Introduction
Gallbladder cancer (GBC) is the most common
malignancy of the biliary tract originating from epithelial cells
of the gallbladder with a particularly high incidence (up to 7.5
per 100,000 for men and 23 per 100,000 for women) in native
American and South American populations (1). It ranks sixth among malignancies of the
alimentary tract and its prevalence is higher in women than in men
(2). The global incidence rate of
GBC has recently increased, notably in India, Eastern Europe and
China (3). GBC is closely associated
with genetic and environmental factors, such as chronic infection
of the gallbladder, exposure to toxic compounds and heavy metals
and dietary habits (1,4). GBC is highly heterogeneous and
invasive, which develops liver metastases due to its adjacency to
the liver and lack of serosa layer (2). The diagnosis of early-stage GBC is
considerably difficult and the disease develops lymphatic
metastasis (5). Patients with GBC
have a high mortality rate, with an overall 5-year survival rate of
~5% (6). Notably, the metastatic
spread of GBC is the main reason for its high morbidity (2). Although radical surgery is a preferred
method for the treatment of GBC, its resection rate is low
(1). Current treatment methods for
GBC are ineffective, with very poor treatment efficacy (2). Thus, this cancer type is considered a
serious threat to public health. Based on this evidence, it is
important to identify specific and efficient drugs for the
treatment of GBC.
Melatonin or N-acetyl-5-methoxytrypamine is a
neurohormone predominantly synthesized and secreted by the pineal
gland, with potent antioxidant, immunomodulatory and anticancer
effects (7). In vivo and
in vitro data have indicated that melatonin can inhibit
tumor growth (8–10). However, the function and mechanism of
action of melatonin vary in different types of cancer. For example,
melatonin induces the inhibition of leiomyosarcoma cell
proliferation and invasion, which is mediated by aerobic glycolysis
(known as the Warburg effect) (11).
However, in lung cancer cells, melatonin suppresses cancer
metastasis by inhibiting epithelial-to-mesenchymal transition (EMT)
by targeting Twist (12). In renal
cell carcinoma, melatonin inhibits matrix metalloproteinase-9 by
suppressing the AKT/MAPK pathway and the NF-κB DNA-binding activity
(13). In human epidermal growth
factor receptor 2-positive human breast cancer cells, melatonin
represses metastasis by suppressing the expression of ribosomal
protein S6 kinase 2 (14). Despite
its extensive antimetastatic function, the effects of melatonin on
GBC remain unknown.
In the present study, the antimetastatic effects of
melatonin on GBC were investigated. In addition, the molecular
mechanism of melatonin involved in gallbladder cancer invasion and
metastasis was investigated. This hormone may be considered as a
potential candidate drug in the treatment of metastatic GBC.
Materials and methods
Cell culture
The human GBC cell line, GBC-SD, was purchased from
the Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. The NOZ cell line was gifted by Dr Jianwen Ye, Department
of Hepatobiliary and Pancreatic Surgery, The First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China). Cells were
maintained in RPMI-1640 medium (Beijing Solarbio Science &
Technology Co., Ltd.) supplemented with 10% fetal bovine serum
(FBS, Gibco; Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin/streptomycin, at 37°C with 5% CO2 (Thermo
Fisher Scientific, Inc.).
Drug treatment
Melatonin (>99%) was purchased from
MedChemExpress and dissolved in DMSO (Sigma-Aldrich; Merck KGAa).
GBC-SD and NOZ cells were treated with melatonin (0.00, 0.25, 0.50,
1.00, 2.00 and 3.00 mM) for 24 or 48 h, and DMSO was used as the
control. Tert-Butylhydroquinone (tBHQ) was purchased from
MedChemExpress and dissolved in DMSO. For detection of the ERK
pathway, GBC-SD cells were treated with 2 mM melatonin and 50 µM
tBHQ for 3, 6 and 12 h. Cell morphology was assessed using an
inverted fluorescence microscope (IX71; Olympus Corporation)
(magnification ×100).
Cell Counting Kit-8 (CCK-8) assay
Cell viability was assessed via the CCK-8 assay
(Suzhou Yuheng Biological Technology Co., Ltd.). Briefly, GBC-SD
cells were seeded into 96-well plates at a density of
5×103 cells/well. Cells were treated with different
concentrations of melatonin (0.25, 0.5, 1, 2 and 3 mM) for 24 and
48 h. Melatonin was diluted in DMSO (0.01%). The control group
contained DMSO, which was adjusted according to the corresponding
concentration of the highest melatonin dosage (3 mM) to exclude the
possibility of the solvent causing effects on the cells.
Subsequently, 10 µl CCK-8 solution was added to each well and the
plates were incubated at 37°C for 1 h. The absorbance was measured
at a wavelength of 450 nm, using the Multiskan Spectrum (Varioskan
LUX; Thermo Fisher Scientific, Inc.).
Wound healing assay
GBC-SD and NOZ cells were seeded into 6-well plates
at a density of 1×106 cells/well and cultured at 37°C
with 5% CO2. Once cells reached 100% confluence, the
monolayers were scratched to create a linear wound, using a 100 µl
pipette tip. Cells were washed three times with PBS, and RPMI-1640
medium (1% FBS) with 3 mM melatonin or DMSO were added to each
well. Following the indicated treatment periods (24 or 48 h), cells
were observed under an inverted fluorescence microscope (IX71;
Olympus Corporation) (magnification ×100) and the images were
captured. The proportion of wound closure was calculated according
to the migration over the denuded area.
Cell invasion assay
The invasion assay was performed using Transwell
chambers (Corning, Inc.). Matrigel (BD Biosciences) was diluted at
a ratio of 1:10. A total of 100 µl diluted Matrigel was added
vertically in the upper chambers and cultured at 37°C with 5%
CO2 overnight. Once the cell monolayer reached 90%
confluence, GBC-SD and NOZ cells were digested with trypsin. Cells
were subsequently suspended in serum-free medium with or without
melatonin (2 mM) and the concentration was adjusted to
2×105/ml. Subsequently 100 µl cell suspension was added
to the upper chamber. A total of 600 µl RPMI-1640 medium with 10%
FBS was plated in the lower chambers, and cells were incubated at
37°C for 24 h. Non-invasive cells were removed, while invasive
cells were fixed in 4% formalin at room temperature for 20 min and
subsequently stained with 0.1% crystal violet at room temperature
for 15 min. Stained cells were counted in five randomly selected
fields using a light microscope (magnification ×200).
Western blotting
GBC-SD and NOZ cells were lysed with RIPA lysis
buffer (Beijing Solarbio Science & Technology Co., Ltd.) on ice
for 20 min. The cell lysate was collected and centrifuged at 13,000
× g for 15 min. Total protein was quantified using Bicinchoninic
acid (Beijing Solarbio Science & Technology Co., Ltd.).
Proteins (50 µg/lane) were separated by 10% SDS-PAGE. The separated
proteins were subsequently transferred onto polyvinylidene fluoride
membranes and blocked with non-fat milk for 3 h at room
temperature. The membranes were incubated with primary antibodies
against E-cadherin (1:5,000; cat. no. 20874-1-AP); N-cadherin
(1:5,000; cat. no. 22018-1-AP); vimentin (1:5,000; cat. no.
10366-1-AP); Snail (1:800; cat. no. 13099-1-AP); ERK (1:1,000; cat.
no. AB3373); phosphorylated (p)-ERK (1:1,000; cat. no. AB3355); AKT
(1:1,000; cat. no. AY0420); p-AKT (1:1,000; cat. no. AY0421);
NF-κB-p65 (1:1,000; cat. no. AY5034), p-NF-κB-p65 (1:1,000; cat.
no. CY6372); PI3K (1:1,000; cat. no. CY5355) and p-PI3K (1:1,000;
cat. no. CY6427), overnight at 4°C. E-cadherin, N-cadherin,
Vimentin and Snail polyclonal antibodies were purchased from
ProteinTech Group, Inc. ERK, p-ERK, AKT, p-AKT, NF-κB-p65,
p-NF-κB-p65, PI3K and p-PI3K antibodies were purchased from
Shanghai Abways Biotechnology Co., Ltd. Membranes were washed three
times with TBS and Polysorbate 20, and subsequently incubated with
Rabbit Anti-Goat IgG (H+L) horseradish peroxidase (HRP) (1:5,000;
cat. no. AB0103; Shanghai Abways Biotechnology Co., Ltd.) for 1 h
at room temperature. Protein bands were visualized using an ECL kit
(Suzhou Xinsaimei Biotechnology Co., Ltd.). The gray levels of the
protein bands were analyzed using ImageJ 1.8.0 software (National
Institutes of Health) and the relative protein levels were
normalized to GAPDH.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from GBC-SD cells using
TRIzol® reagent (Gibco; Thermo Fisher Scientific, Inc.),
according to the manufacturers instruction. cDNA was synthesized
using the HiScript II Reverse Transcriptase kit (Vazyme Biotech
Co., Ltd.) according to the manufacturer's protocol. The reverse
transcription conditions used were as follows: 25°C for 5 min, 50°C
for 15 min and 85°C for 2 min. qPCR was subsequently performed
using the PowerUp™ SYBR-Green Master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.), with specific primer sequences.
The thermocycling conditions used were as follows: Pre-denaturation
at 95°C for 30 sec, denaturation at 95°C for 5 sec and annealing at
59.5°C for 30 sec for a total of 40 cycles. The fold change in
relative mRNA levels was expressed as the relative quantification
calculated by the average of the standardized gene expression
levels 2−ΔΔCq (15).
GAPDH was used as the housekeeping gene. The primer sequences used
for qPCR are listed in Table I.
 | Table I.Primer sequences used for
quantitative PCR. |
Table I.
Primer sequences used for
quantitative PCR.
| Number | Name | Primer
sequences |
|---|
| Primer 1 | h-GAPDH | F:
GGAGCGAGATCCCTCCAAAAT |
|
|
| R:
GGCTGTTGTCATACTTCTCATGG |
| Primer 2 | h-E-Cadherin | F:
ATTTTTCCCTCGACACCCGAT |
|
|
| R:
TCCCAGGCGTAGACCAAGA |
| Primer 3 | h-N-Cadherin | F:
AGCCAACCTTAACTGAGGAGT |
|
|
| R:
GGCAAGTTGATTGGAGGGATG |
| Primer 4 | h-Vimentin | F:
TGCCGTTGAAGCTGCTAACTA |
|
|
| R:
CCAGAGGGAGTGAATCCAGATTA |
Phalloidin staining
GBC-SD cells were seeded into 24-well plates with
carry sheet glass and treated with melatonin (2 mM) for 6 h. Cells
were fixed with 4% paraformaldehyde for 15 min at room temperature.
Cells were re-washed 3 times with PBS and YE Dye phalloidin
conjugates (US Everbright) staining was performed according to the
manufacturer's instructions. DAPI was used for nuclear staining at
room temperature for 5 min. The images were acquired using a laser
scanning confocal microscope (Nikon A1R/A1; Nikon Corporation)
(magnification ×400).
Flow cytometry
The Annexin-V assay was performed to assess the
detection of exposed phosphatidylserine, which provides a highly
sensitive method for detecting apoptosis (16). A total of 3×105 GBC-SD
cells were seeded into 6-well plates and treated with different
concentrations of melatonin (0, 0.25, 0.5, 1, 2 and 3 mM) for 48 h.
The Annexin-V/PI double staining kit (BD Biosciences) was used to
detect apoptotic cells, according to the manufacturer's
instructions. Apoptosis cells were detected by flow cytometry (BD
Canto II; BD Biosciences). Cell apoptosis was analyzed by BD
FACSDiva Software v.8.0.1 (BD Biosciences). Cells that were
positive for Annexin-V and negative for PI were considered viable
cells, while cells that were in the early apoptotic phase were
Annexin-V positive and PI negative. Cells that were non-viable or
late apoptotic were Annexin-V and PI-positive.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc.). All experiments were performed in triplicate
and data are presented as the mean ± SD. Paired Student's t-test
followed by the Shapiro-Wilk W test were used to compare
differences between two groups, while one-way ANOVA followed by
Bonferroni's test were used to compare differences between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cytotoxicity of melatonin in GBC
cells
Melatonin induces apoptosis in various human cancer
cells, such as hepatocellular carcinoma, lung cancer,
cholangiocarcinoma and pancreatic cancer cells (11–14).
Thus, the present study initially investigated whether melatonin
promotes cellular apoptosis in human GBC cells. Treatment of GBC-SD
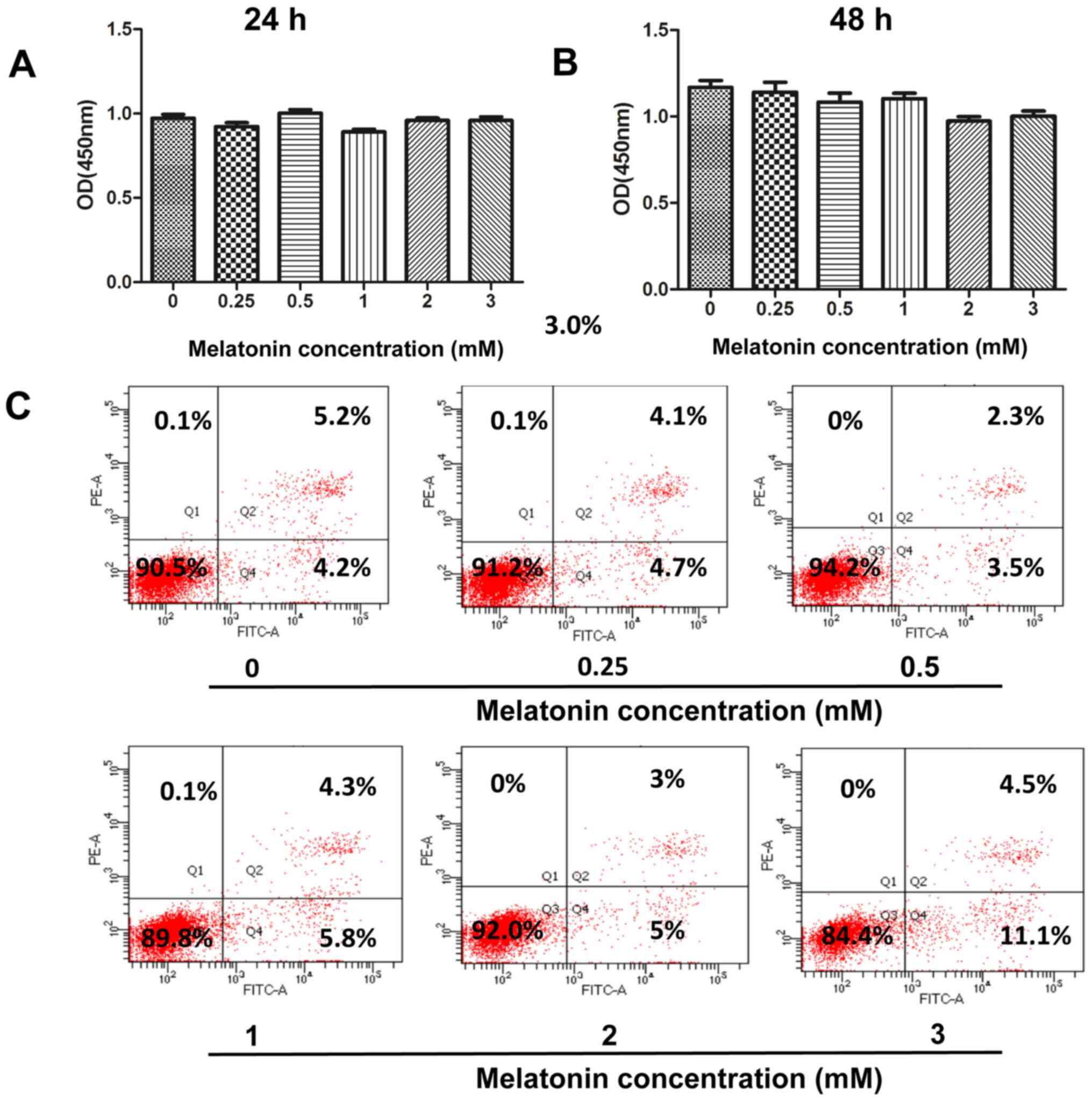
cells with melatonin (0.25, 0.5, 1, 2 and 3 mM) for 24 or 48 h did
not significantly affect cell viability, according to the CCK-8
assay (Fig. 1A and B). To further
confirm the cytotoxicity of melatonin, Annexin V-FITC/PI double
staining was performed to determine the induction of apoptosis via
flow cytometry. The number of early and late apoptotic cells did
not significantly increase following treatment with melatonin (0–2
mM) for 48 h (Fig. 1C). However, the
apoptotic rate of GBC-SD cells increased to 15.6% (4.5+11.1%)
following an increase in the concentration of melatonin to 3 mM.
Thus, the concentration range of melatonin (0–2 mM) was adjusted in
subsequent experiments to investigate its antimetastatic
properties.
Melatonin inhibits GBC cell migration
and invasion
Metastasis plays a significant role in the malignant
status of GBC (2). Thus, the effects
of melatonin on GBC cell invasion and migration were examined in
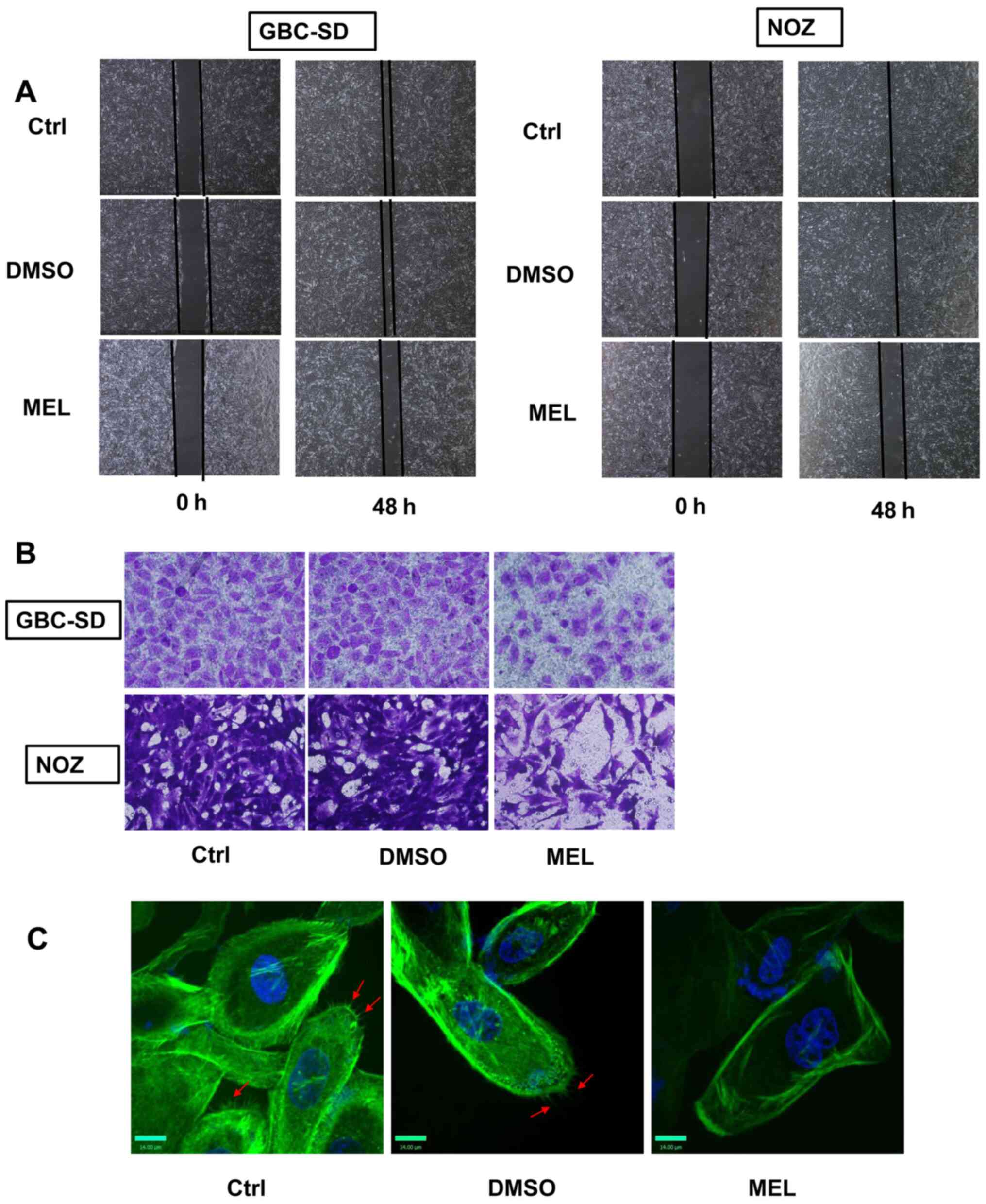
the present study. The wound healing assay was performed to assess
cell migratory, and the results demonstrated that melatonin (2 mM)
significantly inhibited the rate of wound closure compared with the
control group (Fig. 2A). The effects
of melatonin were also verified using Matrigel chambers. Melatonin
significantly decreased the number of cells that passed through the
membrane (Fig. 2B). To assess the
effects of melatonin on cell movement, YF dye phalloidin conjugates
were used to stain the actin filaments of GBC cells. Phalloidin is
found in amanita phalloides, and can specificity bind to
polymerized microfilaments (17). By
binding to the polymerized microfilaments, the cyclic peptides
inhibit the disintegration of the microfilaments, and destroy the
dynamic balance of polymerization and depolymerization of the
microfilaments (17). Treatment of
GBC cells with melatonin (2 mM) decreased the number of
microfilaments compared with the control group (Fig. 2C). Taken together, these results
suggest that melatonin mitigates the invasive and migratory
abilities of GBC cells.
Melatonin inhibits EMT in GBC
cells
EMT promotes migration and invasion of human cancer
cells (9). Studies have demonstrated
that melatonin can downregulate EMT (9,12). Thus,
it was speculated that melatonin exerts its inhibitory effect by
blocking EMT in gallbladder carcinoma cells. To assess the
underlying molecular mechanisms of the antimetastatic effects of
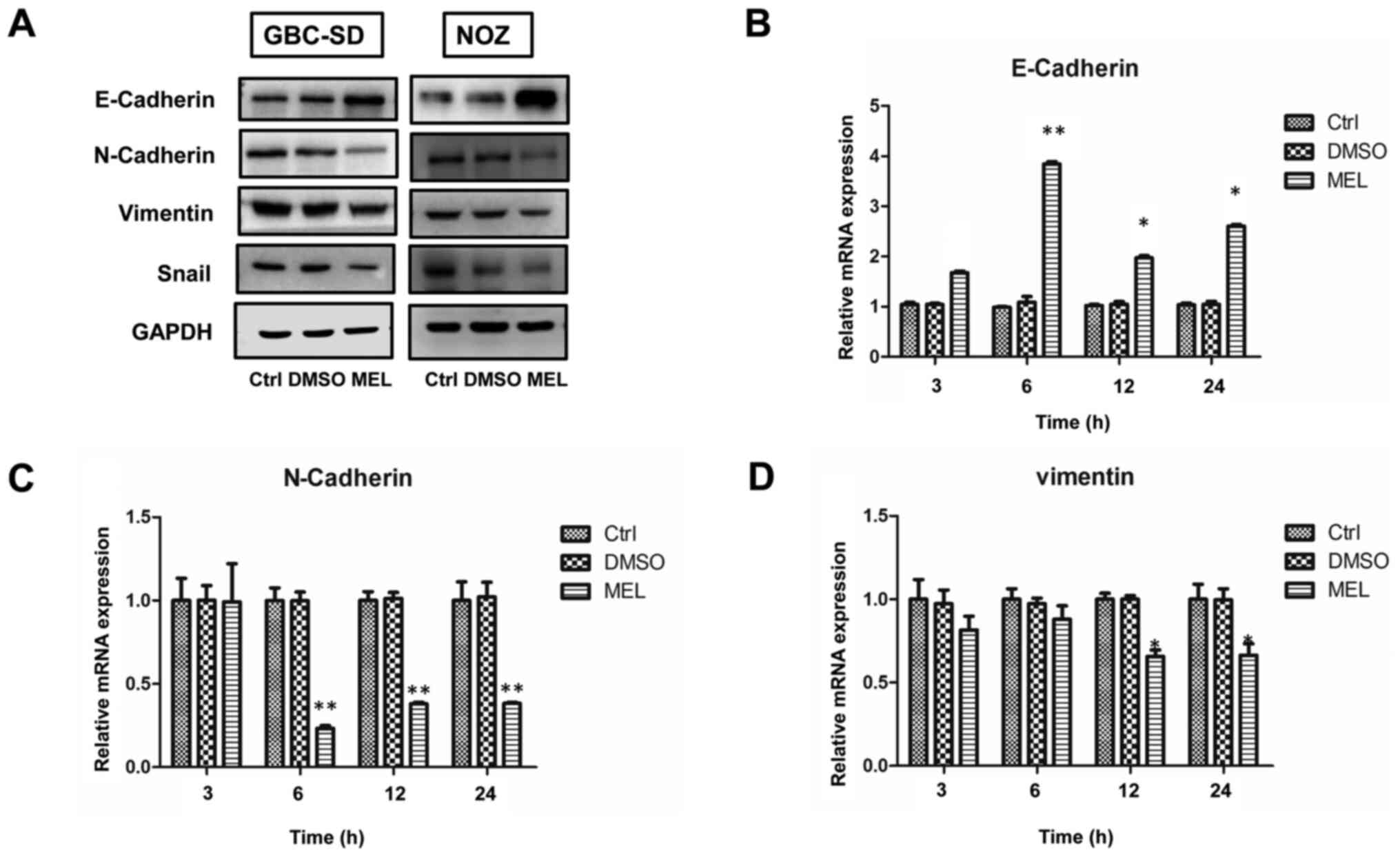
melatonin in GBC, cells were treated with 2 mM melatonin for
specific time periods. The expression levels of the epithelial
marker, E-cadherin, in the melatonin-treated group significantly
increased, whereas the expression levels of the mesenchymal
phenotype markers, N-cadherin and vimentin, significantly decreased
(Fig. 3A). These results were
further confirmed via RT-qPCR analysis (Fig. 3B-D). However, the PCR result of Snail
was not shown as the gene expression level was much lower compared
with the other markers assessed (data not shown). Collectively,
these results suggest that melatonin exerts its inhibitory effect
by blocking EMT.
Melatonin attenuates EMT and exerts
antimetastatic effects by inhibiting the ERK1/2 pathway in GBC
cells
Increasing evidence suggest that the AKT, NF-κB,
PI3K, ERK and Wnt/β-catenin signaling pathways are involved in the
regulation of metastasis caused by melatonin (9). Thus, the present study investigated
whether these signaling pathways are involved in melatonin-induced
inhibition of GBC-SD cell motility. The results demonstrated that
cells treated with melatonin (2 mM) diminished activation of ERK,
whereas the phosphorylation levels of AKT, PI3K and NF-κB were
unaffected. To confirm the role of melatonin in downregulating the
ERK signaling pathway, NOZ cells were treated with melatonin, and
the results demonstrated that the expression of phosphorylated ERK
decreased (Fig. 4A). These findings
suggest that the ERK signaling pathway may be involved in
melatonin-mediated inhibition of GBC cells.
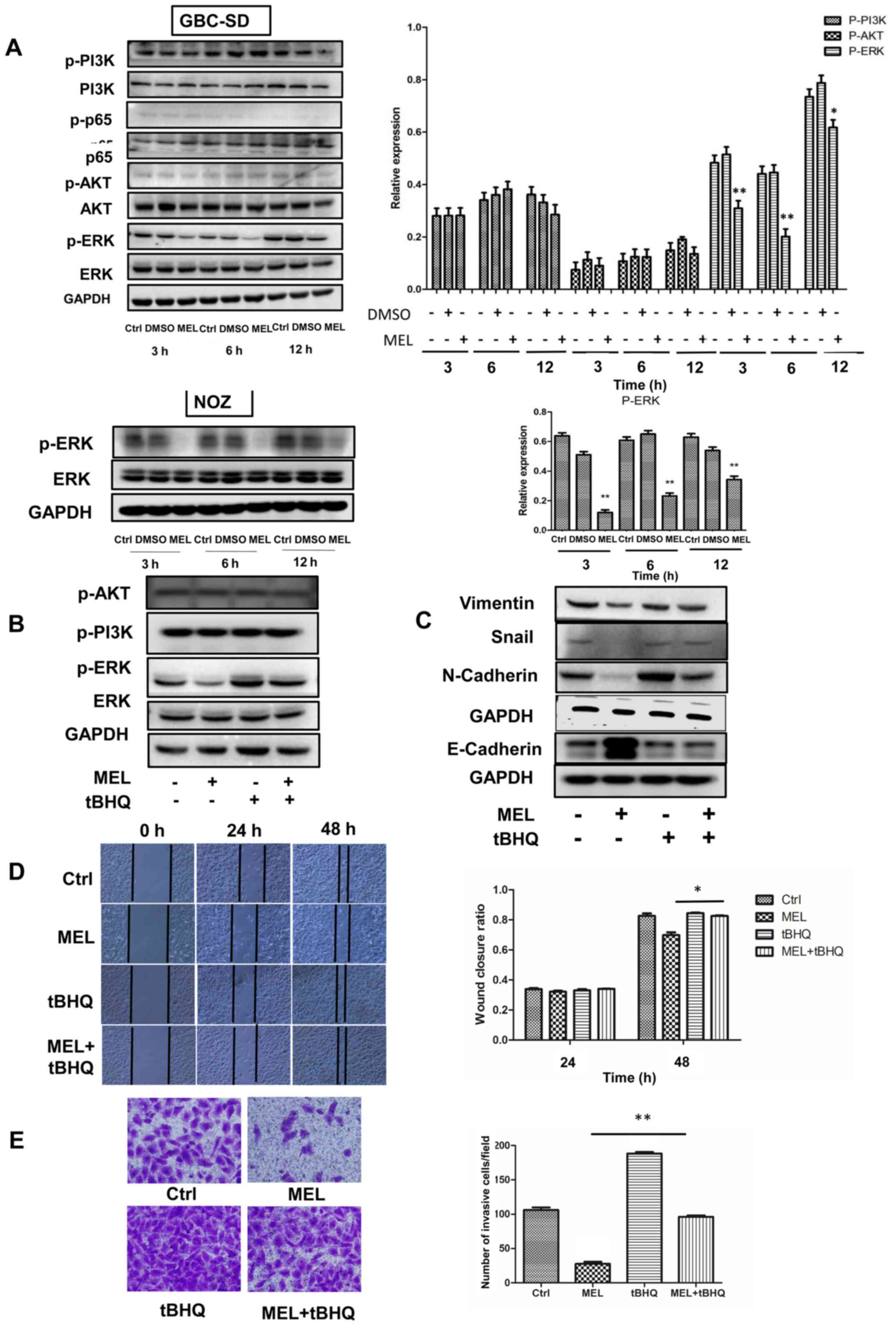 | Figure 4.Effects of the ERK signaling pathway
on the invasion and epithelial-to-mesenchymal transition of GBC
cells. (A) Western blot analysis was performed to detect the
protein expression levels of AKT, p-AKT, p65, p-p65, PI3K, p-PI3K,
ERK and p-ERK in GBC and NOZ cells treated with melatonin (2 mM)
for different time points. (B and C) Western blot analysis was
performed to detect the protein expression levels of ERK, p-ERK,
N-cadherin, E-cadherin, snail and vimentin in GBC cells following
treatment with melatonin and/or tBHQ (50 µM) for 48 h. GAPDH was
used as the control. (D) The wound healing assay was performed to
assess cell migration (magnification ×200) following treatment with
melatonin and/or tBHQ (50 µM) for 48 h. The wound closure rate was
analyzed. (E) The Transwell assay was performed to assess cell
invasion (magnification ×200). Data are presented as the mean ± SD.
*P<0.05; **P<0.01 vs. the MEL group. GBC, gallbladder cancer;
p, phosphorylated; tBHQ, tert-Butylhydroquinone; MEL, melatonin;
Ctrl, control. |
To verify the role of the ERK signaling pathway in
regulating the migratory potential and EMT, tBHQ (an activator of
ERK1/2) was added to GBC cells (18–20).
Notably, tBHQ (50 µM) reversed the effects of melatonin in
inhibiting phosphorylation of ERK (Fig.
4B). In addition, the expression levels of E-cadherin
significantly decreased following treatment with tBHQ, whereas the
expression levels of Snail, vimentin and N-cadherin significantly
increased compared with cells treated with melatonin (Fig. 4C). Accordingly, the inhibitory
effects of melatonin on migration (Fig.
4D) and invasion (Fig. 4E) of
GBC cells was partially reversed following treatment with tBHQ.
Taken together, these results suggest that melatonin downregulates
activity of the ERK signaling pathway, which in turn inhibits EMT
and attenuates the metastatic effect observed in GBC cells
(Fig. S1).
Discussion
GBC is the most common malignancy of the biliary
tract that exhibits poor prognosis (3). Metastasis remains an important factor
that affects the prognosis of patients with GBC (1). Currently, the therapeutic outcome of
GBC treatment is extremely poor (21). Melatonin is a naturally occurring
hormone secreted by the pineal gland that exhibits anticancer
effects on different types of cancer, including glioma, uterine
neck, ovarian, colon, liver and lung cancers (12,22–26).
Previous studies have demonstrated reduced cytotoxicity of
melatonin over a range of doses (9,26–28). The
oral administration of 0.5 mg or more of melatonin instantaneously
makes itself available in blood plasma, but it does not mimic the
endogenous profile (29). However,
the effects of melatonin on migration and invasion of GBC cells
have not yet been investigated. The results of the present study
demonstrated that melatonin inhibited the invasion of GBC cells by
blocking activity of the ERK signaling pathway.
To achieve the desired therapeutic effects,
endogenous substances are usually administered at considerably high
concentrations compared with those corresponding to the
physiological levels (30).
Melatonin concentrations at >1 µM are described as
pharmacological, while the physiological concentrations of this
hormone include those <1 nM (31,32). In
the present study, only high concentrations (2 and 3 mM) of
melatonin were effective in decreasing GBC cell metastasis.
However, treatment of cells with melatonin (3 mM) induced partial
apoptosis. Thus, subsequent experiments were performed using 2 mM
melatonin. The results of the present study demonstrated that
melatonin decreased the invasive and migratory abilities of GBC
cells.
EMT is a process during which epithelial cells shift
towards the mesenchymal state, which is considered a principal
process in tumor metastasis (33,34). The
molecular and cellular changes observed in EMT are characterized by
upregulation of specific proteins, such as N-cadherin, Snail and
vimentin and by downregulation of E-cadherin (35). Thus, these proteins are used as EMT
markers. It has been reported that EMT may be associated with the
prognosis and clinicopathological characteristics of patients with
GBC (36–38). Loss of epithelial markers and
dysfunction of EMT-associated transcription factors promotes
metastasis of GBC cells (39,40). To
further investigate the mechanism of action of melatonin on GBC
cells, the expression levels of the EMT-associated markers were
assessed in the present study. The results demonstrated that
melatonin promoted the expression levels of E-cadherin, while
attenuating the levels of N-cadherin, Snail and vimentin. Thus,
melatonin may inhibit GBC cell migration and/or invasion by
influencing EMT.
Signaling pathways, such as the ERK, NF-κB, Wnt,
Notch, Hedgehog, activator protein 1 and the growth factor
signaling pathways, induce or modulate the EMT process (9,41).
Evaluation of the phosphorylated levels of ERK, AKT, p65 and PI3K
in the present study demonstrated that only the ERK signaling
pathway was inhibited by melatonin. Overactivation of the ERK
signaling pathway has been reported in both GBC tissues and cells
(42). Melatonin inhibits cancer
migration by blocking activation of ERK in different types of human
cancer (11–14,42,43).
Thus, the present study investigated whether the induction of EMT
by melatonin was mediated by regulating the ERK signaling pathway
in GBC cells. tBHQ is an ERK activator that induces the
phosphorylation of ERK protein (43). The results of the present study
demonstrated that the anti-invasive effects of melatonin were
reversed by tBHQ, suggesting that this hormone can inhibit EMT via
the ERK signaling pathway in GBC cells.
In conclusion, the results of the present study
demonstrated that melatonin inhibited the invasion of GBC cells by
blocking the ERK signaling pathway. This study lays a foundation
for future studies investigating the mechanisms of melatonin in GBC
and may provide insights into a new therapeutic agent for GBC.
However, the lack of further validation in vivo is a
limitation of the present study. Further in vivo studies
will help to elucidate the role of melatonin in the antitumor
therapy of gallbladder cancer.
Supplementary Material
Supporting Data
Acknowledgements
The authors acknowledge Dr Jianwen Ye (Department of
Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital
of Zhengzhou University, Zhengzhou, China) for gifting the NOZ
cells.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant. no. 81971881), the
Foundation of Henan Charity Federation (grant. no. GDXZ2019002) and
the Henan Science and Technology Project (grant. no.
SBGJ2020003043).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HWT, XYS and PFZ performed the experiments. WZG, SJZ
and JL designed the study. HWT and BY analyzed and interpreted the
data. HWT and SJZ wrote the article. HWT, SJZ and XYS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Misra S, Chaturvedi A, Misra NC and Sharma
ID: Carcinoma of the gallbladder. Lancet Oncol. 4:167–176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
3
|
Sharma A, Sharma KL, Gupta A, Yadav A and
Kumar A: Gallbladder cancer epidemiology, pathogenesis and
molecular genetics: Recent update. World J Gastroenterol.
23:3978–3998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andia ME, Hsing AW, Andreotti G and
Ferreccio C: Geographic variation of gallbladder cancer mortality
and risk factors in chile: A population-based ecologic study. Int J
Cancer. 123:1411–1416. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheth S, Bedford A and Chopra S: Primary
gallbladder cancer: Recognition of risk factors and the role of
prophylactic cholecystectomy. Am J Gastroenterol. 95:1402–1410.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levy AD, Murakata LA and Rohrmann CA Jr:
Gallbladder carcinoma: Radiologic-pathologic correlation.
Radiographics. 21:295–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cipolla-Neto J and Amaral FGD: Melatonin
as a hormone: New physiological and clinical insights. Endocr Rev.
39:990–1028. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhattacharya S, Patel KK, Dehari D,
Agrawal AK and Singh S: Melatonin and its ubiquitous anticancer
effects. Mol Cell Biochem. 462:133–155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su SC, Hsieh MJ, Yang WE, Chung WH, Reiter
RJ and Yang SF: Cancer metastasis: Mechanisms of inhibition by
melatonin. J Pineal Res. 62:622017. View Article : Google Scholar
|
|
10
|
Wei JY, Li WM, Zhou LL, Lu QN and He W:
Melatonin induces apoptosis of colorectal cancer cells through
HDAC4 nuclear import mediated by CaMKII inactivation. J Pineal Res.
58:429–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao L, Dauchy RT, Blask DE, Dauchy EM,
Slakey LM, Brimer S, Yuan L, Xiang S, Hauch A, Smith K, et al:
Melatonin suppression of aerobic glycolysis (Warburg effect),
survival signalling and metastasis in human leiomyosarcoma. J
Pineal Res. 60:167–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chao CC, Chen PC, Chiou PC, Hsu CJ, Liu
PI, Yang YC, Reiter RJ, Yang SF and Tang CH: Melatonin suppresses
lung cancer metastasis by inhibition of epithelial-mesenchymal
transition through targeting to twist. Clin Sci (Lond).
133:709–722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin YW, Lee LM, Lee WJ, Chu CY, Tan P,
Yang YC, Chen WY, Yang SF, Hsiao M and Chien MH: Melatonin inhibits
MMP-9 transactivation and renal cell carcinoma metastasis by
suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J
Pineal Res. 60:277–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao L, Summers W, Xiang S, Yuan L, Dauchy
RT, Reynolds A, Wren-Dail MA, Pointer D, Frasch T, Blask DE, et al:
Melatonin represses metastasis in Her2-postive human breast cancer
cells by suppressing RSK2 expression. Mol Cancer Res. 14:1159–1169.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bubner B and Baldwin IT: Use of real-time
PCR for determining copy number and zygosity in transgenic plants.
Plant Cell Rep. 23:263–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar R, Saneja A and Panda AK: An annexin
V-FITC-propidium iodide-based method for detecting apoptosis in a
non-small cell lung cancer cell line. Methods Mol Biol.
2279:213–223. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Faulstich H, Zobeley S, Rinnerthaler G and
Small JV: Fluorescent phallotoxins as probes for filamentous actin.
J Muscle Res Cell Motil. 9:370–383. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu H, Dong Z, Wang X, Bai L, Lei Q, Yang
J, Li L, Li Q, Liu L, Zhang Y, et al: Dehydrocorydaline inhibits
cell proliferation, migration and invasion via suppressing
MEK1/2-ERK1/2 cascade in melanoma. Onco Targets Ther. 12:5163–5175.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi H, Bi H, Sun X, Dong H, Jiang Y, Mu H,
Liu G, Kong W, Gao R and Su J: Antitumor effects of tubeimoside-1
in NCI-H1299 cells are mediated by microRNA-126-5p-induced
inactivation of VEGF-A/VEGFR-2/ERK signaling pathway. Mol Med Rep.
17:4327–4336. 2018.PubMed/NCBI
|
|
20
|
Yu R, Tan TH and Kong AN: Butylated
hydroxyanisole and its metabolite tert-butylhydroquinone
differentially regulate mitogen-activated protein kinases. The role
of oxidative stress in the activation of mitogen-activated protein
kinases by phenolic antioxidants. J Biol Chem. 14:28962–28970.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baiu I and Visser B: Gallbladder cancer.
JAMA. 320:12942018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Hao H, Yao L, Zhang X, Zhao S,
Ling EA, Hao A and Li G: Melatonin suppresses migration and
invasion via inhibition of oxidative stress pathway in glioma
cells. J Pineal Res. 53:180–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farriol M, Venereo Y, Orta X, Castellanos
JM and Segovia-Silvestre T: In vitro effects of melatonin on cell
proliferation in a colon adenocarcinoma line. J Appl Toxicol.
20:21–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Papazisis KT, Kouretas D, Geromichalos GD,
Sivridis E, Tsekreli OK, Dimitriadis KA and Kortsaris AH: Effects
of melatonin on proliferation of cancer cell lines. J Pineal Res.
25:211–218. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petranka J, Baldwin W, Biermann J, Jayadev
S, Barrett JC and Murphy E: The oncostatic action of melatonin in
an ovarian carcinoma cell line. J Pineal Res. 26:129–136. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ordoñez R, Carbajo-Pescador S,
Prieto-Dominguez N, García-Palomo A, González-Gallego J and Mauriz
JL: Inhibition of matrix metalloproteinase-9 and nuclear factor
kappa B contribute to melatonin prevention of motility and
invasiveness in HepG2 liver cancer cells. J Pineal Res. 56:20–30.
2014. View Article : Google Scholar
|
|
27
|
Cutando A, López-Valverde A,
Arias-Santiago S, DE Vicente J and DE Diego RG: Role of melatonin
in cancer treatment. Anticancer Res. 32:2747–2753. 2012.PubMed/NCBI
|
|
28
|
Nordlund JJ and Lerner AB: The effects of
oral melatonin on skin color and on the release of pituitary
hormones. J Clin Endocrinol Metab. 45:768–774. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malhotra S, Sawhney G and Pandhi P: The
therapeutic potential of melatonin: A review of the science.
MedGenMed. 3:462004.PubMed/NCBI
|
|
30
|
Yerneni LK and Jayaraman S:
Pharmacological action of high doses of melatonin on B16 murine
melanoma cells depends on cell number at time of exposure. Melanoma
Res. 13:113–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dubocovich ML, Delagrange P, Krause DN,
Sugden D, Cardinali DP and Olcese J: International union of basic
and clinical pharmacology. LXXV. Nomenclature, classification, and
pharmacology of G protein-coupled melatonin receptors. Pharmacol
Rev. 62:343–380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Juszczak M, Roszczyk M, Kowalczyk E and
Stempniak B: The influence od melatonin receptors antagonists,
luzindole and 4-phenyl-2-propionamidotetralin (4-P-PDOT), on
melatonin-dependent vasopressin and adrenocorticotropic hormone
(ACTH) release from the rat hypothalamo-hypophysial system. In
vitro and in vivo studies. J Physiol Pharmacol. 65:777–784.
2014.PubMed/NCBI
|
|
33
|
Baum B, Settleman J and Quinlan MP:
Transitions between epithelial and mesenchymal states in
development and disease. Semin Cell Dev Biol. 19:294–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen T, You Y, Jiang H and Wang ZZ:
Epithelial-mesenchymal transition (EMT): A biological process in
the development, stem cell differentiation, and tumorigenesis. J
Cell Physiol. 232:3261–3272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu S, Zhan M and Wang J:
Epithelial-to-mesenchymal transition in gallbladder cancer: From
clinical evidence to cellular regulatory networks. Cell Death
Discov. 3:170692017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Puhalla H, Herberger B, Soleiman A,
Filipits M, Laengle F, Gruenberger T and Wrba F: E-cadherin and
beta-catenin expression in normal, inflamed and cancerous
gallbladder tissue. Anticancer Res. 25:4249–4254. 2005.PubMed/NCBI
|
|
38
|
Kai K, Masuda M and Aishima S: Inverse
correlation between CD8+ inflammatory cells and
E-cadherin expression in gallbladder cancer: Tissue microarray and
imaging analysis. World J Clin Cases. 5:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong P, He XW, Gu J, Wu WG, Li ML, Yang
JH, Zhang L, Ding QC, Lu JH, Mu JS, et al: Vimentin significantly
promoted gallbladder carcinoma metastasis. Chin Med J (Engl).
124:4236–4244. 2011.PubMed/NCBI
|
|
40
|
Lee DG, Lee SH, Kim JS, Park J, Cho YL,
Kim KS, Jo DY, Song IC, Kim N, Yun HJ, et al: Loss of NDRG2
promotes epithelial-mesenchymal transition of gallbladder carcinoma
cells through MMP-19-mediated Slug expression. J Hepatol.
63:1429–1439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Buchegger K, Silva R, López J, Ili C,
Araya JC, Leal P, Brebi P, Riquelme I and Roa JC: The ERK/MAPK
pathway is overexpressed and activated in gallbladder cancer.
Pathol Res Pract. 213:476–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu KH, Su SC, Lin CW, Hsieh YH, Lin YC,
Chien MH, Reiter RJ and Yang SF: Melatonin attenuates osteosarcoma
cell invasion by suppression of C-C motif chemokine ligand 24
through inhibition of the c-Jun N-terminal kinase pathway. J Pineal
Res. 65:e125072018. View Article : Google Scholar : PubMed/NCBI
|