Introduction
Neuroglioma is the most common primary central
nervous system tumor, accounting for ~50% of intracranial tumors,
with high morbidity and mortality rates (1). Surgical resection is currently the main
treatment method. However, since tumor cells often infiltrate into
normal brain tissue, and the boundary with normal brain tissue is
not obvious, it is often difficult to completely remove the tumor
during surgery (2,3). Therefore, it is extremely necessary to
actively explore more effective treatment methods (3). With the development of medical
technology, the mode of medical development has changed from
symptomatic treatment to treatment of the cause. Therefore,
studying the pathogenesis of glioma may help to identify more
effective treatments.
Long non-coding RNAs (lncRNAs) serve an important
role in cellular and biological processes (4), and their abnormal expression is
associated with a variety of diseases, including colorectal cancer
(5) and gastric cancer (6). LncRNAs, such as HOTAIR (7) and H19 (8), can act as tumor suppressors or
oncogenes, and they may serve as competitive endogenous RNAs.
Therefore, the interaction between lncRNA and microRNA (miRNA/miR)
has gradually become a research hotspot (9–12). In a
previous study, He et al (13) found that downregulation of miR-383
promotes glioma cell invasion, and identified that miR-383
functions as a tumor suppressor in glioma, targeting peroxiredoxin
3 (PRDX-3). However, the reason for the low miR-383 expression in
glioma remains unknown.
Narcolepsy candidate region 1 gene C (NLC1-C), also
known as RNA162 (LINC00162), PRED74, C21orf113 and NCRNA00162, is
involved in tumor development and is expressed in specific tissues.
NLC1-C reportedly targets miR-383, and the accumulation of
lnc-NLC1-C in the nucleus can inhibit the transcriptional levels of
miR-320a and miR-383 (14–19). Nevertheless, lnc-NLC1-C has rarely
been studied in tumor cells, and its binding with miR-383 in glioma
has not been reported.
The present study hypothesized that lnc-NLC1-C may
regulate the proliferation, migration, invasion and apoptosis of
glioma cells by regulating the expression levels of miR-383-5p,
PRDX-3, autophagy-related protein 9 (ATG9), LC3II/I, Rab1 and P62.
The aim of the study was to investigate the association between the
lnc-NLC1-C/miR-383/PRDX-3 axis and the occurrence of glioma, and to
investigate the possible underlying mechanism. The current study
may provide a future basis for the clinical treatment of
glioma.
Materials and methods
Cell lines
The human glioma U251, SHG44, U87MG (glioblastoma of
unknown origin, HTB-14) and U118MG cell lines were purchased from
the American Type Culture Collection. All cells were verified by
STR profiling. U87MG cells were cultured in RPMI-1640 (cat. no.
SH30809.01B; HyClone; Cytiva) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) at 5% CO2 and 37°C, and U251,
SHG44 and U118MG cells were cultured in DMEM (cat. no. c11095500bt;
Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS at 5%
CO2 and 37°C.
Cell transfection
Lnc-NLC1-C-short hairpin (sh)RNA plasmids and
pcDNA3.1-lnc-NLC1-C plasmids were purchased from Wuhan Hualianke
Biotechnology Co., Ltd. The primer sequences were as follows:
Lnc-NLC1-C-shRNA sense,
5′-GATCCGGAAGAGGCAGACACGGAAGGTTCAAGAGACCTTCCGTGTCTGCCTCTTCCTTTTTG-3′
and antisense,
5′-AATTCAAAAAGGAAGAGGCAGACACGGAAGGTCTCTTGAACCTTCCGTGTCTGCCTCTTCCG-3′;
pcDNA3.1-lnc-NLC1-C forward, 5′-CGGAATTCCGTGAAGTGCTGACGGG-3′ and
reverse, 5′-GCTCTAGAGCTTTTTTTTTTTTTTTTTT-3′. All primers were
synthesized by Nanjing Genscript Biotechnology Co., Ltd.
Lnc-NLC1-C-shRNA and pcDNA3.1 (ov)-lnc-NLC1-C plasmids (4 µg) were
transfected into U87MG cells using the Lipofectamine®
2000 liposome transfection kit (Thermo Fisher Scientific, Inc.) at
37°C for 48 h before subsequent experiments. Negative controls
(ov-NC and sh-NC; scramble sequence; Wuhan Hualianke Biotechnology
Co., Ltd.) were established. The control mimic (5 nmol; cat. no.
miR1N0000001-1-5) and control inhibitor (5 nmol; cat. no.
miR2N0000001-1-5) were bought from Guangzhou RiboBio Co., Ltd.
miR-383 mimics (5 nmol; cat. no. MC10353;
5′-AGAUCAGAAGGUGAUUGUGGCU-3′) and inhibitors (5 nmol; cat. no.
MH10353; 5′-AGAUCAGAAGGUGAUUGUGGCU-3′) were bought from Thermo
Fisher Scientific, Inc., and transfection was performed as
aforementioned for the plasmids.
Cell proliferation
Cells in the logarithmic growth phase (100 µl) were
seeded into a 96-well plate at 5×103 cells/ml and
cultured overnight, after which the culture supernatant was
discarded. The cells were divided into five groups (Control, ov-NC,
ov-lnc-NLC1-C, sh-NC and sh-lnc-NLC1-C) according to the
experiment. After 0, 24, 48 and 72 h of culture, 10 µl CCK-8
reagent (cat. no. PAB180031; Bioswamp; Wuhan Bienle Biotechnology
Co., Ltd.) was added to each well, and the plates were incubated at
37°C for 4 h. The optical density was measured at 450 nm using a
plate reader, and the cell proliferative activity was
calculated.
Cell migration and invasion
U87MG cells (2×104) were cultured in
serum-free medium and seeded into the upper chamber of a Transwell
plate without coating (for migration) or coated with
Matrigel® (for invasion; cat. no. 354230; BD
Biosciences) at 37°C for 30 min. Subsequently, 750 µl culture
medium with 10% FBS was added to the bottom chamber. After 48 and
72 h of culture at 37°C, cells in the upper chamber were removed,
and those in the lower chamber were fixed with 4% paraformaldehyde
at 4°C for 10 min and stained with 0.1% crystal violet at 4°C for
30 min. The numbers of migrating and invading cells were counted
using an inverted fluorescence microscope (magnification, ×200; MIL
LED; Leica Microsystems GmbH).
Reactive oxygen species (ROS)
assay
ROS production was detected using an active oxygen
detection kit (Beijing Solarbio Science & Technology Co.,
Ltd.). A DCFH-DA probe was diluted with a dimethyl sulfoxide
solution at 1:1,000, resulting in a final concentration of 20
µmol/l. Cells were collected and suspended in diluted DCFH-DA at a
density of 1×107 cells/ml and incubated at 37°C for 1 h.
The cells were washed twice with PBS, and the ROS levels were
measured using flow cytometry (FC500 MCL; Beckman Coulter, Inc.),
and the data were analyzed with the CytExpert software (Beckman
Coulter, Inc.; version 2.0)
Apoptosis and cell cycle analysis
To evaluate cell apoptosis, a single suspension of
transfected U87MG cells was prepared using 0.25% trypsin. A total
of 5×105 cells were centrifuged at 1,000 × g and 4°C for
5 min and washed twice with 1 ml precooled PBS. After 200 µl
binding buffer was added to the cells, 10 µl Annexin V-fluorescein
isothiocyanate and 10 µl propidium iodide (part of the
AnnexinV-FITC/PI Apoptosis Detection kit; cat. no. 556547; BD
Biosciences) were added, and the cells were incubated at 4°C in the
dark for 30 min and subjected to flow cytometry (FC500 MCL; Beckman
Coulter, Inc.). Data were analyzed with the CytExpert software
(Beckman Coulter, Inc.; version 2.0).
To evaluate cell cycle progression, transfected
U87MG cells were fixed for 24 h with 70% cold ethanol at 4°C. The
fixed cells were washed twice with PBS and incubated with 10 µg/ml
RNase A and 20 µg/ml propidium iodide (Sigma-Aldrich; Merck KGaA)
at 37°C for 30 min. The cell cycle was analyzed using flow
cytometry (FC500 MCL; Beckman Coulter, Inc.), and the results were
analyzed using ModFit LT software (BD Biosciences).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cultured cells using
TRIzol® (Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. RNA concentration was measured using a
spectrophotometer (Thermo Fisher Scientific, Inc.) and total RNA
(500 ng) was reverse transcribed into cDNA at a final volume of 10
µl using 5X reaction buffer (Takara Bio, Inc.), 10 mM dNTP Mix,
oligodT primers and SuperScript II reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.), and the temperature
protocol is 42°C for 60 min, 70°C for 15 min and hold at 16°C. qPCR
was performed using the SYBR Green reaction mix (Qiagen GmbH). The
primer sequences were as follows: Lnc-NLC1-C forward (F),
5′-GCCAACACCCTACCCA-3′ and reverse (R), 5′-TGCCCATCTTCACCACA-3′;
GAPDH F, 5′-CCACTCCTCCACCTTTG-3′ and R, 5′-CACCACCCTGTTGCTGT-3′;
miR-383 F, 5′-GGGAGATCAGAAGGTGA-3′ and R,
5′-AACTGGTGTCGTGGAGTCGGC−3′; U6 F, 5′-CTCGCTTCGGCAGCACA-3′ and R,
5′-AACGCTTCACGAATTTGCGT-3′; PRDX-3 F, 5′-GCCTGGATAAATACACC-3′ and
R, 5′-ACTGGGAGATCGTTGA-3′. GAPDH and U6 were used as internal
controls for mRNA and miRNA, respectively. The reaction proceeded
according to the manufacturer's protocol. The relative mRNA
expression was calculated using the 2−∆∆Cq method
(20).
Western blotting
U87MG cells were lysed using
radioimmunoprecipitation assay buffer, and the total protein was
extracted. A bicinchoninic acid assay was performed to determine
the protein concentration, and the proteins were heated to the
thermal denaturation temperature. SDS-PAGE (12%) was performed, and
the proteins (20 µg/lane) were transferred to polyvinylidene
fluoride membranes (EMD Millipore). The membranes were blocked at
37°C for 1 h using 5% skimmed milk powder and then incubated at 4°C
overnight with rabbit antibodies against PRDX-3 (1:1,000; cat. no.
PAB31341; Bioswamp; Wuhan Bienle Biotechnology Co., Ltd.), LC3II/I
(1:3,000; cat. no. ab51520R; Abcam), ATG9 (1:1,000; cat. no.
PAB35248; Bioswamp; Wuhan Bienle Biotechnology Co., Ltd.), Rab1
(1:1,000; cat. no. PAB32093; Bioswamp; Wuhan Bienle Biotechnology
Co., Ltd.), p62 (1:1,000; ab455686; Abcam) and GAPDH (1:10,000;
cat. no. PAB36269; Bioswamp; Wuhan Bienle Biotechnology Co., Ltd.).
Thereafter, the membranes were incubated with secondary goat
anti-rabbit IgG antibodies (1:10,000; cat. no. SAB43711; Bioswamp;
Wuhan Bienle Biotechnology Co., Ltd.) at 37°C for 1 h. An enhanced
chemiluminescence kit (Beijing Solarbio Science & Technology
Co., Ltd.) was used for membrane development and images were
processed using Image J software (version 1.8.0; National
Institutes of Health).
Statistical analysis
Data were analyzed using SPSS 19.0 (IBM Corp.)
statistical software and expressed as the mean ± standard
deviation. Comparisons between two or multiple groups were
performed using the unpaired t-test or one-way ANOVA followed by
Tukey's post-hoc test, respectively. P<0.05 was considered to
indicate a statistically significant difference. All experiments
were repeated three times.
Results
Cell screening and lnc-NLC1-C
expression in U87MG cells
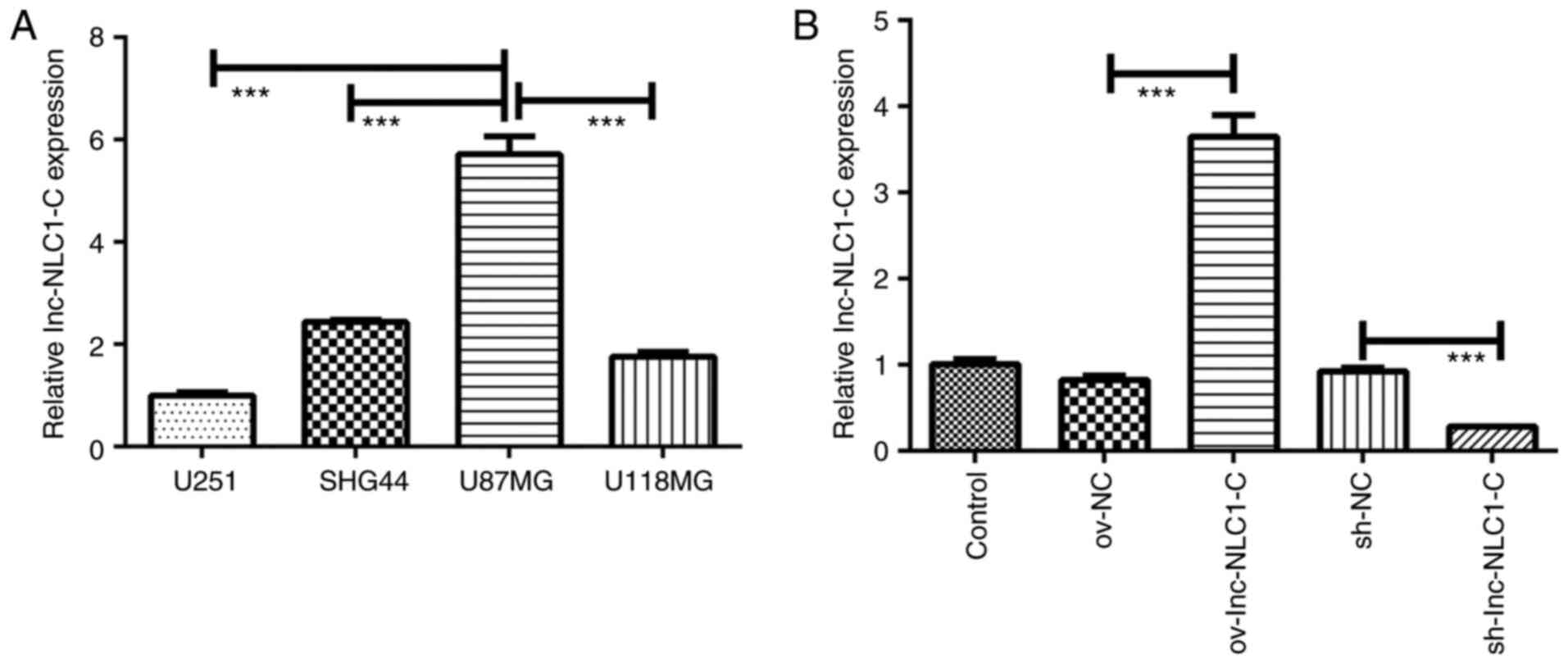
Compared with U251, SHG44 and U118MG glioma cells,
lnc-NLC1-C expression was significantly increased in U87MG cells
(P<0.001; Fig. 1A). Thus, U87MG
cells were selected for subsequent experiments. Compared with the
control, ov-NC and sh-NC showed no difference in lnc-NLC1-C
expression (Fig. 1B). Compared with
ov-NC, ov-lnc-NLC1-C significantly increased lnc-NLC1-C expression
(P<0.001), while compared with sh-NC, sh-lnc-NLC1-C
significantly decreased lnc-NLC1-C expression (P<0.001)
(Fig. 1B).
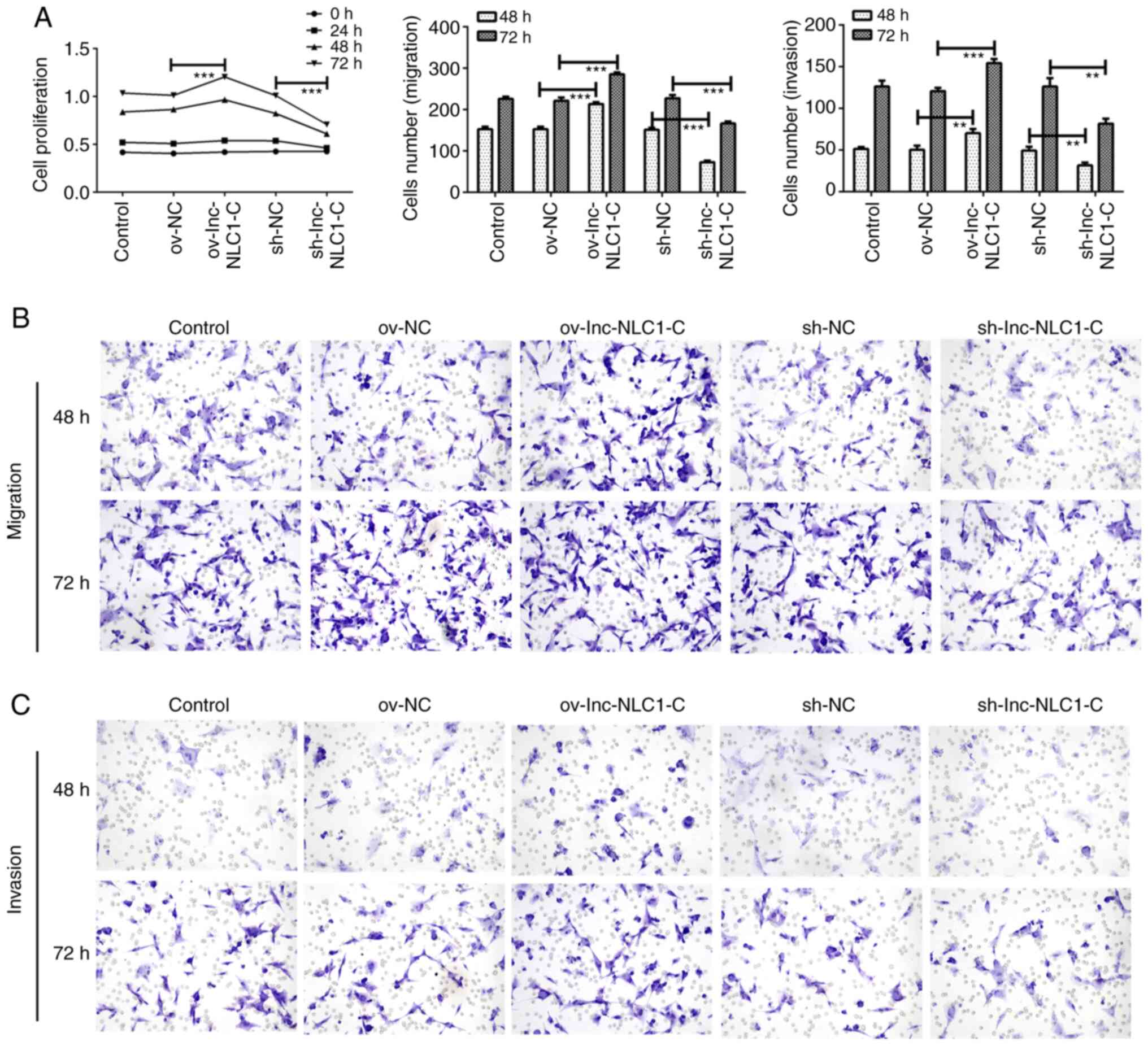
Lnc-NLC1-C promotes the proliferation,
migration and invasion of U87MG cells
Compared with ov-NC, ov-lnc-NLC1-C significantly
enhanced the proliferation (P<0.001; Fig. 2A) and significantly promoted the
migration (Fig. 2B) and invasion
(Fig. 2C) of U87MG cells at 48 and
72 h. Compared with sh-NC, sh-lnc-NLC1-C significantly decreased
the proliferation (P<0.001; Fig.
2A) and inhibited the migration (Fig. 2B) and invasion (Fig. 2C) of U87MG cells at 48 and 72 h.
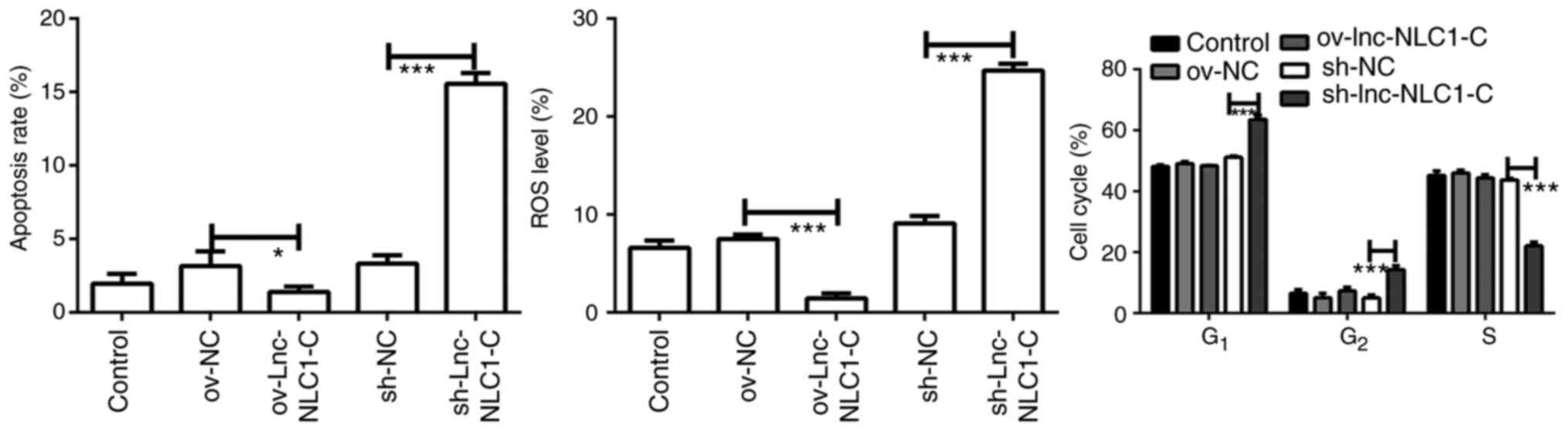
Lnc-NLC1-C inhibits ROS generation and
promotes cell cycle blocking in U87MG cells
Compared with ov-NC, ov-lnc-NLC1-C significantly
decreased the ROS production (P<0.001; Figs. 3 and S1B). Compared with sh-NC, sh-lnc-NLC1-C
significantly promoted ROS generation and apoptosis of U87MG cells
(P<0.001; Figs. 3 and S1A and B). Compared with the sh-NC group,
U87MG cells in the G1 phase were significantly higher in
the sh-lnc-NLC1-C group (P<0.001), whereas cells in the S phase
were significantly decreased (P<0.001) (Figs. 3 and S1C). There was no significant effect on
apoptosis or cell cycle following lnc-NLC1-C overexpression
compared with the ov-NC group (P>0.05).
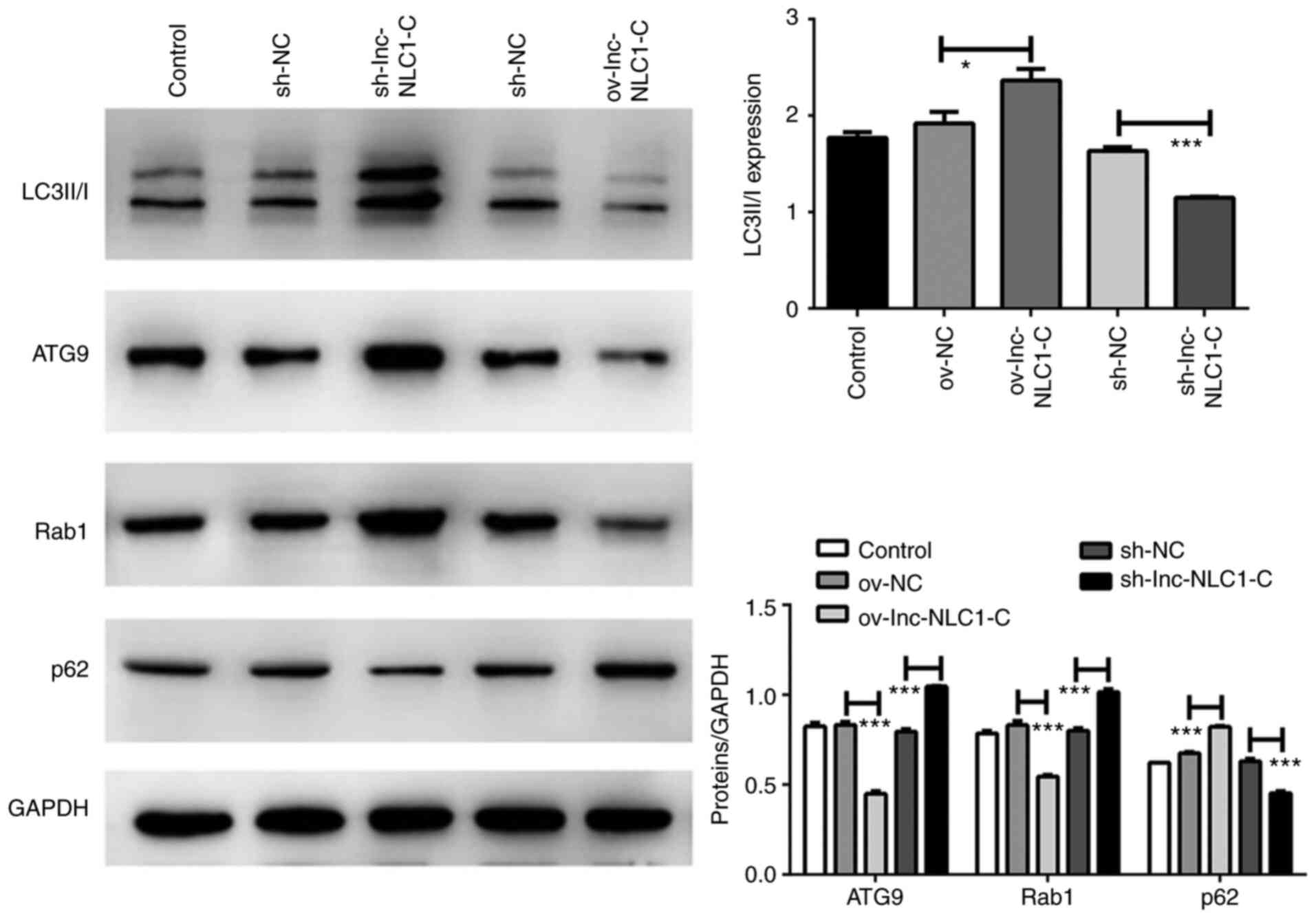
Lnc-NLC1-C inhibits autophagy in U87MG
cells
Compared with ov-NC, the protein expression levels
of LC3II/I (P<0.05) and p62 (P<0.001) were significantly
increased by ov-lnc-NLC1-C, whereas those of ATG9 and Rab1 were
significantly decreased (P<0.001) (Fig. 4). Compared with sh-NC, the protein
expression levels of LC3II/I and p62 were significantly decreased
by sh-lnc-NLC1-C (P<0.001), whereas those of ATG9 and Rab1 were
significantly increased (P<0.001) (Fig. 4).
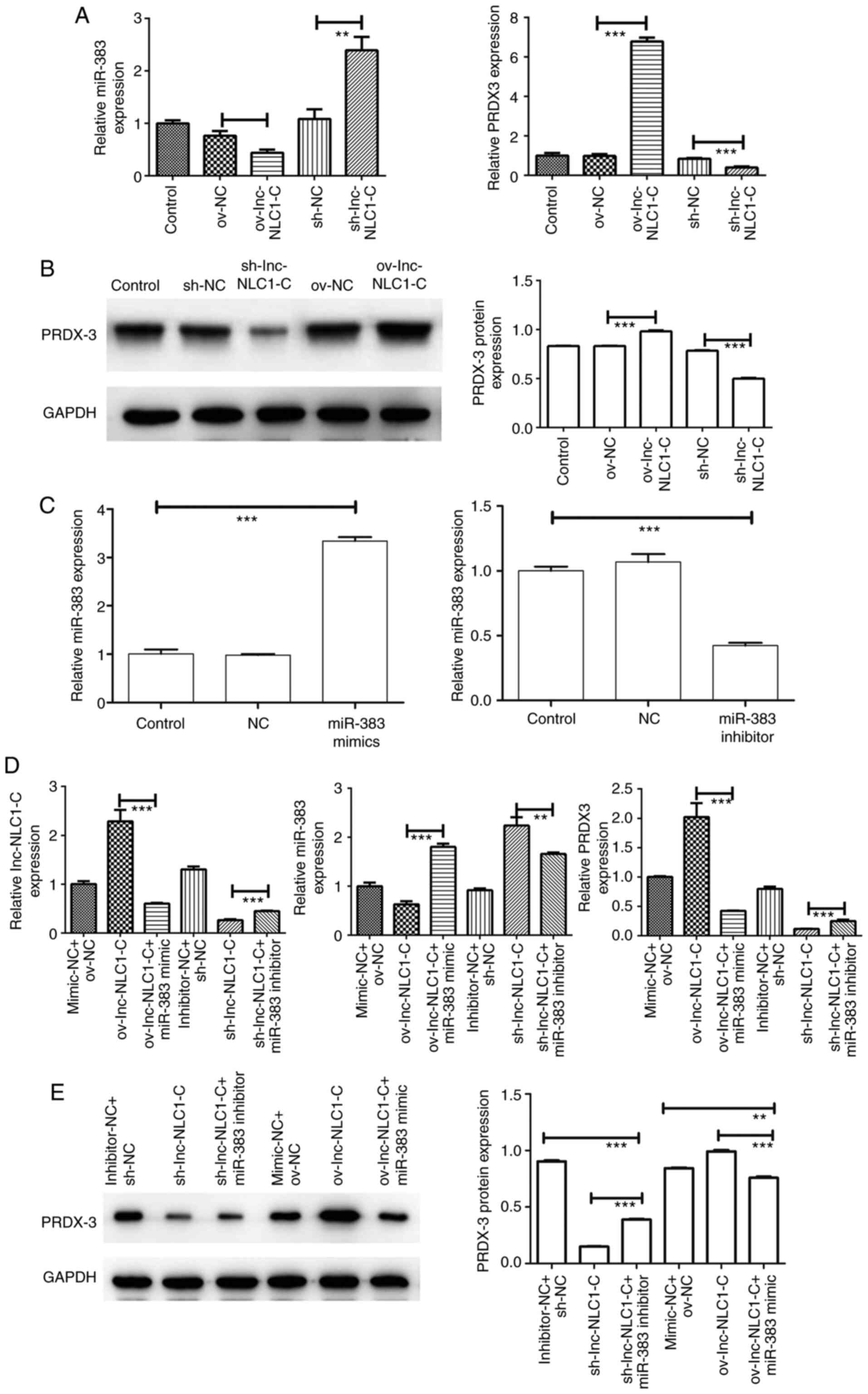
Expression of the
lnc-NLC1-C/miR-383/PRDX-3 axis in U87MG cells
Compared with ov-NC, ov-lnc-NLC1-C significantly
increased PRDX-3 expression (P<0.001) and significantly
decreased miR-383 expression (P<0.01) (Fig. 5A and B). Compared with sh-NC,
sh-lnc-NLC1-C significantly decreased PRDX-3 expression
(P<0.001) and increased miR-383 expression (P<0.01) (Fig. 5A and B). As shown in Fig. 5C, miR-383 expression was
significantly increased in the miR-383 mimic group (P<0.001) and
was significantly decreased in the miR-383 inhibitor group compared
with in the control group (P<0.001), indicating that the miR-383
mimic and inhibitor were successfully transfected. Compared with
ov-lnc-NLC1-C, ov-lnc-NLC1-C + miR-383 mimic significantly
decreased the expression levels of lnc-NLC1-C and PRDX-3, and
significantly increased miR-383 expression (P<0.001; Fig. 5D and E). Compared with sh-lnc-NLC1-C,
sh-lnc-NLC1-C + miR-383 inhibitor significantly increased the
expression levels of lnc-NLC1-C and PRDX-3 (P<0.001), and
decreased miR-383 expression (P<0.01) (Fig. 5D and E).
Discussion
Carcinogenic factors affecting glioma include
high-risk congenital genetic and environmental factors. Previous
studies have shown that glioma accounts for ~80% of all malignant
tumors of the central nervous system (21,22).
Glioma has a high incidence, a high recurrence rate and a poor
prognosis (23,24). Tumor heterogeneity and infection pose
great challenges to the clinical treatment of glioma (25,26). In
recent years, with the development of treatment for glioma, the
mechanism of lncRNAs has attracted the attention of experts and
scholars.
LncRNAs are non-coding RNAs composed of 200–100,000
nucleotides (27) that are closely
associated with the occurrence and development of a number of
tumors, such as gastric and colon cancer (28). A previous study has found that
lncRNAs regulate gene expression, mainly by interacting with DNA,
RNA and proteins (29). For example,
lncRNAs activate or deactivate protein complexes by binding to
chromosomal DNA (30). Currently
known lncRNAs associated with glioma include HOTAIR, H19, CRNDE,
MEG3 and ADAMTS9-AS2 (31–35). The present study revealed that
lnc-NLC1-C expression was highest in U87MG cells compared with that
in U251, SHG44 and U118MG cells. Lnc-NLC1-C overexpression promoted
the proliferation, cell cycle blocking, migration and invasion of
U87MG cells, and inhibited apoptosis and autophagy, indicating that
lnc-NLC1-C may be a cancer-promoting factor in glioma.
NLC1-C is weakly expressed in cutaneous squamous
cell carcinoma and highly expressed in the breasts, testis and
parotid gland, thus participating in tumor development (19,36).
Lnc-NLC1-C targets miR-383, and the accumulation of NLC1-C in the
nucleus inhibits the transcription of miR-320a and miR-383, and
promotes the proliferation of embryonic testicular cancer cells by
binding to nucleolin (19). These
results indicate that lnc-NLC1-C can target miR-383 and affect the
expression of downstream genes and proteins. It has been previously
demonstrated that miR-383 reverses mitochondrial ROS inhibition
caused by PRDX-3 overexpression, promotes autophagy and oxidative
stress in cancer cells, and enhances cancer cell apoptosis
(37). Tian et al (38) showed that PRDX3 is a potential target
gene of miR-383, and there is a targeting relationship between the
two genes. miR-383 can inhibit the proliferation, migration and
invasion of human hepatocellular carcinoma HepG2 cells by
down-regulating PRDX3 expression (39). Wang et al (40) observed that miR-383 inhibited the
proliferation of human medulloblastoma cells and promoted their
apoptosis by downregulating the expression levels of PRDX3.
Currently, to the best of our knowledge, there are no studies on
the role of the PRDX3/miR-383 axis in glioma, and based on the fact
that lnc-NLC1-C targets miR-383, the present study predicted that
lnc-NLC1-C may affect the development of glioma through the
miR-383/PRDX-3 axis. The present results revealed that lnc-NLC1-C
downregulated miR-383 expression and upregulated PRDX-3 expression.
miR-383 inhibition rescue experiments confirmed that miR-383
reversed the effect of lnc-NLC1-C and downregulated PRDX-3
expression. Li et al (41)
observed that miR-383 acts as a regulator controlling cell
proliferation of medulloblastoma via targeting PRDX3; although the
studied diseases were different, they both belong to the
neurological system, which indirectly confirmed the current
results. In the present study, only one cell line was used for
experimental manipulation, and there were no in vivo
experiments, which are the main limitations of the study.
Therefore, multiple cell lines and animal experiments should be
used in follow-up experiments to further verify the conclusions of
the present study. In conclusion, the lnc-NLC1-C/miR-383/PRDX-3
axis may serve an important role in glioma development.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX and QC performed the experiments and confirmed
the authenticity of all the raw data. XZ, ML and JL analyzed the
data. ZX drafted the manuscript. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lai NS, Wu DG, Fang XG, Lin YC, Chen SS,
Li ZB and Xu SS: Serum microRNA-210 as a potential noninvasive
biomarker for the diagnosis and prognosis of glioma. Br J Cancer.
112:1241–1246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fabian C, Han M, Bjerkvig R and Niclou SP:
Novel facets of glioma invasion. Int Rev Cell Mol Bio. 360:33–64.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mansouri A, Mansouri S, Hachem LD,
Klironomos G, Vogelbaum MA, Bernstein M and Zadeh G: The role of
5-aminolevulinic acid in enhancing surgery for high-grade glioma,
its current boundaries, and future perspectives: A systematic
review. Cancer. 122:2469–2478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moran VA, Perera RJ and Khalil AM:
Emerging functional and mechanistic paradigms of mammalian long
non-coding RNAs. Nucleic Acids Res. 40:6391–6400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Cho KB, Li Y, Tao G, Xie Z and Guo
B: Long noncoding RNA (lncRNA)-mediated competing endogenous RNA
networks provide novel potential biomarkers and therapeutic targets
for colorectal cancer. Int J Mol Sci. 20:57582019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei GH and Wang X: LncRNA MEG3 inhibit
proliferation and metastasis of gastric cancer via p53 signaling
pathway. Eur Rev Med Pharmacol Sci. 21:3850–3856. 2017.PubMed/NCBI
|
|
7
|
Yu GJ, Sun Y, Zhang DW and Zhang P: Long
non-coding RNA HOTAIR functions as a competitive endogenous RNA to
regulate PRAF2 expression by sponging miR-326 in cutaneous squamous
cell carcinoma. Cancer Cell Int. 19:2702019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang K, Luo Z, Zhang Y, Zhang L, Wu L,
Liu L, Yang J, Song X and Liu J: Circulating lncRNA H19 in plasma
as a novel biomarker for breast cancer. Cancer Biomark. 17:187–194.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paraskevopoulou MD and Hatzigeorgiou AG:
Analyzing miRNA-lncRNA interactions. Methods Mol Biol.
1402:271–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ballantyne MD, McDonald RA and Baker AH:
LncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol
Ther. 99:494–501. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Zhou Y and Li H: LncRNA, miRNA and
lncRNA-miRNA interaction in viral infection. Virus Res. 257:25–32.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei L, Wang X, Lv L, Liu J, Xing H, Song
Y, Xie M, Lei T, Zhang N and Yang M: The emerging role of microRNAs
and long noncoding RNAs in drug resistance of hepatocellular
carcinoma. Mol Cancer. 18:1472019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Z, Cen D, Luo X, Li D, Li P, Liang L
and Meng Z: Downregulation of miR-383 promotes glioma cell invasion
by targeting insulin-like growth factor 1 receptor. Med Oncol.
30:5572013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawashima M, Tamiya G, Oka A, Hohjoh H,
Juji T, Ebisawa T, Honda Y, Inoko H and Tokunaga K: Genomewide
association analysis of human narcolepsy and a new resistance gene.
Am J Hum Genet. 79:252–263. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mills JD, Kavanagh T, Kim WS, Chen BJ,
Kawahara Y, Halliday GM and Janitz M: Unique transcriptome patterns
of the white and grey matter corroborate structural and functional
heterogeneity in the human frontal lobe. PLoS One. 8:e784802013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zong L, Hattori N, Yasukawa Y, Kimura K,
Mori A, Seto Y and Ushijima T: LINC00162 confers sensitivity to
5-Aza-2′-deoxycytidine via modulation of an RNA splicing protein,
HNRNPH1. Oncogene. 38:5281–5293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lou X, Li J, Yu D, Wei YQ, Feng S and Sun
JJ: Comprehensive analysis of five long noncoding RNAs expression
as competing endogenous RNAs in regulating hepatoma carcinoma.
Cancer Med. 8:5735–5749. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piipponen M, Nissinen L, Farshchian M,
Riihila P, Kivisaari A, Kallajoki M, Peltonen J, Peltonen S and
Kähäri VM: Long noncoding RNA PICSAR promotes growth of cutaneous
squamous cell carcinoma by regulating ERK1/2 activity. J Invest
Dermatol. 136:1701–1710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu M, Tian H, Cao YX, He X, Chen L, Song
X, Ping P, Huang H and Sun F: Downregulation of
miR-320a/383-sponge-like long non-coding RNA NLC1-C (narcolepsy
candidate-region 1 genes) is associated with male infertility and
promotes testicular embryonal carcinoma cell proliferation. Cell
Death Dis. 6:e19602015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morgan LL, Miller AB, Sasco A and Davis
DL: Mobile phone radiation causes brain tumors and should be
classified as a probable human carcinogen (2A) (review). Int J
Oncol. 46:1865–1871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu W, Long H, Zhang M, Wang Y, Lu Q, Yuan
H, Qu Q and Qu J: Glutathione S-transferase genes variants and
glioma risk: A case-control and meta-analysis study. J Cancer.
10:4679–4688. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng M and Liu L: MUC15 promotes growth
and invasion of glioma cells by activating Raf/MEK/ERK pathway.
Clin Exp Pharmacol Physiol. 47:1041–1048. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu L, Zhao T, Wang J, Wang R, Bu H, Zheng
S, Li Y, Liu J and Wang S: Beauvericin inhibits the growth of U251
glioma cells and promotes apoptosis in vitro. J Biobased Materials
Bioenergy. 14:639–644. 2020. View Article : Google Scholar
|
|
25
|
Hanaei S, Afshari K, Hirbod-Mobarakeh A,
Mohajer B, Amir Dastmalchi D and Rezaei N: Therapeutic efficacy of
specific immunotherapy for glioma: A systematic review and
meta-analysis. Rev The Neurosci. 29:443–461. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baumert BG, Hegi ME, van den Bent MJ, von
Deimling A, Gorlia T, Hoang-Xuan K, Brandes AA, Kantor G, Taphoorn
MJB, Hassel MB, et al: Temozolomide chemotherapy versus
radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): A
randomised, open-label, phase 3 intergroup study. Lancet Oncol.
17:1521–1532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jathar S, Kumar V, Srivastava J and
Tripathi V: Technological developments in lncRNA biology. Adv Exp
Med Biol. 1008:283–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar MM and Goyal R: LncRNA as a
therapeutic target for angiogenesis. Curr Top Med Chem.
17:1750–1757. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jarroux J, Morillon A and Pinskaya M:
History, discovery, and classification of lncRNAs. Adv Exp Med
Biol. 1008:1–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren J, Zhang X, Cao J, Tian J, Luo J, Yu
Y, Wang F, Li J and Zhao Q: Radiosynthesis of a novel antisense
imaging probe targeting lncRNA HOTAIR in malignant glioma. Res Sq.
doi: 10.21203/rs.3.rs-152169/v1. PubMed/NCBI
|
|
32
|
Shi Y, Wang Y, Luan W, Wang P, Tao T,
Zhang J, Qian J, Liu N and You Y: Long Non-Coding RNA H19 promotes
glioma cell invasion by deriving miR-675. PLoS One. 9:e862952014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jing SY, Yang ZG, Cheng ZH, Fang JS and Li
X: Changes in expression levels of long-chain non-coding RNA CRNDE
in glioma and its association with clinical and pathological
characteristics. J Clin Neurosurgery. 16:11–16. 2019.PubMed/NCBI
|
|
34
|
Wang D, Fu CW and Fan DQ: Participation of
tumor suppressors long non-coding RNA MEG3, microRNA-377 and PTEN
in glioma cell invasion and migration. Pathol Res Pract.
215:1525582019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang JL, Ge HM, Yan ZJ and Yan H:
Correlativity between low expression level of long non-coding RNA
ADAMTS9-AS2 and poor prognosis in patients with gliomas. Chin J
Clin Neurosurgery. 24:278. 2019.
|
|
36
|
Luo Y, Morgan SL and Wang KC: PICSAR: Long
noncoding RNA in cutaneous squamous cell carcinoma. J Invest
Dermatol. 136:1541–1542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li KK, Pang JC, Lau KM, Zhou L, Mao Y,
Wang Y, Poon WS and Ng HK: MiR-383 is downregulated in
medulloblastoma and targets peroxiredoxin 3 (PRDX3). Brain Pathol.
23:413–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tian S, Jiang H, Liu H, Cao X, Zhang D,
Mou SD and Li Z: The effects of miR-383 targeting PRDX3 on the
proliferation and invasion of HepG2 cells. J Gastroenterol Hepatol.
29:1335–1340. 2020.PubMed/NCBI
|
|
39
|
Ng HK: Hsa-miR-383 and its Target
Peroxiredoxin 3 (PRDX3) Have Major Roles Controlling Cell Growth in
Medulloblastoma. Proceedings of the 88th Annual Meeting of the
American Association of Neuropathologists. AANP; Chicago, IL:
2012
|
|
40
|
Wang SZ, Gao ML and Hou JH: Regulation of
PRDX3 expression by miR383 and its effect on proliferation and
apoptosis of human medulloblastoma. Labeled Immunoassays Clin Med.
26:139–142. 2019.(In Chinese).
|
|
41
|
Li KK, Pang JC, Lau KM, Zhou L, Mao Y,
Wang Y, Poon WS and Ng HK: miR-383 is downregulated in
medulloblastoma and targets peroxiredoxin 3 (PRDX3). Brain Pathol.
23:413–425. 2013. View Article : Google Scholar : PubMed/NCBI
|