Introduction
Pigment epithelium-derived factor (PEDF), a serine
protease inhibitor, is first identified from conditioned medium of
retinal pigment epithelium and now recognized as one of the
adipocytokines with multifaceted functions (1–11); it
not only promotes neuronal cell differentiation, but also inhibits
pathological angiogenesis and suppresses inflammatory and
thrombotic reactions through its anti-oxidative properties in
numerous cell culture and animal models (1–11).
Moreover, we, along with others, have shown that decreased
expression levels of PEDF in tumor tissues are associated with
growth expansion, aggressiveness, and metastasis in various types
of tumors, such as breast cancer and colorectal cancer, thereby
being a poor prognostic marker in tumor-bearing patients (12–20).
Since overexpression of PEDF or administration of PEDF-derived
peptides have been shown to inhibit the growth and metastasis of a
variety of tumors, including breast cancer in animal models, PEDF
may be a novel therapeutic target for breast cancer (12–20).
Non-enzymatic modification of amino groups of
proteins, lipids, and nucleic acids by sugars has progressed under
diabetic conditions, which could alter their structural and
functional properties via formation and accumulation of aging
molecules called advanced glycation end products (AGEs) (21–25).
Accumulating evidence has suggested the pathological involvement of
AGEs in aging-related diseases, such as cancer, cardiovascular
disease, diabetes, osteoporosis, Alzheimer's disease (21–25).
Indeed, we have previously found that AGEs stimulate proliferation
and gene expression of vascular endothelial growth factor (VEGF) in
MCF-7 human breast cancer cells, which may contribute to the tumor
expansion and metastasis (26).
Although PEDF exerted anti-angiogenic effects on endothelial cells
through the interaction of a non-integrin laminin receptor (LR)
(11), it remains unclear whether
and how PEDF could inhibit the AGE-induced growth and VEGF
expression in MCF-7 breast cancer cells. In this study, we
addressed the issue.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) and MCF-7
human breast cancer cells were obtained from Sigma-Aldrich; Merck
KGaA and American Type Culture Collection, respectively.
Neutralizing monoclonal antibody (Ab) raised against LR (clone
MLuC5; LR-Ab) was purchased from Abcam (cat. no. ab3099). Normal
mouse IgG was purchased from WAKO Pure Chemical Co. (cat. no.
140-09511).
Preparation of PEDF proteins
PEDF proteins were purified as described previously
(27). SDS-PAGE analysis of purified
PEDF proteins revealed a single band with a molecular mass of about
50-kDa, which showed positive reactivity with monoclonal Ab raised
against human PEDF (TransGenic Inc.; cat. no. KM037).
Preparation of AGEs
AGEs were prepared as described previously (28). In brief, BSA (25 mg/ml) was incubated
under sterile conditions with 0.1 M glyceraldehyde in 0.2 M
NaPO4 buffer (pH 7.4) for 7 days. Then unincorporated
sugars were removed by PD-10 column chromatography and dialysis
against phosphate-buffered saline. Control non-glycated BSA was
incubated in the same conditions except for the absence of reducing
sugars.
Cells
MCF-7 cells were maintained in DMEM supplemented
with 10% heat-inactivated fetal bovine serum. MCF-7 cells were
treated with or without 100 µg/ml non-glycated BSA or AGE-modified
BSA (AGEs) in the presence or absence of the indicated
concentrations of PEDF, 5 µg/ml LR-Ab, or 5 µg/ml mouse IgG in DMEM
with 1% fetal bovine serum for 24 h.
NADPH oxidase activity
NADPH oxidase activity of MCF-7 cells was measured
by luminescence assay as described previously (6).
Reactive oxygen species (ROS)
generation
Intracellular ROS production in MCF-7 cells was
measured with a fluorescent probe,
5-(and-6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate
(carboxy-H2DFFDA) purchased from Thermo Fisher
Scientific (29).
Real-time reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells with NucleoSpin
RNA Plus (Takara Bio Inc.) according to the manufacturer's
instructions. cDNA was obtained using the PrimeScript RT reagent
kit (Takara Bio, Inc.). Quantitative real-time RT-PCR was performed
using Assay-on-Demand and TaqMan 5 fluorogenic nuclease chemistry
(Applied Biosystems) according to the supplier's recommendation.
IDs of primers for human cytochrome b-245 α chain (p22phox),
cytochrome b-245 β chain (gp91phox), receptor for AGE (RAGE), VEGF,
matrix metalloproteinase-9 (MMP-9) and β-actin gene were
Hs00609145_m1, Hs00166163_m1, Hs00542592_g1, Hs00900055_m1,
Hs00234579_m1 and Hs99999903_m1 respectively. Expression levels of
RAGE, VEGF, MMP-9 and β-actin were measured using the
2−ΔΔCq method. Data were normalized by the intensity of
internal control β-actin-derived signals. ΔCq and ΔΔCq values were
calculated using the following mathematical formulas:
ΔCq=Cq(p22phox/ gp91phox/RAGE/VEGF/MMP-9)-Cq(β-actin), and
ΔΔCq=ΔCq(target sample)-ΔCq(control sample).
Cell proliferation
Cell proliferation was measured with a Cell
Proliferation Reagent WST-1 by measuring the absorbance at
wavelength 450 nm according to the supplier's recommendations
(Darmstadt, Germany).
Measurements of VEGF
VEGF levels in MCF-7 cell culture medium were
measured with an enzyme-linked immunosorbent assay kit (Proteintech
Group, Inc.).
Western blot analysis
Proteins were extracted from MCF-7 cells using lysis
buffer (27). The samples were then
separated by SDS-PAGE and transferred to nitrocellulose membranes
(Life Technologies Japan, Ltd.). Membranes were probed with LR-Ab
(1:100 dilution; Santa Cruz Biotechnology, Inc.; cat. no. sc-20979)
for 12 h at room temperature, and then incubated with
peroxidase-conjugated polyclonal donkey anti-rabbit IgG Ab
(1:20,000 dilution; GE Healthcare UK Ltd.; cat. no. NA934-100UL).
Tubulin was visualized with peroxidase-conjugated anti-α-tubulin
antibody (1:10,000 dilution; Abcam; cat. no. ab40742). Immune
complexes were visualized using an enhanced chemiluminescence
detection system (Amersham Bioscience). Protein signals were
quantified using ImageJ software (version 1.53; National Institutes
of Health, Bethesda, MD, USA). Expression levels of LR were
normalized by those of α-tubulin.
Statistical analysis
All values were presented as mean ± standard
deviation. Post hoc comparison of means was carried out using
Tukey's honestly significant difference test after one-way ANOVA by
R software (version 4.0.3. The R Foundation for Statistical
Computing Platform). P<0.05 was considered significant.
Results
We first examined the effects of PEDF on NADPH
oxidase activity, mRNA levels of p22phox and gp91phox, two membrane
components of NADPH oxidase, ROS generation, and RAGE gene
expression in AGE-exposed MCF-7 cells. As shown in Fig. 1A-C, compared with non-glycated BSA,
AGE-modified BSA (AGEs) significantly increased NADPH oxidase
activity, gp91phox mRNA levels, and ROS generation in MCF-7 cells,
all of which were dose-dependently inhibited by the treatment with
PEDF. Furthermore, the inhibitory effects of 100 nM PEDF on NADPH
oxidase activity, gp91phox mRNA levels, and ROS generation in
AGE-exposed MCF-7 cells were significantly blocked by LR-Ab
(Fig. 1D-F). As is the case in NADPH
oxidase activity, gp91phox mRNA levels, and ROS generation, PEDF at
100 nM significantly suppressed the AGE-induced up-regulation of
RAGE mRNA level in MCF-7 cells, which was also inhibited by LR-Ab
(Fig. 1G and H). LR-Ab alone did not
affect NADPH oxidase activity, mRNA levels of p22phox and gp91phox,
ROS generation, or RAGE gene expression in non-glycated BSA-exposed
MCF-7 cells.
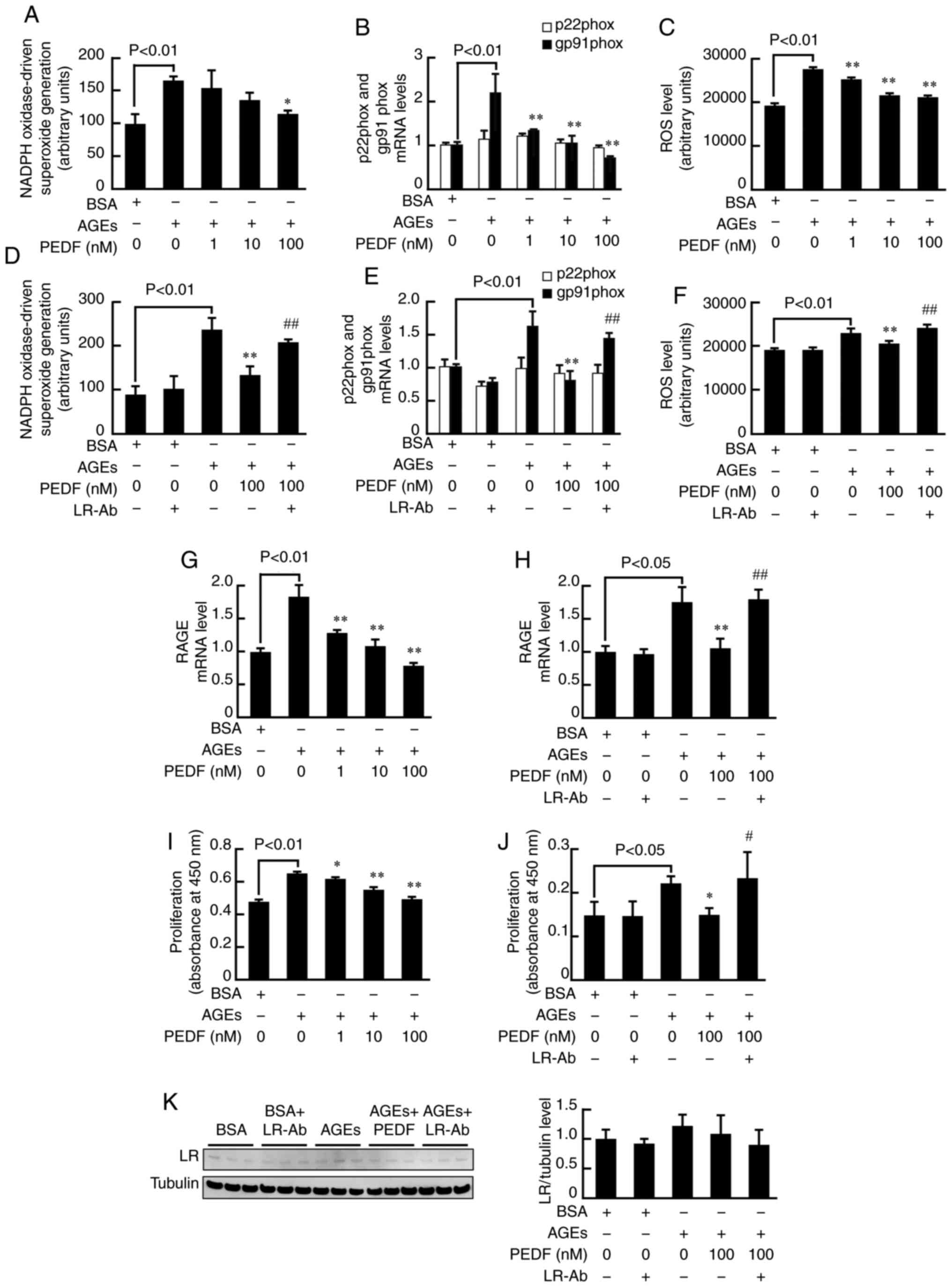 | Figure 1.Effects of PEDF on NADPH
oxidase-driven superoxide generation, mRNA expression levels of
p22phox and gp91phox, ROS production, RAGE mRNA expression,
proliferation and LR expression in MCF-7 breast cancer cells
exposed to AGEs or BSA. (A) NADPH oxidase-driven superoxide
generation (n=4), (B) mRNA expression levels of p22phox and
gp91phox (n=3), and (C) ROS production (n=4) in MCF-7 cells treated
with 100 µg/ml non-glycated BSA or AGEs in the presence or absence
of the indicated concentrations of PEDF. (D) NADPH oxidase-driven
superoxide generation (n=4), (E) mRNA expression levels of p22phox
and gp91phox (n=3), and (F) ROS production (n=4) in MCF-7 cells
treated with 100 µg/ml non-glycated BSA or AGEs in the presence or
absence of the indicated concentrations of PEDF or 5 µg/ml LR-Ab.
(G) RAGE mRNA expression in MCF-7 cells treated with 100 µg/ml
non-glycated BSA or AGEs in the presence or absence of the
indicated concentrations of PEDF or (H) 5 µg/ml LR-Ab (n=3). (I)
Proliferation of MCF-7 cells treated with 100 µg/ml non-glycated
BSA or AGEs in the presence or absence of the indicated
concentrations of PEDF or (J) 5 µg/ml LR-Ab (n=4). (K) LR protein
expression in MCF-7 cells treated with 100 µg/ml non-glycated BSA
or AGEs in the presence or absence of the indicated concentrations
of PEDF or 5 µg/ml LR-Ab analyzed by western blotting (n=3).
*P<0.05 and **P<0.01 vs. AGEs; #P<0.05 and
##P<0.01 vs. AGEs and 100 nM PEDF. AGEs, advanced
glycation end products; LR, laminin receptor; Ab, antibody; PEDF,
pigment epithelium-derived factor; ROS, reactive oxygen species;
BSA, bovine serum albumin; p22phox, cytochrome b-245 α chain;
gp91phox, cytochrome b-245 β chain; RAGE, receptor for AGE. |
AGEs significantly stimulated proliferation of MCF-7
cells, which was suppressed by PEDF in a dose-dependent manner
(Fig. 1I). LR-Ab blocked the growth
inhibitory effects of 100 nM PEDF in AGE-exposed MCF-7 cells
(Fig. 1J). Although western blot
analysis revealed that LR was actually expressed in MCF-7 cells,
expression levels of LR were not changed by the treatment of AGEs,
100 nM PEDF, or LR-Ab (Fig. 1K). We
have already confirmed that PEDF directly binds to LR, which is
significantly blocked by LR-Ab (27).
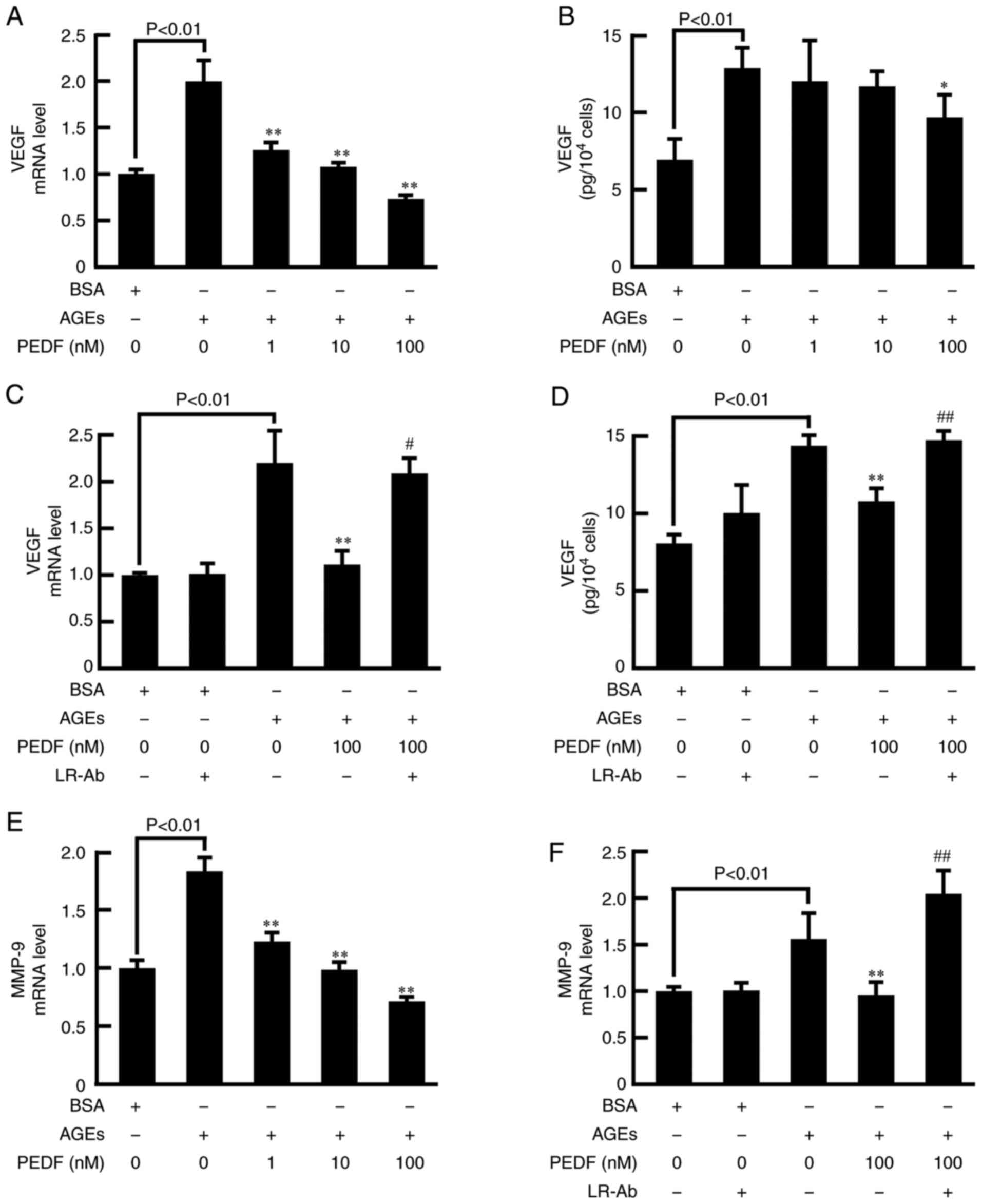
We next examined the effects of PEDF on VEGF and
MMP-9 expression in AGE-exposed MCF-7 cells. As shown in Fig. 2A and B, VEGF gene and protein
expression were significantly stimulated by AGEs, which were
inhibited by PEDF in a dose-dependently manner. LR-Ab significantly
blocked the effects of PEDF in VEGF expression in MCF-7 cells
exposed to AGEs (Fig. 2C and D).
Furthermore, PEDF dose-dependently inhibited the AGE-induced
increase in MMP-9 gene expression in MCF-7 cells (Fig. 2E). LR-Ab also significantly blocked
the PEDF-induced MMP-9 gene suppression in AGE-exposed MCF-7 cells
(Fig. 2F).
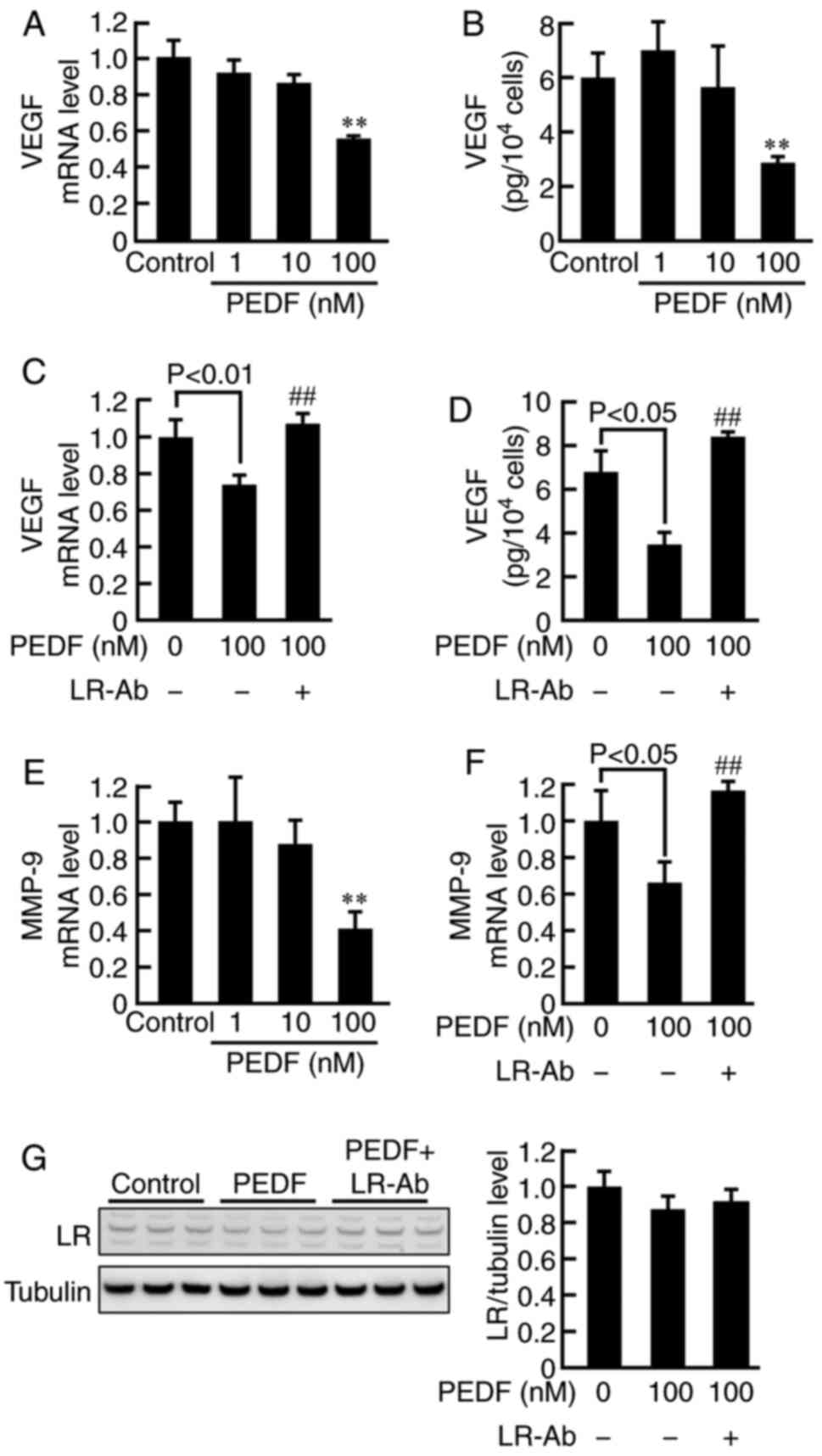
We further investigated the effects of PEDF in NADPH
oxidase activity, mRNA levels of p22phox and gp91phox, ROS
generation, RAGE, VEGF, and MMP-9 expression, and proliferation in
MCF-7 cells not exposed to BSA or AGEs. As is the case in
non-glycated BSA- or AGE-exposed MCF-7 cells, PEDF dose-dependently
inhibited the NADPH oxidase activity, gp91phox mRNA levels, ROS
generation, RAGE mRNA levels, proliferation, VEGF gene and protein
expression, and MMP-9 mRNA levels in MCF-7 cells (Figs. 3, 4A, B
and E). LR-Ab significantly blocked all these effects of 100 nM
PEDF in MCF-7 cells (Figs. 3 and
4C, D and F). LR expression levels
were not affected by PEDF or LR-Ab (Fig.
4G).
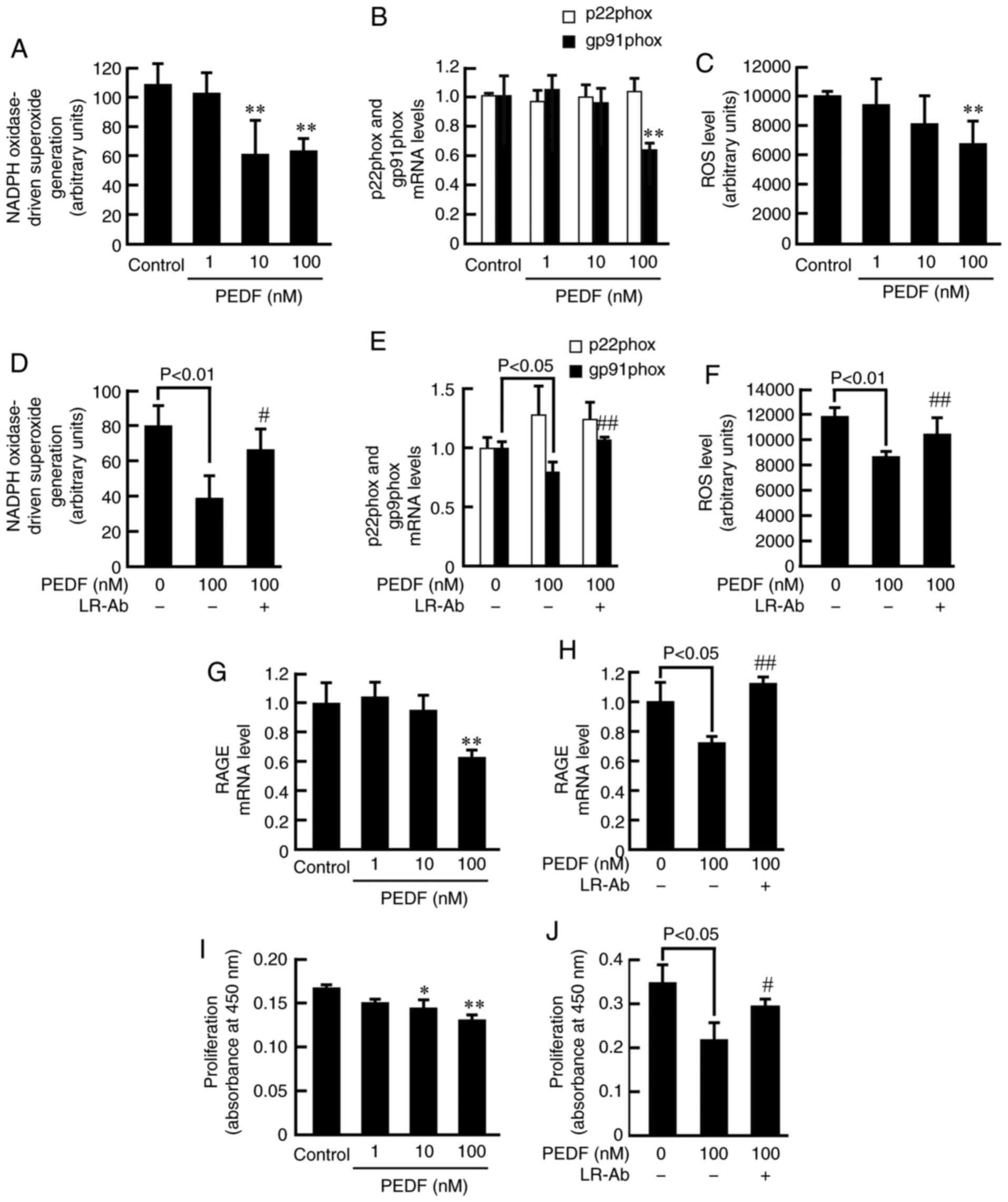 | Figure 3.Effects of PEDF on NADPH
oxidase-driven superoxide generation, mRNA expression levels of
p22phox and gp91phox, ROS production, RAGE mRNA expression and
proliferation in MCF-7 breast cancer cells. (A) NADPH
oxidase-driven superoxide generation (n=4), (B) mRNA expression
levels of p22phox and gp91phox (n=3), and (C) ROS production (n=4)
in MCF-7 cells treated with the indicated concentrations of PEDF.
(D) NADPH oxidase-driven superoxide generation (n=4), (E) mRNA
expression levels of p22phox and gp91phox (n=3), and (F) ROS
production (n=4) in MCF-7 cells treated with the indicated
concentrations of PEDF in the presence or absence of 5 µg/ml LR-Ab.
(G and H) RAGE mRNA expression in MCF-7 cells treated with the
indicated concentrations of PEDF in the presence or absence of 5
µg/ml LR-Ab (n=3). (I and J) Proliferation of MCF-7 cells treated
with the indicated concentrations of PEDF in the presence or
absence of 5 µg/ml LR-Ab (n=4). *P<0.05 and **P<0.01 vs.
control; #P<0.05 and ##P<0.01 vs. 100
nM PEDF. AGEs, advanced glycation end products; LR, laminin
receptor; Ab, antibody; PEDF, pigment epithelium-derived factor;
ROS, reactive oxygen species; p22phox, cytochrome b-245 α chain;
gp91phox, cytochrome b-245 β chain; RAGE, receptor for AGE. |
Discussion
Breast cancer is the most common cancer in women all
over the world, accounting for 10–15% of all cancer deaths in
developed countries (30,31). There is a growing body of evidence to
show the clinical link between diabetes and breast cancer (20,32,33).
Diabetes is associated with the higher incidence and more advanced
stage of breast cancer, thereby increasing the mortality rate in
these patients compared with non-diabetic individuals (32,33).
Moreover, we, along with others, have shown the pathological role
of AGEs, which are formed during a physiological aging process and
at an accelerated rate under diabetic conditions, in the
development and progression of breast cancer (34–37).
Indeed, AGEs have been reported to stimulate proliferation,
migration, invasion, and VEGF gene expression in cultured human
breast cancer cells in association with tamoxifen resistance
(26). Moreover, dietary intake of
AGEs is associated with the increased risk of breast cancer in two
independent cohort studies (38,39).
These are reasons why we focused on the effects of PEDF on
AGE-exposed human breast cancer cells.
In the present study, we found that AGEs
significantly increased NADPH oxidase activity, gp91phox mRNA
levels, ROS generation, RAGE gene expression, proliferation, gene
and protein expression of VEGF, and MMP-9 mRNA levels in cultured
breast cancer cells, all of which were dose-dependently inhibited
by the treatment with PEDF. Furthermore, these beneficial effects
of PEDF on AGE-exposed MCF-7 breast cancer cells were significantly
blocked by neutralizing LR-Ab. In addition, PEDF exerted similar
anti-tumor effects in non-glycated BSA- or AGE-exposed MCF-7 cells,
whose actions were also inhibited by LR-Ab. VEGF and MMP-9 are
crucial factors for tumor growth and invasion, respectively, whose
expression levels are associated with breast cancer progression and
metastasis (40–42). Given that PEDF expression levels are
decreased in breast cancer tissues (15,20), our
present study suggests that pharmacological up-regulation or
restoration of PEDF may inhibit the growth and metastasis of breast
cancer via the interaction with LR in two distinct pathways; one is
a direct inhibition of tumor growth, and the other is the
suppression of VEGF and MMP-9 expression, which could lead to
attenuate tumor angiogenesis, invasion, and metastasis.
We have previously shown that AGEs stimulate growth
and VEGF expression in both endothelial cells and malignant
melanoma cells by inducing the ROS generation through the
interaction with RAGE (29,43). Oxidative stress and redox-sensitive
transcriptional factor are involved in VEGF and MMP-9 gene
expression in various kinds of cells, including tumor cells
(29,43–46).
Moreover, p66ShcA has recently been shown to play a role in breast
cancer metastasis, while quercetin, an anti-oxidant suppresses the
mobility as well as VEGF and MMP-9 expression of breast cancer
cells (47,48). Since PEDF has been reported to
attenuate the AGE-RAGE-induced proliferative, inflammatory and
thrombotic reactions in a variety of cells and tissues through the
suppression of NADPH oxidase-driven superoxide generation (6,11), the
present findings suggest that PEDF may exert anti-tumor effects on
AGE-exposed breast cancer cells by suppressing the NADPH
oxidase-induced ROS generation via interaction with LR via
down-regulation of gp91phox mRNA levels. In support of our
speculation, we have found previously that (1) LR-Ab actually inhibits the binding of
PEDF to LR and resultantly restores VEGF mRNA levels in
PEDF-exposed myeloma cells and (2)
PEDF inhibits tumor necrosis factor-α-induced inflammatory
reactions in endothelial cells via inhibition of NADPH oxidase
activity through the interaction of ca. 60-kDa receptor,
which is considered to be LR (16,49).
Taken together, although the anti-tumorigenic ability and the
anti-invasiveness are different aspects, suppression of oxidative
stress by PEDF may connect these two phenomena. We did not know the
exact reason why our present results contradicted the effect of
PEDF in hepatocellular carcinoma cells; PEDF was found to play a
role in metastasis and angiogenesis in this cell type (50). However, there is accumulating
evidence that decreased expression levels of PEDF are associated
with angiogenesis and metastasis in various types of tumors,
whereas PEDF inhibits the growth and metastasis of tumors in animal
models (12–20). Differences in expression levels or
pattern of PEDF and LR between breast cancer cells and
hepatocellular carcinoma cells may partly explain the discrepant
results.
Results of all the parameters we examined here were
almost the same regardless of whether the cells were treated with
AGEs or not. Therefore, AGEs may not affect the anti-tumor effects
of PEDF. However, AGEs have been shown to decrease PEDF expression
in endothelial cells, mesangial cells, and podocytes via oxidative
stress generation, further potentiating the deleterious effects by
attenuating the protective action of PEDF against AGEs (51–53).
Therefore, there is a bi-directional interaction between AGEs and
PEDF. In many aging-related diseases, such as cancer and diabetic
vascular complications, the balance may shift towards AGEs.
Amelioration of the balance of two molecules may be a novel
therapeutic strategy for aging-related diseases, including breast
cancer in patients with diabetes.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by
Grants-in-Aid for Scientific Research (grant nos. 20K06475 and
19K06461) from the Ministry of Education, Culture, Sports, Science
and Technology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SIY conceptualized and designed the study, acquired,
analyzed and interpreted the data, drafted the manuscript and takes
responsibility for all the integrity of the data and accuracy of
the data analysis. ST, TM, YK, AS and MY conceptualized and
designed the study, as well as acquired, analyzed and interpreted
the data. MY revised the manuscript critically for intellectual
content. ST and TM confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tombran-Tink J, Chader CG and Johnson LV:
PEDF: A pigment epithelium-derived factor with potent neuronal
differentiative activity. Exp Eye Res. 53:411–414. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dawson DW, Volpert OV, Gillis P, Crawford
SE, Xu H, Benedict W and Bouck NP: Pigment epithelium-derived
factor: A potent inhibitor of angiogenesis. Science. 285:245–248.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duh EJ, Yang HS, Suzuma I, Miyagi M,
Youngman E, Mori K, Katai M, Yan L, Suzuma K, West K, et al:
Pigment epithelium-derived factor suppresses ischemia-induced
retinal neovascularization and VEGF-induced migration and growth.
Invest Ophthalmol Vis Sci. 43:821–829. 2002.PubMed/NCBI
|
|
4
|
Yamagishi S, Amano S, Inagaki Y, Okamoto
T, Takeuchi M and Inoue H: Pigment epithelium-derived factor
inhibits leptin-induced angiogenesis by suppressing vascular
endothelial growth factor gene expression through anti-oxidative
properties. Microvasc Res. 65:186–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doll JA, Stellmach VM, Bouck NP, Bergh AR,
Lee C, Abramson LP, Cornwell ML, Pins MR, Borensztajn J and
Crawford SE: Pigment epithelium-derived factor regulates the
vasculature and mass of the prostate and pancreas. Nat Med.
9:774–780. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamagishi S, Nakamura K, Matsui T, Inagaki
Y, Takenaka K, Jinnouchi Y, Yoshida Y, Matsuura T, Narama I,
Motomiya Y, et al: Pigment epithelium-derived factor inhibits
advanced glycation end product-induced retinal vascular
hyperpermeability by blocking reactive oxygen species-mediated
vascular endothelial growth factor expression. J Biol Chem.
281:20213–20220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujimura T, Yamagishi S, Ueda S, Fukami K,
Shibata R, Matsumoto Y, Kaida Y, Hayashida A, Koike K, Matsui T, et
al: Administration of pigment epithelium-derived factor (PEDF)
reduces proteinuria by suppressing decreased nephrin and increased
VEGF expression in the glomeruli of adriamycin-injected rats.
Nephrol Dial Transplant. 24:1397–1406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsui T, Higashimoto Y, Taira J and
Yamagishi S: Pigment epithelium-derived factor (PEDF) binds to
caveolin-1 and inhibits the pro-inflammatory effects of caveolin-1
in endothelial cells. Biochem Biophys Res Commun. 441:405–410.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsui T, Nishino Y, Ojima A, Maeda S,
Tahara N and Yamagishi S: Pigment epithelium-derived factor
improves metabolic derangements and ameliorates dysregulation of
adipocytokines in obese type 2 diabetic rats. Am J Pathol.
184:1094–1103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takenaka K, Yamagishi S, Matsui T,
Nakamura K, Jinnouchi Y, Yoshida Y, Ueda S, Katsuki Y, Katsuda Y
and Imaizumi T: Pigment epithelium-derived factor (PEDF)
administration inhibits occlusive thrombus formation in rats: A
possible participation of reduced intraplatelet PEDF in thrombosis
of acute coronary syndromes. Atherosclerosis. 197:25–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamagishi SI and Matsui T: Pigment
epithelium-derived factor: A novel therapeutic target for
cardiometabolic diseases and related complications. Curr Med Chem.
25:1480–1500. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abe R, Shimizu T, Yamagishi S, Shibaki A,
Amano S, Inagaki Y, Watanabe H, Sugawara H, Nakamura H, Takeuchi M,
et al: Overexpression of pigment epithelium-derived factor
decreases angiogenesis and inhibits the growth of human malignant
melanoma cells in vivo. Am J Pathol. 164:1225–1232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takenaka K, Yamagishi S, Jinnouchi Y,
Nakamura K, Matsui T and Imaizumi T: Pigment epithelium-derived
factor (PEDF)-induced apoptosis and inhibition of vascular
endothelial growth factor (VEGF) expression in MG63 human
osteosarcoma cells. Life Sci. 77:3231–3241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mirochnik Y, Aurora A, Schulze-Hoepfner
FT, Deabes A, Shifrin V, Beckmann R, Polsky C and Volpert OV: Short
pigment epithelial-derived factor-derived peptide inhibits
angiogenesis and tumor growth. Clin Cancer Res. 15:1655–1663. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou D, Cheng SQ, Ji HF, Wang JS, Xu HT,
Zhang GQ and Pang D: Evaluation of protein pigment
epithelium-derived factor (PEDF) and microvessel density (MVD) as
prognostic indicators in breast cancer. J Cancer Res Clin Oncol.
136:1719–1727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seki R, Yamagishi S, Matsui T, Yoshida T,
Torimura T, Ueno T, Sata M and Okamura T: Pigment
epithelium-derived factor (PEDF) inhibits survival and
proliferation of VEGF-exposed multiple myeloma cells through its
anti-oxidative properties. Biochem Biophys Res Commun. 431:693–697.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Becerra SP and Notario V: The effects of
PEDF on cancer biology: Mechanisms of action and therapeutic
potential. Nat Rev Cancer. 13:258–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gong Q, Qiu S, Li S, Ma Y, Chen M, Yao Y,
Che D, Feng J, Cai W, Ma J, et al: Proapoptotic PEDF functional
peptides inhibit prostate tumor growth-a mechanistic study. Biochem
Pharmacol. 92:425–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou D, Zhang M, Xu P, Yu Y, Ye G, Zhang L
and Wu A: Expression of pigment epithelium-derived factor is
associated with a good prognosis and is correlated with
epithelial-mesenchymal transition-related genes in infiltrating
ductal breast carcinoma. Oncol Lett. 11:116–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamagishi SI, Koga Y, Sotokawauchi A,
Hashizume N, Fukahori S, Matsui T and Yagi M: Therapeutic potential
of pigment epithelium-derived factor in cancer. Curr Pharm Des.
25:313–324. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamagishi S: Potential clinical utility of
advanced glycation end product cross-link breakers in age- and
diabetes-associated disorders. Rejuvenation Res. 15:564–572. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sadowska-Bartosz I and Bartosz G: Effect
of glycation inhibitors on aging and age-related diseases. Mech
Ageing Dev. 160:1–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rowan S, Bejarano E and Taylor A:
Mechanistic targeting of advanced glycation end-products in
age-related diseases. Biochim Biophys Acta Mol Basis Dis.
1864:3631–3643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamagishi SI and Matsui T: Therapeutic
potential of DNA-aptamers raised against AGE-RAGE axis in
diabetes-related complications. Curr Pharm Des. 24:2802–2809. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rungratanawanich W, Qu Y, Wang X, Essa MM
and Song BJ: Advanced glycation end products (AGEs) and other
adducts in aging-related diseases and alcohol-mediated tissue
injury. Exp Mol Med. 53:168–188. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishibashi Y, Matsui T, Takeuchi M and
Yamagishi S: Metformin inhibits advanced glycation end products
(AGEs)-induced growth and VEGF expression in MCF-7 breast cancer
cells by suppressing AGEs receptor expression via AMP-activated
protein kinase. Horm Metab Res. 45:387–390. 2013.PubMed/NCBI
|
|
27
|
Matsui T, Higashimoto Y and Yamagishi S:
Laminin receptor mediates anti-inflammatory and anti-thrombogenic
effects of pigment epithelium-derived factor in myeloma cells.
Biochem Biophys Res Commun. 443:847–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsui T, Higashimoto Y, Nishino Y,
Nakamura N, Fukami K and Yamagishi SI: RAGE-aptamer blocks the
development and progression of experimental diabetic nephropathy.
Diabetes. 66:1683–1695. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakamura N, Matsui T, Ishibashi Y,
Sotokawauchi A, Fukami K, Higashimoto Y and Yamagishi SI:
RAGE-aptamer attenuates the growth and liver metastasis of
malignant melanoma in nude mice. Mol Med. 23:295–306. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saika K and Sobue T: Epidemiology of
breast cancer in Japan and the US. JMAJ. 52:39–44. 2009.
|
|
31
|
Akram M, Iqbal M, Daniyal M and Khan AU:
Awareness and current knowledge of breast cancer. Biol Res.
50:332017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peairs KS, Barone BB, Snyder CF, Yeh HC,
Stein KB, Derr RL, Brancati FL and Wolff AC: Diabetes mellitus and
breast cancer outcomes: A systematic review and meta-analysis. J
Clin Oncol. 29:40–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang M, Yang Y and Liao Z: Diabetes and
cancer: Epidemiological and biological links. World J Diabetes.
11:227–238. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamagishi S, Matsui T and Fukami K: Role
of receptor for advanced glycation end products (RAGE) and its
ligands in cancer risk. Rejuvenation Res. 18:48–56. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matou-Nasri S, Sharaf H, Wang Q, Almobadel
N, Rabhan Z, Al-Eidi H, Yahya WB, Trivilegio T, Ali R, Al-Shanti N
and Ahmed N: Biological impact of advanced glycation endproducts on
estrogen receptor-positive MCF-7 breast cancer cells. Biochim
Biophys Acta Mol Basis Dis. 1863:2808–2820. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee KJ, Yoo JW, Kim YK, Choi JH, Ha TY and
Gil M: Advanced glycation end products promote triple negative
breast cancer cells via ERK and NF-κB pathway. Biochem Biophys Res
Commun. 495:2195–2201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walter KR, Ford ME, Gregoski MJ, Kramer
RM, Knight KD, Spruill L, Nogueira LM, Krisanits BA, Phan V, La Rue
AC, et al: Advanced glycation end products are elevated in estrogen
receptor-positive breast cancer patients, alter response to
therapy, and can be targeted by lifestyle intervention. Breast
Cancer Res Treat. 173:559–571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Omofuma OO, Turner DP, Peterson LL,
Merchant AT, Zhang J and Steck SE: Dietary advanced glycation
end-products (AGE) and risk of breast cancer in the prostate, lung,
colorectal and ovarian cancer screening trial (PLCO). Cancer Prev
Res (Phila). 13:601–610. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peterson LL, Park S, Park Y, Colditz GA,
Anbardar N and Turner DP: Dietary advanced glycation end products
and the risk of postmenopausal breast cancer in the national
institutes of health-AARP diet and health study. Cancer.
126:2648–2657. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hao L, Zhang C, Qiu Y, Wang L, Luo Y, Jin
M and Zhang Y, Guo TB, Matsushima K and Zhang Y: Recombination of
CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human
breast cancer. Cancer Lett. 253:34–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schneider BP and Sledge GW Jr: Drug
insight: VEGF as a therapeutic target for breast cancer. Nat Clin
Pract Oncol. 4:181–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dofara SG, Chang SL and Diorio C: Gene
polymorphisms and circulating levels of MMP-2 and MMP-9: A review
of their role in breast cancer risk. Anticancer Res. 40:3619–3631.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nakamura N, Matsui T, Nishino Y,
Sotokawauchi A, Higashimoto Y and Yamagishi SI: Long-term local
injection of RAGE-aptamer suppresses the growth of malignant
melanoma in nude mice. J Oncol. 2019:73876012019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamagishi S, Abe R, Inagaki Y, Nakamura K,
Sugawara H, Inokuma D, Nakamura H, Shimizu T, Takeuchi M, Yoshimura
A, et al: Minodronate, a newly developed nitrogen-containing
bisphosphonate, suppresses melanoma growth and improves survival in
nude mice by blocking vascular endothelial growth factor signaling.
Am J Pathol. 165:1865–1874. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamagishi S and Imaizumi T: Diabetic
vascular complications: Pathophysiology, biochemical basis and
potential therapeutic strategy. Curr Pharm Des. 11:2279–2299. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gutsche K, Randi EB, Blank V, Fink D,
Wenger RH, Leo C and Scholz CC: Intermittent hypoxia confers
pro-metastatic gene expression selectively through NF-κB in
inflammatory breast cancer cells. Free Radic Biol Med. 101:129–142.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lewis K, Kiepas A, Hudson J, Senecal J, Ha
JR, Voorand E, Annis MG, Sabourin V, Ahn R, La Selva R, et al:
p66ShcA functions as a contextual promoter of breast cancer
metastasis. Breast Cancer Res. 22:72020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jia L, Huang S, Yin X, Zan Y, Guo Y and
Han L: Quercetin suppresses the mobility of breast cancer by
suppressing glycolysis through Akt-mTOR pathway mediated autophagy
induction. Life Sci. 208:123–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yamagishi S, Inagaki Y, Nakamura K, Abe R,
Shimizu T, Yoshimura A and Imaizumi T: Pigment epithelium-derived
factor inhibits TNF-alpha-induced interleukin-6 expression in
endothelial cells by suppressing NADPH oxidase-mediated reactive
oxygen species generation. J Mol Cell Cardiol. 37:497–506. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hou J, Ge C, Cui M, Liu T, Liu X, Tian H,
Zhao F, Chen T, Cui Y, Yao M, et al: Pigment epithelium-derived
factor promotes tumor metastasis through an interaction with
laminin receptor in hepatocellular carcinomas. Cell Death Dis.
8:e29692017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamagishi S, Matsui T and Inoue H:
Inhibition by advanced glycation end products (AGEs) of pigment
epithelium-derived factor (PEDF) gene expression in microvascular
endothelial cells. Drugs Exp Clin Res. 31:227–232. 2005.PubMed/NCBI
|
|
52
|
Ide Y, Matsui T, Ishibashi Y, Takeuchi M
and Yamagishi S: Pigment epithelium-derived factor inhibits
advanced glycation end product-elicited mesangial cell damage by
blocking NF-kappaB activation. Microvasc Res. 80:227–232. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ishibashi Y, Matsui T, Ohta K, Tanoue R,
Takeuchi M, Asanuma K, Fukami K, Okuda S, Nakamura K and Yamagishi
S: PEDF inhibits AGE-induced podocyte apoptosis via PPAR-gamma
activation. Microvasc Res. 85:54–58. 2013. View Article : Google Scholar : PubMed/NCBI
|