Introduction
Tumors escape immune surveillance by developing an
immunosuppressive microenvironment that induces immune tolerance
(1). This tumor microenvironment
contains various immunosuppressive cells, including
tumor-associated macrophages (TAMs), myeloid-derived suppressor
cells (MDSCs) and tumor-associated neutrophils (TANs), which
contribute to immune tolerance and tumor progression (2–4). Tumor
immune tolerance is characterized by tumor myelopoiesis (1), which is not only characterized by the
accumulation of myeloid precursors, but is also associated with
defective cellular differentiation that results in the accumulation
and persistence of immunosuppressive myeloid cells (5). These myeloid cells promote tumor
progression by regulating the antitumor immune activity of T
lymphocytes, natural killer T (NKT) cells, natural killer (NK)
cells, dendritic cells (DCs) and various other cell types (6). Previous studies have demonstrated that
the spleen is an important site of extramedullary hematopoiesis,
which generates immunosuppressive myeloid cells in tumor-bearing
mice (7,8). Therefore, investigation of the
association between the spleen and cancer immunology is crucial to
an improved understanding of tumor immune tolerance.
The role of splenectomy in tumor behavior has
recently received increasing attention (9). Clinical studies have demonstrated that
traumatic splenectomy in healthy individuals can increase the risk
of cancer, while splenectomy in patients with post-hepatitis
cirrhosis is associated with a reduced risk of hepatocellular
carcinoma (10,11). Previous studies have demonstrated
that the spleen is an origin of MDSCs in tumor-bearing mice
(7,12). Additionally, our previous studies
indicated that a large number of myeloid cells accumulate in the
spleens of tumor-bearing mice (13,14), and
the spleen has been reported as a site of tumor immune tolerance
(1). As such, an increasing number
of studies have reported the inhibitory effects of splenectomy on
tumor progression (9,15,16).
However, to the best of our knowledge, the relevance of spleen
weight and volume in tumor progression remains unclear. Although a
decrease in spleen volume has been observed in patients with
locally advanced non-small cell lung cancer receiving
chemo-radiotherapy (17), the
association between spleen weight and tumor weight also remains to
be clarified. Specifically, the spleen is an origin of myeloid
cells (5,7); however, little is known of the
association between spleen weight and the immune response in
tumor-bearing mice. Clarifying the relevance of spleen weight in
tumor progression may provide a novel strategy for anticancer
therapy. In the present study, dynamic changes in the percentages
of immune cells in the spleen and peripheral blood, as well as the
association between spleen weight and immune cells in the spleen
and peripheral blood of tumor-bearing mice, were evaluated.
Materials and methods
Cell line and cell culture
H22 murine hepatoma cells were purchased from China
Center for Type Culture Collection (cat. no. GDC0091). The cells
were cultured in RPMI-1640 (HyClone; Cytiva) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin, and maintained at 37°C with 5% CO2 in a
humidified atmosphere.
Mice
A total of 36 male C57BL/6 mice (age, 6–8 weeks;
weight, 23.9±1.9 g) were purchased from the Experimental Animal
Center of Xi'an Jiaotong University (Xi'an, China). All animals
were housed at the animal facility under specific pathogen-free
conditions, at 26°C with a relative humidity of 50%, with a 12-h
light/dark cycle and free access to food and water. The study was
approved by the Ethics Committee of Xi'an Jiaotong University
College of Medicine (Xi'an, China).
Antibodies
The following antibodies were purchased from
BioLegend, Inc.: FITC anti-CD3 (dilution, 1:80; cat. no. 100204),
phycoerythrin (PE) anti-CD4 (dilution, 1:80; cat. no. 100407),
allophycocyanin (APC) anti-CD8a (dilution, 1:80; cat. no. 100712),
Brilliant Violet 421™ anti-CD279 [programmed cell death protein 1
(PD1); dilution, 1:50; cat. no. 135218], FITC anti-CD11c (dilution,
1:200; cat. no. 117306), purified anti-CD16/32 (dilution, 1:80;
cat. no. 101302), APC anti-CD11b (dilution, 1:80; cat. no. 101212),
APC anti-adhesion G protein-coupled receptor E1 (F4/80; dilution,
1:80; cat. no. 123116), FITC anti- lymphocyte antigen 6 (Ly6)G
(dilution, 1:400; cat. no. 127606), PE/Cy7 anti-CD11b (dilution,
1:160; cat. no. 101216) and PE anti-Ly6C (dilution, 1:80; cat. no.
128008). The following antibodies were purchased from eBioscience;
Thermo Fisher Scientific, Inc.: PE anti-major histocompatibility
complex (MHC)II (dilution, 1:500; cat. no. 12-5321-81) and
peridinin-chlorophyll-protein-Cyanine5.5 anti-natural killer 1.1
(dilution, 1:60; NK1.1; cat. no. 45-5941-82). PE/Cy7 anti-CD11b and
APC anti-F4/80 were used for macrophages. APC anti-CD11b, FITC
anti-Ly6G and PE anti-Ly6C were used for MDSCs. The biomarkers used
to identify immune cells are shown in Table I.
 | Table I.Biomarkers of immune cells. |
Table I.
Biomarkers of immune cells.
| Immune cells | Biomarkers |
|---|
| CD4+ T
lymphocytes |
CD4+ |
| CD8+ T
lymphocytes |
CD8+ |
| Myeloid cells |
CD11b+ |
| M-MDSCs |
CD11b+Ly6ChiLy6G− |
| Macrophages |
CD11b+F4/80+ |
| PMN-MDSCs |
CD11b+Ly6ClowLy6G+ |
| DCs |
CD11c+MHCII+ |
| NK |
CD3−NK1.1+ |
| NKT |
CD3+NK1.1+ |
Animal model
Tumor models were performed as previously described
(18). The entire duration of the
experiment was 21 days. Briefly, H22 cells (5×105 cells in 100 µl
normal saline) were subcutaneously injected into the right flanks
of male C57BL/6 mice. Control mice were not injected with H22
cells. The spleen weight was recorded at days 7 (4 control mice and
6 tumor-bearing mice), 14 (5 control mice and 8 tumor-bearing mice)
and 21 (5 control mice and 8 tumor-bearing mice), and the tumor
weight was recorded at days 14 (5 control mice and 8 tumor-bearing
mice) and 21 (5 control mice and 8 tumor-bearing mice) post-tumor
cell injection. The maximum tumor diameter was 1.35 cm. Spleen and
peripheral blood samples were also collected and immediately used
for flow cytometric analysis at 7, 14 and 21 days after injection.
Mouse health and behavior, including exercise, diet and weight of
mice, were monitored every day. The mice were anesthetized using an
intraperitoneal injection of sodium pentobarbital (50 mg/kg; Merck
KGaA) and subsequently sacrificed by cervical dislocation at 7, 14
and 21 days after tumor cell injection.
Generation of single-cell
suspensions
Single-cell suspensions were generated as previously
described (18,19). Briefly, ~1 ml peripheral blood was
collected from each mouse into EDTA-coated tubes and diluted 1:5 in
NH4Cl lysing buffer (0.16 M NH4Cl, 10 mM KHCO3, 0.13 mM EDTA, pH
7.2) for 5 min on ice. The samples were then centrifuged at 350 × g
for 5 min at 4°C. The pelleted cells were washed twice with PBS/5%
FBS buffer (Beyotime Institute of Biotechnology), in which they
were then resuspended and quantified (1×106 cells/ml).
To obtain single-cell suspensions, the spleen tissues were
resuspended in ice-cold PBS/5% FBS buffer and disrupted
mechanically. Similarly, red blood cells were lysed by adding 5 ml
NH4Cl lysing buffer for 5 min on ice, followed by centrifugation at
350 × g for 5 min at 4°C. The pelleted cells were then washed twice
and resuspended (both in PBS/5% FBS buffer), and the concentration
was adjusted to 1×106 cells/ml.
Flow cytometric analysis
Flow cytometry was performed as previously described
(19,20). The single-cell suspensions were
collected in tubes and blocked with a CD16/32 antibody for 15 min,
followed by incubation with the appropriate antibodies for 30 min
at 4°C. Data were acquired using the FACSCanto II flow cytometer
iva 7.0 software (BD Biosciences). FlowJo software 7.6.1 (Tree
Star, Inc.) was used for data analysis.
Calculation of spleen index (SI)
The SI was calculated according to the following
formula: SI = spleen weight (g)/body weight (g).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. All experiments were repeated three times. Data were
analyzed with SPSS 17.0 software (SPSS, Inc.). One-way ANOVA
followed by Bonferroni's test was used to compare the means among
multiple samples. Pearson's correlation analysis was used for
correlation analysis and linear regression analysis was performed
to confirm the association. P<0.05 was considered to indicate a
statistically significant difference.
Results
Spleen weight is positively correlated
with tumor weight
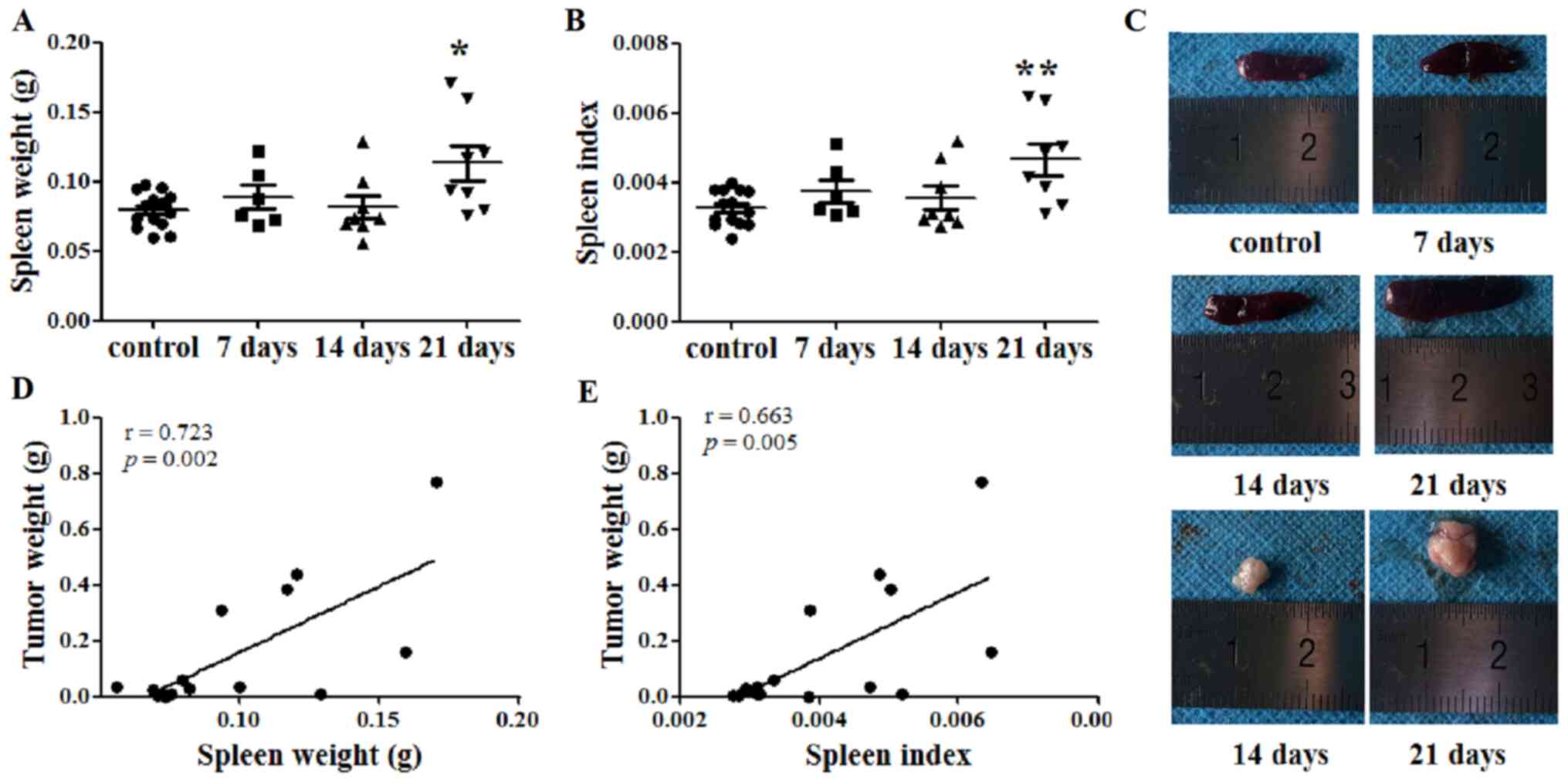
The spleen weight of each mouse was recorded, and
the SI was calculated at days 7, 14 and 21 after tumor cell
injection. At day 21, the spleen weights of the tumor-bearing mice
were significantly increased compared with those of the control
mice (P<0.05; Fig. 1A and C).
Similarly, the SI of the tumor-bearing mice was increased compared
with that of the control mice at 21 days after tumor cell
inoculation (P<0.01; Fig. 1B).
Additionally, the tumor weight was recorded at days 14 and 21.
Subsequently, the correlation between spleen and tumor weight was
investigated. Notably, both spleen weight and SI were identified to
be positively correlated with tumor weight, with Pearson's r values
of 0.723 (P=0.002; Fig. 1D) and
0.663 (P=0.005; Fig. 1E),
respectively.
Immune cell balance is disrupted in
the spleens of tumor-bearing mice
Flow cytometry was used to investigate changes in
immune cell populations in the spleens of tumor-bearing mice. The
results indicated that at day 21 after tumor cell inoculation, the
percentages of CD4+ splenic T lymphocytes were lower
than those of the control group (P<0.01; Figs. 2A and S1A). Similarly, the percentages of
CD8+ splenic T lymphocytes were decreased at days 14 and
21 post-injection compared with those in the normal control mice
(P<0.05; Figs. 2B and S1A). As an immunosuppressive marker of T
lymphocytes, PD1 expression on T lymphocytes was also investigated
in tumor-bearing mice (3). The
results indicated no significant differences in the expression
levels of PD1 on CD4+ (P>0.05; Figs. 2C and S1B) and CD8+ T lymphocytes
(P>0.05; Figs. 2D and S1C) between the tumor-bearing and control
mice.
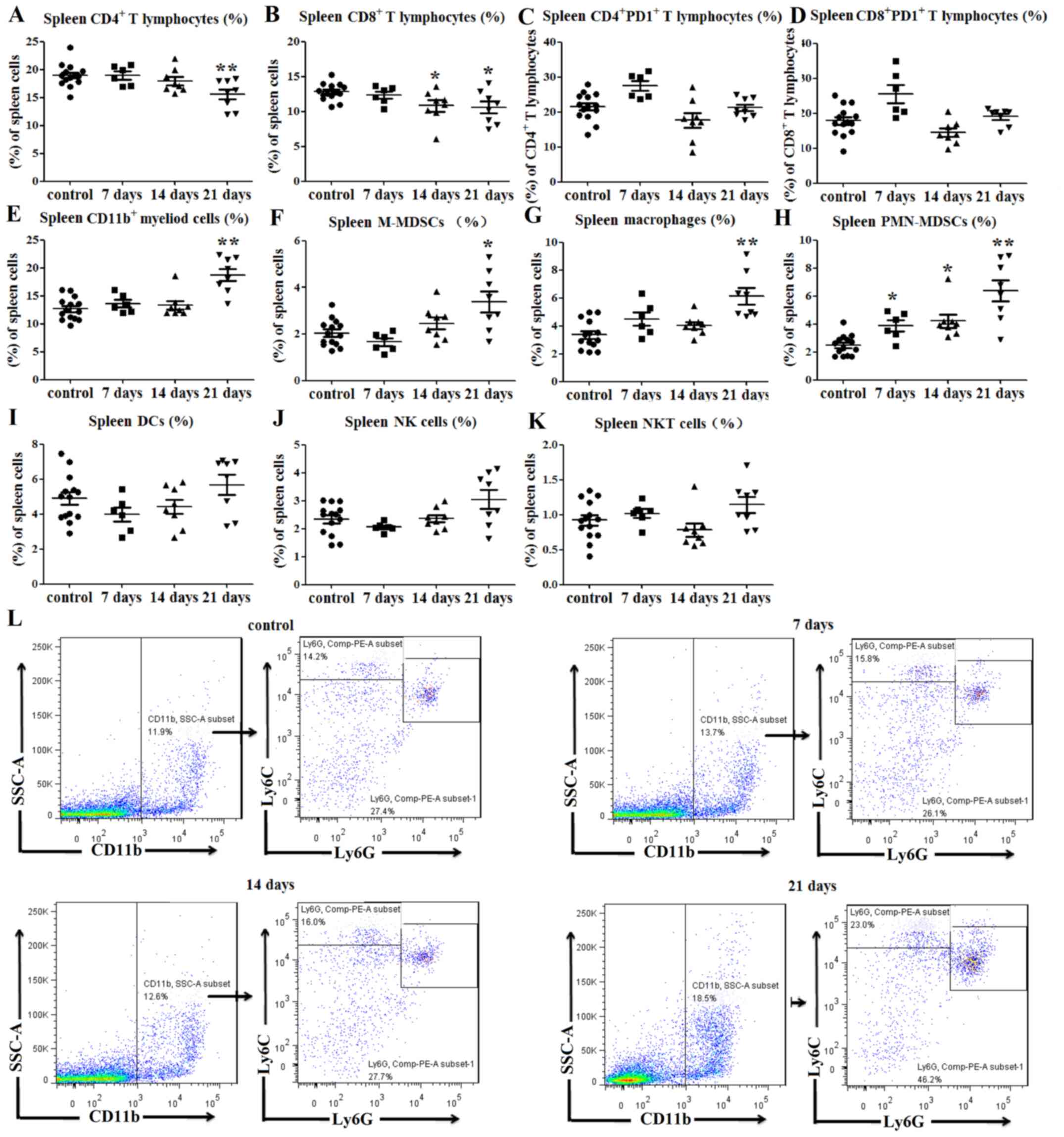 | Figure 2.Immune cell balance is disrupted in
the spleens of tumor-bearing mice. (A) Percentages of
CD4+ splenic T lymphocytes were decreased at 21 days
post-tumor cell inoculation. (B) Percentages of CD8+
splenic T lymphocytes were decreased at 14 and 21 days post-tumor
cell inoculation. No significant differences in PD1 expression on
(C) CD4+ and (D) CD8+ T lymphocytes were
observed between tumor-bearing and control mice. Percentages of (E)
CD11b+ splenic myeloid cells, (F)
CD11b+Ly6ChiLy6G− M-MDSCs and (G)
CD11b+F4/80+ splenic macrophages (among total
cells) were increased at 21 days after tumor cell inoculation. (H)
Percentages of CD11b+Ly6ClowLy6G+
PMN-MDSCs in the spleens (among total cells) of tumor-bearing mice
were increased at 7, 14 and 21 days after tumor cell injection.
There were no significant differences in the percentages of (I)
CD11c+MHCII+ DCs, (J)
CD3−NK1.1+NK cells and (K)
CD3+NK1.1+ NKT cells between the
tumor-bearing mice and control mice. (L) Representative flow
cytometry dot plots of splenic MDSCs. *P<0.05 and **P<0.01
vs. control. F4/80, adhesion G protein-coupled receptor E1; hi,
high; Ly6, lymphocyte antigen 6; MDSC, myeloid-derived suppressor
cell; MHC, major histocompatibility complex; NK1.1, natural killer
1.1; NKT, natural killer T; M-MDSCs, monocytic-like myeloid-derived
suppressor cells; PMN-MDSCs, polymorphonuclear-like myeloid-derived
suppressor cells; DCs, dendritic cells; PD1, programmed cell death
protein 1; SSC-A, side scatter area. |
As predicted, the percentage of CD11b+
splenic myeloid cells at 21 days post-inoculation was notably
higher than that of the control group (P<0.01; Fig. 2E). Since myeloid cells include MDSCs
and macrophages (5,6), flow cytometry was used to evaluate
alterations in the levels of these cell subgroups. Monocytic-like
MDSCs (M-MDSCs) can be defined as CD11b+Ly6C-high
(Ly6Chi)Ly6G− and polymorphonuclear-like
MDSCs (PMN-MDSCs) can be defined as
CD11b+Ly6ClowLy6G+ (21). The percentages of
CD11b+Ly6ChiLy6G− M-MDSCs
(P<0.05; Fig. 2F and L) and
CD11b+F4/80+ splenic macrophages (P<0.01;
Figs. 2G and S1D) in the spleens of tumor-bearing mice
were higher than those in the control mice at 21 days
post-inoculation. Similarly, the percentage of
CD11b+Ly6ClowLy6G+ PMN-MDSCs in
the spleen of tumor-bearing mice was increased at days 7
(P<0.05; Fig. 2H), 14 (P<0.05;
Fig. 2H) and 21 (P<0.01; Fig. 2H and L) compared with the control
mice.
The percentages of
CD11c+MHCII+ DCs,
CD3−NK1.1+ NK and
CD3+NK1.1+ NKT cells in the spleens of
tumor-bearing mice were also determined. The results indicated no
significant differences in the percentages of DCs (P>0.05;
Figs. 2I and S1E), NK cells (P>0.05; Figs. 2J and S1F) and NKT cells (P>0.05; Figs. 2K and S1F) between the tumor-bearing and control
mice. Therefore, these data indicated that the percentages of T
lymphocytes were decreased, while those of myeloid cells were
increased in the spleens of tumor-bearing mice compared with those
of control mice.
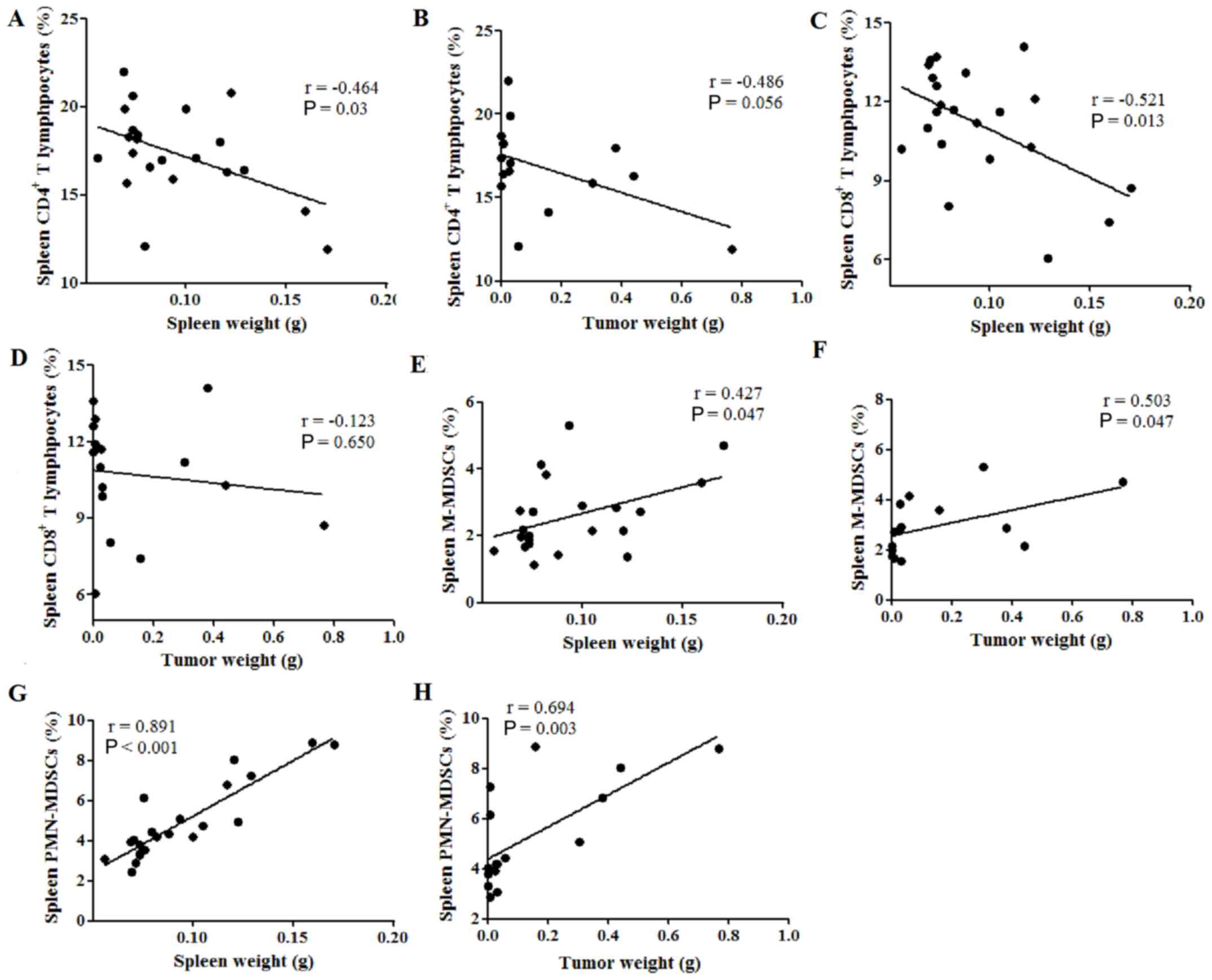
Spleen weight is correlated with
cellular immune response in the tumor-bearing mouse spleen
Therefore, the potential correlations between spleen
weight or tumor weight and the percentages of immune cells in the
spleen were subsequently investigated. The percentage of
CD4+ T lymphocytes was found to be negatively correlated
with spleen weight (P=0.03; Fig.
3A), while no correlation was observed between CD4+
T cell percentage and tumor weight (P=0.056; Fig. 3B). Similarly, spleen weight was
negatively correlated with the percentage of CD8+ T
lymphocytes (P=0.013; Fig. 3C),
while the tumor weight was not (P=0.650; Fig. 3D). Furthermore, the spleen and tumor
weight were both positively correlated with the percentage of
M-MDSCs in the spleen, with Pearson's r values of 0.427 (P=0.047;
Fig. 3E) and 0.503 (P=0.047;
Fig. 3F), respectively.
Additionally, both the spleen and tumor weight were positively
correlated with the percentage of PMN-MDSCs in the spleen, with
Pearson's r values of 0.891 (P<0.001; Fig. 3G) and 0.694 (P=0.003; Fig. 3H), respectively. These results
indicate that spleen weight was negatively correlated with the
percentages of tumor-suppressive immune cells, such as
CD4+ and CD8+ T lymphocytes, while spleen and
tumor weight were both positively correlated with the percentages
of tumor-promoting immune cells, such as MDSCs, in the spleen of
tumor-bearing mice.
Immune cell balance is disrupted in
the blood of tumor-bearing mice
Changes in the proportions of immune cells in the
peripheral blood of tumor-bearing mice were also investigated, and
the results indicated no significant difference in the percentage
of CD4+ T lymphocytes (P>0.05; Fig. 4A and B) between the tumor-bearing and
control mice. The percentages of CD8+ T lymphocytes in
the peripheral blood of tumor-bearing mice were decreased at days 7
(P<0.01; Fig. 4C), 14 (P<0.01;
Fig. 4C) and 21 (P<0.01; Fig. 4A and C) after tumor cell injection,
compared with those in the control group. There were no significant
differences in the expression of PD1 on CD4+ T
lymphocyte between tumor-bearing mice and the control mice
(P>0.05; Figs. 4D and S2A), while PD1 expression on
CD8+ T lymphocytes was increased in tumor-bearing mice
at day 21 post-tumor cell injection (P<0.05; Figs. 4E and S2B).
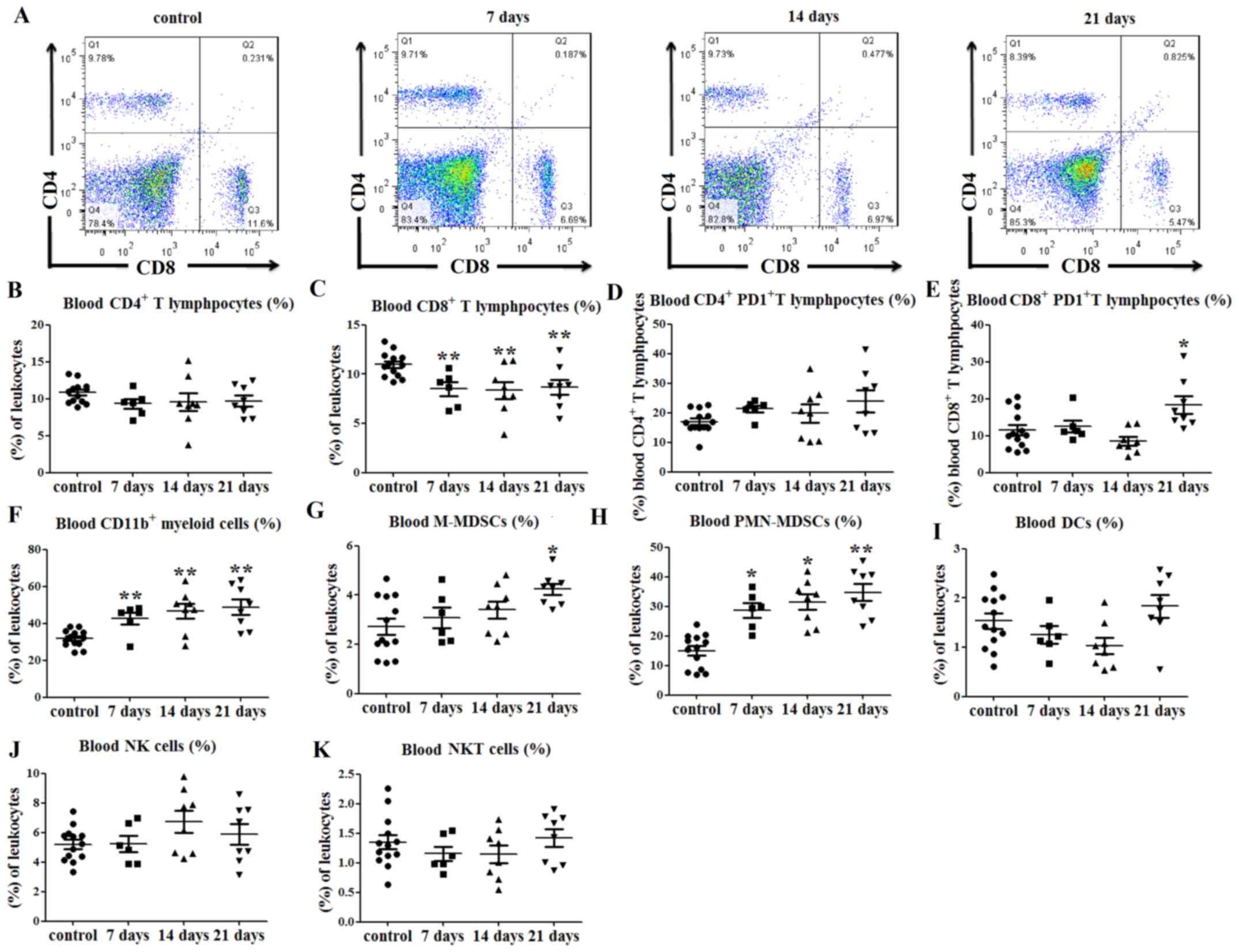 | Figure 4.Immune cell balance is disrupted in
the blood of tumor-bearing mice. (A) Representative flow cytometry
dot plots of blood CD4+ and CD8+ T lymphocyte
levels. (B) No differences in the percentages of blood
CD4+ T lymphocytes were observed between tumor-bearing
and control mice. (C) Percentages of CD8+ T lymphocytes
in the peripheral blood were decreased at days 7, 14 and 21
post-tumor cell injection. (D) No significant differences in PD1
expression on CD4+ T lymphocytes were observed between
the tumor-bearing and control mice, (E) while PD1 expression on
CD8+ T lymphocytes was increased at day 21. (F)
Percentages of CD11b+ myeloid cells in the peripheral
blood were increased at 7, 14 and 21 days after tumor cell
injection. (G) Percentages of peripheral blood
CD11b+Ly6ChiLy6G− M-MDSCs (among
total cells) were increased at 21 days after tumor cell
inoculation, and those of (H)
CD11b+Ly6ClowLy6G+ PMN-MDSCs among
total cells) were increased at days 7, 14 and 21. There were no
significant differences in the percentages of (I)
CD11c+MHCII+ DCs, (J)
CD3−NK1.1+ NK and (K)
CD3+NK1.1+ NK T cells in the peripheral blood
of tumor-bearing mice compared with control mice. *P<0.05 and
**P<0.01 vs. control. hi, high; Ly6, lymphocyte antigen 6; NKT,
natural killer T; M-MDSCs, monocytic-like myeloid-derived
suppressor cells; PMN-MDSCs, polymorphonuclear-like myeloid-derived
suppressor cells; DCs, dendritic cells; MHC, major
histocompatibility complex; NK1.1, natural killer 1.1; PD1,
programmed cell death protein 1. |
Similarly, the percentage of CD11b+
myeloid cells in the peripheral blood of tumor-bearing mice was
increased at days 7, 14 and 21 after tumor cell injection, compared
with that in the control mice (P<0.01; Figs. 4F and S2C). The percentage of
CD11b+Ly6ChiLy6G− M-MDSCs in the
peripheral blood was also higher than that in the control group at
day 21 (P<0.05; Figs. 4G and
S2C). Furthermore, the percentages
of peripheral blood
CD11b+Ly6ClowLy6G+ PMN-MDSCs were
increased at days 7 (P<0.05; Figs.
4H and S2C), 14 (P<0.05;
Figs. 4H and S2C) and 21 (P<0.01; Figs. 4H and S2C) in tumor-bearing mice compared with
those in control mice. There were no significant differences in the
percentages of DCs (P>0.05; Figs.
4I and S2D), NK cells
(P>0.05; Figs. 4J and S2E) and NKT cells (P>0.05; Figs. 4K and S2E) in the peripheral blood of the
tumor-bearing mice compared with the control mice.
These data indicated that the percentages of
CD8+ T lymphocytes were decreased, while those of
myeloid cells were increased, in the peripheral blood of
tumor-bearing mice compared with those in control mice.
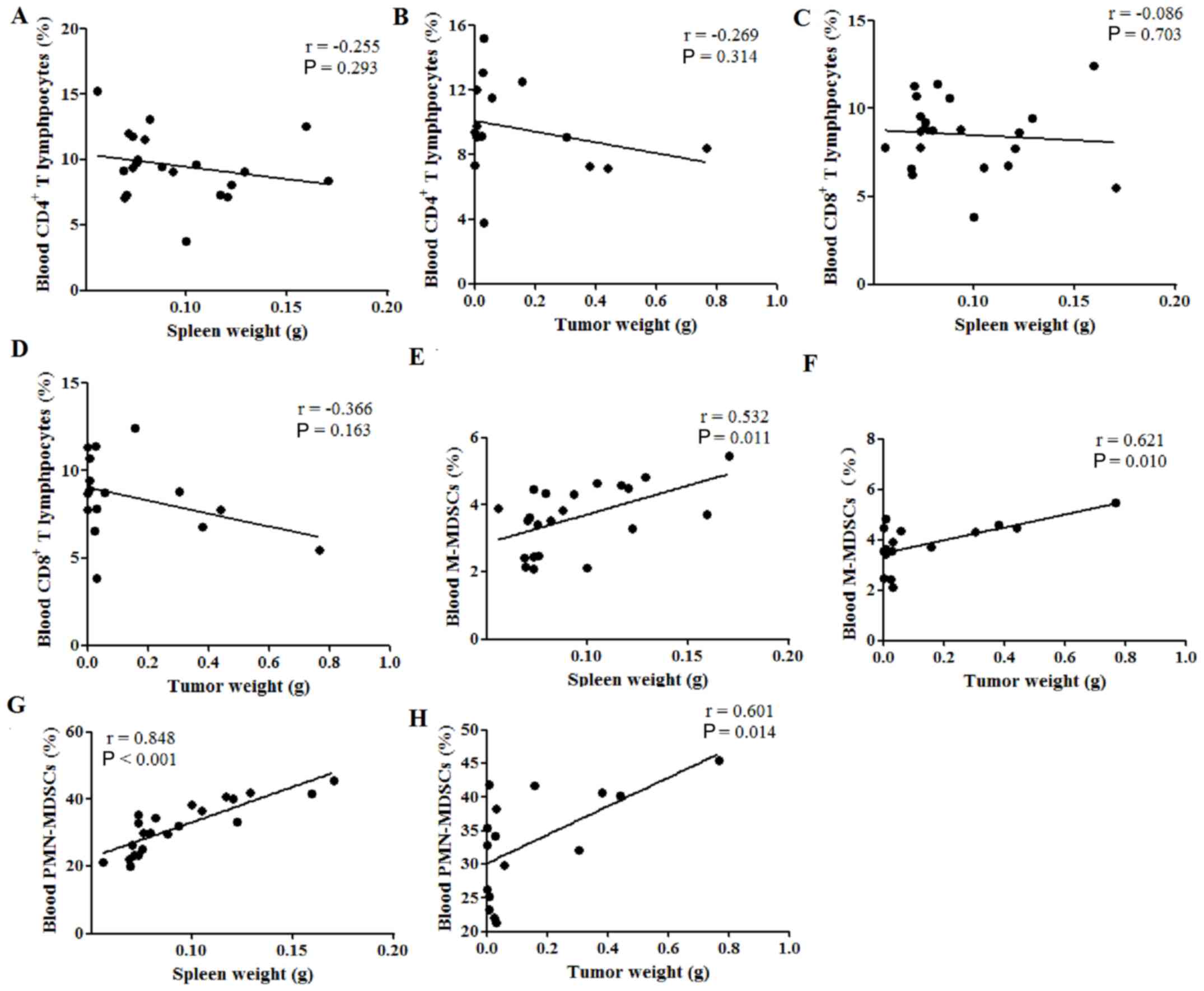
Spleen weight is correlated with
cellular immune response in the peripheral blood of tumor-bearing
mice
Finally, the correlation between spleen or tumor
weight and the percentages of immune cells in the peripheral blood
was investigated. Neither spleen nor tumor weight were correlated
with the percentages of CD4+ (P>0.05; Fig. 5A and B) and CD8+
(P>0.05; Fig. 5C and D) T
lymphocytes in the peripheral blood; however, both were positively
correlated with the percentage of M-MDSCs, with Pearson's r values
of 0.532 (P=0.011; Fig. 5E) and
0.621 (P=0.010; Fig. 5F),
respectively. Furthermore, both the spleen and tumor weight were
also positively correlated with the percentages of PMN-MDSCs in the
peripheral blood, with Pearson's r values of 0.848 (P<0.001;
Fig. 5G) and 0.601 (P<0.014;
Fig. 5H), respectively. These
results indicated that both the spleen weight and tumor weight were
positively correlated with the percentages of MDSCs in the
peripheral blood of tumor-bearing mice.
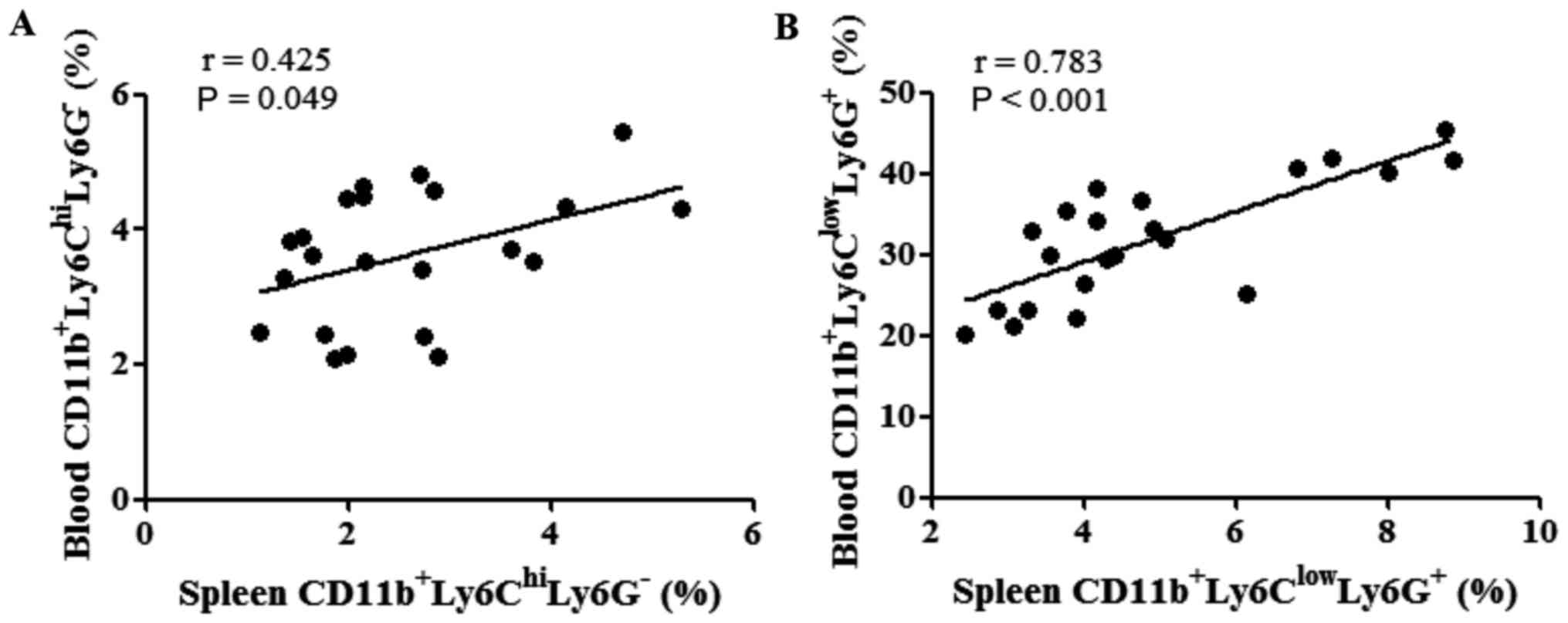
The correlations between the percentages of MDSCs in
the spleen and peripheral blood were evaluated following tumor cell
inoculation. The percentages of M-MDSCs in the spleen were found to
be positively correlated with those of M-MDSCs in the peripheral
blood (P=0.049; Fig. 6A). Similarly,
the percentage of PMN-MDSCs in the spleen was also positively
correlated with that of PMN-MDSCs in the peripheral blood
(P<0.001; Fig. 6B).
Discussion
The results of the present study demonstrated a
novel role for the spleen in predicting the cellular immune
response of tumor-bearing mice. Firstly, the spleen weight and SI
of tumor-bearing mice at day 21 were increased compared with those
of the control group. Secondly, the spleen weight and SI were
positively correlated with tumor weight. Thirdly, the percentages
of T lymphocytes were decreased, while those of myeloid cells were
increased in the spleen and peripheral blood of tumor-bearing mice
at day 21 after tumor cell inoculation. Finally, spleen weight was
negatively correlated with the percentages of tumor-suppressive
immune cells, such as T lymphocytes, in the spleen, while it was
positively correlated with the percentages of tumor-promoting
immune cells, such as MDSCs, in the spleen and peripheral blood of
tumor-bearing mice.
Although the spleen weight and SI of tumor-bearing
mice were revealed to be increased compared with those of the
controls, the result was not significant until 21 days after tumor
cell inoculation. These findings were consistent with our previous
results that spleen weight was observed to increase, and was
significantly higher at week 2, in a murine H22 orthotopic hepatoma
model (14). Additionally, tumor
weight was demonstrated to be positively correlated with both
spleen weight and SI. These findings suggested that the spleen may
be a suitable marker for cancer diagnosis, as well as a follow-up
marker for patients with cancer.
An increasing number of animal studies have
demonstrated that the spleen is an important site of extramedullary
hematopoiesis and an origin of myeloid cell genesis (22–24). In
those with cancer, splenic extramedullary hematopoiesis is
generally perceived as a supplementary mechanism to fulfill an
increased myeloid cell demand (16),
and is characterized by splenic hematopoietic stem/progenitor
cells, which are primed and committed to generate immunosuppressive
myeloid cells (5). Our previous
studies, as well as other previous studies, have indicated that a
large number of myeloid cells accumulate in the spleens of
tumor-bearing mice (8,13,14,25).
Additionally, our previous study has indicated that the immune
functions of splenic macrophages are impaired in the advanced stage
of cancer (26). The results of the
present study suggested that the percentages of T lymphocytes were
decreased, while those of myeloid cells were increased in the
spleen and peripheral blood of tumor-bearing mice.
MDSCs are a class of immune suppressor cells
comprising a heterogenous population of immature granulocytes and
monocytes (2,27–29). In
mice, MDSCs are defined as CD11b+Ly6G+, and
numerous studies have further characterized
CD11b+Ly6ChiLy6G− M-MDSC and
CD11b+Ly6ClowLy6G+ PMN-MDSC
subsets (30,31). In tumor tissues, MDSCs differentiate
into TAMs or TANs (6,32), and promote tumor progression by
suppressing antitumor immunity (6,33). A
previous study has also reported that CD11b+granulocyte
receptor 1intLy6Chi myeloid cells induce the
immunotolerance of memory CD8+ T cells in the spleen
(1). A recent study revealed that
tumor tissues were heavily infiltrated by MDSCs with the ability to
inhibit cytotoxic T cell expansion (34). The results of the present study
indicated that the percentages of
CD11b+Ly6G−Ly6Chi PMN-MDSCs
increased at days 7, 14 and 21 and
CD11b+Ly6ChiLy6G− M-MDSCs
increased at day 21 post-tumor cell administration. Notably, PD1
expression on CD8+ T lymphocytes in the peripheral blood
of tumor-bearing mice was also increased at day 21, indicating that
the mice were immunosuppressed.
Spleen volume can be determined by computed
tomography or magnetic resonance imaging, which may be used as a
predictor of disease. For example, patients with primary
myelofibrosis and a low spleen volume experience improved
leukemia-free survival and overall survival times compared with
those with a high spleen volume (35). Post-stroke infections, as well as a
decrease in lymphocytes and various lymphocyte subsets, are
associated with a reduction in spleen volume following acute
ischemic stroke (36). Notably,
spleen size may also be a predictor of tumor prognosis. A recent
study indicated that a larger spleen volume is a predictor of
hepatocellular carcinoma occurrence and poor overall survival rates
in patients with compensated chronic liver disease resulting from
chronic hepatitis B infection (37).
In patients with hepatocellular carcinoma, a larger spleen volume
is associated with a higher rate of liver failure and worse overall
survival rates after hepatectomy (38), and spleen volumetry has been
considered to be a predictor of persistent post-hepatectomy
decompensation in patients with hepatocellular carcinoma (39). Furthermore, a previous study
indicated that spleen length and stiffness are predictors for the
development of hepatocellular carcinoma (40). A decrease in spleen volume has been
observed in patients with locally advanced non-small cell lung
cancer receiving chemo-radiotherapy (17).
The spleen is an origin of MDSC genesis and tumor
immune tolerance (1,16). In patients with hepatocellular
carcinoma, splenectomy combined with hepatectomy positively
influences the recovery of T-lymphocyte subsets and the maintenance
of the T helper (Th)l/Th2 cytokine balance (41). The results of the present study
revealed that spleen weight was negatively correlated with the
percentages of tumor-suppressive immune cells, such as
CD4+ and CD8+ T lymphocytes, in the spleen,
but positively correlated with the percentages of tumor-promoting
immune cells, such as M-MDSC and PMN-MDSC, in the spleen and
peripheral blood of tumor-bearing mice. To the best of our
knowledge, the present study was the first to investigate whether
spleen weight was a predictor of the cellular immune response in
tumor-bearing mice. Therefore, the present results require
verification in further studies of patients with cancer. In future
clinical research, more attention should be paid to the association
between spleen volume and the immune status and prognosis of
patients with tumors.
In conclusion, spleen weight may be a predictor for
tumor prognosis, since it is directly correlated with tumor weight
and the percentages of M-MDSC and PMN-MDSCs in tumor-bearing
mice.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shaanxi Province (grant no. 2021JQ-410), the
National Natural Science Foundation of China (grant no. 91842307),
the Youth Science Foundation of the Second Affiliated Hospital of
Xi'an Jiaotong University [grant no. YJ (QN) 2018.07], and the
Program for Changjiang Scholars and Innovative Research Team in
University (grant no. IRT1171).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WJ and ZL conceived and designed the experiments. WJ
and YL conducted the animal studies. WJ, GK and YL conducted the
flow cytometric analysis. WJ and SZ analyzed the data. WJ, GK and
ZL wrote the manuscript. WJ and GK modified the manuscript. WJ and
ZL confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xi'an Jiaotong University College of Medicine (Xi'an,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ugel S, Peranzoni E, Desantis G, Chioda M,
Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S and
Bronte V: Immune tolerance to tumor antigens occurs in a
specialized environment of the spleen. Cell Rep. 2:628–639. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marvel D and Gabrilovich DI:
Myeloid-derived suppressor cells in the tumor microenvironment:
Expect the unexpected. J Clin Invest. 125:3356–3364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang W, Li Y, Wei W, Li JW, Li L, Zhang
C, Zhang SQ, Kong GY and Li ZF: Spleen contributes to restraint
stress induced hepatocellular carcinoma progression. Int
Immunopharmacol. 83:1064202020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia Y, Wei Y, Li Z-Y, Cai XY, Zhang LL,
Dong XR, Zhang S, Zhang RG, Meng R, Zhu F, et al: Catecholamines
contribute to the neovascularization of lung cancer via
tumor-associated macrophages. Brain Behav Immun. 81:111–121. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu C, Ning H, Liu M, Lin J, Luo S, Zhu W,
Xu J, Wu WC, Liang J, Shao CK, et al: Spleen mediates a distinct
hematopoietic progenitor response supporting tumor-promoting
myelopoiesis. J Clin Invest. 128:3425–3438. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar V, Patel S, Tcyganov E and
Gabrilovich DI: The nature of myeloid-derived suppressor cells in
the tumor microenvironment. Trends Immunol. 37:208–220. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cortez-Retamozo V, Etzrodt M, Newton A,
Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B,
Gorbatov R, et al: Origins of tumor-associated macrophages and
neutrophils. Proc Natl Acad Sci USA. 109:2491–2496. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cortez-Retamozo V, Etzrodt M, Newton A,
Ryan R, Pucci F, Sio SW, Kuswanto W, Rauch PJ, Chudnovskiy A,
Iwamoto Y, et al: Angiotensin II drives the production of
tumor-promoting macrophages. Immunity. 38:296–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han Y, Liu Q, Hou J, Gu Y, Zhang Y, Chen
Z, Fan J, Zhou W, Qiu S, Zhang Y, et al: Tumor-Induced Generation
of Splenic Erythroblast-like Ter-Cells Promotes Tumor Progression.
Cell. 173:634–648.e12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kristinsson SY, Gridley G, Hoover RN,
Check D and Landgren O: Long-term risks after splenectomy among
8,149 cancer-free American veterans: A cohort study with up to 27
years follow-up. Haematologica. 99:392–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv X, Yang F, Guo X, Yang T, Zhou T, Dong
X, Long Y, Xiao D and Chen Y: Hypersplenism is correlated with
increased risk of hepatocellular carcinoma in patients with
post-hepatitis cirrhosis. Tumour Biol. 37:8889–8900. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dubeykovskaya Z, Si Y, Chen X, Worthley
DL, Renz BW, Urbanska AM, Hayakawa Y, Xu T, Westphalen CB,
Dubeykovskiy A, et al: Neural innervation stimulates splenic TFF2
to arrest myeloid cell expansion and cancer. Nat Commun.
7:105172016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li B, Zhang S, Huang N, Chen H, Wang P,
Yang J and Li Z: CCL9/CCR1 induces myeloid derived suppressor cell
recruitment to the spleen in a murine H22 orthotopic hepatoma
model. Oncol Rep. 41:608–618. 2019.PubMed/NCBI
|
|
14
|
Li B, Zhang S, Huang N, Chen H, Wang P, Li
J, Pu Y, Yang J and Li Z: Dynamics of the spleen and its
significance in a murine H22 orthotopic hepatoma model. Exp Biol
Med (Maywood). 241:863–872. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller MR, Mandell JB, Beatty KM, Harvey
SA, Rizzo MJ, Previte DM, Thorne SH and McKenna KC: Splenectomy
promotes indirect elimination of intraocular tumors by
CD8+ T cells that is associated with IFNγ- and
Fas/FasL-dependent activation of intratumoral macrophages. Cancer
Immunol Res. 2:1175–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bronte V and Pittet MJ: The spleen in
local and systemic regulation of immunity. Immunity. 39:806–818.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen SW, Everitt SJ, Bedő J, Chabrot M,
Ball DL, Solomon B, MacManus M, Hicks RJ, Möller A and Leimgruber
A: Spleen volume variation in patients with locally advanced
non-small cell lung cancer receiving platinum-based
chemo-radiotherapy. PLoS One. 10:e01426082015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang W, Li Y, Li ZZ, Sun J, Li JW, Wei W,
Li L, Zhang C, Huang C, Yang SY, et al: Chronic restraint stress
promotes hepatocellular carcinoma growth by mobilizing splenic
myeloid cells through activating β-adrenergic signaling. Brain
Behav Immun. 80:825–838. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang W, Li Y, Sun J, Li L, Li JW, Zhang
C, Huang C, Yang J, Kong GY and Li ZF: Spleen contributes to
restraint stress induced changes in blood leukocytes distribution.
Sci Rep. 7:65012017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Jiang W, Li ZZ, Zhang C, Huang C,
Yang J, Kong GY and Li ZF: Repetitive restraint stress changes
spleen immune cell subsets through glucocorticoid receptor or
β-adrenergic receptor in a stage dependent manner. Biochem Biophys
Res Commun. 495:1108–1114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabrilovich DI: Myeloid-derived suppressor
sells. Cancer Immunol Res. 5:3–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L, Duan M, Chen W, Jiang A, Li X, Yang
J and Li Z: The spleen in liver cirrhosis: Revisiting an old enemy
with novel targets. J Transl Med. 15:1112017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McKim DB, Patterson JM, Wohleb ES, Jarrett
BL, Reader BF, Godbout JP and Sheridan JF: Sympathetic release of
splenic monocytes promotes recurring anxiety following repeated
social defeat. Biol Psychiatry. 79:803–813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swirski FK, Nahrendorf M, Etzrodt M,
Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler
RH, Chudnovskiy A, Waterman P, et al: Identification of splenic
reservoir monocytes and their deployment to inflammatory sites.
Science. 325:612–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jordan KR, Kapoor P, Spongberg E, Tobin
RP, Gao D, Borges VF and McCarter MD: Immunosuppressive
myeloid-derived suppressor cells are increased in splenocytes from
cancer patients. Cancer Immunol Immunother. 66:503–513. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Li ZF, Pan D, Huang C, Zhou R and
Liu ZW: Changes of splenic macrophage during the process of liver
cancer induced by diethylnitrosamine in rats. Chin Med J (Engl).
122:3043–3047. 2009.PubMed/NCBI
|
|
27
|
Mundy-Bosse BL, Thornton LM, Yang HC,
Andersen BL and Carson WE: Psychological stress is associated with
altered levels of myeloid-derived suppressor cells in breast cancer
patients. Cell Immunol. 270:80–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Zou C, Zhao W, Yu Y, Cui Y, Zhang
H, e F, Qiu Z, Zou C and Gao X: Juglone eliminates MDSCs
accumulation and enhances antitumor immunity. Int Immunopharmacol.
73:118–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng R and Chen S and Chen S: Correlation
between myeloid-derived suppressor cells and S100A8/A9 in tumor and
autoimmune diseases. Int Immunopharmacol. 29:919–925. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohammadpour H, MacDonald CR, Qiao G, Chen
M, Dong B, Hylander BL, McCarthy PL, Abrams SI and Repasky EA: β2
adrenergic receptor-mediated signaling regulates the
immunosuppressive potential of myeloid-derived suppressor cells. J
Clin Invest. 129:5537–5552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geng J, Yuan Y, Jiao X, Wang R, Liu N,
Chen H, Griffin N and Shan F: Novel modulation on myeloid-derived
suppressor cells (MDSCs) by methionine encephalin (MENK). Int
Immunopharmacol. 68:193–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ostrand-Rosenberg S: Myeloid
derived-suppressor cells: Their role in cancer and obesity. Curr
Opin Immunol. 51:68–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma M, Huang W and Kong D: IL-17 inhibits
the accumulation of myeloid-derived suppressor cells in breast
cancer via activating STAT3. Int Immunopharmacol. 59:148–156. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang K, Li J, Zhang J, Wang L, Zhang Q,
Ge J, Guo Y, Wang B, Huang Y, Yang T, et al: SDF-1/CXCR4 axis
facilitates myeloid-derived suppressor cells accumulation in
osteosarcoma microenvironment and blunts the response to anti-PD-1
therapy. Int Immunopharmacol. 75:1058182019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song MK, Chung JS, Lim SN, Lee GW, Lee SM,
Lee NK, Choi JC and Oh SY: Usefulness of spleen volume measured by
computed tomography for predicting clinical outcome in primary
myelofibrosis. Int J Hematol. 104:476–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nous A, Peeters I, Nieboer K, Vanbinst AM,
De Keyser J and De Raedt S: Post-stroke infections associated with
spleen volume reduction: A pilot study. PLoS One. 15:e02324972020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoo J, Kim SW, Lee DH, Bae JS and Cho EJ:
Prognostic role of spleen volume measurement using computed
tomography in patients with compensated chronic liver disease from
hepatitis B viral infection. Eur Radiol. 31:1432–1442. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bae JS, Lee DH, Yoo J, Yi NJ, Lee KW, Suh
KS, Kim H and Lee KB: Association between spleen volume and the
post-hepatectomy liver failure and overall survival of patients
with hepatocellular carcinoma after resection. Eur Radiol.
31:2461–2471. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fernández-Placencia R, Golse N, Cano L,
Allard MA, Pittau G, Ciacio O, Cunha AS, Castaing D, Salloum C,
Azoulay D, et al: Spleen volumetry and liver transient
elastography: Predictors of persistent posthepatectomy
decompensation in patients with hepatocellular carcinoma. Surgery.
168:17–24. 2020. View Article : Google Scholar
|
|
40
|
Marasco G, Colecchia A, Colli A, Ravaioli
F, Casazza G, Reggiani ML, Cucchetti A, Cescon M and Festi D: Role
of liver and spleen stiffness in predicting the recurrence of
hepatocellular carcinoma after resection. J Hepatol. 70:440–448.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao ZX, Chen XP and Wu ZD: Effects of
splenectomy in patients with cirrhosis undergoing hepatic resection
for hepatocellular carcinoma. World J Gastroenterol. 9:2460–2463.
2003.PubMed/NCBI
|