In 1979, multiple laboratories simultaneously
discovered a highly expressed ~53 kDa protein in cancer cells and
named this protein the p53 protein. Initially, the p53 gene was
considered an oncogene (1,2), but it was later demonstrated to be a
tumor suppressor gene by several groups (3,4).
The role of p53 as a tumor suppressor gene is mainly
realized through the transcriptional regulatory mechanism of
apoptosis and senescence. Under normal conditions, wild-type p53
(p53wt) in cells is maintained at a low level due to its
interaction with the E3 ubiquitin ligase MDM2. However, once DNA is
damaged by radiation, the ataxia telangiectasia mutated (ATM) gene
may be activated and ATM directly or indirectly phosphorylates p53
(5–8). In this circumstance, the p53 protein is
subjected to numerous posttranslational modifications, such as
phosphorylation, acetylation, ubiquitination, SUMOylation,
neddylation and methylation. These modifications inhibit p53 from
binding to MDM2, making p53 a stable transcription factor that
binds to specific DNA sequences, transactivates multiple target
genes [such as p21, Bax and p53-upregulated modulator of apoptosis
(PUMA)], repairs DNA through homologous recombination and maintains
the integrity of the genome (9–11). In
addition, p53 also inhibits the inflammatory response (12), repairs broken DNA double strands
(13) and maintains metabolic
homeostasis (14).
p53 function depends on its structure. The p53
protein is composed of three major domains. The N-terminal domain
is related to the transactivation capacity of p53, and it is a
disordered and inherently unfolded region consisting of a
trans-activated domain and a proline-rich region. The central
domain is the key component of p53, controlling the
sequence-specific DNA-binding activity of p53 and promoting the
DNA-p53 protein interaction. This region is also the most
susceptible to mutations, which are known to occur in >90% of
human cancers. The C-terminal domain is also a disordered
structural domain that controls the posttranslational modification
activity of p53 (15).
The p53 protein status is correlated with the p53
gene status. Cancer cells may have three different p53 gene states:
p53wt, mutant-type p53 (p53mt) and p53
deletion (null-type p53 or p53null) (16). p53 mutation is common, occurring in
~50% of human cancers (17,18). The most common and well-characterized
p53 mutations are missense mutations (75%). Other alterations
include frameshift insertion and deletion (9%), nonsense mutation
(7%), silent mutation (5%) and rare changes (19,20).
p53mt gene encodes p53mt protein.
P53mt prevents apoptosis and promotes the proliferation
and metastasis of cancer cells (21). p53mt deactivates
sequence-specific DNA-binding activity, thereby causing the loss of
normal p53 tumor suppressor function and the inability of the
p53wt heterodimer to form a tetramer complex, thereby
damaging dimer function (22). In
addition, p53mt causes another ‘gain of function’ (GOF)
that not only promotes tumor progression, migration and metabolism
but also increases tumor cell resistance to radiotherapy (23,24).
This outcome is ascribed to the upregulation of multiple genes by
p53mt, including phosphatase and tensin homolog, c-myc,
NF-κB, p16INK4A, p63 and p73 (25–30).
Radiotherapy is one of the most common cancer
treatments and it may be used in combination with chemotherapy or
surgery to treat various malignant tumor types (35). Radiation kills tumor cells by causing
DNA damage. Once DNA damage is identified, cellular sensor
proteins, such as ATM, deliver damage signals to effectors and
phosphorylate certain targets, such as p53 (7).
Although p53 mutations are observed in the whole
gene, there are six mutation hotspots distributed in the
DNA-binding domain. These six hotspots are located in codons 175,
245, 248, 249, 273 and 282 (60).
Different p53 mutation types may lead to distinctly different
cellular functions and effects on radiotherapeutic efficacy even
when the mutations are in the same domain. Menendez et al
(61) indicated that different p53
mutations produce different cellular phenotypes after transfection,
leading to cell survival or apoptosis. After cells are exposed to
radiation, the expression levels of target genes, such as p21,
MDM2, Bax and mutS homolog 2, which correlate with the cell cycle
and cell apoptosis, induced by different p53mt mutants
are different; therefore, the biological activities of these
transfectants are completely different. Okaichi et al
(62) transferred four types of
mutant p53 genes distributed separately at codons 123 (Thr→Ala),
143 (Val→Ala), 175 (Arg→His) and 273 (Arg→His) into human
osteosarcoma Saos-2 cells (p53null) and irradiated the
cells with X-rays. The results indicated that the radiosensitivity
of cells expressing mutant p53 proteins (123Thr→Ala) is higher than
that of cells expressing proteins with other mutation types, while
the expression of p53 proteins mutated at amino acids 143, 175 and
273 does not affect cellular radiosensitivity. In addition, the
same group performed a similar experiment with 12 different types
of p53 mutations and determined that mutations in hotspots may
cause loss of p53 binding ability to DNA and, thus, loss of
transcription function. However, mutations in loop domains do not
change the conformation of the p53 protein and its normal functions
are retained. They also demonstrated that mutations occurring at
the N-terminus of the p53 protein affect downstream
p21WAF1 expression and radiosensitivity (63,64).
Radiation disrupts the DNA structure and activates
certain protein kinase pathways, which block cell cycle progression
and initiate DNA repair processes. p53 is the major downstream
effector in DNA damage-activated kinase pathways and the main role
of p53 is to promote cellular apoptosis induced by radiation.
Activated p53 triggers G1 phase arrest by transactivating
p21Waf1, allowing cells additional time for DNA repair
(65). In addition, p53 upregulates
the proapoptotic molecules PUMA and NADPH oxidase activator 1, and
activates the Bcl family member Bax to promote the apoptosis of
nonrepairable cells and the recovery of tissue homeostasis
(66).
miRNAs constitute a class of small noncoding RNA
molecules involved in the regulation of multiple cell behaviors,
such as gene expression, cell division, differentiation, apoptosis,
metabolism and cancer development (75–77). The
communication of miRNAs with various signaling factors provides an
ingenious entry point for improving the efficacy of radiotherapy.
Further understanding the role of miRNAs in tumor radiosensitivity,
particularly their relationship with p53, is helpful for forming
novel ideas to solve the ubiquitous radiation resistance problem in
cancer treatment and for providing more effective therapeutic
strategies for patients who are suitable for radiotherapy.
p53 and miRNA interactions have key roles in
modulating the cellular response to radiation. For instance, p53
induces the expression of miR-34 in cells with DNA damage and
overexpression of miR-34 enhances radiosensitivity (78,79).
miRNAs bind to the 3′UTR of p53 mRNA and suppress p53 translation,
negatively regulating p53-related genes, such as p21 and MDM2, and
inducing cell cycle arrest and radioresistance. Liu et al
(80) reported that the presence of
miR-375, which is associated with the recurrence of gastric cancer,
reduces the radiosensitivity of tumor cells by regulating the
expression of p53. He et al (81) observed that in human lung cancer
cells, radiation upregulates the expression level of miR-300 and
reduces radiosensitivity by inducing p53/apaf1-related G2-phase
cell cycle arrest and apoptosis. By contrast, miRNAs may stimulate
the expression of p53 and increase tumor radiosensitivity (82,83).
Song et al (84) indicated
that in patients with cervical cancer, miR-375 is overexpressed
after radiotherapy. miR-375 inhibits the degradation of p53 and
leads to cell cycle arrest in G1-phase, thereby increasing
radiosensitivity.
Radiation-induced p53 overexpression also inhibits
the level of miRNAs and negatively affects radiation efficacy
(85). miRNA let-7 family members
function differently in different cells after irradiation (86). One of the targets of let-7 is KRAS,
which induces radioresistance (87).
In certain cancer cells, miRNAs such as let-7a and let-7b (let-7
family members) are inhibited after irradiation and their
inhibition is correlated with p53wt expression. Saleh
et al (88) irradiated
p53null and p53wt mouse colon cancer cell
lines and observed that let-7 decreases the radiosensitivity of
p53wt cells but not p53null cells. In
addition, experiments have revealed that functional p53 directly
interacts with Drosha, a miRNA-processing enzyme, and inhibits the
expression of let-7a and let-7b through transcriptional regulation
in cells after irradiation.
LncRNAs constitute a class of noncoding RNAs with a
length of >200 bp that have regulatory roles in chromatin
modification, transcription and posttranscriptional gene
regulation. An increasing number of lncRNAs have been indicated to
have important roles in the development and treatment of tumors.
This increase in lncRNAs may be used as a cancer marker to detect
specific diseases and improve the rate of early diagnosis of tumors
(89).
LncRNAs are involved in various mechanisms in cells
to regulate radiosensitivity, such as DNA damage repair, cell cycle
arrest and apoptosis. LncRNA and p53 interactions may regulate
cellular radiosensitivity. Zhang et al (90) and Wang et al (91) indicated that lncRNAs participate in
the nonhomologous end joining pathway after radiation-induced DNA
damage, which accelerates damage repair and reduces radiation
sensitivity. Yang et al (92)
reported that the lncRNA regulator of reprogramming (ROR) increases
radioresistance in colorectal cancer cells by inhibiting the
p53/miR-145 axis, which is known to be an important pathway for
tumor inhibition; they determined that the levels of p53 protein
and miR-145, as well as the degree of radiosensitivity of
colorectal cells, are increased after X-ray irradiation compared to
lncRNA ROR-deficient cells. In esophageal cancer, Wang et al
(93) observed that lncRNA colon
cancer-associated transcript 2 promotes radioresistance by
inhibiting the p53 proapoptotic signaling pathway. Beer et
al (94) indicated that p53 has
a central role in the modulation of radiation-induced cellular
apoptosis by regulating the expression of several lncRNAs,
including tumor protein p53 pathway corepressor 1 (Trp53cor1) and
taurine-upregulated (Tug1). They treated human peripheral blood
mononuclear cells with high-dose γ radiation and determined that
Trp53cor1, as a downstream target of p53, was significantly
upregulated after cell irradiation and that the expression of
caspase-3 was increased; they also observed that the expression
level of lncRNA Tug1 in cells was increased after IR. As a
downstream target gene of p53, lncRNA Tug1 increases cell
radiosensitivity by promoting cell cycle arrest and apoptosis
through the regulation of various miRNAs, including miR-222-3p,
miR-221-3p, miR-132 and miR-29a.
Over the past 40 years, with the enhanced
understanding of the structure, role and mutations of the p53 tumor
suppressor gene, it has become increasingly evident that p53 has an
important role in tumor inhibition and treatment, and its
importance underlies the carcinogenicity of its deletion or
mutation. Deletion or mutation of p53 in tumor cells leads to the
loss of normal p53 antitumor function and promotes the occurrence
and metastasis of cancer. Summary of the research results of the
past 30 years supports that the antiapoptotic properties of
p53mt enhance tumor resistance to radiotherapy, which
inhibits the efficacy of cancer treatment. The p53 status, to a
certain degree, may be considered as a useful biomarker for
radiosensitivity, and it is advocated to elevate the irradiation
dose, prolong the irradiation time, or select a more suitable
treatment strategy, i.e. chemotherapy, targeted therapy and
immunotherapy for those patients with p53mt.
Identifying the mechanism of p53 in promoting
radioresistance may help doctors develop accurate treatment
strategies for patients and improve the radiotherapeutic outcomes
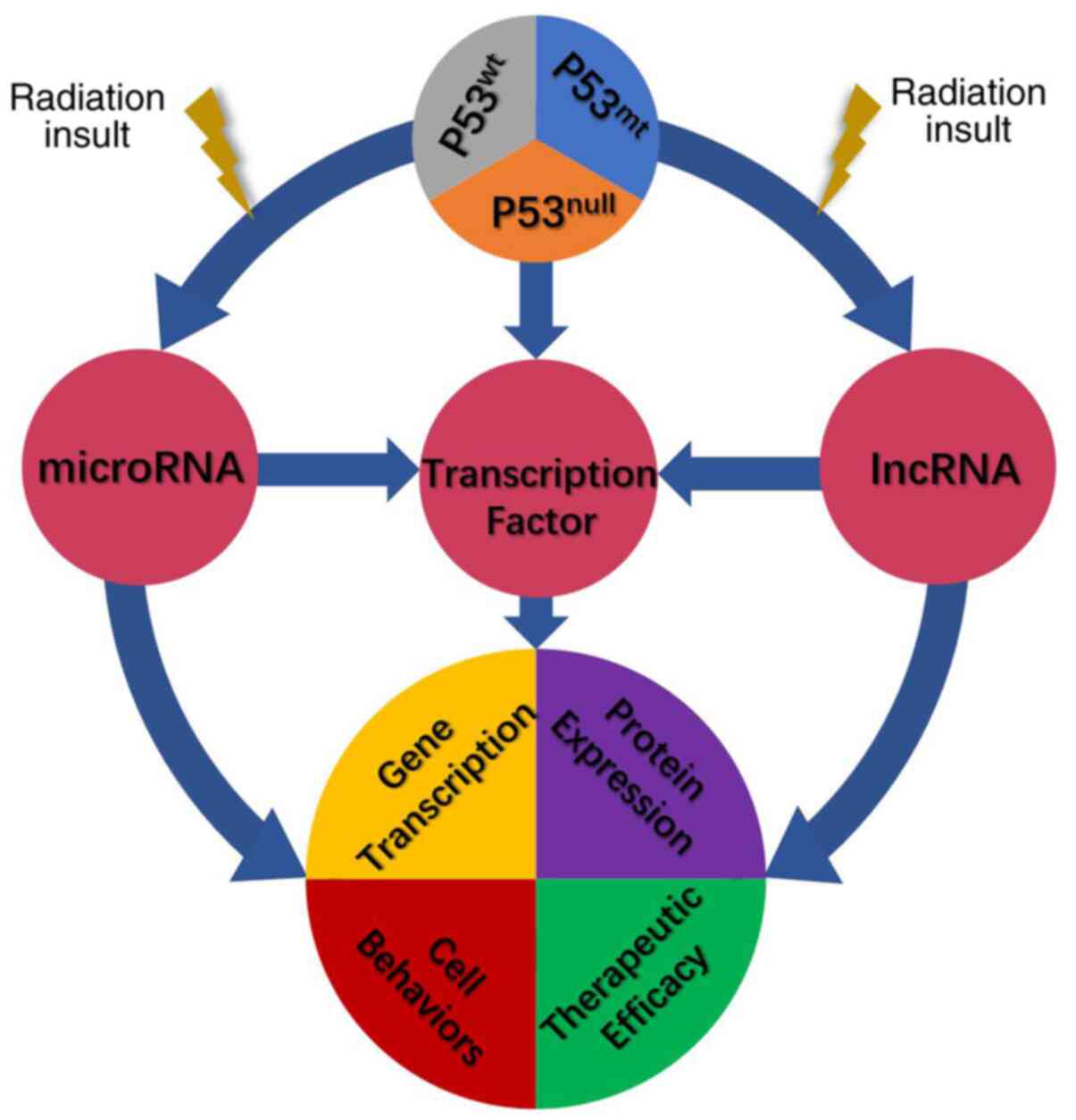
and prognosis for patients. As part of the present study, a summary
of the history of p53 research and the association between p53
expression and radiation response (Table
I) and a schematic of the mechanisms related to p53 and
radioresistance (Fig. 1) are
provided. Specifically, the role of non-coding RNAs in p53-induced
radioresistance was summarized, which is rarely mentioned in
previous reports and similar reviews. The present review is an
extension and supplement to the traditional theories. It is
esteemed that these mechanisms serve as a basis to guide further
analysis, laboratory studies and clinical practice for cancer
patients receiving radiotherapy.
Not applicable.
This work was supported in part by the Subject
Arrangement Program from the Science and Technology Department of
Jilin Province (grant nos. 20190201209JC to SL and 20200201123JC to
DY).
Data sharing is not applicable.
DY and SL contributed to the conception and design
of the article. XK drafted the manuscript. ZW drafted the figure
and table and revised the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Lane DP and Crawford LV: T antigen is
bound to a host protein in SV40-transformed cells. Nature.
278:261–263. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Linzer DI and Levine AJ: Characterization
of a 54K dalton cellular SV40 tumor antigen present in
SV40-transformed cells and uninfected embryonal carcinoma cells.
Cell. 17:43–52. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eliyahu D, Michalovitz D, Eliyahu S,
Pinhasi-Kimhi O and Oren M: Wild-type p53 can inhibit
oncogene-mediated focus formation. Proc Natl Acad Sci USA.
86:8763–8767. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baker SJ, Fearon ER, Nigro JM, Hamilton
SR, Preisinger AC, Jessup JM, vanTuinen P, Ledbetter DH, Barker DF,
Nakamura Y, et al: Chromosome 17 deletions and p53 gene mutations
in colorectal carcinomas. Science. 244:217–221. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Canman CE, Lim DS, Cimprich KA, Taya Y,
Tamai K, Sakaguchi K, Appella E, Kastan MB and Siliciano JD:
Activation of the ATM kinase by ionizing radiation and
phosphorylation of p53. Science. 281:1677–1679. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Q, Guo Y, Liu X, Czauderna F, Carr MI,
Zenke FT, Blaukat A and Vassilev LT: Therapeutic implications of
p53 status on cancer cell fate following exposure to ionizing
radiation and the DNA-PK inhibitor M3814. Mol Cancer Res.
17:2457–2468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui D, Xiong X, Shu J, Dai X, Sun Y and
Zhao Y: FBXW7 confers radiation survival by targeting p53 for
degradation. Cell Rep. 30:497–509.e4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venkata Narayanan I, Paulsen MT, Bedi K,
Berg N, Ljungman EA, Francia S, Veloso A, Magnuson B, di Fagagna
FD, Wilson TE and Ljungman M: Transcriptional and
post-transcriptional regulation of the ionizing radiation response
by ATM and p53. Sci Rep. 7:435982017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marcel V, Catez F and Diaz JJ: p53, a
translational regulator: Contribution to its tumour-suppressor
activity. Oncogene. 34:5513–5523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shirai Y, Shiba H, Iwase R, Haruki K,
Fujiwara Y, Furukawa K, Uwagawa T, Ohashi T and Yanaga K: Dual
inhibition of nuclear factor kappa-B and Mdm2 enhance the antitumor
effect of radiation therapy for pancreatic cancer. Cancer Lett.
370:177–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bechill J, Zhong R, Zhang C, Solomaha E
and Spiotto MT: A high-throughput cell-based screen identified a
2-[(E)-2-Phenylvinyl]-8-quinolinol core structure that activates
p53. PLoS One. 11:e01541252016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uehara I and Tanaka N: Role of p53 in the
regulation of the inflammatory tumor microenvironment and tumor
suppression. Cancers (Basel). 10:2192018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menon V and Povirk L: Involvement of p53
in the repair of DNA double strand breaks: Multifaceted Roles of
p53 in homologous recombination repair (HRR) and non-homologous end
joining (NHEJ). Subcell Biochem. 85:321–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moulder DE, Hatoum D, Tay E, Lin Y and
McGowan EM: The roles of p53 in mitochondrial dynamics and cancer
metabolism: The pendulum between survival and death in breast
cancer? Cancers (Basel). 10:1892018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fischbach A, Krüger A, Hampp S, Assmann G,
Rank L, Hufnagel M, Stöckl MT, Fischer JMF, Veith S, Rossatti P, et
al: The C-terminal domain of p53 orchestrates the interplay between
non-covalent and covalent poly(ADP-ribosyl)ation of p53 by PARP1.
Nucleic Acids Res. 46:804–822. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamp WM, Wang PY and Hwang PM: TP53
mutation, mitochondria and cancer. Curr Opin Genet Dev. 38:16–22.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu G, Pan C, Bei JX, Li B, Liang C, Xu Y
and Fu X: Mutant p53 in cancer progression and targeted therapies.
Front Oncol. 10:5951872020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Long S, Loureiro JB, Carvalho C, Gales L,
Saraiva L, Pinto MMM, Puthongking P and Sousa E: Semi-synthesis of
small molecules of aminocarbazoles: Tumor growth inhibition and
potential impact on p53. Molecules. 26:16372021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olotu FA and Soliman MES: Dynamic
perspectives into the mechanisms of mutation-induced p53-DNA
binding loss and inactivation using active perturbation theory:
Structural and molecular insights toward the design of potent
reactivators in cancer therapy. J Cell Biochem. 120:951–966. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petitjean A, Achatz MI, Borresen-Dale AL,
Hainaut P and Olivier M: TP53 mutations in human cancers:
Functional selection and impact on cancer prognosis and outcomes.
Oncogene. 26:2157–2165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mantovani F, Collavin L and Del Sal G:
Mutant p53 as a guardian of the cancer cell. Cell Death Differ.
26:199–212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Milner J, Medcalf EA and Cook AC: Tumor
suppressor p53: Analysis of wild-type and mutant p53 complexes. Mol
Cell Biol. 11:12–19. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarado-Ortiz E, de la Cruz-López KG,
Becerril-Rico J, Sarabia-Sánchez MA, Ortiz-Sánchez E and
García-Carrancá A: Mutant p53 gain-of-function: Role in cancer
development, progression, and therapeutic approaches. Front Cell
Dev Biol. 8:6076702021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Zhang J, Tong JHM, Chan AWH, Yu J,
Kang W and To KF: Targeting the oncogenic p53 mutants in colorectal
cancer and other solid tumors. Int J Mol Sci. 20:59992019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Guessous F, Kwon S, Kumar M, Ibidapo
O, Fuller L, Johnson E, Lal B, Hussaini I, Bao Y, et al: PTEN has
tumor-promoting properties in the setting of gain-of-function p53
mutations. Cancer Res. 68:1723–1731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang F, Li K, Yao X, Wang H, Li W, Wu J,
Li M, Zhou R, Xu L and Zhao L: A miR-567-PIK3AP1-PI3K/AKT-c-Myc
feedback loop regulates tumour growth and chemoresistance in
gastric cancer. EBioMedicine. 44:311–321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vaughan CA, Singh S, Windle B, Sankala HM,
Graves PR, Andrew Yeudall W, Deb SP and Deb S: p53 mutants induce
transcription of NF-κB2 in H1299 cells through CBP and STAT binding
on the NF-κB2 promoter and gain of function activity. Arch Biochem
Biophys. 518:79–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Pickering CR, Holst CR, Gauthier
ML and Tlsty TD: p16INK4a modulates p53 in primary human mammary
epithelial cells. Cancer Res. 66:10325–10331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gaiddon C, Lokshin M, Ahn J, Zhang T and
Prives C: A subset of tumor-derived mutant forms of p53
down-regulate p63 and p73 through a direct interaction with the p53
core domain. Mol Cell Biol. 21:1874–1887. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA,
Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, et
al: Gain of function of a p53 hot spot mutation in a mouse model of
Li-Fraumeni syndrome. Cell. 119:861–872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marusyk A, Porter CC, Zaberezhnyy V and
DeGregori J: Irradiation selects for p53-deficient hematopoietic
progenitors. PLoS Biol. 8:e10003242010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wouters A, Pauwels B, Lambrechts HA,
Pattyn GG, Ides J, Baay M, Meijnders P, Peeters M, Vermorken JB and
Lardon F: Retention of the in vitro radiosensitizing potential of
gemcitabine under anoxic conditions, in p53 wild-type and
p53-deficient non-small-cell lung carcinoma cells. Int J Radiat
Oncol Biol Phys. 80:558–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tchelebi L, Ashamalla H and Graves PR:
Mutant p53 and the response to chemotherapy and radiation. Subcell
Biochem. 85:133–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fuentes-Orrego JM and Sahani DV: Low-dose
CT in clinical diagnostics. Expert Opin Med Diagn. 7:501–510. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Poon DJJ, Tay LM, Ho D, Chua MLK, Chow EK
and Yeo ELL: Improving the therapeutic ratio of radiotherapy
against radioresistant cancers: Leveraging on novel artificial
intelligence-based approaches for drug combination discovery.
Cancer Lett. 511:56–67. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu C, Guo E, Ming J, Sun W, Nie X, Sun L,
Peng S, Luo M, Liu D, Zhang L, et al: Radiation-induced DNMT3B
promotes radioresistance in nasopharyngeal carcinoma through
methylation of p53 and p21. Mol Ther Oncolytics. 17:306–319. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
da Costa Araldi IC, Bordin FPR, Cadoná FC,
Barbisan F, Azzolin VF, Teixeira CF, Baumhardt T, da Cruz IBM,
Duarte MMMF and Bauermann LF: The in vitro radiosensitizer
potential of resveratrol on MCF-7 breast cancer cells. Chem Biol
Interact. 282:85–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fei P and El-Deiry WS: P53 and radiation
responses. Oncogene. 22:5774–5783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gudkov AV and Komarova EA: The role of p53
in determining sensitivity to radiotherapy. Nat Rev Cancer.
3:117–129. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brachman DG, Beckett M, Graves D, Haraf D,
Vokes E and Weichselbaum RR: p53 mutation does not correlate with
radiosensitivity in 24 head and neck cancer cell lines. Cancer Res.
53:3667–3669. 1993.PubMed/NCBI
|
|
42
|
Hinata N, Shirakawa T, Zhang Z, Matsumoto
A, Fujisawa M, Okada H, Kamidono S and Gotoh A: Radiation induces
p53-dependent cell apoptosis in bladder cancer cells with
wild-type-p53 but not in p53-mutated bladder cancer cells. Urol
Res. 31:387–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Williams KJ, Boyle JM, Birch JM, Norton JD
and Scott D: Cell cycle arrest defect in Li-Fraumeni Syndrome: A
mechanism of cancer predisposition? Oncogene. 14:277–282. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ribeiro JC, Barnetson AR, Fisher RJ,
Mameghan H and Russell PJ: Relationship between radiation response
and p53 status in human bladder cancer cells. Int J Radiat Biol.
72:11–20. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Biard DS, Martin M, Rhun YL, Duthu A,
Lefaix JL, May E and May P: Concomitant p53 gene mutation and
increased radiosensitivity in rat lung embryo epithelial cells
during neoplastic development. Cancer Res. 54:3361–3364.
1994.PubMed/NCBI
|
|
46
|
Kawashima K, Mihara K, Usuki H, Shimizu N
and Namba M: Transfected mutant p53 gene increases X-ray-induced
cell killing and mutation in human fibroblasts immortalized with
4-nitroquinoline 1-oxide but does not induce neoplastic
transformation of the cells. Int J Cancer. 61:76–79. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weber KJ and Wenz F: p53, apoptosis and
radiosensitivity-experimental and clinical data. Onkologie.
25:136–141. 2002.PubMed/NCBI
|
|
48
|
Concin N, Zeillinger C, Stimpfel M,
Schiebel I, Tong D, Wolff U, Reiner A, Leodolter S and Zeillinger
R: p53-dependent radioresistance in ovarian carcinoma cell lines.
Cancer Lett. 150:191–199. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheng G, Kong D, Hou X, Liang B, He M,
Liang N, Ma S and Liu X: The tumor suppressor, p53, contributes to
radiosensitivity of lung cancer cells by regulating autophagy and
apoptosis. Cancer Biother Radiopharm. 28:153–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pirollo KF, Hao Z, Rait A, Jang YJ, Fee WE
Jr, Ryan P, Chiang Y and Chang EH: p53 mediated sensitization of
squamous cell carcinoma of the head and neck to radiotherapy.
Oncogene. 14:1735–1746. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gallardo D, Drazan KE and McBride WH:
Adenovirus-based transfer of wild-type p53 gene increases ovarian
tumor radiosensitivity. Cancer Res. 56:4891–4893. 1996.PubMed/NCBI
|
|
52
|
Servomaa K, Kiuru A, Grénman R,
Pekkola-Heino K, Pulkkinen JO and Rytömaa T: p53 mutations
associated with increased sensitivity to ionizing radiation in
human head and neck cancer cell lines. Cell Prolif. 29:219–230.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lowe SW, Bodis S, McClatchey A, Remington
L, Ruley HE, Fisher DE, Housman DE and Jacks T: p53 status and the
efficacy of cancer therapy in vivo. Science. 266:807–810. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Merritt AJ, Potten CS, Kemp CJ, Hickman
JA, Balmain A, Lane DP and Hall PA: The role of p53 in spontaneous
and radiation-induced apoptosis in the gastrointestinal tract of
normal and p53-deficient mice. Cancer Res. 54:614–617.
1994.PubMed/NCBI
|
|
55
|
Matsui Y, Tsuchida Y and Keng PC: Effects
of p53 mutations on cellular sensitivity to ionizing radiation. Am
J Clin Oncol. 24:486–490. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shi Q, Sutariya V, Varghese Gupta S and
Bhatia D: GADD45α-targeted suicide gene therapy driven by synthetic
CArG promoter E9NS sensitizes NSCLC cells to cisplatin,
resveratrol, and radiation regardless of p53 status. Onco Targets
Ther. 12:3161–3170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cuneo KC, Morgan MA, Davis MA, Parcels LA,
Parcels J, Karnak D, Ryan C, Liu N, Maybaum J and Lawrence TS: Wee1
kinase inhibitor AZD1775 radiosensitizes hepatocellular carcinoma
regardless of TP53 mutational status through induction of
replication stress. Int J Radiat Oncol Biol Phys. 95:782–790. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tada M, Matsumoto R, Iggo RD, Onimaru R,
Shirato H, Sawamura Y and Shinohe Y: Selective sensitivity to
radiation of cerebral glioblastomas harboring p53 mutations. Cancer
Res. 58:1793–1797. 1998.PubMed/NCBI
|
|
59
|
Koch WM, Brennan JA, Zahurak M, Goodman
SN, Westra WH, Schwab D, Yoo GH, Lee DJ, Forastiere AA and
Sidransky D: p53 mutation and locoregional treatment failure in
head and neck squamous cell carcinoma. J Natl Cancer Inst.
88:1580–1586. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mello SS and Attardi LD: Not all p53
gain-of-function mutants are created equal. Cell Death Differ.
20:855–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Menendez D, Inga A and Resnick MA: The
biological impact of the human master regulator p53 can be altered
by mutations that change the spectrum and expression of its target
genes. Mol Cell Biol. 26:2297–2308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Okaichi K, Wang LH, Ihara M and Okumura Y:
Sensitivity to ionizing radiation in Saos-2 cells transfected with
mutant p53 genes depends on the mutation position. J Radiat Res.
39:111–118. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Okaichi K, Nose K, Kotake T, Izumi N and
Kudo T: Phosphorylation of p53 modifies sensitivity to ionizing
radiation. Anticancer Res. 31:2255–2258. 2011.PubMed/NCBI
|
|
64
|
Okaichi K, Ide-Kanematsu M, Izumi N,
Morita N, Okumura Y and Ihara M: Variations in sensitivity to
ionizing radiation in relation to p53 mutation point. Anticancer
Res. 28:2687–2690. 2008.PubMed/NCBI
|
|
65
|
Mazzatti DJ, Lee YJ, Helt CE, O'Reilly MA
and Keng PC: p53 modulates radiation sensitivity independent of p21
transcriptional activation. Am J Clin Oncol. 28:43–50. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Aubrey BJ, Kelly GL, Janic A, Herold MJ
and Strasser A: How does p53 induce apoptosis and how does this
relate to p53-mediated tumour suppression? Cell Death Differ.
25:104–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Brosh R and Rotter V: When mutants gain
new powers: News from the mutant p53 field. Nat Rev Cancer.
9:701–713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Stein Y, Rotter V and Aloni-Grinstein R:
Gain-of-function mutant p53: All the roads lead to tumorigenesis.
Int J Mol Sci. 20:61972019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bellazzo A, Sicari D, Valentino E, Del Sal
G and Collavin L: Complexes formed by mutant p53 and their roles in
breast cancer. Breast Cancer (Dove Med Press). 10:101–112.
2018.PubMed/NCBI
|
|
70
|
Zhang C, Liu J, Xu D, Zhang T, Hu W and
Feng Z: Gain-of-function mutant p53 in cancer progression and
therapy. J Mol Cell Biol. 12:674–687. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang X, Zhang Y, Tang Y, Butler N, Kim J,
Guessous F, Schiff D, Mandell J and Abounader R: A novel
PTEN/mutant p53/c-Myc/Bcl-XL axis mediates context-dependent
oncogenic effects of PTEN with implications for cancer prognosis
and therapy. Neoplasia. 15:952–965. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ganci F, Pulito C, Valsoni S, Sacconi A,
Turco C, Vahabi M, Manciocco V, Mazza EMC, Meens J, Karamboulas C,
et al: PI3K inhibitors curtail MYC-dependent mutant p53
gain-of-function in head and neck squamous cell carcinoma. Clin
Cancer Res. 26:2956–2971. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kim SH, Lee WH, Seong D, An JH, Je HU, Nam
HY, Kim SY, Kim SW and Han MW: The role of CIP2A as a therapeutic
target of rapamycin in radioresistant head and neck cancer with
TP53 mutation. Head Neck. 41:3362–3371. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Matsumoto H, Hayashi S, Hatashita M,
Ohnishi K, Shioura H, Ohtsubo T, Kitai R, Ohnishi T and Kano E:
Induction of radioresistance by a nitric oxide-mediated bystander
effect. Radiat Res. 155:387–396. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bajan S and Hutvagner G: RNA-based
therapeutics: From antisense oligonucleotides to miRNAs. Cells.
9:1372020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bajan S and Hutvagner G: Regulation of
miRNA processing and miRNA mediated gene repression in cancer.
Microrna. 3:10–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Adams BD, Parsons C, Walker L, Zhang WC
and Slack FJ: Targeting noncoding RNAs in disease. J Clin Invest.
127:761–771. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hermeking H: p53 enters the microRNA
world. Cancer Cell. 12:414–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Balça-Silva J, Sousa Neves S, Gonçalves
AC, Abrantes AM, Casalta-Lopes J, Botelho MF, Sarmento-Ribeiro AB
and Silva HC: Effect of miR-34b overexpression on the
radiosensitivity of non-small cell lung cancer cell lines.
Anticancer Res. 32:1603–1609. 2012.
|
|
80
|
Liu Y, Xing R, Zhang X, Dong W, Zhang J,
Yan Z, Li W, Cui J and Lu Y: miR-375 targets the p53 gene to
regulate cellular response to ionizing radiation and etoposide in
gastric cancer cells. DNA Repair (Amst). 12:741–750. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
He J, Feng X, Hua J, Wei L, Lu Z, Wei W,
Cai H, Wang B, Shi W, Ding N, et al: miR-300 regulates cellular
radiosensitivity through targeting p53 and apaf1 in human lung
cancer cells. Cell Cycle. 16:1943–1953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xu R, Li H, Wu S, Qu J, Yuan H, Zhou Y and
Lu Q: MicroRNA-1246 regulates the radio-sensitizing effect of
curcumin in bladder cancer cells via activating P53. Int Urol
Nephrol. 51:1771–1779. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ye C, Sun NX, Ma Y, Zhao Q, Zhang Q, Xu C,
Wang SB, Sun SH, Wang F and Li W: MicroRNA-145 contributes to
enhancing radiosensitivity of cervical cancer cells. FEBS Lett.
589:702–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Song L, Liu S, Zeng S, Zhang L and Li X:
miR-375 modulates radiosensitivity of HR-HPV-positive cervical
cancer cells by targeting UBE3A through the p53 pathway. Med Sci
Monit. 21:2210–2217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kumar A and Chandna S: Evidence for a
radiation-responsive ‘p53 gateway’ contributing significantly to
the radioresistance of lepidopteran insect cells. Sci Rep. 8:22018.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Metheetrairut C and Slack FJ: MicroRNAs in
the ionizing radiation response and in radiotherapy. Curr Opin
Genet Dev. 23:12–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Saleh AD, Savage JE, Cao L, Soule BP, Ly
D, DeGraff W, Harris CC, Mitchell JB and Simone NL: Cellular stress
induced alterations in microRNA let-7a and let-7b expression are
dependent on p53. PLoS One. 6:e244292011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang Y, He Q, Hu Z, Feng Y, Fan L, Tang
Z, Yuan J, Shan W, Li C, Hu X, et al: Long noncoding RNA LINP1
regulates repair of DNA double-strand breaks in triple-negative
breast cancer. Nat Struct Mol Biol. 23:522–530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang X, Liu H, Shi L, Yu X, Gu Y and Sun
X: LINP1 facilitates DNA damage repair through non-homologous end
joining (NHEJ) pathway and subsequently decreases the sensitivity
of cervical cancer cells to ionizing radiation. Cell Cycle.
17:439–447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yang P, Yang Y, An W, Xu J, Zhang G, Jie J
and Zhang Q: The long noncoding RNA-ROR promotes the resistance of
radiotherapy for human colorectal cancer cells by targeting the
p53/miR-145 pathway. J Gastroenterol Hepatol. 32:837–845. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang M, Wang L, He X, Zhang J, Zhu Z,
Zhang M and Li X: lncRNA CCAT2 promotes radiotherapy resistance for
human esophageal carcinoma cells via the miR-145/p70S6K1 and p53
pathway. Int J Oncol. 56:327–336. 2020.PubMed/NCBI
|
|
94
|
Beer L, Nemec L, Wagner T, Ristl R,
Altenburger LM, Ankersmit HJ and Mildner M: Ionizing radiation
regulates long non-coding RNAs in human peripheral blood
mononuclear cells. J Radiat Res. 58:201–209. 2017. View Article : Google Scholar : PubMed/NCBI
|