Introduction
Non-small cell lung cancer (NSCLC) is one of the
deadliest diseases worldwide; however, its prognosis has been
improved by the discovery of driver oncogenes and the development
of corresponding molecular targeted therapies. In particular,
epidermal growth factor receptor (EGFR) gene mutations are
the most frequent driver mutations in never-smokers or individuals
with Asian ethnicity. EGFR tyrosine kinase inhibitors (TKIs) are
standard therapies for patients with lung cancer harboring EGFR
mutations (1,2); however, their inhibitory effects are
insufficient to achieve complete remission and acquired resistance
usually develops within two years (2). The third-generation EGFR-TKI
osimertinib is the standard of care for patients with NSCLC
harboring EGFR T790M, which is the most common mechanism of
resistance for first- or second-generation EGFR-TKIs. Although
osimertinib has been approved to treat patients with untreated
EGFR-mutant lung cancers (3,4), resistance is ultimately inevitable
(5).
Multiple factors have been reported to negatively
impact the progression-free survival or time-to-treatment failure
of EGFR-TKIs, such as co-occurring gene mutations, tumor mutation
burden, pre-existing clonal MET amplification, or HER2 expression
(6–9). Studies have also demonstrated that
clinical characteristics like tumor volume (10) or cavity wall thickness (11) correlate negatively with EGFR-TKI
efficacy. Consequently, therapies combining EGFR-TKIs and other
agents could be more effective than EGFR-TKI monotherapy in
EGFR-mutant lung cancer with negative predictive factors.
Combination therapies aiming to achieve the deep
remission of EGFR-mutant lung cancers have been an active area of
investigation, with clinical trials demonstrating the benefits of
EGFR-TKIs combined with antiangiogenetic agents such as the
anti-vascular endothelial growth factor (VEGF) antibody bevacizumab
(12,13) or the anti-VEGF receptor (VEGFR)
antibody ramucirumab (14). In
addition, studies have investigated intensive EGFR inhibition using
EGFR-TKIs with anti-EGFR antibodies such as cetuximab or
necitumumab (15,16); however, the optimal combination
therapy remains unclear.
In this study, we investigated the effect of tumor
volume on osimertinib efficacy in a preclinical in vivo
model and assessed the potential of combining osimertinib with
bevacizumab and/or cetuximab to produce greater remission in lung
tumors harboring EGFR T790M mutations.
Materials and methods
Cell lines
RPC-9 gefitinib-resistant lung adenocarcinoma cells
harboring EGFR exon 19 deletion mutation and T790M were established
in our laboratory (17). H1975
pulmonary adenocarcinoma cells harboring L858R and T790M were
purchased from the American Type Culture Collection.
Cell culture and growth inhibition in
vitro
Cells were cultured in RPMI 1640 medium supplemented
with 10% heat-inactivated fetal bovine serum in a 37°C incubator
with a humidified 5% CO2 atmosphere, where the oxygen
levels were maintained at either 21% (normoxia) or 1% (hypoxia).
Growth inhibition was determined using a modified
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, as described previously (17). Each assay was performed in
triplicate.
Crystal violet assay
Cells were seeded in 6-well plates at a density of
5×104 cells/well, grown under normoxic or hypoxic
conditions for 48 h, and then grown with or without various
concentrations of osimertinib. After three days, the cells were
fixed with 10% formalin for 10 min, stained with crystal violet
solution (Sigma-Aldrich) for 10 min, and then washed with
H2O. After the plates had been dried overnight, stained
cells were quantified using ImageJ software (version 1.52a,
National Institute of Health).
Immunoblot analysis
Cells and frozen tissues were lysed using
radioimmunoprecipitation assay buffer [1% Triton X-100, 0.1% SDS,
50 mmol·L-1 Tris/HCl (pH 7.4), 150 mmol·L-1 NaCl, 1 mmol·L-1
ethylenediaminetetraacetic acid, 1 mmol·L-1 ethylene glycol
tetraacetic acid, 10 mmol·L-1 β-glycerol phosphate, 10 mmol·L-1
NaF, 1 mmol·L-1 sodium orthovanadate-containing protease inhibitor
tablets (Roche Applied Sciences)]. Proteins were separated by
electrophoresis on polyacrylamide gels, transferred onto
nitrocellulose membranes, and probed with specific antibodies that
were detected using Enhanced Chemiluminescence Plus (GE Healthcare
Biosciences). Bands were detected using an ImageQuant LAS-4000
imager (GE Healthcare Biosciences).
Reagents and antibodies
Gefitinib, cetuximab, and bevacizumab were purchased
from EVERLTH. Osimertinib was purchased from Selleck Chemicals.
Antibodies against phospho-EGFR (#3777), EGFR (#2232), phospho-ERK
(#9101), ERK (#9102), phospho-AKT (#9271), AKT (#9272), hypoxia
inducible factor-1α (HIF-1α; #36169), GAPDH (#2118), and CD31
(#77699) were purchased from Cell Signaling Technology. Anti-TGF-α
antibodies (#ab9585) were purchased from Abcam.
Phospho-RTK array
A Human Phospho-RTK Array Kit (R&D Systems) was
used according to the manufacturer's instructions. Bands and dots
were detected using an ImageQuant LAS-4000 imager (GE Healthcare
Biosciences). Mean pixel density was measured using ImageJ (version
1.52a, National Institute of Health).
mRNA expression analysis
RNA was extracted from cells using an RNeasy Mini
Kit (Qiagen) according to the manufacturer's protocol. Total cDNA
was synthesized and amplified using a PrimeScript RT Reagent Kit
(Perfect Real Time; TaKaRa). RNA expression was analyzed using
real-time quantitative reverse transcription-PCR (qRT-PCR) with
SYBR Premix Ex Taq II (Tli RNase H Plus; TaKaRa), according to the
manufacturer's protocol. PCR amplification was performed using a
LightCycler Real-Time PCR System (Roche Applied Science), and gene
dosage was calculated using a standard curve analysis. PCR was
carried out using primers (forward,
5′-AGATTCCCACACTCAGTTCTGCTTC-3′; reverse,
5′-ACAGCGTGCACCAACGTACC-3′).
Xenograft model
Female 5–7-week-old athymic mice were purchased from
Charles River Laboratories. All mice were provided with sterile
food and water and were housed in a barrier facility under a 12 h
light/dark cycle. Cells (5×106) were injected
bilaterally into the back of each mouse. After 7–21 days, the mice
were randomly divided into groups and then treated either with a
mono-, double, or triple therapy (3–4 mice per group) consisting of
a vehicle, osimertinib (per os 5 mg/kg, five times a week),
cetuximab [intraperitoneal (i.p.) 1 mg/mouse, twice a week], and
bevacizumab (i.p. 5 mg/kg, twice a week). Each drug was
administered for 28 days, with a 28 day follow-up period. Tumor
volume (width2 × length/2) was measured twice a week.
Euthanasia was then induced via administration of 5% isoflurane via
an anesthesia machine. In addition to the assessment of inhibitory
effect, the mice were treated with each drug similar to above
mentioned regimen for 3 days, following which the tumor samples
were collected for immunohistochemical analysis after euthanasia.
Experimental protocols were approved by the Animal Care and Use
Committee of Okayama University, Okayama, Japan (OKU-2017084,
OKU-2020152).
Immunohistochemical analysis
Tissue samples were fixed using formalin, embedded
in paraffin, and cut to a thickness of 5 µm before being placed on
glass slides and deparaffinized in Hemo-De (FALMA) and graded
alcohol. For antigen retrieval, sections were incubated in 10
mmol/l sodium citrate buffer, pH 6.0, for 10 min in a 95°C water
bath, after which the sections were incubated with 0.3% hydrogen
peroxide in methanol to block endogenous peroxidase activity. The
slides were rinsed with Tris-buffered saline containing 0.1%
Tween-20, and the sections were blocked with goat serum for 60 min.
The sections were incubated with anti-CD31, anti-HIF-1α, or
anti-TGF-α antibodies overnight at 4°C (dilution factors described
in the data sheets) and amplified using biotinylated anti-rabbit
antibodies and avidin-biotinylated horseradish peroxidase (HRP)
conjugate for 30 min (SignalStain Boost IHC Detection Reagent (HRP,
rabbit) #8114, Cell Signaling Technology, Inc.), reacted with
3,3-diaminobenzidine, and counterstained with hematoxylin.
Statistical analysis
Statistical analyses were performed using STATA
software version 15.1 (StataCorp.). Differences between two groups
were compared using two-tailed paired Student's t-tests.
Differences between three or more groups were compared using
one-way ANOVA followed by Bonferroni's test. P-values of <0.05
were considered statistically significant. The Pearson correlation
coefficient was calculated using STATA software version 15.1
(StataCorp.).
Results
Effect of tumor volume on osimertinib
monotherapy in lung cancer harboring EGFR mutations in vivo
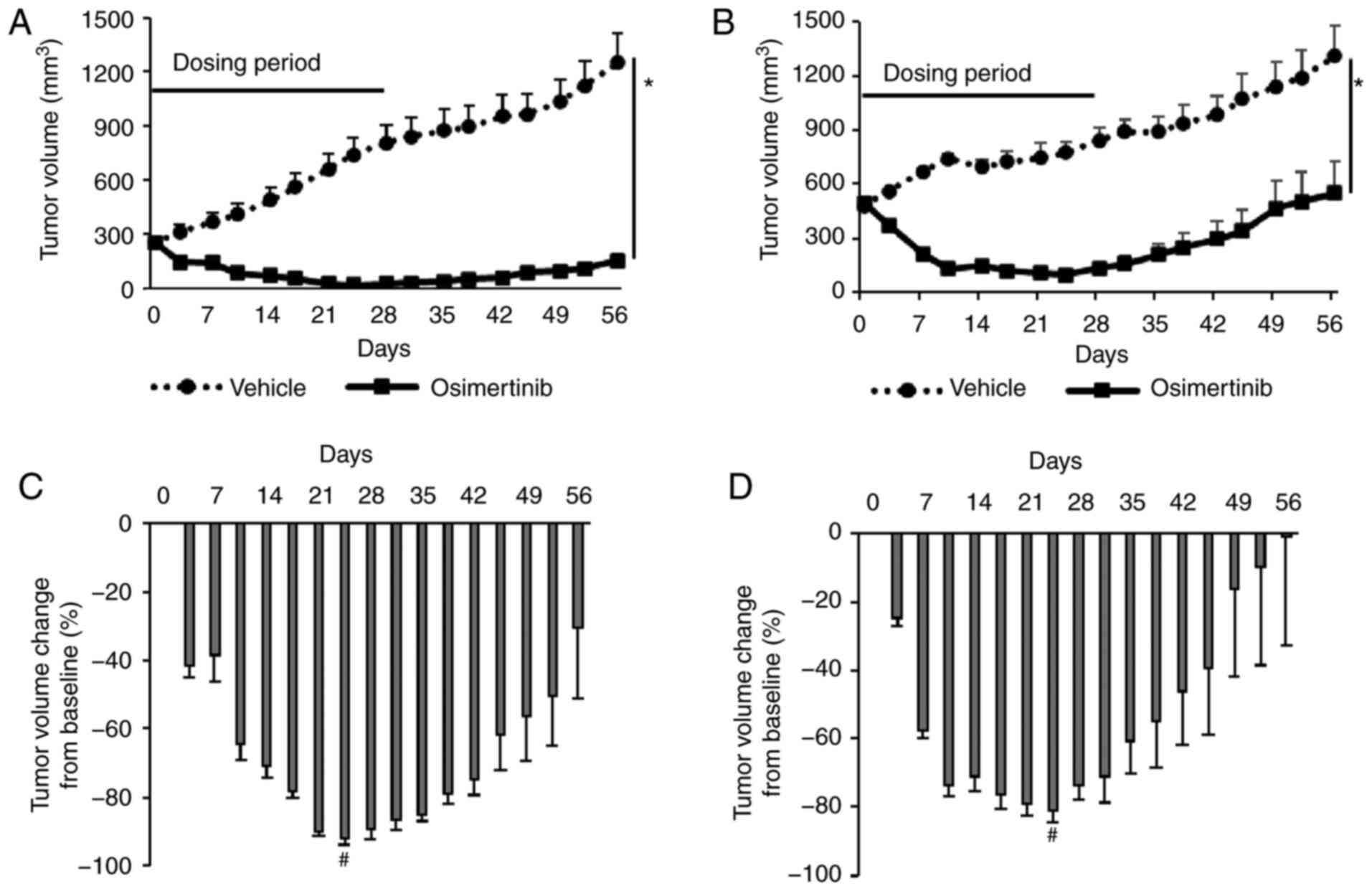
First, we assessed the inhibitory effect of
osimertinib monotherapy on tumor growth in a mouse xenograft model
derived from RPC-9 cells harboring EGFR exon 19 deletion and T790M
mutation. Two types of xenograft lung cancer models were prepared
using the RPC-9 cells to achieve different starting tumor volumes
(conventional: 200 mm3 or large: 500 mm3).
Both models were administered with osimertinib (5 mg/kg, 5
times/week by gavage) for 28 days and then observed for another 28
days. Consistent with the in vitro data (Fig. S1A), osimertinib monotherapy
significantly inhibited tumor growth compared to the vehicle in
both the conventional (maximum tumor diameter at day 56; vehicle:
15.4 mm ± 0.67 vs. osimertinib: 6.9 mm ± 0.74, mean ± SE) and large
models (maximum tumor diameter at day 56; vehicle: 15.0 mm ± 0.97
vs. osimertinib: 8.7 mm ± 2.2, mean ± SE) (Fig. 1A and B); however, maximum tumor
regression was significantly lower in the large model than in the
conventional model (−73.8% vs. −89.3% on day 24, P=0.015, t-test;
Fig. 1C and D). These results
suggest that although osimertinib monotherapy is effective, the
magnitude of its antitumor effect in the xenograft model is
affected by tumor volume.
Increased HIF-1α and TGF-α expression
may attenuate the efficacy of osimertinib
To explore the cause of distinct tumor inhibition
between the conventional and large models, we performed
pathological examinations on tumors from both models. Since we
suspected that hypoxia may have been induced by the increase in
tumor volume, we measured HIF-1α expression. As expected, we
observed higher HIF-1α expression in tumors from the large model
than in the conventional model (Fig.
2A). HIF-1α regulates the transcription of various growth
factors (18); therefore, we
investigated the phosphorylation status of receptor tyrosine kinase
(RTK) using a Phospho-RTK array (Fig.
S1B). The phosphorylation of most RTKs, including EGFR, was
lower in tumors from the conventional model treated with
osimertinib for 7 days (Fig. 2B),
whereas the inhibition of RTK phosphorylation (other than EGFR) was
generally limited in the large model (Fig. 2C). In addition, the inhibitory effect
of osimertinib on EGFR phosphorylation was lower in tumors from the
large model than the conventional model. Due to the observed
increase in HIF-1α expression and the modest inhibition of EGFR
phosphorylation, we also measured TGF-α expression, finding that
TGF-α expression was higher in tumors from the large model than the
conventional model (Fig. 2A). As
expected, a significant correlation was observed between HIF-1α and
TGF-α expression levels in these tumors (Fig. S1C).
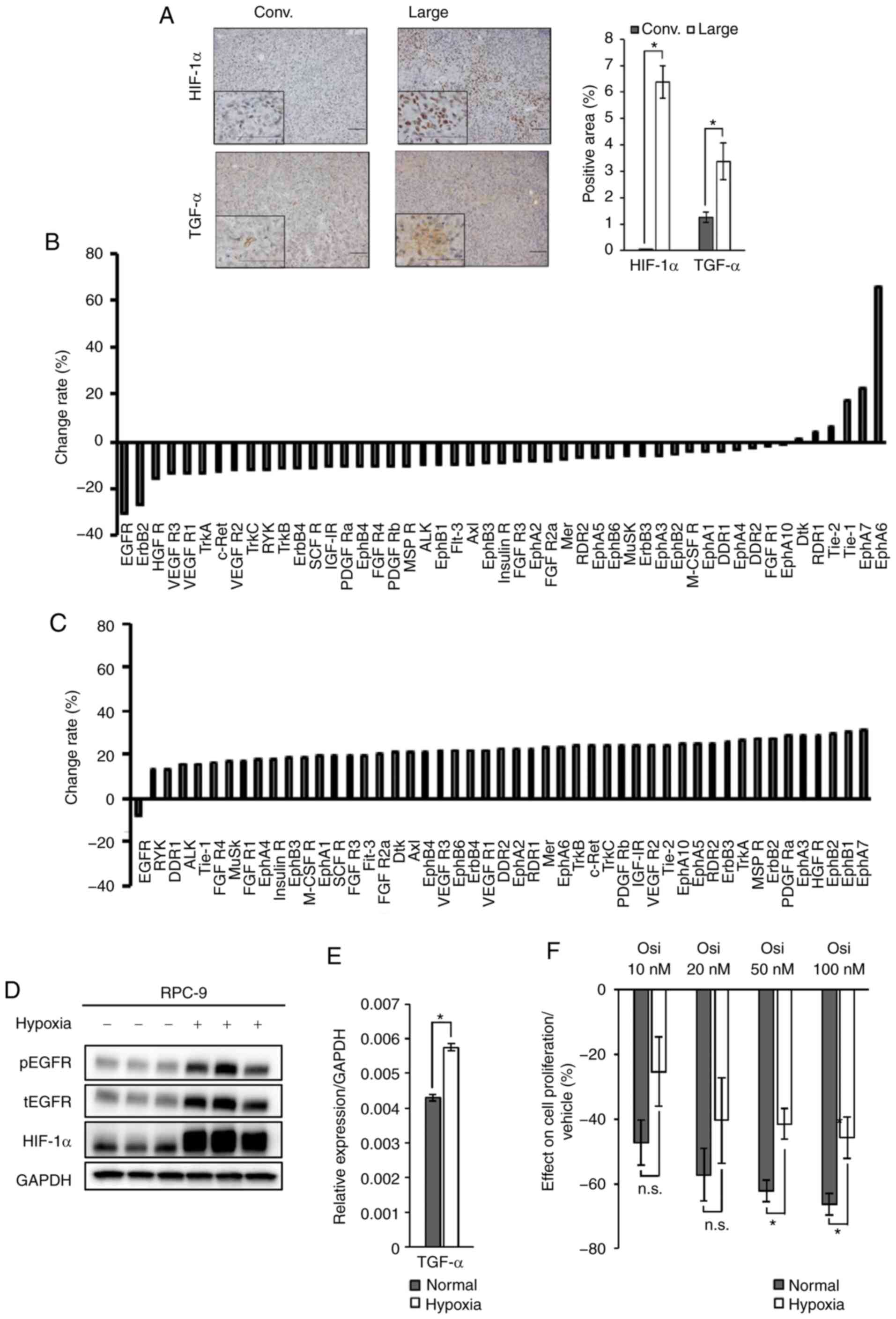 | Figure 2.HIF-1α/TGF-α expression attenuates
sensitivity to osimertinib in RPC-9 cells harboring EGFR mutations.
(A) Immunohistochemical analysis of HIF-1α and TGF-α expression in
xenograft tumors from the large and conventional models. Scale bar,
100 µm. Magnification of the zoomed in squares on the bottom left,
800 fold. Positive cells were quantified using ImageJ software.
Data are presented as the mean ± SEM. Effect of osimertinib (5
mg/kg/day, day 7) on receptor tyrosine kinase phosphorylation in
RPC-9 cell xenograft tumors from the (B) conventional or (C) large
models. Mean pixel density was measured using ImageJ software. (D)
HIF-1α protein expression and EGFR phosphorylation in RPC-9 cells
cultured under hypoxic or normoxic conditions for 48 h. (E) TGF-α
RNA expression in RPC-9 cells cultured under hypoxic or normoxic
conditions for 48 h. (F) Inhibitory effect of osimertinib (72 h) on
the viability of RPC-9 cells pre-incubated under hypoxic or
normoxic conditions for 48 h. Crystal violet assay data were
quantified using ImageJ software. Data are presented as the mean ±
SEM. *P<0.05. HIF-1α, hypoxia-inducible factor-1α; TGF-α,
transforming growth factor-α; EGFR, epidermal growth factor
receptor; Conv., conventional model; Osi, osimertinib, n.s., not
significant; t, total; p, phosphorylated. |
Consequently, we assessed the effect of HIF-1α
expression on sensitivity to osimertinib in vitro. As
reported previously (19), HIF-1α
and TGF-α expression were induced in RPC-9 cells cultured under
hypoxic conditions (Fig. 2D and E).
In addition, sensitivity to osimertinib was significantly lower in
RPC-9 cells pre-incubated under hypoxic conditions than in cells
pre-incubated under normoxic conditions (Figs. 2F and S1D). Together, these results suggest that
the HIF-1α/TGF-α axis may account for the differing osimertinib
sensitivity observed in the conventional and large models.
Combination therapy with osimertinib
and bevacizumab exhibits limited efficacy in the large model
Next, we tested the effect of combination therapy
with osimertinib and bevacizumab (OsiBev) in both the conventional
and large models derived from RPC-9 cells, since EGFR-TKI plus
bevacizumab is one of the most clinically relevant combination
therapies (12). In the conventional
model, combination therapy with OsiBev inhibited tumor growth to
the same extent as osimertinib monotherapy during the treatment
period (Fig. 3A) and the maximum
tumor regression did not differ significantly between therapies
(osimertinib: −93.3% vs. OsiBev: −96.6% at day 24, P=0.16, t-test).
However, the combination therapy significantly delayed tumor
re-growth compared to osimertinib monotherapy. The regression rate
of the tumor treated with OsiBev was significantly higher than that
of the tumor treated with osimertinib monotherapy at day 56
(osimertinib: 57.3% vs. OsiBev: −64.0%, P=0.01, t-test) (maximum
tumor diameter at day 56; osimertinib: 10.1 mm ± 1.9 vs. OsiBev:
3.5 mm ± 1.5, mean ± SE).
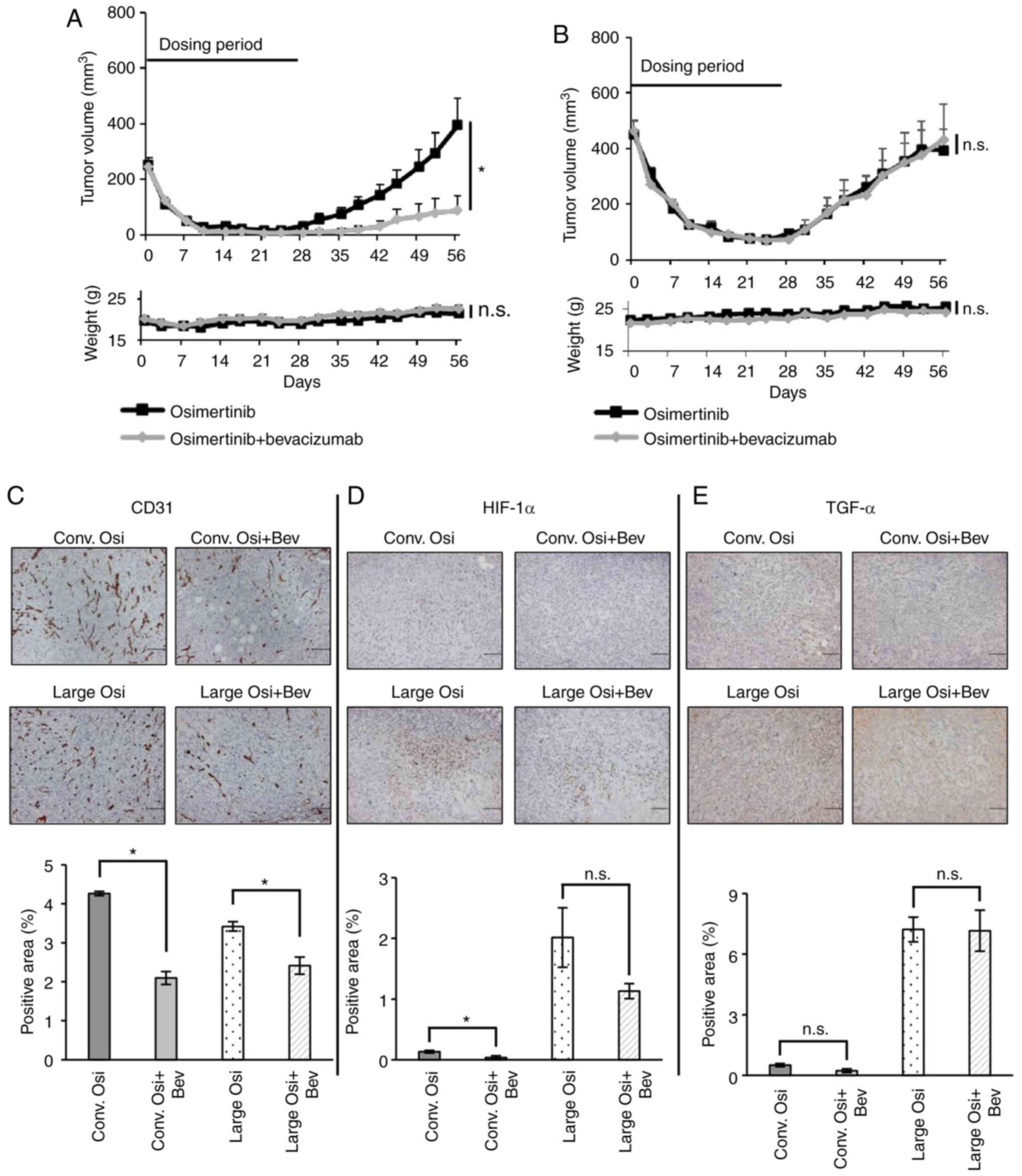 | Figure 3.Combination therapy with osimertinib
and bevacizumab exhibits limited efficacy in xenograft tumors with
HIF-1α/TGF-α expression. Effect of osimertinib monotherapy (5
mg/kg, 5 times/week) or its combination with bevacizumab (5 mg/kg,
twice/week) on RPC-9 cell xenograft tumors from the (A)
conventional (starting tumor volume, 200 mm3; n=8) or
(B) large (starting tumor volume, 500 mm3; n=6) models
for 28 days, with a 28-day observation period. Body weight loss was
not observed in the mice. The combination therapy inhibited tumor
growth compared with osimertinib monotherapy at day 56 in the
conventional model but not the large model. Immunohistochemical
analysis in xenograft tumors from the conventional and large models
treated with the combination therapy or osimertinib. Scale bar, 100
µm. Positive cells were quantified using ImageJ software. (C) CD31,
(D) HIF-1α and (E) TGF-α expression levels in xenograft tumors from
the large model. Data are presented as the mean ± SEM. *P<0.05.
HIF-1α, hypoxia-inducible factor-1α; TGF-α, transforming growth
factor-α; Osi, osimertinib; Bev, bevacizumab; n.s., not
significant. |
In the large model, no significant difference in the
maximum tumor regression rate was observed between the osimertinib
monotherapy and OsiBev (osimertinib: −84.4% vs. OsiBev: −83.6% on
day 24, P=0.86, t-test; Fig. 3B).
Moreover, combination therapy with OsiBev did not delay tumor
re-growth compared to osimertinib monotherapy (tumor regression
rate at day 56, osimertinib: −7.9% vs. OsiBev: −0.9%, P=0.87,
t-test) (maximum tumor diameter at day 56; osimertinib: 9.6 mm ±
0.77 vs. OsiBev: 10.3 mm ± 1.8, mean ± SE) unlike in the
conventional model (Fig. 3B).
To evaluate the effects of bevacizumab, we measured
the expression of the vascular endothelial marker, CD31. As
expected, there were significantly fewer CD31-positive cells in
tumors treated with the combination therapy than osimertinib
monotherapy in both the conventional and large models (Fig. 3C). Although HIF-1α expression was
significantly lower and TGF-α expression did not change in the
conventional model treated with the combination therapy (Fig. 3D and E), neither HIF-1α nor TGF-α
expression decreased significantly in tumors from the large model
treated with the combination therapy (Fig. 3D and E). Therefore, OsiBev only
achieved a limited decline in HIF-1α expression and relatively high
TGF-α expression was maintained in tumors from the large model
compared to the conventional model.
Cetuximab restores the inhibitory
effects of osimertinib against EGFR-mutant lung cancer cells
stimulated by TGF-α in vitro
Having observed relatively high TGF-α expression in
tumors from the large model, we decided to assess the effect of
TGF-α on the inhibitory function of osimertinib in vitro. As
expected, adding TGF-α (100 ng/ml) to the culture medium
significantly reduced the inhibitory effect of osimertinib on RPC-9
or H1975 cell viability (Fig. 4A and
D). Moreover, western blotting revealed that TGF-α activated
the phosphorylation of the EGFR downstream signaling protein ERK
and increased HIF-1α expression in RPC-9 and H1975 cells (Fig. 4B, C, E and F). ERK phosphorylation
and HIF-1α protein expression were decreased in cells treated with
osimertinib and not TGF-α, but were partially restored in RPC-9 or
H1975 cells treated with osimertinib and TGF-α.
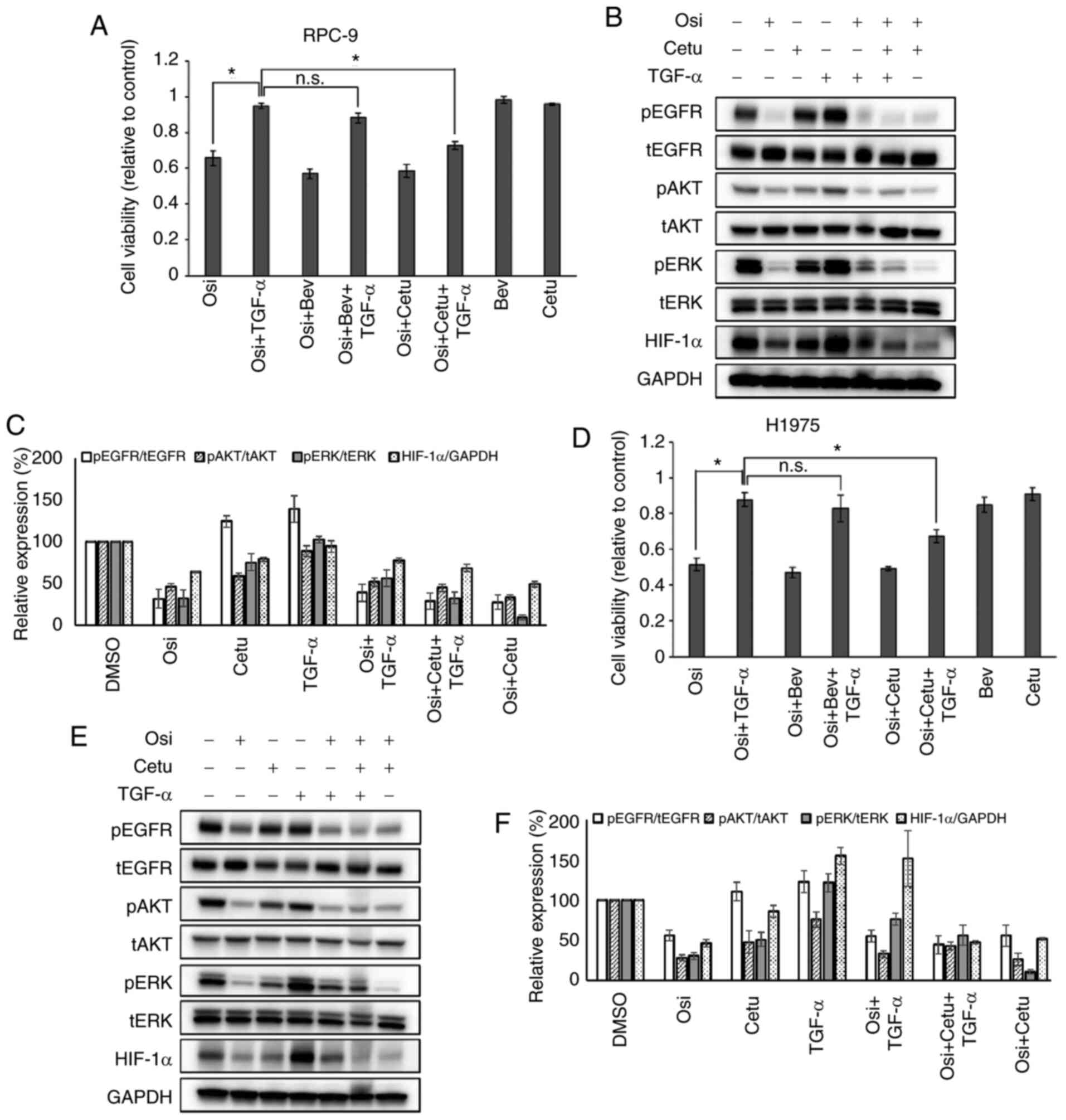 | Figure 4.Cetuximab restores osimertinib
efficacy in epidermal growth factor receptor-mutant lung cancer
cells stimulated with TGF-α in vitro. (A) Effect of
osimertinib on the viability of RPC-9 cells incubated with the
indicated drugs and/or TGF-α (100 ng/ml) for 96 h (Osi, 10 nM; Bev,
150 µg/ml and Cetu, 5 µg/ml). (B) Effect of osimertinib and
cetuximab on HIF-1α expression in RPC-9 cells incubated with the
indicated drugs for 4 h [Osi, 10 nM; Cetu, 5 µg/ml and TGF-α (50
ng/ml)]. (C) Effect of the osimertinib/cetuximab combination on
HIF-1α expression in RPC-9 cells. Data from (B) were quantified to
determine the relative values of phosphorylation and protein
expression to the corresponding expression of total protein or
GAPDH; these values were then graphed for comparison against
relative values for the DMSO control group. Mean pixel density was
measured using ImageJ software. (D) Effect of osimertinib on the
viability of H1975 cells incubated with the indicated drugs and/or
TGF-α (100 ng/ml) for 96 h (Osi, 10 nM; Bev, 150 µg/ml and Cetu, 5
µg/ml). (E) Effect of osimertinib and cetuximab on HIF-1α
expression in H1975 cells incubated with the indicated drugs for 4
h [Osi, 10 nM; Cetu, 5 µg/ml and TGF-α (50 ng/ml)]. (F) Effect of
the osimertinib/cetuximab combination on HIF-1α expression in H1975
cells. Data from (E) were quantified to determine the relative
values of phosphorylation and protein expression to the
corresponding expression of total protein or GAPDH; these values
were then graphed for comparison against relative values for the
DMSO control group. Mean pixel density was measured using ImageJ
software. Data are presented as the mean ± SEM. *P<0.05. HIF-1α,
hypoxia-inducible factor-1α; TGF-α, transforming growth factor-α;
Osi, osimertinib; Bev, bevacizumab; Cetu, cetuximab; n.s., not
significant; p, phosphorylated; t, total. |
We also assessed the effect of bevacizumab in
vitro, finding that bevacizumab alone had little inhibitory
effect on cell viability and its combination with osimertinib did
not restore sensitivity to osimertinib in RPC-9 or H1975 cells
incubated with TGF-α in vitro (Fig. 4A and D). Cetuximab, which inhibits
EGFR activation by blocking ligand binding, had little inhibitory
effect on cell viability and partially inhibited ERK
phosphorylation and HIF-1α protein expression in cells treated
without TGF-α. Interestingly, the combination of osimertinib plus
cetuximab (OsiCet) exerted a similar inhibitory effect on cell
vaibility to osimertinib monotherapy in RPC-9 or H1975 cells
without TGF-α stimulation, but exhibited a superior inhibitory
effect after TGF-α stimulation (Fig. 4A
and D). Consistent with this, cetuximab alone had little effect
but the combination of osimertinib and cetuximab showed a tendency
of superior inhibitory effect on cell viability in RPC-9 cells
pre-incubated under hypoxic conditions (Fig. S2). Furthermore, ERK phosphorylation
and HIF-1α were lower in RPC-9 or H1975 cells treated with OsiCet
and TGF-α compared to osimertinib alone and TGF-α (Fig. 4B, C, E and F). Taken together, these
findings suggest that TGF-α plays an important role in mediating
the effect of osimertinib, and cetuximab could restore cellular
sensitivity to osimertinib.
Triple therapy with osimertinib,
bevacizumab, and cetuximab exerts beneficial effects in the large
model
Finally, we confirmed the effect of cetuximab in the
large model. Although cetuximab monotherapy exhibited a modest
effect similar to bevacizumab (Fig.
S3A), OsiCet tended to exert a superior inhibitory effect on
tumor growth; however, this effect was not significantly higher
than for osimertinib monotherapy (Fig.
S3B).
Therefore, we examined the effect of triple therapy
with osimertinib, bevacizumab, and cetuximab in vivo.
Although triple therapy did not inhibit tumor growth more than
OsiBev in the conventional model (maximum tumor diameter at day 56;
osimertinib: 8.4 mm ± 1.7 vs. OsiBev: 3.4 mm ± 1.7 vs. triplet: 3.7
mm ± 1.3, mean ± SE) (Fig. 5A), it
exerted a greater tumor inhibitory effect than osimertinib
monotherapy, OsiBev, or OsiCet in the large model (maximum tumor
diameter at day 56; osimertinib: 11.2 mm ± 1.9 vs. OsiBev: 11.4 mm
± 0.49 vs. triplet: 4.7 mm ± 1.2, mean ± SE) (Figs. 5B and S3B). Importantly, no decrease in body
weight was observed in any of the mice (Fig. 5A and B). Pathological assessment of
the tumors revealed that the triple therapy tended to reduce CD31,
HIF-1α, and TGF-α expression compared to the double therapies
(Fig. 5C-E).
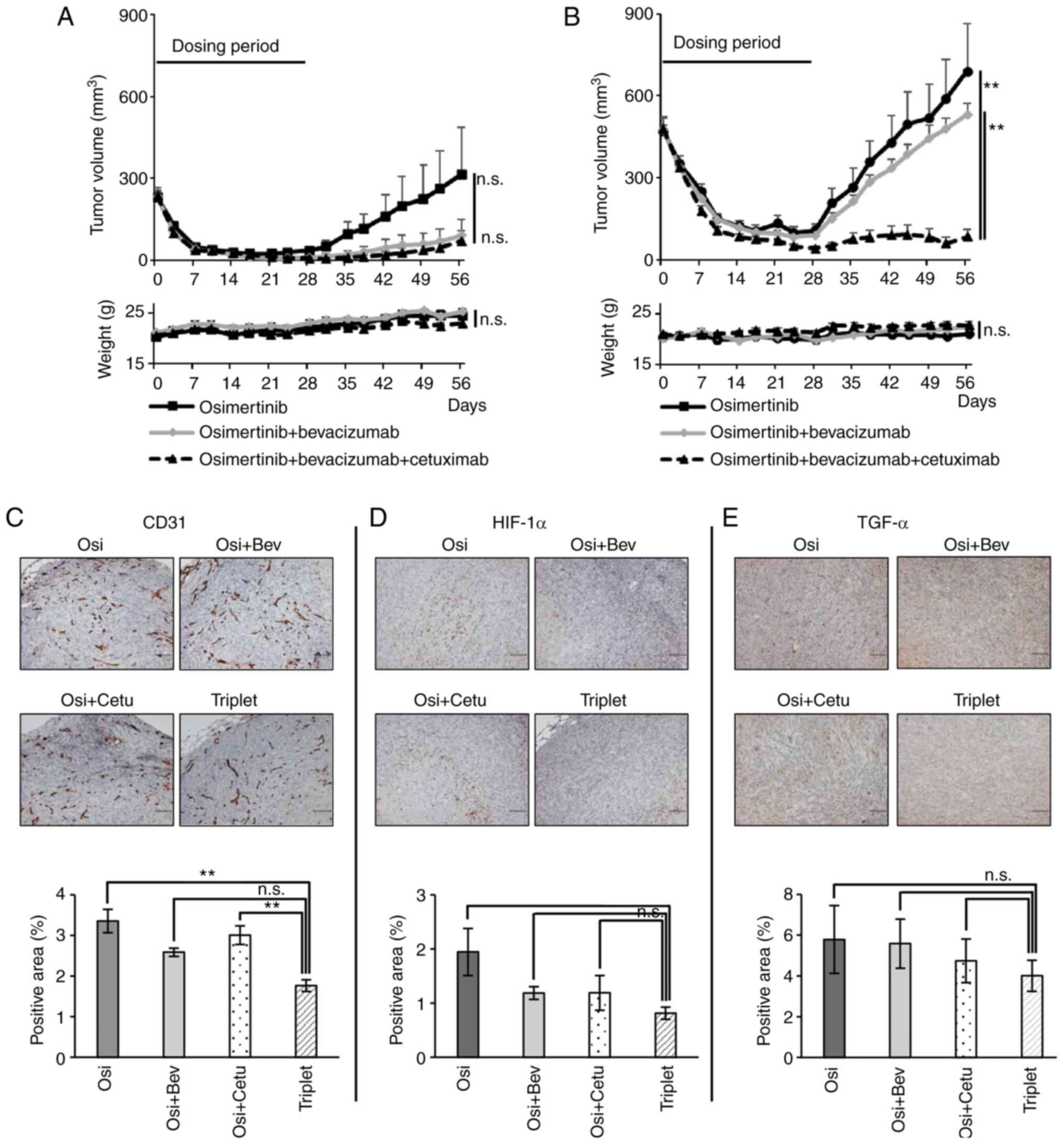 | Figure 5.Effect of triple therapy with
osimertinib, bevacizumab and cetuximab in xenograft tumors with
HIF-1α/TGF-α expression. Effect of osimertinib monotherapy (5
mg/kg, 5 times/week), its combination with bevacizumab (5 mg/kg,
twice/week) or triple therapy with bevacizumab (5 mg/kg,
twice/week) and cetuximab (1 mg/body, twice/week) for 28 days on
RPC-9 cell xenograft tumors from the (A) conventional (starting
tumor volume, 200 mm3; n=8) or (B) large (starting tumor
volume, 500 mm3; n=8) models, over a 28-day observation
period. Body weight loss was not observed among the mice. The
triple therapy did not inhibit tumor growth more than combination
therapy in the conventional model at day 56 (P=1.00, one-way ANOVA
with Bonferroni's test), but the triple therapy inhibited tumor
growth more than osimertinib or the combination therapy in the
large model. Immunohistochemical analysis in xenograft tumors from
the large model treated with the triple therapy, osimertinib or
osimertinib plus cetuximab. (C) CD31, (D) HIF-1α and (E) TGF-α
expression levels in xenograft tumors. Scale bar; 100 µm. Positive
cells were quantified using ImageJ software. Data are presented as
the mean ± SEM. **P<0.01. HIF-1α, hypoxia-inducible factor-1α;
TGF-α, transforming growth factor-α; Osi, osimertinib; Bev,
bevacizumab; Cetu, cetuximab; n.s., not significant. |
Together, the findings of this study suggest that
osimertinib or its combination with bevacizumab exerted limited
efficacy in lung cancer with EGFR T790M mutation and HIF-1α/TGF-α
expression; however, triple therapy with osimertinib, bevacizumab,
and cetuximab induces an even greater inhibitory effect (Fig. S4).
Discussion
In this study, we found that: i) TGF-α attenuated
sensitivity to osimertinib and increased HIF-1α expression in EGFR
mutant NSCLC; ii) OsiCet restored sensitivity to osimertinib and
decreased HIF-1α expression, but OsiBev did not; iii) triple
therapy with osimertinib, bevacizumab, and cetuximab effectively
inhibited the tumor growth of NSCLC with HIF-1α/TGF-α. Previous
studies show that HIF-1α expression is an indicator of poor
prognosis in patients with NSCLC and HIF-1α overexpression
attenuates the effect of bevacizumab, whereas HIF-1α inhibitors
improve its effect (20–22). Moreover, cetuximab is reported to
decrease HIF-1α protein synthesis (23). Taken together, these findings suggest
that cetuximab may be required to counteract the activation loop
via the HIF-1α/TGF-α axis (Fig.
S4); therefore, combining cetuximab with osimertinib and
bevacizumab may allow osimeritinib or bevacizumab to function
effectively.
The efficacy of EGFR-TKI treatment has been found to
vary, potentially due to the heterogeneity in the tumor or its
microenvironment (24). In fact, the
expression of HIF-1α/TGF-α was distinct and heterogenous in central
and peripheral areas in the xenograft tumors, and the degree of
heterogeneity was greater in the large model than in the small
model. A higher dose of osimertinib might be able to provide
benefits in cases of EGFR-mutant lung cancer in which the standard
dose of osimertinib does not confer effective inhibition (25). Although some EGFR-mutant lung cancers
may be suitable for EGFR-TKI monotherapy, others may require
intensive treatment with combination or triple therapies involving
EGFR-TKIs and other agents. The toxicity of combination therapy
must also be considered, but recent preclinical studies suggested a
beneficial effect of three- or four-drug combination with low-dose
therapy for EGFR-mutant lung cancer (26,27).
Tomoshige et al reported that TGF-α
expression is relatively high in EGFR mutated lung cancer compared
to EGFR wild-type lung cancer in the Cancer Genome Atlas (28). In addition, they used preclinical
models to demonstrate that TGF-α promoted the progression of
EGFR-mutated, but not KRAS-mutated, lung cancer and correlated with
poor prognosis in patients with lung cancer harboring EGFR
mutations (28). Although these
findings suggest that combination therapy with EGFR-TKI and
cetuximab could be a reasonable strategy for treating patients with
EGFR-mutant lung cancer, clinical trials have failed to show that
the combination of afatinib and cetuximab is superior to afatinib
monotherapy (15).
EGFR-TKIs have been successfully combined with
anti-angiogenic agents such as bevacizumab or ramucirumab to treat
patients with lung cancer harboring EGFR mutations (12,14);
however, a recent randomized clinical trial revealed that the
combination of osimertinib and bevacizumab failed to show superior
progression-free survival compared to osimertinib monotherapy
(29). Multiple negative predictive
biomarkers have been reported for the effect of EGFR-TKIs (6–11);
however, the predictive factor for combination therapies including
EGR-TKIs and bevacizumab has not yet been identified. Although the
reason for this negative result remains unknown, some negative
predictive factors (for example HIF-1α/TGF-α expression) may be
unbalanced between the combination and monotherapy groups. These
results may indicate that biomarker-driven patient selection is
required for clinical trials of combination therapy. The expression
of HIF-1α is reported to be associated with T factor of the TNM
staging system, lymph node metastasis, and poorly differentiated
tumors (30,31). Therefore, a patient with lung cancer
harboring such a clinical characteristic might benefit from triple
therapy.
HIF-1 inhibitors were actively investigated through
clinical trials, but an HIF-1 inhibitor has not been clinically
approved yet (32,33). The main obstacle to the development
of HIF-1 inhibitors as therapies is their lack of specificity;
therefore, identifying more specific inhibitors is warranted. In
this study, we used clinically available drugs, such as cetuximab,
but the combination of osimertinib and specific HIF1 inhibitors
might be a promising therapeutic strategy for EGFR-mutant lung
cancer with HIF-1α high expression.
In summary, the HIF-1α/TGF-α axis may be involved in
the effect of osimertinib and its combination with bevacizumab in
lung cancer harboring EGFR T790M mutation. Furthermore, triple
therapy with osimertinib, bevacizumab, and cetuximab may be able to
achieve deep remission in EGFR-mutant lung cancers with
HIF-1α/TGF-α expression; therefore, clinical trials are required to
explore this combination further.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Hiromi Nakashima
and Ms. Kyoko Maeda (Department of Hematology, Oncology and
Respiratory Medicine, Okayama University Graduate School of
Medicine, Dentistry and Pharmaceutical Sciences) for their
technical support, and Dr Takehiro Matsubara (Okayama University
Hospital Biobank, Okayama University Hospital) for his technical
support with hypoxic culture.
Funding
The present study was supported by the JSPS
Grants-in-Aid for Scientific Research [Grant-in-Aid for Young
Scientists (B): Grant no. KAKEN 16K19454], the JSPS Grants-in-Aid
for Scientific Research [Scientific Research (C): Grant no. KAKEN
19K08625] and the JSPS Grants-in-Aid for Scientific Research
[Scientific Research (B): Grant no. KAKEN 15H04830].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KaN and KO had full access to all data and assume
responsibility for data integrity and the accuracy of data
analysis. KaN and KO contributed to the study design, and
manuscript writing. KaN, KO, HW and GM performed the experiments
and collected the data. KaN, KO, HW, GM, TN, HH, KiN, YK, TK, KR,
EI, KH, MT, YM and KK performed data analysis. KaN and KO confirmed
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Care and Use Committee of Okayama University (Okayama, Japan;
approval no. OKU-2017084, OKU-2020152).
Patient consent for publication
Not applicable.
Competing interests
Dr Kadoaki Ohashi reports research funding from
Boehringer Ingelheim, Novartis, AstraZeneca, Eli Lilly, MSD, and
Daiichi-Sankyo outside the submitted work. Dr Kadoaki Ohashi
reports personal fees from AstraZeneca, MSD, and Chugai
pharmaceutical outside the submitted work. Dr Kiichiro Ninomiya has
received honoraria from AstraZeneca, Boehringer Ingelheim, Eli
Lilly, MSD, Ono Pharmaceutical, Nippon Kayaku, Taiho
pharmaceutical, Kyowa-Kirin, and Chugai pharmaceutical outside the
submitted work. Dr Katsuyuki Hotta received honoraria from
AstraZeneca and MSD; research funding from Chugai Pharmaceutical,
Eli Lilly Japan, Bristol-Myers Squibb, Astellas Pharma and
AstraZeneca outside the submitted work. Dr Katsuyuki Kiura received
honoraria from MSD; research funding from Ono Pharmaceutical,
Boehringer Ingelheim, Taiho Pharmaceutical, Chugai Pharmaceutical,
Nippon Kayaku, Bristol-Myers Squibb, and Shionogi & Co., Ltd.,
outside the submitted work.
References
|
1
|
Ninomiya K, Teraoka S, Zenke Y, Kenmotsu
H, Nakamura Y, Okuma Y, Tamiya A, Nosaki K, Morise M, Aokage K, et
al: Japanese Lung Cancer Society Guidelines for Stage IV NSCLC With
EGFR Mutations. JTO Clin Res Rep. 2:1001072021.
|
|
2
|
Ohashi K, Maruvka YE, Michor F and Pao W:
Epidermal growth factor receptor tyrosine kinase
inhibitor-resistant disease. J Clin Oncol. 31:1070–1080. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piper-Vallillo AJ, Sequist LV and
Piotrowska Z: Emerging treatment paradigms for EGFR-mutant lung
cancers progressing on osimertinib: A review. J Clin Oncol. Jun
18–2020.(Epub ahead of print). doi: 10.1200/JCO.19.03123.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blakely CM, Watkins TBK, Wu W, Gini B,
Chabon JJ, McCoach CE, McGranahan N, Wilson GA, Birkbak NJ, Olivas
VR, et al: Evolution and clinical impact of co-occurring genetic
alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet.
49:1693–1704. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lai GGY, Lim TH, Lim J, Liew PJR, Kwang
XL, Nahar R, Aung ZW, Takano A, Lee YY, Lau DPX, et al: Clonal MET
amplification as a determinant of tyrosine kinase inhibitor
resistance in epidermal growth factor receptor-mutant
non-small-cell lung cancer. J Clin Oncol. 37:876–884. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Offin M, Rizvi H, Tenet M, Ni A,
Sanchez-Vega F, Li BT, Drilon A, Kris MG, Rudin CM, Schultz N, et
al: Tumor mutation burden and efficacy of EGFR-tyrosine kinase
inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer
Res. 25:1063–1069. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohashi K, Ninomiya K, Yoshioka H, Bessho
A, Shibayama T, Aoe K, Ishikawa N, Kozuki T, Kawai H, Kuyama S, et
al: Impact of HER2 expression on EGFR-TKI treatment outcomes in
lung tumors harboring EGFR mutations: A HER2-CS study subset
analysis. Lung Cancer. 150:83–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan Y, Gao G, Chen X, Tian Q, Wu F, Liu Q,
Wang Y, Jiang T, Liu Y, Li X, et al: Larger tumors are associated
with inferior progression-free survival of first-line EGFR-tyrosine
kinase inhibitors and a lower abundance of EGFR mutation in
patients with advanced non-small cell lung cancer. Thorac Cancer.
10:686–694. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou F, Ma W, Li W, Ni H, Gao G, Chen X,
Zhang J and Shi J: Thick-wall cavity predicts worse
progression-free survival in lung adenocarcinoma treated with
first-line EGFR-TKIs. BMC Cancer. 18:10332018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito H, Fukuhara T, Furuya N, Watanabe K,
Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori
K, et al: Erlotinib plus bevacizumab versus erlotinib alone in
patients with EGFR-positive advanced non-squamous non-small-cell
lung cancer (NEJ026): Interim analysis of an open-label,
randomised, multicentre, phase 3 trial. Lancet Oncol. 20:625–635.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ninomiya T, Ishikawa N, Inoue K, Kubo T,
Yasugi M, Shibayama T, Maeda T, Fujitaka K, Kodani M, Yokoyama T,
et al: Phase 2 study of afatinib alone or combined with bevacizumab
in chemonaive patients with advanced non-small-cell lung cancer
harboring EGFR mutations: AfaBev-CS study protocol. Clin Lung
Cancer. 20:134–138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakagawa K, Garon EB, Seto T, Nishio M,
Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, et
al: Ramucirumab plus erlotinib in patients with untreated,
EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 20:1655–1669. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldberg SB, Redman MW, Lilenbaum R,
Politi K, Stinchcombe TE, Horn L, Chen EH, Mashru SH, Gettinger SN,
Melnick MA, et al: Randomized trial of afatinib plus cetuximab
versus afatinib alone for first-line treatment of EGFR-mutant
non-small-cell lung cancer: Final results from SWOG S1403. J Clin
Oncol. 38:4076–4085. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kudo K, Ohashi K, Makimoto G, Higo H, Kato
Y, Kayatani H, Kurata Y, Takami Y, Minami D, Ninomiya T, et al:
Triplet therapy with afatinib, cetuximab, and bevacizumab induces
deep remission in lung cancer cells harboring EGFR T790M in vivo.
Mol Oncol. 11:670–681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogino A, Kitao H, Hirano S, Uchida A,
Ishiai M, Kozuki T, Takigawa N, Takata M, Kiura K and Tanimoto M:
Emergence of epidermal growth factor receptor T790M mutation during
chronic exposure to gefitinib in a non small cell lung cancer cell
line. Cancer Res. 67:7807–7814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minakata K, Takahashi F, Nara T, Hashimoto
M, Tajima K, Murakami A, Nurwidya F, Yae S, Koizumi F, Moriyama H,
et al: Hypoxia induces gefitinib resistance in non-small-cell lung
cancer with both mutant and wild-type epidermal growth factor
receptors. Cancer Sci. 103:1946–1954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Q, Hu DF, Rui Y, Jiang AB, Liu ZL and
Huang LN: Prognosis value of HIF-1α expression in patients with
non-small cell lung cancer. Gene. 541:69–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hartwich J, Orr WS, Ng CY, Spence Y,
Morton C and Davidoff AM: HIF-1α activation mediates resistance to
anti-angiogenic therapy in neuroblastoma xenografts. J Pediatr
Surg. 48:39–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rapisarda A, Hollingshead M, Uranchimeg B,
Bonomi CA, Borgel SD, Carter JP, Gehrs B, Raffeld M, Kinders RJ,
Parchment R, et al: Increased antitumor activity of bevacizumab in
combination with hypoxia inducible factor-1 inhibition. Mol Cancer.
8:1867–1877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Lu Y, Liang K, Pan T, Mendelsohn J
and Fan Z: Requirement of hypoxia-inducible factor-1alpha
down-regulation in mediating the antitumor activity of the
anti-epidermal growth factor receptor monoclonal antibody
cetuximab. Mol Cancer Ther. 7:1207–1217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roper N, Brown AL, Wei JS, Pack S,
Trindade C, Kim C, Restifo O, Gao S, Sindiri S, Mehrabadi F, et al:
Clonal evolution and heterogeneity of osimertinib acquired
resistance mechanisms in EGFR mutant lung cancer. Cell Rep Med.
1:1000072020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park S, Lee MH, Seong M, Kim ST, Kang JH,
Cho BC, Lee KH, Cho EK, Sun JM, Lee SH, et al: A phase II,
multicenter, two cohort study of 160 mg osimertinib in EGFR
T790M-positive non-small-cell lung cancer patients with brain
metastases or leptomeningeal disease who progressed on prior EGFR
TKI therapy. Ann Oncol. 31:1397–1404. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romaniello D, Mazzeo L, Mancini M,
Marrocco I, Noronha A, Kreitman M, Srivastava S, Ghosh S, Lindzen
M, Salame TM, et al: A Combination of approved antibodies overcomes
resistance of lung cancer to osimertinib by blocking bypass
pathways. Clin Cancer Res. 24:5610–5621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernandes Neto JM, Nadal E, Bosdriesz E,
Ooft SN, Farre L, McLean C, Klarenbeek S, Jurgens A, Hagen H, Wang
L, et al: Multiple low dose therapy as an effective strategy to
treat EGFR inhibitor-resistant NSCLC tumours. Nat Commun.
11:31572020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomoshige K, Guo M, Tsuchiya T, Fukazawa
T, Fink-Baldauf IM, Stuart WD, Naomoto Y, Nagayasu T and Maeda Y:
An EGFR ligand promotes EGFR-mutant but not KRAS-mutant lung cancer
in vivo. Oncogene. 37:3894–908. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akamatsu H, Toi Y, Hayashi H, Fujimoto D,
Tachihara M, Furuya N, Otani S, Shimizu J, Katakami N, Azuma K, et
al: Efficacy of osimertinib plus bevacizumab vs osimertinib in
patients with EGFR T790M-mutated non-small cell lung cancer
previously treated with epidermal growth factor receptor-tyrosine
kinase inhibitor: West Japan oncology Group 8715L Phase 2
randomized clinical trial. JAMA Oncol. 7:386–394. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Swinson DE, Jones JL, Cox G, Richardson D,
Harris AL and O'Byrne KJ: Hypoxia-inducible factor-1 alpha in non
small cell lung cancer: Relation to growth factor, protease and
apoptosis pathways. Int J Cancer. 111:43–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren W, Mi D, Yang K, Cao N, Tian J, Li Z
and Ma B: The expression of hypoxia-inducible factor-1α and its
clinical significance in lung cancer: A systematic review and
meta-analysis. Swiss Med Wkly. 143:w138552013.PubMed/NCBI
|
|
32
|
Fallah J and Rini BI: HIF inhibitors:
Status of current clinical development. Curr Oncol Rep. 21:62019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Z, Xiang X, Li S, Xie P, Gong Q, Goh BC
and Wang L: Targeting hypoxia-inducible factor-1, for cancer
treatment: Recent advances in developing small-molecule inhibitors
from natural compounds. Semin Cancer Biol. Sep 28–2020.(Epub ahead
of print). doi: 10.1016/j.semcancer.2020.09.011. View Article : Google Scholar
|