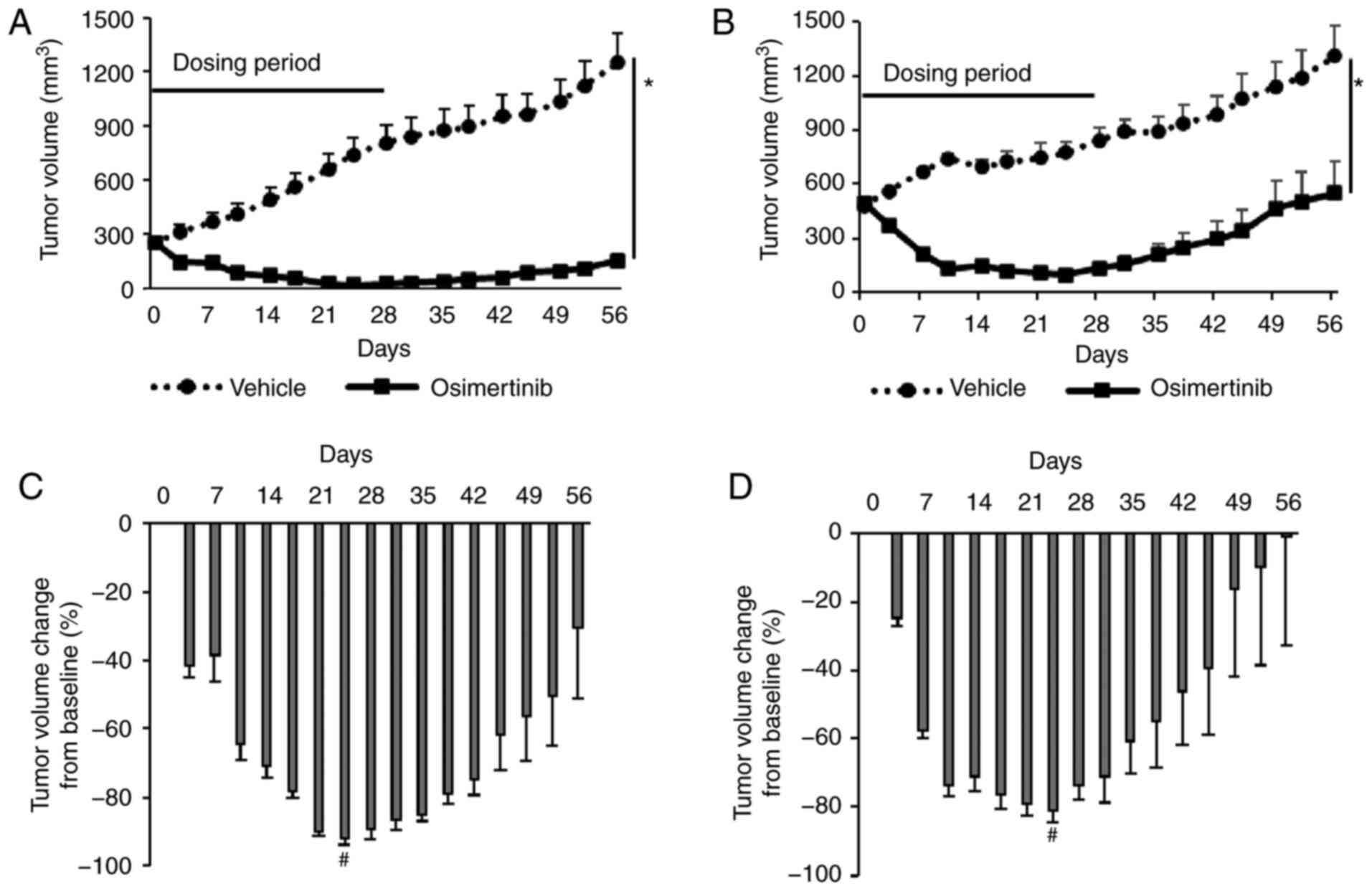
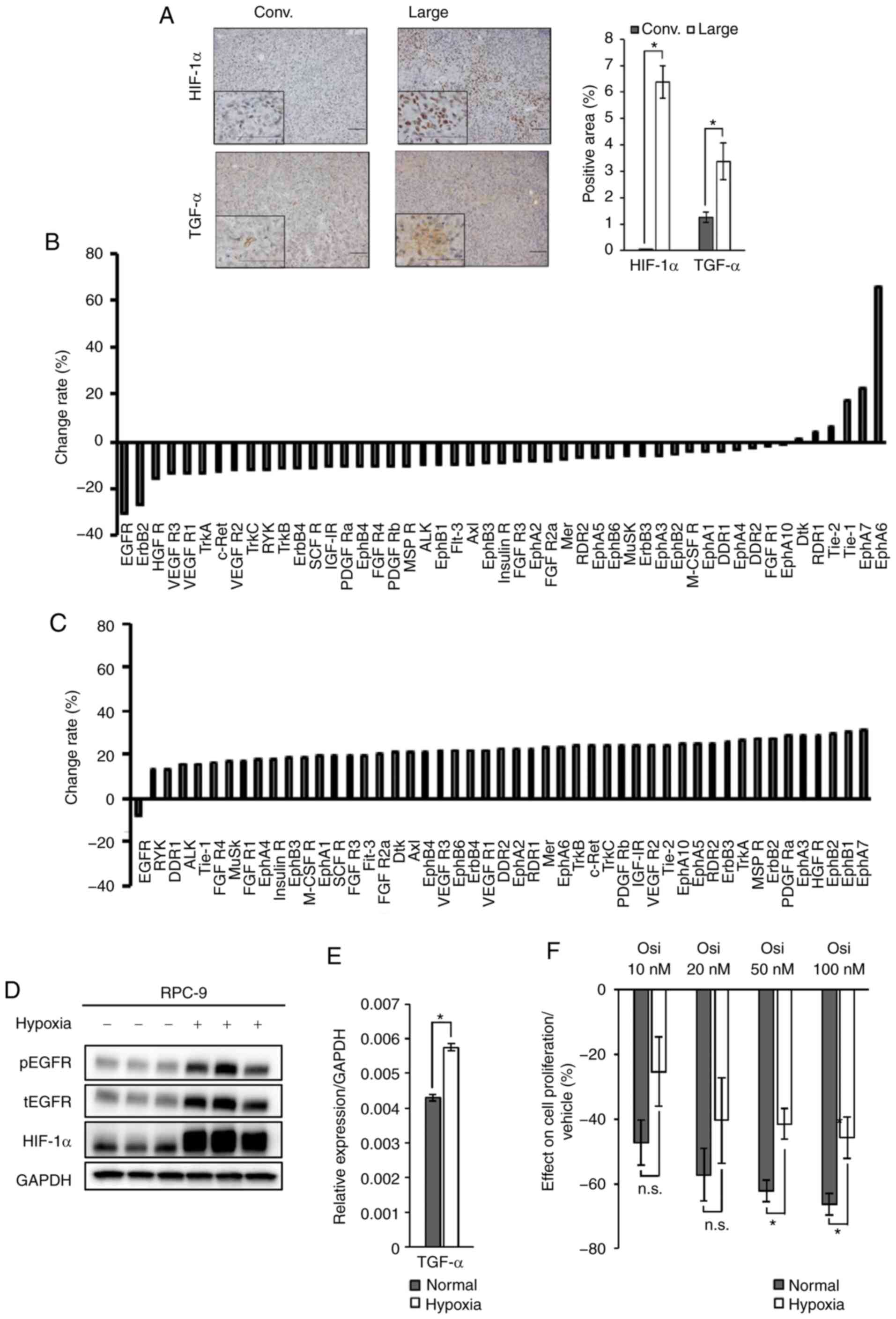
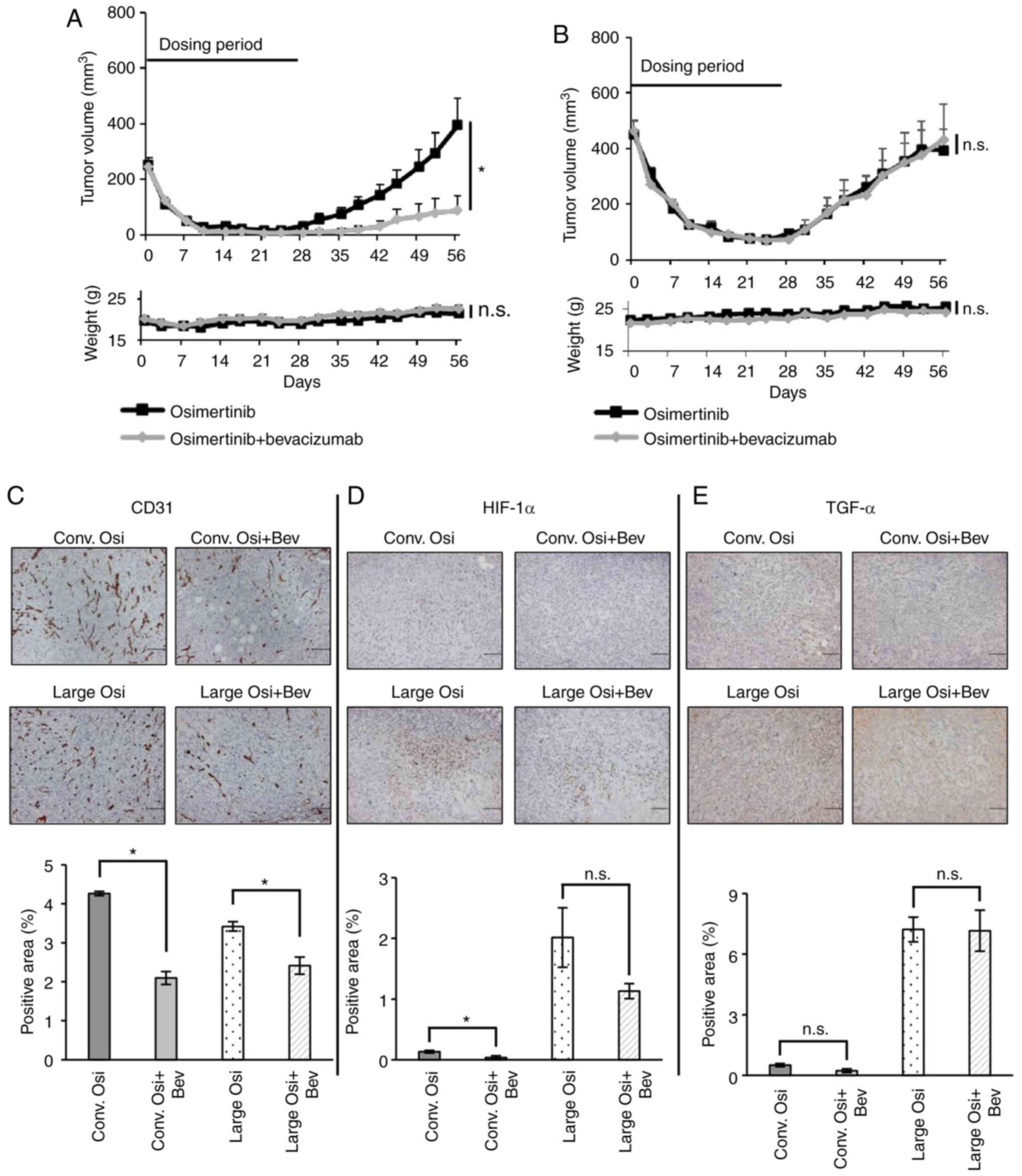
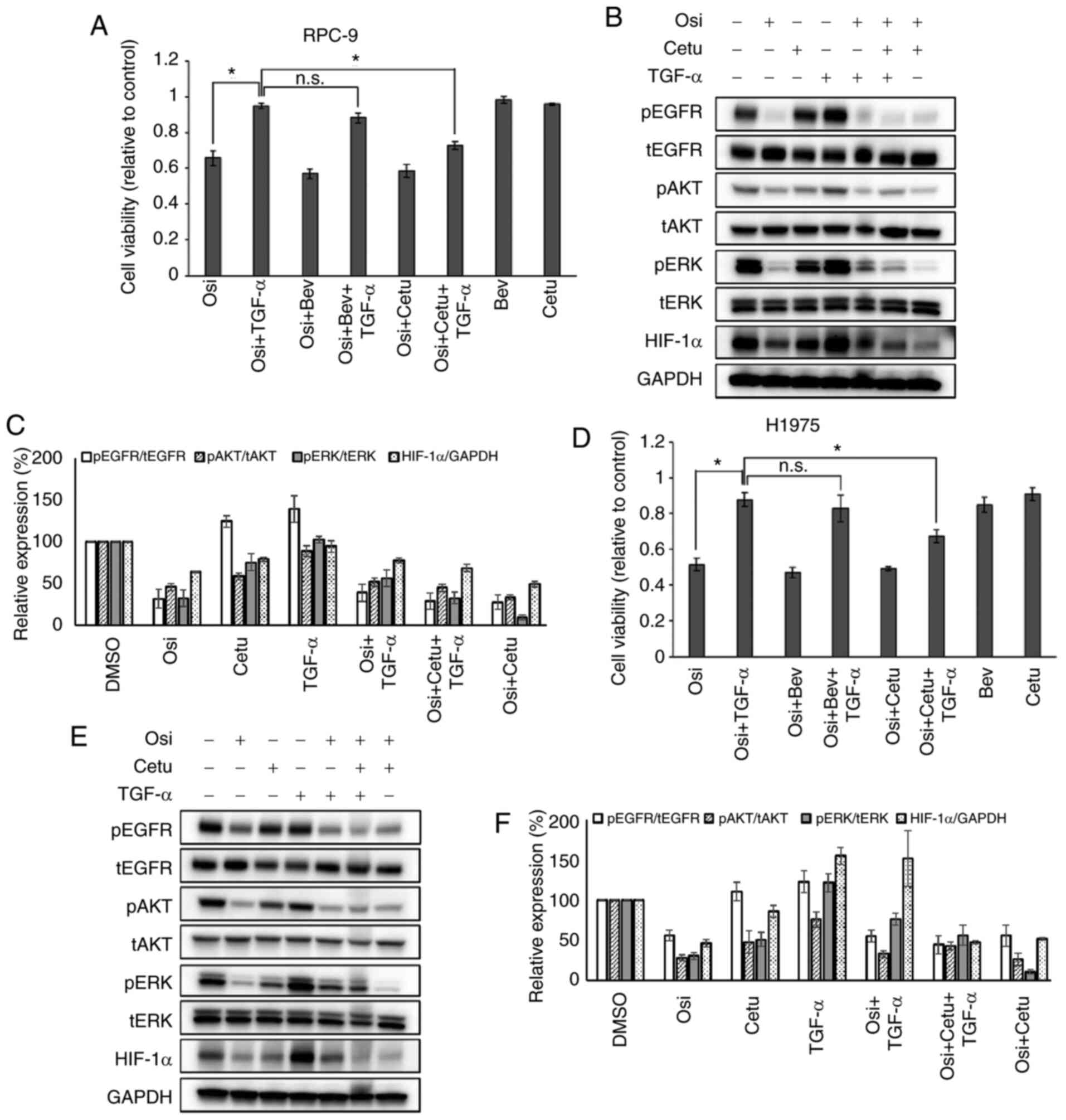
|
1
|
Ninomiya K, Teraoka S, Zenke Y, Kenmotsu
H, Nakamura Y, Okuma Y, Tamiya A, Nosaki K, Morise M, Aokage K, et
al: Japanese Lung Cancer Society Guidelines for Stage IV NSCLC With
EGFR Mutations. JTO Clin Res Rep. 2:1001072021.
|
|
2
|
Ohashi K, Maruvka YE, Michor F and Pao W:
Epidermal growth factor receptor tyrosine kinase
inhibitor-resistant disease. J Clin Oncol. 31:1070–1080. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piper-Vallillo AJ, Sequist LV and
Piotrowska Z: Emerging treatment paradigms for EGFR-mutant lung
cancers progressing on osimertinib: A review. J Clin Oncol. Jun
18–2020.(Epub ahead of print). doi: 10.1200/JCO.19.03123.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blakely CM, Watkins TBK, Wu W, Gini B,
Chabon JJ, McCoach CE, McGranahan N, Wilson GA, Birkbak NJ, Olivas
VR, et al: Evolution and clinical impact of co-occurring genetic
alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet.
49:1693–1704. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lai GGY, Lim TH, Lim J, Liew PJR, Kwang
XL, Nahar R, Aung ZW, Takano A, Lee YY, Lau DPX, et al: Clonal MET
amplification as a determinant of tyrosine kinase inhibitor
resistance in epidermal growth factor receptor-mutant
non-small-cell lung cancer. J Clin Oncol. 37:876–884. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Offin M, Rizvi H, Tenet M, Ni A,
Sanchez-Vega F, Li BT, Drilon A, Kris MG, Rudin CM, Schultz N, et
al: Tumor mutation burden and efficacy of EGFR-tyrosine kinase
inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer
Res. 25:1063–1069. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohashi K, Ninomiya K, Yoshioka H, Bessho
A, Shibayama T, Aoe K, Ishikawa N, Kozuki T, Kawai H, Kuyama S, et
al: Impact of HER2 expression on EGFR-TKI treatment outcomes in
lung tumors harboring EGFR mutations: A HER2-CS study subset
analysis. Lung Cancer. 150:83–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan Y, Gao G, Chen X, Tian Q, Wu F, Liu Q,
Wang Y, Jiang T, Liu Y, Li X, et al: Larger tumors are associated
with inferior progression-free survival of first-line EGFR-tyrosine
kinase inhibitors and a lower abundance of EGFR mutation in
patients with advanced non-small cell lung cancer. Thorac Cancer.
10:686–694. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou F, Ma W, Li W, Ni H, Gao G, Chen X,
Zhang J and Shi J: Thick-wall cavity predicts worse
progression-free survival in lung adenocarcinoma treated with
first-line EGFR-TKIs. BMC Cancer. 18:10332018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito H, Fukuhara T, Furuya N, Watanabe K,
Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori
K, et al: Erlotinib plus bevacizumab versus erlotinib alone in
patients with EGFR-positive advanced non-squamous non-small-cell
lung cancer (NEJ026): Interim analysis of an open-label,
randomised, multicentre, phase 3 trial. Lancet Oncol. 20:625–635.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ninomiya T, Ishikawa N, Inoue K, Kubo T,
Yasugi M, Shibayama T, Maeda T, Fujitaka K, Kodani M, Yokoyama T,
et al: Phase 2 study of afatinib alone or combined with bevacizumab
in chemonaive patients with advanced non-small-cell lung cancer
harboring EGFR mutations: AfaBev-CS study protocol. Clin Lung
Cancer. 20:134–138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakagawa K, Garon EB, Seto T, Nishio M,
Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, et
al: Ramucirumab plus erlotinib in patients with untreated,
EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 20:1655–1669. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldberg SB, Redman MW, Lilenbaum R,
Politi K, Stinchcombe TE, Horn L, Chen EH, Mashru SH, Gettinger SN,
Melnick MA, et al: Randomized trial of afatinib plus cetuximab
versus afatinib alone for first-line treatment of EGFR-mutant
non-small-cell lung cancer: Final results from SWOG S1403. J Clin
Oncol. 38:4076–4085. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kudo K, Ohashi K, Makimoto G, Higo H, Kato
Y, Kayatani H, Kurata Y, Takami Y, Minami D, Ninomiya T, et al:
Triplet therapy with afatinib, cetuximab, and bevacizumab induces
deep remission in lung cancer cells harboring EGFR T790M in vivo.
Mol Oncol. 11:670–681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogino A, Kitao H, Hirano S, Uchida A,
Ishiai M, Kozuki T, Takigawa N, Takata M, Kiura K and Tanimoto M:
Emergence of epidermal growth factor receptor T790M mutation during
chronic exposure to gefitinib in a non small cell lung cancer cell
line. Cancer Res. 67:7807–7814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minakata K, Takahashi F, Nara T, Hashimoto
M, Tajima K, Murakami A, Nurwidya F, Yae S, Koizumi F, Moriyama H,
et al: Hypoxia induces gefitinib resistance in non-small-cell lung
cancer with both mutant and wild-type epidermal growth factor
receptors. Cancer Sci. 103:1946–1954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Q, Hu DF, Rui Y, Jiang AB, Liu ZL and
Huang LN: Prognosis value of HIF-1α expression in patients with
non-small cell lung cancer. Gene. 541:69–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hartwich J, Orr WS, Ng CY, Spence Y,
Morton C and Davidoff AM: HIF-1α activation mediates resistance to
anti-angiogenic therapy in neuroblastoma xenografts. J Pediatr
Surg. 48:39–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rapisarda A, Hollingshead M, Uranchimeg B,
Bonomi CA, Borgel SD, Carter JP, Gehrs B, Raffeld M, Kinders RJ,
Parchment R, et al: Increased antitumor activity of bevacizumab in
combination with hypoxia inducible factor-1 inhibition. Mol Cancer.
8:1867–1877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Lu Y, Liang K, Pan T, Mendelsohn J
and Fan Z: Requirement of hypoxia-inducible factor-1alpha
down-regulation in mediating the antitumor activity of the
anti-epidermal growth factor receptor monoclonal antibody
cetuximab. Mol Cancer Ther. 7:1207–1217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roper N, Brown AL, Wei JS, Pack S,
Trindade C, Kim C, Restifo O, Gao S, Sindiri S, Mehrabadi F, et al:
Clonal evolution and heterogeneity of osimertinib acquired
resistance mechanisms in EGFR mutant lung cancer. Cell Rep Med.
1:1000072020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park S, Lee MH, Seong M, Kim ST, Kang JH,
Cho BC, Lee KH, Cho EK, Sun JM, Lee SH, et al: A phase II,
multicenter, two cohort study of 160 mg osimertinib in EGFR
T790M-positive non-small-cell lung cancer patients with brain
metastases or leptomeningeal disease who progressed on prior EGFR
TKI therapy. Ann Oncol. 31:1397–1404. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romaniello D, Mazzeo L, Mancini M,
Marrocco I, Noronha A, Kreitman M, Srivastava S, Ghosh S, Lindzen
M, Salame TM, et al: A Combination of approved antibodies overcomes
resistance of lung cancer to osimertinib by blocking bypass
pathways. Clin Cancer Res. 24:5610–5621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernandes Neto JM, Nadal E, Bosdriesz E,
Ooft SN, Farre L, McLean C, Klarenbeek S, Jurgens A, Hagen H, Wang
L, et al: Multiple low dose therapy as an effective strategy to
treat EGFR inhibitor-resistant NSCLC tumours. Nat Commun.
11:31572020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomoshige K, Guo M, Tsuchiya T, Fukazawa
T, Fink-Baldauf IM, Stuart WD, Naomoto Y, Nagayasu T and Maeda Y:
An EGFR ligand promotes EGFR-mutant but not KRAS-mutant lung cancer
in vivo. Oncogene. 37:3894–908. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akamatsu H, Toi Y, Hayashi H, Fujimoto D,
Tachihara M, Furuya N, Otani S, Shimizu J, Katakami N, Azuma K, et
al: Efficacy of osimertinib plus bevacizumab vs osimertinib in
patients with EGFR T790M-mutated non-small cell lung cancer
previously treated with epidermal growth factor receptor-tyrosine
kinase inhibitor: West Japan oncology Group 8715L Phase 2
randomized clinical trial. JAMA Oncol. 7:386–394. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Swinson DE, Jones JL, Cox G, Richardson D,
Harris AL and O'Byrne KJ: Hypoxia-inducible factor-1 alpha in non
small cell lung cancer: Relation to growth factor, protease and
apoptosis pathways. Int J Cancer. 111:43–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren W, Mi D, Yang K, Cao N, Tian J, Li Z
and Ma B: The expression of hypoxia-inducible factor-1α and its
clinical significance in lung cancer: A systematic review and
meta-analysis. Swiss Med Wkly. 143:w138552013.PubMed/NCBI
|
|
32
|
Fallah J and Rini BI: HIF inhibitors:
Status of current clinical development. Curr Oncol Rep. 21:62019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Z, Xiang X, Li S, Xie P, Gong Q, Goh BC
and Wang L: Targeting hypoxia-inducible factor-1, for cancer
treatment: Recent advances in developing small-molecule inhibitors
from natural compounds. Semin Cancer Biol. Sep 28–2020.(Epub ahead
of print). doi: 10.1016/j.semcancer.2020.09.011. View Article : Google Scholar
|