Introduction
Osteosarcoma is a highly aggressive sarcoma that
originates from mesenchymal stem cells with osteoblastic lineage
commitment, for which the treatment regimen has remained
essentially unchanged for >40 years (1,2). The
5-year overall survival (OS) of advanced osteosarcoma refractory to
multiagent chemotherapy has remained <20% over decades according
to recent European and American prospective trial results (1,2).
Although anti-angiogenesis tyrosine kinase inhibitors (TKIs) have
been shown to prolong 6-month progression-free survival rate (PFSR)
in heavily treated patients in multiple trials, patients have a
median overall survival of <12 months, and a standard management
strategy has yet to be established (3–7).
Apatinib, a multikinase inhibitor used in gastric, non-small cell
lung and oesophageal cancer, has been shown to prevent the
proliferation and migration/invasion of cell lines and subsequent
growth and metastasis in various osteosarcoma preclinical studies
(8–10). These findings led to two phase 2
trials to explore the activity of apatinib-based treatment in
patients with relapsed, unresectable high-grade osteosarcoma after
standard treatment (11). In this
setting, apatinib showed signs of antitumor activity in terms of
the proportion of patients who achieved a response (43%). However,
these encouraging results were short lived, with only 36.8% of
patients were progression free at 6 months. The combination regimen
of camrelizumab, a humanized IgG4-κ PD-1 monoclonal antibody, and
apatinib did not significantly prolong PFS compared with apatinib
alone (11–13). From these prospective trials, it was
observed that different target lesions at different locations
responded distinctly to apatinib, with a varied duration of
response. Moreover, musculoskeletal lesions were found to quickly
develop secondary resistance to apatinib, with a median PFS time of
only 2.1 [95% confidence interval (CI), 1.8–5.7] months compared
with pulmonary lesions (12,13). Furthermore, nearly one-third of the
cases of progression were due to musculoskeletal lesions, while
another one-third were due to the appearance of new lesions outside
the lung (11–13). Thus, overcoming disease progression
outside the lung during TKI-based treatment has become an important
clinical challenge.
Ifosfamide (IFO) is one of the most active systemic
agents for the treatment of patients with sarcoma (14,15). In
addition to its anticancer activity, ifosfamide is a
radiosensitizing agent that can be given concurrently with
radiotherapy (15). Etoposide (VP16)
was one of the first identified topoisomerase II inhibitors and has
been studied for decades in combination with IFO for treating
osteosarcoma (16). However, the
findings of the European and American Osteosarcoma Study did not
support the addition of ifosfamide and etoposide (IE) to
postoperative chemotherapy in poorly responding osteosarcoma
because of increased toxicity and no improvement in event-free
survival (17). Despite this
finding, the status of IE in osteosarcoma is ambiguous between
first- and second-line chemotherapy (18–22), and
several sarcoma centres have still merged this agent into
first-line chemotherapy regimens for various reasons. Gaspar et
al (23), at the 2019 Congress
of the European Society for Medical Oncology (ESMO), proposed a
combination of lenvatinib and IE as a second-line therapy for
recurrent osteosarcoma; this therapy was found to prolong the
median PFS from 3 (95% CI, 1.8–5.5) months with single lenvatinib
to 11.1 (95% CI, 4.5–12.6) months. As a result, it was speculated
that the addition of chemotherapy overcame the disadvantage of the
poor disease control of musculoskeletal lesions with TKIs.
Therefore, the present study sought to evaluate the combination
strategy of apatinib + IE in patients with recurrent or refractory
osteosarcoma from retrospective data, especially those with
metastatic lesions both in and outside the lung. The current study
was designed to review our experience and investigate the activity
of this combination therapy in these patients in two sarcoma
centres in China and to further characterize the toxicity profile
of this combination in Asian patients.
Patients and methods
Patients
From June 3 2017 to July 17 2020, patients who
received apatinib + IE were included if they met the following
criteria: i) Histologically confirmed high-grade osteosarcoma
according to Enneking Grading System (24); ii) initial treatment in the
Orthopaedic/Oncology Departments of Peking University People's
Hospital or Peking University Shougang Hospital (both Beijing,
China); iii) progression <6 months after first-line chemotherapy
(25) with a combination of
high-dose methotrexate, doxorubicin, cisplatin and ifosfamide
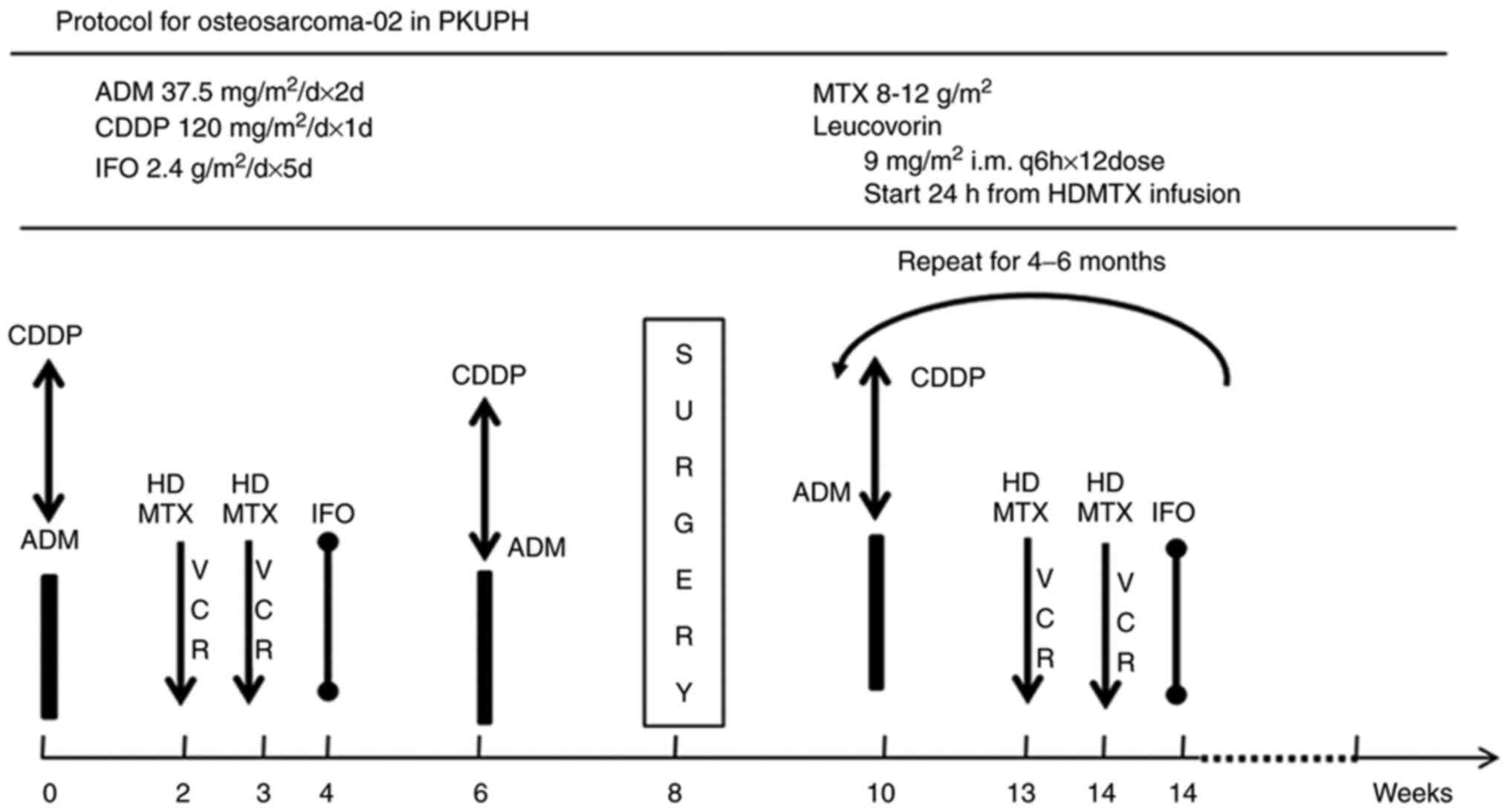
(Fig. 1) (MAPI), including high-dose
methotrexate (12 g/m2), doxorubicin (75
mg/m2), cisplatin (100–120 mg/m2) with or
without ifosfamide (12 g/m2). We usually defined these
four agents as first-line chemotherapy while choose IE as the
second-line chemotherapy, which included ifosfamide 1.8
g/m2/d d1-5 and etoposide 100
mg/m2/d d1-5 Q3W (IE); iv) measurable lesions
according to the Response Evaluation Criteria for Solid Tumours
(RECIST 1.1) (26); v) Eastern
Cooperative Oncology Group (ECOG) performance status ≤2 (27); and vi) acceptable haematological,
hepatic and renal function. The following patients were excluded:
i) Those who did not have intact clinical evaluation radiographic
materials or complete follow-up information after chemotherapy; ii)
those who did not receive more than two courses of the regimens due
to reasons other than tumour progression or toxicity; and iii)
those who had severe or uncontrolled medical disorders that could
jeopardize the outcomes of the study. Notably, patients who had
previously shown progression upon single apatinib or IE and then
later adopted combination therapy were included to analyse whether
this combination had synergistic effects. All patients who had been
assessed by the joint sarcoma board of the aforementioned
hospitals, which contains medical, paediatric, surgical and
radiology oncologists in both hospitals, for eligibility for
metastasectomy were also included. It was possible for the included
patients to undergo surgery with curative intent only if they had
been stable for at least 4 months.
This trial was retrospectively registered in The
Medical Ethics Committees of Peking University People's Hospital
and Peking University Shougang Hospital on December 28 2020
(registration no. 2020PHB388-02). The trial was registered at
ClinicalTrials.gov under identifier no.
NCT04690231. Both hospitals obtained approval from The Medical
Ethics Committee of Peking University People's Hospital and The
Medical Ethics Committee of Peking University Shougang Hospital
(Beijing, China) to review the patients' medical records and
radiographic materials. The outcome data were then retrospectively
combined. Written informed consent from patients was not
required.
During systemic treatment, patients underwent chest
computed tomography (CT) or CT/magnetic resonance imaging of the
musculoskeletal tumour sites every 2 months. Following the
completion of chemotherapy, patients were followed-up every 2
months for the first 2 years and then every 3 months for the next 3
years with median follow-up time of 19.8 (IQR, 9.4,51.8) months.
Radionuclide bone scans or positron emission tomography/CT was used
to assess metastatic disease every 6 months during treatment and
for the first 5 years after the completion of systemic treatment.
All the adverse events (AE) which were deemed to be associated with
study drugs were recorded according to according to Common
Terminology Criteria for Adverse Events (CTCAE) version 5.0
(28). For this retrospective study,
we recorded severe AEs related to various dose combinations in
Table II, of which we sometimes
hardly verdicted whether the AEs were related to TKIs or
chemotherapy.
 | Table II.Main dose combination (for more than
3/4 of the whole treatment time) for apatinib+IE (n=33) and main
AEs (according to CTCAE 5.0) for the present retrospective
study. |
Table II.
Main dose combination (for more than
3/4 of the whole treatment time) for apatinib+IE (n=33) and main
AEs (according to CTCAE 5.0) for the present retrospective
study.
|
| Apatinib dose | Target AE, n
(%) |
|---|
|
|
|
|
|---|
| IE dose for
combination | 250 mg QD po (BSA
>1.0), n=11 | 500 mg QD po or 250
mg QD po (BSA <1.0), n=21 | 375 mg QD po,
n=1 | Neutrophil count
decreased and thrombocytopenia, grade 4, for >3 days | Bronchial
infection, more than grade 3 | Reversible
posterior leukoencephalopathy syndrome, grade 3 | Anorexia, grade
3 | Pneumothorax, grade
3 |
|---|
| IFO 2.4
g/m2/d d1-5, n=3 | 1 | 2 |
| 3 (100.0) |
| 1 (33.3) |
|
|
| IFO 2
g/m2/d d1-5, n=1 | 1 |
|
|
| 1 (100.0) |
|
| 1 (100.0) |
| IFO 1.8
g/m2/d d1-5, VP16 100 mg/m2/d | 4 | 9 | 1 | 8 (57.1) | 2 (14.3) |
| 1 (7.1) | 1 (7.1) |
| d1-5, n=14 |
|
|
|
|
|
|
|
|
| IFO 1.8
g/m2/d d1-3, VP16 100 mg/m2/d | 4 | 9 |
| 3 (23.1) | 2 (15.4) |
|
|
|
| d1-3, n=13 |
|
|
|
|
|
|
|
|
| IFO 1.8
g/m2/d d1-3, n=2 | 1 | 1 |
|
| 1 (50.0) | 1 (50.0) |
|
|
| Target AE:
Neutrophil count decreased and thrombocytopenia, grade 4, for more
than 3 day | 4 (36.4%) | 10 (47.6%) | 1 (100.0%) |
|
|
|
|
|
| Target AE:
Bronchial infection, more than grade 3 | 3 (27.3%) | 3 (14.3%) |
|
|
|
|
|
|
| Target AE:
Reversible posterior leukoencephalopathy syndrome, grade 3 |
| 1 (4.8%) |
|
|
|
|
|
|
| Target AE:
Anorexia, grade 3 |
| 1 (4.8%) |
|
|
|
|
|
|
| Target AE:
pneumothorax, grade 3 |
| 2 (9.5%) |
|
|
|
|
|
|
Treatment protocols
Initially, IE chemotherapy was routinely
administered as follows: Ifosfamide 1.8 g/m2/d
d1-5 and etoposide 100 mg/m2/d
d1-5 once every 2 weeks (Q2w), with sufficient hydration
and mesna protection. Given the variability in the disease status
and haematopoietic function of the patients, for the combination
therapy, patients were retrospectively divided into five groups for
IE dosing (more than half the duration of the whole treatment
course) and three groups for apatinib dosing as follows: 250 mg
once daily (QD) orally (po) [body surface area (BSA) ≥1.0]; 500 mg
QD po (BSA ≥1.0) or 250 mg QD po (BSA <1.0); and 375 mg QD po
(BSA ≥1.0). For the combination therapy we had tried the following
combinations: i) Ifosfamide 2.4 g/m2/d d1-5
Q3W, ii) ifosfamide 2.0 g/m2/d d1-5 Q3W and
iii) ifosfamide 1.8 g/m2/d d1-5, etoposide
100 mg/m2/d d1-5 Q3W. Next, the following
combinations were attempted: iv) Ifosfamide 1.8 g/m2/d
d1-3; etoposide 100 mg/m2/d d1-3
Q2w and in some patients v) ifosfamide 1.8 g/m2/d
d1-3 Q2w. Severe haematological toxicity was classified
as the following: i) Haematological toxicity: Absolute neutrophil
count <500/mm3 for ≥3 days and platelets
<25,000/mm3 for ≥3 days despite platelet transfusion
or grade ≥3 thrombocytopenic bleeding and ii) non-haematological
grade ≥3 toxicities. If these were observed in patients then VP16
was removed from the treatment regime following the dose reduction
protocols of COG protocol-AOST0331 as well as EURAMOUS-1 (17).
Statistical analysis
All analyses were performed with SPSS 19.0 software
(IBM Corp.). The data were presented as n (%) or median,
interquartile range. Given that some patients underwent lesion
resection, event-free survival (EFS) was calculated from the date
at which apatinib + IE (not from first-line chemotherapy) was
started until disease progression or death, whichever came first in
median value as well as interquartile range. PFS was calculated
from the start of target treatment until disease progression or
death, whichever came first. For patients who underwent lesion
resection, the events were calculated as censored for PFS and
recorded the time at the local therapeutic time point. Overall
survival (OS) was calculated from the date of treatment initiation
to death from any cause. Descriptive statistics were used to
display demographic data. Kaplan-Meier plotter was used to
determine OS, EFS and PFS (29). Cox
proportional hazards analysis was subsequently performed on
variables to identify factors associated with survival and local
recurrence. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
In total, 33 patients who received 818 courses of
apatinib + IE were identified, among whom the initial diagnosis was
established between August 2014 and January 2020. The
characteristics of the included patients are summarized in Table I. Patients' age were grouped
according to Collins et al (30) in Table
I because in EURAMOUS-1 trial this factor significantly
influenced the outcomes (17). In
total, 13/33 (39.4%) patients had ECOG performance status scores of
1 or 2, and 17/33 (51.5%) had lesions located in the lungs, as well
as musculoskeletal sites or viscera. In addition, 18/33 (54.6%) had
progressed on two or more lines of systemic therapy at baseline.
Notably, 12/33 (36.4%) patients progressed on IE chemotherapy,
among whom 2/33 (6.1%) had progressed on apatinib alone (Table I). Usually, these cases of
progression manifested as indolent progression patterns, such as PD
over a time window of 4–6 months or even longer based on the RECIST
1.1 or simply oligoprogression of 1–2 lesions (data not shown). Of
the patients who had ever progressed on IE chemotherapy, all still
had the potential to subsequently benefit from the combination.
 | Table I.Demographics of 33 patients with
osteosarcoma treated with apatinib+IE in the study. |
Table I.
Demographics of 33 patients with
osteosarcoma treated with apatinib+IE in the study.
| Variable | Value |
|---|
| Age, median years
(minimum, maximum) | 16.0 (3.0,
48.0) |
| Age
groupa, n (%) |
|
|
Child | 8
(24.2) |
|
Adolescent | 9
(27.3) |
|
Adult | 16 (48.5) |
| Sex, n (%) |
|
|
Male | 14 (42.4) |
|
Female | 19 (57.6) |
| ECOG performance
status before treatment, n (%) |
|
| 0 | 20 (60.6) |
| 1 | 11 (33.3) |
| 2 | 2
(6.1) |
| Sites of target and
non-target lesions before treatment, n (%) |
|
| Lung
only | 13 (39.4) |
|
Musculoskeletal sites
only | 3
(9.1) |
| Lung +
musculoskeletal sites | 16 (48.5) |
| Lung +
bone + other sitesb, n
(%) | 1
(3.0) |
| Lines of previous
chemotherapy including MAP/Ic, n (%) |
|
| 1 | 15 (45.4) |
| 2 | 16 (48.5) |
| 3 | 2
(6.1) |
| High-grade
osteosarcoma subtypes, n (%) |
|
|
Canonical (osteoblastic,
chondroblastic, fibroblastic) | 32 (97.0) |
| Small
cell | 0
(0.0) |
|
Telangiectatic | 1
(3.0) |
| Resistance to MAP/I
chemotherapyd, n
(%) |
|
|
Yes | 33 (100.0) |
| No | 0
(0.0) |
| Resistance to IE
chemotherapye, n
(%) |
|
|
Yes | 12 (36.4) |
| No | 21 (63.6) |
| Resistance to
apatinibf, n (%) |
|
|
Yes | 2
(6.1) |
| No | 31 (93.9) |
Dosing
For the combination therapy we had tried the
following combinations i), ii) and iii). However, only two patients
could tolerate dosing i) and one patient could tolerate dosing ii).
The majority of patients had myelosuppression that was so severe
that the doses were reduced shortly after initiation (after 1–2
cycles). Then we tried dose iv) and v), of which the patients'
tolerance was improved (14/33 and 13/33 of the population,
respectively). It was noticed that 4/33 (12.1%) of patients with
unresectable pulmonary and musculoskeletal lesions had been given
doses iv and iii of IE (one and three patients, respectively) for
>12 months without disease progression or severe toxicity (12.6,
13.9, 18.4 and 19.2 months) (data not shown). However, after 8 to 9
months of treatment, the intervals of their chemotherapy cycles
were sometimes prolonged to 3–4 weeks sometimes due to
myelosuppression and sometimes just patients' preferences. Two
patients had been so heavily treated that dosing iv was attempted,
but treatment was interrupted after just 3 cycles due of
myelosuppression. Then, dose v was chosen for 2 and 6 months (for
each patient). Due to the complexity of the data and given that
this was a retrospective investigation, we then summarized the
dosing for patients who received for more than half of the
treatment course as their recorded dosing into Table II. It was noticed that for the
heavily treated patients with a high tumour burden in multiple
metastatic sites, combinations with a low dose and long dosing
times resulted in improved clinical benefit. Thus, we aimed not to
use aggressive treatment to induce an objective response and then
interrupt treatment because of toxicity but rather to use the
combination of a similar metronomic chemotherapy and TKIs to
prolong overall disease control.
Assessment of efficacy
With a median follow-up of 19.8 [interquartile range
(IQR), 10.1–29.4] months, 20/79 (25.3%) patients showed no evidence
of disease, 27/79 (34.2%) patients were alive with disease, and
32/79 (40.5%) patients died of disease (Table II, Figs.
2 and 3). Thus, the OS data were
estimated using Kaplan-Meier analysis. As of the most recent
follow-up, 21/33 (63.6%) patients had partial responses, while
12/33 (36.4%) had stable disease without any initial disease
progression at the first 2-month evaluation (Table III) according to the RECIST
1.1.
 | Table III.Efficacy of combination treatment of
apatinib+IE in patients 33 with advanced osteosarcoma. |
Table III.
Efficacy of combination treatment of
apatinib+IE in patients 33 with advanced osteosarcoma.
| Efficacy
variable | Value |
|---|
| Complete response,
n (%) | 0 (0.0) |
| Partial response, n
(%) | 21 (63.6) |
| Stable disease, n
(%) | 12 (36.4) |
| Progressive
disease, n (%) | 0 (0.0) |
| CBR of ITT, 6
months (95% CI) | 81.8% (68.7%,
95.0%) |
| ITT event-free
survival |
|
| KM,
median (95% CI) | 11.4 (7.5,
17.1) |
| 4
months, n% (95% CI) | 90.9 (74.4,
97.0) |
| 6
months, n% (95% CI) | 78.5 (60.0,
89.1) |
| ITT overall
survival |
|
| KM,
median, IQR | 19.8 (10.1,
29.4) |
| Patient status at
last follow-up, n (%) |
|
|
NED | 4 (12.1) |
|
AWD | 13 (39.4) |
|
DOD | 16 (48.5) |
| Complete surgical
remission, n (%) | 16 (48.5) |
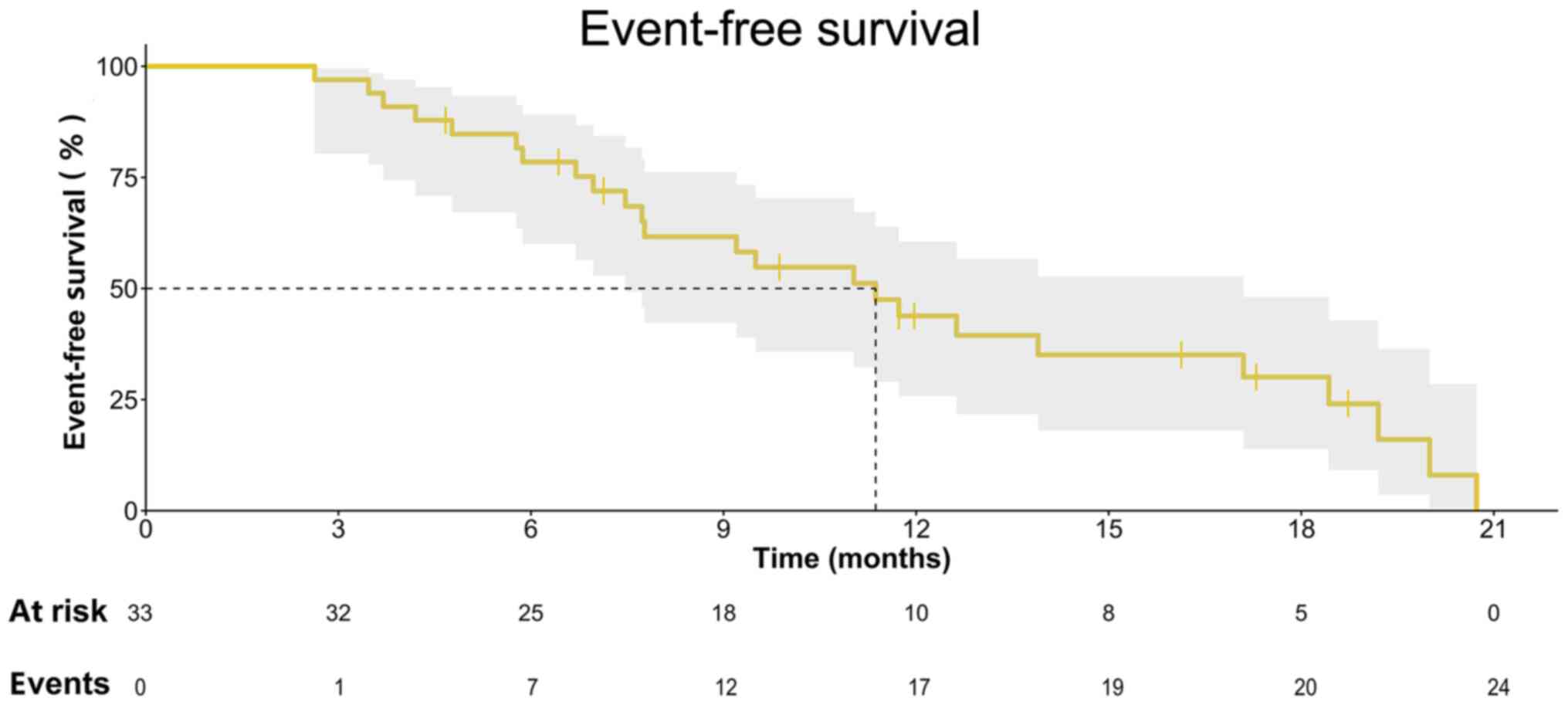
The 4- and 6-month EFS rates were 90.9% (95% CI,
74.4–97.0%) and 78.5% (95% CI, 60.0–89.1%), respectively, with a
median EFS of 11.4 (IQR, 7.5–17.1) months (Fig. 2). The median PFS time was 12.6 (95%
CI, 6.4–18.9) months, with 22/33 (66.7%) events censored (Fig. S1). At the last follow-up, 16/33
patients had tumour downstaging, and all lesions had been
completely resected (Table III).
Thus, EFS was more representative of the real-world conditions of
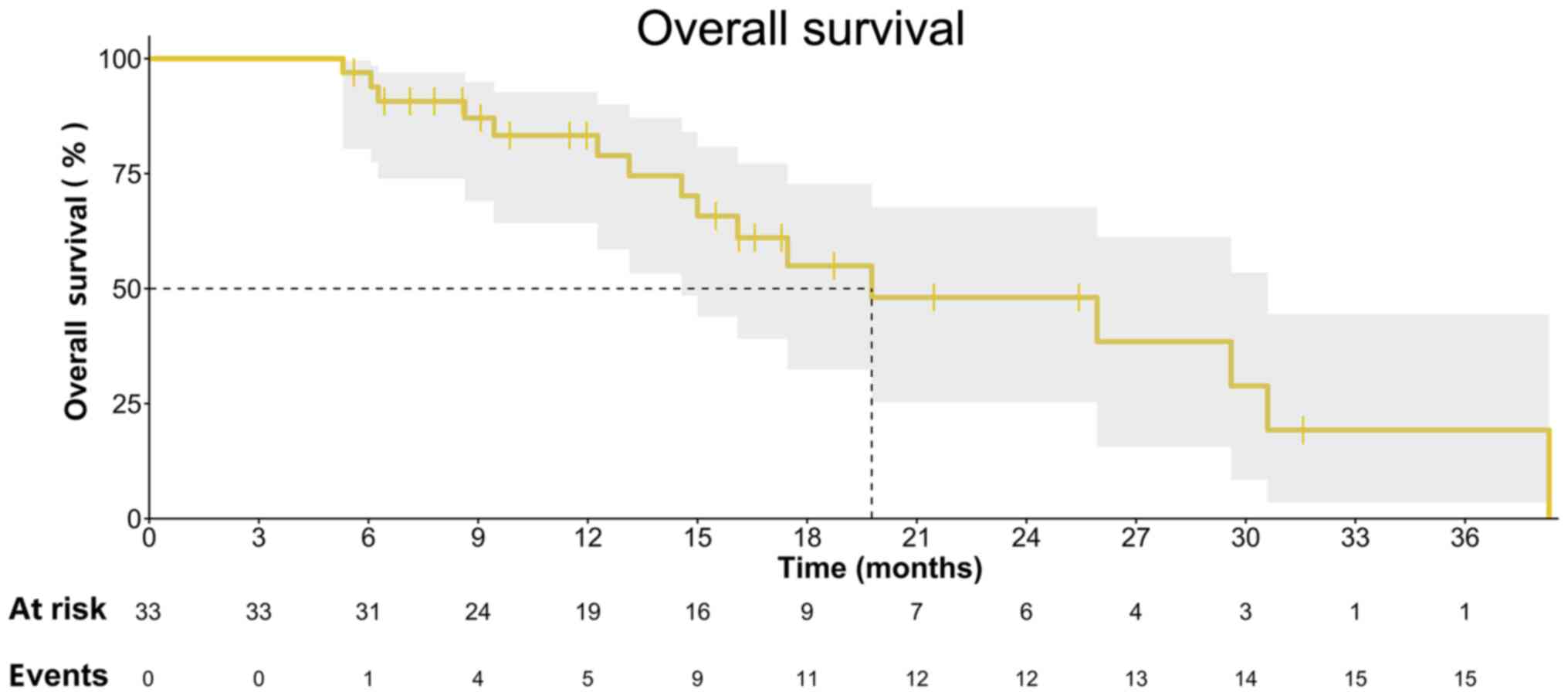
our patients compared with OS. The median OS time of these patients
was 19.8 (IQR, 10.1–29.4) months (Fig.
4). We then compared the data of our previous clinical trials'
data for single apatinib and apatinib in combination with
camrelizumab, as well as our retrospective data of single IE
chemotherapy delivered from 2016 to 2019 into Table IV. However, even for the most
heavily treated patient group with the highest tumour burden, the
combination of apatinib and IE chemotherapy led to a more objective
tumour response (Fig. 4) and a
higher disease control rate (Table
IV) than compared with IE chemotherapy, single apatinib or
apatinib + camrelizumab.
 | Table IV.Comparison of the patients'
demographics and efficacy of different apatinib-based therapeutic
strategies for advanced osteosarcoma. |
Table IV.
Comparison of the patients'
demographics and efficacy of different apatinib-based therapeutic
strategies for advanced osteosarcoma.
| Items | Apatinib+IE,
n=33 | Apatinib (11), n=37 |
Apatinib+Camrelizumab (12,13),
N=41 | IE, n=46 |
|---|
| Study type
(11–13) | Retrospective
study | Prospective
trial | Prospective
trial | Retrospective
study |
| Trial registration
number | NCT04690231 | NCT02711007 | NCT03359018 | NCT04690231 |
| Patient age,
average years ± | 19.1±8.5 | 21.7±11.5 | 19.7±9.0 | 17.7±9.3 |
| standard deviation
(95% CI) | (16.0, 22.2) | (17.9, 25.6) | (17.1, 22.4) | (14.9, 20.5) |
| Target lesions
before treatment, n (%) |
|
|
|
|
|
Pulmonary lesions | 13 (39.39) | 27 (72.97) | 18 (41.86) | 39 (84.78) |
|
Musculoskeletal lesions | 3 (9.09) | 4 (10.81) | 3 (6.98) | 1 (2.17) |
| Lung+
musculoskeletal lesions | 16 (48.48) | 6 (16.22) | 22 (51.16) | 6 (13.04) |
|
Lung+bone+other
lesionsa | 1 (3.03) | 0 (0.00) |
| 0 (0.00) |
| Lines of systemic
therapy, n (%) |
|
|
|
|
| 1st
line MAPIb | 15 (45.45) | 31 (83.78) | 37 (86.05) | 40 (86.96) |
| 2nd
line IE/GT | 16 (48.48) | 5 (13.52) | 6 (13.95) | 5 (10.87) |
| 3rd
line | 2 (6.06) | 1 (2.70) |
| 1 (2.17) |
| ECOG status before
treatment, n (%) |
|
|
|
|
| 0 | 20 (60.60) | 27 (72.97) | 34 (79.07) | 44 (95.65) |
| 1 | 11 (33.33) | 10 (27.03) | 9 (20.93) | 2 (4.35) |
| 2 | 2 (6.06) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Osteosarcoma
subtypes, canonical types, N (%) | 32 (96.97) | 35 (94.59) | 42 (97.67) | 46 (100.00) |
| Best overall
response according to RECIST 1.1 |
|
|
|
|
|
Complete response, N (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Partial
response, N (%) | 21 (63.6) | 16 (43.2) | 9 (20.9) | 13 (26.1) |
| Stable
disease, N (%) | 12 (36.4) | 8 (21.6) | 26 (60.5) | 28 (60.9) |
|
Progressive disease, N
(%) | 0 (0.0) | 13 (35.1) | 8 (18.6) | 5 (10.9) |
| ITT event-free
survival |
|
|
|
|
| Delete
loss rate, n (%) | 9 (27.3) | 11 (29.7) | 7 (16.3) | 12 (42.9) |
| KM
median months (Q1, Q3) | 11.4 (7.5,
17.1) | 4.5 (3.5, 6.3) | 6.2 (3.6, 8.9) | 11.7 (7.6,
15.7) |
| 6
months, % | 78.5% | 36.8% | 50.9% | 71.7% |
| 12
months, % | 39.5% | <10% | <10% | 56.8% |
| ITT overall
survival |
|
|
|
|
| KM
median months (IQR) | 19.8
(9.4,51.8) | 9.9 (8.0,
19.0) | 11.3 (8.1,
14.8) | 30.4 (26.9,
NR) |
|
Complete surgical remission, n
(%) | 16 (48.5) | 0.0 (0.0) | 0.0 (0.0) | 23 (50.0) |
Toxicity and safety
The severe adverse events (AEs) related to various
dose combinations are summarized in Table II according to CTCAE v.5.0, of which
the dosing used for more than half of the treatment course was
recorded. A total of 25 severe AEs were recorded. Despite the fact
that the daily dose of apatinib was lower than that used in the
aforementioned phase II trial (11),
the AEs were both higher grades and different compared with those
in the treatment combination with IE. The majority of grade 3 and 4
toxicities were neutropenia, thrombocytopenia, bronchial infection,
pneumothorax, anorexia, and posterior leukoencephalopathy syndrome
(PLPS). It is worth noting that the records for AEs might not have
been complete given the retrospective nature of the study. However,
physicians are trained to record the most concerning AEs to
empirically deliver regimens safely. From the data shown in
Table II, it is speculated that the
dose combination with apatinib 500 mg QD po for BSA ≥1.0 and IFO
1.8 g/m2/d d1-5 VP16 100 mg/m2/d
d1-3 Q2w could represent a compromise, with acceptable
toxicities in these patients.
Discussion
The prognosis of patients with recurrent or
refractory osteosarcoma progression upon first-line chemotherapy
has been predominantly poor for the past 30 years with 5-year
post-relapse survival in <20% globally (31). Despite evidence of genomic
instability characterized by widespread and recurrent somatic
copy-number alterations and structural rearrangements, osteosarcoma
has few recurrent point mutations in protein-coding genes (32). Moreover, trials of targeted agents
have generally been disappointing with median PFS of only 4 to 6
months and secondary drug resistance seemed to be inevitable
(2,4). We previously showed the promising
anti-angiogenic therapeutic effects of TKIs, including apatinib
(11), cabozantinib (33), lenvatinib (22), regorafenib (34) and sorafenib (35), of which the treatment targets are
thought to include VEGFRs and proto-oncogene tyrosine-protein
kinase receptor RET (a transmembrane receptor protein-tyrosine
kinase that is required for the development of the nervous system
and several other tissues) (5), in
osteosarcoma. However, these TKIs have short-lived activity in the
treatment of musculoskeletal lesions or other metastases outside
the lungs (11,13). It is suspected that the plasma
concentrations of these TKIs might be limited due to their
pharmacokinetic characteristics (7).
Despite this speculation, very little is known about the biology
underlying this poor control of bone lesions. However, it is known
that in the early stages of metastatic lung colonization,
disseminated cancer cells experience a variety of cellular
stresses, including redox/endoplasmic reticulum stress, which
threaten their survival in the distant bone microenvironment
(36). Thus, overcoming cancer
progression outside the lung during TKI-based treatment is
important. Moreover, for heavily treated patients with inoperable
lesions, AEs need to be managed to a tolerable level given that
therapy is potentially lifelong; this leads us to question which
types of dose combinations would be most beneficial to this patient
group.
The rationale for the combination of apatinib and IE
in the present study was built on the theory that the addition of
chemotherapy could overcome the weakness of the cytostatic
properties of these molecular targeted agents. The results on
lenvatinib and IE reported at the 2019 ESMO (23) showed promise for the future use of
this combination, of which the median PFS was prolonged to 11.1
months. The current retrospective study also provided evidence for
exploration of the activity of apatinib + IE in the treatment of
metastatic osteosarcoma with lesions outside the lungs. Indeed,
several prospective trials with apatinib-based treatment have been
completed in the last decade (11,13).
Thus, to compare the efficacy of these regimens, we combined the
data of these non-randomized, historical non-controlled cohorts, as
well as our retrospective data of single IE chemotherapy delivered
from 2016 to 2019 into Table IV. As
a result, we were able to observe that different tumour burdens and
diverse tumour locations have the potential to significantly
influence the treatment outcomes. For advanced osteosarcoma, in our
opinion, the major factor that influences overall survival is
tumour stage. Notably, those who progressed upon more lines of
systemic therapies and those with higher tumour burdens as well as
more metastatic lesions and more advanced ECOG statuses had a
poorer prognosis. Our study showed that apatinib plus IE
chemotherapy had better disease control rate than single apatinib
or apatinib in combination with camrelizumab. It is considered that
these advantages are partly due to the disease control of
musculoskeletal lesions by relatively high concentrations of
chemotherapy drugs in the bone marrow. In the present study, a
limited number of patients progressed on single apatinib and single
IE chemotherapy separately could still benefit from the combination
therapy, demonstrating that synergistic effects likely exist.
It is noteworthy that a considerable number of
patients in the current retrospective study underwent local
radiation or surgical resection during or after treatment. In
particular, for patients treated with apatinib + IE-treated, after
quick tumour regression and downstaging of the locally advanced
lesions, 16/33 (48.5%) patients received local radiation or
surgery. Thus, although EFS instead of PFS was selected to evaluate
disease control, whether these local treatments partly contributed
to the prolonged EFS needs to be further clarified. Based on the
outcomes of patients with recurrent osteosarcoma who were enrolled
in seven phase II trials through the Children's Oncology Group
(2), it was realized that using EFS
as an end point in an osteosarcoma study was reasonable given that
the historical benchmark for tumour shrinkage is difficult to
obtain and detect. However, given that our past two prospective
trials (11–13) used PFS as an end point without local
treatments, caution should be taken when attempting to compare the
outcomes of these cohorts.
Toxicity tends to be partly associated with apatinib
and with IE. Given past experiences of the overall incidence of
toxicity in the phase II trials of advanced osteosarcoma (11,13,18,22,23,33–35,37) and
our routine practice, we prefer to use lower doses of apatinib and
IE instead of bolus dosing. In bolus dosing, toxicities lead to
frequent interruptions that can compromise the therapeutic effect.
The present study observed that a large proportion of patients
could remain on the combination of the 3-day IE Q2w regimen for
>12 months. In our opinion, the ifosfamide 1.8 g/m2/d
d1-3 and etoposide 100 mg/m2/d
d1-3 Q2w regimen with apatinib 500 mg po seemed to be
suitable for most Chinese patients Some AEs, such as PLPS, might
become more severe as a result of the combination treatment given
that both apatinib and IFO can cause leukoencephalopathy.
The present study has several limitations. First,
given that the present study was retrospective, selection bias was
unavoidable in choosing patients who received apatinib + IE.
Moreover, the comparisons of the four cohorts of patients were not
controlled, and the patients had clear differences in disease
status, which made the interpretation and comparison of the data
difficult. Second, OS was not investigated due to the short
follow-up time. Third, the histological comparative end points were
different (EFS and PFS), because unlike the prospective phase 2
trials, some patients underwent lesion resection during or after
treatment in the current real-world study. Moreover, given that
local radiotherapy or surgeries might benefit EFS, this comparison
was relatively promiscuous. We compared our synchronous
retrospective data of patients who received IE chemotherapy alone
with those of controls and found almost the same surgical complete
remission rate (23/46, 50.0%), as shown in Table IV, and EFS was not significantly
different retrospectively. To overcome the influence of other
interventions on the outcome, we are currently performing a
prospective trial to investigate this combination, from which more
accurate data on this treatment strategy are expected. Fourth, the
current study did not strictly perform a dose climbing ‘3 + 3’
study, which made the combination dosing prescribed according to
disorder and toxicity profiles unclear.
For osteosarcoma with multiple sites of metastasis,
apatinib + IE demonstrated clinically meaningful antitumour
activity after failure of high-dose methotrexate, doxorubicin,
cisplatin with or without ifosfamide chemotherapy or failure of
former single apatinib or IE treatment, with a positive effect on
delaying disease progression. The results of the present study
indicated that this combination with manageable toxicity deserves
further investigation in prospective trials.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Danhua Shen
(senior pathologist from Peking University People's Hospital) for
involvement in the review of all pathological slides.
Funding
This work was supported by The Beijing Municipal
Science & Technology Project (grant no. Z181100001718054) and
The Research and Development Fund of Peking University People's
Hospital (Clinical Medicine +X Cultivation Project, grant no.
RDX2019-08).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX and WG conceived and designed the study. LX, JX,
XS, KL, XWL, ZZ and HZ collected the data. KS reviewed the
pathological slides according to RECIST 1.1. YW, JG, LX and JX
clinically evaluated the efficacy of the drugs. KL, XL and XWL
performed the laboratory work and statistical analysis. LX, JX and
WG analysed and interpreted the data. LX wrote the manuscript. All
authors confirmed the authenticity of the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Both hospitals obtained approval from The Medical
Ethics Committee of Peking University People's Hospital and Peking
University Shougang Hospital (Beijing, China) to retrospectively
review the patients' medical records and radiographic materials.
Written informed consent was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AEs
|
adverse events
|
|
BSA
|
body surface area
|
|
CI
|
confidence interval
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
EFS
|
event-free survival
|
|
ESMO
|
European Society for Medical
Oncology
|
|
IE
|
ifosfamide and etoposide
|
|
IFO
|
ifosfamide
|
|
IQR
|
interquartile range
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
PLPS
|
posterior leukoencephalopathy
syndrome
|
|
po
|
orally
|
|
Q2w
|
once every 2 weeks
|
|
Q3W
|
once every 3 weeks
|
|
QD
|
once daily
|
|
RECIST 1.1
|
Response Evaluation Criteria for Solid
Tumours
|
|
TKIs
|
tyrosine kinase inhibitors
|
References
|
1
|
Xie L, Ji T and Guo W: Anti-angiogenesis
target therapy for advanced osteosarcoma (Review). Oncol Rep.
38:625–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lagmay JP, Krailo MD, Dang H, Kim A,
Hawkins DS, Beaty O III, Widemann BC, Zwerdling T, Bomgaars L,
Langevin AM, et al: Outcome of patients with recurrent osteosarcoma
enrolled in seven phase II trials through Children's Cancer Group,
Pediatric Oncology Group, and Children's Oncology Group: Learning
from the past to move forward. J Clin Oncol. 34:3031–3038. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carrle D and Bielack SS: Current
strategies of chemotherapy in osteosarcoma. Int Orthop. 30:445–451.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberts RD, Lizardo MM, Reed DR, Hingorani
P, Glover J, Allen-Rhoades W, Fan T, Khanna C, Sweet-Cordero EA,
Cash T, et al: Provocative questions in osteosarcoma basic and
translational biology: A report from the Children's Oncology Group.
Cancer. 125:3514–3525. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian Z, Niu X and Yao W: Receptor tyrosine
kinases in osteosarcoma treatment: Which is the key target? Front
Oncol. 10:16422020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaikh AB, Li F, Li M, He B, He X, Chen G,
Guo B, Li D, Jiang F, Dang L, et al: Present advances and future
perspectives of molecular targeted therapy for osteosarcoma. Int J
Mol Sci. 17:5062016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
8
|
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang
S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis
through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death
Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng B, Ren T, Huang Y and Guo W:
Apatinib inhibits migration and invasion as well as PD-L1
expression in osteosarcoma by targeting STAT3. Biochem Biophys Res
Commun. 495:1695–1701. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie L, Guo W, Wang Y, Yan T, Ji T and Xu
J: Apatinib for advanced sarcoma: Results from multiple
institutions' off-label use in China. BMC Cancer. 18:3962018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie L, Xu J, Sun X, Tang X, Yan T, Yang R
and Guo W: Apatinib for advanced osteosarcoma after failure of
standard multimodal therapy: An open label phase II clinical trial.
Oncologist. 24:e542–e550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Correction: Apatinib plus camrelizumab
(anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO)
progressing after chemotherapy: A single-arm, open-label, phase 2
trial. J Immunother Cancer. 8:e000798corr0007912020.
|
|
13
|
Xie L, Xu J, Sun X, Guo W, Gu J, Liu K,
Zheng B, Ren T, Huang Y, Tang X, et al: Apatinib plus camrelizumab
(anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO)
progressing after chemotherapy: A single-arm, open-label, phase 2
trial. J Immunother Cancer. 8:e0007982020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosen G, Marcove RC, Caparros B, Nirenberg
A, Kosloff C and Huvos AG: Primary osteogenic sarcoma: The
rationale for preoperative chemotherapy and delayed surgery.
Cancer. 43:2163–2177. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cormier JN, Patel SR, Herzog CE, Ballo MT,
Burgess MA, Feig BW, Hunt KK, Raney RB, Zagars GK, Benjamin RS and
Pisters PW: Concurrent ifosfamide-based chemotherapy and
irradiation. Analysis of treatment-related toxicity in 43 patients
with sarcoma. Cancer. 92:1550–1555. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miser JS, Kinsella TJ, Triche TJ, Tsokos
M, Jarosinski P, Forquer R, Wesley R and Magrath I: Ifosfamide with
mesna uroprotection and etoposide: An effective regimen in the
treatment of recurrent sarcomas and other tumors of children and
young adults. J Clin Oncol. 5:1191–1198. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marina NM, Smeland S, Bielack SS,
Bernstein M, Jovic G, Krailo MD, Hook JM, Arndt C, van den Berg H,
Brennan B, et al: Comparison of MAPIE versus MAP in patients with a
poor response to preoperative chemotherapy for newly diagnosed
high-grade osteosarcoma (EURAMOS-1): An open-label, international,
randomised controlled trial. Lancet Oncol. 17:1396–1408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goorin AM, Harris MB, Bernstein M,
Ferguson W, Devidas M, Siegal GP, Gebhardt MC, Schwartz CL, Link M
and Grier HE: Phase II/III trial of etoposide and high-dose
ifosfamide in newly diagnosed metastatic osteosarcoma: A pediatric
oncology group trial. J Clin Oncol. 20:426–433. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferrari S, Mercuri M, Picci P, Bertoni F,
Brach del Prever A, Tienghi A, Mancini A, Longhi A, Rimondini S,
Donati D, et al: Nonmetastatic osteosarcoma of the extremity:
Results of a neoadjuvant chemotherapy protocol (IOR/OS-3) with
high-dose methotrexate, intraarterial or intravenous cisplatin,
doxorubicin, and salvage chemotherapy based on histologic tumor
response. Tumori. 85:458–464. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salzer-Kuntschik M, Delling G, Beron G and
Sigmund R: Morphological grades of regression in osteosarcoma after
polychemotherapy-study COSS 80. J Cancer Res Clin Oncol. 106
(Suppl):S21–S24. 1983. View Article : Google Scholar
|
|
21
|
Ferrari S, Bielack SS, Smeland S, Longhi
A, Egerer G, Sundby Hall K, Donati D, Kevric M, Brosjo O, Comandone
A, et al: EURO-B.O.S.S.: A European study on chemotherapy in
bone-sarcoma patients aged over 40: Outcome in primary high-grade
osteosarcoma. Tumori. 104:30–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaspar N, Occean BV, Pacquement H, Bompas
E, Bouvier C, Brisse HJ, Castex MP, Cheurfa N, Corradini N, Delaye
J, et al: Results of methotrexate-etoposide-ifosfamide based
regimen (M-EI) in osteosarcoma patients included in the French
OS2006/sarcome-09 study. Eur J Cancer. 88:57–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gaspar N, Sirvent B, Venkatramani R,
Longhi A, Lervat C, Casanova M, Aerts I, Bielack SS, Entz-Werle N,
Strauss S, et al: 1399 Phase 1 combination dose-finding/phase 2
expansion cohorts of lenvatinib + etoposide + ifosfamide in
patients (pts) aged 2 to 25 years with relapsed/refractory (r/r)
osteosarcoma. Ann Oncol. 30 (Suppl 5):v683–v709. 2019. View Article : Google Scholar
|
|
24
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 153:106–120. 1980.PubMed/NCBI
|
|
25
|
Xie L, Xu J, Dong S, Gao J, Tang X, Yan T,
Yang R and Guo W: Gain and loss from transcatheter intra-arterial
limb infusion of cisplatin for extremity osteosarcoma: A
retrospective study of 99 cases in the past six years. Cancer Manag
Res. 11:7183–7195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verger E, Salamero M and Conill C: Can
Karnofsky performance status be transformed to the Eastern
Cooperative Oncology Group scoring scale and vice versa? Eur J
Cancer. 28A:1328–1330. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
U.S. Department of Health and Human
Services: Common Terminology Criteria for Adverse Events (CTCAE)
Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdfNovember
27–2018
|
|
29
|
Nagy A, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Collins M, Wilhelm M, Conyers R, Herschtal
A, Whelan J, Bielack S, Kager L, Kühne T, Sydes M, Gelderblom H, et
al: Benefits and adverse events in younger versus older patients
receiving neoadjuvant chemotherapy for osteosarcoma: Findings from
a meta-analysis. J Clin Oncol. 31:2303–2312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Italiano A, Mir O, Mathoulin-Pelissier S,
Penel N, Piperno-Neumann S, Bompas E, Chevreau C, Duffaud F,
Entz-Werle N, Saada E, et al: Cabozantinib in patients with
advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre,
single-arm, phase 2 trial. Lancet Oncol. 21:446–455. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duffaud F, Mir O, Boudou-Rouquette P,
Piperno-Neumann S, Penel N, Bompas E, Delcambre C, Kalbacher E,
Italiano A, Collard O, et al: Efficacy and safety of regorafenib in
adult patients with metastatic osteosarcoma: A non-comparative,
randomised, double-blind, placebo-controlled, phase 2 study. Lancet
Oncol. 20:120–133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grignani G, Palmerini E, Dileo P, Asaftei
SD, D'Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F,
Casali PG, et al: A phase II trial of sorafenib in relapsed and
unresectable high-grade osteosarcoma after failure of standard
multimodal therapy: An Italian Sarcoma Group study. Ann Oncol.
23:508–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vasudev NS and Reynolds AR:
Anti-angiogenic therapy for cancer: Current progress, unresolved
questions and future directions. Angiogenesis. 17:471–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grignani G, Palmerini E, Ferraresi V,
D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al: Sorafenib and everolimus for
patients with unresectable high-grade osteosarcoma progressing
after standard treatment: A non-randomised phase 2 clinical trial.
Lancet Oncol. 16:98–107. 2015. View Article : Google Scholar : PubMed/NCBI
|