Introduction
Colon cancer (CC) is one of the most common
malignancies of the digestive system, with a high incidence
worldwide (1). According to
statistics, the incidence of CC worldwide is approximately 6.1%,
and the incidence rate in North America, Australia and other
regions is significantly higher than other countries (2). Moreover, in recent years, with the
improvement of living standards and changes in dietary structure,
the incidence of CC has been on the rise (3). CC tends to occur at the junction of
rectum and rectum sigmoid colon, and its male patients are
significantly more prominent than female patients (4). CC, not only has a high incidence, but
also poses a great threat to human health (1). Previous findings have shown that in
2012, 1.4 million new CC cases resulted in almost 700,000 deaths
(5). One of the main reasons for the
poor prognosis of CCC patients is that it is difficult to conduct
early screening in clinic, and there is no obvious special clinical
disease in the early stage of CC. Therefore, patients are often
deprived of the early optimal treatment period due to the lack of
medical and health knowledge, and CC has developed to the middle
and late stage when diagnosed (6).
At this time, the tumor is usually accompanied by metastasis and
invasion, and the commonly used clinical treatment scheme (surgery
or combined chemoradiotherapy) has generally failed to achieve the
optimal effect of tumor lesion removal (7). In the face of the increasingly serious
challenges brought by CC to the clinic, finding an effective,
convenient and accurate tumor marker is a hot and difficult point
in the research of CC.
With the deepening of research, it has been
suggested that the occurrence of tumor diseases is closely related
to microRNAs (8–10). MicroRNAs are conservative non-coding
RNAs that regulate gene expression at the post-transcriptional
level and have certain effects on regulating cell proliferation,
apoptosis and differentiation (11).
MicroRNA-135a (miR-135a) is a member of the miR-135 family
discovered in recent years, located at chromosome 3p21.1.
Currently, miR-135a has been confirmed to have abnormal expression
in prostate and pancreatic cancer (12,13). A
study suggested that lncRNA FOXD3-AS1 affects the development of CC
via regulating miR-135a (14), which
indicates that miR-135a is also correlated with the occurrence of
CC. However, there are few studies on the role of miR-135a in CC.
We speculated that miR-135a may be the key to the potential
diagnosis and treatment of future CC. However, the detection of
microRNA alone may have low specificity, which requires the
combination of other detection indicators to improve its
application value. Matrix metalloproteinase-13 (MMP-13), as a
member of MMPs family, has an important role in degrading and
remodeling the dynamic balance of extracellular matrix, and is
involved in the occurrence of numerous diseases (15). In addition, it was found that MMP-13
has a certain influence on the occurrence of stomach cancer
(16). Consequently, MMP-13 may also
have some potential clinical significance in CC. Moreover, with
advances in research, MMP-13 has been confirmed to play an
important role in colorectal cancer with liver metastasis (17). Rath et al (18) also suggested that MMP-13 has the
potential to be a marker of intestinal disease in the future. CC is
a malignant tumor with extremely high morbidity and mortality in
the clinic. Finding an effective blood marker can, not only
effectively improve the diagnosis rate of early CC, but also assist
clinical judgment of the patient's disease development, and timely
and effective intervention measures can be carried out to assess
the prognosis of patients by evaluating changes in markers, which
is of great significance for the clinical treatment and prognosis
judgment of CC patients. Therefore, by analyzing the diagnostic and
therapeutic significance of miR-135a and MMP-13 on CC, this study
aims to provide a new reference for the clinical diagnosis and
treatment of CC.
Materials and methods
General data
A total of 117 CCC patients admitted to Sheng Li Oil
Field Central Hospital from May 2015 to May 2017 and 120 normal
physical examination subjects were selected for prospective
analysis. Of these, CC patients were taken as the research group
(RG) and healthy physical subjects were taken as the control group
(CG).
This study was approved by the ethics committee of
Sheng Li Oil Field Central Hospital, and all the subjects mentioned
above signed informed consent.
Inclusion and exclusion criteria. Inclusion criteria
for the study were: Patients whose symptoms met the clinical
manifestations of CC and was diagnosed as CC after biopsy by the
pathology department of our hospital, patients received follow-up
treatment in our hospital after diagnosis, patients aged 30–70
years, patients with complete case data, patients who agreed to
co-operate with the investigation of our hospital, patients without
adjuvant treatment prior to admission, patients treated with
radical resection after admission, and the patients who could not
achieve complete resection were treated with postoperative
chemotherapy. Exclusion criteria were: Patients complicated with
other tumors, cardio-cerebral vascular disease, chronic diseases,
mental diseases or autoimmune diseases, organ failure, hepatic and
renal insufficiency, or drug allergy, patients with long-term
physical disability or bedridden and unable to take care of
themselves, patients transferred to other hospitals, patients who
died during treatment. Inclusion and exclusion criteria of the CG:
Subjects without disease, subjects whose examinations were normal
according to physical examination results, subjects aged 30–70
years. All the subjects agreed to participate in the investigation
of our hospital and provided informed consent.
Materials and methods
Treatment methods
All the patients in the RG received tumor removal
surgery (or postoperative chemotherapy) in our hospital. The
surgery was completed by senior digestive surgeons. Postoperative
chemotherapy regimen: Based on 5-FU, tetrahydrofolate was used as a
regulator to enhance the efficacy of 5-FU.
Enzyme linked immunosorbent assay
(ELISA) determination
Fasting venous blood (4 ml) was extracted from
subjects in the RG (before and after treatment) and the CG, placed
at room temperature for 30 min and then centrifuged for 10 min
(2,504 × g, 4°C) to obtain the serum. The concentration of MMP-13
in serum was determined by ELISA. The kit was purchased from
Shanghai Jingkang Biotechnology Co., Ltd., Jk-(a)-5224. The
operation was carried out in a sterile environment in strict
accordance with the kit instructions.
Electrochemiluminescence immunoassay
(ECLI) determination
Fasting venous blood (3 ml) was extracted from
subjects in the RG (before and after treatment) and the CG, placed
at room temperature for 30 min and then centrifuged for 10 min
(2,504 × g, 4°C) to obtain the serum. ECLI was applied to determine
the concentration of tumor marker CEA in serum. The kit was
purchased from Shanghai Yaji Biotechnology Co., Ltd.: No. CL01236.
The assay was completed by the laboratory department of our
hospital.
PCR determination. Fasting venous blood (4 ml) was
extracted from subjects in the RG (before and after treatment) and
the CG, placed at room temperature for 30 min and then centrifuged
for 10 min (2,504 × g, 4°C) to obtain the serum. EasyPure miRNA Kit
(Beijing TransGen Biotech Co., Ltd.: No. ER601-01) was used for
total RNA extraction. The purity of serum extracted RNA was
determined by UV spectrophotometer and agarose gel electrophoresis.
TransScript Green miRNA Two-Step RT-qPCR SuperMix (Beijing TransGen
Co., Ltd.: No. AQ202-01) was used for reverse transcription of the
extracted total RNA. The steps were carried out according to the
kit instructions, and cDNA was collected for PCR amplification.
Primer sequences are shown in Table
I. qPCR amplification system was as follows: cDNA 1 µl, sense
primer 0.4 µl, reverse primer 0.4 µl, 2X TransTaq® Tip
Green qPCR SuperMix 10 µl, Passive Reference Dye (50X) 0.4 µl, and
ddH2O was added to complement to 20 microns, qPCR
amplification conditions were as follows: Pre-denaturation at 94°C
for 30 sec, denaturation at 94°C for 5 sec, annealing and extension
at 60°C for 30 sec, for a total of 40 cycles. Each sample was set
with 3 duplicate holes, and the experiment was carried out 3 times.
In this study, U6 was taken as the internal parameter and
2−ΔΔCq was used to analyze the data (19). ELISA was applied to detect the
concentration of MMP-13 in serum. The kit was purchased from
Shanghai Jingkang Bioengineering Co., Ltd., JK-(a)-5224. The
operation process was conducted in a sterile environment in strict
accordance with the kit instructions. CEA concentration of tumor
markers in serum was detected by electrochemical
immunoluminescence. The kit was purchased from Shanghai Yaji
Biotechnology Co., Ltd., CL01236, and the detection was completed
by the clinical laboratory of our hospital.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
|
| Upstream (5′-3′) | Downstream
(5′-3′) |
|---|
| miR-135a |
ACACTCCAGCTGGGTATGGCTTTTTATTCCT |
GGTGTCGTGGAGTCGGCAA |
| U6 |
GCTCTGTCACCAACACTCACT |
GCTGCCTTTCTTGTGTCGTT |
Patient follow-up
Patients in the RG were followed up for 3 years.
Patient prognosis and 3-year survival were recorded in the form of
hospital review.
Observational indicators
Main indicators were miR-135a and MMP-13 in the RG
and CG before and after treatment. The diagnostic value of miR-135a
and MMP-13 for CC was assessed. Secondary indicators included the
correlation of miR-135a and MMP-13 with the pathological features
of CC. The correlation of miR-135a and MMP-13 with CEA and the
effect of miR-135a and MMP-13 on the prognosis of CC patients were
investigated.
Statistical analysis
The data results were processed by SPSS 22.0
statistical software, and the data results were graphically drawn
by Graphpad8. The enumeration data were expressed by (rate), the
comparison in groups was performed by the Chi-square test. The
measurement data are expressed by mean ± standard deviation. The
data conforming to the normal distribution were qualified by the
independent sample t-test, the data conforming to the non-normal
distribution were qualified by the rank sum test. Paired t-test was
used for comparison before and after treatment. The comparison
between groups was analyzed by univariate analysis of variance and
the LSD back testing. The correlation was analyzed by Pearson's
correlation coefficient. The diagnosis was analyzed by ROC curve.
Binary Logistic Regression Analysis was used to calculate the
combined formula and then conduct ROC curve analysis. Survival rate
was calculated using the Kaplan-Meier method, and was compared
using the log-rank test. P<0.05 was considered statistically
significant.
Results
Comparison of general data
There was no difference in age, BMI, sex, smoking,
drinking, exercise habits, place of residence and ethnicity between
the two groups (P>0.05) as is evident in Table II.
 | Table II.Comparison of clinical data of
patients in the two groups [n (%)]. |
Table II.
Comparison of clinical data of
patients in the two groups [n (%)].
|
| Research group
(n=117) | Control group
(n=120) | t/χ2 | P-value |
|---|
| Age (years) |
54.3±8.2 |
55.1±7.6 | 0.437 | 0.779 |
| BMI
(KG/cm2) | 24.62±2.84 | 24.78±3.38 | 0.694 | 0.394 |
| Sex |
|
| 1.133 | 0.287 |
| Male | 78
(66.67) | 72
(60.00) |
|
|
|
Female | 39
(33.33) | 48
(40.00) |
|
|
| Smoking |
|
| 0.318 | 0.573 |
|
Yes | 64
(54.70) | 70
(58.33) |
|
|
| No | 53
(45.30) | 50
(41.67) |
|
|
| Drinking |
|
| 1.193 | 0.275 |
|
Yes | 81
(69.23) | 75
(62.50) |
|
|
| No | 36
(30.77) | 45
(37.50) |
|
|
| Exercise habit |
|
| 0.830 | 0.362 |
|
With | 42
(35.90) | 50
(41.67) |
|
|
|
Without | 75
(64.10) | 70
(58.33) |
|
|
| Place of
residence |
|
| 1.537 | 0.215 |
|
Cities | 92
(78.63) | 86
(71.67) |
|
|
|
Countryside | 25
(21.37) | 34
(28.33) |
|
|
| Nationality |
|
| 1.404 | 1.185 |
| Han
Chinese | 112 (95.73) | 118 (98.33) |
|
|
|
Minority | 5
(4.27) | 2
(1.67) |
|
|
| Treatment mode |
|
|
|
|
|
Curative resection | 48
(41.03) |
|
|
|
|
Adjuvant or palliative
chemotherapy | 69
(58.97) |
|
|
|
Comparison of miR-135a and MMP-13 in
the two groups
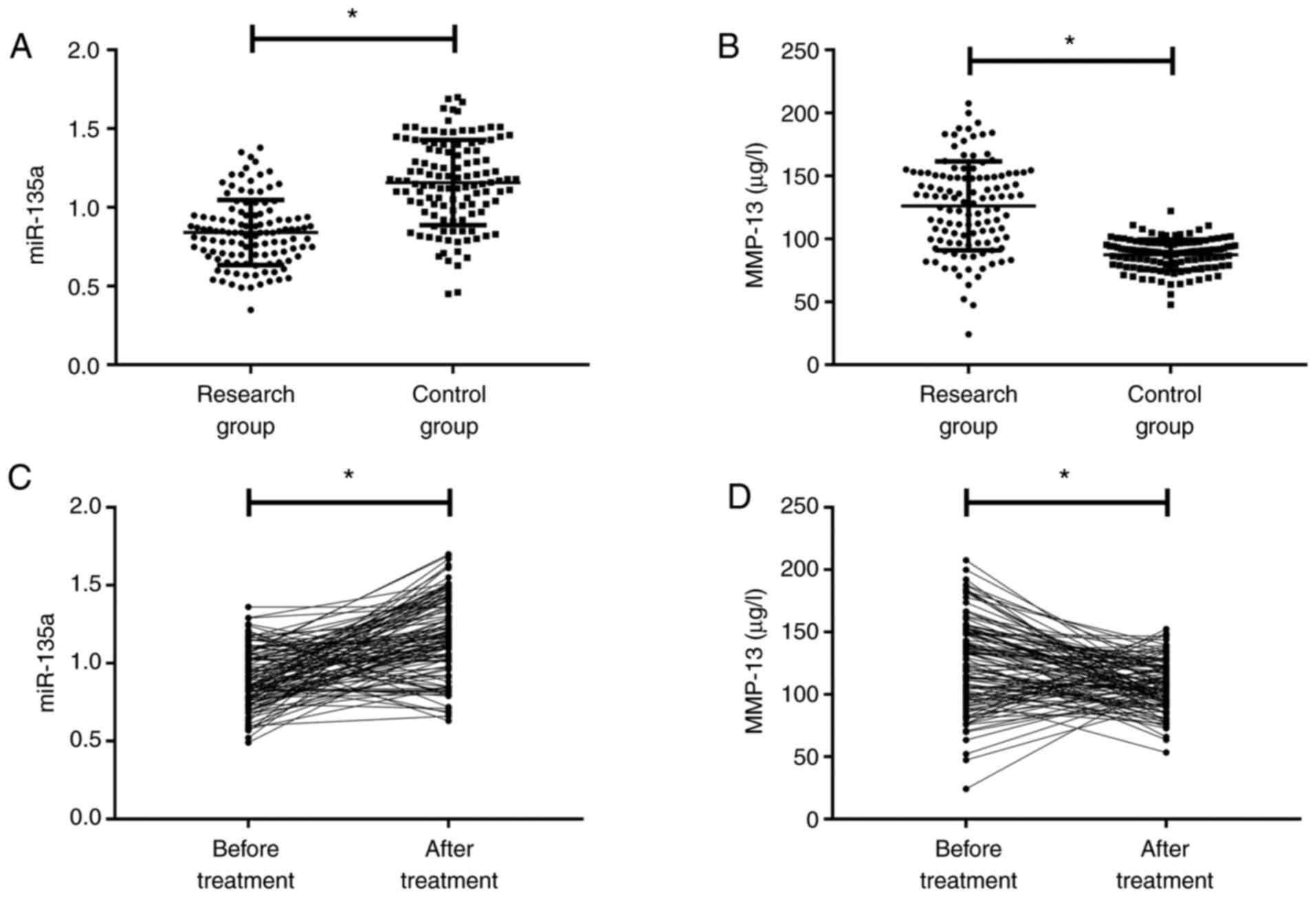
Prior to treatment, miR-135a in the RG was lower
than that in the CG, and MMP-13 was higher than that in the CG
(P<0.050, Fig. 1A and B). After
treatment, miR-135a in the RG was increased compared with that
before treatment, while MMP-13 was decreased (P<0.050, Fig. 1C and D).
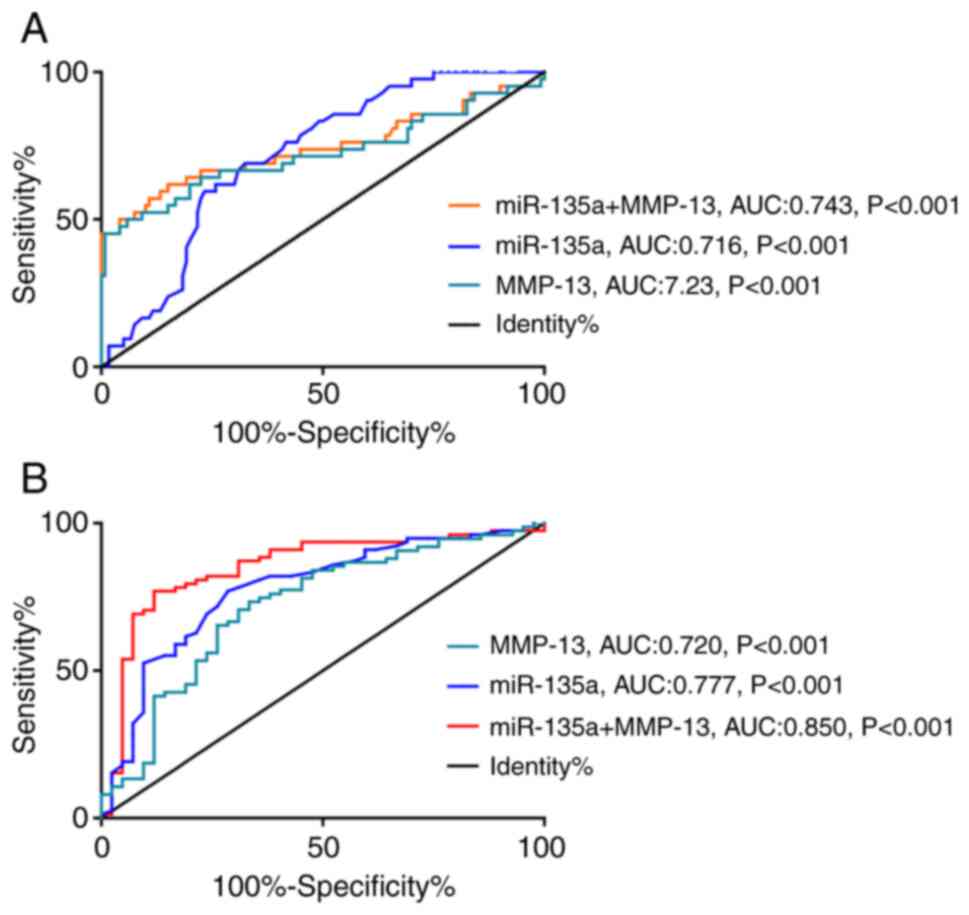
Diagnostic value of miR-135a and
MMP-13 for CC
Among hospital CCC patients, 42 cases were in
clinical stage I or II. ROC curve analysis revealed that the
diagnostic sensitivity and specificity of miR-135a for early CC
were 69.05 and 67.50%, respectively, and the diagnostic sensitivity
and specificity of MMP-13 for early CC were 45.24 and 99.17%,
respectively. Binary logistic regression analysis with miR-135a and
MMP-13 as two independent variables was performed to obtain the
joint detection model Log (P)=1.799+ −0.043 × miR-135a + 3.254 ×
MMP-13, when taking a cut-off value of 0.703, the model had a
diagnostic sensitivity of 61.90% for CC, with a specific of 85.00%
(Fig. 2A, Table III, P<0.001). Then, we drew the
ROC curve based on the detection results of miR-135a and MMP-13 of
CCC patients in stage I and II prior to treatment. In addition,
miR-135a had a sensitivity of 76.92% and a specificity of 71.43%
for predicting CC development into the late stages of CC, and
MMP-13 had a sensitivity of 73.33% and a specificity of 66.67% for
predicting CC development into the late stage. As above, the Log
(P) of miR-135a combined with MMP-13 formula model=27.545 + −0.081
× miR-135a + −19.548 × MMP-13 showed a sensitivity of 76.92% and a
specificity of 88.10% in predicting the development of CC in the
middle and later stages (Fig. 2B and
Table IV, P<0.001).
 | Table III.The diagnostic value of miR-135a and
MMP-13 for CC. |
Table III.
The diagnostic value of miR-135a and
MMP-13 for CC.
| Item | miR-135a | MMP-13 | Joint
detection |
|---|
| AUC | 0.716 | 0.723 | 0.743 |
| Standard error | 0.04142 | 0.05454 | 0.05342 |
| 95% CI | 0.6355 to
0.7979 | 0.6161 to
0.8299 | 0.6383 to
0.8477 |
| Cut-off | <1.045 | >111.000 | <0.703 |
| Sensitivity
(%) | 69.05 | 45.24 | 61.90 |
| Specificity
(%) | 67.50 | 99.17 | 85.00 |
| P-value | <0.001 | <0.001 | <0.001 |
 | Table IV.miR-135a and MMP-13 in the middle and
late stage of CC development. |
Table IV.
miR-135a and MMP-13 in the middle and
late stage of CC development.
| Item | miR-135a | MMP-13 | Joint
detection |
|---|
| AUC | 0.777 | 0.720 | 0.850 |
| Standard error | 0.04531 | 0.05061 | 0.03917 |
| 95% CI | 0.6884 to
0.866 | 0.6208 to
0.8192 | 0.7737 to
0.9272 |
| Cut-off | <0.865 | >117.00 | >0.713 |
| Sensitivity
(%) | 76.92 | 73.33 | 76.92 |
| Specificity
(%) | 71.43 | 66.67 | 88.10 |
| P-value | <0.001 | <0.001 | <0.001 |
The correlation of miR-135a and MMP-13
with the pathological features of CC before treatment
miR-135a was not related to patient age, BMI, sex or
intestinal inflammation history (P>0.05), but was closely
related to T stage, N stage, clinical stage, tumor type, tissue
type, lymphatic metastasis and differentiation degree (P<0.05)
(Table V). MMP-13 was not related to
the patient age, BMI or gender (P>0.05), but was closely related
to the intestinal inflammation history, T stage, N stage, clinical
stage, tumor type, tissue type, lymphatic metastasis,
differentiation degree, and invasion (P<0.05) (Table V).
 | Table V.Relationship of miR-135a and MMP-13
with the pathological features of CC. |
Table V.
Relationship of miR-135a and MMP-13
with the pathological features of CC.
| Item | n | miR-135a | t/F | P-value | MMP-13 (µg/l) | t/F | P-value |
|---|
| Age (years) |
|
| 0.365 | 0.716 |
| 0.645 | 0.520 |
|
<54.3 | 49 | 0.84±0.25 |
|
| 125.98±27.38 |
|
|
|
≥54.3 | 68 | 0.82±0.32 |
|
| 122.12±34.85 |
|
|
| BMI
(KG/cm2) |
|
| 0.181 | 0.857 |
| 1.208 | 0.230 |
|
<24.62 | 34 | 0.81±0.22 |
|
| 135.83±35.54 |
|
|
|
≥24.62 | 83 | 0.80±0.29 |
|
| 127.13±35.30 |
|
|
| Sex |
|
| 0.192 | 0.849 |
| 0.199 | 0.843 |
|
Male | 78 | 0.81±0.29 |
|
| 125.41±38.27 |
|
|
|
Female | 39 | 0.80±0.21 |
|
| 126.77±26.56 |
|
|
| Intestinal
inflammation history (have suffered from colitis or proctitis) |
|
| 0.433 | 0.666 |
| 5.476 | <0.001 |
|
With | 65 | 0.83±0.22 |
|
| 149.62±28.97 |
|
|
|
Without | 52 | 0.81±0.28 |
|
| 121.16±26.58 |
|
|
| T stage |
|
| 15.140 | <0.001 |
| 8.504 | <0.001 |
|
T1+T2 | 49 | 1.04±0.18 |
|
| 118.62±24.62 |
|
|
|
T3+T4 | 68 | 0.62±0.12 |
|
| 156.21±22.82 |
|
|
| N stage |
|
| 4.288 | 0.001 |
| 8.374 | <0.001 |
| N0 | 32 | 1.08±0.25 |
|
| 115.42±25.62 |
|
|
|
N1+N2+N3 | 85 | 0.68±0.16 |
|
| 153.80±20.63 |
|
|
| Clinical stage |
|
| 64.390 | <0.001 |
| 30.880 | <0.001 |
|
I–II | 42 | 1.02±0.16 |
|
| 113.32±28.62 |
|
|
|
III | 49 | 0.79±0.20 |
|
| 142.96±35.83 |
|
|
| IV | 26 | 0.54±0.12 |
|
| 172.62±21.52 |
|
|
| Tumor type |
|
| 34.080 | <0.001 |
| 5.300 | 0.002 |
| Protrud
type of polyps | 14 | 1.13±0.22 |
|
| 118.62±21.52 |
|
|
| Flat
protrud type | 16 | 1.10±0.24 |
|
| 121.42±20.52 |
|
|
| Flat
protrude with ulcer type | 12 | 1.11±0.14 |
|
| 128.62±18.62 |
|
|
| Mass
type | 31 |
0.61±0.24a–c |
|
|
136.24±26.32a–c |
|
|
|
Ulcerative type | 28 |
0.63±0.16a–c |
|
|
146.16±25.62a–c |
|
|
|
Infiltrating type | 16 |
0.51±0.21a–d |
|
|
153.16±28.16a–c |
|
|
| Pattern of
organization |
|
| 29.170 | <0.001 |
| 5.262 | 0.007 |
|
Adenocarcinoma | 62 | 0.96±0.21 |
|
| 124.62±24.16 |
|
|
|
Mucinous carcinoma | 36 |
0.95±0.16e |
|
|
138.62±28.41e |
|
|
|
Undifferentiated
carcinoma | 19 |
0.64±0.25e |
|
|
142.62±26.87e |
|
|
| Lymph node
metastasis |
|
| 13.240 | <0.001 |
| 4.150 | <0.001 |
|
With | 26 | 0.58±0.25 |
|
| 152.13±25.60 |
|
|
|
Without | 91 | 1.12±0.16 |
|
| 122.41±33.81 |
|
|
| Grade of
Differentiation |
|
| 8.444 | <0.001 |
| 4.620 | <0.001 |
| Middle,
high | 85 | 0.94±0.22 |
|
| 118.37±32.14 |
|
|
|
Low | 32 | 0.58±0.16 |
|
| 148.62±29.96 |
|
|
| Invasion |
|
| 6.058 | 0.001 |
| 5.474 | 0.001 |
|
Yes | 22 | 0.62±0.18 |
|
| 152.62±24.86 |
|
|
| No | 95 | 0.95±0.24 |
|
| 116.62±28.41 |
|
|
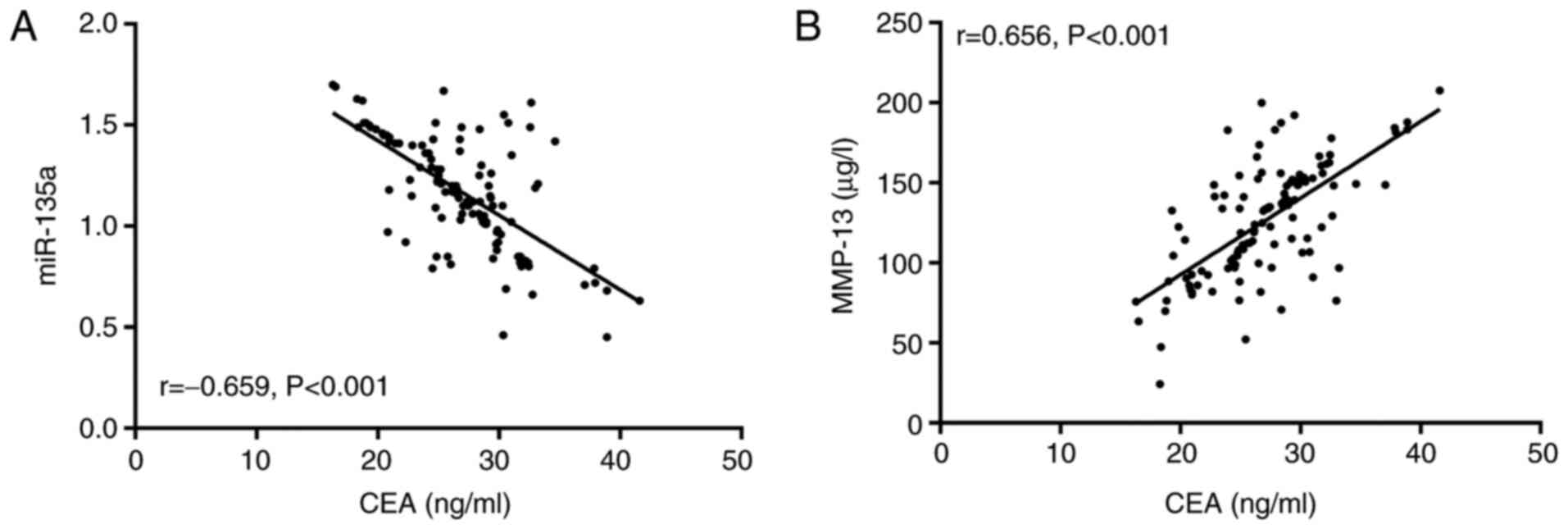
The correlation of miR-135a and MMP-13 with CEA
before treatment. According to Pearson's correlation coefficient
analysis, miR-135a was negatively correlated with CEA in the study
group before treatment (r=−0.659, P<0.001, Fig. 3A), while MMP-13 was negatively
correlated with CEA (r=0.656, P<0.001, Fig. 3B).
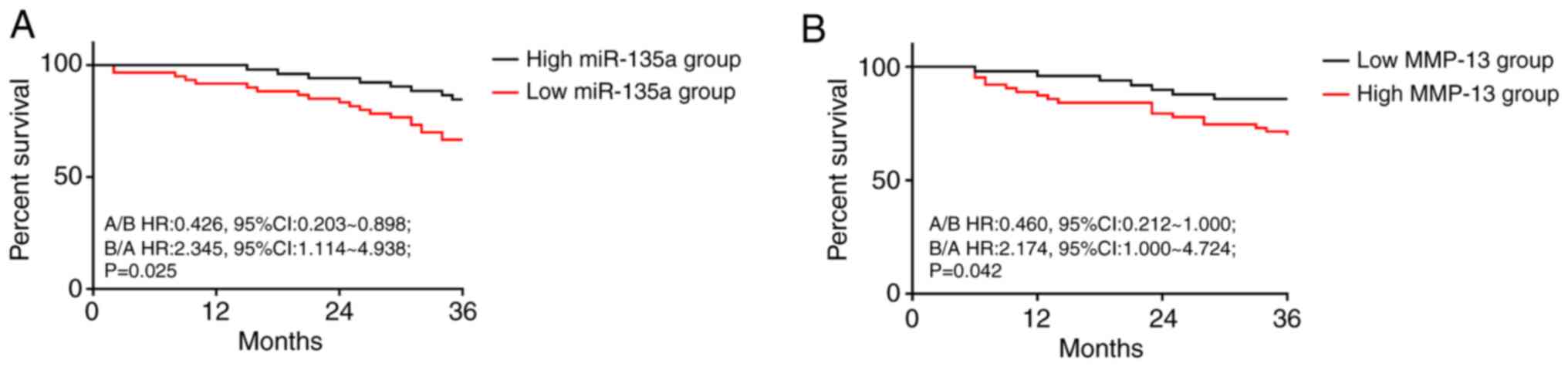
Effect of miR-135a and MMP-13 on prognosis of CCC
patients after treatment. A total of 117 patients in the RG were
successfully followed up for 3 years, and 112 patients were
successfully followed up, with a follow-up success rate of 95.73%.
According to the median expression levels of miR-135a after
treatment, the patients were divided into high-miR-135a group
(miR-135a >0.91, n=60) and low-miR-135a group (miR-135a ≤0.91,
n=52). According to the median expression levels of MMP-13 after
treatment, the patients were divided into high-MMP-13 group (MMP-13
>104.24 µg/l, n=63) and low-MMP-13 group (MMP-13 ≤104.24 µg/l,
n=49). Their prognosis of survival were compared, the prognosis of
the group with high miR-135a was better than that of the group with
low miR-135a (P=0.025, Fig. 4A), and
the prognosis of the group with low MMP-13 was better than that of
the group with high MMP-13 (P=0.042, Fig. 4B).
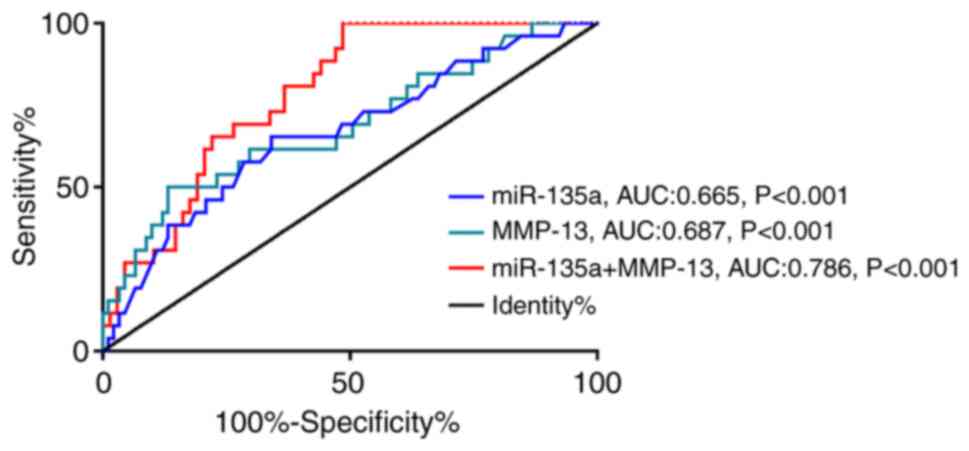
Predictive value of miR-135a and MMP-13 levels in
CCC patients after treatment. The 3-year prognosis of the patients
in the RG was 23.21% (26/112). ROC curve analysis of levels of
miR-135a and MMP-13 of patients after treatment showed that the
sensitivity and specificity of miR-135a in predicting the prognosis
of CCC patients was 65.38 and 65.93% when the cut-off value was
1.215. When the cut-off value was 123.000, the sensitivity and
specificity of MMP-13 in predicting the prognosis of CCC patients
were 50.00 and 86.81%, respectively. With miR-135a and MMP-13 being
regarded as two independent variables, binary Logistic regression
analysis was performed to obtain the joint detection model
Log(P)=8.739 + −0.412 × miR-135a + −1.298 × MMP-13. When the
cut-off value was 0.205, the sensitivity and specificity of the
model for predicting the prognosis of CCC patients were 73.08 and
66.18%, respectively. More details are shown in Fig. 5 and Table
VI.
 | Table VI.Predictive value of miR-135a and
MMP-13 on death in patients with CC after treatment. |
Table VI.
Predictive value of miR-135a and
MMP-13 on death in patients with CC after treatment.
| Item | miR-135a | MMP-13 | Joint
detection |
|---|
| AUC | 0.665 | 0.687 | 0.786 |
| Standard error | 0.061 | 0.062 | 0.046 |
| 95% CI | 0.544–0.785 | 0.564–0.809 | 0.695–0.877 |
| Cut-off | >1.215 | >123.000 | >0.205 |
| Sensitivity [n
(%)] | 65.38 | 50.00 | 73.08 |
| Specificity
(%) | 65.93 | 86.81 | 66.18 |
| Youden index
(%) | 31.31 | 36.81 | 51.47 |
| P | 0.005 | 0.004 | <0.001 |
Discussion
CC is not only the most common malignant tumor in
digestive tract organs, but also one of the most common
malignancies throughout the body (20). In addition, the incidence of the
disease has been on the increase in recent years and is getting
younger, which does harm to the human body increasingly day by day
(21). At present, the pathogenesis
of CC is not clear. It has been suggested that the occurrence of CC
is related to external factors such as diet, smoking, obesity,
diabetes and drinking, as well as internal factors such as cell and
gene changes (22). Therefore, there
is always a lack of specific tumor markers as early diagnostic
criteria for CC in clinical practice. With the application of
microRNAs being regarded as early screening indicators for tumors,
however, microRNAs have gradually become a major research focus in
China and abroad, and it is crucial that potential markers of CC in
clinical practice be identified. This study explored the clinical
significance of miR-135a and MMP-13 for CC, which is of great
significance for the diagnosis and treatment of CC in the
future.
The results showed that miR-135a had a low
expression in CCC patients, while MMP-13 was significantly
increased in CCC patients, suggesting that miR-135a and MMP-13 may
be involved in the occurrence and development of CC. Previous
studies have confirmed miR-135a was decreased in breast cancer and
MMP-13 was increased in lung cancer (23,24),
which also support the results of this study. miR-135a is a
recently discovered miRNA. Xu and Wen (25) proposed that miR-135a participates in
the progress of acute myeloid leukemia by regulating HOXA10. Zhou
et al (26) indicated that
miR-135a affects non-small-cell lung cancer through the pathway of
IGF-1/P13K/Akt. Studies have suggested that miR-135a can bind to
MTSS1, and its synthetic substances can effectively inhibit the
proliferation and invasion ability of tumor cells (27), and MTSS1 has been proven to be
significantly reduced in CC (28).
Thus, we speculated that miR-135a might also bind to MTSS1 in CC
and played a role of tumor suppressor gene. Cheng et al
(29) proved that miR-135a targeting
FAK pathway could inhibit tumor metastasis and angiogenesis, which
may be one of the pathways in which it plays a role in CC. However,
the basic experiment was not conducted in this experiment, and the
mechanism of miR-135a could not be determined. MMPs is a kind of
proteolytic enzyme dependent on calcium ion and zinc ion, which is
composed of 10 exons and 9 introns. MMPs can promote the peripheral
development of tumor cells by enhancing the intercellular adhesion
and can participate in the immune process of tumor cells by
stimulating some potential biological activities (30). MMP-13 belongs to collagenase type I,
which has been confirmed to not only degrade interstitial collagen,
but also degrade extracellular matrix molecules, and regulate the
process of invasion and metastasis of tumor (31). Currently, MMP-13 has been proved to
be abnormally expressed in both osteosarcoma and gastric cancer
(32,33), and its mechanism of action in CC is
speculated to be associated with its ability in promoting
angiogenesis. By analyzing the diagnostic value of miR-135a and
MMP-13 for CC, we found that the combined detection of the two had
a good prediction effect for the occurrence of CC, indicating that
the two could be used as a screening indicator for CC in future
clinical practice, so as to improve the early detection rate of CC.
Compared with traditional imaging methods, miR-135a combined with
MMP-13 has the advantage of convenient detection and intuitive
detection results, without relying on the clinician's previous
judgment experience to analyze the image results. Moreover, the
preservation time of peripheral blood samples is longer, which is
conducive to clinical review at any time. Compared with traditional
tumor markers, the detection advantage of miR-135a combined with
MMP-13 is that it has a higher degree of speciality, which can
assist clinicians to make early judgments on tumor types and
implement relevant interventions.
We further analyzed the correlation of miR-135a and
MMP-13 with traditional cancer marker CEA, and found that miR-135a
was negatively correlated with CEA, while MMP-13 was positively
correlated with CEA, which further confirmed the application value
of miR-135a and MMP-13 in tumor diagnosis in the future. In
addition, according to its relationship with the clinical
pathological features, we found that miR-135a and MMP-13 were bound
to T stage, N stage, clinical stage, tumor type, organization type,
lymphatic metastasis, differentiation degree of CC. However, due to
the limited experimental conditions, we did not analyze the
diagnostic value of miR-135a and MMP-13 in different pathological
features in more detail. A more in-depth analysis and discussion is
required in future research. The results of this study also
confirmed that miR-135a and MMP-13 are related to the tumor
progression of CC, which is a potential therapeutic target of CC.
It is hoped that relevant researchers can conduct experimental
verification and analysis against our conjecture. Through the
follow-up of the patient's prognosis, we found that miR-135a and
MMP-13 are closely related to the prognosis of patients, and the
expression of the two has a good predictive value for the prognosis
and death of patients, suggesting that monitoring of miR-135a and
MMP-13 in patients clinically can help clinicians to judge the
conditions of recovery and prognosis of patients in the future.
Currently, the incidence of CC is on the rise, as is the threat of
death from the disease. By analyzing miR-135a and MMP-13 in CC,
this study confirmed that both the two had great value in
evaluating the prognosis of patients. According to the results of
this study, the lower miR-135a was, the higher MMP-13 was, and the
higher the prognosis and death risk of patients was. Therefore, we
speculated that miR-135a and MMP-13 may also be potential
therapeutic targets of CC, and effective treatment may be achieved
in the future through targeted regulation of the two. Thus, more
experimental analysis is needed to confirm this point, which is the
main direction of our follow-up research.
This study aimed to explore the situation of
miR-135a and MMP-13 in CCC patients. However, due to the limited
experimental conditions, there are still some deficiencies, such as
the study period was short and the impact of miR-135a and MMP-13 on
the long-term prognosis of CCC patients could not be determined. In
addition, this experiment lacked the support of in vitro
experiments, and the mechanism of miR-135a and MMP-13 affecting CC
could not be fully clarified. von Felden et al (34) suggested in the study that miR-135a is
highly expressed in hepatic cancer; the differences between this
study and our results might be attributed to the specific
expression of miR-135a in different tumor diseases and the
different biological effects. Further experiments will be conducted
to verify our view, as well as a more comprehensive and complete
analysis on the application of miR-135a and MMP-13 in CC to obtain
the optimal experimental results. In addition, in this study, we
did not analyze the diagnosis of miR-135a and MMP-13 CC at
different pathological stages, which may lead to certain errors in
the early screening of CC. This study preliminarily confirmed the
clinical significance of miR-135a and MMP-13 in CC, and more
in-depth and detailed experimental analysis is needed to improve
our results.
In conclusion, miR-135a is lowly expressed in CC and
MMP-13 is increased in CC. The combined detection of the two has a
good diagnostic effect on the occurrence of CC, and is closely
related to the prognosis of CCC patients, which may be an excellent
potential indicator for the diagnosis and treatment of CC in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ, DY and PX conceived and designed the study, and
drafted the manuscript. XZ, DY, XD and PX collected, analyzed and
interpreted the experimental data. PX revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Sheng Li Oil Field Central Hospital. Signed written informed
consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marley AR and Nan H: Epidemiology of
colorectal cancer. Int J Mol Epidemiol Genet. 7:105–114.
2016.PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ
and Lai MS: Incidence and survival of adult cancer patients in
Taiwan, 2002–2012. J Formos Med Assoc. 115:1076–1088. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moreno CC, Mittal PK, Sullivan PS,
Rutherford R, Staley CA, Cardona K, Hawk NN, Dixon WT, Kitajima HD,
Kang J, et al: Colorectal cancer initial diagnosis: Screening
colonoscopy, diagnostic colonoscopy, or emergent surgery, and tumor
stage and size at initial presentation. Clin Colorectal Cancer.
15:67–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holch JW, Ricard I, Stintzing S, Modest DP
and Heinemann V: The relevance of primary tumour location in
patients with metastatic colorectal cancer: A meta-analysis of
first-line clinical trials. Eur J Cancer. 70:87–98. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Svoronos AA, Engelman DM and Slack FJ:
OncomiR or tumor suppressor? The duplicity of microRNAs in cancer.
Cancer Res. 76:3666–3670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fabris L, Ceder Y, Chinnaiyan AM, Jenster
GW, Sorensen KD, Tomlins S, Visakorpi T and Calin GA: The potential
of microRNAs as prostate cancer biomarkers. Eur Urol. 70:312–322.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohammadi A, Mansoori B and Baradaran B:
The role of microRNAs in colorectal cancer. Biomed Pharmacother.
84:705–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu B, Tao T, Wang Y, Fang F, Huang Y, Chen
S, Zhu W and Chen M: hsa-miR-135a-1 inhibits prostate cancer cell
growth and migration by targeting EGFR. Tumour Biol.
37:14141–14151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Gao F, Zhou L, Wang H, Shi G and
Tan X: UCA1 regulates the growth and metastasis of pancreatic
cancer by sponging miR-135a. Oncol Res. 25:1529–1541. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Q, Shi M, Meng W, Wang Y, Hui P and Ma
J: Long noncoding RNA FOXD3-AS1 promotes colon adenocarcinoma
progression and functions as a competing endogenous RNA to regulate
SIRT1 by sponging miR-135a-5p. J Cell Physiol. 234:21889–21902.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vafadari B, Salamian A and Kaczmarek L:
MMP-9 in translation: From molecule to brain physiology, pathology,
and therapy. J Neurochem. 139 (Suppl 2):S91–S114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kehlet SN, Harling H, Jørgensen LN,
Karsdal MA and Willumsen N: Effect of MMP-13 degraded SPARC on type
I collagen and its biomarker potential in colorectal cancer. J Clin
Oncol. 36 (Suppl 4):S7172018. View Article : Google Scholar
|
|
17
|
Yamada T, Oshima T, Yoshihara K, Tamura S,
Kanazawa A, Inagaki D, Yamamoto N, Sato T, Fujii S, Numata K, et
al: Overexpression of MMP-13 gene in colorectal cancer with liver
metastasis. Anticancer Res. 30:2693–2699. 2010.PubMed/NCBI
|
|
18
|
Rath T, Roderfeld M, Graf J, Wagner S,
Vehr AK, Dietrich C, Geier A and Roeb E: Enhanced expression of
MMP-7 and MMP-13 in inflammatory bowel disease: A precancerous
potential? Inflamm Bowel Dis. 12:1025–1035. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aran V, Victorino AP, Thuler LC and
Ferreira CG: Colorectal cancer: Epidemiology, disease mechanisms
and interventions to reduce onset and mortality. Clin Colorectal
Cancer. 15:195–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levin TR, Corley DA, Jensen CD,
Schottinger JE, Quinn VP, Zauber AG, Lee JK, Zhao WK, Udaltsova N,
Ghai NR, et al: Effects of organized colorectal cancer screening on
cancer incidence and mortality in a large community-based
population. Gastroenterology. 155:1383–1391.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Drewes JL, Housseau F and Sears CL:
Sporadic colorectal cancer: Microbial contributors to disease
prevention, development and therapy. Br J Cancer. 115:273–280.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tribollet V, Barenton B, Kroiss A, Vincent
S, Zhang L, Forcet C, Cerutti C, Périan S, Allioli N, Samarut J and
Vanacker JM: miR-135a inhibits the invasion of cancer cells via
suppression of ERRα. PLoS One. 11:e01564452016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan HQ, Zhang D, Shi YY, You X, Shi L, Li
Q and Gao FG: Ataxia-telangiectasia mutated activation mediates
tumor necrosis factor-alpha induced MMP-13 up-regulation and
metastasis in lung cancer cells. Oncotarget. 7:62070–62083. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu H and Wen Q: Downregulation of miR-135a
predicts poor prognosis in acute myeloid leukemia and regulates
leukemia progression via modulating HOXA10 expression. Mol Med Rep.
18:1134–1140. 2018.PubMed/NCBI
|
|
26
|
Zhou Y, Li S, Li J, Wang D and Li Q:
Effect of microRNA-135a on cell proliferation, migration, invasion,
apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt
signaling pathway in non-small cell lung cancer. Cell Physiol
Biochem. 42:1431–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin L, He Y, Xi BL, Zheng HC, Chen Q, Li
J, Hu Y, Ye MH, Chen P and Qu Y: miR-135a suppresses calcification
in senescent VSMCs by regulating KLF4/STAT3 pathway. Curr Vasc
Pharmacol. 14:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agarwal E, Robb CM, Smith LM, Brattain MG,
Wang J, Black JD and Chowdhury S: Role of Akt2 in regulation of
metastasis suppressor 1 expression and colorectal cancer
metastasis. Oncogene. 36:3104–3118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng Z, Liu F, Zhang H, Li X, Li Y, Li J,
Liu F, Cao Y, Cao L and Li F: miR-135a inhibits tumor metastasis
and angiogenesis by targeting FAK pathway. Oncotarget.
8:31153–31168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jabłońska-Trypuć A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31 (Suppl
1):S177–S183. 2016. View Article : Google Scholar
|
|
31
|
Louka ML and Ramzy MM: Involvement of
fibroblast-specific protein 1 (S100A4) and matrix
metalloproteinase-13 (MMP-13) in CCl4-induced reversible liver
fibrosis. Gene. 579:29–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng L, Rong XF, Li RH and Wu XY: Icariin
inhibits MMP-1, MMP-3 and MMP-13 expression through MAPK pathways
in IL-1β-stimulated SW1353 chondrosarcoma cells. Mol Med Rep.
15:2853–2858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sheibani S, Mahmoudian RA, Abbaszadegan
MR, Chamani J, Memar B and Gholamin M: Expression analysis of
matrix metalloproteinase-13 in human gastric cancer in the presence
of Helicobacter Pylori infection. Cancer Biomark. 18:349–356. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
von Felden J, Heim D, Schulze K, Krech T,
Ewald F, Nashan B, Lohse AW and Wege H: High expression of micro
RNA-135A in hepatocellular carcinoma is associated with recurrence
within 12 months after resection. BMC Cancer. 17:602017. View Article : Google Scholar : PubMed/NCBI
|