Introduction
Gastric cancer (GC) was reported the fifth most
commonly diagnosed malignancy and the third leading cause of
cancer-related deaths worldwide in 2018 (1). East Asia has the highest incidence and
mortality rates of GC (2). Although
advances have been made in GC treatment in recent years, the 5-year
survival rate for patients with advanced GC is <15% (3,4). Thus,
the identification of novel drugs and therapeutic targets for GC
treatment is urgently needed.
Palbociclib (PD0332991) is a potent and highly
selective inhibitor of cyclin-dependent kinase 4/6 (CDK4/6)
(5), which was approved by the Food
and Drug Administration in 2015 for the treatment of advanced
breast cancer (6). In addition,
increasing evidence demonstrates that palbociclib also plays an
important role in the treatment of multiple cancer types, including
hepatocellular carcinoma, head and neck cancer, colorectal cancer
and GC (7–10). However, the potential role of
palbociclib in GC has not been extensively analyzed.
The Notch signaling pathway is evolutionarily
conserved and can direct cell fate decisions by regulating
proliferation, cell cycle progression, apoptosis, differentiation,
senescence and metastasis (11,12).
Previous studies have demonstrated that the Notch pathway plays an
important role in tumorigenesis (13,14).
Moreover, activation of the Notch pathway could promote the
progression of GC (15). Jena et
al (16) reported that CDK6
kinase activity plays a key role in the onset of T cell acute
lymphoblastic leukemia via Notch1 activation in a mouse model.
However, whether palbociclib affects GC by modulating the Notch
pathway remains unknown. The present study aimed to determine the
effect, if any, of palbociclib on the Notch pathway in GC.
Materials and methods
Cell culture and drug treatment
The AGS and HGC-27 GC cell lines were obtained from
the American Type Culture Collection. All cells were cultured in
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (HyClone; Cytiva) and maintained at 37°C in
a humidified incubator containing 5% CO2. Palbociclib
was purchased from Pfizer (PD0332991), dissolved in DMSO and added
into the culture medium at the indicated concentration. Cells were
exposed to drug treatment for 2 days at 37°C, unless otherwise
indicated. Cells were randomly divided into seven groups: i)
Control (0 µM palbociclib); ii) 0.25 µM palbociclib; iii) 0.5 µM
palbociclib; iv) 1 µM palbociclib; v) 2 µM palbociclib; vi) 4 µM
palbociclib and vii) 0.5 µM palbociclib + 10 µM Jagged-1/FC. The
activator of Notch pathway (Jagged-1/FC) was supplied by Abcam.
MTT assay
The proliferation of AGS and HGC-27 cells was
measured using an MTT assay. After 48 h of palbociclib treatment,
cells (2×103 cells/well) were seeded in 96-well plates
for 24, 48, 72, 96 and 120 h at room temperature. At each time
point, a volume of 20 µl MTT solution (5 mg/ml) was then added to
each well, and the cells were incubated for an additional 4 h.
Finally, the absorbance at 495 nm was measured using a microplate
reader (Bio-Rad Laboratories, Inc.).
Colony formation assay
Colony-forming capability was measured using a
colony formation assay. After 48 h of palbociclib treatment, AGS
and HGC-27 cells were seeded into 6-well plates at a density of
1×103 cells/well and cultured with complete medium for 2
weeks. The cells were then fixed with 4% paraformaldehyde for 20
min at room temperature and stained with 0.1% crystal violet for 25
min at room temperature. The stained colonies were counted under a
light microscope.
Cell apoptosis assay
Apoptosis was assessed in AGS and HGC-27 cells using
an Annexin V-EGFP apoptosis detection kit (cat. no. KGA101; Nanjing
KeyGen Biotech Co., Ltd.). After 48 h of palbociclib treatment, AGS
and HGC-27 cells were collected and resuspended in binding buffer.
Annexin V-EGFP (5 µl) and propidium iodide (5 µl, PI; Beyotime
Institute of Biotechnology) were added to the cell suspension and
maintained at room temperature in the dark for 15 min. Early and
late apoptosis in the second and fourth quadrants were detected
using FACSCalibur, using CellQuest Pro software (version 5.1; BD
Biosciences).
Cell cycle analysis
Cell cycle progression was evaluated using PI
staining. After 48 h of palbociclib treatment, AGS and HGC-27 cells
were harvested and washed in PBS. Subsequently, the cells were
fixed in cold 75% ethanol at 4°C overnight. After washing with PBS
twice, the cells were stained with PI (40 µg/ml; Beyotime Institute
of Biotechnology) containing RNase A (20 ng/ml; Sigma-Aldrick;
Merck KGaA) for 30 min at 37°C in the dark. Cell cycle distribution
was then determined using FACSCalibur (BD Biosciences), using
ModFit LT software (version 3.1; Verity Software House).
Senescence-associated β-galactosidase
(SA-β-gal) staining
Cell senescence was measured using a Senescence
β-Galactosidase Staining kit (Beyotime Institute of Biotechnology),
as previously described (17). After
5 days of palbociclib treatment, AGS and HGC-27 cells were fixed
with 4% paraformaldehyde for 10 min at room temperature, then
incubated overnight at 37°C in freshly prepared β-gal staining
solution (1 mg/ml X-Gal, 5 mM potassium ferricyanide, 5 mM
potassium ferrocyanide, 40 mM Na2HPO4, 150 mM
NaCl and 2 mM MgCl2). The senescent cells were observed
and counted under a light microscope.
Reverse transcription-quantitative PCR
(RT-qPCR)
After 48 h of palbociclib treatment, total RNA from
AGS and HGC-27 cells was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA using TransScript One-Step gDNA Removal and
cDNA Synthesis SuperMix (TransGen Biotech Co., Ltd.). The
conditions for RT were as follows: 25°C for 5 min, 55°C for 20 min
and 85°C for 5 min. qPCR was then performed using the TransStart
TipTop Green qPCR SuperMix (TransGen Biotech Co., Ltd.). The primer
sequences (Sangon Biotech Co., Ltd.) were as follows: i) Notch1
sense, 5′-GACATCACGGATCATATGGA-3′ and antisense,
5′-CTCGCATTGACCATTCAAAC-3′; ii) Notch2 sense,
5′-TGCCAAGCTCAGTGGTGTTGTA-3′ and antisense,
5′-TGCTAGGCTTTGTGGGATTCAG-3′; iii) Hes1 sense,
5′-ACGTGCGAGGGCGTTAATAC-3′ and antisense,
5′-ATTGATCTGGGTCATGCAGTTG-3′; iv) β-actin sense,
5′-AGGCACCAGGGCGTGAT-3′ and antisense,
5′-GCCCACATAGGAATCCTTCTGAC-3′. The following thermocycling
conditions were used for qPCR: 95°C, 10 sec (denaturation); 55°C,
30 sec (annealing); 72°C, 30 sec (extension) for 40 cycles.
Relative expression levels were calculated using the
2−ΔΔCq method (18) and
normalized to the internal reference gene β-actin.
Western blot analysis
After 48 h of palbociclib treatment, total protein
was extracted from AGS and HGC-27 cells using RIPA lysis buffer
(Beyotime Institute of Biotechnology), as previously described
(19) and maintained at −80°C until
use. Protein concentrations were determined using a bicinchoninic
acid assay kit (Thermo Fisher Scientific, Inc.). Protein samples
(50 µg) were resolved by SDS-PAGE on 10% gels, then transferred
onto a polyvinylidene difluoride membrane and blocked for 1.5 h in
5% skimmed milk at room temperature. The membranes were then
incubated with primary antibodies against p16 (1:1,000; cat. no.
ab220800), p21 (1:1,000; cat. no. ab109199), p53 (1:1,000; cat. no.
ab131442), Bax (1:1,000; cat. no. ab77566) Caspase-3 (1:500; cat.
no. ab4051), Bcl-2 (1:1,000; cat. no. ab196495), Notch1 (1:500;
cat. no. ab52301), Notch2 (1:500; cat. no. ab8926), Hes1 (1:1,000;
cat. no. ab221788) and β-actin (1:1,000; cat. no. ab115777) (all
from Abcam) overnight at 4°C. Subsequently, the membranes were
incubated with the horseradish peroxidase-conjugated secondary
antibody (1:5,000; cat. no. 5961; Cell Signaling Technology, Inc.)
for 2 h at room temperature. Finally, the protein bands were
visualized with an ECL reagent (Amersham Biosciences) and analyzed
using Quantity One 1-D Analysis software (Bio-Rad Laboratories,
Inc.). The density of the protein bands was quantitated using
ImageJ software (version 1.8.0; National Institutes of Health).
Statistical analysis
Data are presented as the mean ± SD of three
independent measurements. Statistical analysis was performed using
GraphPad Prism 8.0 (GraphPad Software, Inc.) and SPSS 22.0 software
(IBM Corp.). One-way ANOVA followed by Tukey's post hoc test was
used to assess the differences between the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Palbociclib inhibits the proliferation
of AGS and HGC-27 cells
To evaluate the effect of palbociclib on cell
proliferation, AGS and HGC-27 cells were treated with different
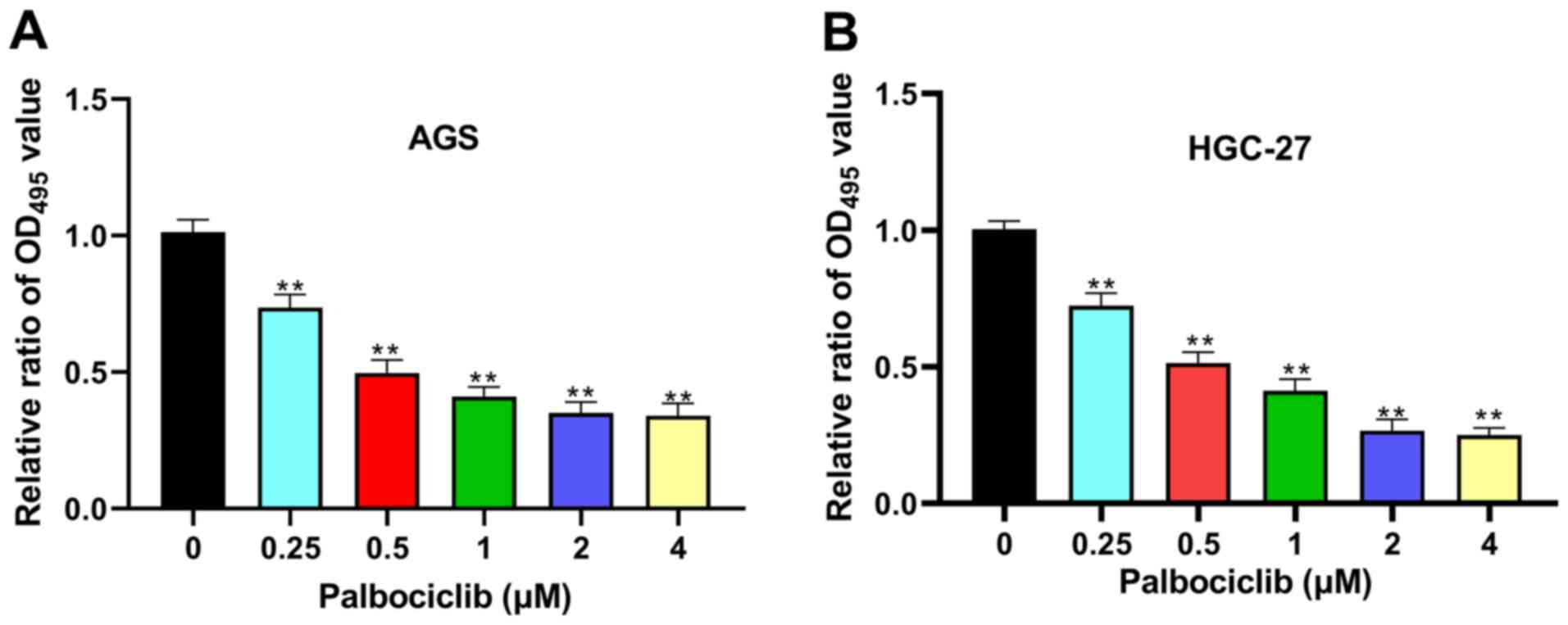
doses of palbociclib. Palbociclib significantly inhibited the
proliferation of AGS and HGC-27 cells in a dose-dependent manner
(Fig. 1). Palbociclib was used at
concentrations of 0.5, 1 and 2 µM for subsequent experiments. The
results of MTT assays indicated that palbociclib (0.5, 1 and 2 µM)
significantly inhibited the proliferation of AGS and HGC-27 cells
at day 1, 2, 3, 4 and 5, compared with the control group
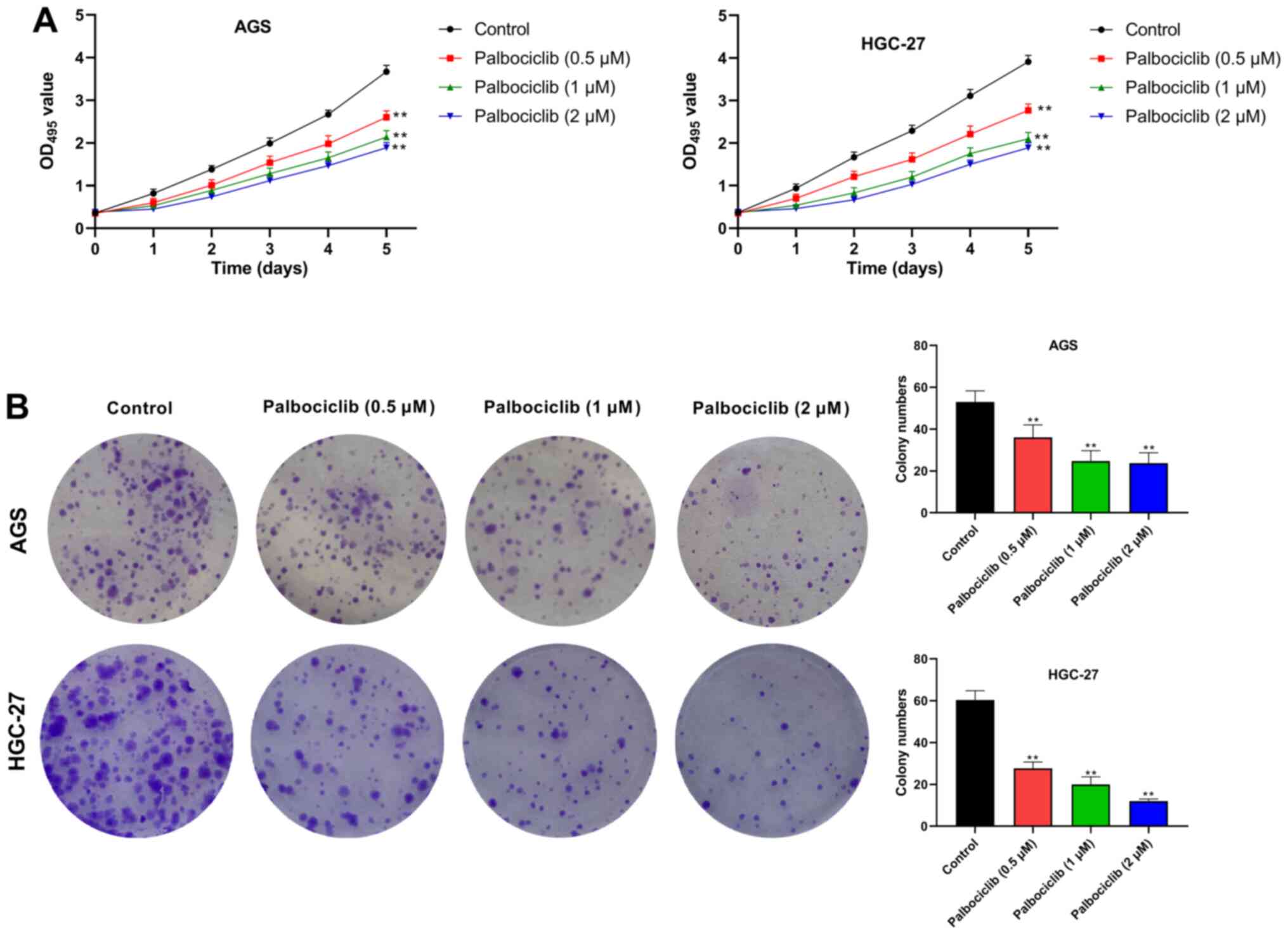
(P<0.01) (Fig. 2A). Moreover, in
colony formation assays, the number of AGS and HGC-27 cell clones
were significantly reduced by palbociclib in a dose-dependent
manner (P<0.01) (Fig. 2B). Thus,
palbociclib inhibited the proliferation of AGS and HGC-27
cells.
Palbociclib promotes the apoptosis of
AGS and HGC-27 cells
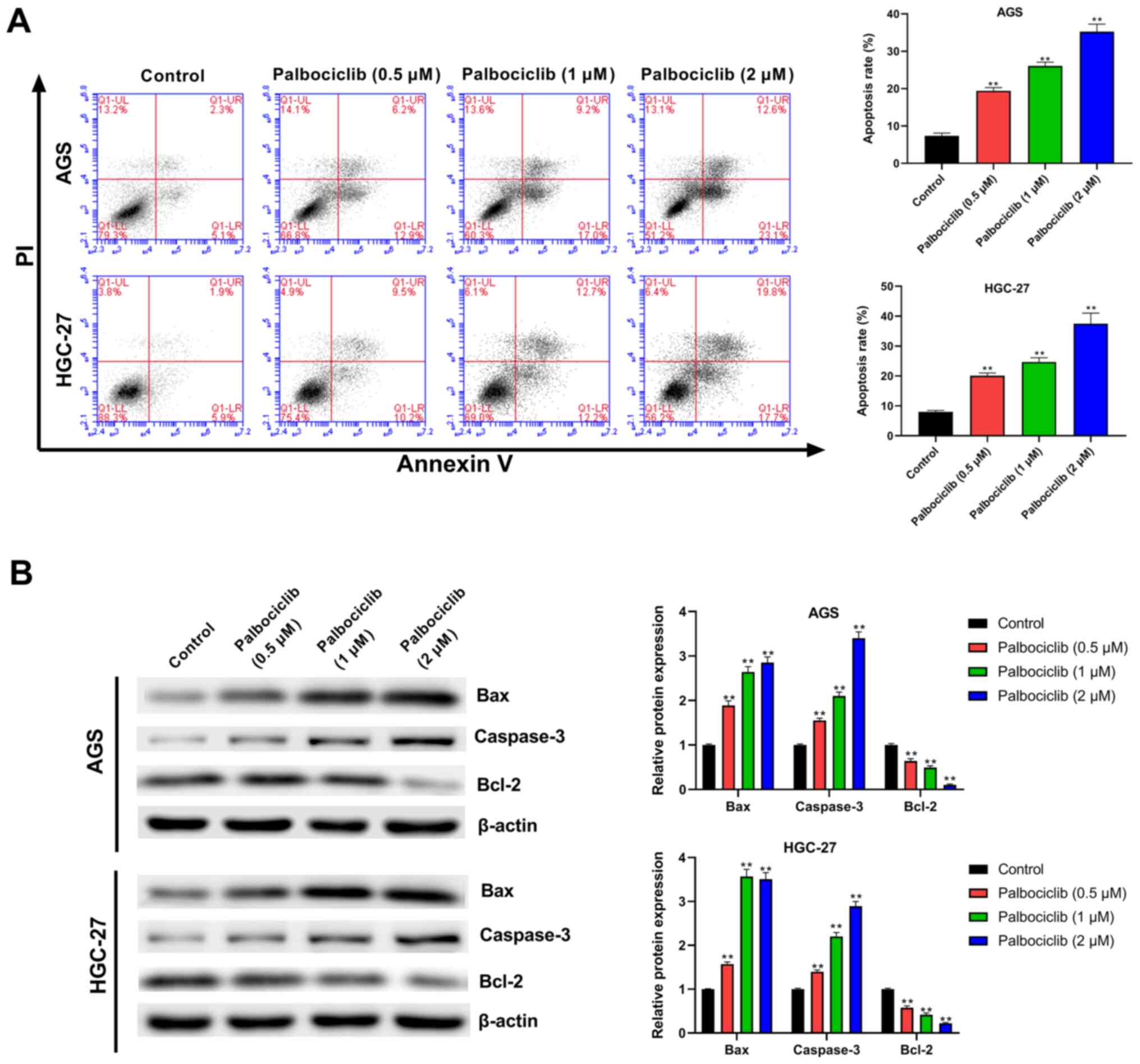
As shown in Fig. 3A,
palbociclib (0.5, 1 and 2 µM) significantly increased the apoptosis
of AGS and HGC-27 cells relative to the control group (P<0.01).
To confirm the effect of palbociclib on apoptosis, the expression
of apoptosis-related proteins was detected using western blotting.
Palbociclib significantly reduced Bcl-2 expression in AGS and
HGC-27 cells in a dose-dependent manner compared with the control
group, but increased the expression levels of Bax and Caspase-3
(P<0.01 in all cases; Fig. 3B).
This finding suggested that palbociclib promoted the apoptosis of
AGS and HGC-27 cells.
Palbociclib induces cell senescence
and cell cycle arrest in AGS and HGC-27 cells
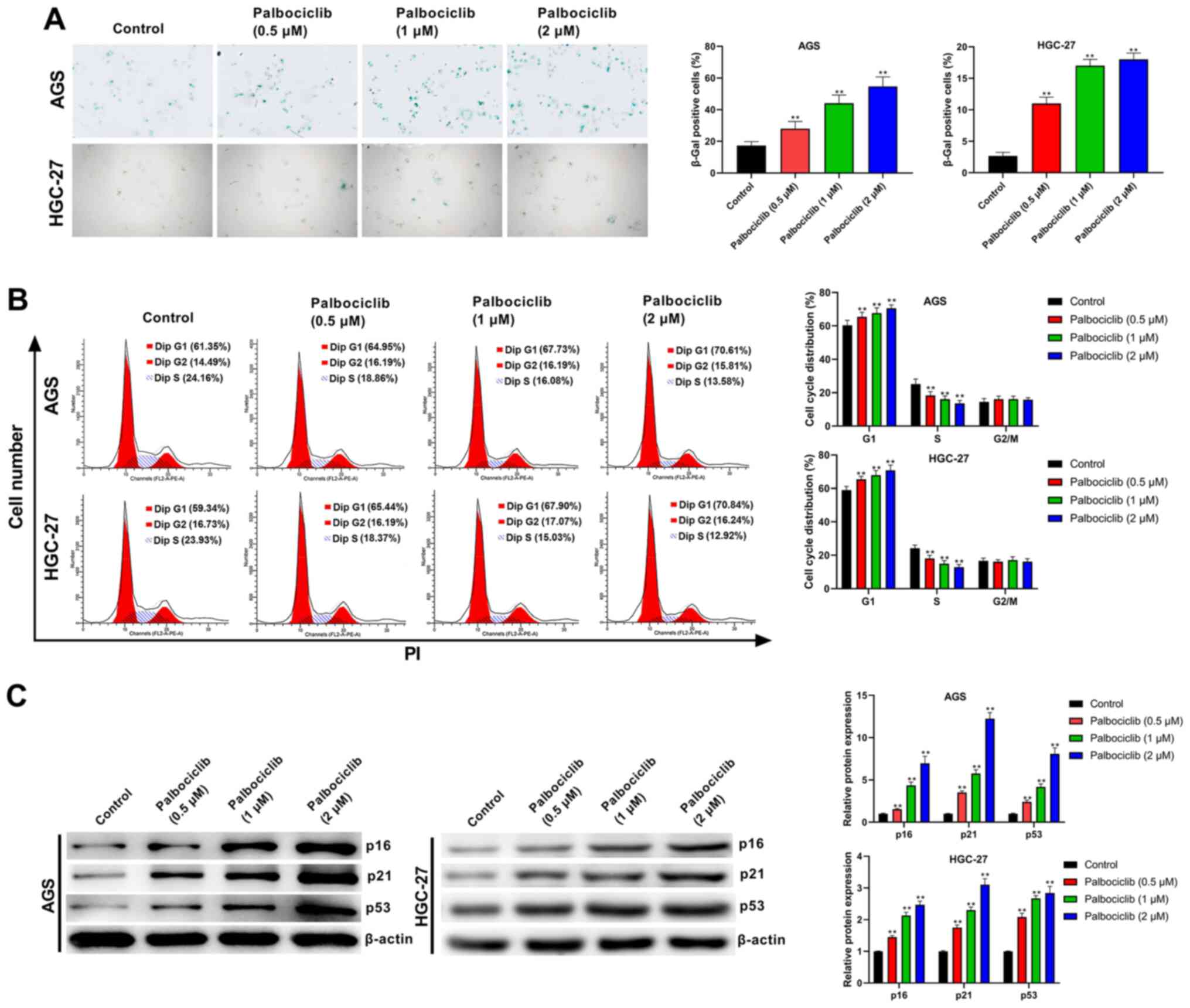
As shown in Fig. 4A,
palbociclib (0.5, 1 and 2 µM) significantly increased the
senescence of AGS and HGC-27 cells compared with the control group
(P<0.01). In addition, the cell cycle analysis revealed that
palbociclib (0.5, 1 and 2 µM) increased the proportion of cells in
the G1 phase in both AGS and HGC-27 cells, but decreased
the proportion of cells in the S phase (P<0.01) (Fig. 4B), suggesting that palbociclib could
induce an accumulation of AGS and HGC-27 cells in the G1
phase of the cell cycle. Furthermore, palbociclib significantly
increased the expression levels of p16, p21 and p53 in AGS and
HGC-27 cells compared with the control group, and this effect was
dose-dependent (P<0.01 in all cases; Fig. 4C). These results indicated that
palbociclib induced cell senescence and cell cycle arrest in AGS
and HGC-27 cells.
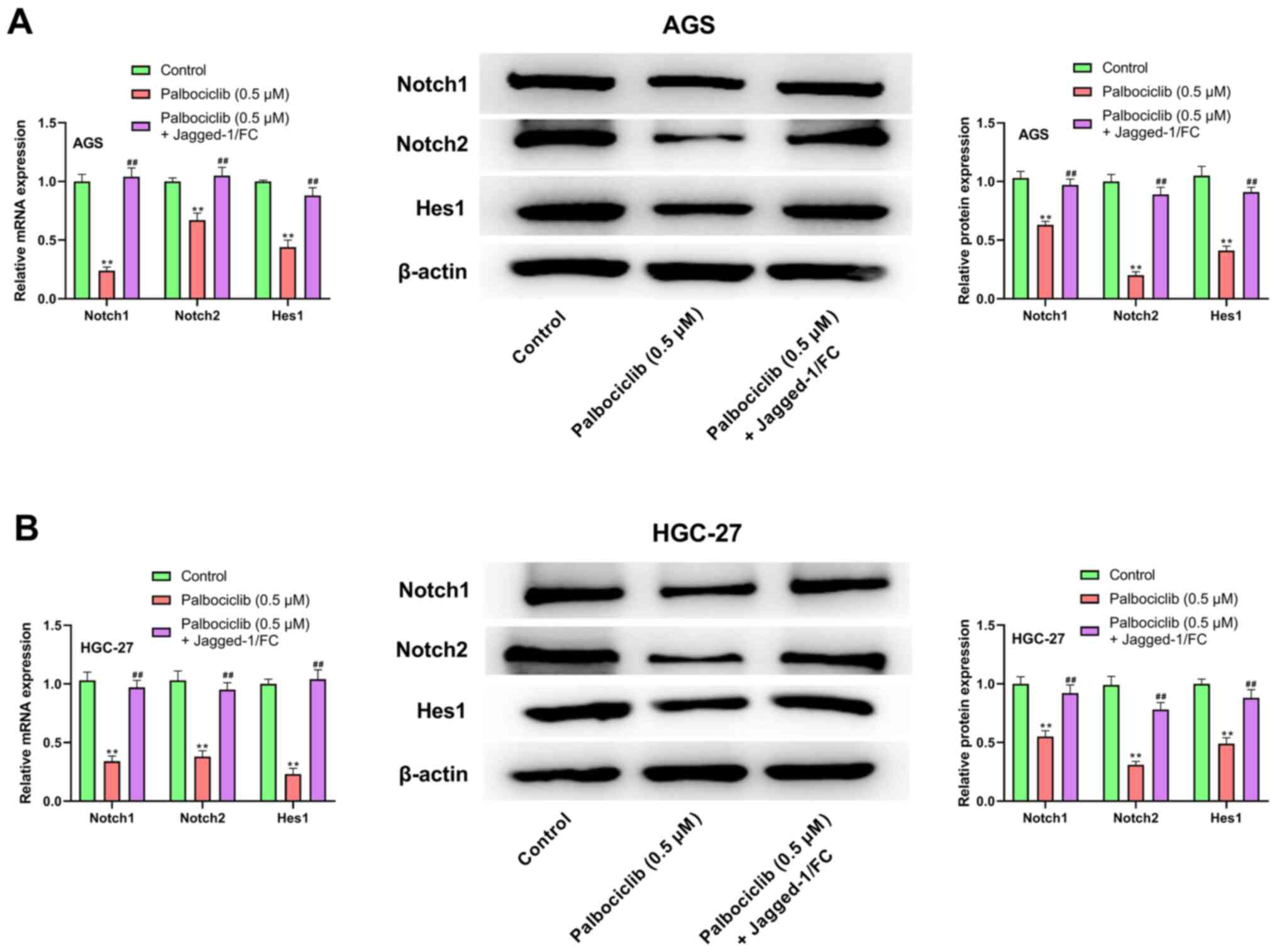
Palbociclib inhibits the Notch
signaling pathway in AGS and HGC-27 cells
As shown in Fig. 5,
palbociclib (0.5 µM) significantly reduced the mRNA and protein
expression levels of Notch1, Notch2 and Hes1 in AGS and HGC-27
cells compared with the control group (P<0.01 in all cases).
However, compared with the palbociclib (0.5 µM) group, the mRNA and
protein expression levels of Notch1, Notch2 and Hes1 in AGS and
HGC-27 cells were significantly increased following treatment with
palbociclib (0.5 µM) + Jagged-1/FC (P<0.01 in all cases). These
results suggested that palbociclib inhibited the Notch signaling
pathway in AGS and HGC-27 cells.
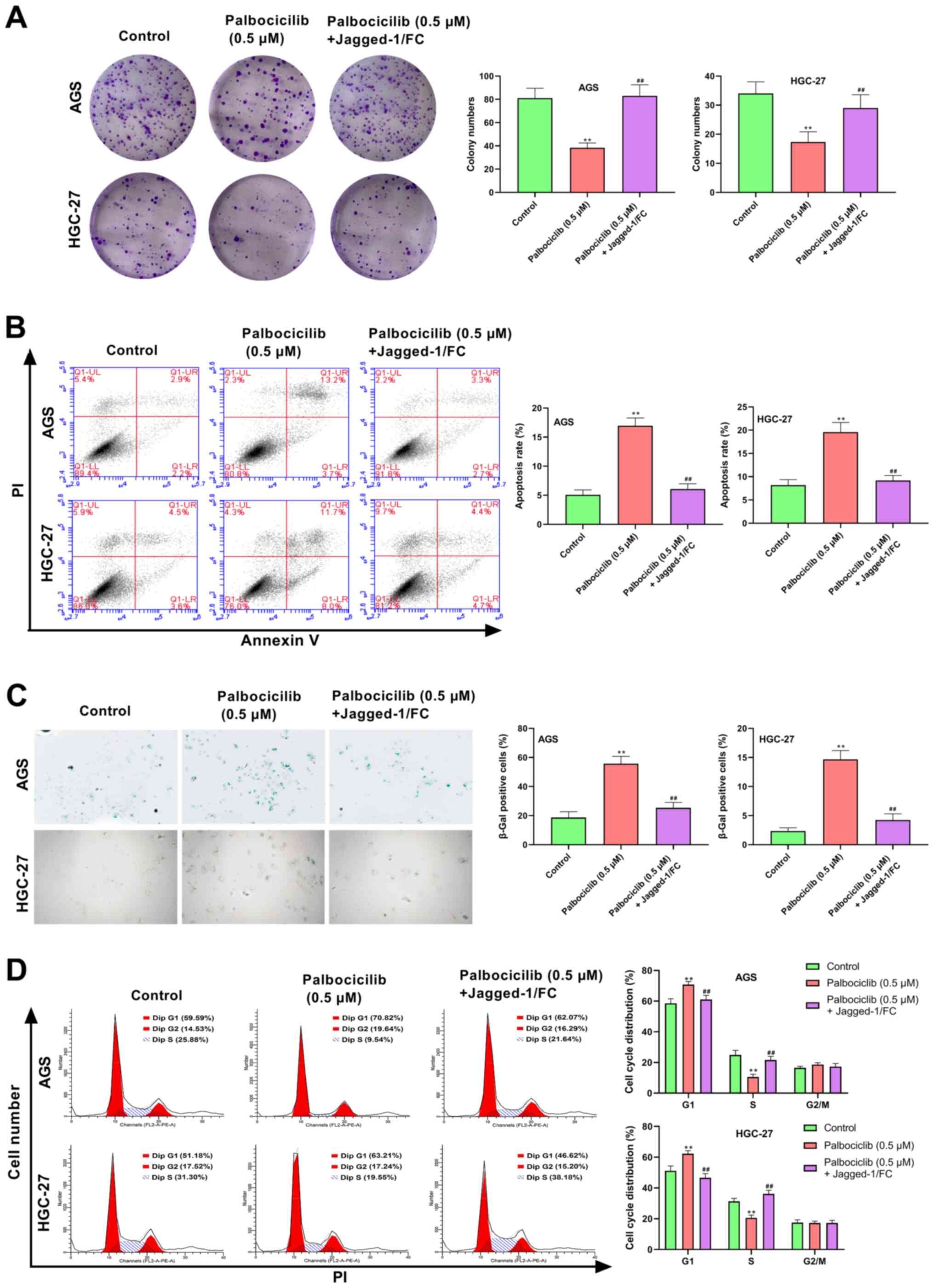
Jagged-1/FC reverses the effect of
palbociclib on the proliferation, apoptosis, cell senescence and
cell cycle progression of AGS and HGC-27 cells
As shown in Fig. 6,
AGS and HGC-27 cells treated with palbociclib (0.5 µM) displayed
significantly inhibited proliferation, increased apoptosis,
increased cell senescence and cell cycle arrest compared with the
control (P<0.01). Compared with palbociclib (0.5 µM) treatment,
the proliferation of AGS and HGC-27 cells was markedly increased in
the palbociclib (0.5 µM) + Jagged-1/FC group (P<0.01), whereas
apoptosis, senescence and cell cycle arrest were notably decreased
(P<0.01). Altogether, these results suggested that palbociclib
could inhibit cell proliferation and induce senescence, cell cycle
arrest and apoptosis by inhibiting the Notch pathway in AGS and
HGC-27 cells.
Discussion
Most patients with GC are diagnosed at an advanced
stage and usually show extensive tumor infiltration and distant
lymph node metastasis (20,21). The overall 5-year survival rate for
patients with advanced GC is <10% (4). Thus, the identification of novel drugs
and therapeutic targets for the treatment of GC is urgently needed.
In the present study, palbociclib inhibited proliferation and
induced senescence, cell cycle arrest and apoptosis by inhibiting
the Notch pathway in GC cells.
Cell division depends on the cell cycle, which is
regulated by CDKs (22,23). The interaction of cyclin D with CDK4
and CDK6 promotes the phosphorylation of the retinoblastoma gene
product, which in turn leads the cell cycle from the G1
phase to the S phase (22). There is
currently much interest in CDK6 and the closely related CDK4 kinase
as targets for cancer therapy. A previous study reported that
palbociclib is a highly selective CDK4/6 inhibitor that can block
the transition from the G1 to the S phase of the cell
cycle (24). The present study also
confirmed that palbociclib could inhibit cell proliferation and
induce cell cycle arrest at the G1 phase in GC cells in
a dose-dependent manner.
In addition, a previous study also indicated that
palbociclib could suppress the progression of nasopharyngeal
carcinoma by mediating tumor cell apoptosis (24). Zhang et al (25) have reported that palbociclib could
trigger cell apoptosis by upregulating Caspase-3 and downregulating
Bcl-xl (25). The present study
indicated that Palbociclib might facilitate GC cell apoptosis in a
dose-dependent manner. Moreover, palbociclib decreased Bcl-2
expression and increased the expression of Bax and Caspase-3, which
is a new finding that contrasts with a study by Min et al
(26).
Cell senescence plays an important role against the
progression of cancers and represents another intracellular
tumor-suppressive mechanism (27).
Evidence indicates that cell senescence is associated with the
induction of several genes, including p16, p21 and p53 (28). Previous studies have confirmed that
palbociclib can induce cell senescence in several types of cancer,
including hepatocellular carcinoma, breast cancer, lung cancer and
glioma (7,29,30). In
the present study, SA-β-gal staining results suggested that
palbociclib promoted GC cell senescence in a dose-dependent manner.
In addition, western blot results also showed that palbociclib
increased the expression levels of p16, p21 and p53 in GC cells,
further confirming that palbociclib could promote GC cell
senescence.
In mammals, the Notch pathway is mediated by four
Notch receptors, Notch 1–4, and five ligands, delta-like ligand
(DLL) 1, DLL3, DLL4, Jagged-1 and Jagged-2 (31,32). The
Notch pathway is activated when one of these ligands binds to a
Notch receptor. Once the Notch pathway is activated, the Notch
receptors are cleaved, and the Notch Intracellular Domain (NICD)
translocates into the nucleus to regulate downstream target genes,
such as Hes1, for instance (33).
Previous studies have shown Notch activation leads to activation of
CDK6 in the setting of mouse or human T-cell acute lymphoblastic
leukemia (34,35). The Notch pathway plays an oncogenic
role in multiple cancer types, including hepatocellular carcinoma,
glioma and breast cancer, by modulating proliferation, cell cycle,
apoptosis, differentiation, senescence and metastasis (12,36).
Hibdon et al (37) have
suggested that the activation of the Notch pathway could facilitate
the proliferation of GC cells. Moreover, Notch1 and Notch2
signaling is essential for gastric stem cell proliferation in the
stomach tissue of mice and humans (38,39). In
the present study, palbociclib significantly inhibited
proliferation and induced senescence, cell cycle arrest and
apoptosis by inhibiting the Notch pathway in AGS and HGC-27 cells,
which highlights a new regulatory mechanism for palbociclib in GC
that contrasts with a previous study (26). One of the limitations of the present
study was that healthy cell lines were not used as a control.
Furthermore, additional studies are required to determine whether
palbociclib produces the same effect in other GC cell lines.
In conclusion, this study demonstrated that
palbociclib could inhibit proliferation and induce senescence, cell
cycle arrest and apoptosis in GC cells. In addition, the
tumor-suppressive mechanism of palbociclib may be associated with
the inhibition of the Notch pathway in GC. By revealing a new
mechanism whereby palbociclib, regulates the Notch pathway in GC,
the findings presented here not only add more data to the
anticancer mechanisms of palbociclib, but also provide another
option for the treatment of GC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YH made substantial contributions to conception and
design. HB, JS, XZ and JX prepared the experimental materials and
performed the experiments. HB, XZ and JX interpreted the data,
performed the statistical analysis and analyzed the results. HB
revised and approved the final version of the manuscript. HB, XZ
and JX confirmed the authenticity of all the raw data. All authors
have read and approved the final manuscript and agree to be
accountable for all aspects of the work in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song YW, Lim Y and Cho SK:
2,4-Di-tert-butylphenol, a potential HDAC6 inhibitor, induces
senescence and mitotic catastrophe in human gastric adenocarcinoma
AGS cells. Biochim Biophys Acta Mol Cell Res. 1865:675–683. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goscinski MA, Larsen SG, Warloe T, Stoldt
S, Nesland JM, Suo ZH and Giercksky KE: Adenocarcinomas on the
rise-does it influence survival from oesophageal cancer? Scand J
Surg. 98:214–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruf C, Thomusch O, Goos M, Makowiec F,
Illerhaus G and Ruf G: Impact of neoadjuvant chemotherapy with
PELF-protocoll versus surgery alone in the treatment of advanced
gastric carcinoma. BMC Surg. 14:52014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finn RS, Dering J, Conklin D, Kalous O,
Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al: PD
0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially
inhibits proliferation of luminal estrogen receptor-positive human
breast cancer cell lines in vitro. Breast Cancer Res. 11:R772009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beaver JA, Amiri-Kordestani L, Charlab R,
Chen W, Palmby T, Tilley A, Zirkelbach JF, Yu J, Liu Q, Zhao L, et
al: FDA approval: Palbociclib for the treatment of postmenopausal
patients with estrogen receptor-positive, HER2-negative metastatic
breast cancer. Clin Cancer Res. 21:4760–4766. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bollard J, Miguela V, Ruiz de Galarreta M,
Venkatesh A, Bian CB, Roberto MP, Tovar V, Sia D, Molina-Sánchez P,
Nguyen CB, et al: Palbociclib (PD-0332991), a selective CDK4/6
inhibitor, restricts tumour growth in preclinical models of
hepatocellular carcinoma. Gut. 66:1286–1296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michel L, Ley J, Wildes TM, Schaffer A,
Robinson A, Chun SE, Lee W, Lewis J Jr, Trinkaus K and Adkins D:
Phase I trial of palbociclib, a selective cyclin dependent kinase
4/6 inhibitor, in combination with cetuximab in patients with
recurrent/metastatic head and neck squamous cell carcinoma. Oral
Oncol. 58:41–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Zhou L, Zhao S, Dicker DT and
El-Deiry WS: The CDK4/6 inhibitor palbociclib synergizes with
irinotecan to promote colorectal cancer cell death under hypoxia.
Cell Cycle. 16:1193–1200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valenzuela CA, Vargas L, Martinez V, Bravo
S and Brown NE: Palbociclib-induced autophagy and senescence in
gastric cancer cells. Ex Cell Res. 360:390–396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bolós V, Grego-Bessa J and de la Pompa JL:
Notch signaling in development and cancer. Endocr Rev. 28:339–363.
2007. View Article : Google Scholar
|
|
12
|
Giovannini C, Gramantieri L, Minguzzi M,
Fornari F, Chieco P, Grazi GL and Bolondi L: CDKN1C/P57 is
regulated by the notch target gene hes1 and induces senescence in
human hepatocellular carcinoma. Am J Pathol. 181:413–422. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu J, Yu J, Gan J, Song N, Shi L, Liu J,
Zhang Z and Du J: Notch1/2/3/4 are prognostic biomarker and
correlated with immune infiltrates in gastric cancer. Aging (Albany
NY). 12:2595–2609. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Revandkar A, Perciato ML, Toso A, Alajati
A, Chen J, Gerber H, Dimitrov M, Rinaldi A, Delaleu N, Pasquini E,
et al: Inhibition of Notch pathway arrests PTEN-deficient advanced
prostate cancer by triggering p27-driven cellular senescence. Nat
Commun. 7:137192016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piao HY, Guo S, Wang Y and Zhang J: Long
noncoding RNA NALT1-induced gastric cancer invasion and metastasis
via NOTCH signaling pathway. World J Gastroenterol. 25:6508–6526.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jena N, Sheng J, Hu JK, Li W, Zhou W, Lee
G, Tsichlis N, Pathak A, Brown N, Deshpande A, et al: CDK6-mediated
repression of CD25 is required for induction and maintenance of
Notch1-induced T-cell acute lymphoblastic leukemia. Leukemia.
30:1033–1043. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong X, Hu X, Chen J, Hu D and Chen LF:
BRD4 regulates cellular senescence in gastric cancer cells via
E2F/miR-106b/p21 axis. Cell Death Dis. 9:2032018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Q, Xu S, Li X, Wang B, Wang X, Ma D,
Yang L, Peng J and Hou M: Pathway of Toll-like receptor 7/B cell
activating factor/B cell activating factor receptor plays a role in
immune thrombocytopenia in vivo. PLoS One. 6:e227082011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dassen AE, Dikken JL, van de Velde CJ,
Wouters MW, Bosscha K and Lemmens VE: Changes in treatment patterns
and their influence on long-term survival in patients with stages
I–III gastric cancer in The Netherlands. Int J Cancer.
133:1859–1866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie X, Zheng W, Chen T, Lin W, Liao Z, Liu
J and Ding Y: CDK4/6 inhibitor palbociclib amplifies the
radiosensitivity to nasopharyngeal carcinoma cells via mediating
apoptosis and suppressing DNA damage repair. Onco Targets Ther.
12:11107–11117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang G, Ma F, Li L, Li J, Li P, Zeng S,
Sun H and Li E: Palbociclib triggers apoptosis in bladder cancer
cells by Cdk2-induced Rad9-mediated reorganization of the
Bak.Bcl-xl complex. Biochem Oharmacol. 163:133–141. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Min A, Kim JE, Kim YJ, Lim JM, Kim S, Kim
JW, Lee KH, Kim TY, Oh DY, Bang YJ and Im SA: Cyclin E
overexpression confers resistance to the CDK4/6 specific inhibitor
palbociclib in gastric cancer cells. Cancer Lett. 430:123–132.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hornsby PJ: Senescence as an anticancer
mechanism. J Clin Oncol. 25:1852–1857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kovatcheva M, Liu DD, Dickson MA, Klein
ME, O'Connor R, Wilder FO, Socci ND, Tap WD, Schwartz GK, Singer S,
et al: MDM2 turnover and expression of ATRX determine the choice
between quiescence and senescence in response to CDK4 inhibition.
Oncotarget. 6:8226–8243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Puyol M, Martin A, Dubus P, Mulero F,
Pizcueta P, Khan G, Guerra C, Santamaría D and Barbacid M: A
synthetic lethal interaction between K-Ras oncogenes and Cdk4
unveils a therapeutic strategy for non-small cell lung carcinoma.
Cancer Cell. 18:63–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ntziachristos P, Lim JS, Sage J and
Aifantis I: From Fly wings to targeted cancer therapies: A
centennial for notch signaling. Cancer Cell. 25:318–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Espinoza I and Miele L: Notch inhibitors
for cancer treatment. Pharmacol Ther. 139:95–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S,
Wu GS and Wu K: Notch signaling: An emerging therapeutic target for
cancer treatment. Cancer Lett. 369:20–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li N, Fassl A, Chick J, Inuzuka H, Li X,
Mansour MR, Liu L, Wang H, King B, Shaik S, et al: Cyclin C is a
haploinsufficient tumour suppressor. Nat Cell Biol. 16:1080–1091.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Joshi I, Minter LM, Telfer J, Demarest RM,
Capobianco AJ, Aster JC, Sicinski P, Fauq A, Golde TE and Osborne
BA: Notch signaling mediates G1/S cell-cycle progression in T cells
via cyclin D3 and its dependent kinases. Blood. 113:1689–1698.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiu MX, Liu YM and Kuang BH: The oncogenic
role of Jagged1/Notch signaling in cancer. Biomed Pharmacother.
129:1104162020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hibdon ES, Razumilava N, Keeley TM, Wong
G, Solanki S, Shah YM and Samuelson LC: Notch and mTOR signaling
pathways promote human gastric cancer cell proliferation.
Neoplasia. 21:702–712. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Demitrack ES, Gifford GB, Keeley TM,
Horita N, Todisco A, Turgeon DK, Siebel CW and Samuelson LC: NOTCH1
and NOTCH2 regulate epithelial cell proliferation in mouse and
human gastric corpus. Am J Physiol Gastrointest Liver Physiol.
312:G133–G144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gifford GB, Demitrack ES, Keeley TM, Tam
A, La Cunza N, Dedhia PH, Spence JR, Simeone DM, Saotome I, Louvi
A, et al: Notch1 and Notch2 receptors regulate mouse and human
gastric antral epithelial cell homoeostasis. Gut. 66:1001–1011.
2017. View Article : Google Scholar : PubMed/NCBI
|