Introduction
As a common primary tumor in adults, uveal melanoma
(UM) accounts for 3.7% of all melanomas (1). Numerous risk factors are associated
with UM, including age, sex, genetic or phenotypic predisposition,
work environment and dermatological conditions (2). UM has a strong propensity for fatal
metastasis (3) and frequently
metastasizes to the liver via the hematogenous route; according to
data from 2005, 90% of UM metastases occur in the liver (4), which results in a dismal prognosis
(5). Additionally, severe
inflammation has been identified in UM cells (6,7),
particularly those with mutations of G protein subunit α (GNA)11 or
GNAQ, which trigger a wide range of cell signaling cascades,
including the PI3K/Akt/mTOR and YAP/TAZ pathways (2); however, limited approaches are
available to alleviate inflammation in UM, as the eye is an
immunologically privileged site, which provides UM with a
protective niche (8). To date,
several strategies have been adopted to treat UM in the clinic,
including surgical resection, immunotherapy (9) and gamma knife radiosurgery (10). For example, ipilimumab, an
anti-cytotoxic T-lymphocyte-associated protein 4 antibody, elicits
a positive response in 40–60% of patients with metastatic cutaneous
melanoma (2), providing a potential
new treatment for UM in the clinic. Recently, small molecules, such
as selumetinib (11), nivolumab
(12), ipilimumab (12) and selumetinib in combination with
dacarbazine (13), have been
identified as potential inhibitors of tumor progression able to
improve the prognosis of patients with UM, although the underlying
mechanisms remain elusive. Overall, there is a need to identify
novel drugs for UM treatment and explore their underlying
mechanisms.
Artemisinin is a natural product derived from the
Chinese herb Artemisia annua (14). As a stable derivative of artemisinin,
artesunate exhibits robust antimalarial activity in mammals
(15,16), as well as various physiological
activities, including inhibiting inflammation (17) and nervous system protection (18). For example, Zeng et al
(17) have reported that artesunate
inhibits the Toll-like receptor 4/tumor necrosis factor
receptor-associated factor 6 and phosphoinositide-specific
phospholipase C1/Ca2+/nuclear factor of activated T
cells 1 signaling pathways and improves lipopolysaccharide-induced
osteoclastogenesis in RAW264.7 cells and mice. Another study used
electrophysiological assays and histopathological examination to
demonstrate that topical artesunate treatment has a positive effect
on peripheral nerve regeneration (18). In addition, artesunate exhibits
antitumor effects against several types of cancer cells, including
lymphoma (19,20), head and neck cancer (21) and hepatocellular carcinoma (22), and suppresses the MEK/ERK and
PI3K/Akt signaling pathways in HL-60 cells, inducing apoptosis and
inhibiting leukemia cell proliferation (23). Additionally, artesunate induces
apoptosis by activating reactive oxygen species (ROS)- and p38
MAPK-mediated signaling and suppresses embryonal rhabdomyosarcoma
cell proliferation (24); however, a
limited number of studies have focused on the effect of artesunate
on UM progression (25,26).
Apoptosis is a form of programmed cell death that
occurs in multicellular organisms and is characterized by various
cellular changes, ranging from nuclear fragmentation to global mRNA
decay (27). In mammals, apoptosis
exerts positive effects on cell self-renewal (28). In addition, apoptosis serves critical
roles in tumor growth, including colon cancer (29,30),
pancreatic ductal adenocarcinoma (31) and acute myeloid leukemia (32). For example, Nangia et al
(33) have demonstrated that
treatment with a combination of MEK and myeloid cell leukemia-1
(MCL1) inhibitors induces apoptosis by regulating MCL1, leading to
inhibition of cell proliferation in a KRAS-mutant non-small cell
lung cancer model. In addition, the proliferation of hepatocellular
carcinoma cells is suppressed by elevating the levels of apoptosis
following CBX2 knockdown (34).
Notably, artesunate exerts antitumor activity by enhancing
apoptosis; Zhou et al (35)
have reported that artesunate upregulates ROS levels and activates
the AMP-activated protein kinase/mTOR/unc-51-like
autophagy-activating kinase 1 axis in T24 cells, resulting in
autophagy-dependent apoptosis of human bladder cancer. However,
whether artesunate treatment enhances UM cell apoptosis and the
potential underlying mechanisms of this effect are poorly
understood. The metastasis-associated lung adenocarcinoma
transcript 1 (MALAT1)/yes-associated protein (YAP) signaling
pathway is involved in mediating apoptosis, particularly in tumor
cells (36). Zhou et al
(36) have demonstrated that the
MALAT1/YAP signaling pathway is activated in pancreatic
cancer cells, whereas downregulation of MALAT1 suppresses
the development of pancreatic cancer by inhibiting the Hippo/YAP
signaling pathway and affecting apoptosis, which may be attributed
to the inhibition of YAP translocation from the nucleus to the
cytoplasm (37). Therefore, whether
artesunate exhibits antitumor effects by regulating the
MALAT1/YAP signaling pathway warrants further study.
The present study aimed to determine the effects of
artesunate on the proliferation of UM cells and to explore the
underlying mechanism. First, the effects of artesunate treatment on
C918 cell proliferation and apoptosis were assessed. In addition,
the role of the MALAT1/YAP axis in mediating the effects of
artesunate was evaluated. Furthermore, the present study tested
whether combination therapy was more efficient compared with single
treatment, and described a potential novel strategy for treating UM
in the clinic.
Materials and methods
Cell culture and chemicals
The human UM cell lines C918 and M619 were obtained
from the Type Culture Collection of the Chinese Academy of Medical
Sciences. C918 or M619 cells were incubated in DMEM (cat. no.
C11995500BT; Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (cat. no. P30-3301; PAN-Biotech GmbH)
and 1% penicillin-streptomycin (cat. no. 15140-122; Gibco; Thermo
Fisher Scientific, Inc.). All cells were maintained in an incubator
at 37°C with 5% CO2 in a water-saturated atmosphere.
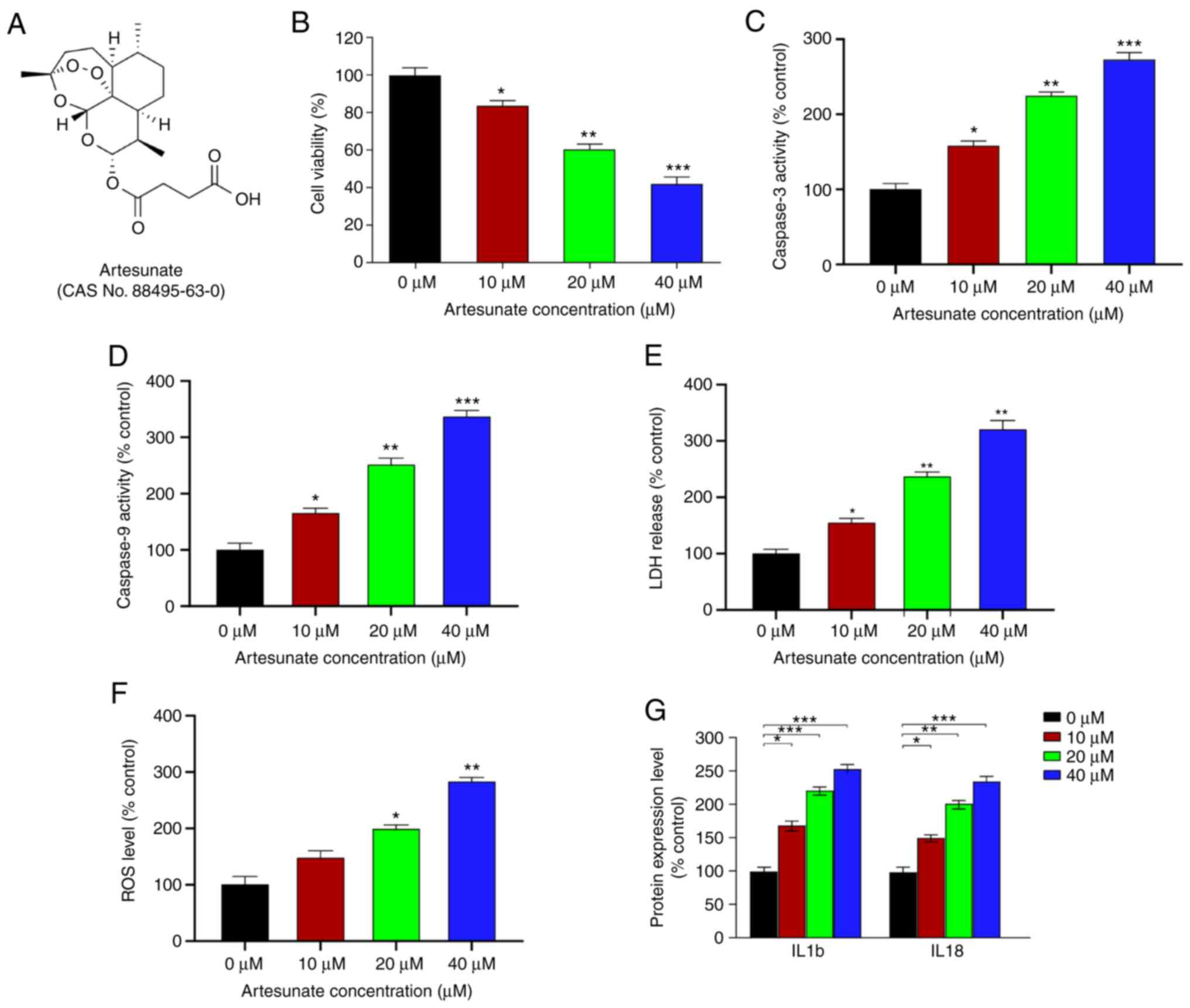
Artesunate (Fig. 1A) and verteporfin
were procured from Selleck Chemicals (cat. nos. S2265 and S1786,
respectively) and used to treat the cells for 48 h. Dimethyl
sulfoxide (DMSO; cat. no. 276855; Sigma-Aldrich; Merck KGaA) was
used as a negative control.
Cell viability assay
Methyl thiazolyl tetrazolium (MTT) assays were used
to analyze cell viability in the present study. C918 or M619 cells
(1×104 cells/well) were cultured in 96-well plates at
37°C with 5% CO2. Following treatment with artesunate
(0, 10, 20 and 40 µM) or a drug combination (40 µM artesunate and 5
µM verteporfin), MTT reagent (cat. no. M1020; Beijing Solarbio
Science & Technology Co., Ltd.) was added to each well and
incubated for 4 h at 37°C. A microplate reader was used to measure
absorbance at 490 nm. Experiments were repeated three times.
Caspase activity assay
C918 cells were plated at a density of
1×106 cells/well in 6-well plates. Following treatment
with artesunate (0, 10, 20 and 40 µM) or a drug combination (40 µM
artesunate and 5 µM verteporfin), cells were lysed using RIPA
buffer (cat. no. R0020, Beijing Solarbio Science & Technology
Co., Ltd.), and protein concentration was determined using a
commercial BCA kit (cat. no. P1511-1; Applygen Technologies, Inc.).
Caspase-3 and caspase-9 activity levels were detected using
Caspase3 and Caspase9 Activity kits (cat. nos. BC3830 and BC3890,
respectively; Beijing Solarbio Science & Technology Co., Ltd.)
according to the manufacturer's instructions. Absorbance of the
resulting solution at 405 nm was determined using a microplate
reader.
ROS level detection
C918 cells were exposed to various concentrations
(0, 10, 20, and 40 µM) of artesunate for 48 h and resuspended in
culture medium (without serum) containing 10 µM DCFH-DA (cat. no.
CA1410; Beijing Solarbio Science & Technology Co., Ltd.)
(38). The cells were harvested, and
a microplate reader was used to detect the relative level of ROS at
488 and 525 nm. Cells treated with DMSO served as the negative
control.
Lactate dehydrogenase (LDH) release
assay
C918 cells (1×104 cells/well) were seeded
into 96-well plates and subsequently treated with 0, 10, 20 and 40
µM artesunate for 48 h at 37°C. LDH assays were performed using a
commercial assay kit (cat. no. BC0685; Beijing Solarbio Science
& Technology Co., Ltd.), according to the manufacturer's
protocol.
ELISA
Following treatment with artesunate (0, 10, 20 and
40 µM) or a drug combination (40 µM artesunate and 5 µM
verteporfin), C918 cells were lysed in RIPA lysis buffer (cat. no.
R0020; Beijing Solarbio Science & Technology Co., Ltd.). The
lysates were centrifuged at 12,000 × g for 30 min at 4°C, and the
supernatants were collected. Then, the concentrations of IL-1b and
IL18 were determined using ELISA kits, according to the
accompanying instructions (cat. nos. ab214025 and ab215539; Abcam).
Absorption was determined using a microplate reader, and
experiments were repeated three times.
Reverse transcription-quantitative
(RT-q) PCR assay
Relative levels of target RNA were determined
following treatment with artesunate (0, 10, 20 and 40 µM) or a drug
combination (40 µM artesunate and 5 µM verteporfin). Total RNA of
C918 cells was extracted from cells using TRIzol®
reagent (cat. no. 15596-026; Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions, and the
concentration of the extracted RNA was measured using a
NanoDrop2000 (Thermo Fisher Scientific, Inc.). Subsequently, RNA
was reverse-transcribed into cDNA using a reverse transcription kit
(cat. no. 04896866001; Roche Molecular Systems, Inc.).
SYBR® Green (TransGen Biotech Co., Ltd.) was used to
perform RT-qPCR on the ABI-Quant Studio 5 system (Thermo Fisher
Scientific, Inc.) to determine the relative expression levels of
the target genes. Relative expression level of YAP was
normalized to β-actin, and MALAT1 was normalized to
U6. The primer pairs used in this study are listed in the
Table SI. The thermocycling
conditions were as follows: Activation at 95°C for 10 min; 40
cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30
sec (data collected during each cycle) and extension at 72°C for 30
sec; and a melting curve between 55°C and 95°C (data collected at
each temperature).
Cell transfection
Plasmids overexpressing YAP and MALAT1
(constructed in pEGFP-N2) and negative controls (empty pEGFP-N2
vector) were synthesized by Shanghai GenePharma Co., Ltd.. Cells
were transfected with 2 µg plasmid using Lipofectamine®
2000 transfection reagent (cat. no. 11668-027, Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions and cultured for 72 h at 37°C. Subsequently, cells
were harvested for further analysis.
Western blot assay
Following treatment with artesunate (0, 10, 20 and
40 µM) or overexpression plasmids, C918 cells were lysed on ice
using RIPA lysis buffer (cat. no. R0020, Beijing Solarbio Science
& Technology Co., Ltd.). The lysates were centrifuged at 12,000
× g for 30 min at 4°C, the supernatants were collected, and total
protein concentration was determined using a BCA quantitation kit
(cat. no. P1511-1; Applygen Technologies, Inc.). Proteins were
separated by 10% SDS-PAGE and transferred to PVDF membranes (cat.
no. IPVH00010; MilliporeSigma). Following blocking with 5% milk at
room temperature for 2 h, the membranes were incubated with primary
antibodies at 4°C overnight. Following three washes with 0.1%
TBS-Tween 20, the membranes were incubated with secondary
HRP-conjugated antibodies at room temperature for 2 h. ECL
High-Signal reagent (170-5060; Bio-Rad Laboratories, Inc.) was used
to visualize protein bands on a Bio-Rad System (Bio-Rad
Laboratories, Inc.). β-actin served as a loading control. The
primary antibodies were diluted 1:2,000, and the secondary
antibodies were diluted 1:5,000. The antibody against YAP (cat. no.
ab76252) was obtained from Abcam, and the actin antibody (cat. no.
AC026) was purchased from ABclonal Biotech Co., Ltd. Secondary
antibodies were obtained from Suzhou Biodragon Immunotechnologies
Co., Ltd..
Statistical analysis
Data are presented as the mean ± SEM of ≥3
independent experiments for each assay. All experimental data were
analyzed using SPSS software (version 24.0; IBM Corp.). Data were
evaluated using an unpaired two-tailed Student's t-test or one-way
analysis of variance followed by Bonferroni (for data meeting
homogeneity of variance) or Tamhane's T2 (for data demonstrating
heteroscedasticity) post hoc tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Artesunate alleviates UM cell
apoptosis and inflammation, and inhibits UM cell viability
Previous studies have demonstrated that artesunate
reduces the number of viable C2C12 cells in a dose-dependent manner
following a 48-h treatment (23).
Therefore, the present study tested whether artesunate (Fig. 1A) exerted similar effects in UM
cells. The results demonstrated that, relative to that in the
control group, artesunate significantly reduced the viability of
C918 cells, particularly at doses ≥10 µM (Fig. 1B). The effects of artesunate on M619
cells were also determined, and similar inhibition of cell
viability was observed (Fig. S1).
Due to more pronounced inhibitory effects of artesunate on C918
cells compared with those on M619 cells, this cell line was
selected for further experiments.
The caspase-3 and caspase-9 activity levels were
significantly increased in C918 cells following treatment with
artesunate (Fig. 1C and D). In
addition, cells treated with artesunate exhibited elevated ROS
levels and LDH release compared with those in the control group
(Fig. 1E and F). Furthermore, the
effects of artesunate on the expression levels of inflammatory
factors IL1b and IL18, which are positively associated with
apoptosis (39), were assessed.
Compared with those in the control cells, the levels of IL1b and
IL18 were significantly increased following artesunate treatment
(Fig. 1G), which suggested that
artesunate exerted a proinflammatory effect on C918 cells. Overall,
these results demonstrated that artesunate induced elevated
apoptosis and an enhanced the inflammatory response, particularly
at 40 µM, resulting in inhibition of C918 cell viability.
Therefore, 40 µM artesunate was selected for use in further
experiments.
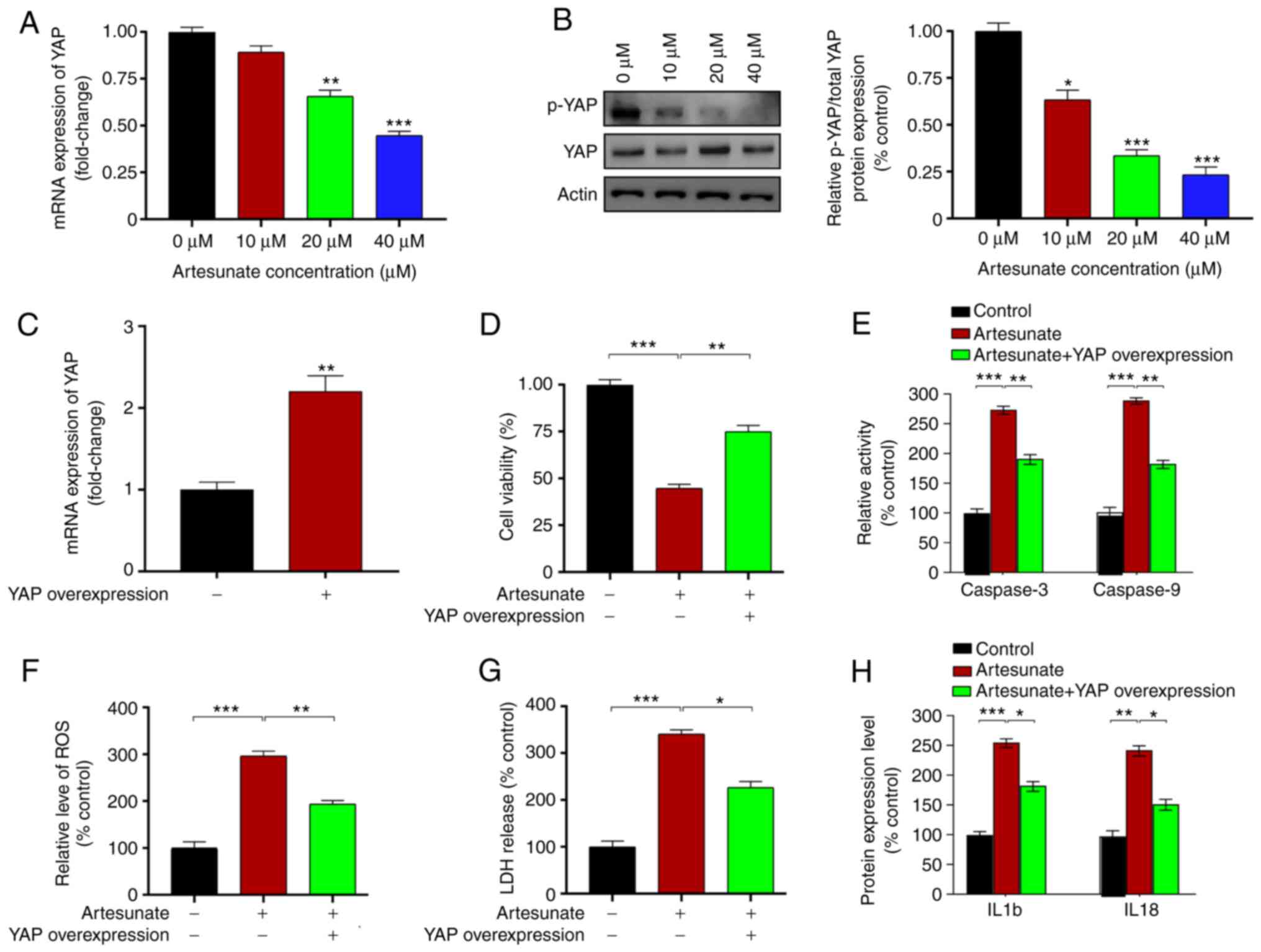
Overexpression of YAP ameliorates the
effects of artesunate in C918 cells
YAP is a crucial factor in UM progression (40). Therefore, the present study assessed
whether may YAP serve a role in mediating the effects of artesunate
on UM cell apoptosis. Cells were treated with 40 µM artesunate for
48 h. The results of qPCR and western blot assays demonstrated that
YAP expression and phosphorylation levels were significantly
decreased following artesunate treatment compared with those in the
control cells (Fig. 2A and B).
Subsequently, C918 cells were transfected with a plasmid
overexpressing YAP to assess the role of YAP in mediating the
effects of artesunate. Plasmid transfection significantly enhanced
the expression levels of YAP compared with those in the cells
transfected with the empty vector (Fig.
2C). Compared with cells treated with artesunate alone, those
overexpressing YAP exhibited reversal of the inhibitory effects of
artesunate, as elevated C918 cell viability was observed (Fig. 2D). In addition, the caspase-3 and
caspase-9 activity levels were decreased following YAP
overexpression compared with those in control cells treated with
artesunate (Fig. 2E). YAP
overexpression plasmid transfection also reduced the levels of
intracellular ROS and LDH release (Fig.
2F and G), which were accompanied by decreased levels of IL1b
and IL18 (Fig. 2H) compared with
those in artesunate-treated control cells. Overall, these results
demonstrated that YAP was involved in mediating C918 cell apoptosis
induced by artesunate.
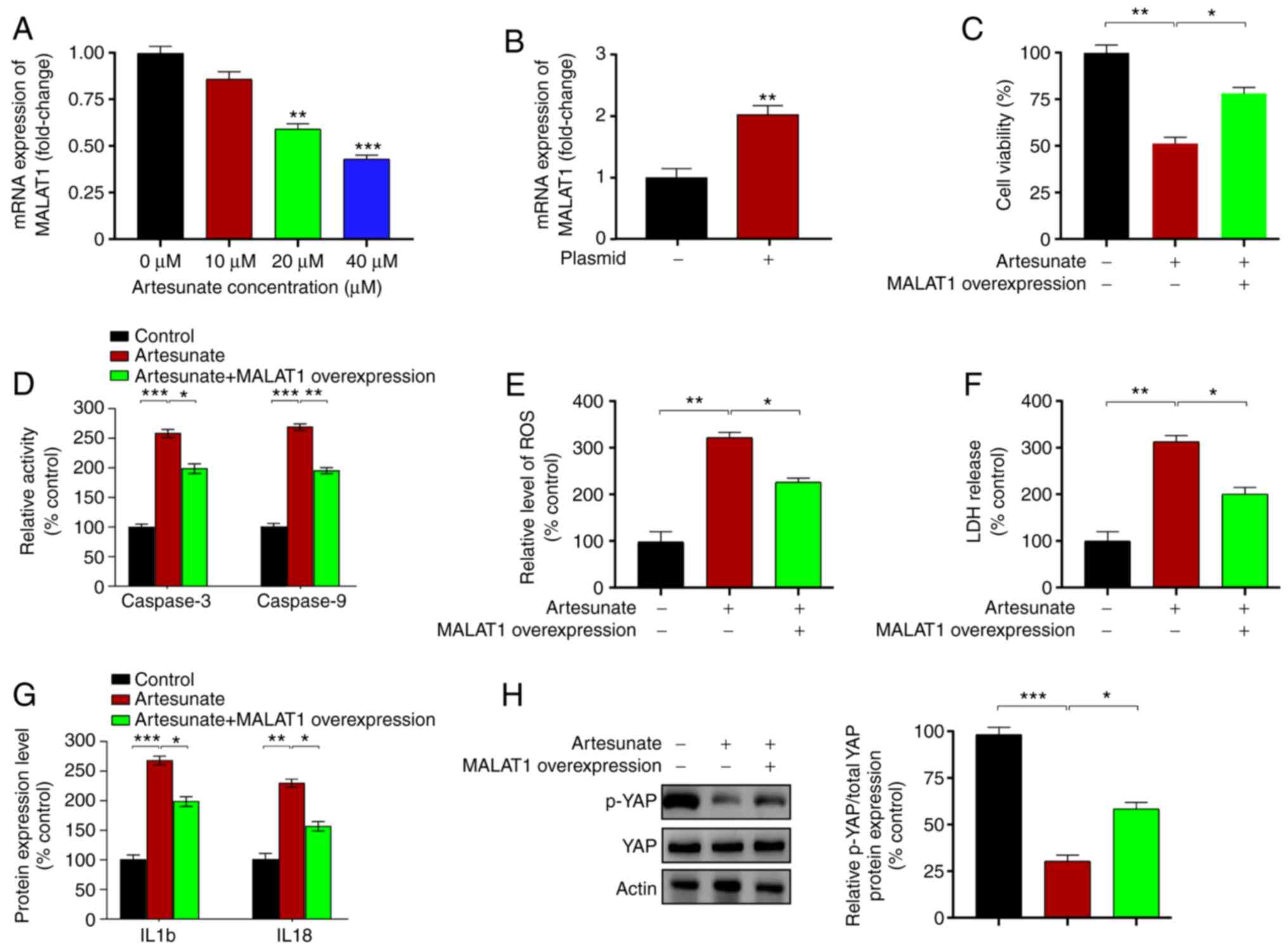
Upregulation of MALAT1 reverses the
effects of artesunate by regulating YAP levels in C918 cells
The present study next analyzed the effects of
artesunate treatment on the upstream regulator of YAP
MALAT1, which binds to the pro-metastatic transcription
factor TEA domain (TEAD), blocking TEAD from associating with its
coactivator YAP (41). The results
demonstrated that artesunate significantly downregulated the mRNA
expression levels of MALAT1 in C918 cells compared with
those in the control group (Fig.
3A). MALAT1 was overexpressed by transfecting a plasmid
into C918 cells, which significantly enhanced the expression levels
of MALAT1 compared with those in the empty
vector-transfected cells (Fig. 3B).
Compared with those in the control C918 cells treated with
artesunate alone, MALAT1 overexpression led to increased
cell viability (Fig. 3C), reduced
caspase-3 and caspase-9 activity levels (Fig. 3D), and decreased levels of LDH
release and intracellular ROS (Fig. 3E
and F). In addition, the levels of IL1b and IL18 were assessed
following transfection and were significantly reduced following
MALAT1 overexpression in C918 cells compared with those in
the control cells treated with artesunate (Fig. 3G). Notably, YAP phosphorylation
levels were upregulated in response to MALAT1 overexpression
compared with those in the control cells treated with artesunate
(Fig. 3H). These results suggested
that MALAT1/YAP signaling may serve an important role in
mediating the effects of artesunate on C918 cells.
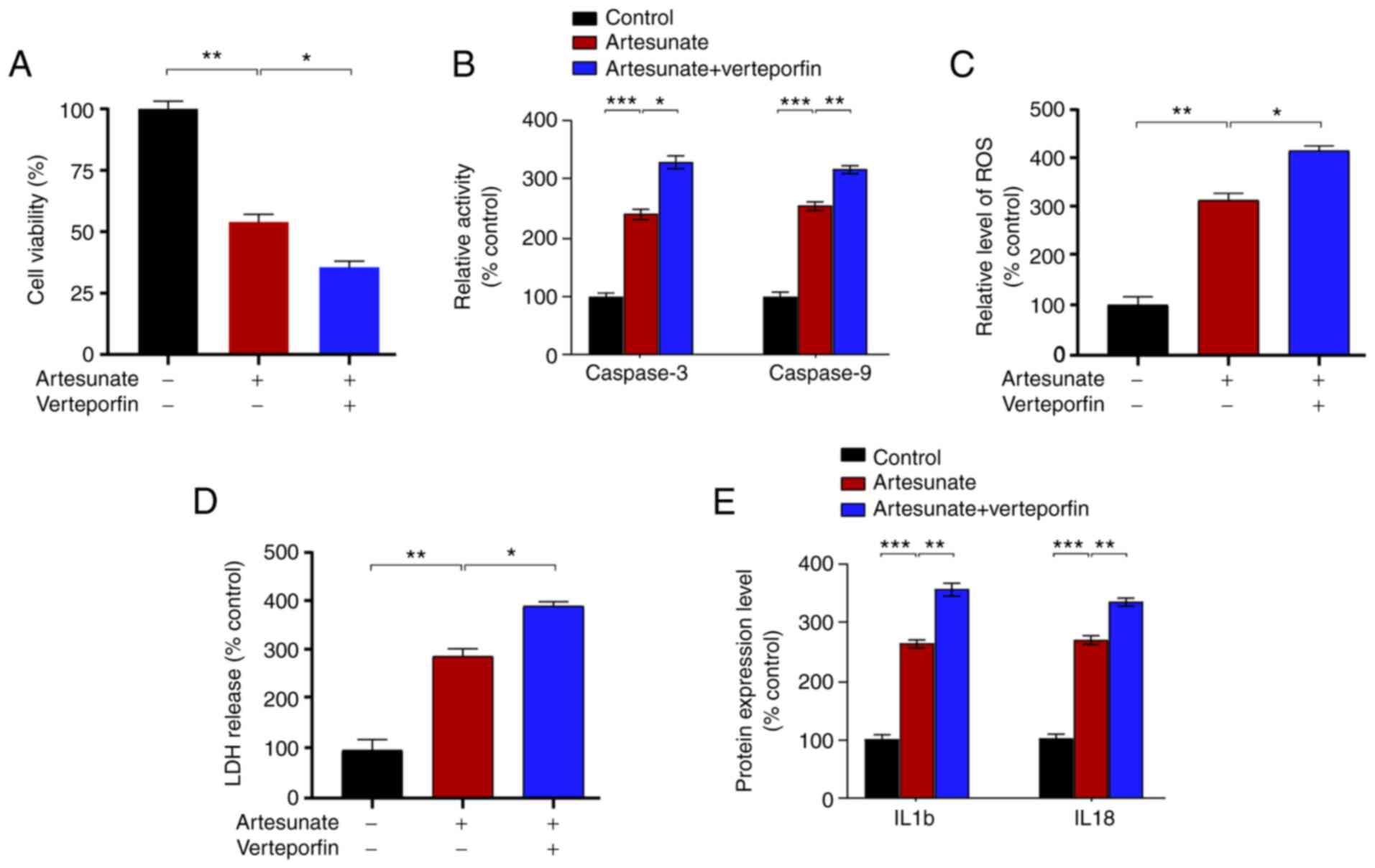
Verteporfin enhances the antitumor
effects of artesunate in C918 cells
The present study further assessed the ability of
combination therapy to treat UM. Combination therapy has strong
potential for application in clinical treatment of diseases, such
as diabetes (42), glioblastoma
(43), and rheumatoid arthritis
(44), as well as various types of
tumor (45–47). As an inhibitor of YAP, verteporfin
suppresses the interaction between YAP and TEAD (48). Therefore, the present study
determined the effects of combined artesunate and verteporfin
treatment in C918 cells. The results demonstrated that verteporfin
enhanced the inhibitory effects of artesunate on C918 cells,
further inhibiting cell viability (Fig.
4A), elevating caspase-3 and caspase-9 activity levels
(Fig. 4B), and increasing
intracellular ROS and LDH release (Fig.
4C and D) compared with those in the cells treated with
artesunate alone. In addition, combination therapy significantly
increased the levels of IL18 and IL1b compared with those following
treatment with artesunate alone (Fig.
4E). In conclusion, combination therapy exerted stronger
effects on C918 cells compared with treatment with artesunate
alone.
Discussion
UM is a primary malignant intraocular tumor in
adults, which represents 5–6% of all melanoma diagnoses (49); however, effective therapies for UM
are lacking, although certain advances have been achieved in local
ocular treatments, such as chemotherapy and immunotherapy (50). In addition, various small molecules
have been demonstrated to exhibit potent antitumor activity in
vivo and in vitro, including sorafenib (51), crotepoxide (52) and luteolin (53). Notably, combination therapies exhibit
higher efficacy compared with single-treatment approaches (54,55). For
instance, Matsunaga et al (55) have demonstrated that combination
therapy exhibits higher treatment efficacy for Alzheimer's disease
compared with that of a single cholinesterase inhibitor. In the
present study, artesunate was identified as a potential candidate
to inhibit UM cell viability by enhancing apoptosis. Furthermore,
the results of the present study demonstrated that the
MALAT1/YAP signaling pathway was involved in mediating the
effects of artesunate. In addition, verteporfin enhanced the
artesunate treatment-mediated induction of apoptosis in C918 cells,
providing evidence for its potential for application in treating
UM.
ROS serves dual roles in biological processes,
including the maintenance of normal physiological conditions, as
well as exerting pathogenic effects by inducing cell damage and
destruction during pathophysiology (56). Under physiological conditions, ROS
preferentially triggers redox signaling rather than inducing
oxidative damage to macromolecules, such as proteins, lipids and
DNA (57); however, previous studies
have suggested that ROS serves a crucial role in tumor
proliferation and progression (57,58).
Pelicano et al (59) have
demonstrated that moderate mitochondrial ROS levels promote breast
cancer cell motility in a CXCL14-dependent manner. Notably, a
positive association has also been identified between apoptosis and
ROS production (60,61). Similar to previous reports (35,62), the
results of the present study revealed that artesunate induced
apoptosis in a ROS-dependent manner. In addition, the present study
demonstrates that overexpression of MALAT1 or YAP reversed
the antitumor effects of artesunate on ROS induction and apoptosis
in C918 cells, indicating that MALAT1 or YAP may be
potential drug targets for UM treatment.
YAP is a transcriptional coactivator that shuttles
between the cytoplasm and the nucleus (63). YAP recognizes cognate cis-regulatory
elements by interacting with other transcription factors in the
nucleus, particularly TEAD family members (64). As a transcriptional regulator of
TEAD, YAP activates the transcription of genes involved in cell
proliferation, leading to a suppression of apoptosis (65). YAP is regulated by the Hippo
signaling pathway to control tumor progression (66). In addition, YAP is an oncogene and
serves crucial roles in various types of human cancer (65,67),
such as kidney and blood cancer (68,69).
White et al (68) have
reported that inhibition of the YAP/TAZ signaling pathway
suppresses glycolysis-dependent proliferation and enhances
mitochondrial respiration as well as ROS buildup, resulting in the
death of kidney tumor cells when challenged by nutrient stress.
Additionally, neratinib suppresses the proliferation of pancreatic
and blood cancer cells by inhibiting the Hippo/YAP signaling
pathway and mutant KRAS expression (69). Neratinib upregulates the
phosphorylation of YAP and TAZ by 30% and promotes YAP
translocation into the cytosol, resulting in a reduction of YAP/TAZ
protein levels. In addition, knockdown of YAP enhances the
lethality of neratinib (69). These
previous studies indicate that YAP serves a crucial role in
mediating the antitumor effects of small molecules by regulating
apoptosis. Notably, a previous study has demonstrated an activation
of YAP in UM compared with that in patients without metastasis
(70). Therefore, as an enhanced
effect of artesunate on UM cell apoptosis was observed in the
present study, we hypothesized that YAP may be involved in
mediating the effects of artesunate in C918 cells. The results of
the present study demonstrated that artesunate treatment
significantly reduced YAP expression. Furthermore, YAP
overexpression ameliorated the inhibitory effects of artesunate in
C918 cells, reducing caspase-3 and caspase-9 activity levels, as
well as inhibiting the levels of IL1b and IL18. Compared with those
in the control group, artesunate treatment led to elevated levels
of IL1b and IL18 in C918 cells, whereas previous studies have
reported that it exerts an anti-inflammatory effect (71,72).
This may be attributed to enhanced of caspase-1 activity, which is
a factor upstream of pyroptosis (73). In the present study, caspase-1
activity was increased following artesunate treatment compared with
that in the control cells (data not shown). During the process of
pyroptosis, caspase-1 specifically cleaves the linker between the
N- and C-terminal domains of gasdermin D (GSDMD), leading to
release of GSDMD N-terminal domain (74). GSDMD is an executor of pyroptosis and
is required for the secretion of IL1β and IL18 (75). Additionally, YAP is a suppressor of
inflammation (76); thus, a high
level of inflammation may be associated with low YAP expression
levels. In our future studies, the effects of artesunate on the
regulation of pyroptosis in UM cells will be assessed.
As an infrequently spliced non-coding RNA,
MALAT1 is highly conserved amongst mammals and strongly
expressed in the nucleus (77).
MALAT1 contributes to various physiological processes,
including alternative splicing, nuclear organization, and
epigenetic modulation of gene expression (78). In addition, previous studies have
provided evidence that MALAT1 is crucial for the regulation
of tumor cell proliferation. For example, methyltransferase-like 3
initiates m6A mRNA methylation and promotes YAP mRNA
translation by regulating the MALAT1/microRNA
(miR)-1914-3p/YAP axis, which increases YAP mRNA stability
and induces non-small cell lung cancer drug resistance and
metastasis (79). In addition, Sun
et al (80) have reported
that silencing of MALAT1 upregulates miR-181a-5p levels by
activating the Hippo-YAP signaling pathway, leading to inhibition
of myeloma cell proliferation and adhesion. These results suggest
an association between YAP and MALAT1 in the regulation of
tumor cell proliferation (81).
Therefore, the present study aimed to determine how MALAT1
may contribute to mediating the effects of artesunate, since YAP
expression levels were reduced in response to artesunate treatment.
The results demonstrated that artesunate suppressed C918 cell
viability by inhibiting the MALAT1/YAP signaling pathway,
whereas the inhibitory effect was ameliorated by MALAT1
overexpression, which supported the role of the MALAT1/YAP
axis in mediating the effects of artesunate on UM cells. The
present study also assessed the feasibility of combination therapy
for UM. Compared with cells treated with artesunate alone, cells
administered a combination of artesunate and verteporfin exhibited
lower viability and higher levels of apoptosis, indicating that
combination therapy may be more effective.
However, there were certain limitations to the
present study. For instance, the present study did not assess
whether artesunate may alleviate UM by regulating the
MALAT1/YAP signaling pathway in a mouse UM model.
Additionally, how pyroptosis is involved in mediating the effects
of artesunate remains elusive. In the future, multiple cell lines
will be used to verify the results of the present study, and the
antitumor effects of artesunate will be analyzed in animal models
of UM. In addition, other drugs with the potential to inhibit UM
cell proliferation will be assessed, with the aim of advancing UM
treatment in the clinic.
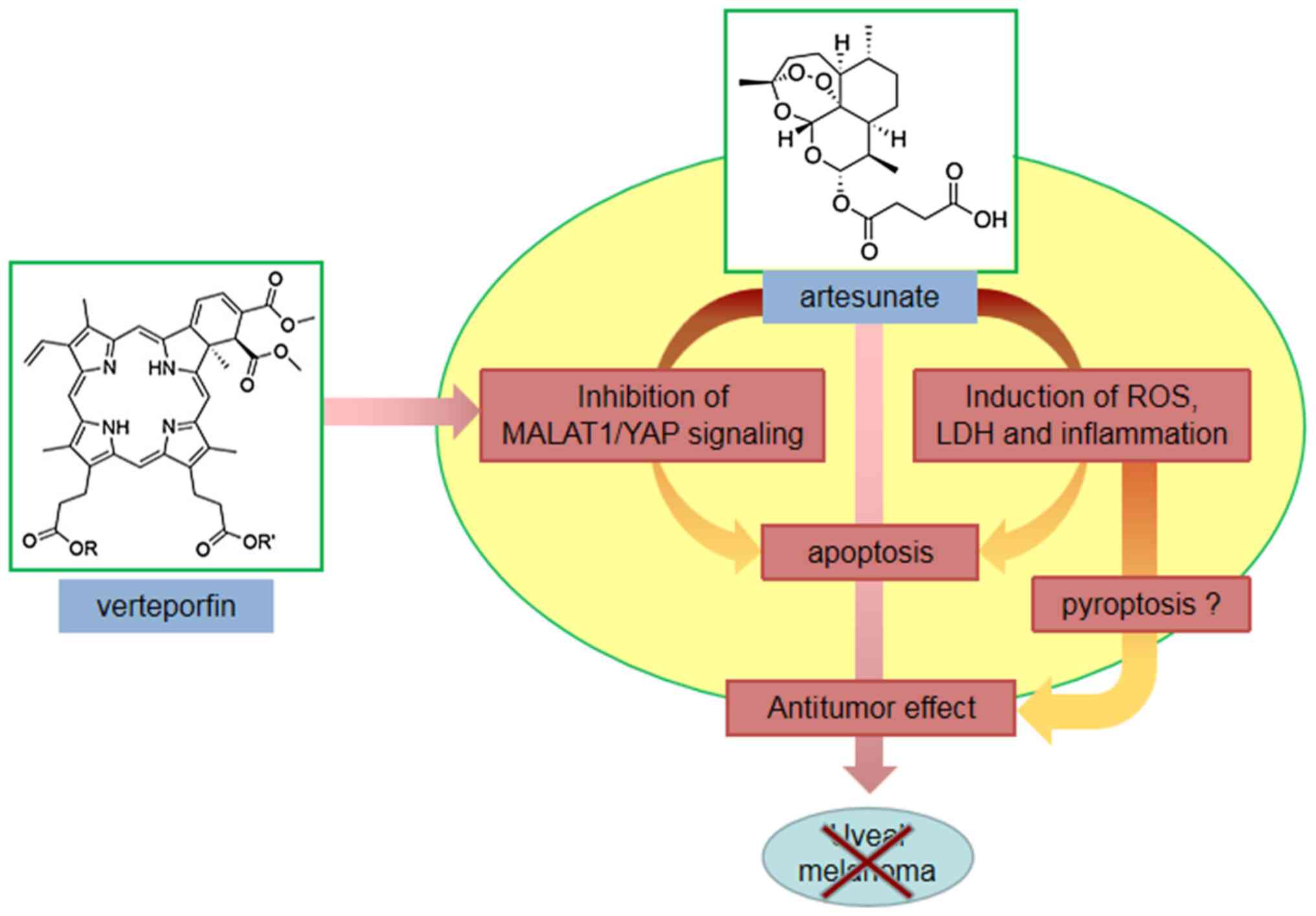
In conclusion, the results of the present study
identified artesunate as a potent small molecule that inhibited UM
cell proliferation. In addition, the MALAT1/YAP signaling
pathway was demonstrated to mediate the effects of artesunate.
Notably, combination therapy exerted a stronger inhibitory effect
on C918 cells compared with treatment with artesunate alone
(Fig. 5). To the best of our
knowledge, this is the first report of assessment of the role of
MALAT1/YAP signaling in mediating the effects of artesunate
in UM cells, and it provides evidence supporting artesunate
combined with verteporfin as a candidate clinical treatment for
UM.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and XJ designed and performed the study, analyzed
the data and wrote the manuscript. YL contributed to the writing
the manuscript and data analysis. JW, XJ and YL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McLaughlin CC, Wu XC, Jemal A, Martin HJ,
Roche LM and Chen VW: Incidence of noncutaneous melanomas in the
U.S. Cancer. 103:1000–1007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ortega MA, Fraile-Martinez O,
Garcia-Honduvilla N, Álvarez-Mon M, Buján J and Teus MA: Update on
uveal melanoma: Translational research from biology to clinical
practice (review). Int J Oncol. 57:1262–1279. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Landreville S, Agapova OA and Harbour JW:
Emerging insights into the molecular pathogenesis of uveal
melanoma. Future Oncol. 4:629–636. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh AD, Bergman L and Seregard S: Uveal
melanoma: Epidemiologic aspects. Ophthalmol Clin North Am. 1875–84.
(viii)2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chandran SS, Somerville RPT, Yang JC,
Sherry RM, Klebanoff CA, Goff SL, Wunderlich JR, Danforth DN, Zlott
D, Paria BC, et al: Treatment of metastatic uveal melanoma with
adoptive transfer of tumour-infiltrating lymphocytes: A
single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol.
18:792–802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Basile MS, Mazzon E, Russo A, Mammana S,
Longo A, Bonfiglio V, Fallico M, Caltabiano R, Fagone P, Nicoletti
F, et al: Differential modulation and prognostic values of
immune-escape genes in uveal melanoma. PLoS One. 14:e02102762019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petralia MC, Mazzon E, Fagone P, Russo A,
Longo A, Avitabile T, Nicoletti F, Reibaldi M and Basile MS:
Characterization of the pathophysiological role of CD47 in uveal
melanoma. Molecules. 24:24502019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basile MS, Mazzon E, Fagone P, Longo A,
Russo A, Fallico M, Bonfiglio V, Nicoletti F, Avitabile T and
Reibaldi M: Immunobiology of uveal melanoma: State of the art and
therapeutic targets. Front Oncol. 9:11452019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naing A, Papadopoulos KP, Autio KA, Ott
PA, Patel MR, Wong DJ, Falchook GS, Pant S, Whiteside M, Rasco DR,
et al: Safety, antitumor activity, and immune activation of
pegylated recombinant human interleukin-10 (AM0010) in patients
with advanced solid tumors. J Clin Oncol. 34:3562–3569. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parker T, Rigney G, Kallos J, Stefko ST,
Kano H, Niranjan A, Green AL, Aziz T, Rath P and Lunsford LD: Gamma
knife radiosurgery for uveal melanomas and metastases: A systematic
review and meta-analysis. Lancet Oncol. 21:1526–1536. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Selumetinib shows promise in metastatic
uveal melanoma. Cancer Discov. 3:OF82013. View Article : Google Scholar
|
|
12
|
Pelster MS, Gruschkus SK, Bassett R,
Gombos DS, Shephard M, Posada L, Glover MS, Simien R, Diab A, Hwu
P, et al: Nivolumab and ipilimumab in metastatic uveal melanoma:
Results from a single-arm phase II study. J Clin Oncol. 39:599–607.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carvajal RD, Piperno-Neumann S, Kapiteijn
E, Chapman PB, Frank S, Joshua AM, Piulats JM, Wolter P, Cocquyt V,
Chmielowski B, et al: Selumetinib in combination with dacarbazine
in patients with metastatic uveal melanoma: A phase III,
multicenter, randomized trial (SUMIT). J Clin Oncol. 36:1232–1239.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Judd R, Bagley MC, Li M, Zhu Y, Lei C,
Yuzuak S, Ekelöf M, Pu G, Zhao X, Muddiman DC and Xie DY:
Artemisinin biosynthesis in non-glandular trichome cells of
artemisia annua. Mol Plant. 12:704–714. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dondorp AM, Fanello CI, Hendriksen IC,
Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N,
Maitland K, et al: Artesunate versus quinine in the treatment of
severe falciparum malaria in African children (AQUAMAT): An
open-label, randomised trial. Lancet. 376:1647–1657. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vivas L, Rattray L, Stewart L, Bongard E,
Robinson BL, Peters W and Croft SL: Anti-malarial efficacy of
pyronaridine and artesunate in combination in vitro and in vivo.
Acta Trop. 105:222–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng XZ, Zhang YY, Yang Q, Wang S, Zou BH,
Tan YH, Zou M, Liu SW and Li XJ: Artesunate attenuates LPS-induced
osteoclastogenesis by suppressing TLR4/TRAF6 and
PLCγ1-Ca2+-NFATc1 signaling pathway. Acta Pharmacol Sin.
41:229–236. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uzun T, Toptas O, Saylan A, Carver H and
Turkoglu SA: Evaluation and comparison of the effects of
artesunate, dexamethasone, and tacrolimus on sciatic nerve
regeneration. J Oral Maxillofac Surg. 77:1092.e1–1092.e12. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vatsveen TK, Myhre MR, Steen CB, Wälchli
S, Lingjærde OC, Bai B, Dillard P, Theodossiou TA, Holien T, Sundan
A, et al: Artesunate shows potent anti-tumor activity in B-cell
lymphoma. J Hematol Oncol. 11:232018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishikawa C, Senba M and Mori N: Evaluation
of artesunate for the treatment of adult T-cell leukemia/lymphoma.
Eur J Pharmacol. 872:1729532020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roh JL, Kim EH, Jang H and Shin D: Nrf2
inhibition reverses the resistance of cisplatin-resistant head and
neck cancer cells to artesunate-induced ferroptosis. Redox Biol.
11:254–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao X, Zhao CR, Yin H, Wang K and Gao JJ:
Synergistic antitumor activity of sorafenib and artesunate in
hepatocellular carcinoma cells. Acta Pharmacol Sin. 41:1609–1620.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Gan S, Han L, Li X, Xie X, Zou D
and Sun H: Artesunate induces apoptosis and inhibits the
proliferation, stemness, and tumorigenesis of leukemia. Ann Transl
Med. 8:7672020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beccafico S, Morozzi G, Marchetti MC,
Riccardi C, Sidoni A, Donato R and Sorci G: Artesunate induces ROS-
and p38 MAPK-mediated apoptosis and counteracts tumor growth in
vivo in embryonal rhabdomyosarcoma cells. Carcinogenesis.
36:1071–1083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng L and Pan J: The anti-malarial drug
artesunate blocks Wnt/β-catenin pathway and inhibits growth,
migration and invasion of uveal melanoma cells. Curr Cancer Drug
Targets. 18:988–998. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berger TG, Dieckmann D, Efferth T, Schultz
ES, Funk JO, Baur A and Schuler G: Artesunate in the treatment of
metastatic uveal melanoma-first experiences. Oncol Rep.
14:1599–1603. 2005.PubMed/NCBI
|
|
27
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin J, Zhang D, Fan Y, Chao Y, Chang J, Li
N, Han L and Han C: Regulation of cancer stem cell self-renewal by
HOXB9 antagonizes endoplasmic reticulum stress-induced melanoma
cell apoptosis via the miR-765-FOXA2 axis. J Invest Dermatol.
138:1609–1619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roberti MP, Yonekura S, Duong CPM, Picard
M, Ferrere G, Tidjani Alou M, Rauber C, Iebba V, Lehmann CHK, Amon
L, et al: Chemotherapy-induced ileal crypt apoptosis and the ileal
microbiome shape immunosurveillance and prognosis of proximal colon
cancer. Nat Med. 26:919–931. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh A, Sweeney MF, Yu M, Burger A,
Greninger P, Benes C, Haber DA and Settleman J: TAK1 inhibition
promotes apoptosis in KRAS-dependent colon cancers. Cell.
148:639–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ogawa S, Fukuda A, Matsumoto Y, Hanyu Y,
Sono M, Fukunaga Y, Masuda T, Araki O, Nagao M, Yoshikawa T, et al:
SETDB1 inhibits p53-mediated apoptosis and is required for
formation of pancreatic ductal adenocarcinomas in mice.
Gastroenterology. 159:682–696.e13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reyna DE, Garner TP, Lopez A, Kopp F,
Choudhary GS, Sridharan A, Narayanagari SR, Mitchell K, Dong B,
Bartholdy BA, et al: Direct activation of BAX by BTSA1 overcomes
apoptosis resistance in acute myeloid leukemia. Cancer Cell.
32:490–505.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nangia V, Siddiqui FM, Caenepeel S,
Timonina D, Bilton SJ, Phan N, Gomez-Caraballo M, Archibald HL, Li
C, Fraser C, et al: Exploiting MCL1 dependency with combination MEK
+ MCL1 inhibitors leads to induction of apoptosis and tumor
regression in KRAS-mutant non-small cell lung cancer. Cancer
Discov. 8:1598–1613. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao J, Tian Y, Wang C, Jiang K, Li R, Yao
Y, Zhang R, Sun D, Liang R, Gao Z, et al: CBX2 regulates
proliferation and apoptosis via the phosphorylation of YAP in
hepatocellular carcinoma. J Cancer. 10:2706–2719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou X, Chen Y, Wang F, Wu H, Zhang Y, Liu
J, Cai Y, Huang S, He N, Hu Z and Jin X: Artesunate induces
autophagy dependent apoptosis through upregulating ROS and
activating AMPK-mTOR-ULK1 axis in human bladder cancer cells. Chem
Biol Interact. 331:1092732020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou Y, Shan T, Ding W, Hua Z, Shen Y, Lu
Z, Chen B and Dai T: Study on mechanism about long noncoding RNA
MALAT1 affecting pancreatic cancer by regulating Hippo-YAP
signaling. J Cell Physiol. 233:5805–5814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu X, Wang Y, Zhong W, Cheng H and Tian Z:
The long non-coding RNA MALAT1 enhances ovarian cancer cell
stemness by inhibiting YAP translocation from nucleus to cytoplasm.
Med Sci Monit. 26:e9220122020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang C, Guan Y, Lv M, Zhang R, Guo Z, Wei
X, Du X, Yang J, Li T, Wan Y, et al: Manganese increases the
sensitivity of the cGAS-STING pathway for double-stranded DNA and
is required for the host defense against DNA viruses. Immunity.
48:675–687.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li H, Li Q, Dang K, Ma S, Cotton JL, Yang
S, Zhu LJ, Deng AC, Ip YT, Johnson RL, et al: YAP/TAZ activation
drives uveal melanoma initiation and progression. Cell Rep.
29:3200–3211.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang
Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, et al: Long noncoding RNA
MALAT1 suppresses breast cancer metastasis. Nat Genet.
50:1705–1715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matthews DR, Paldanius PM, Proot P, Chiang
Y, Stumvoll M and Del Prato S; VERIFY study group, : Glycaemic
durability of an early combination therapy with vildagliptin and
metformin versus sequential metformin monotherapy in newly
diagnosed type 2 diabetes (VERIFY): A 5-year, multicentre,
randomised, double-blind trial. Lancet. 394:1519–1529. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Herrlinger U, Tzaridis T, Mack F,
Steinbach JP, Schlegel U, Sabel M, Hau P, Kortmann RD, Krex D,
Grauer O, et al: Lomustine-temozolomide combination therapy versus
standard temozolomide therapy in patients with newly diagnosed
glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A
randomised, open-label, phase 3 trial. Lancet. 393:678–688. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hazlewood GS, Barnabe C, Tomlinson G,
Marshall D, Devoe D and Bombardier C: Methotrexate monotherapy and
methotrexate combination therapy with traditional and biologic
disease modifying antirheumatic drugs for rheumatoid arthritis:
Abridged Cochrane systematic review and network meta-analysis. BMJ.
353:i17772016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wong PP, Demircioglu F, Ghazaly E,
Alrawashdeh W, Stratford MR, Scudamore CL, Cereser B,
Crnogorac-Jurcevic T, McDonald S, Elia G, et al: Dual-action
combination therapy enhances angiogenesis while reducing tumor
growth and spread. Cancer Cell. 27:123–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Q, Feng L, Liu J, Zhu W, Dong Z, Wu Y
and Liu Z: Intelligent albumin-MnO2 nanoparticles as pH-/H2
O2-responsive dissociable nanocarriers to modulate tumor hypoxia
for effective combination therapy. Adv Mater. 28:7129–7136. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wei H, Wang F, Wang Y, Li T, Xiu P, Zhong
J, Sun X and Li J: Verteporfin suppresses cell survival,
angiogenesis and vasculogenic mimicry of pancreatic ductal
adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci.
108:478–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Singh AD, Turell ME and Topham AK: Uveal
melanoma: Trends in incidence, treatment, and survival.
Ophthalmology. 118:1881–1885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alvarez-Rodriguez B, Latorre A, Posch C
and Somoza A: Recent advances in uveal melanoma treatment. Med Res
Rev. 37:1350–1372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Prasad S, Yadav VR, Sundaram C, Reuter S,
Hema PS, Nair MS, Chaturvedi MM and Aggarwal BB: Crotepoxide
chemosensitizes tumor cells through inhibition of expression of
proliferation, invasion, and angiogenic proteins linked to
proinflammatory pathway. J Biol Chem. 291:169212016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chian S, Thapa R, Chi Z, Wang XJ and Tang
X: Luteolin inhibits the Nrf2 signaling pathway and tumor growth in
vivo. Biochem Biophys Res Commun. 447:602–608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wilding JP: Combination therapy for
obesity. J Psychopharmacol. 31:1503–1508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Matsunaga S, Kishi T and Iwata N:
Combination therapy with cholinesterase inhibitors and memantine
for Alzheimer's disease: A systematic review and meta-analysis. Int
J Neuropsychopharmacol. 18:pyu1152014.PubMed/NCBI
|
|
56
|
Mijatović S, Savić-Radojević A,
Plješa-Ercegovac M, Simić T, Nicoletti F and Maksimović-Ivanić D:
The double-faced role of nitric oxide and reactive oxygen species
in solid tumors. Antioxidants (Basel). 9:3742020. View Article : Google Scholar
|
|
57
|
Assi M: The differential role of reactive
oxygen species in early and late stages of cancer. Am J Physiol
Regul Integr Comp Physiol. 313:R646–R653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pelicano H, Lu W, Zhou Y, Zhang W, Chen Z,
Hu Y and Huang P: Mitochondrial dysfunction and reactive oxygen
species imbalance promote breast cancer cell motility through a
CXCL14-mediated mechanism. Cancer Res. 69:2375–2383. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mao X, Yu CR, Li WH and Li WX: Induction
of apoptosis by shikonin through a ROS/JNK-mediated process in
Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell
Res. 18:879–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Takahashi M, Higuchi M, Makokha GN,
Matsuki H, Yoshita M, Tanaka Y and Fujii M: HTLV-1 Tax oncoprotein
stimulates ROS production and apoptosis in T cells by interacting
with USP10. Blood. 122:715–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Qin G, Wu L, Liu H, Pang Y, Zhao C, Wu S,
Wang X and Chen T: Artesunate induces apoptosis via a
ROS-independent and Bax-mediated intrinsic pathway in HepG2 cells.
Exp Cell Res. 336:308–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Koo JH and Guan KL: Interplay between
YAP/TAZ and metabolism. Cell Metab. 28:196–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang J, Wu S, Barrera J, Matthews K and
Pan D: The Hippo signaling pathway coordinately regulates cell
proliferation and apoptosis by inactivating yorkie, the drosophila
homolog of YAP. Cell. 122:421–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:12405–12410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
White SM, Avantaggiati ML, Nemazanyy I, Di
Poto C, Yang Y, Pende M, Gibney GT, Ressom HW, Field J, Atkins MB
and Yi C: YAP/TAZ inhibition induces metabolic and signaling
rewiring resulting in targetable vulnerabilities in NF2-deficient
tumor cells. Dev Cell. 49:425–443.e9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dent P, Booth L, Roberts JL, Liu J,
Poklepovic A, Lalani AS, Tuveson D, Martinez J and Hancock JF:
Neratinib inhibits Hippo/YAP signaling, reduces mutant K-RAS
expression, and kills pancreatic and blood cancer cells. Oncogene.
38:5890–5904. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Brouwer NJ, Konstantinou EK, Gragoudas ES,
Marinkovic M, Luyten GPM, Kim IK, Jager MJ and Vavvas DG: Targeting
the YAP/TAZ pathway in uveal and conjunctival melanoma with
verteporfin. Invest Ophthalmol Vis Sci. 62:32021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kumar VL, Verma S and Das P: Artesunate
suppresses inflammation and oxidative stress in a rat model of
colorectal cancer. Drug Dev Res. 80:1089–1097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Feng FB and Qiu HY: Effects of artesunate
on chondrocyte proliferation, apoptosis and autophagy through the
PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid
arthritis. Biomed Pharmacother. 102:1209–1220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
He WT, Wan H, Hu L, Chen P, Wang X, Huang
Z, Yang ZH, Zhong CQ and Han J: Gasdermin D is an executor of
pyroptosis and required for interleukin-1β secretion. Cell Res.
25:1285–1298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lv Y, Kim K, Sheng Y, Cho J, Qian Z, Zhao
YY and Hu G, Pan D, Malik AB and Hu G: YAP controls endothelial
activation and vascular inflammation through TRAF6. Circ Res.
123:43–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hutchinson JN, Ensminger AW, Clemson CM,
Lynch CR, Lawrence JB and Chess A: A screen for nuclear transcripts
identifies two linked noncoding RNAs associated with SC35 splicing
domains. BMC Genomics. 8:392007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang X, Hamblin MH and Yin KJ: The long
noncoding RNA Malat1: Its physiological and pathophysiological
functions. RNA Biol. 14:1705–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jin D, Guo J, Wu Y, Du J, Yang L, Wang X,
Di W, Hu B, An J, Kong L, et al: m6A mRNA methylation
initiated by METTL3 directly promotes YAP translation and increases
YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to
induce NSCLC drug resistance and metastasis. J Hematol Oncol.
12:1352019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sun Y, Jiang T, Jia Y, Zou J, Wang X and
Gu W: LncRNA MALAT1/miR-181a-5p affects the proliferation and
adhesion of myeloma cells via regulation of Hippo-YAP signaling
pathway. Cell Cycle. 18:2509–2523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang J, Wang H, Zhang Y, Zhen N, Zhang L,
Qiao Y, Weng W, Liu X, Ma L, Xiao W, et al: Mutual inhibition
between YAP and SRSF1 maintains long non-coding RNA, Malat1-induced
tumourigenesis in liver cancer. Cell Signal. 26:1048–1059. 2014.
View Article : Google Scholar : PubMed/NCBI
|