Introduction
Lung cancer is the most common of the major types of
malignant diseases, presenting the greatest threat to the health
and lives of individuals worldwide (1). Among the various types of cancer, lung
cancer ranks first in terms of its incidence and mortality rate in
men, whereas it ranks second for these parameters in women
(1). An estimated 2.09 million
individuals worldwide were newly diagnosed with lung cancer in
2018, the highest number among all types of cancer (2,3). In
China, the lung cancer incidence and mortality rate have also
increased rapidly in recent years, attributable in large parts to
smoking habits and severe air pollution (4). Although surgery is considered the most
effective treatment for lung cancer (5), more than half of afflicted patients are
already in an advanced metastatic stage of the disease at the time
of diagnosis, which is beyond the optimal period for surgical
intervention (6). Despite great
improvements in therapeutic strategies, the 5-year survival rate of
patients with lung cancer is still unsatisfactory (7). Therefore, the identification of novel
and efficient molecular markers and clinical targets for the
prevention and treatment of the disease remains a critical
goal.
A previous study suggested that the occurrence and
development of lung cancer are associated with the dysregulated
expression and mutation of oncogenes and tumor suppressor genes
(8), and a number of proteins and
non-coding RNAs have been demonstrated to be involved in the
pathogenesis of the disease (9,10).
Previous studies have demonstrated that Musashi 2 and fibroblast
growth factor 19 act as independent prognostic biomarkers in
non-small cell lung cancer (NSCLC) (11,12).
Mitotic spindle organizing protein 2A overexpression promotes NSCLC
cell proliferation and invasion, which is associated with poor
NSCLC prognosis (13). However, the
pathological processes leading to lung cancer development are
complex and still not fully understood. Therefore, further
exploration of the functions and underlying mechanisms of other
cancer-related genes is still essential. Calponin 3 (CNN3), a
member of the calponin family comprising actin filament-associated
proteins (14), acts as a regulator
of actin cytoskeleton reorganization and dynamics (15). During embryonic development, CNN3 is
expressed in the myoblasts and serves an important role in
trophoblast fusion through its regulation of actin cytoskeleton
rearrangement (16,17). Furthermore, CNN3 expression is
increased in cervical cancer cells, promoting the growth and
metastasis of the disease (18). In
gastric cancer, elevated CNN3 expression contributes to cancer cell
resistance to doxorubicin (19).
Additionally, CNN3 has been revealed to be a potential diagnostic
marker of lymph node metastasis in colorectal cancer (20). In a more recent study, CNN3 was
identified to be associated with a poor prognosis in patients with
osteosarcoma, and could act as a potential prognostic biomarker
(21). These aforementioned findings
suggest that CNN3 serves various important roles in the
pathogenesis of different types of cancer. However, to the best of
our knowledge, its clinical significance and functions in NSCLC
have not yet been investigated. Therefore, the present study aimed
to explore the function of CNN3 in NSCLC and whether the protein
could be used as a prognostic marker of this disease.
The present study focused on the role of CNN3 in
NSCLC, including lung squamous cell carcinoma (LUSC), lung
adenocarcinoma (LUAD) and large-cell lung carcinoma, which
comprises all lung cancer types other than the small cell
carcinomas (22). The relative
expression levels of CNN3 in NSCLC and its association with disease
prognosis were first analyzed using bioinformatics. Subsequently,
the roles of CNN3 in NSCLC cell proliferation, migration and
invasion were assessed. Finally, the mechanisms underlying the
regulatory activity of CNN3 were investigated.
Materials and methods
Bioinformatics analysis
The GSE2514 (23),
GSE10072 (24), GSE72094 (25), GSE41271 (26) and GSE31210 (27) datasets were selected on Gene
Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/), and the basic
clinical information of the patients is shown in Tables SI and SII. CNN3 expression in LUAD and LUSC
tissues was analyzed using the GEO2R tools available on GEO,
including the GSE2514 and GSE10072 datasets. CNN3 expression in
LUAD and LUSC tissues was also analyzed on the Gene Expression
Profiling Interactive Analysis 2 (GEPIA2; http://gepia2.cancer-pku.cn/#index) bioinformatics
website (28), which includes data
from The Cancer Genome Atlas (TCGA; http://portal.gdc.cancer.gov/) and Genotype Tissue
Expression (GTEx; http://gtexportal.org/). Additionally, the diagnostic
value of CNN3 was determined according to the data from the GSE2514
dataset, the GSE10072 dataset, and TCGA and GTEx. The prognostic
value of CNN3 in LUAD and LUSC was assessed using the Kaplan-Meier
plotter (http://www.kmplot.com), the GEPIA2
website, and the GSE72094, GSE41271 and GSE31210 datasets. The
groups with high and low CNN3 expression were established according
to the ‘Auto select best cutoff’ using Kaplan-Meier plotter. For
the analysis of the prognostic value of CNN3 in LUAD, the follow-up
period was restricted to 150 months to exclude the late crossover
event when using Kaplan-Meier plotter analysis.
Cell culture and transfection
A total of two NSCLC cell lines (LUAD cells, A549;
and LUSC cells, SK-MES-1) and a human bronchial epithelial cell
line (BEAS-2B) were obtained from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences. Another three NSCLC
cell lines (LUAD cells, PC9 and NCI-H1975; and LUSC cells,
NCI-H520) were obtained from Procell Life Science & Technology
Co., Ltd. The culture and passage of these cells were performed
according to the methods provided by the suppliers. The BEAS-2B
cells were cultured in bronchial epithelial growth medium (Lonza
Group, Ltd.). The A549 and PC3 cells were cultured in F-12K medium,
while the NCI-H1975 and NCI-H520 cell lines were cultured in
RPMI-1640 medium and the SK-MES-1 cell line was cultured in Eagle's
minimum essential medium (all from Gibco; Thermo Fisher Scientific,
Inc.). All media was supplemented with 10% FBS, 100 U/ml penicillin
and 100 µg/ml streptomycin (all from Gibco; Thermo Fisher
Scientific, Inc.). All the cell lines were maintained at 37°C in a
humidified incubator with 5% CO2.
The CNN3-coding sequence, synthesized by Guangzhou
IGE Biotechnology LTD, was cloned into the pcDNA 3.1 vector
(Invitrogen; Thermo Fisher Scientific, Inc.) to construct a CNN3
overexpression recombinant plasmid, referred to as CNN3-OV. The
pcDNA 3.1 empty vector, referred to as negative control (NC), was
used as a control. A total of 5 µg CNN3-OV and NC were transfected
into the A549, SK-MES-1, NCI-H1975 and NCI-H520 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 24 h, then used for the subsequent
experimentation. The images of cell morphology were captured under
a light microscope (Olympus Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). After its quantification, 1 µg total RNA was reverse
transcribed into first-strand cDNA using the PrimeScript™ II 1st
Strand cDNA Synthesis Kit (Takara Biotechnology Co., Ltd.), at 30°C
for 10 min, 42°C for 60 min, then 85°C for 10 min. PCR was
performed using an ABI 7500 RT-PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and the SYBR® Premix Ex
Taq™ Kit (Takara Biotechnology Co., Ltd.). The following
thermocycling conditions were used: Initial denaturation at 95°C
for 5 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 32
sec. The primer sequences used were as follows: CNN3 forward,
5′-CCCAGAAAGGAATGAGTGTGT-3′ and reverse,
5′-CTCGCCATGATACTCATCAG-3′; and 18S ribosomal RNA (18S rRNA)
forward, 5′-CCTGGATACCGCAGCTAGGA-3′ and reverse,
5′-GCGGCGCAATACGAATGCCCC-3′. The 18S rRNA sequence was used as an
internal control to normalize CNN3 expression. The relative
expression levels of CNN3 were calculated using the
2−ΔΔCq method (29). The
experiment was performed in triplicate.
Cell proliferation assay
The CellTiter 96® AQueous One Solution
Cell Proliferation Assay (MTS assay; Promega Corporation) was used
to evaluate cell proliferation, which does not require the formazan
product to be dissolved prior to measuring the absorbance. In
brief, A549 and SK-MES-1 cells (1×104 cells/well in 100
µl) transfected with the CNN3-OV or NC recombinant plasmids for 24
h were seeded into 96-well plates. After culture for 0, 24, 48 and
72 h, AQueous One Solution reagent (10 µl) was added to the wells,
and the cells were further incubated at 37°C for 2 h. The optical
density at 490 nm (OD490 nm) was determined using a
microplate reader (Bio-Rad Laboratories, Inc.). The experiment was
performed in triplicate.
Cell cycle analysis
A549 and SK-MES-1 cells transfected with the CNN3-OV
or NC recombinant plasmids for 48 h were collected and washed
twice. Subsequently, the cells were fixed in ice-cold 70% ethanol
at 25°C for 2 h. Next, the cells were washed twice and then stained
according to the instructions of the PI Cell Cycle Detection kit
(Nanjing KeyGen Biotech Co., Ltd.). Cell cycle distribution was
assessed using a BD FACSCanto flow cytometer (BD Biosciences) and
the cells were analyzed using ModFit LT v4.1 (Verity Software
House). The experiment was performed in triplicate.
Cell apoptosis assay
A549 and SK-MES-1 cells transfected with the CNN3-ov
or NC recombinant plasmids for 48 h were collected and washed twice
with ice-cold PBS. Subsequently, the cells were resuspended and
stained according to the instructions of the Annexin V-FITC/PI
Apoptosis Detection kit (Nanjing KeyGen Biotech Co., Ltd.). The
percentage of apoptotic cells was assessed using a BD FACSCanto
flow cytometer (BD Biosciences) and cells were analyzed using
FACSDiva v8.0 (BD Biosciences). This experiment was performed in
triplicate.
Transwell migration and invasion
assays
A549 or SK-MES-1 cells were transfected with the
CNN3-OV or NC recombinant plasmids for 24 h. Subsequently,
2×105 cells in 200 µl serum-free medium were seeded into
the upper chamber of the polycarbonate filter Transwell chamber
(8-µm; BD Biosciences). The lower chamber was filled with culture
medium containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
The Transwell chamber was then incubated at 37°C in a humidified
incubator with 5% CO2 for 48 h. Next, the cells unable
to pass through the filter were removed with a cotton swab.
Finally, after washing with PBS, the filter membrane from the upper
chamber was first fixed with precooled 4% paraformaldehyde at 25°C
for 10 min, then stained with crystal violet (0.5%) at 25°C for 15
min. Subsequently, the numbers of migratory or invasive cells were
counted under a light microscope (Olympus Corporation). For the
Transwell invasion assay, 8-µm polycarbonate filter Transwell
chambers were coated at 37°C for 30 min with Matrigel (BD
Biosciences) and all other protocol steps were the same as those
for the Transwell migration assay. The experiment was repeated five
times.
Western blotting
Total protein was extracted from the cells using
RIPA lysis buffer with protease and phosphatase inhibitors (all
from Beyotime Institute of Biotechnology). The protein
concentration in the supernatant was quantified using a BCA assay
(Beyotime Institute of Biotechnology). After quantification of the
total protein, 30 µg of each protein sample was separated using 10%
SDS-PAGE and then transferred onto polyvinylidene fluoride
membranes. Subsequently, all membranes were blocked with 5% skimmed
milk diluted in TBS. After the membranes were blocked at 37°C for 1
h, they were incubated at 4°C for 12 h with the following primary
antibodies: Anti-CNN3 (dilution, 1:1,000; cat. no. ab93593),
anti-PI3K p85 α (dilution, 1:1,000; cat. no. ab191606),
anti-phosphorylated AKT1 (p-AKT1 Ser473) (dilution, 1:5,000; cat.
no. ab81283), anti-AKT1 (dilution, 1:1,000; cat. no. ab233755) and
anti-GAPDH (dilution, 1:3,000; cat. no. ab181602) (all from Abcam).
Subsequently, the membranes were incubated with HRP-conjugated
secondary antibodies (both at dilution, 1:10,000) (cat. nos.
ab205718 and ab6708) (both from Abcam) at 25°C for 40 min. Finally,
an enhanced chemiluminescence detection kit (Nanjing KeyGen Biotech
Co., Ltd.) was used for visualization of the target proteins, and
the ECL signal was exposed to X-rays. Densitometry of the bands was
measured with ImageJ software (v6; National Institutes of Health).
The experiment was repeated twice.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
statistical software (IBM Corp.). Data are presented as the mean ±
standard deviation. The diagnostic value of CNN3 was determined
using receiver operating characteristic (ROC) curve analysis, and
then the cutoff value, sensitivity and specificity were calculated
according to the Youden index. Survival curves were plotted using
the Kaplan-Meier method and compared using the log-rank test.
Differences between two groups were analyzed using an unpaired
t-test. However, the differences in the CNN3 expression level
between LUAD and normal paired adjacent lung tissues in the GSE2514
dataset were analyzed using a paired t-test. Differences in the
CNN3 expression levels among the NSCLC and BEAS-2B cell lines were
analyzed using one-way ANOVA followed by Dunnett's post hoc
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Association between CNN3 expression
and prognosis in patients with NSCLC
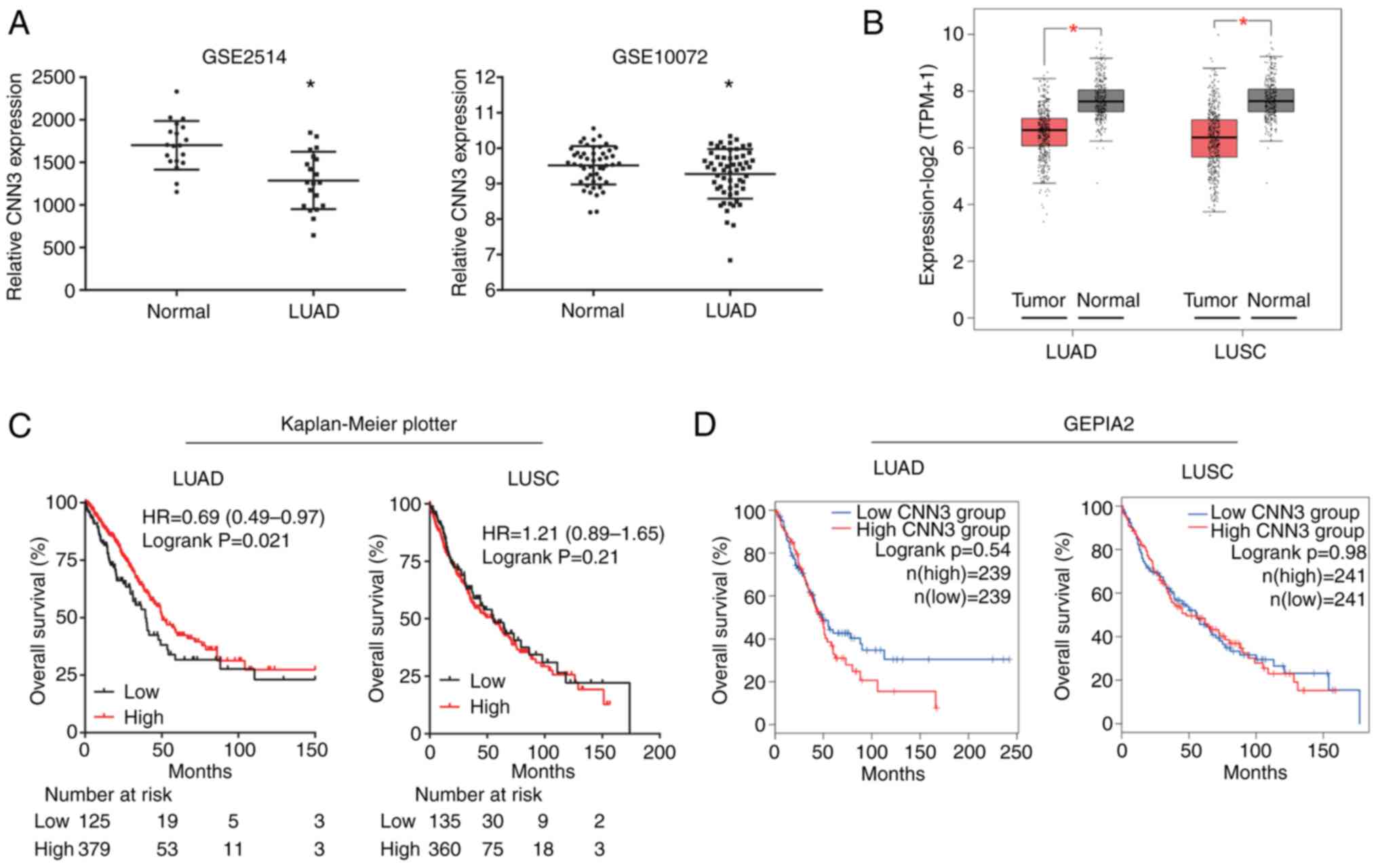
The analyses using the bioinformatics tools GEO and
GEPIA2 revealed that the expression levels of CNN3 were
significantly downregulated in both LUAD and LUSC tissues compared
with in normal lung tissues (Fig. 1A and
B, respectively). Additionally, the diagnostic value of CNN3
was determined using ROC curve analysis. The area under the curve
values for CNN3 were 0.824 and 0.596 for LUAD according to the
GSE2514 and GSE10072 datasets, respectively (Fig. S1A), whereas they were 0.830 for LUSC
and 0.819 for LUAD according to data from TCGA and the GTEx project
database (Fig. S1A). Therefore,
except for the GSE10072 dataset, CNN3 was determined to have a high
diagnostic value based on data from the GSE2514 dataset and the
LUAD and LUSC data from TCGA and the GTEx database. The
Kaplan-Meier plotter tool was used to investigate the prognostic
potential of CNN3 in NSCLC and the results revealed that low CNN3
expression was associated with a shorter survival time for LUAD,
while it exhibited no association for patients with LUSC (Fig. 1C). However, CNN3 expression was not
associated with the survival rate of patients with LUAD and LUSC
according to GEPIA2 (Fig. 1D). In
addition, CNN3 expression was also not associated with the
prognosis of patients according to data from GEO (GSE72094,
GSE41271 and GSE31210 datasets; Fig.
S1B).
CNN3 expression is downregulated in
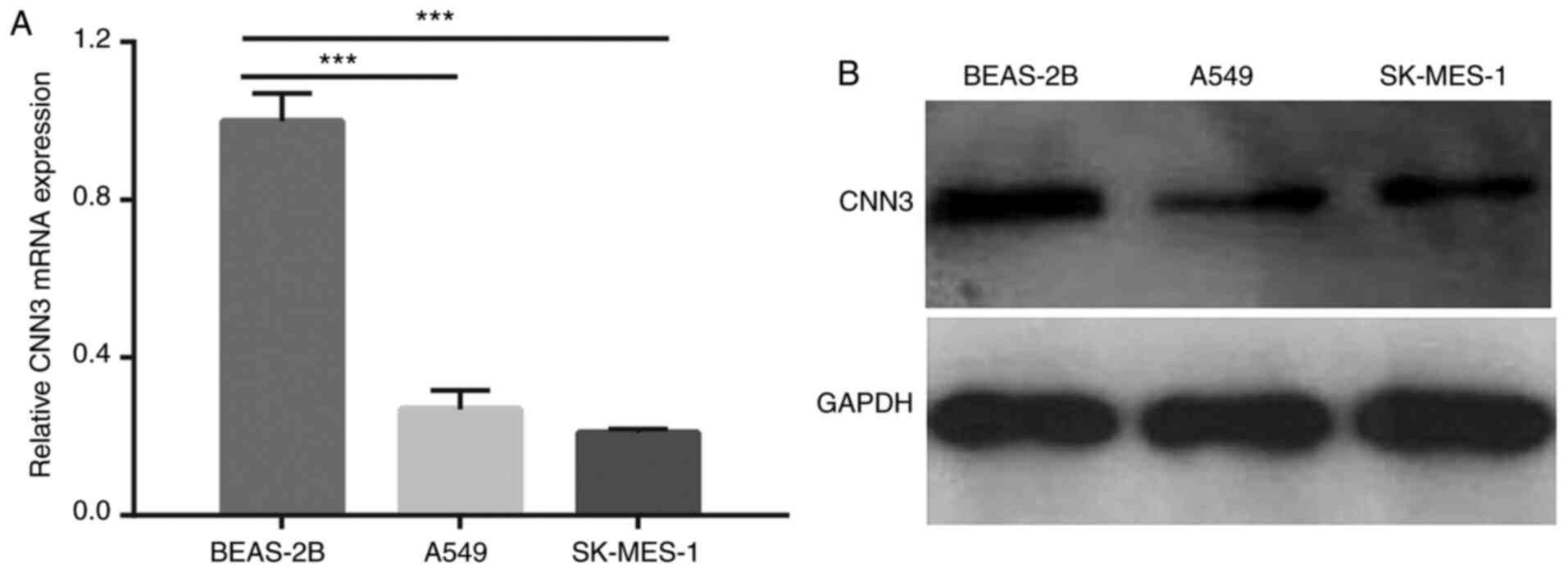
NSCLC cell lines
The mRNA and protein expression levels of CNN3 in
NSCLC cells were assessed by RT-qPCR and western blotting,
respectively. The results demonstrated that both the mRNA and
protein expression levels of CNN3 were notably downregulated in the
A549 and SK-MES-1 cells compared with in BEAS-2B cells (Fig. 2A and B). Additionally, CNN3 mRNA
expression was significantly downregulated in NCI-H1975, PC9 and
NCI-H520 cells compared with in BEAS-2B cells (Fig. S2).
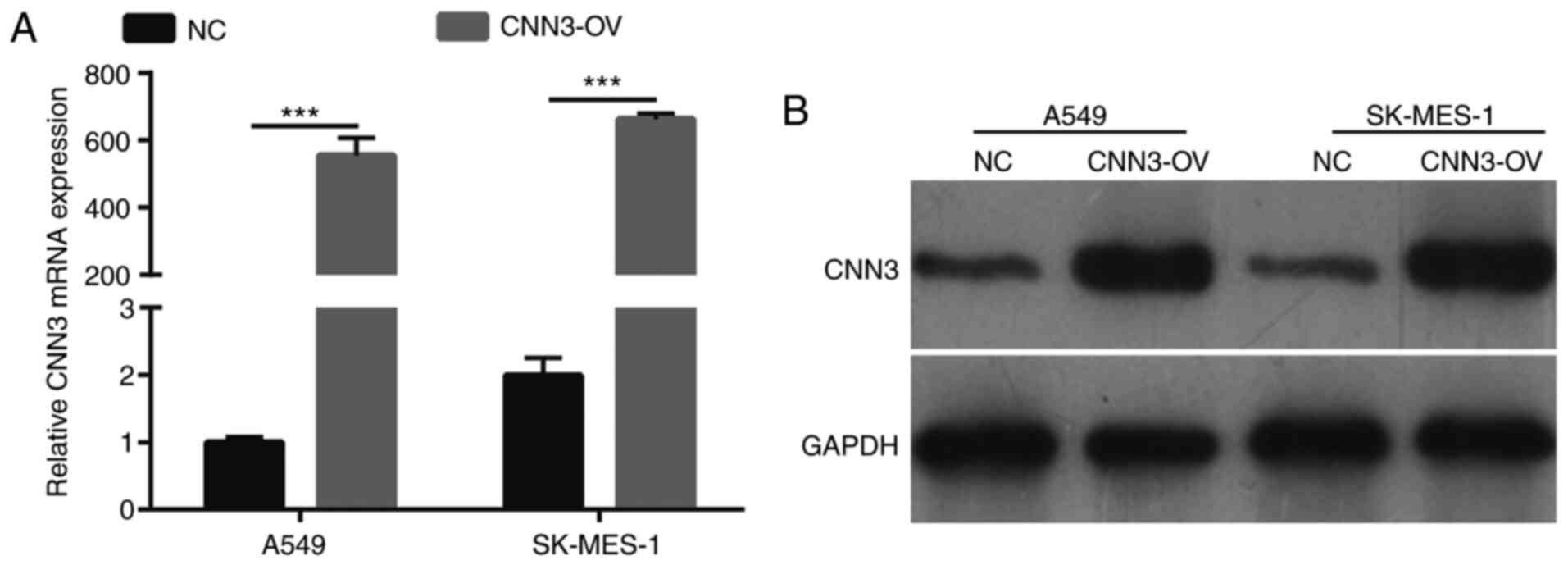
CNN3 was successfully overexpressed in
NSCLC cell lines
The expression levels of CNN3 in A549 and SK-MES-1
cells, which were transfected with the CNN3-OV or NC recombinant
plasmids for 48 h, were measured using RT-qPCR and western
blotting. In both cell lines, CNN3 mRNA and protein expression was
notably higher in the CNN3-OV group than in the NC group (Fig. 3A and B).
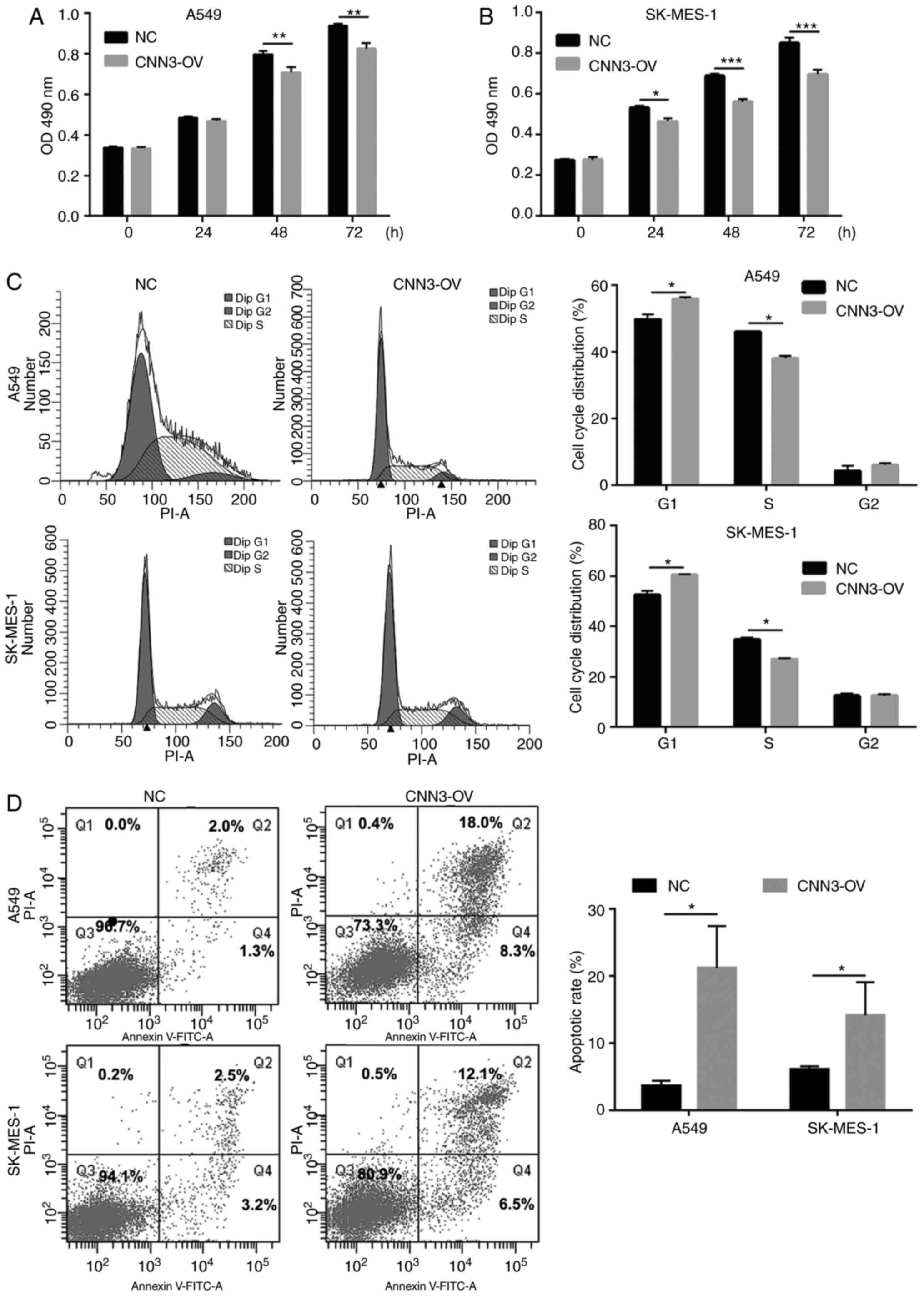
CNN3 overexpression suppresses NSCLC
cell proliferation and promotes apoptosis
To investigate the role of CNN3 in the pathogenesis
of NSCLC, the present study analyzed the effects of its
overexpression on proliferation, the cell cycle and apoptosis in
both A549 and SK-MES-1 cells. As shown in Fig. 4A and B, in both cell lines, the
OD490 nm value was significantly lower in the CNN3-OV
group than in the NC group after culture for 48 and 72 h.
Additionally, compared with that in the NC group, the percentage of
cells in the G1 phase was higher, whereas that in the S
phase was lower, in the CNN3-OV group (Fig. 4C). Furthermore, compared with that in
the NC group the number of apoptotic cells in the CNN3-OV group was
also high: 3.60 to 21.23% and 6.07 to 13.80% for the A549 and
SK-MES-1 cell lines, respectively (Fig.
4D). These results indicated that CNN3 overexpression could
suppress proliferation, induce G1-phase arrest and
promote apoptosis in NSCLC cells. Additionally, CNN3 mRNA and
protein expression level was notably higher in the CNN3-OV group
compared with that in the NC group in both the NCI-H1975 and
NCI-H520 cell lines (Fig. S3A and
B). In both the NCI-H1975 and NCI-H520 cell lines, cell
proliferation was significantly lower in the CNN3-OV group than in
the NC group after culture for 24, 48 and 72 h (Fig. S3C and D).
CNN3 overexpression suppresses NSCLC
cell migration and invasion
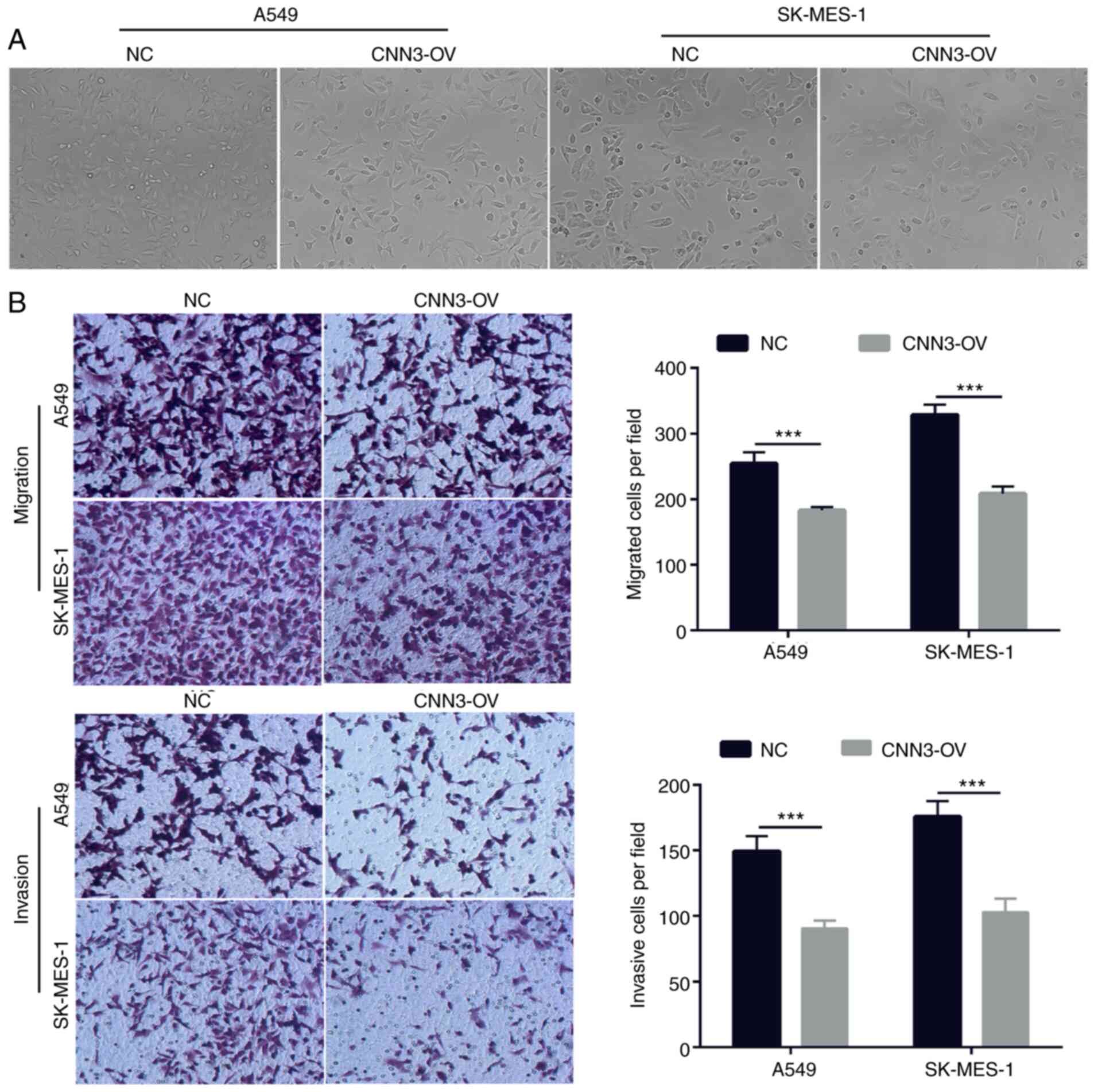
Next, the cell morphology was not markedly altered
between the NC and CNN3-OV groups according to the results of light
microscope and Transwell analysis (Fig.
5A and B). To investigate the effect of CNN3 overexpression on
cell migration and invasion, Transwell migration and invasion
assays were performed using the transfected A549 and SK-MES-1
cells. In both NSCLC cell lines, the CNN3-OV group had a lower
number of migratory and invasive cells than the NC group (Fig. 5B). The inhibitory effect of CNN3
overexpression on A549 and SK-MES-1 cell migration was 33.00 and
36.58%, respectively. The inhibitory effect of CNN3 overexpression
on A549 and SK-MES-1 cell invasion was 39.28 and 41.52%,
respectively. These results suggested that CNN3 overexpression
could suppress NSCLC cell migration and invasion.
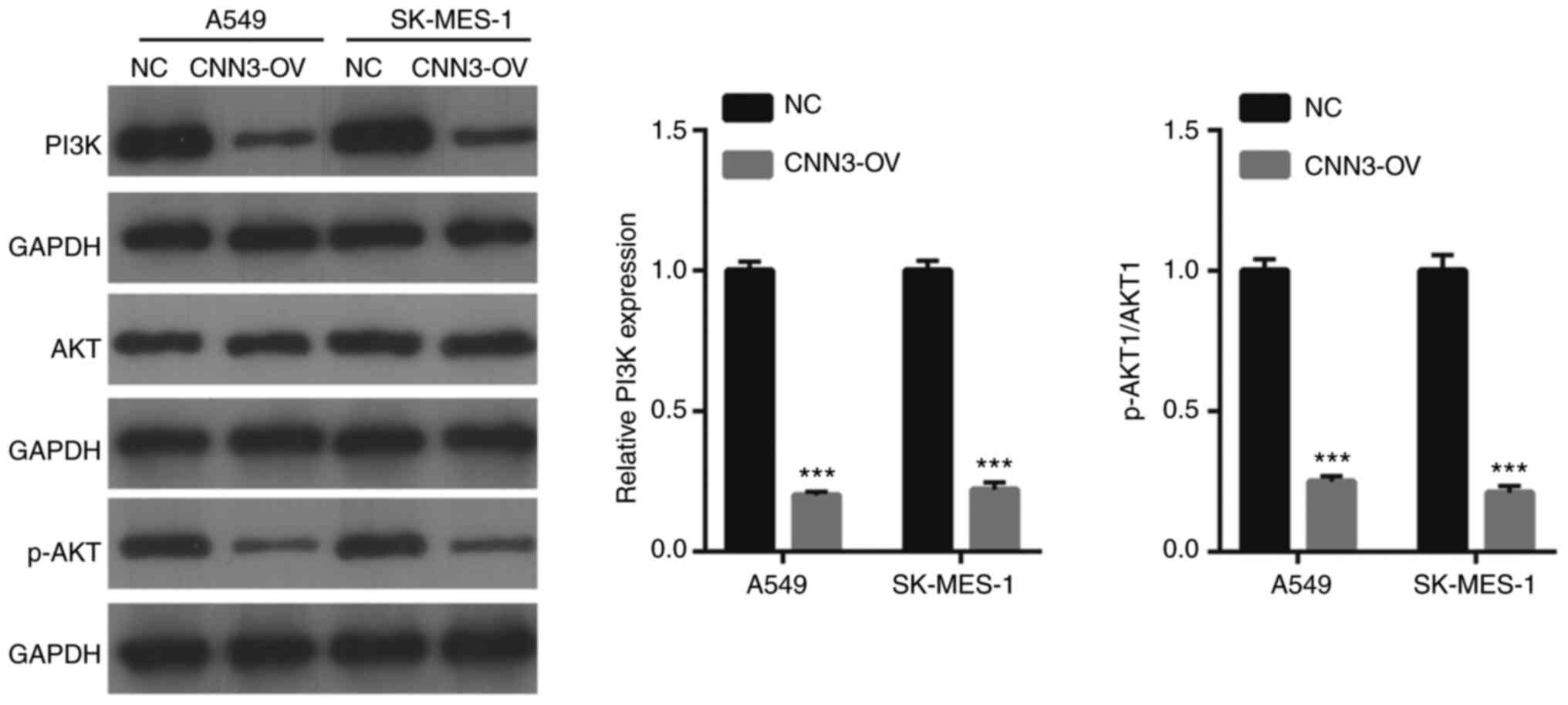
CNN3 overexpression suppresses the
PI3K/AKT signaling pathway in NSCLC cells
Western blot results revealed that the levels of
PI3K p85 α and the ratio of p-AKT1/AKT1 were lower in the CNN3-OV
group than in the NC group (Fig. 6).
This suggested that CNN3 overexpression could suppress the PI3K/AKT
signaling pathway in NSCLC cells.
Discussion
In the present study, bioinformatics analysis
revealed that CNN3 expression was significantly downregulated in
both LUAD and LUSC tissues compared with its expression in normal
lung tissue, suggesting that CNN3 dysregulation may serve a key
regulatory role in the pathogenesis of NSCLC. Therefore, the
present study verified this hypothesis by investigating the effect
of CNN3 overexpression on cell proliferation, migration, invasion
and apoptosis, and the cell cycle distribution of A549 and SK-MES-1
NSCLC cell lines.
The present study revealed that CNN3 expression was
significantly downregulated in both LUAD and LUSC tissues. However,
the identified profile of downregulated CNN3 expression in NSCLC
tissues was inconsistent with the expression profile in
osteosarcoma and cervical cancer tissues (18,21),
which suggests that CNN3 may serve different roles in different
types of cancer. Additionally, CNN3 expression had a high
diagnostic value based on the GSE2514 dataset and the LUAD and LUSC
data from TCGA and the GTEx database, whereas it had a low
diagnostic value when based on the GSE10072 dataset. Furthermore,
CNN3 expression was associated with overall survival rate in
patients with LUAD, while it was not associated with overall
survival rate in patients with LUSC according to the Kaplan-Meier
plotter results. According to the data from TCGA, and the GSE72094,
GSE41271 and GSE31210 datasets, CNN3 expression was not associated
with the prognosis of patients with LUAD and LUSC. These results
implied that CNN3 could not act as prognostic marker for monitoring
patients with NSCLC, specifically for improving prognostic
assessment in LUSC. The aforementioned results indicated that
whether CNN3 can be used as a marker for diagnosis and prognosis of
NSCLC requires further collection of NSCLC specimens for
research.
The present study also demonstrated that the
overexpression of CNN3 suppressed proliferation, migration and
invasion in NSCLC cells. These in vitro results indicated
that CNN3 may serve a tumor-suppressive role in NSCLC. Although
significant apoptosis occurred in the CNN3-overexpressing cells,
the inhibitory effect of CNN3 overexpression on cell migration and
invasion was greater than its effect on cell apoptosis. The results
of the in vitro cell function assay in the present study
were also supported by the present findings that patients with LUAD
in the high CNN3 expression group had a longer survival time than
those in the low CNN3 expression group, according to Kaplan-Meier
plotter results. CNN3 has been reported to serve a tumor-promoting
role in osteosarcoma, gastric cancer and colon cancer (19,21,30).
Actin cytoskeleton reorganization promotes cancer metastasis
(31). CNN3 is one of the actin
filament-associated proteins and functions to reorganize the actin
cytoskeleton (14), suggesting that
CNN3 may enhance the metastasis of cancer cells by promoting actin
cytoskeleton reorganization in osteosarcoma, gastric cancer and
colon cancer. However, no studies have demonstrated that there is
an association between the effect of CNN3 on cell proliferation and
migration and the changes in cell morphology. The present study
revealed that CNN3 expression was lower in LUSC and LUAD tissues
and cell lines, and CNN3 overexpression had no effect on the cell
morphology, suggesting that CNN3 did not affect the proliferation
and metastasis of cancer cells by promoting actin cytoskeleton
reorganization. These results further support the hypothesis that
CNN3 may serve different roles in different cancer types.
To investigate the regulatory mechanism underlying
the tumor-suppressive role of CNN3 in NSCLC, the present study
investigated the effect of CNN3 overexpression on the PI3K/AKT
signaling pathway, the abnormal activation of which is known to be
involved in numerous human diseases, including tumorigenesis
(32–34). It was revealed that the
overexpression of CNN3 decreased the levels of PI3K p85 α and the
ratio of p-AKT1/AKT1 in A549 and SK-MES-1 cells. Human PI3K
comprises a 110 kDa catalytic subunit and a 85 kDa regulatory
subunit, the latter of which is PI3K p85 α, also known as
phosphoinositide 3-kinase regulatory subunit 1 (35). A total of 3 AKT isomers (AKT1, AKT2
and AKT3) mediate numerous downstream signaling pathways regulated
by PI3K, which phosphorylates AKT at Ser473 (36,37).
PI3K overexpression mediates downstream AKT phosphorylation, while
not affecting the expression of total Akt protein (36,37).
Therefore, the present results demonstrated that the overexpression
of CNN3 could inactivate the PI3K/AKT signaling pathway. The
activation of this signaling pathway promotes the growth and
metastasis of a number of cancer types, including NSCLC (34,38).
Given that CNN3 had suppressive effects on NSCLC cell
proliferation, migration and invasion, it was hypothesized that the
effects of this actin filament-associated protein in NSCLC cells
may be mediated through its inhibition of the PI3K/AKT signaling
pathway. To the best of our knowledge, the present study was the
first to report that CNN3 is involved in the regulation of this
important signaling pathway.
The present study had certain limitations. First,
the diagnostic and prognostic functions of CNN3 were analyzed using
GEO and TCGA data, leading to differences in results. Therefore, a
large number of NSCLC tissues should be collected and follow-up
analyses should be conducted to verify the possibility of its use
as a prognostic and diagnostic marker for patients with NSCLC in
China or worldwide. Additionally, the effect of CNN3 overexpression
on the proliferation and metastasis of NSCLC cells still needs to
be verified in in vivo experiments. Finally, the present
study only examined the effect of CNN3 overexpression on the
PI3K/AKT signaling pathway, and whether it can affect other
signaling pathways has not been studied. Therefore, further
experiments are required to analyze the role of CNN3 in the
diagnosis and prognosis of NSCLC and the regulatory mechanism of
CNN3 in the growth and metastasis of NSCLC.
In conclusion, the results revealed that CNN3 had a
downregulated expression profile in NSCLC tissues and cells. CNN3
overexpression suppressed NSCLC cell proliferation, migration and
invasion in vitro. Therefore, CNN3 may be a potential
therapeutic target for NSCLC. However, whether CNN3 can be used as
a diagnostic and prognostic marker remains to be studied
further.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY conceived and designed the present study,
developed the methodology, and drafted the manuscript. CY and SZ
performed the experiments and collected the data. WF and XC
analyzed and interpreted the data. SZ, WF and XC revised the
manuscript. All authors read and approved the final manuscript. CY
and XC confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. Ca Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in Europe: Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao M and Chen W: Epidemiology of lung
cancer in China. Thorac Cancer. 10:3–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richard PJ and Rengan R: Oligometastatic
non-small-cell lung cancer: Current treatment strategies. Lung
Cancer (Auckl). 7:129–140. 2016.PubMed/NCBI
|
|
6
|
Fischer C, Leithner K, Wohlkoenig C,
Quehenberger F, Bertsch A, Olschewski A, Olschewski H and Hrzenjak
A: Panobinostat reduces hypoxia-induced cisplatin resistance of
non-small cell lung carcinoma cells via HIF-1α destabilization. Mol
Cancer. 14:42015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lakshmi SP, Reddy AT, Banno A and Reddy
RC: PPAR agonists for the prevention and treatment of lung cancer.
Ppar Res. 2017:82527962017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohamad N, Jayalakshmi P, Rhodes A, Liam
CK, Tan JL and Yousoof S: Anaplastic lymphoma kinase (ALK)
mutations in patients with adenocarcinoma of the lung. Br J Biomed
Sci. 74:176–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Virmani AK and Gazdar AF: Tumor suppressor
genes in lung cancer. Methods Mol Biol. 222:97–115. 2003.PubMed/NCBI
|
|
10
|
Ghafouri-Fard S, Shoorei H, Branicki W and
Taheri M: Non-coding RNA profile in lung cancer. Exp Mol Pathol.
114:1044112020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Topchu I, Karnaukhov N, Mazitova A, Yugai
V, Voloshin M, Tikhomirova M, Kit O, Frantsiyants E, Kharin L,
Airapetova T, et al: Musashi 2 (MSI2) expression as an independent
prognostic biomarker in non-small cell lung cancer (NSCLC). J
Thorac Dis. 13:1370–1379. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Shao J, Shen A, Zhu X, Zhang X,
Sun H, Wei S and Ling Y: Enhanced expression of FGF19 predicts poor
prognosis in patients with non-small cell lung cancer. J Thorac
Dis. 13:1769–1784. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Jiang X, Cheng Y, Ren H, Hu Y,
Zhang Y, Su H, Zou Z, Wang Q, Liu Z, et al: MZT2A promotes NSCLC
viability and invasion by increasing Akt phosphorylation via the
MOZART2 domain. Cancer Sci. 112:2210–2222. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi K and Nadal-Ginard B: Molecular
cloning and sequence analysis of smooth muscle calponin. J Biol
Chem. 266:13284–13288. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rami G, Caillard O, Medina I, Pellegrino
C, Fattoum A, Ben-Ari Y and Ferhat L: Change in the shape and
density of dendritic spines caused by overexpression of acidic
calponin in cultured hippocampal neurons. Hippocampus. 16:183–197.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daimon E, Shibukawa Y and Wada Y: Calponin
3 regulates stress fiber formation in dermal fibroblasts during
wound healing. Arch Dermatol Res. 305:571–584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shibukawa Y, Yamazaki N, Daimon E and Wada
Y: Rock-dependent calponin 3 phosphorylation regulates myoblast
fusion. Exp Cell Res. 319:633–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia L, Yue Y, Li M, Zhang YN, Zhao L, Lu
W, Wang X and Xie X: CNN3 acts as a potential oncogene in cervical
cancer by affecting RPLP1 mRNA expression. Sci Rep. 10:24272020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong KS, Kim H, Kim SH, Kim M and Yoo J:
Calponin 3 regulates cell invasion and doxorubicin resistance in
gastric cancer. Gastroenterol Res Pract. 2019:30249702019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakarai C, Osawa K, Akiyama M, Matsubara
N, Ikeuchi H, Yamano T, Hirota S, Tomita N, Usami M and Kido Y:
Expression of AKR1C3 and CNN3 as markers for detection of lymph
node metastases in colorectal cancer. Clin Exp Med. 15:333–341.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai F, Luo F, Zhou R, Zhou Q, Xu J, Zhang
Z, Xiao J and Song L: Calponin 3 is associated with poor prognosis
and regulates proliferation and metastasis in osteosarcoma. Aging
(Albany NY). 12:14037–14049. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stearman RS, Dwyer-Nield L, Zerbe L,
Blaine SA, Chan Z, Bunn PA Jr, Johnson GL, Hirsch FR, Merrick DT,
Franklin WA, et al: Analysis of orthologous gene expression between
human pulmonary adenocarcinoma and a carcinogen-induced murine
model. Am J Pathol. 167:1763–1775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Landi MT, Dracheva T, Rotunno M, Figueroa
JD, Liu H, Dasgupta A, Mann FE, Fukuoka J, Hames M, Bergen AW, et
al: Gene expression signature of cigarette smoking and its role in
lung adenocarcinoma development and survival. PLoS One.
3:e16512008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schabath MB, Welsh EA, Fulp WJ, Chen L,
Teer JK, Thompson ZJ, Engel BE, Xie M, Berglund AE, Creelan BC, et
al: Differential association of STK11 and TP53 with KRAS
mutation-associated gene expression, proliferation and immune
surveillance in lung adenocarcinoma. Oncogene. 35:3209–3216. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato M, Larsen JE, Lee W, Sun H, Shames
DS, Dalvi MP, Ramirez RD, Tang H, DiMaio JM, Gao B, et al: Human
lung epithelial cells progressed to malignancy through specific
oncogenic manipulations. Mol Cancer Res. 11:638–650. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okayama H, Kohno T, Ishii Y, Shimada Y,
Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S,
et al: Identification of genes upregulated in ALK-positive and
EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res.
72:100–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47:W556–W560. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nair VA, Al-Khayyal NA, Sivaperumal S and
Abdel-Rahman WM: Calponin 3 promotes invasion and drug resistance
of colon cancer cells. World J Gastrointest Oncol. 11:971–982.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang X, Qin Y, Kun L and Zhou Y: The
significant role of the microfilament system in tumors. Front
Oncol. 11:6203902021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, He X, Liu Y, Zhang H, Chen H, Guo
S and Liang Y: MiR-101-3p inhibits the growth and metastasis of
non-small cell lung cancer through blocking PI3K/AKT signal pathway
by targeting MALAT-1. Biomed Pharmacother. 93:1065–1073. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang J, Cron P, Thompson V, Good VM, Hess
D, Hemmings BA and Barford D: Molecular mechanism for the
regulation of protein kinase B/Akt by hydrophobic motif
phosphorylation. Mol Cell. 9:1227–1240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Calera MR, Martinez C, Liu H, Jack AK,
Birnbaum MJ and Pilch PF: Insulin increases the association of
Akt-2 with Glut4-containing vesicles. J Biol Chem. 273:7201–7204.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao X, Wang N, Wu S, Cui H, An X and Yang
Y: Long noncoding RNA FER1L4 inhibits cell proliferation and
metastasis through regulation of the PI3K/AKT signaling pathway in
lung cancer cells. Mol Med Rep. 20:182–190. 2019.PubMed/NCBI
|