Introduction
Colorectal cancer (CRC) is the third most common
cancer and the fourth most common cause of cancer-associated
mortality worldwide; it is estimated that ~135,430 new cases of
colon cancer are diagnosed and 50,260 deaths occur each year in the
United States of America (1,2). Despite recent advancements in the
therapeutic treatments for patients with CRC, the clinical outcome
of this disease remains unsatisfactory (3). Thus, it is important to investigate the
molecular mechanisms underlying the development of CRC to identify
effective therapeutic targets.
Autophagy is a biological process involving several
key genes named autophagy-related genes (ATGs), and is involved in
cellular metabolism, which depends on the lysosomal degradation of
cargo (viruses, bacteria and proteins) (4). The main role of autophagy is to
eliminate misfolded proteins, organelles or other factors that
cannot be degraded by the proteasome (4). Autophagy protects cells from stress and
helps cells survive under stressed conditions (4). Previous studies have demonstrated that
autophagy mediates CRC metastasis and proliferation (5,6).
Increasing the autophagy level promotes the growth of advanced
cancer and enhances drug resistance of cells in different types of
cancer (7). However, the molecular
mechanisms underlying autophagy in CRC remain unclear.
Long non-coding RNAs (lncRNAs) are a class of
non-protein coding RNAs >200 nucleotides in length (8). Previous studies have reported the
unique association between lncRNAs and the development of CRC
(8,9). For example, it has been demonstrated
that lncRNA small nucleolar RNA host gene 8 (SNHG8) mediates
autophagy by upregulating autophagy-related gene 7 (ATG7)
expression in hepatic ischaemia/reperfusion injury (10). In addition, lncRNA CDKN2B antisense
RNA 1 promotes tumor growth and metastasis in hepatocellular
carcinoma by upregulating the mRNA expression of nucleosome
assembly protein 1-like 1 (11).
Several studies have reported that the regulatory network of
lncRNAs is both conventional and crucial (8,9). SNHG8
is a novel lncRNA (12) that has
been investigated in a number of studies (10,12–14);
however, its underlying molecular mechanisms in CRC progression and
autophagy remain unclear.
MicroRNAs (miRNAs/miRs) are non-coding RNAs that
regulate gene expression by altering the 3′-untranslated region
(UTR) of specific genes, resulting in mRNA degradation (15). Increasing evidence suggest that
miRNAs can mediate carcinogenesis by regulating tumor-related genes
(16). For example, a study
demonstrated that miR-588 expression is downregulated in lung
cancer, and acts as a tumor suppressor that targets with
progranulin (17). In addition,
miR-588 has been reported to regulate breast cancer, CRC and
gastric cancer (18,19). However, the underlying molecular
mechanisms of miR-588 in CRC remain unclear, particularly in
autophagy. Thus, the present study aimed to investigate the role of
miR-588 in CRC.
Materials and methods
Cell culture
Colorectal cell lines, HCT116, FHC, HCT8, HT29 and
SW480, were purchased from the Cell Bank of Type Culture Collection
of The Chinese Academy of Sciences. Cross-contamination of the cell
lines was excluded via short tandem repeat profiling (20,21). CRC
cells were maintained in DMEM supplemented with 10% fetal bovine
serum (both purchased from Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.), at 37°C with 5% CO2.
Cell transfection
Plasmids, including SNHG8 cDNA and pcDNA-3.1 (5
µg/1×104 cells), and miR-588 mimics and negative control
(mimic-NC) (3 µg/1×104 cells) were purchased from
Shanghai GeneChem Co., Ltd. Small interfering RNA (siRNA) and NC
oligonucleotides of ATG7 were purchased from Sigma-Aldrich (Merck
KGaA) (3 µg/1×104 cells). HCT116 and SW480 cells were
transfected using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) for 6 h at 37°C,
according to the manufacturer's protocol. Subsequent experiments
were performed 24 h after transfection. The following sequences
were used: mimic-NC forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
reverse, 5′-ACGUGACACGUUCGGAGAATT-3′; mimic-miR-588 forward,
5′-GCUUCCAAAGAUCAGGUAACATT-3′ and reverse,
5′-UGUUACCUGAUCUUUGGAAGCTT-3′; si-ATG7 forward,
5′-CAGCCUGGCAUUUGAUAAATT-3′ and reverse,
5′-UUUAUCAAAUGCCAGGCUGTT-3′; and si-NC forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from HCT116 and SW480 cells
using the TRIzol® reagent kit (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Total
RNA concentration was detected using a NanoDrop spectrophotometer
(ND-1,000; Thermo Fisher Scientific, Inc.), at a wavelength of 260
nm. Total RNA (0.5 µg) was reverse transcribed into cDNA using the
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.) at 25°C
for 10 min, 48°C for 30 min and 95°C for 5 min. qPCR was
subsequently performed using the Power SYBR-Green PCR Master Mix
(Thermo Fisher Scientific, Inc.), at a final volume of 10 µl,
containing 0.04 µg cDNA. U6 was used as the internal control for
miR-588. The following thermocycling conditions were used: Initial
denaturation at 95°C for 10 min, followed by 40 cycles for 15 sec
at 95°C and 60°C for 1 min. The subsequent melt curve stage
consisted of 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec.
Relative expression levels were calculated using the
2−ΔΔCq method (22). The
following primer sequences were used: SNHG8 forward,
5′-AAGTTTACAAGCATGCGCGG-3′ and reverse, 5′-TCAAACTGACGGTTCTCGGG-3′;
ATG3 forward, 5′-GAGCGGCTCCTCAAGGAA-3′ and reverse,
5′-TGTAGCCCATTGCCATGTTGG-3′; ATG5 forward,
5′-AAAGATGTGCTTCGAGATGTGT-3′ and reverse,
5′-CACTTTGTCAGTTACCAACGTCA-3′; ATG7 forward,
5′-GAACAAGCAGCAAATGA-3′ and reverse, 5′-GACAGAGGGCAGGATAG-3′; ATG10
forward, 5′-AATGGAAGGGCGACAGTGAG-3′ and reverse,
5′-AGTCCTACACGCCACTTGAC-3′; ATG12 forward,
5′-CTGCTGGCGACACCAAGAAA-3′ and reverse, 5′-CGTGTTCGCTCTACTGCCC-3′;
GAPDH forward, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′; miR-588 forward,
5′-TACTCAACTCACTACTGCATGG-3′ and reverse,
5′-TATCGAAGTTCTGCTCTCTGTC-3′; and U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
Western blotting
Total protein was extracted from CRC cells (HCT116
and SW480) using RIPA lysis buffer (Cell Signaling Technology,
Inc.). Protein concentration was quantified using the bicinchoninic
acid assay kit (Beyotime Institute of Biotechnology), according to
the manufacturer's protocol, and 5 µl protein/lane was separated by
10% SDS-PAGE gels. The separated proteins were subsequently
transferred onto PVDF membranes (Sigma-Aldrich; Merck KGaA) and
blocked with non-fat milk dissolved in TBST (10 mM Tris-HCl, pH
7.4, 150 mM NaCl, 0.1% Tween-20) for 2 h at room temperature. The
membranes were incubated with primary antibodies against
microtubule-associated protein 1 light chain 3B (LC3) (cat. no.
ab192890), ATG7 (cat. no. ab52472) and β-actin (cat. no. ab8226)
for 16 h at 4°C (all 1:1,000 and purchased from Abcam). Membranes
were washed three times with TBST (0.01% Tween) (5 min each) and
subsequently incubated with HRP-conjugated goat anti-rabbit IgG
(1:5,000; Cell Signaling Technology, Inc.; cat. no. 7074) and
anti-mouse IgG (1:10,000; Cell Signaling Technology, Inc.; cat. no.
7076S) secondary antibodies at room temperature for 1.5–2 h.
Membranes were re-washed three times with TBST and the protein
bands were visualized using an electrogenerated chemiluminescence
detection system (Tanon-5200; Tanon Science and Technology Co.,
Ltd.). The rescue assays were performed by co-transfection with
SNHG8 and mimics-miR-588.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) for 20 min at 20°C. Cells were
subsequently permeabilized with 0.4% Triton X-100 for 5 min at room
temperature, and blocked with 5% BSA at 20°C for 30 min. Cells were
washed three times with PBS and incubated with LC3 primary antibody
(1:100; Abcam; cat. no. ab192890) overnight at 4°C. Cells were
re-washed three times with PBS (5 min each) and subsequently
incubated with AlexaFluor® 488-conjugated secondary
antibodies (1:100; Abcam; cat. no. ab150077) for 1 h at 37°C.
Finally, cells were re-washed three times with PBS. Nuclei were
stained with DAPI for 3 min at room temperature and cells were
washed three times with PBS, prior to observation under a confocal
microscope (magnification, ×400; Olympus Corporation).
Immunofluorescence analysis was performed using ImageJ v1.52
(National Institutes of Health).
Cell proliferation assay
Cell proliferation was assessed via the Cell
Counting Kit-8 (CCK-8) assay (Beijing Solarbio Science &
Technology Co., Ltd.). HCT116 and SW480 cells were seeded into
96-well plates at a density of 0.5–1×104 cells/well.
Following transfection, cells were cultured for 24, 48 and 72 h.
Cells were subsequently incubated with 10 µl CCK-8 reagent for 2 h
at 37°C and cell proliferation was analyzed at a wavelength of 450
nm.
Dual-luciferase reporter assay
The binding sites were constructed using the
StarBase database (http://starbase.sysu.edu.cn/). The luciferase reporter
vector pmirGLO was purchased from Promega Corporation. Wild-type
(WT) ATG7 (ATG7-WT) and the mutant (MUT) sequence of the miR-588
binding site of the 3′-UTR of ATG7 (ATG7-MUT) were cloned and
inserted into the luciferase gene sequence. The WT SNHG8 luciferase
reporter construct (SNHG8-WT) and mutant site (SNHG8-MUT) were
constructed by Shanghai GenePharma Co., Ltd. The following
sequences were used: ATG7-WT, 5′-CUGCUGCCCAGGAGUGGCCAG-3′ and
ATG7-MUT, 5′-CUGGAGCGGAGGAGCACCGGUG-3′; and SNHG8-WT,
5′-CGUCUGUGUCAUGUGGCCAU-3′; and SNHG8-MUT,
5′-CGUCUGUGUCAACACCGGUU-3′. Luciferase activity was measured 24 h
after transfection and was detected using the Luciferase Assay
System kit (Promega Corporation), according to the manufacturer's
protocol. Firefly luciferase activity was normalized to
Renilla luciferase activity.
Pull-down assay
Cellular lysates (50 µl) were pulled down using
biotinylated control (NC-Bio), miR-588 (miR-588-Bio) or miR-588
probes containing mutations in the SNHG8-binding site
(miR-588-Bio-MUT). CRC cells (HCT116 and SW480) were cultivated
with miR-588-Bio or miR-588-Bio-MUT (Thermo Fisher Scientific,
Inc.). Cells were lysed in lysis buffer (Thermo Fisher Scientific,
Inc.) and the lysate was subsequently cultivated with magnetic
beads (50 µl; Thermo Fisher Scientific, Inc.; cat. no. 65305) and
RNA probe. The immunoprecipitate was obtained via magnetic forces
and centrifugation at 1,000 × g for 20 min at room temperature, and
was washed using the Pierce™ Magnetic RNA Pull-Down kit (Thermo
Fisher Scientific, Inc.; cat. no. 20164). RNA was prepared using
TRIzol® reagent, and SNHG8 enrichment was measured via
PCR analysis. PCR was performed as aforementioned (RT-qPCR). The
following probes were used: miR-588-Bio,
3′-CAAGAUUGGGUAACACCGGUU-5′ and miR-588-Bio-MUT,
3′-CAUCUUUGGGUAUGUGGCCAU-5′.
Fluorescence in situ hybridization
(FISH)
Visualization of lncRNA localization in HCT116 and
SW480 cells was assessed via FISH. HCT116 and SW480 cells were
seeded into 6-wells plates and cultured until they reached 70%
confluence. Cells were washed three times with PBS and subsequently
fixed with 4% paraformaldehyde for 30 min at room temperature. FISH
was performed using the BersinBio™ RNA FISH kit (BersinBio; cat.
no. Bes-nRRF-HSA000002) according to the manufacturer's protocol.
The RNase inhibitor was purchased from Thermo Fisher Scientific,
Inc. (cat. no. AM2694). T7 RNA polymerase was purchased from Xinhai
Gene Testing Co., Ltd. (cat. no. A3601A). Restriction enzyme (T7)
was used to linearize plasmid. RT-qPCR was performed as
aforementioned. The SNHG8 FISH probe (length, 2,067 nt) (DIG-dUTP
and DIG RNA Labeling kit; Wolcavi Biotech; cat. no. 11175025910)
was constructed. Cells were maintained in prehybridization solution
(BersinBio) and hybridized with RNA FISH solution (Wuhan Servicebio
Technology Co., Ltd.), containing SNHG8 FISH probe
(3′-AATTCAAATGTTCGTACG-5′). Subsequently, cells were stained with
6-diamidino-2-phenylindole dye solution for 5 min at room
temperature (5 µg/ml; Beyotime Institute of Biotechnology) and
observed under a fluorescence microscope (magnification, ×400;
Olympus Corporation).
Bioinformatics analysis
The Cancer Genome Atlas (TCGA) dataset [colon
adenocarcinoma (COAD)] was obtained and analyzed using the
bioinformatics website UALCAN (http://ualcan.path.uab.edu/), including 41 normal
tissues and 286 primary CRC tumor tissues. TargetScan (http://www.targetscan.org/mamm_31/) and StarBase
(http://www.sysu.edu.cn/) were used to predict
potential binding sites.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corp.). All experiments were performed in triplicate
and data are presented as the mean ± standard deviation. Unpaired
Student's t-test was used to compare differences between two
groups, while one-way ANOVA and Bonferroni post hoc test were used
to compare differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
SNHG8 expression is upregulated in CRC
tissues and cell lines
To further investigate SNHG8 expression in CRC, TCGA
database was analyzed (41 normal tissues and 286 primary CRC tumor
tissues). The results demonstrated that SNHG8 expression was
significantly upregulated in CRC tissues compared with in normal
tissues (P<0.001; Fig. 1A),
indicating that abnormal SNHG8 expression may serve an important
role in patients with CRC. RT-qPCR analysis was performed to detect
SNHG8 expression in colorectal cell lines (HCT116, FHC, HCT8, HT29
and SW480). The results demonstrated that SNHG8 expression was
significantly upregulated in CRC cells compared with FHC cells
(P<0.05; Fig. 1B). In addition,
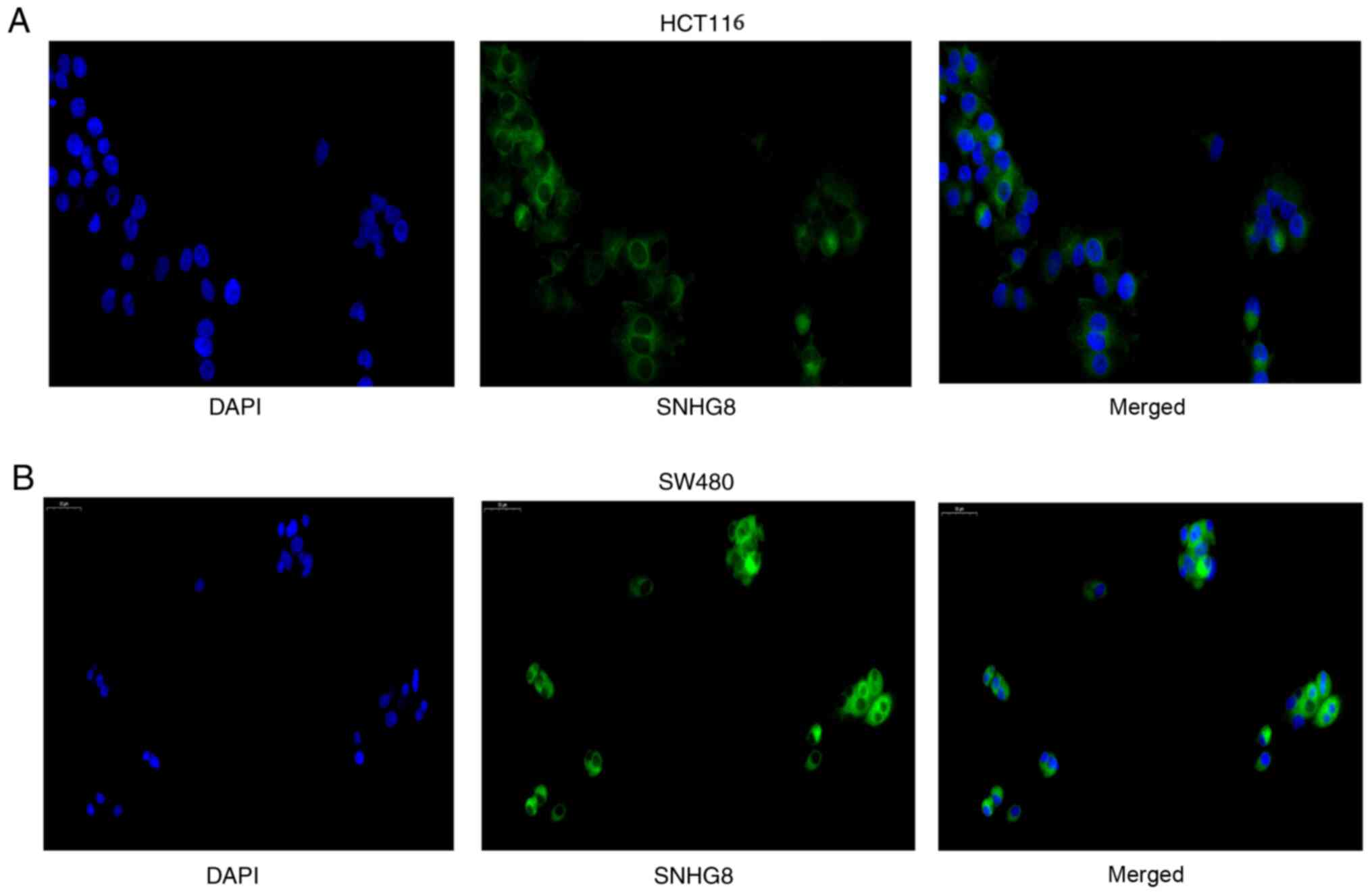
the subcellular distribution of SNHG8 in CRC cells was assessed.
The results indicated that SNHG8 was predominantly located in the
cytoplasm of HCT116 and SW480 cells (Fig. 2A and B).
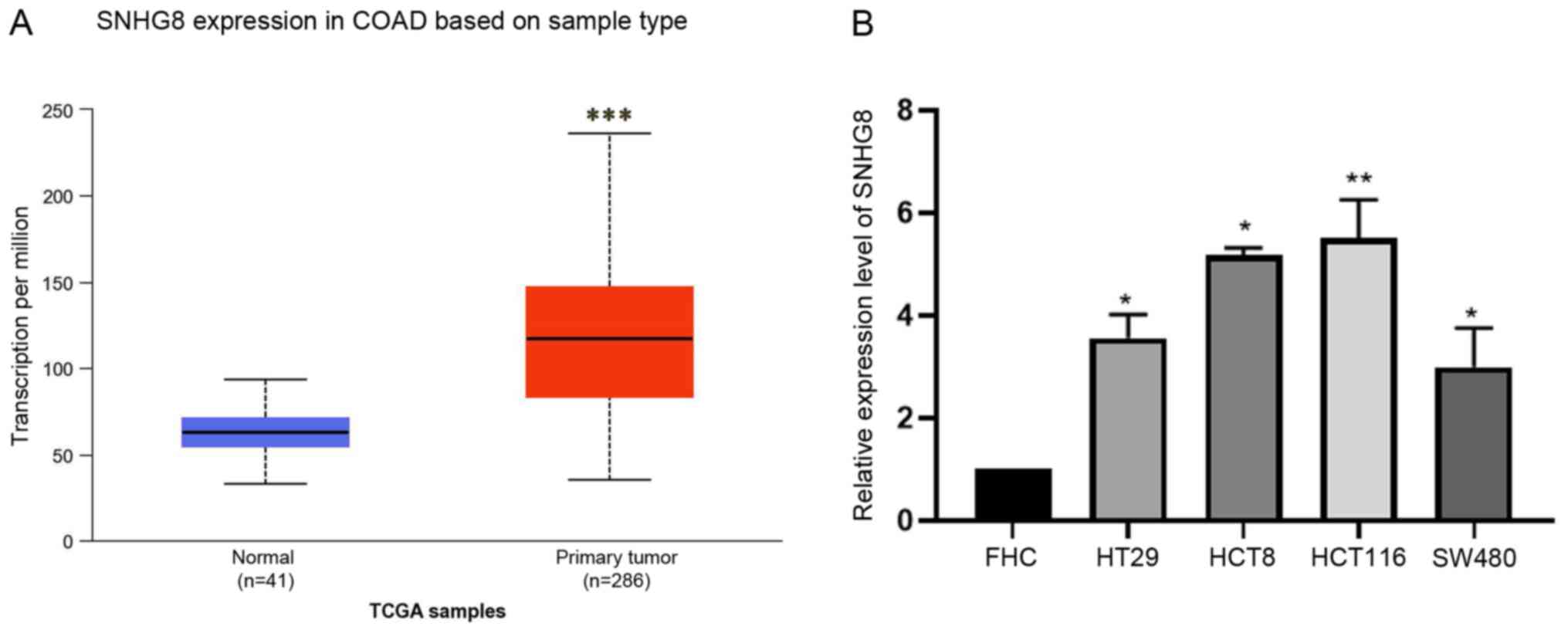 | Figure 1.SNHG8 expression in COAD based on
sample type. (A) lncRNA SNHG8 expression was significantly
upregulated in primary CRC tumor tissues compared with normal
tissues in The Cancer Genome Atlas COAD dataset (41 normal tissues
and 286 primary CRC tumor tissues). (B) Reverse
transcription-quantitative PCR analysis was performed to detect
SNHG8 expression in CRC cell lines, HT29, HCT8, HCT116 and SW480,
and FHC cells were used as the control group. Data are presented as
the mean ± standard deviation (n=3). *P<0.05, **P<0.01,
***P<0.001 vs. control. lncRNA, long non-coding RNA; SNHG8,
small nucleolar RNA host gene 8; CRC, colorectal cancer; COAD,
colon adenocarcinoma. |
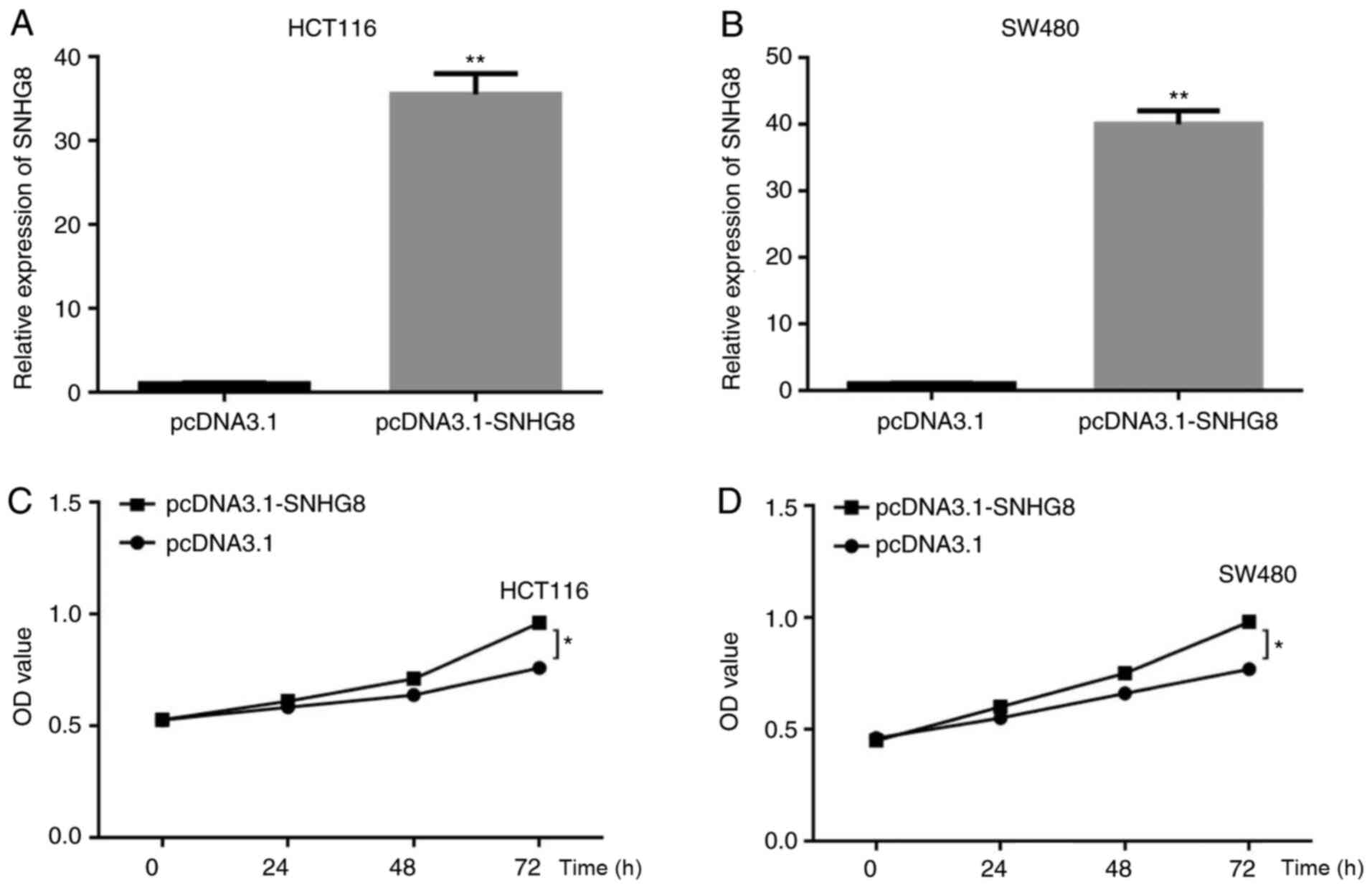
SNHG8 promotes the proliferation of
CRC cells
To determine the effect of SNHG8 on the
proliferation of CRC cells, SNHG8 overexpressing cell lines were
constructed using transfecting plasmids, and the transfection
increased efficiency was 35-fold and 42-fold in HCT116 and SW480
cells, respectively (P<0.01; Fig. 3A
and B). The CCK-8 assay was performed to assess the effect of
SNHG8 on the proliferation of CRC cells. The result demonstrated
that overexpression of SNHG8 increased the proliferation of HCT116
and SW480 cells (P<0.05; Fig. 3C and
D).
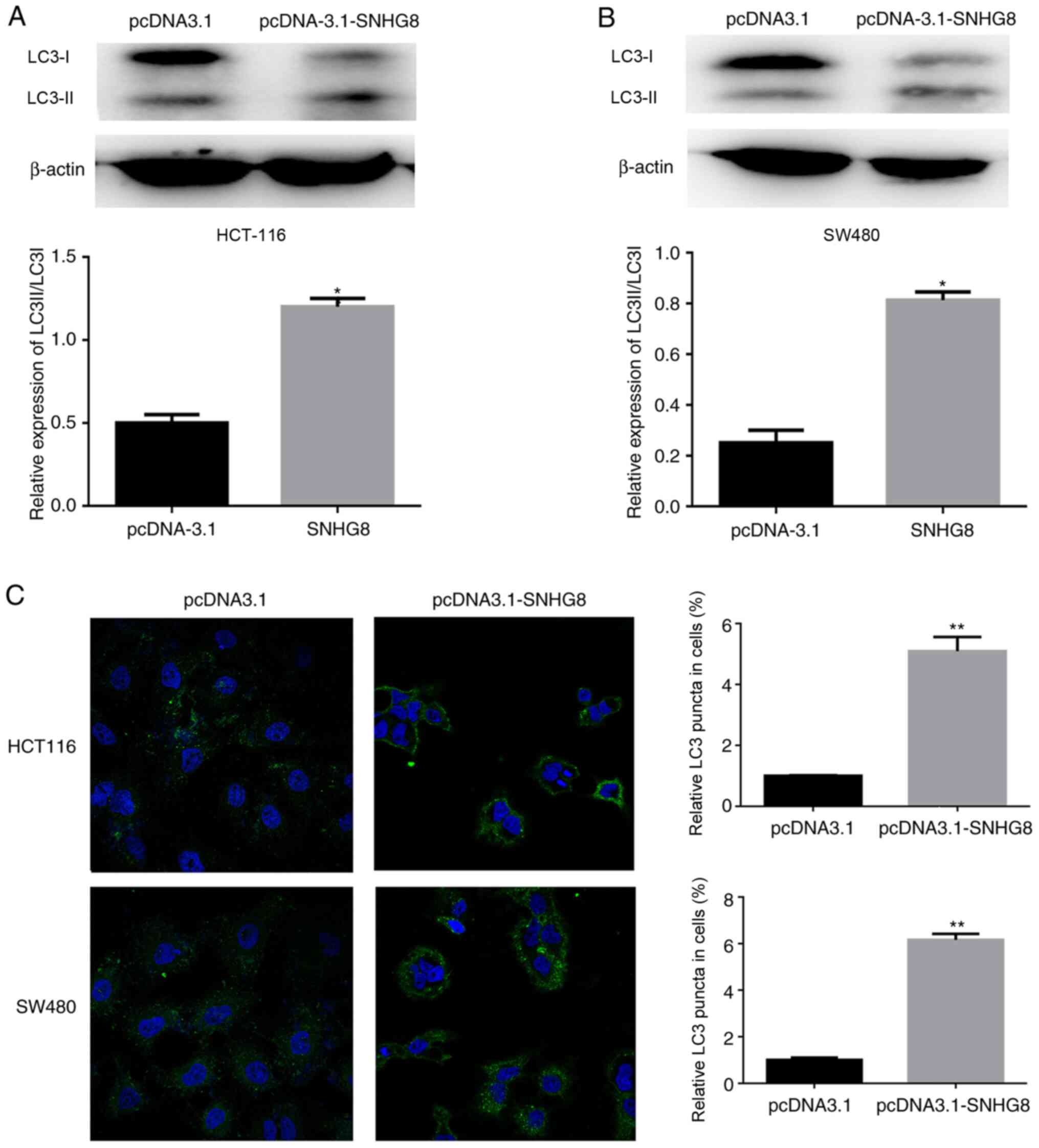
SNHG8 promotes autophagy in CRC
cells
Increasing evidence suggest that autophagy plays a
vital role in tumor progression (23). To investigate the association between
SNHG8 and autophagy, ATGs were detected in the CRC cell lines. The
results demonstrated that overexpression of SNHG8 enhanced the
conversion of LC3-I to LC3-II in HCT116 and SW480 cells and
quantitative analysis was performed, respectively (P<0.05;
Fig. 4A and B). In addition,
immunofluorescence analysis demonstrated that overexpression of
SNHG8 increased LC3 puncta in both HCT116 and SW480 cells
(P<0.01; Fig. 4C). Taken
together, these results suggest that SNHG8 promotes autophagy in
CRC cells.
SNHG8 regulates autophagy by
upregulating ATG7 expression in CRC cells
Previous studies have demonstrated that autophagy is
a dynamic and continuous process that involves ATGs (24,25),
with several ATGs participating in autophagosome formation
(23). To further investigate the
molecular mechanisms, RT-qPCR analysis was performed to detect the
potential targets, including ATG3, ATG5, ATG7, ATG10 and ATG12. The
results demonstrated that overexpression of SNHG8 significantly
upregulated ATG7 expression in HCT116 and SW480 cells (P<0.01;
Fig. 5A). Consistent with these
results, ATG7 protein expression was markedly upregulated following
overexpression of SNHG8 (Fig. 5B and
C). Subsequently, si-ATG7 cell lines were constructed, and
RT-qPCR and western blot analyses were performed to detect ATG7
mRNA and protein expression levels following transfection with
si-ATG7 or si-NC, respectively (HCT116, P<0.001; SW480,
P<0.01; Fig. 5D and E). Specific
siRNA for ATG7 was used to perform the rescue experiments, and
quantitative analysis was performed (P<0.05; Fig. 5F and G). Collectively, these results
suggest that SNHG8 promotes autophagy in CRC cells by upregulating
ATG7 expression.
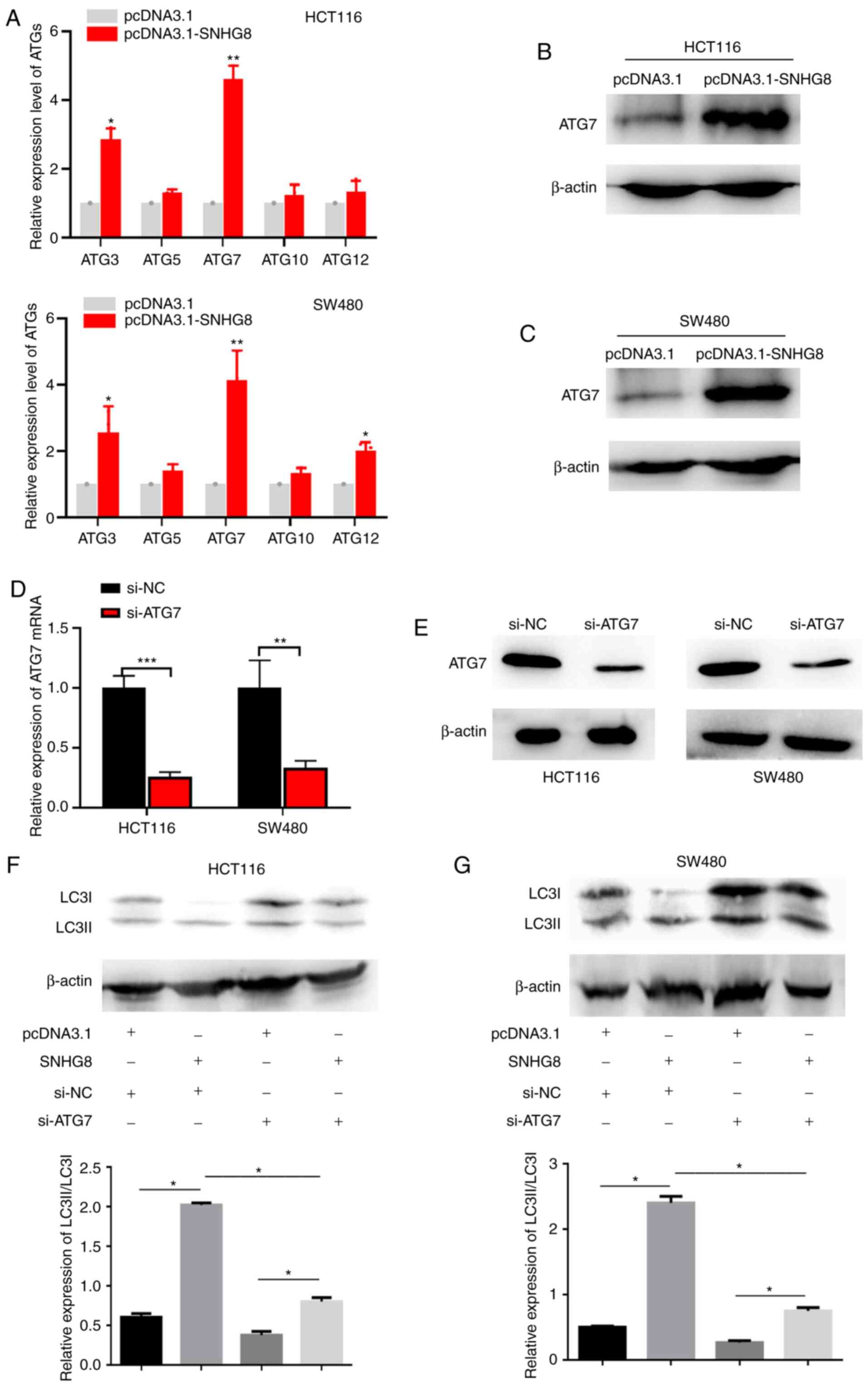 | Figure 5.SNHG8 regulates autophagy by
upregulating ATG7 expression in CRC cells. (A) RT-qPCR analysis was
performed to detect potential regulatory targets, including ATG3,
ATG5, ATG7, ATG10 and ATG12. Western blot analysis was performed to
detect ATG7 protein expression following overexpression of SNHG8 in
(B) HCT116 and (C) SW480 cells. (D) RT-qPCR and (E) western blot
analyses were performed to detect ATG7 mRNA and protein expression
levels following transfection with si-NC and si-ATG7, respectively.
Rescue experiments demonstrated that SNHG8 promoted autophagy by
upregulating ATG7 expression in (F) HCT116 and (G) SW480 cells.
Data are presented as the mean ± standard deviation (n=3).
*P<0.05, **P<0.01, ***P<0.001 vs. control. SNHG8, small
nucleolar RNA host gene 8; ATG, autophagy-related gene; CRC,
colorectal cancer; RT-qPCR, reverse transcription-quantitative PCR;
si, small interfering; NC, negative control; LC3,
microtubule-associated protein 1 light chain 3B. |
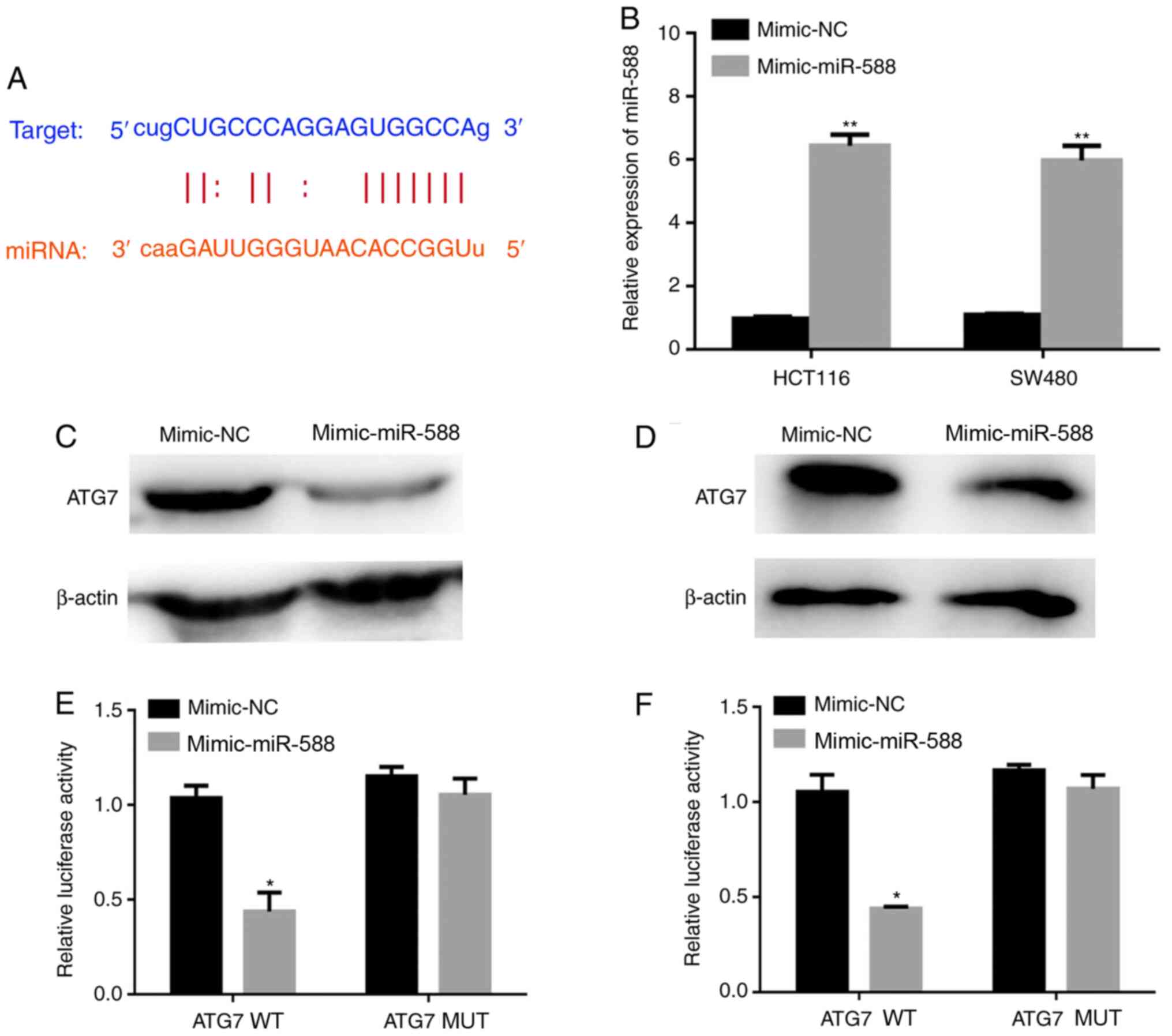
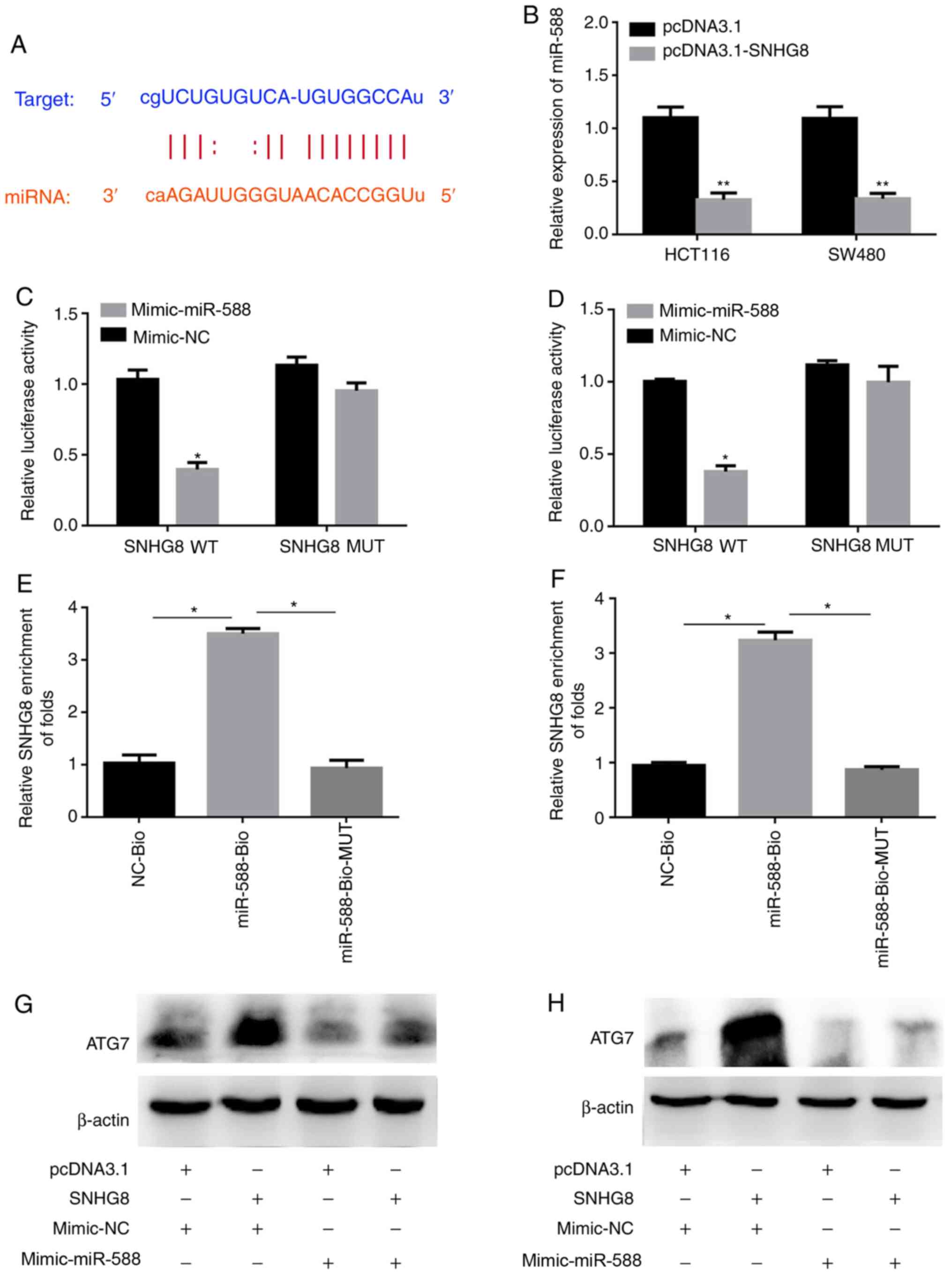
miR-588 inhibits ATG7 expression
lncRNA SNHG8 has been reported to regulate gene
expression as a ceRNA by sponging specific miRNAs (26). To further investigate the molecular
mechanisms underlying SNHG8 in autophagy, the potential binding
sites between ATG7 and miRNAs were analyzed. The TargetScan and
StarBase databases demonstrated that miR-588 may have a potential
binding site with SNHG8 or the 3′-UTR of ATG7 mRNA (Fig. 6A). The present study aimed to
investigate whether miR-588 can inhibit ATG7 expression, and thus
miR-588 overexpressing cell lines were constructed using miR-588
mimics. The transfection efficiency resulted in 6.3-fold and
5.9-fold increases in HCT116 and SW480 cells, respectively
(P<0.01; Fig. 6B). The results
demonstrated that miR-588 significantly inhibited ATG7 expression
(Fig. 6C and D). The dual-luciferase
reporter assay was performed to confirm the binding sites between
miR-588 and ATG7 (P<0.05; Fig. 6E and
F).
SNHG8 mediates ATG7 expression via
miR-588
To further verify the underlying molecular mechanism
of SNHG8 in CRC cells, the present study investigated the molecular
mechanisms of SNHG8 in autophagy and analyzed the potential binding
sites between SNHG8 and miR-588 using the StarBase database
(Fig. 7A). RT-qPCR analysis was
performed to detect miR-588 expression following overexpression of
SNHG8. The results demonstrated that miR-588 expression was
inhibited following overexpression of SNHG8 (P<0.01; Fig. 7B). The dual-luciferase reporter assay
was performed to confirm the association between miR-588 and SNHG8
(P<0.05; Fig. 7C and D). In
addition, the pull-down assay was performed to assess the effect of
miR-588 on SNHG8. The results demonstrated that miR-588 can bind
with SNHG8 (P<0.05; Fig. 7E and
F). Western blot analysis was performed to detect ATG7 protein
expression in cells under different conditions (Fig. 7G and H). The results demonstrated
that overexpression of miR-588 inhibited upregulation of ATG7
expression via SNHG8. Taken together, these results suggest that
SNHG8 regulates the occurrence of autophagy through the regulation
of ATG7 via miR-588.
Discussion
It is estimated that 135,430 new cases of CRC are
diagnosed and 50,260 CRC-associated mortalities are reported per
year in the United States (1,2). Despite
recent advancements in the treatment methods for CRC, the prognosis
for patients with CRC remains unsatisfactory (27). Thus, the identification of novel
biomarkers and therapeutic targets for CRC are urgently
required.
Autophagy is a self-digestion process that
eliminates harmful cargo to protect cells from stress. lncRNAs are
a class of RNA molecules that play important roles in tumor
progression (28). Increasing
evidence suggest that lncRNAs are involved in the tumor cell
biology (29,30). SNHG8 is a novel oncogene that acts as
a target to prevent tumor progression, and some studies have
indicated that SNHG8 induces cell proliferation, invasion and
metastasis in different types of tumors (13,14,31).
Thus, the present study aimed to investigate the molecular
mechanism of SNHG8 in CRC to provide a target for future clinical
treatment.
The present study analyzed SNHG8 expression in CRC
tissues using TCGA dataset. The results demonstrated that SNHG8
expression was abnormally expressed in CRC tissues compared with
normal tissues, suggesting the involvement of SNHG8 in tumor
progression. Previous studies had demonstrated that SNHG8 serves as
an oncogene, whereby its expression is upregulated in several types
of solid tumors, such as breast cancer and osteosarcoma (13,14,31). In
the present study, immunofluorescence and western blot analyses
were performed to determine the molecular mechanisms of SNHG8 in
CRC cells. The results demonstrated that SNHG8 promoted CRC cell
proliferation and autophagy. Recently, it has been demonstrated
that autophagy is a protective process in cells, particularly under
stress; however, the excessive activation of autophagy may result
in cell death (13). Autophagy is a
highly conserved process that exerts a protective mechanism to
promote drug resistance of cells (32). Furthermore, autophagy activation can
promote malignant migratory and invasive abilities (33,34).
However, the role of SNHG8 on CRC cellular autophagy remains
unclear. Thus, the present study aimed to investigate the role of
SNHG8 in autophagy.
In the present study, western blot and
immunofluorescence analyses demonstrated that the level of
autophagy was enhanced following overexpression of SNHG8. Autophagy
is a complex process involving several ATGs, such as ATG3, ATG5,
ATG7, ATG10 and ATG12, which are associated with tumor progression
(35). A previous study demonstrated
that lncRNAs can mediate ATG expression to promote autophagy in
tumors (36). For example, lncRNA
KCNQ1OT1 promotes autophagy via ATG3 in lung cancer (37). In addition, lncRNA MEG3 promotes
autophagy via the miR-21/PTEN axis in nasopharyngeal carcinoma
cells (38). Taken together, these
findings suggest that lncRNAs play important roles in tumor-related
autophagy.
To further investigate the molecular mechanisms of
SNHG8 in autophagy, the expression levels of ATGs were measured,
and ATG7 expression was upregulated in CRC cells following
transfection with the SNHG8 plasmid. Taken together, the results of
the present study demonstrated that ATG7 can be regulated by
SNHG8.
LncRNAs sponge miRNAs to increase mRNA expression
(39). miR-588 is an important miRNA
that has been reported to regulate different types of tumors, such
as breast cancer, and it a well-known prognostic marker (18,19).
However, the association between SNHG8 and miR-588 remains unclear.
To further investigate the potential molecular mechanisms, the
binding sites between SNHG8 and miR-588 were predicted using the
StarBase database. Bioinformatics analysis revealed that SNHG8 is a
target of miR-588, and miR-588 can target ATG7 mRNA. The
dual-luciferase reporter assay was performed to confirm the
association between miR-588 and ATG7 mRNA. The results of the
dual-luciferase reporter and pull-down assays verified the
association between SNHG8 and miR-588. Collectively, these results
suggest that SNHG8 can target miR-588 and ATG7 is a target of
miR-588. To further clarify the regulatory network, a rescue assay
was performed, which proved that SNHG8 promotes autophagy via the
miR-588/ATG7 axis. Taken together, the results of the present study
suggest that SNHG8 can target miR-588 to inhibit its expression,
which enhances ATG7 mRNA expression.
The present study is not without limitations. For
example, only two CRC cell lines were used to prove the generality
of these results and investigate the molecular mechanisms in
vivo. However, the results provided a novel target in the
tumorigenesis of CRC.
In conclusion, the results of the present study
demonstrated that SNHG8 expression was substantially upregulated in
CRC cells and tissues. Furthermore, overexpression of SNHG8
enhanced autophagy in CRC cells via the miR-588/ATG7 axis. Notably,
the present study identified a target of autophagy, which may
provide a novel therapeutic target for CRC and a promising
autophagy-related therapeutic method.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CH, YF and YC designed the present study. XL revised
the manuscript for important intellectual content and provided
final approval of the version to be published. CH performed the
experiments and drafted the initial manuscript. XL and CH analyzed
and interpreted the data, and confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
ATGs
|
autophagy-related genes
|
|
LC3
|
microtubule-associated protein 1 light
chain 3B
|
|
lncRNA
|
long non-coding RNA
|
|
SNHG8
|
small nucleolar RNA host gene 8
|
|
NC
|
negative control
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Liu A and Liu L: Long non-coding RNA
ZEB2-AS1 promotes proliferation and inhibits apoptosis of colon
cancer cells via miR-143/bcl-2 axis. Am J Transl Res. 11:5240–5248.
2019.PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Zhou Y, Li Y, Yang L, Ma Y, Peng X,
Yang S, Liu J and Li H: Autophagy: A novel mechanism of
chemoresistance in cancers. Biomed Pharmacother. 119:1094152019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li C, Lei B, Huang S, Zheng M, Liu Z, Li Z
and Deng Y: H19 derived microRNA-675 regulates cell proliferation
and migration through CDK6 in glioma. Am J Transl Res. 7:1747–1764.
2015.PubMed/NCBI
|
|
6
|
Liu J, Tian W, Zhang W, Jia Y, Yang X,
Wang Y and Zhang J: MicroRNA-142-3p/MALAT1 inhibits lung cancer
progression through repressing β-catenin expression. Biomed
Pharmacother. 114:1088472019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amaravadi R, Kimmelman AC and White E:
Recent insights into the function of autophagy in cancer. Genes
Dev. 30:1913–1930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma H, Yuan L, Li W, Xu K and Yang L: The
lncRNA H19/miR-193a-3p axis modifies the radio-resistance and
chemotherapeutic tolerance of hepatocellular carcinoma cells by
targeting PSEN1. J Cell Biochem. 119:8325–8335. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang B, Bao N, He G and Wang J: Long
noncoding RNA HOTAIR regulates autophagy via the miR-20b-5p/ATG7
axis in hepatic ischemia/reperfusion injury. Gene. 686:56–62. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Si Y, Yang Z, Ge Q, Yu L, Yao M, Sun X,
Ren Z and Ding C: Long non-coding RNA Malat1 activated autophagy,
hence promoting cell proliferation and inhibiting apoptosis by
sponging miR-101 in colorectal cancer. Cell Mol Biol Lett.
24:502019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang P, Li S, Chen Z, Lu Y and Zhang H:
lncRNA SNHG8 promotes proliferation and invasion of gastric cancer
cells by targeting the miR-491/PDGFRA axis. Hum Cell. 33:123–130.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan D, Qiu B, Yang XJ, Tang HL, Peng SJ,
Yang P, Dong YM, Yang L, Bao GQ and Zhao HD: lncRNA SNHG8 promotes
cell migration and invasion in breast cancer cell through
miR-634/ZBTB20 axis. Eur Rev Med Pharmacol Sci. 24:11639–11649.
2020.PubMed/NCBI
|
|
14
|
Miao W, Lu T, Liu X, Yin W and Zhang H:
lncRNA SNHG8 induces ovarian carcinoma cells cellular process and
stemness through Wnt/β-catenin pathway. Cancer Biomark. 28:459–471.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ying SY, Chang DC and Lin SL: The microRNA
(miRNA): Overview of the RNA genes that modulate gene function. Mol
Biotechnol. 38:257–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
17
|
Qian L, Lin L, Du Y, Hao X, Zhao Y and Liu
X: MicroRNA-588 suppresses tumor cell migration and invasion by
targeting GRN in lung squamous cell carcinoma. Mol Med Rep.
14:3021–3028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu M, Zhang X, Li H, Zhang P and Dong W:
MicroRNA-588 is downregulated and may have prognostic and
functional roles in human breast cancer. Med Sci Monit.
23:5690–5696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kohlhapp FJ, Mitra AK, Lengyel E and Peter
ME: MicroRNAs as mediators and communicators between cancer cells
and the tumor microenvironment. Oncogene. 34:5857–5868. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masters JR, Thomson JA, Daly-Burns B, Reid
YA, Dirks WG, Packer P, Toji LH, Ohno T, Tanabe H, Arlett CF, et
al: Short tandem repeat profiling provides an international
reference standard for human cell lines. Proc Natl Acad Sci USA.
98:8012–8017. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li HR, Shagisultanova EI, Yamashita K,
Piao Z, Perucho M and Malkhosyan SR: Hypersensitivity of tumor cell
lines with microsatellite instability to DNA double strand break
producing chemotherapeutic agent bleomycin. Cancer Res.
64:4760–4767. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mukhopadhyay S, Biancur DE, Parker SJ,
Yamamoto K, Banh RS, Paulo JA, Mancias JD and Kimmelman AC:
Autophagy is required for proper cysteine homeostasis in pancreatic
cancer through regulation of SLC7A11. Proc Natl Acad Sci USA.
118:e20214751182021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu JL, Yuan L, Tang YC, Xu ZY, Xu HD,
Cheng XD and Qin JJ: The role of autophagy in gastric cancer
chemoresistance: Friend or foe? Front Cell Dev Biol. 8:6214282020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yun CW, Jeon J, Go G, Lee JH and Lee SH:
The dual role of autophagy in cancer development and a therapeutic
strategy for cancer by targeting autophagy. Int J Mol Sci.
22:1792020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi Z, Zhang H, Jie S, Yang X, Huang Q,
Mao Y and Zhang Y: Long non-coding RNA SNHG8 promotes prostate
cancer progression through repressing miR-384 and up-regulating
HOXB7. J Gene Med. e33092021.PubMed/NCBI
|
|
27
|
Yu X, Zhu L, Liu J, Xie M, Chen J and Li
J: Emerging role of immunotherapy for colorectal cancer with liver
metastasis. Onco Targets Ther. 13:11645–11658. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin KT, Tao XH, Fan YB and Wang SB:
Crosstalk between oncolytic viruses and autophagy in cancer
therapy. Biomed Pharmacother. 134:1109322021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao
SD, Zhang Y, Yang L, Shan W, He Q, et al: Comprehensive genomic
characterization of long Non-coding RNAs across human cancers.
Cancer Cell. 28:529–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li CF, Li YC, Wang Y and Sun LB: The
effect of lncRNA H19/miR-194-5p axis on the epithelial-mesenchymal
transition of colorectal adenocarcinoma. Cell Physiol Biochem.
50:196–213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu X, Li Y, Wang L, Yuan N, Ma M and Chen
Y: lncRNA SNHG8 accelerates proliferation and inhibits apoptosis in
HPV-induced cervical cancer through recruiting EZH2 to
epigenetically silence RECK expression. J Cell Biochem.
121:4120–4129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dikic I and Elazar Z: Mechanism and
medical implications of mammalian autophagy. Nat Rev Mol Cell Biol.
19:349–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kroemer G: Autophagy: A druggable process
that is deregulated in aging and human disease. J Clin Invest.
125:1–4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo C, Peng X, Song L, Ying M, Wu Y, Chang
R, Li J, Feng D, Zhan L and Zhan X: Autophagy promotes malignant
migration and invasion via miR-224-5p/BCL2 in pancreatic mucinous
cystadenocarcinoma MCC1 cells. Oncol Lett. 20:2762020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao QH, Liu F, Yang ZL, Fu XH, Yang ZH,
Liu Q, Wang L, Wan XB and Fan XJ: Prognostic value of autophagy
related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10,
ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am J Transl Res.
8:3831–3847. 2016.PubMed/NCBI
|
|
36
|
Yang L, Peng X, Jin H and Liu J: Long
non-coding RNA PVT1 promotes autophagy as ceRNA to target ATG3 by
sponging microRNA-365 in hepatocellular carcinoma. Gene.
697:94–102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang Y, Jia Y, Wang Q, Zhao Q, Song M, Ni
R and Wang J: Long noncoding RNA KCNQ1OT1 promotes the progression
of non-small cell lung cancer via regulating miR-204-5p/ATG3 axis.
Onco Targets Ther. 12:10787–10797. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin L, Liu X and Lv B: Long non-coding RNA
MEG3 promotes autophagy and apoptosis of nasopharyngeal carcinoma
cells via PTEN up-regulation by binding to microRNA-21. J Cell Mol
Med. 25:61–72. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li XM, Jiao YY, Luan BH, Wu HX, Wang RR
and Zhong J: Long non-coding RNA MIAT promotes gastric cancer
proliferation and metastasis via modulating the miR-331-3p/RAB5B
pathway. Oncol Lett. 20:3552020. View Article : Google Scholar : PubMed/NCBI
|