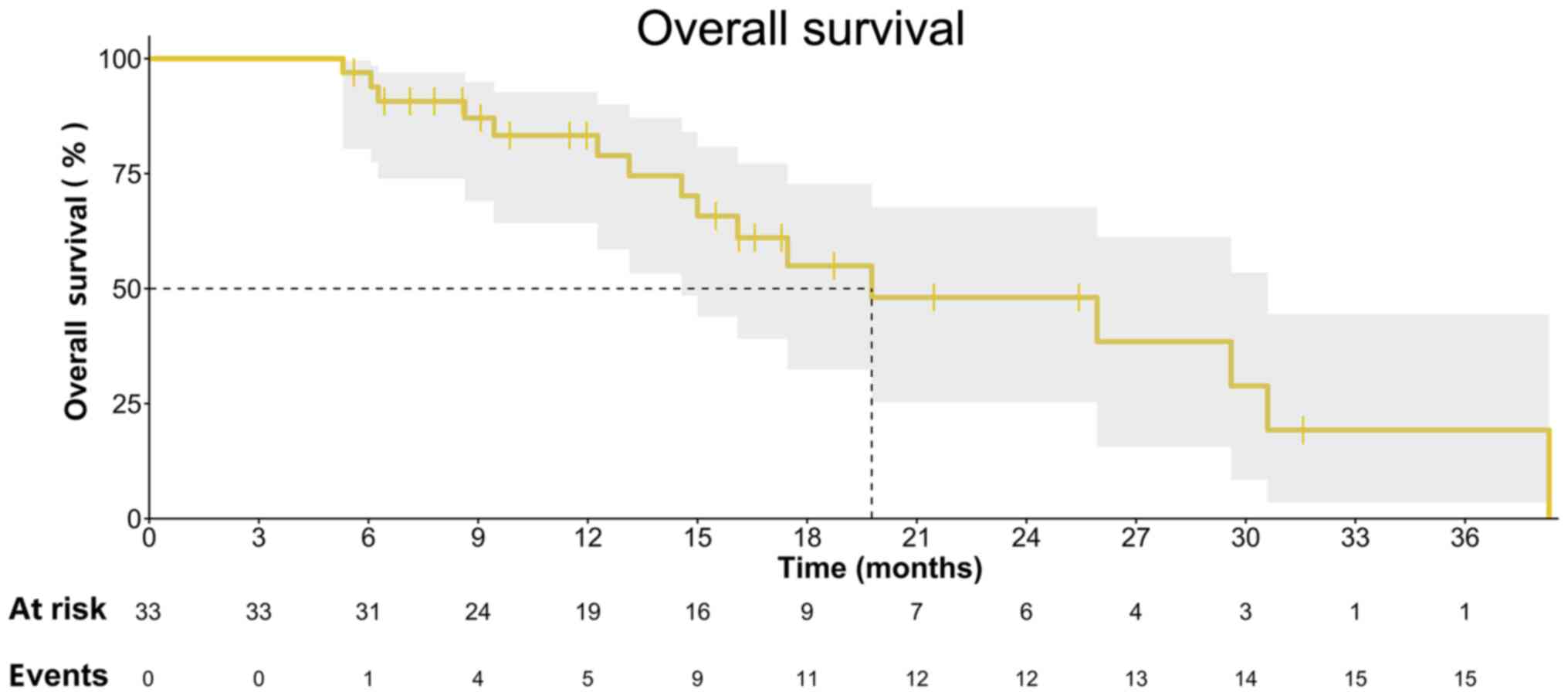
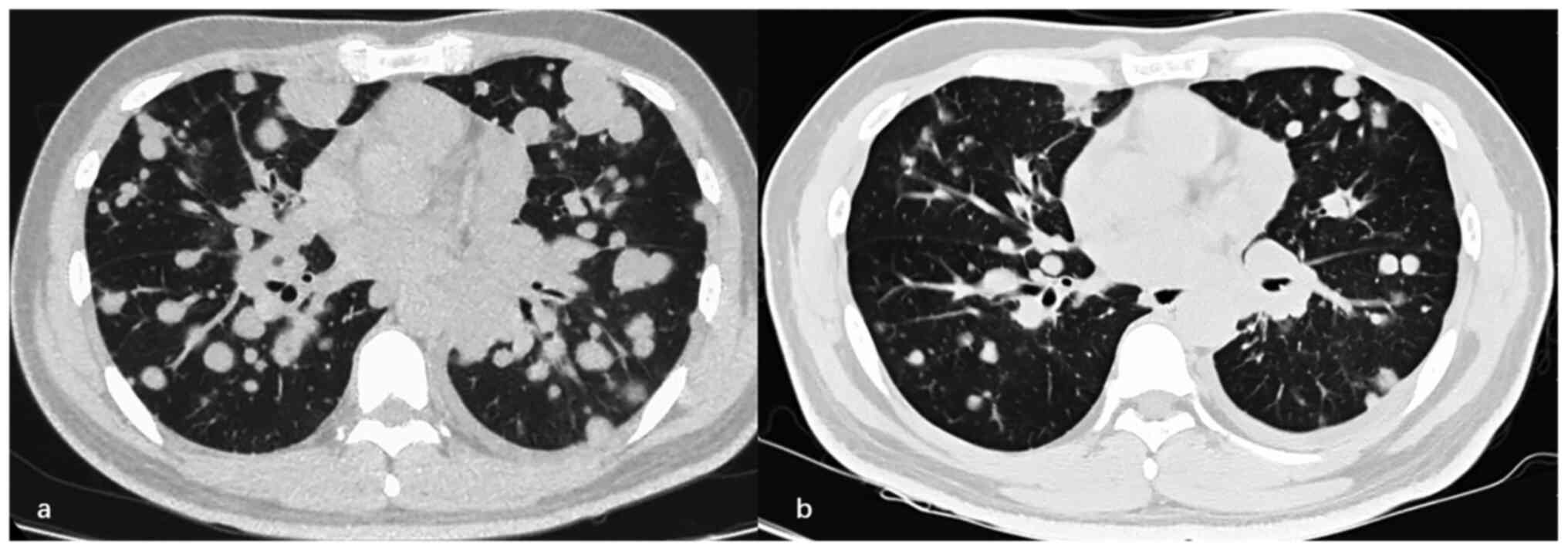
|
1
|
Xie L, Ji T and Guo W: Anti-angiogenesis
target therapy for advanced osteosarcoma (Review). Oncol Rep.
38:625–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lagmay JP, Krailo MD, Dang H, Kim A,
Hawkins DS, Beaty O III, Widemann BC, Zwerdling T, Bomgaars L,
Langevin AM, et al: Outcome of patients with recurrent osteosarcoma
enrolled in seven phase II trials through Children's Cancer Group,
Pediatric Oncology Group, and Children's Oncology Group: Learning
from the past to move forward. J Clin Oncol. 34:3031–3038. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carrle D and Bielack SS: Current
strategies of chemotherapy in osteosarcoma. Int Orthop. 30:445–451.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberts RD, Lizardo MM, Reed DR, Hingorani
P, Glover J, Allen-Rhoades W, Fan T, Khanna C, Sweet-Cordero EA,
Cash T, et al: Provocative questions in osteosarcoma basic and
translational biology: A report from the Children's Oncology Group.
Cancer. 125:3514–3525. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian Z, Niu X and Yao W: Receptor tyrosine
kinases in osteosarcoma treatment: Which is the key target? Front
Oncol. 10:16422020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaikh AB, Li F, Li M, He B, He X, Chen G,
Guo B, Li D, Jiang F, Dang L, et al: Present advances and future
perspectives of molecular targeted therapy for osteosarcoma. Int J
Mol Sci. 17:5062016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
8
|
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang
S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis
through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death
Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng B, Ren T, Huang Y and Guo W:
Apatinib inhibits migration and invasion as well as PD-L1
expression in osteosarcoma by targeting STAT3. Biochem Biophys Res
Commun. 495:1695–1701. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie L, Guo W, Wang Y, Yan T, Ji T and Xu
J: Apatinib for advanced sarcoma: Results from multiple
institutions' off-label use in China. BMC Cancer. 18:3962018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie L, Xu J, Sun X, Tang X, Yan T, Yang R
and Guo W: Apatinib for advanced osteosarcoma after failure of
standard multimodal therapy: An open label phase II clinical trial.
Oncologist. 24:e542–e550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Correction: Apatinib plus camrelizumab
(anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO)
progressing after chemotherapy: A single-arm, open-label, phase 2
trial. J Immunother Cancer. 8:e000798corr0007912020.
|
|
13
|
Xie L, Xu J, Sun X, Guo W, Gu J, Liu K,
Zheng B, Ren T, Huang Y, Tang X, et al: Apatinib plus camrelizumab
(anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO)
progressing after chemotherapy: A single-arm, open-label, phase 2
trial. J Immunother Cancer. 8:e0007982020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosen G, Marcove RC, Caparros B, Nirenberg
A, Kosloff C and Huvos AG: Primary osteogenic sarcoma: The
rationale for preoperative chemotherapy and delayed surgery.
Cancer. 43:2163–2177. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cormier JN, Patel SR, Herzog CE, Ballo MT,
Burgess MA, Feig BW, Hunt KK, Raney RB, Zagars GK, Benjamin RS and
Pisters PW: Concurrent ifosfamide-based chemotherapy and
irradiation. Analysis of treatment-related toxicity in 43 patients
with sarcoma. Cancer. 92:1550–1555. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miser JS, Kinsella TJ, Triche TJ, Tsokos
M, Jarosinski P, Forquer R, Wesley R and Magrath I: Ifosfamide with
mesna uroprotection and etoposide: An effective regimen in the
treatment of recurrent sarcomas and other tumors of children and
young adults. J Clin Oncol. 5:1191–1198. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marina NM, Smeland S, Bielack SS,
Bernstein M, Jovic G, Krailo MD, Hook JM, Arndt C, van den Berg H,
Brennan B, et al: Comparison of MAPIE versus MAP in patients with a
poor response to preoperative chemotherapy for newly diagnosed
high-grade osteosarcoma (EURAMOS-1): An open-label, international,
randomised controlled trial. Lancet Oncol. 17:1396–1408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goorin AM, Harris MB, Bernstein M,
Ferguson W, Devidas M, Siegal GP, Gebhardt MC, Schwartz CL, Link M
and Grier HE: Phase II/III trial of etoposide and high-dose
ifosfamide in newly diagnosed metastatic osteosarcoma: A pediatric
oncology group trial. J Clin Oncol. 20:426–433. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferrari S, Mercuri M, Picci P, Bertoni F,
Brach del Prever A, Tienghi A, Mancini A, Longhi A, Rimondini S,
Donati D, et al: Nonmetastatic osteosarcoma of the extremity:
Results of a neoadjuvant chemotherapy protocol (IOR/OS-3) with
high-dose methotrexate, intraarterial or intravenous cisplatin,
doxorubicin, and salvage chemotherapy based on histologic tumor
response. Tumori. 85:458–464. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salzer-Kuntschik M, Delling G, Beron G and
Sigmund R: Morphological grades of regression in osteosarcoma after
polychemotherapy-study COSS 80. J Cancer Res Clin Oncol. 106
(Suppl):S21–S24. 1983. View Article : Google Scholar
|
|
21
|
Ferrari S, Bielack SS, Smeland S, Longhi
A, Egerer G, Sundby Hall K, Donati D, Kevric M, Brosjo O, Comandone
A, et al: EURO-B.O.S.S.: A European study on chemotherapy in
bone-sarcoma patients aged over 40: Outcome in primary high-grade
osteosarcoma. Tumori. 104:30–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaspar N, Occean BV, Pacquement H, Bompas
E, Bouvier C, Brisse HJ, Castex MP, Cheurfa N, Corradini N, Delaye
J, et al: Results of methotrexate-etoposide-ifosfamide based
regimen (M-EI) in osteosarcoma patients included in the French
OS2006/sarcome-09 study. Eur J Cancer. 88:57–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gaspar N, Sirvent B, Venkatramani R,
Longhi A, Lervat C, Casanova M, Aerts I, Bielack SS, Entz-Werle N,
Strauss S, et al: 1399 Phase 1 combination dose-finding/phase 2
expansion cohorts of lenvatinib + etoposide + ifosfamide in
patients (pts) aged 2 to 25 years with relapsed/refractory (r/r)
osteosarcoma. Ann Oncol. 30 (Suppl 5):v683–v709. 2019. View Article : Google Scholar
|
|
24
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 153:106–120. 1980.PubMed/NCBI
|
|
25
|
Xie L, Xu J, Dong S, Gao J, Tang X, Yan T,
Yang R and Guo W: Gain and loss from transcatheter intra-arterial
limb infusion of cisplatin for extremity osteosarcoma: A
retrospective study of 99 cases in the past six years. Cancer Manag
Res. 11:7183–7195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verger E, Salamero M and Conill C: Can
Karnofsky performance status be transformed to the Eastern
Cooperative Oncology Group scoring scale and vice versa? Eur J
Cancer. 28A:1328–1330. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
U.S. Department of Health and Human
Services: Common Terminology Criteria for Adverse Events (CTCAE)
Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdfNovember
27–2018
|
|
29
|
Nagy A, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Collins M, Wilhelm M, Conyers R, Herschtal
A, Whelan J, Bielack S, Kager L, Kühne T, Sydes M, Gelderblom H, et
al: Benefits and adverse events in younger versus older patients
receiving neoadjuvant chemotherapy for osteosarcoma: Findings from
a meta-analysis. J Clin Oncol. 31:2303–2312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Italiano A, Mir O, Mathoulin-Pelissier S,
Penel N, Piperno-Neumann S, Bompas E, Chevreau C, Duffaud F,
Entz-Werle N, Saada E, et al: Cabozantinib in patients with
advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre,
single-arm, phase 2 trial. Lancet Oncol. 21:446–455. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duffaud F, Mir O, Boudou-Rouquette P,
Piperno-Neumann S, Penel N, Bompas E, Delcambre C, Kalbacher E,
Italiano A, Collard O, et al: Efficacy and safety of regorafenib in
adult patients with metastatic osteosarcoma: A non-comparative,
randomised, double-blind, placebo-controlled, phase 2 study. Lancet
Oncol. 20:120–133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grignani G, Palmerini E, Dileo P, Asaftei
SD, D'Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F,
Casali PG, et al: A phase II trial of sorafenib in relapsed and
unresectable high-grade osteosarcoma after failure of standard
multimodal therapy: An Italian Sarcoma Group study. Ann Oncol.
23:508–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vasudev NS and Reynolds AR:
Anti-angiogenic therapy for cancer: Current progress, unresolved
questions and future directions. Angiogenesis. 17:471–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grignani G, Palmerini E, Ferraresi V,
D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al: Sorafenib and everolimus for
patients with unresectable high-grade osteosarcoma progressing
after standard treatment: A non-randomised phase 2 clinical trial.
Lancet Oncol. 16:98–107. 2015. View Article : Google Scholar : PubMed/NCBI
|